Abstract
Resistance exercise is a potent stimulator of muscle protein synthesis and muscle cell growth, with the increase in protein synthesis being detected within 2–3 h post-exercise and remaining elevated for up to 48 h. However, during exercise, muscle protein synthesis is inhibited. An increase in AMP-activated protein kinase (AMPK) activity has recently been shown to decrease mammalian target of rapamycin (mTOR) signalling to key regulators of translation initiation. We hypothesized that the cellular mechanism for the inhibition of muscle protein synthesis during an acute bout of resistance exercise in humans would be associated with an activation of AMPK and an inhibition of downstream components of the mTOR pathway (4E-BP1 and S6K1). We studied 11 subjects (seven men, four women) before, during, and for 2 h following a bout of resistance exercise. Muscle biopsy specimens were collected at each time point from the vastus lateralis. We utilized immunoprecipitation and immunoblotting methods to measure muscle AMPKα2 activity, and mTOR-associated upstream and downstream signalling proteins, and stable isotope techniques to measure muscle fractional protein synthetic rate (FSR). AMPKα20 activity (pmol min−1 (mg protein)−1) at baseline was 1.7 ± 0.3, increased immediately post-exercise (3.0 ± 0.6), and remained elevated at 1 h post-exercise (P < 0.05). Muscle FSR decreased during exercise and was significantly increased at 1 and 2 h post-exercise (P < 0.05). Phosphorylation of 4E-BP1 at Thr37/46 was significantly reduced immediately post-exercise (P < 0.05). We conclude that AMPK activation and a reduced phosphorylation of 4E-BP1 may contribute to the inhibition of muscle protein synthesis during resistance exercise. However, by 1–2 h post-exercise, muscle protein synthesis increased in association with an activation of protein kinase B, mTOR, S6K1 and eEF2.
AMP-activated protein kinase (AMPK) is often referred to as the fuel gauge of the cell because of its ability to ‘sense’ cellular energy status (Hardie & Carling, 1997; Winder & Hardie, 1999). In particular, when activated, AMPK is responsible for inhibiting anabolic processes that require ATP (e.g. fatty acid synthesis and gluconeogenesis), and for stimulating catabolic processes that generate ATP (e.g. fatty acid oxidation) (Winder & Hardie, 1999; Hardie, 2005). For example, early studies have shown that a change in cellular energy status within rodent skeletal muscle cells during exercise increases AMPK activity, resulting in a stimulation of fatty acid oxidation during exercise (Winder & Hardie, 1996; Rasmussen & Winder, 1997) and the post-exercise recovery period (Rasmussen et al. 1998). AMPK activity is also increased during aerobic exercise in humans (Fujii et al. 2000; Stephens et al. 2002; Yu et al. 2003; Wojtaszewski et al. 2003; Roepstorff et al. 2005; Coffey et al. 2006), although the regulation of fuel selection by AMPK during aerobic exercise in endurance trained subjects is uncertain (McConell et al. 2005).
However, the ATP-consuming process of muscle protein synthesis has been much less studied. Muscle contractions in rodents result in a decrease in muscle protein synthesis (Dohm et al. 1980; Bylund-Fellenius et al. 1984; Davis & Karl, 1986). Prolonged aerobic exercise in humans also results in a reduction in whole-body protein synthesis (Rennie et al. 1981) and a trend for muscle protein synthesis to be decreased (Carraro et al. 1990). The mechanisms responsible for the decrease in muscle protein synthesis during aerobic exercise are not known; however, recent work provides evidence that one mechanism may be the inhibition of the elongation phase of translation, since the phosphorylation of eukaryotic elongation factor 2 (eEF2) is rapidly induced by aerobic exercise (Rose et al. 2005). However, we and others have shown that AMPK activity is increased to a greater extent with an increase in exercise intensity (Rasmussen et al. 1998; Chen et al. 2003) and an early report suggests that an altered energy state in rodent muscle during muscle contractions may be responsible for the reduced protein synthesis (Bylund-Fellenius et al. 1984). In addition, more recent work has shown that artificially activating AMPK in rodent muscle suppresses muscle protein synthesis by inhibiting the mammalian target of rapamycin (mTOR) signalling pathway (Bolster et al. 2002). Specifically, AMPK activation inhibits translation initiation by phosphorylating a protein known as tuberin or tuberous sclerosis complex (TSC2), an upstream regulator and inhibitor of mTOR activity (Inoki et al. 2003), as well as phosphorylating and inactivating mTOR directly (Cheng et al. 2004). However, AMPK can also inhibit translation elongation by directly phosphorylating eEF2 kinase (Horman et al. 2002; Browne et al. 2004).
Muscle protein synthesis during resistance exercise also appears to be suppressed in human muscle (Rasmussen et al. 2000; Durham et al. 2004). Resistance exercise training causes muscle cell hypertrophy, and therefore the signalling pathways regulating muscle cell growth and protein synthesis are expected to be different from the adaptations associated with aerobic exercise training (Rasmussen & Phillips, 2003; Atherton et al. 2005). In fact, an acute bout of resistance exercise results in an increase in muscle protein synthesis within 2–3 h, and remains elevated for 24–48 h post-exercise (Biolo et al. 1995; MacDougall et al. 1995; Phillips et al. 1997). The mechanisms responsible for the delayed activation of muscle protein synthesis during the early post-exercise recovery period following resistance exercise are not known; however, recent work in rodent muscle suggests that activation of the mTOR pathway, and hence translation initiation, become gradually activated after resistance exercise (Baar & Esser, 1999; Bolster et al. 2003).
Therefore, we hypothesized that the cellular mechanism for the decrease in muscle protein synthesis during an acute bout of resistance exercise in humans would be associated with an activation of AMPK and an inhibition of the downstream components of the mTOR signalling pathway (4E-BP1 Thr37/46 and S6K1 Thr389), which are key proteins involved in the regulation of translation initiation.
Methods
Subjects
We studied 11 young subjects (seven men and four women). All subjects were healthy and physically active, but were not currently engaged in an exercise training programme. All subjects gave informed written consent before participating in the study, which was approved by the Institutional Review Board of the University of Texas Medical Branch (which is in compliance with the Declaration of Helsinki). Screening of subjects were performed with clinical history, physical examination, and laboratory tests, including complete blood count with differential, liver and kidney function tests, coagulation profile, fasting blood glucose and oral glucose tolerance test (OGTT), hepatitis B and C screening, HIV test, thyroid stimulating hormone, lipid profile, pregnancy test in women, urinalysis, drug screening and ECG. All women were not taking oral contraceptives and were studied during their follicular phase. The subjects' characteristics are summarized in Table 1.
Table 1.
Physical characteristics of the subjects
| Characteristic | Mean ±s.e.m. (n = 11) |
|---|---|
| Age (years) | 27 ± 2 |
| Height (cm) | 168 ± 3 |
| Weight (kg) | 71 ± 5 |
| Body mass index (kg m−2) | 25.3 ± 1.3 |
| Lean body mass (kg) | 53 ± 3 |
| Body fat (%) | 23 ± 2 |
| Leg lean mass (kg) | 8.9 ± 0.6 |
Study design
On two separate occasions (>5 days apart), and more than 5 days prior to conducting the study, each subject was tested for muscle strength by measuring their 1 repetition maximum (1RM) on a leg extension machine (Cybex-VR2; Cybex International, Inc., Medway, MA, USA) located within the Exercise Laboratory of the General Clinical Research Center (GCRC). The higher of the two 1RM values obtained was used to determine the starting weight (70% of 1RM) for the resistance exercise portion of our study. On the second visit a dual-energy X-ray absorptiometry (DEXA) scan (Hologic QDR 4500 W, Bedford, MA, USA) was performed to measure body composition and lean mass, and a pregnancy test was repeated in women.
Each subject was admitted to the GCRC of the University of Texas Medical Branch the day prior to the exercise study. The subjects were then fed a standard dinner, and a snack was given at 22.00 h. The subjects were studied following an overnight fast under basal conditions and refrained from exercise for 24 h prior to study participation. On the morning of the study, polyethylene catheters were inserted into a forearm vein for tracer infusion, in the contralateral hand vein which was heated for arterialized blood sampling, and in the femoral artery and vein (retrograde placement) of the leg for blood sampling. The femoral lines were placed in the same leg from which muscle biopsy samples were obtained. The arterial catheter was also used for the infusion of indocyanine green (ICG; Akorn, Inc., Buffalo Grove, IL, USA) to determine blood flow.
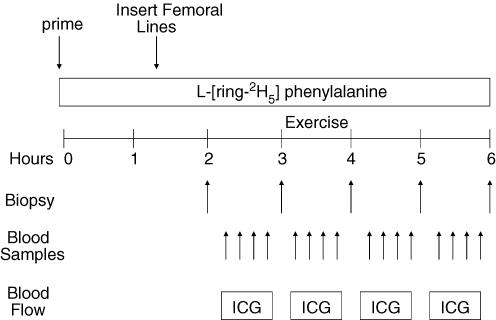
After drawing a background blood sample, a primed continuous infusion of l-[ring-2H5]phenylalanine (Cambridge Isotope Laboratories, Andover, MA, USA) was begun (time, 0 h at 08.00 h) and maintained at a constant rate until the end of the experiment (Fig. 1). The priming dose for the labelled phenylalanine was 2 μmol kg−1 and the infusion rates was 0.05 μmol kg−1 min−1. All subjects were studied at the exact same time (i.e. between 08.00 and 14.00 h).
Figure 1. Schematic displaying the study design used to measure the effect of resistance exercise on the regulation of muscle protein synthesis in human subjects.
The study design consisted of a basal period (hours 2–3), an exercise period (hours 3–4), and two post-exercise periods (hours 4–5, and 5–6). ICG, indocyanine green was infused to measure blood flow in each of the periods. Blood samples were collected to measure blood glucose uptake, lactate concentration and pH. Muscle biopsy samples were used to measure muscle protein synthesis, AMP-activated protein kinase (AMPK) enzyme activity, and signalling pathways involved in translation initiation and elongation.
Subjects were studied during four time periods: 1st period (basal), 2nd period (during exercise), 3rd period (the first hour post-exercise), and 4th period (the second hour post-exercise). The 2nd period was performed in the exercise laboratory within the GCRC, and the 1st, 3rd and 4th periods were all conducted in the special procedures room, also within the GCRC.
Marking the beginning of the basal period, and 2 h following the initiation of the tracer infusions, the first muscle biopsy sample was obtained from the lateral portion of the vastus lateralis of the leg, with the biopsy site between 15 and 25 cm from the mid-patella. The biopsy was performed using a 5 mm Bergström biopsy needle, under sterile procedure and local anaesthesia (1% lidocaine (lignocaine)). Once harvested, the muscle tissue was immediately blotted, and frozen in liquid nitrogen (within seconds), and stored at −80°C until analysis. Immediately after the first biopsy, a continuous infusion of ICG was started in the femoral artery (0.5 mg min−1) and maintained for 50 min. Ten minutes after ICG infusion was started, blood samples were drawn four times, at 10 min intervals, from the femoral vein and the arterialized hand vein to measure ICG concentration (Fig. 1). In addition to the blood obtained for ICG measurement, blood samples were also taken from the femoral artery and vein, and from the arterialized hand vein to measure blood pH, and glucose and lactate concentrations. At the end of baseline, a second biopsy sample was obtained; however, the biopsy needle was inclined at a different angle so that the second biopsy sample was taken approximately 2 in (∼5 cm) away from the first sample.
Following the second biopsy, the subject was transported to the exercise laboratory within the GCRC for the entire 2nd period. There the subjects performed 10 sets of 10 repetitions of leg extension exercises on a Cybex leg extension machine set to 70% of their 1RM. All subjects started out at 70% of 1RM; however, for a few of the subjects, the weight was slightly reduced (60–65% of 1RM) in order to achieve 10 repetitions per set. The rest period between sets was 3 min, except during blood collection which required a few more minutes. As during the basal period, ICG was continually infused during exercise in order to accurately measure leg blood flow. Blood samples were again drawn for blood pH, glucose and lactate concentrations immediately after the 3rd, 6th, 8th and 10th sets. Following the last blood collection, subjects performed one final set of 10 repetitions and a third muscle biopsy sample was obtained within seconds of completing the last muscle contraction. The subjects were then transported back to the special procedures room of the GCRC for the duration of the study.
During the 3rd period (the first hour post-exercise), ICG was again infused continuously (as during the 1st and 2nd periods) to measure leg blood flow, and blood was drawn for the measurement of blood gas, electrolytes, and glucose and lactate concentrations. Samples were obtained every 10 min (as during the 1st and 2nd periods). At the end of the first hour post-exercise, a fourth muscle biopsy sample was obtained through a new incision site, approximately 5 cm from the first incision.
During the 4th period (second hour post-exercise), blood samples were collected in the same manner as during the previous periods. At the end of the second hour post-exercise, a final muscle biopsy sample was collected, as described above, from the second incision; however, the biopsy needle was inclined at a different angle again so that the biopsy sample was taken approximately ∼5 cm away from the prior biopsy sample. Each biopsy sample was taken a mean of 73 ± 7 min apart.
Blood flow, pH, glucose uptake and lactate concentration
Serum ICG concentration for the determination of leg blood flow was measured spectrophotometrically (Beckman Coulter, Fullerton, CA, USA) at a λ value of 805 nm (Jorfeldt & Wahren, 1971). Plasma glucose and lactate concentration was measured using an automated glucose and lactate analyser (YSI, Yellow Springs, OH, USA). Blood pH was measured at the UTMB core laboratory using standard procedure. Leg glucose utilization was calculated as net glucose uptake across the leg: leg glucose uptake = (CA−CV) blood flow (BF), where CA and CV are the blood glucose concentrations in the femoral artery and vein, respectively, and it was expressed as micromoles of glucose utilized per minute per kilogram of fat free mass (FFM) of the leg (μmol min−1 (kg leg FFM)−1).
AMPK activity assay
AMPKα1 and α2 activities were measured using ∼70 mg per biopsy sample per subject of muscle tissue. After homogenization, total protein concentrations were determined using the Bradford assay (Bio-Rad Smartspec plus spectrophotometer). The immunoprecipitation method and the enzyme activity measurements were completed as previously described (Hardie et al. 2000). Slight adaptations to this method included performing the immunoprecipitation overnight and the AMPK assay on resuspended immunoprecipitates in the medium previously described by Winder & Hardie (1996a). Tissue samples were homogenized (1:9, w/v) in a buffer containing: 50 mm Tris-HCl, 250 mm mannitol, 50 mm NaF, 5 mm sodium pyrophosphate, 1 mm EDTA, 1 mm EGTA, 1% Triton X-100, pH 7.4, 1 mm DTT, 1 mm benzamidine, 0.1 mm PMSF and 5 μg ml−1 soybean trypsin inhibitor (SBTI). DTT, benzamidine, PMSF, and SBTI were added to the buffer immediately prior to use. Supernatant was collected after centrifugation at 3500 g for 10 min at 4°C. AMPKα1 and α2 isoform specific activities were measured in immunoprecipitates. Immunoprecipitation was achieved by incubating 40 μl of supernatant with protein-G-Sepharose beads (Sigma, St Louis, MO, USA) complexed with antibodies against either the AMPKα1 peptide corresponding to amino acids 376–392 (ARHTLDELNPQKSKHQG) or the AMPKα2 peptide corresponding to amino acids 353–367 (DDSAMHIPPGLKPHP) (Upstate Cell Signalling Solutions, Charlottesville, VA, USA) overnight on a roller mixer at 4°C. The suspension was washed twice in a buffer containing 50 mm Tris-HCl, 1 m NaCl, 50 mm NaF, 5 mm sodium pyrophosphate, 1 mm EDTA, 1 mm EGTA, 1 mm DTT, 1 mm benzamidine, 0.1 mm PMSF, 5 μg ml−1 soybean trypsin inhibitor, pH 7.4, and washed once in a buffer containing 62.5 mm Hepes, 62.5 mm NaCl, 62.5 mm NaF, 6.25 mm sodium pyrophosphate, 1.25 mm EDTA, 1.25 mm EGTA, 1 mm DTT, 1 mm benzamidine, 0.1 mm PMSF, 1 μg ml−1 SBTI. Immunoprecipitated AMPKα1 and α2 were then suspended in 30 μl of Hepes-brij buffer containing 25 mm Hepes, 0.02% brij, 1 mm DTT, pH 7.0. AMPKα1 and α2 activities were measured by adding 15 μl of working assay cocktail containing 40 mm Hepes, 8% glycerol, 80 mm NaCl, 0.8 mm EDTA, 0.8 mm MgCl2, pH 7.0, 0.2 mm AMP, 0.2 mm ATP, 0.8 mm DTT, 0.2 mm SAMS peptide (Zinsser Analytic, Maidenhead, Berkshire, UK) (and 10 mCi ml−1[γ-32P]ATP; MP Biomedicals, Aurora, OH, USA). The mixture was then incubated for 10 min in a water bath (30°C and 60 r.p.m. agitation) to facilitate mixing. At the end of the incubation period, a 15 μl aliquot was spotted onto a 1 cm2 P81 Whatman filter paper (Whatman International Ltd, Maidstone, UK) and immediately placed in 200 ml of 1% phosphoric acid to stop the kinase reaction. Each filter paper was washed six times in 1% phosphoric acid for 5 min. After air drying, they were added to 3 ml of Ecolite liquid scintillation fluid (MP Biomedicals) and counted for 10 min in a LS 6500 multi-purpose scintillation counter (Beckman Coulter). Activity is expressed as picomoles of phosphate incorporated per milligram of muscle protein subjected to immunoprecipitation per minute (pmol mg−1 min−1).
Muscle fractional synthetic rate
Muscle tissue samples were ground, and intracellular free amino acids and muscle proteins were extracted as previously described (Wolfe & Chinkes, 2005). Muscle intracellular free concentration and enrichment of phenylalanine and leucine were determined by gas chromatography-mass spectrometry (GCMS; 6890 Plus GC, 5973N MSD, 7683 autosampler; Agilent Technologies, Palo Alto, CA, USA) using appropriate internal standards (Wolfe & Chinkes, 2005). Mixed muscle protein-bound phenylalanine enrichment was analysed by GCMS, after protein hydrolysis and amino acid extraction (Wolfe & Chinkes, 2005), using the external standard curve approach (Calder et al. 1992). We calculated the fractional synthetic rate of mixed muscle proteins (FSR) by measuring the incorporation rate of the phenylalanine tracer into the proteins (ΔEp/t) and using the precursor-product model to calculate the synthesis rate:
where ΔEp is the increment in protein-bound phenylalanine enrichment between two sequential biopsy samples, t is the time between the two sequential biopsies, and EM(1) and EM(2) are the phenylalanine enrichments in the free intracellular pool in the two sequential biopsy samples. Our mean percentage change in muscle protein-bound phenylalanine enrichments (between hourly biopsy samples) ranged between 27 and 63%, indicating that we were able to sufficiently measure the change in enrichment between biopsy samples, and this enabled us to calculate valid fractional synthetic rates. Data are expressed as percentages per hour.
SDS PAGE and immunoblotting
Aliquots from homogenates (described above) were boiled at 100°C for 3 min in 2× sample buffer (SB) containing 125 mm Tris, pH 6.8, 25% glycerol, 2.5% SDS, 2.5%β-mercaptoethanol, and 0.002% bromophenol blue; except for aliquots used to detect 4E-BP1 which were initially boiled at 100°C for 10 min, spun for 30 min at 6000 g before combining the supernatants with 2× SB. Total protein (100 μg per sample) was loaded per lane in duplicate and separated by SDS-PAGE. For mTOR separation, 7.5% gels were run for 90 min at 150 V; protein kinase B (PKB)/Akt separation was done using a 7.5% gel run for 45 min at 150 V; TSC2 separation was performed using 7.5% gels run for 90 min at 100 V; p70 S6K1 separation was achieved using 7.5% gels run for 60 min at 100 V; 4E-BP1 separation was accomplished with 15% gels run overnight; and eEF2 was separated using a 7.5% gel run for 80–90 min at 100 V. Following SDS PAGE, proteins were transferred to polyvinylidene difluoride membranes (PVDF) (Hybond-P; Amersham Biosciences, Piscataway, NJ, USA) at 50 V for 1 h. Once transferred, PVDF membranes were placed in blocking buffer (5% non-fat dry milk (NFDM) in TBST (Tris-buffered saline and 0.1% Tween-20)) for 1 h. Blots were then serially washed two times in deionized water and two more times in TBST, and incubated with primary antibody in 5% NFDM in TBST (except 4E-BP1 and TSC2, which were incubated in 5% bovine serum albumin) overnight at 4°C with constant agitation. The next morning, the blots were washed in TBST twice, and incubated with secondary antibody for 1 h in 5% NFDM in TBST at room temperature, with constant agitation. After secondary incubation, the blots were washed for 15 min, and then serially washed (3 × 5 min) with TBST. Blots were then incubated for 5 min with enhanced chemiluminescence reagent (ECL plus Western Blotting Detection System; Amersham Biosciences) to detect horseradish peroxidase activity. Optical density measurements were obtained with a CCD camera mounted in a ChemiDoc XRS imaging system (Bio-Rad, Hercules, CA, USA). Once the appropriate image was captured, densitometric analysis was performed using Quantity One 1-D analysis Software (Version 4.5.2; Bio-Rad). Preliminary experiments were performed to assess if protein abundance changed over the 5 h of the experiment. We found that protein abundance did not change over the short time frame of the study; therefore, all data were expressed as the change in phosphorylation in arbitrary units.
Antibodies
The primary antibodies used were all purchased from Cell Signaling (Beverly, MA, USA): phospho-mTOR (Ser2448; 1:1000), phospho-p70 S6K1 (Thr389; 1 : 500), phospho-Akt (Ser473; 1 : 500), phospho-Tuberin/TSC2 (Thr1462; 1:500), phospho-4E-BP1 (Thr37/46; 1 : 500), and phospho-eEF2 (Thr56; 1 : 1000). Anti-rabbit IgG horseradish-peroxidase-conjugated secondary antibody was purchased from Amersham Bioscience (1 : 2000).
Statistical analysis
All values are expressed as means ±s.e.m. Comparisons were performed using analysis of variance with repeated measures, the effects being subject and time (basal, exercise, 1 h post, and 2 h post). Post hoc testing was performed using the Dunnett's test for multiple comparisons; however, if a test of normality or equal variance failed, then analysis of variance on ranks followed by a Dunn's post hoc multiple comparisons test was performed. Significance was set at P < 0.05.
Results
Blood flow
The blood flow results are shown in Table 2. Blood flow was significantly elevated during exercise (P < 0.05). Blood flow returned to baseline values during the first and second hours post-exercise.
Table 2.
Blood flow, glucose uptake across the leg, plasma lactate concentration, blood pH, and muscle intracellular phenylalanine and leucine concentrations at baseline, during resistance exercise, and 1 h and 2 h post-exercise
| Basal | Exercise | 1 h Post | 2 h Post | |
|---|---|---|---|---|
| Blood flow (ml min−1 (100 ml leg vol)−1) | 3.55 ± 0.4 | 13.10 ± 1.6* | 4.60 ± 0.5 | 3.92 ± 0.5 |
| Leg glucose uptake (μmol min−1 (kg leg FFM)−1) | 3.5 ± 0.7 | 24.1 ± 3.2* | 8.2 ± 2.9 | 9.3 ± 2.7 |
| Lactate (mmol l−1) | ||||
| Femoral artery | 0.7 ± 0.1 | 9.7 ± 0.6* | 2.0 ± 0.2* | 0.8 ± 0.1 |
| Femoral vein | 0.8 ± 0.1 | 11.5 ± 0.7* | 2.3 ± 0.2* | 1.0 ± 0.1 |
| Blood pH | ||||
| Femoral artery | 7.42 ± 0.01 | 7.30 ± 0.01* | 7.40 ± 0.01 | 7.42 ± 0.01 |
| Femoral vein | 7.38 ± 0.01 | 7.17 ± 0.01* | 7.36 ± 0.01 | 7.37 ± 0.01 |
| Muscle intracellular (μmol l−1) | ||||
| Phenylalanine | 77 ± 5 | 86 ± 5 | 72 ± 5 | 64 ± 3* |
| Leucine | 184 ± 13 | 239 ± 28* | 149 ± 18 | 132 ± 11* |
Values are means ±s.e.m. (n = 11) and represent the mean of four blood samples taken ∼10 min apart.
P < 0.05 versus baseline.
Glucose uptake, lactate concentration, and blood pH
Glucose uptake was significantly elevated during exercise (P < 0.05; Table 2). Glucose uptake during the first and second hour post-exercise periods were not different from baseline. Lactate in the femoral vein and artery was significantly increased during the exercise period and remained elevated for 1 h post-exercise (P < 0.05). During the second hour post-exercise, femoral arterial and venous lactate concentrations returned to values not different from baseline (P > 0.05). Blood pH was significantly reduced from basal during exercise (P < 0.05), and returned to basal values at one and two hours post-exercise (Table 2).
Muscle amino acid intracellular concentrations
Phenylalanine concentration within the muscle free pool was unchanged immediately following exercise, however, by 2 h post-exercise, muscle free phenylalanine concentrations decreased below basal values (P < 0.05; Table 2).
Leucine concentration increased by 30% within the muscle free pool immediately post-exercise (P < 0.05), and eventually fell to a concentration below basal values at 2 h post-exercise (P < 0.05; Table 2).
Muscle protein synthesis
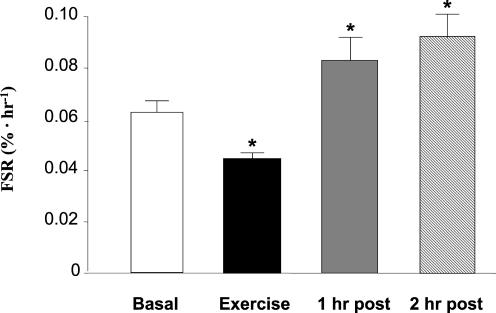
Muscle protein fractional synthetic rate (FSR) decreased immediately following resistance exercise (P < 0.05; Fig. 2). FSR was significantly increased (as compared with basal) at both 1 and 2 h post-exercise (P < 0.05).
Figure 2. Muscle protein synthesis as expressed by the mixed muscle fractional synthetic rate (FSR) before, during, and after a bout of resistance exercise.
Data are expressed as means ±s.e.m., n = 11. *Significantly different from basal (P < 0.05).
AMPKα2 activity
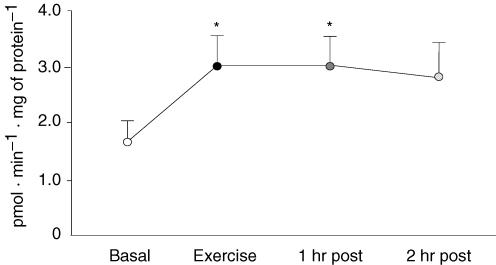
AMPKα2 activity was significantly increased by 75% immediately following resistance exercise (P < 0.05; Fig. 3). AMPKα2 activity remained significantly elevated at 1 h post-exercise (P < 0.05), and became slightly non-significant at 2 h post-exercise (P > 0.05). AMPKα1 activity was unchanged following exercise (data not shown).
Figure 3. Muscle AMPKα2 activity before, during and after a bout of resistance exercise.
Data are expressed as means ±s.e.m., n = 11. *Significantly different from basal (P < 0.05).
TSC2 and PKB: upstream regulators of mTOR signalling
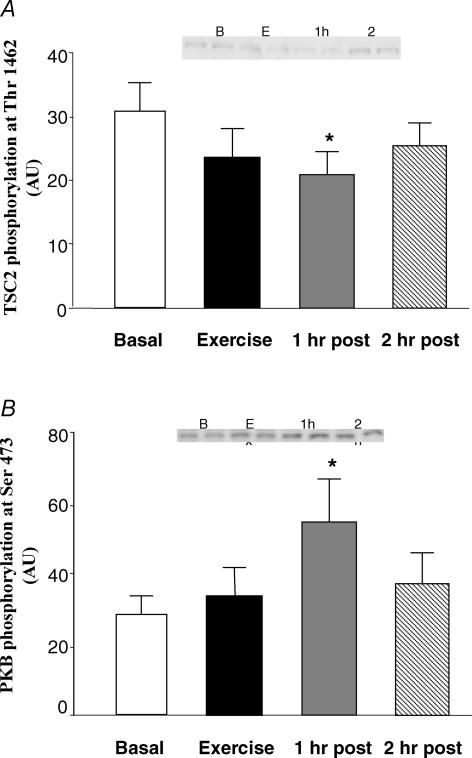
The phosphorylation of TSC2 at Thr1462 was significantly reduced at 1 h post-exercise (P < 0.05; Fig. 4A), but did not quite reach significance immediately post-exercise. Phosphorylation status at 2 h post-exercise was not different from basal.
Figure 4. Phosphorylation of TSC2 and PKB: upstream regulators of mTOR signalling before, during and after a bout of resistance exercise.
Data are expressed as means ±s.e.m.*Significantly different from basal (P < 0.05). Inset shows duplicate samples for each time point.
The phosphorylation of PKB at Ser473 was unchanged immediately post-exercise, and was significantly elevated at 1 h post-exercise (P < 0.05; Fig. 4B). PKB Ser473 phosphorylation at 2 h post-exercise also tended to be elevated above baseline, but did not reach significance (P > 0.05).
mTOR, and downstream indicators of mTOR signalling (4E-BP1 and S6K1)
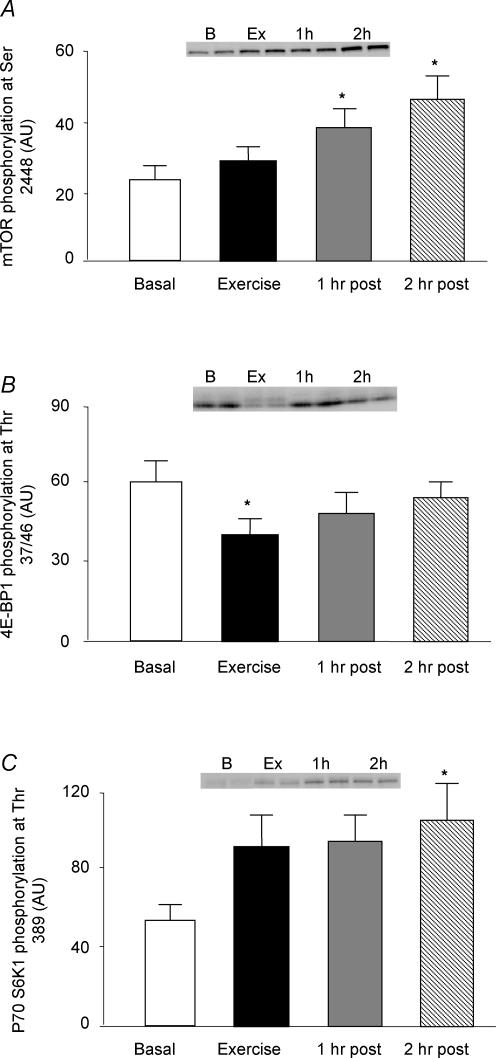
The phosphorylation of mTOR at Ser2448 was unchanged immediately following exercise, and was significantly increased at 1 and 2 h post-exercise, respectively (P < 0.05; Fig. 5A).
Figure 5. Phosphorylation of mTOR and downstream indicators of mTOR signalling (4E-BP1 and S6K1) before, during and after a bout of resistance exercise.
Data are expressed as means ±s.e.m.*Significantly different from basal (P < 0.05). Inset shows duplicate samples for each time point.
The phosphorylation of 4E-BP1 at Thr37/46 was significantly reduced immediately following exercise (P < 0.05), and gradually returned to baseline values over the next 2 h (Fig. 5B).
The phosphorylation of S6K1 at Thr389 tended to increase immediately and at 1 h post-exercise, although not quite reaching significance (P > 0.05). S6K1 phosphorylation was significantly increased (P < 0.05) at 2 h post-exercise (Fig. 5C).
eEF2 phosphorylation
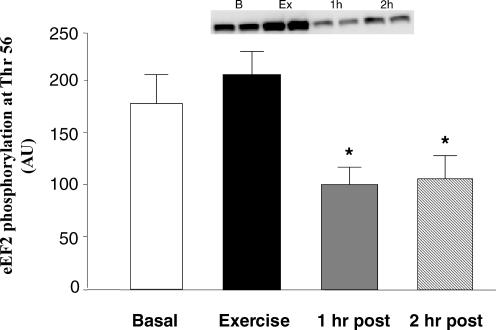
Immediately following resistance exercise, eEF2 phosphorylation at Thr56 tended to increase, although not quite reaching significance (P > 0.05; Fig. 6). The phosphorylation of eEF2 Thr56 at 1 and 2 h post-exercise was significantly reduced from baseline levels (P < 0.05).
Figure 6. Phosphorylation of eEF2: indicator of translation elongation status before, during and after a bout of resistance exercise.
Data are expressed as means ±s.e.m.*Significantly different from basal (P < 0.05). Inset shows duplicate samples for each time point.
Discussion
The primary and novel finding from our study is that the decrease in muscle protein synthesis during resistance exercise is associated with an increase in AMPK activity and a reduction in the phosphorylation of 4E-BP1 (a downstream component of the mTOR signalling pathway, and a key event in controlling translation initiation). Our findings are consistent with previous work showing that AMPK acts as a cellular energy sensor by inhibiting ATP-consuming anabolic processes (such as muscle protein synthesis in our study) and activating ATP-generating catabolic processes. Specifically, we found that immediately following a bout of resistance exercise AMPK activity increased by 75%, and 4E-BP1 phosphorylation at Thr 37/46 was reduced by 36%. These events may have contributed to the 32% reduction in muscle protein synthesis that occurred during resistance exercise.
A potential role for AMPK taking part in the reduced muscle protein synthesis during resistance exercise is supported by our finding of reduced 4E-BP1 phosphorylation during resistance exercise, which probably led to the subsequent inhibition of translation initiation. However, mTOR Ser2448 phosphorylation was unchanged immediately after exercise during the time when 4E-BP1 Thr36/47 phosphorylation was reduced. In addition, during post-exercise recovery, when muscle protein synthesis was stimulated, the increase in mTOR Ser2448 phosphorylation was not associated with an increase in 4E-BP1 Thr36/47 phosphorylation. The apparent disconnect between mTOR Ser2448 phosphorylation and 4E-BP1 Thr36/47 phosphorylation was puzzling; however, recent work has revealed an alternate regulation of 4E-BP1, as shown by the fact that mTOR output to 4E-BP1 Thr36/47 is rapamycin insensitive whereas mTOR signalling to S6K1 is rapamycin sensitive (Wang et al. 2005). It is unclear exactly how mTOR exerts this rapamycin-insensitive effect on 4E-BP1 Thr36/47 phosphorylation; however, phosphorylation of 4E-BP1 Thr36/47 is dependent on the N-terminal motif RAIP (Tee & Proud, 2002; Wang et al. 2005). It has generally been accepted that mTOR directly phosphorylates and regulates both 4E-BP1 and S6K1; therefore, the discovery that the regulation of 4E-BP1 36/47 phosphorylation is insensitive to rapamycin, that amino acids and insulin stimulate mTOR signalling to 4E-BP1 differently, and that output from mTOR to 4E-BP1 and S6K1 is distinct, suggest that the regulation of mTOR signalling to its downstream components is much more complicated than previously thought (Wang et al. 2005; Smith et al. 2005). In any event, our data suggest that resistance exercise reduces 4E-BP1 Thr36/47 phosphorylation. Furthermore, our finding of reduced 4E-BP1 Thr36/47 phosphorylation, despite an increase in phosphorylation of mTOR at Ser2448 during post-exercise recovery, provides additional evidence that the regulation of downstream signalling of mTOR to 4E-BP1 and S6K1 is complex and may be controlled by other mechanisms such as upstream regulation by TSC2 or directly via phosphorylation and/or binding by raptor (Kim et al. 2002; Inoki et al. 2003; Cheng et al. 2004; Long et al. 2005). Finally, it is important to point out that we only measured mTOR Ser2448 phosphorylation (not mTOR activity), and therefore we cannot rule out the possibility that during exercise distinct mTOR output to 4E-BP1 may have been reduced, despite there being no change in mTOR Ser2448 phosphorylation.
The precise mechanism by which AMPK may be reducing 4E-BP1 Thr36/47 phosphorylation remains to be determined; however, it is well documented that mTOR signalling can be influenced by phosphorylation–dephosphorylation at various sites (Sarbassov et al. 2005). Recent work also suggests other modes of regulation exist for controlling mTOR signalling. For example, PKB may directly phosphorylate TSC2 (one site being Thr1462 as used in our study), which leads to an inactivation of TSC2 promoting the binding of Rheb-GTP to mTOR (which activates mTOR) (Long et al. 2005). Interestingly, the phosphorylation of TSC2 at Thr1227 or Ser1345 by AMPK improves the ability of TSC2 to inhibit mTOR activity (Inoki et al. 2003). Although we did not have access to the antibodies for the AMPK phosphorylation sites for TSC2, we did find that TSC2 phosphorylation on Thr1462 tended to decrease immediately and significantly at 1 h post-exercise (P < 0.05). This may indicate that mTOR activity is also regulated by additional mechanisms that are not associated with TSC2. This is supported by recent data showing that when Rat1a-mAkt cells are depleted of ATP, the ability of PKB to activate mTOR is attenuated even though TSC2 phosphorylation at Thr1462 is unaltered (Hahn-Windgassen et al. 2005). In addition, it has recently been shown that amino acids regulate mTOR independently of TSC2 (Smith et al. 2005). In the present study the gradual increase in PKB and mTOR activation following resistance exercise may be responsible for the gradual return of 4E-BP1 Thr37/46 phosphorylation to baseline values, and for the increase in S6K1 Thr389 phosphorylation, despite continued activation of AMPK.
In addition to the upstream regulation of mTOR signalling by TSC2, control of mTOR signalling by its binding to a protein known as the regulatory associated protein to mTOR (raptor) has also been documented (Kim et al. 2002). In particular, the increased stabilization of the interaction between raptor and mTOR during nutrient deprivation or exposure to reducing agents is associated with an inactivation of mTOR (Kim et al. 2002; Sarbassov & Sabatini, 2005). Furthermore, it has been reported that AMPK can directly phosphorylate mTOR at Thr2446, which can prevent the PKB phosphorylation of mTOR at Ser2448 (Cheng et al. 2004). Therefore, the increase in AMPK activity during exercise may also be influencing 4E-BP1 Thr36/47 phosphorylation by either regulating raptor binding to mTOR and/or via direct phosphorylation. Future research is required to determine the precise mechanism by which AMPK may be influencing phosphorylation of 4E-BP1 Thr36/47 in human skeletal muscle.
Although the primary focus of this study was to determine whether mTOR signalling associated with translation initiation was inhibited during resistance exercise, it is also relevant to mention that the elongation phase of translation also plays a vital role in determining muscle protein synthesis rates, and a recent report has shown that the elongation phase of translation is also inhibited rapidly during aerobic exercise (Rose et al. 2005). We found that eEF2 phosphorylation tended to increase during exercise and was significantly reduced at 1 and 2 h post-exercise. It appears that eEF2 phosphorylation directly mirrors muscle FSR in our study. In fact, it has recently been suggested that mTOR may regulate eEF2 kinase (Browne & Proud, 2002) since S6K1 can phosphorylate eEF2 kinase, leading to enhanced elongation (Wang et al. 2001). In our study, the post-exercise increase in mTOR and S6K1 phosphorylation may potentially have overridden the effect of AMPK on eEF2 kinase, leading to a reduction in eEF2 phosphorylation. On the other hand, it has also been suggested that during exercise calcium–calmodulin is most likely to be the major regulator of eEF2 in vivo (Rose et al. 2005). Although recent work suggests that calmodulin-dependent protein kinase kinaseβ can phosphorylate and activate AMPK (Hawley et al. 2005; Woods et al. 2005), its role in skeletal muscle is probably minor because the expression of this kinase in muscle is extremely low. Therefore, additional experiments are required to determine the potential interactions of Ca2+, AMPK and mTOR signalling on controlling eEF2 phosphorylation in human muscle.
Another interesting finding was the change in muscle intracellular amino acid concentrations during the time course of our study. Leucine was increased by 30% immediately post-exercise (probably because of the decrease in protein synthesis and/or to an increase in proteolysis), and both phenylalanine and leucine were significantly reduced at 2 h post-exercise (most probably because of the increase in muscle protein synthesis). These results indirectly suggest that amino acid concentrations are not regulating mTOR signalling during and after resistance exercise, since mTOR phosphorylation was increasing while amino acid concentrations were decreasing. Other potential mechanisms, such as enhanced signalling through PKB and/or other regulators of mTOR signalling, are most likely to be responsible for the increase in muscle protein synthesis post-exercise (Anthony et al. 2002).
While we have focused on the evidence supporting a role for AMPK in inhibiting muscle protein synthesis during resistance exercise, we cannot exclude the possibility that other factors such as acidosis may also be inhibiting key steps of translation initiation. In our subjects, plasma lactate concentrations increased greater than 10-fold, and blood pH significantly decreased during the resistance exercise bout. Both pH and lactate normalized rapidly following exercise (although they were still significantly different from basal 1 h post-exercise). Recent work in rodent muscle has shown 24 h of acute metabolic acidosis (Caso et al. 2004) and 1 h of respiratory acidosis (Caso et al. 2005) significantly decrease skeletal muscle protein synthesis. It has also been reported that 48 h of metabolic acidosis in humans suppresses muscle protein synthesis (Kleger et al. 2001). The mechanism(s) by which acidosis inhibits muscle protein synthesis is not known (Caso & Garlick, 2005). It is also unknown whether short-term acidosis, as reported in our study, decreases human skeletal muscle protein synthesis. Therefore, the role of acidosis in suppressing muscle protein synthesis during resistance exercise requires further investigation.
The current study specifically targeted the early post-exercise recovery phase because we were interested in determining the cellular mechanisms involved in the control of muscle protein synthesis during cellular stress. However, it must be kept in mind that this short ‘refractory’ period immediately following resistance exercise is later reversed, thus explaining the significant anabolic effect of resistance exercise on skeletal muscle. Our study has definitively shown that a single bout of resistance exercise stimulates muscle protein synthesis within 1–2 h, and shown that the cellular mechanisms associated with the stimulation of muscle protein synthesis include an enhanced phosphorylation of mTOR at Ser2448, PKB at Ser473 (significantly elevated at 1 h post), and S6K1 at Thr389 (significantly elevated by 2 h post) and a reduced phosphorylation of eEF2 Thr56. Therefore, it appears that during the early recovery phase following resistance exercise the enhanced phosphorylation of PKB, mTOR and S6K1, and the reduced phosphorylation of eEF2, promoted translation initiation and elongation, leading to an increase in muscle protein synthesis at both 1 and 2 h post-exercise. This occurred despite the fact that AMPK activity was still elevated at 1 h post-exercise. Our data suggest that during exercise, when ATP is being utilized for muscle contraction, the ATP-consuming process of muscle protein synthesis is inhibited. However, the need to suppress muscle protein synthesis following exercise is minimized, and the exercise-induced activation of mTOR signalling to promote muscle protein synthesis is enhanced. The activation of PKB, mTOR, S6K1 and eEF2 post-exercise may be overriding the negative effects of AMPK on muscle protein synthesis; however, the continued activation of AMPK may be important for promoting fatty acid oxidation, which can supply the needed ATP for muscle protein synthesis, as well as muscle glycogen repletion (Rasmussen et al. 1998).
The stimulation of muscle protein synthesis can remain elevated for as long as 24–48 h post-exercise (MacDougall et al. 1995; Phillips et al. 1997). In addition, work in rodent muscle has shown that S6K1 phosphorylation and activation are not increased immediately following a bout of resistance exercise, but they increase by 3 h post-exercise (Baar & Esser, 1999). Although an early time course in rodent muscle has shown a gradual activation of the mTOR pathway following resistance exercise in rats (Bolster et al. 2003), prior to our study much less was known about the response in human muscle. One study has shown that during shortening and lengthening leg exercises (i.e. stepping onto box while carrying 25% of their body weight for 12 min) increased PKB and S6K1 phosphorylation 6 and 24 h post-exercise (Cuthbertson et al. 2006). However, the subjects in that study received a large amount of amino acids and carbohydrate 1 h following the exercise bout, and again before each of the subsequent biopsies. Moreover, the exercise intensity for that study was mild in comparison with ours, and both factors (nutrition and intensity) complicate the interpretation and comparison of results. Another study, using a less intense resistance exercise protocol as compared with the current study, has shown that resistance exercise increases Ser424/Thr421 phosphorylation of S6K1 and that ingestion of branched chain amino acids further enhances phosphorylation (Karlsson et al. 2004). In addition, this early increase in S6K1 phosphorylation following resistance exercise appears to be more pronounced in type II muscle fibres (Koopman et al. 2006). Future studies are necessary to determine a longer time course for the activation of the mTOR pathway in human muscle following resistance exercise, and the effect of nutrient provision on AMPK activity and muscle protein synthesis.
In conclusion, we provide additional data supporting the role of AMPK as an energy sensor, and evidence that it may be involved in the inhibition of the ATP-consuming process of muscle protein synthesis. Specifically, our findings support the hypothesis that muscle protein synthesis is decreased during resistance exercise (a condition of high energy expenditure within contracting muscle cells) in humans because the increase in AMPK activity is associated with a reduced 4E-BP1 Thr36/47 phosphorylation, a downstream component of the mTOR signalling pathway and a key regulator of translation initiation. Furthermore, the increase in muscle protein synthesis during early post-exercise recovery is associated with a reduction in eEF2 phosphorylation, and an increase in PKB, mTOR and S6K1 phosphorylation.
Acknowledgments
We wish to thank the nurses and personnel of the General Clinical Research Center of the University of Texas Medical Branch for their help with the conduct of the clinical portion of this study. We would also like to thank Drs Yoshizawa and Bolster for their advice with the Western blotting procedures, and Erin Glynn and Jessica Lee for technical assistance. This study was supported by grant R01 AR049877 from the National Institute for Arthritis and Musculoskeletal and Skin Diseases, grant S10 RR16650 from the Shared Instrumentation Grant Program, and grant M01 RR00073 from the General Clinical Research Branch, National Center for Research Resources, National Institutes of Health, and National Institute on Aging grant P30 AG024832. H.C. Dreyer was supported by grant H133P040003 from the National Institute on Disability and Rehabilitation Research, Department of Education.
References
- Anthony JC, Lang CH, Crozier SJ, Anthony TG, MacLean DA, Kimball SR, Jefferson LS. Contribution of insulin to the translational control of protein synthesis in skeletal muscle by leucine. Am J Physiol Endocrinol Metab. 2002;282:E1092–E1101. doi: 10.1152/ajpendo.00208.2001. [DOI] [PubMed] [Google Scholar]
- Atherton PJ, Babraj J, Smith K, Singh J, Rennie MJ, Wackerhage H. Selective activation of AMPK-PGC-1alpha or PKB-TSC2-mTOR signaling can explain specific adaptive responses to endurance or resistance training-like electrical muscle stimulation. FASEB J. 2005;19:786–788. doi: 10.1096/fj.04-2179fje. [DOI] [PubMed] [Google Scholar]
- Baar K, Esser K. Phosphorylation of p70 (S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol. 1999;276:C120–C127. doi: 10.1152/ajpcell.1999.276.1.C120. [DOI] [PubMed] [Google Scholar]
- Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol Endocrinol Metab. 1995;268:E514–E520. doi: 10.1152/ajpendo.1995.268.3.E514. [DOI] [PubMed] [Google Scholar]
- Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem. 2002;277:23977–23980. doi: 10.1074/jbc.C200171200. [DOI] [PubMed] [Google Scholar]
- Bolster DR, Kubica N, Crozier SJ, Williamson DL, Farrell PA, Kimball SR, Jefferson LS. Immediate response of mammalian target of rapamycin (mTOR)-mediated signalling following acute resistance exercise in rat skeletal muscle. J Physiol. 2003a;553:213–220. doi: 10.1113/jphysiol.2003.047019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne GJ, Finn SG, Proud CG. Stimulation of the AMP-activated protein kinase leads to activation of eukaryotic elongation factor 2 kinase and to its phosphorylation at a novel site, serine 398. J Biol Chem. 2004;279:12220–12231. doi: 10.1074/jbc.M309773200. [DOI] [PubMed] [Google Scholar]
- Browne GJ, Proud CG. Regulation of peptide-chain elongation in mammalian cells. Eur J Biochem. 2002;269:5360–5368. doi: 10.1046/j.1432-1033.2002.03290.x. [DOI] [PubMed] [Google Scholar]
- Bylund-Fellenius AC, Ojamaa KM, Flaim KE, Li JB, Wassner SJ, Jefferson LS. Protein synthesis versus energy state in contracting muscles of perfused rat hindlimb. Am J Physiol Endocrinol Metab. 1984;246:E297–E305. doi: 10.1152/ajpendo.1984.246.4.E297. [DOI] [PubMed] [Google Scholar]
- Calder AG, Anderson SE, Grant I, McNurlan MA, Garlick PJ. The determination of low d5-phenylalanine enrichment (0.002–0.09 atom percent excess), after conversion to phenylethylamine, in relation to protein turnover studies by gas chromatography/electron ionization mass spectrometry. Rapid Commun Mass Spectrom. 1992;6:421–424. doi: 10.1002/rcm.1290060704. [DOI] [PubMed] [Google Scholar]
- Carraro F, Stuart CA, Hartl WH, Rosenblatt J, Wolfe RR. Effect of exercise and recovery on muscle protein synthesis in human subjects. Am J Physiol Endocrinol Metab. 1990;259:E470–E476. doi: 10.1152/ajpendo.1990.259.4.E470. [DOI] [PubMed] [Google Scholar]
- Caso G, Garlick PJ. Control of muscle protein kinetics by acid–base balance. Curr Opin Clin Nutr Metab Care. 2005;8:73–76. doi: 10.1097/00075197-200501000-00011. [DOI] [PubMed] [Google Scholar]
- Caso G, Garlick BA, Casella GA, Sasvary D, Garlick PJ. Acute metabolic acidosis inhibits muscle protein synthesis in rats. Am J Physiol Endocrinol Metab. 2004;287:E90–E96. doi: 10.1152/ajpendo.00387.2003. [DOI] [PubMed] [Google Scholar]
- Caso G, Garlick BA, Casella GA, Sasvary D, Garlick PJ. Response of protein synthesis to hypercapnia in rats: independent effects of acidosis and hypothermia. Metabolism. 2005;54:841–847. doi: 10.1016/j.metabol.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Chen ZP, Stephens TJ, Murthy S, Canny BJ, Hargreaves M, Witters LA, Kemp BE, McConell GK. Effect of exercise intensity on skeletal muscle AMPK signaling in humans. Diabetes. 2003;52:2205–2212. doi: 10.2337/diabetes.52.9.2205. [DOI] [PubMed] [Google Scholar]
- Cheng SW, Fryer LG, Carling D, Shepherd PR. Thr2446 is a novel mammalian target of rapamycin (mTOR) phosphorylation site regulated by nutrient status. J Biol Chem. 2004;279:15719–15722. doi: 10.1074/jbc.C300534200. [DOI] [PubMed] [Google Scholar]
- Coffey VG, Zhong Z, Shield A, Canny BJ, Chibalin AV, Zierath JR, Hawley JA. Early signaling responses to divergent exercise stimuli in skeletal muscle from well-trained humans. FASEB J. 2006;20:190–192. doi: 10.1096/fj.05-4809fje. [DOI] [PubMed] [Google Scholar]
- Cuthbertson DJ, Babraj JA, Smith K, Wilkes E, Fedele MJ, Esser K, Rennie MJ. Anabolic signalling and protein synthesis in human skeletal muscle after dynamic shortening or lengthening exercise. Am J Physiol Endocrinol Metab. 2006;290:E731–E738. doi: 10.1152/ajpendo.00415.2005. [DOI] [PubMed] [Google Scholar]
- Davis TA, Karl IE. Response of muscle protein turnover to insulin after acute exercise and training. Biochem J. 1986;240:651–657. doi: 10.1042/bj2400651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohm GL, Kasperek GJ, Tapscott EB, Beecher GR. Effect of exercise on synthesis and degradation of muscle protein. Biochem J. 1980;188:255–262. doi: 10.1042/bj1880255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham WJ, Miller SL, Yeckel CW, Chinkes DL, Tipton KD, Rasmussen BB, Wolfe RR. Leg glucose and protein metabolism during an acute bout of resistance exercise in humans. J Appl Physiol. 2004;97:1379–1386. doi: 10.1152/japplphysiol.00635.2003. [DOI] [PubMed] [Google Scholar]
- Fujii N, Hayashi T, Hirshman MF, Smith JT, Habinowski SA, Kaijser L, Mu J, Ljungqvist O, Birnbaum MJ, Witters LA, Thorell A, Goodyear LJ. Exercise induces isoform-specific increase in 5′AMP-activated protein kinase activity in human skeletal muscle. Biochem Biophys Res Commun. 2000;273:1150–1155. doi: 10.1006/bbrc.2000.3073. [DOI] [PubMed] [Google Scholar]
- Hahn-Windgassen A, Nogueira V, Chen CC, Skeen JE, Sonenberg N, Hay N. Akt activates the mammalian target of rapamycin by regulating cellular ATP level and AMPK activity. J Biol Chem. 2005;280:32081–32089. doi: 10.1074/jbc.M502876200. [DOI] [PubMed] [Google Scholar]
- Hardie DG. New roles for the LKB1 → AMPK pathway. Curr Opin Cell Biol. 2005;17:167–173. doi: 10.1016/j.ceb.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Carling D. The AMP-activated protein kinase – fuel gauge of the mammalian cell? Eur J Biochem. 1997;246:259–273. doi: 10.1111/j.1432-1033.1997.00259.x. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Salt IP, Davies SP. Analysis of the role of the AMP-activated protein kinase in the response to cellular stress. Methods Mol Biol. 2000;99:63–74. doi: 10.1385/1-59259-054-3:63. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Horman S, Browne G, Krause U, Patel J, Vertommen D, Bertrand L, Lavoinne A, Hue L, Proud C, Rider M. Activation of AMP-activated protein kinase leads to the phosphorylation of elongation factor 2 and an inhibition of protein synthesis. Curr Biol. 2002;12:1419–1423. doi: 10.1016/s0960-9822(02)01077-1. [DOI] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- Jorfeldt L, Wahren J. Leg blood flow during exercise in man. Clin Sci. 1971;41:459–473. doi: 10.1042/cs0410459. [DOI] [PubMed] [Google Scholar]
- Karlsson HKR, Nilsson P-A, Nilsson J, Chibalin AV, Zierath JR, Blomstrand E. Branched-chain amino acids increase p70S6K phosphorylation in human skeletal muscle after resistance exercise. Am J Physiol Endocrinol Metab. 2004;287:E1–E7. doi: 10.1152/ajpendo.00430.2003. [DOI] [PubMed] [Google Scholar]
- Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- Kleger GR, Turgay M, Imoberdorf R, McNurlan MA, Garlick PJ, Ballmer PE. Acute metabolic acidosis decreases muscle protein synthesis but not albumin synthesis in humans. Am J Kidney Dis. 2001;38:1199–1207. doi: 10.1053/ajkd.2001.29215. [DOI] [PubMed] [Google Scholar]
- Koopman R, Zorenc AH, Gransier RJ, Cameron-Smith D, van Loon LJ. Increase in S6K1 phosphorylation in human skeletal muscle following resistance exercise occurs mainly in type II muscle fibers. Am J Physiol Endocrinol Metab. 2006;290:E1245–E1252. doi: 10.1152/ajpendo.00530.2005. [DOI] [PubMed] [Google Scholar]
- Long X, Lin Y, Ortiz-Vega S, Yonezawa K, Avruch J. Rheb binds and regulates the mTOR kinase. Curr Biol. 2005;15:702–713. doi: 10.1016/j.cub.2005.02.053. [DOI] [PubMed] [Google Scholar]
- MacDougall JD, Gibala MJ, Tarnopolsky MA, MacDonald JR, Interisano SA, Yarasheski KE. The time course for elevated muscle protein synthesis following heavy resistance exercise. Can J Appl Physiol. 1995;20:480–486. doi: 10.1139/h95-038. [DOI] [PubMed] [Google Scholar]
- McConell GK, Lee-Young RS, Chen ZP, Stepto NK, Huynh NN, Stephens TJ, Canny BJ, Kemp BE. Short-term exercise training in humans reduces AMPK signalling during prolonged exercise independent of muscle glycogen. J Physiol. 2005;568:665–676. doi: 10.1113/jphysiol.2005.089839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol Endocrinol Metab. 1997;273:E99–E107. doi: 10.1152/ajpendo.1997.273.1.E99. [DOI] [PubMed] [Google Scholar]
- Rasmussen BB, Hancock CR, Winder WW. Postexercise recovery of skeletal muscle malonyl-CoA, acetyl-CoA carboxylase, and AMP-activated protein kinase. J Appl Physiol. 1998;85:1629–1634. doi: 10.1152/jappl.1998.85.5.1629. [DOI] [PubMed] [Google Scholar]
- Rasmussen BB, Phillips SM. Contractile and nutritional regulation of human muscle growth. Exerc Sport Sci Rev. 2003;31:127–131. doi: 10.1097/00003677-200307000-00005. [DOI] [PubMed] [Google Scholar]
- Rasmussen BB, Tipton KD, Miller SL, Wolf SE, Wolfe RR. An oral essential amino acid–carbohydrate supplement enhances muscle protein anabolism after resistance exercise. J Appl Physiol. 2000;88:386–392. doi: 10.1152/jappl.2000.88.2.386. [DOI] [PubMed] [Google Scholar]
- Rasmussen BB, Winder WW. Effect of exercise intensity on skeletal muscle malonyl-CoA and acetyl-CoA carboxylase. J Appl Physiol. 1997;83:1104–1109. doi: 10.1152/jappl.1997.83.4.1104. [DOI] [PubMed] [Google Scholar]
- Rennie MJ, Edwards RH, Krywawych S, Davies CT, Halliday D, Waterlow JC, Millward DJ. Effect of exercise on protein turnover in man. Clin Sci (Lond) 1981;61:627–639. doi: 10.1042/cs0610627. [DOI] [PubMed] [Google Scholar]
- Roepstorff C, Halberg N, Hillig T, Saha AK, Ruderman NB, Wojtaszewski JF, Richter EA, Kiens B. Malonyl-CoA and carnitine in regulation of fat oxidation in human skeletal muscle during exercise. Am J Physiol Endocrinol Metab. 2005;288:E133–E142. doi: 10.1152/ajpendo.00379.2004. [DOI] [PubMed] [Google Scholar]
- Rose AJ, Broholm C, Kiillerich K, Finn SG, Proud CG, Rider MH, Richter EA, Kiens B. Exercise rapidly increases eukaryotic elongation factor 2 phosphorylation in skeletal muscle of men. J Physiol. 2005;569:223–228. doi: 10.1113/jphysiol.2005.097154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Sabatini DM. Redox regulation of the nutrient-sensitive raptor–mTOR pathway and complex. J Biol Chem. 2005;25:39505–39509. doi: 10.1074/jbc.M506096200. [DOI] [PubMed] [Google Scholar]
- Smith EM, Finn SG, Tee AR, Browne GJ, Proud CG. The tuberous sclerosis protein TSC2 is not required for the regulation of the mammalian target of rapamycin by amino acids and certain cellular stresses. J Biol Chem. 2005;280:18717–18727. doi: 10.1074/jbc.M414499200. [DOI] [PubMed] [Google Scholar]
- Stephens TJ, Chen ZP, Canny BJ, Michell BJ, Kemp BE, McConell GK. Progressive increase in human skeletal muscle AMPKalpha2 activity and ACC phosphorylation during exercise. Am J Physiol Endocrinol Metab. 2002;282:688–694. doi: 10.1152/ajpendo.00101.2001. [DOI] [PubMed] [Google Scholar]
- Tee AR, Proud CG. Caspase cleavage of initiation factor 4E-binding protein 1 yields a dominant inhibitor of cap-dependent translation and reveals a novel regulatory motif. Mol Cell Biol. 2002;22:1674–1683. doi: 10.1128/MCB.22.6.1674-1683.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Beugnet A, Murakami M, Yamanaka S, Proud CG. Distinct signaling events downstream of mTOR cooperate to mediate the effects of amino acids and insulin on initiation factor 4E-binding proteins. Mol Cell Biol. 2005;25:2558–2572. doi: 10.1128/MCB.25.7.2558-2572.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Li W, Williams M, Terada N, Alessi DR, Proud CG. Regulation of elongation factor 2 kinase by p90RSK1 and p70, S6 kinase. EMBO J. 2001;20:4370–4379. doi: 10.1093/emboj/20.16.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder WW, Hardie DG. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol Endocrinol Metab. 1996;270:E299–E304. doi: 10.1152/ajpendo.1996.270.2.E299. [DOI] [PubMed] [Google Scholar]
- Winder WW, Hardie DG. AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol Endocrinol Metab. 1999;277:E1–E10. doi: 10.1152/ajpendo.1999.277.1.E1. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, MacDonald C, Nielsen JN, Hellsten Y, Hardie DG, Kemp BE, Kiens B, Richter EA. Regulation of 5′AMP-activated protein kinase activity and substrate utilization in exercising human skeletal muscle. Am J Physiol Endocrinol Metab. 2003;284:E813–E822. doi: 10.1152/ajpendo.00436.2002. [DOI] [PubMed] [Google Scholar]
- Wolfe RR, Chinkes DL. Isotope Tracers in Metabolic Research: Principles and Practice of Kinetic Analysis. 2. Hoboken, New Jersey: Wiley-Liss; 2005. [Google Scholar]
- Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Yu M, Stepto NK, Chibalin AV, Fryer LG, Carling D, Krook A, Hawley JA, Zierath JR. Metabolic and mitogenic signal transduction in human skeletal muscle after intense cycling exercise. J Physiol. 2003;546:327–335. doi: 10.1113/jphysiol.2002.034223. [DOI] [PMC free article] [PubMed] [Google Scholar]