Abstract
The hippocampus, a key structure in learning and memory processes, receives a powerful cholinergic innervation from the septum and contains nicotinic acetylcholine receptors (nAChRs). Early in postnatal development, activation of nAChRs by nicotine or endogenous acetylcholine contributes to enhance synaptic signalling. Here, the patch-clamp technique was used to assess the contribution of α7 and β2-containing (α7* and β2*) nAChRs to nicotine-elicited modulation of GABAergic and glutamatergic activity at the network and single-cell level in the immature hippocampus of wild-type (WT), α7−/− and β2−/− mice. We found that α7* and β2* nAChRs were sufficient to modulate nicotine-induced increase in frequency of spontaneously occurring giant depolarizing potentials (GDPs), which are generated at the network level by the synergistic action of glutamate and depolarizing GABA, and thought to play a crucial role in neuronal wiring. However, α7* but not β2* receptors were essential in nicotine-induced increase of interictal discharge frequency recorded after postnatal day 3 in the presence of bicuculline, when GABA shifted from the depolarizing to the hyperpolarizing direction. To correlate these observations with nicotine-elicited changes in synaptic transmission, we recorded spontaneous GABAergic and glutamatergic postsynaptic currents in pyramidal cells and interneurons localized in stratum oriens, stratum pyramidale and stratum radiatum, in slices obtained from WT and knock-out animals. We found that early in postnatal life α7* and β2* nAChRs exert a fine regional modulation of GABAergic and glutamatergic transmission that underlies nicotine-elicited changes in network synchronization.
Neuronal nicotinic acetylcholine receptors (nAChRs), which belong to the large family of ligand-gated ion channels, are made up of five subunits organized in a variety of allosteric oligomers (Changeux & Edelstein, 2005). nAChRs are widely distributed within the brain, where they contribute to the regulation of higher cognitive functions (Rezvani & Levin, 2001). In particular, the adequate activation of nAChRs contributes to the functional maturation of the brain (Chang & Berg, 1999; Aramakis et al. 2000; Rossi et al. 2001; Kawa, 2002).
The hippocampus, a key structure in learning and memory processes, receives a large cholinergic innervation (Kasa, 1986) and is endowed with a variety of nAChRs (Alkondon & Albuquerque, 2004), which increase the release of several neurotransmitters and modulate synaptic plasticity processes (McGehee, 2002). Of the four classes of nAChRs described in the central nervous system (Zoli et al. 1998), the main receptor subtypes present in the hippocampus are the homomeric α7 and the heteromeric α4β2-containing receptors (Alkondon & Albuquerque, 2004). Interestingly, in the rat hippocampus during the first postnatal week, α7 nAChR mRNA and α bungarotoxin binding sites have been shown to be expressed at high levels (Adams et al. 2002; Tribollet et al. 2004), suggesting that α7 may have a role in the development of the immature hippocampus. Moreover, several studies have demonstrated that perinatal exposure to nicotine in rodents and humans impairs cognitive functions, supporting the idea that an excessive activation of nAChRs by nicotine during brain maturation interferes with the development of brain areas involved in learning and memory (Johns et al. 1982; Levin et al. 1993; Ernst et al. 2001; Linnet et al. 2003). However, the molecular and cellular mechanisms underlying these processes remain largely unknown. In a previous study, nicotine was shown to increase the frequency of synchronous network-driven membrane oscillations present in the hippocampus at early postnatal stages of development, and called ‘giant depolarizing potentials’ (GDPs; Maggi et al. 2001). GDPs are characterized by recurrent membrane depolarization with superimposed fast action potentials. They are generated spontaneously by the synergistic action of glutamate and GABA and, in the perinatal period, this exerts a depolarizing and excitatory action (Ben Ari et al. 1989; Cherubini et al. 1991; Sipila et al. 2005). GDPs are reminiscent of correlated network activity observed in the retina (Wong et al. 1995), the spinal cord (Gu et al. 1994) and the neocortex (Garaschuk et al. 2000) during development, thought to contribute to circuit wiring (Ben Ari, 2002).
In the present study, the whole-cell patch-clamp technique was used to assess the contribution of α7-containing (α7*) and β2-containing (β2*) nAChRs to nicotine-elicited modulation of network and synaptic activity in immature hippocampal slices obtained from wild-type (WT) mice and mice lacking the gene coding for the α7 or the β2 subunits of nAChRs. In WT, α7 and β2 knock-out (KO) mice, nicotine increased the frequency of GDPs. Moreover, in β2 KO, but not in α7 KO mice, in the absence of GABAA-mediated synaptic transmission, nicotine potentiated interictal-like discharges (Wong et al. 1986), indicating a different regulation of GABAergic and glutamatergic signalling by β2* and α7* nAChRs. To investigate the changes in synaptic activity underpinning these findings, we studied the effect of nicotine on GABAergic and glutamatergic spontaneous synaptic transmission in pyramidal cells and interneurons of the stratum oriens, stratum pyramidale and stratum radiatum, in neonatal WT and KO mice.
Methods
Slice preparation
Transverse hippocampal slices (400 μm thick) were prepared from mice that were 2–10 postnatal days old (P2–P10), using a method previously described (Maggi et al. 2003). In Italy, the procedure was in accordance with the regulations of the Italian Animal Welfare Act, and was approved by the local authority veterinary service. In France, the procedure was in accordance with the Centre National de la Recherche Scientifique guidelines for care and use of laboratory animals. Briefly, animals were decapitated and the brain was quickly removed from the skull, then sectioned with a vibratome (DTK 1000; DSK, Kyoto, Japan) using ice-cold artificial cerebrospinal fluid (ACSF) containing (mm): NaCl 130, KCl 3.5, NaH2PO4 1.2, NaHCO3 25, MgCl2 1.3, CaCl2 2, and glucose 11, saturated with 95% O2 and 5% CO2 (pH 7.3–7.4). After 1 h, an individual slice was transferred to the recording chamber where it was continuously superfused with oxygenated ACSF at a rate of 2–3 ml min−1.
KO animals
The generation of β2−/− and α7−/− mice has been previously described (Picciotto et al. 1995; Orr-Urtreger et al. 1997). KO and matching WT colonies were bred separately. For each colony, at least four couples of homozygous breeders were produced by mating heterozygous mice, obtained after 10–12 (α7) or 12–19 (β2) backcrosses with C57Bl/6J mice. For control experiments, WT C57Bl/6J, α7+/+ and β2+/+ mice were used. Animal care was in line with institutional guidelines.
Electrophysiology
Neurons were visually identified using an upright microscope and infrared differential interference contrast videomicroscopy (Axioskop; Zeiss, Oberkochen, Germany). CA1 pyramidal neurons were voltage clamped at −70 mV using the whole-cell configuration of the patch-clamp technique. Series resistance was compensated (60–80%) and checked regularly during the experiment. Cells exhibiting more than 20% changes were excluded from the analysis.
Patch electrodes, formed from thin borosilicate glass (Hilgenberg, Malsfeld, Germany) had a resistance of 3–6 MΩ. Network and synaptic activity were recorded with an intracellular solution containing (mm): KCl 140, Hepes 10, EGTA 1, MgCl2 1, MgATP 4 and NaGTP 0.3. The pH was adjusted to 7.25 with CsOH or KOH, and the osmolarity was 280–290 mosmol l−1.
Spontaneous α amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate (KA) receptor-mediated excitatory postsynaptic currents (EPSCs) were routinely recorded from CA1 principal cells and interneurons at a holding potential of −60 mV in the presence of bicuculline (10 μm) to block GABAA receptors.
Spontaneous γ aminobutyric acid (GABA)A-mediated postsynaptic currents (PSCs) were routinely recorded from CA1 principal cells and interneurons at a holding potential of −60 mV in the presence of DNQX (20 μm) to block AMPA/KA-mediated responses.
Drugs were applied to the bath via a three-way tap system. Drugs used were: tetrodotoxin (TTX), purchased from Latoxan, Valence, France; bicuculline methiodide and 6,7-dinitroquinoxaline-2,3-dione (DNQX), from Tocris, Bristol, UK; nicotine, methyllycaconitine (MLA) and dihydro-β-erythroidine (DHβE), purchased from Sigma, Milan, Italy. Membrane potentials were corrected for liquid junction potential. Experiments were performed at 32–33°C.
Data acquisition and analysis
Data were acquired using pCLAMP 9 software (Axon Instruments, Union City, CA, USA) and currents recorded using an Axopatch 1D amplifier and a Multiclamp 700A amplifier (Axon Instruments). Current signals were transferred to a computer after digitization with an A/D converter (Digidata 1200 and Digidata 1322; Axon Instruments). Data were sampled at 10 kHz, and filtered with a cut-off frequency of 2 kHz.
Spontaneous EPSCs, GABAA-mediated PSCs, GDPs and interictal bursts were analysed off-line with Clampfit 9 software (Axon Instruments).
The rise time of GDPs and interictal discharges was estimated as the time needed for a 10–90% increase of the peak current responses regardless of unclamped action potentials riding on the top.
To examine whether nicotine affected the frequency of EPSCs/GABAA-mediated PSCs/GDPs/interictal bursts, firstly the mean frequency of all events occurring in 6–10 min pre-drug control periods was calculated. Then, synaptic and network events recorded during or after drug application (3 min for EPSCs/GABAA-mediated PSCs and 5 min for GDPs/interictal bursts, starting 2 min after the onset of drug application) were normalized to control values and expressed as percentage changes (see figures).
Interneurons localized in stratum pyramidale were distinguished from pyramidal cells on the basis of their different morphology (rounded soma for interneurons, spindle-shaped for pyramidal cells), and their different firing patterns in response to a steady depolarizing current applied from −60 mV. Pyramidal neurons were characterized by a slow ramp potential before firing initiation, marked spike-frequency accommodation, and small single-spike after-hyperpolarizations (AHPs) in response to depolarizing current steps. In contrast, stratum pyramidale interneurons showed rapid firing initiation, minor spike-frequency accommodation, and big single-spike AHPs.
Data are expressed as means ± s.e.m. Statistical comparisons were made using Student's two-tailed t test. The Kolmogorov-Smirnov test was applied to compare distributions. P < 0.05 was taken as significant.
Results
Nicotine increases the frequency of GDPs via α7* and β2* nAChRs
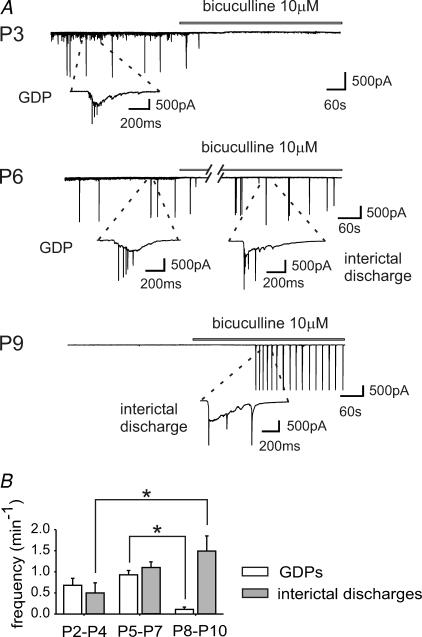
In the present study, GDPs were recorded in voltage-clamped CA1 hippocampal neurons in slices obtained from P2–P10 WT mice (n = 30). GDPs were characterized by slow inward currents giving rise to high-frequency unclamped spikes (see insets of Fig. 1A and B). As illustrated in the summary graph of Fig. 1B, while GDP frequency remained constant between P2 and P4, and P5 and P7 (0.68 ± 0.16 and 0.92 ± 0.10 min−1, respectively; P > 0.05), it sharply decreased from P8 onwards (0.11 ± 0.06 at P8–P10; P < 0.05 compared with P2–P7). GDPs were rarely observed after P9. Application of the GABAA receptor antagonist bicuculline (10 μm) blocked GDPs and induced interictal discharges whose frequency significantly increased with age. GDPs were also blocked by the AMPA/KA receptor antagonist DNQX (20 μm) (not shown), indicating that GABAergic and glutamatergic neurons both contribute to their generation (Ben Ari et al. 1989). In comparison with GDPs, interictal discharges were not observed at any time before P3, despite the prolonged exposure of slices to bicuculline (10–15 min, see Khazipov et al. 2004), and were characterized by a faster rising phase (47 ± 5 ms for interictal discharges, n = 13, versus 153 ± 14 ms for GDPs, n = 12 cells, see Methods). They were blocked by DNQX (20 μm), suggesting that they were mediated by AMPA/KA ionotropic glutamate receptors (n = 3, not shown). They were generated in the CA3 area, since cutting the slices between the CA3 and CA1 regions prevented the propagation of discharges (n = 3, not shown; see Wong & Traub, 1983). After P3, the frequency of bicuculline-elicited interictal discharges increased significantly (from 0.50 ± 0.24 min−1 at P2–P4, to 1.49 ± 0.36 min−1 at P8–P10; P < 0.05; Fig. 1). The age-dependent reduction in the frequency of GDPs and increase in the frequency of interictal discharges between P3 and P7 (Fig. 1B) can be attributed to the gradual increase in the expression of KCC2 (Rivera et al. 1999), followed by the progressive shift of GABA from the depolarizing to the hyperpolarizing direction.
Figure 1. Synchronized patterns of network activity on the immature hippocampus.
A, top, representative trace recorded at P3 from a CA1 pyramidal neuron in a hippocampal slice obtained from a wild-type (WT) mouse in control conditions and during bath application of bicuculline (bar). Inset, giant depolarizing potential (GDP) shown at an expanded time scale. Note the disappearance of GDPs with bicuculline. A, middle, representative trace recorded at P6. Blockade of GABAA receptors with bicuculline (bar) induces interictal discharges. Insets, a GDP and an interictal discharge are shown at an expanded time scale. A, bottom, representative trace recorded at P9. GDPs are absent. Blockade of GABAA receptors with bicuculline (bar) induces interictal discharges (see inset). B, frequency histograms of GDPs (white columns) and interictal bursts (grey columns) recorded at different times of postnatal development (GDPs: P2–P4, n = 10; P5–P7, n = 11; P8–P10, n = 9. Bursts: P2–P4, n = 7; P5–P7, n = 12; P8–P10, n = 9). *P < 0.05.
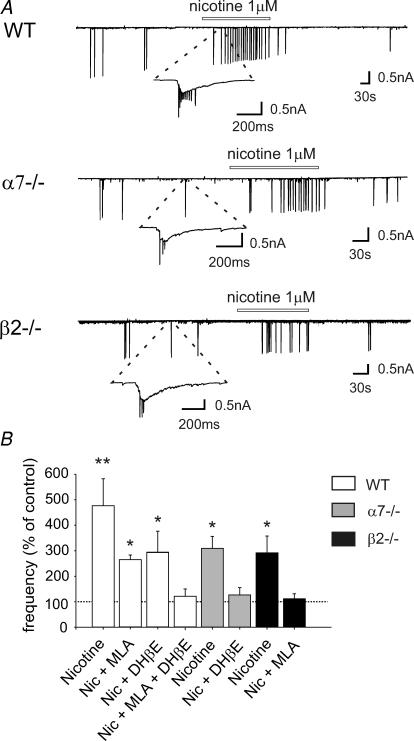
In agreement with a previous study on the rat hippocampus (Maggi et al. 2001), in P2–P7 mice, a brief application of nicotine, 1 μm for 3 min, a concentration close to that present in the smoker's blood after smoking few cigarettes (Dani & Heinemann, 1996), reversibly increased GDP frequency by 477 ± 105% (from 0.70 ± 0.10 min−1 in control; n = 12, P < 0.01), an effect that was partially antagonized by MLA (10 nm), a selective antagonist of α7* nAChRs (230 ± 20% change in the presence of MLA; n = 6, P < 0.05) or DHβE 1 μm, a selective antagonist of β2* nAChRs (292 ± 85% change in the presence of DHβE; n = 7, P < 0.05). In the presence of both MLA and DHβE, nicotine did not elicit any significant increase (120 ± 30% change; n = 6, P > 0.05; Fig. 2).
Figure 2. Nicotine increases the frequency of GDPs in WT mice, as well as in α7−/− and β2−/− mice.
A, top, representative trace recorded at P6 from a CA1 pyramidal neuron in a hippocampal slice obtained from a WT mouse in control conditions and during bath application of nicotine (bar). The inset represents a GDP at an expanded time scale. A, middle and bottom, representative traces recorded from a CA1 pyramidal neuron in hippocampal slices obtained from an α7−/− (P5) and a β2−/− mouse (P6), respectively. Note that nicotine increased GDP frequency in WT, α7−/− and β2−/− mice. B, each column represents nicotine-induced changes of GDP frequency as a percentage of control (dashed line); n = 6–12; **P < 0.01; *P < 0.05.
Similar results were obtained in mice lacking the nAChR β2 or α7-subunit gene. In α7−/− mice, nicotine caused a 309 ± 47% increase in the frequency of GDPs (from 0.83 ± 0.16 min−1; n = 7, P < 0.05), an effect that disappeared when slices were first treated with DHβE (1 μm). In this case, GDP frequency remained unchanged (127 ± 29% change; n = 9, P > 0.05). In β2−/− mice, nicotine elicited a 292 ± 66% increase in frequency (from 0.84 ± 0.20 min−1; n = 8, P < 0.05), which was blocked by 10 nm MLA (112 ± 20% change; n = 10, P > 0.05). These results suggest that α7* and β2* nAChRs can independently increase GDP frequency (Fig. 2).
Nicotine did not modify the shape or charge transfer associated with GDPs: the charge transfer measured before and after nicotine application was 60.4 ± 10.3 and 63.8 ± 13.6 pA s in WT mice (P > 0.05, n = 12), 61.9 ± 21.8 and 63.2 ± 19.9 pA s in α7−/− mice (P > 0.05, n = 7), 46.1 ± 8.1 and 50.7 ± 11.1 pA s in β2−/− mice (P > 0.05, n = 8). Moreover, nicotine (1 μm) did not change the membrane potential or the input conductance of the recorded neurons.
Nicotine increases the frequency of interictal discharges via α7* but not β2* nAChRs
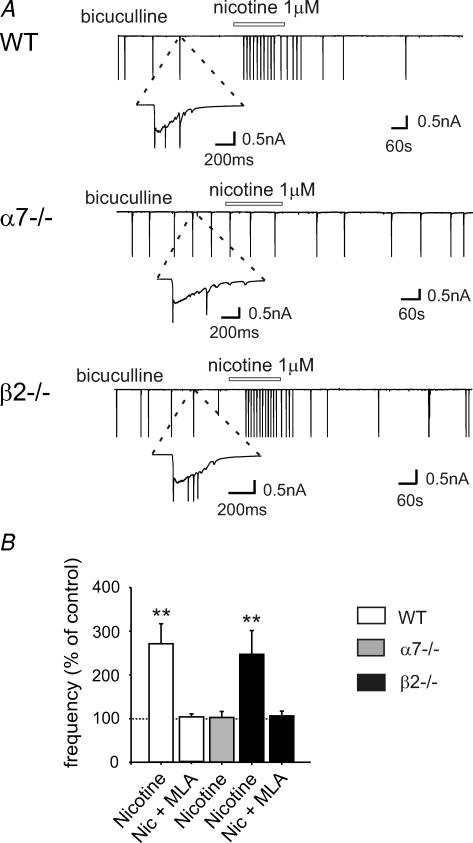
Figure 3 presents the effect of nicotine on interictal discharges caused by bicuculline in WT, α7−/− and β2−/− mice at P5–P9. Nicotine (1 μm) reversibly increased the frequency of interictal discharges in WT mice (271 ± 46% of control, from 1.08 ± 0.17 min−1 in control; n = 13, P < 0.01), an effect that was prevented by MLA 10 nm (111 ± 11% of control; n = 3). Nicotine did not affect the frequency of interictal bursts in slices from α7−/− (103 ± 14% change, from 1.38 ± 0.29 min−1; n = 11, P > 0.05), but increased interictal burst frequency in β2−/− mice (247 ± 54% of control, from 1.02 ± 0.15 min−1; n = 8, P < 0.01). However, in slices from β2−/− mice preincubated in MLA, the frequency of interictal bursts was unaltered (106 ± 11% change; n = 6, P > 0.05). These results demonstrate that α7* nAChRs mediate nicotine-elicited change in interictal burst frequency.
Figure 3. Nicotine increases the frequency of interictal discharges in WT and β2−/− mice, but not in α7−/− mice.
A, from top to bottom, representative traces recorded from CA1 pyramidal neurons in hippocampal slices obtained from WT (P10), α7−/− (P9) and β2−/− (P10) mice, respectively, in the presence of bicuculline and in the presence of bicuculline plus nicotine (bars). Insets, interictal discharges are shown at an expanded time scale. B, each column represents nicotine-induced changes in the frequency of interictal discharge as percentage of control (dashed line) (n = 3–13; **P < 0.01).
In WT and KO mice, nicotine did not affect the shape or charge transfer associated with interictal bursts. On average, the charge transfer measured before and after nicotine application was 141.4 ± 39.5 and 121.3 ± 36.9 pA s in WT mice (n = 13, P > 0.05), 148.0 ± 78.6 and 146.2 ± 81.1 pA s in β2−/− mice (P > 0.05, n = 8), 75.9 ± 17.1 and 60.7 ± 13.0 pA s in α7−/− mice (n = 11, P > 0.05). As in the case of GDPs, nicotine did not change the membrane potential or the input conductance of the recorded cells.
To correlate these results with nicotine-elicited changes in early postnatal synaptic transmission in the hippocampus, the effects of nicotine were studied on spontaneous GABAergic and glutamatergic signalling in pyramidal cells and interneurons localized in stratum oriens, stratum pyramidale and stratum radiatum from WT, α7−/− and β2−/− mice between P4 and P8.
Nicotine transiently increases the frequency of miniature glutamatergic PSCs in pyramidal cells from WT and β2−/− mice, but not those from α7−/− mice
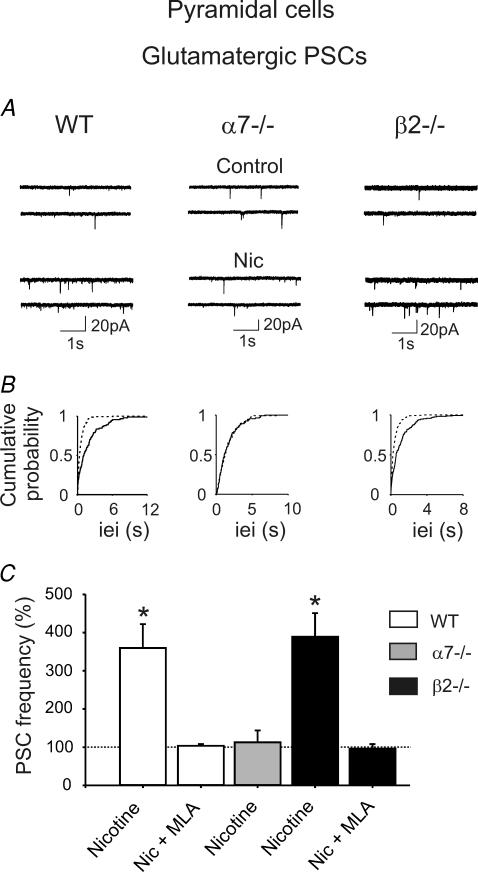
The lack of nicotine-elicited increases in interictal discharges in α7−/− mice and in β2−/− mice in the presence of MLA suggests that α7 but not β2-containing nAChRs are crucial for increasing the frequency of epileptiform bursts observed in the absence of GABAA-mediated inhibition. This may be triggered at the level of recurrent collaterals between principal cells in the CA3 area and propagate to the CA1 region via Schaffer collaterals, which originate from the same axon as recurrent collaterals (Miles & Wong, 1987). Moreover, we have previously shown that activation of presynaptic α7* nAChRs enhance glutamatergic transmission in the immature rat hippocampus (Maggi et al. 2003). Other studies have shown that in the adult rat, nicotine increases the frequency of miniature excitatory postsynaptic currents (mEPSCs) recorded from CA1 and CA3 pyramidal cells in the presence of TTX (1 μm) (Gray et al. 1996). Here, the effect of nicotine was tested on mEPSCs recorded from CA1 pyramidal cells in the presence of TTX (1 μm) and bicuculline (10 μm). mEPSCs were mediated by AMPA/KA receptors since they were blocked by the selective AMPA/KA receptor antagonist DNQX (20 μm, n = 2, not shown). In six neurons nicotine (1 μm for 3 min) enhanced the frequency of mEPSCs by 360 ± 62% (from 0.31 ± 0.11 Hz in control conditions; P < 0.05, Fig. 4) in the absence of any change in their amplitude (12.05 ± 2.83 and 9.82 ± 1.45 pA before and after nicotine application, respectively). A nicotine-elicited increase in frequency of mEPSCs was prevented by MLA 10 nm (102 ± 3% change in the presence of MLA, n = 5) and was not associated with changes in holding current or in membrane input conductance. The increase in mEPSC frequency was transient, and reversed to control values a few minutes after nicotine was washed out. Similar effects were produced when nicotine was applied to CA1 pyramidal neurons in slices obtained from β2−/− mice (n = 6). Also in this case, nicotine produced a transient increase in the frequency of miniature events by 389 ± 62% (from 0.32 ± 0.08 Hz; P < 0.05), which was blocked by preincubation of the slices with MLA (96 ± 12% of control, n = 5). No change in the amplitude of miniature events was observed before and after nicotine application (10.05 ± 1.74 and 9.81 ± 1.58 pA in the absence and presence of nicotine, respectively).
Figure 4. Nicotine enhances mini glutamatergic postsynaptic current (PSC) frequency in CA1 pyramidal neurons from WT and α7−/− mice, but not β2−/− mice.
A, traces recorded from CA1 pyramidal neurons, in the presence of TTX and bicuculline, from WT, α7−/− and β2−/− mice, respectively, before and after the application of nicotine (1 μm). B, cumulative distribution of inter-event intervals of mEPSCs recorded before (continuous line) and during nicotine application (dashed line). Note nicotine-induced changes in inter-event intervals in WT and β2−/− mice (P < 0.0001, Kolmogorov-Smirnov test) but not α7−/− mice (P > 0.05). C, each column represents nicotine-induced changes of PSC frequency as a percentage of control (horizontal line); n = 5–8; *P < 0.05. The effect in β2−/− mice is blocked by MLA but not TTX, indicating that it is mediated by presynaptic (not preterminal) α7* nAChRs.
At variance with WT and β2−/− mice, the frequency of mEPSCs recorded from the hippocampus of α7−/− mice (n = 8) was unaffected by nicotine (113 ± 31% of control, from 0.40 ± 0.19 Hz; P > 0.05). As in WT and β2−/− mice, the amplitude of mEPSCs was unaltered by nicotine (the mean amplitude values of mEPSCs recorded before and during nicotine application were 9.67 ± 0.57 and 9.32 ± 0.59 pA, respectively).
In pyramidal cells from WT and β2−/− mice, but not those from α7−/− mice, nicotine increases the frequency of spontaneous GABAA-mediated PSCs
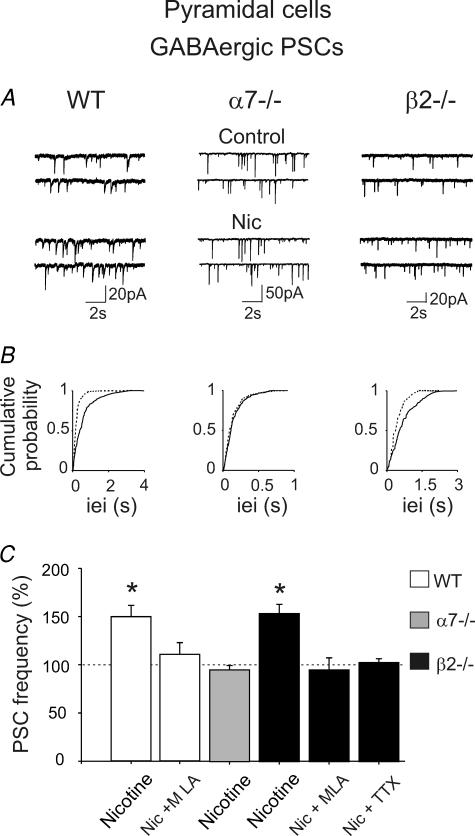
GABAergic neurons in various brain structures have been shown to express presynaptic, preterminal axonal (Lena et al. 1993) or somato-dendritic nAChRs (Frazier et al. 1998). To discriminate between presynaptic (action-potential independent) or preterminal receptors (action-potential dependent, either axonal or somato-dendritic), the frequency of spontaneous GABAA-mediated PSCs in pyramidal cells was studied in the presence and absence of TTX. These PSCs were mediated by GABAA receptors since they were blocked by bicuculline (10 μm, n = 6, not shown).
In the presence of DNQX, nicotine (1 μm for 3 min) increased the frequency of spontaneous GABAergic PSCs in WT mice (150 ± 12% of control; from 2.86 ± 0.31 Hz during control; n = 5, P < 0.05; Fig. 5). This effect was prevented by MLA 10 nm (109 ± 13% of control; n = 13). Similar results were obtained in β2−/− mice (154 ± 10% of control; from 1.78 ± 0.33 Hz during control; n = 5, P < 0.05). The effect of nicotine in β2−/− mice was blocked by MLA or TTX (95 ± 13% change, n = 5, and 103 ± 4% change, n = 5, respectively; P > 0.05), suggesting that preterminal α7* receptors were involved. Consistent with these results, in α7−/− mice, spontaneous GABA release onto pyramidal cells was not affected by nicotine (nicotine-elicited change in PSC frequency: 95 ± 5% of control, from 4.20 ± 0.54 Hz; n = 9, P > 0.05).
Figure 5. Preterminal α7* nAChR nicotine enhancement of GABAergic PSC frequency in pyramidal cells.
A, traces recorded from CA1 pyramidal neurons, in the presence of DNQX, from WT, α7−/− and β2−/− mice, respectively, before and after the application of nicotine (1 μm). B, cumulative distribution of inter-event intervals of mEPSCs recorded before (continuous line) and during nicotine application (dashed line). Note nicotine-induced changes in inter-event intervals in WT and β2−/− mice (P < 0.0001, Kolmogorov-Smirnov test), but not α7−/− mice (P > 0.05). C, each column represents nicotine-induced changes of PSC frequency as a percentage of control (horizontal line); n = 5–9; *P < 0.05. The effect in β2−/− mice is blocked by MLA and TTX, indicating that it is mediated by preterminal α7* nAChRs.
The amplitude of PSCs was not changed by nicotine. It was 11.89 ± 1.38 pA before and 10.48 ± 0.71 pA after nicotine in WT mice (n = 5, P > 0.05), 28.41 ± 4.26 pA before and 29.57 ± 4.13 pA after nicotine in α7−/− mice (n = 9, P > 0.05), 19.36 ± 2.65 pA before and 19.91 ± 2.96 pA after nicotine in β2−/− mice (n = 9, P > 0.05).
Nicotine does not affect the frequency of spontaneous glutamatergic PSCs in interneurons from WT or KO mice
In contrast with pyramidal cells, nicotine (1 μm for 3 min) did not modify the frequency of spontaneous EPSCs recorded in the presence of bicuculline from stratum oriens, stratum pyramidale and stratum radiatum interneurons (in slices obtained from WT, α7−/− and β2−/− mice of the same age; data not shown). In stratum oriens interneurons, the frequency of spontaneous EPSCs in the presence of nicotine was 100 ± 2, 106 ± 4 and 117 ± 11% of control in WT, α7−/− mice and β2−/− mice, respectively (average frequencies during control: 1.04 ± 0.12 Hz, n = 5, 0.98 ± 0.08 Hz, n = 7, 1.11 ± 0.59 Hz, n = 5, respectively; P > 0.05). In stratum pyramidale interneurons, this frequency was 112 ± 8, 103 ± 1 and 102 ± 1% of control in WT, α7−/− mice and β2−/− mice, respectively (average frequencies in control: 0.99 ± 0.41 Hz, n = 5, 1.05 ± 0.08 Hz, n = 5, 0.83 ± 0.09 Hz, n = 7, respectively; P > 0.05). In stratum radiatum interneurons, the frequency was 103 ± 19, 111 ± 7 and 102 ± 1% of control in WT, α7−/− mice and β2−/− mice, respectively (average frequencies during control: 0.78 ± 0.23 Hz, n = 6, 0.96 ± 0.10 Hz, n = 11, 0.76 ± 0.04 Hz, n = 5, respectively; P > 0.05).
Moreover, in stratum oriens, stratum pyramidale and stratum radiatum interneurons, nicotine did not modify the amplitude of the EPSCs in WT mice (from 9.00 ± 0.53 pA, n = 5, 7.94 ± 1.45 pA, n = 6, and 7.87 ± 1.32 pA, n = 6, during control, to 8.44 ± 0.92, 7.77 ± 1.21 and 8.14 ± 0.83 pA after nicotine, respectively; P > 0.05), α7−/− mice (from 9.74 ± 0.78 pA, n = 7, 8.14 ± 0.83 pA, n = 5, and 13.14 ± 2.43 pA, n = 5, during control, to 9.42 ± 1.00, 8.97 ± 0.67 and 12.57 ± 0.82 pA after nicotine, respectively; P > 0.05) and β2−/− mice (from 10.28 ± 1.54, 11.01 ± 1.81 and 12.02 ± 1.41 pA during control to 9.49 ± 1.59, 10.30 ± 1.58 and 12.10 ± 1.45 pA after nicotine, respectively; P > 0.05).
Nicotine affects the frequency of spontaneous GABAergic PSCs differently in interneurons from WT and KO mice
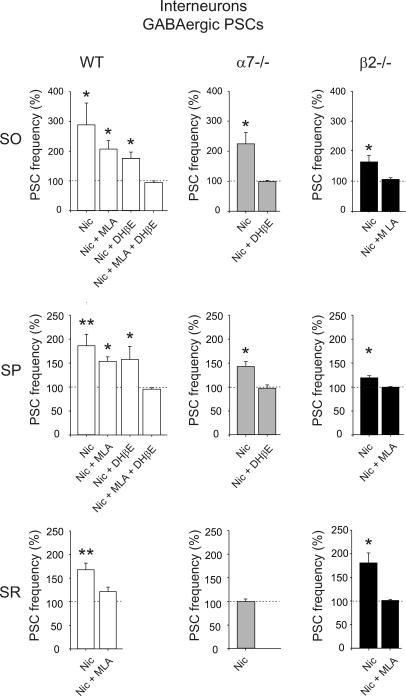
The effect of nicotine on spontaneous GABAergic PSCs (recorded in the presence of DNQX, 20 μm) was compared in interneurons from stratum oriens, stratum pyramidale and stratum radiatum in both WT and KO mice. As shown in the graphs of Fig. 6, in WT mice, nicotine enhanced the frequency of spontaneous PSCs to 289 ± 74% (P < 0.05; n = 6), to 186 ± 24% (P < 0.01; n = 11) and to 167 ± 13% (P < 0.001; n = 6) of controls in stratum oriens, stratum pyramidale and stratum radiatum, respectively. These effects were partially or completely antagonized by MLA 10 nm (207 ± 29% change after nicotine, n = 5, P < 0.05, in stratum oriens; 153 ± 10%, n = 5, P < 0.05, in stratum pyramidale; 120 ± 11%, n = 5, P > 0.05 in stratum radiatum) and DHβE 1 μm (176 ± 22% change after nicotine, n = 5, in stratum oriens; 157 ± 2%, n = 6, in stratum pyramidale, P < 0.05), indicating that they were mediated by both α7* and β2* nAChRs. Preincubating the slices with MLA and DHβE together prevented the effect of nicotine in the stratum oriens and stratum pyramidale (95 ± 6% change after nicotine, n = 5, and 96 ± 3% change, n = 4, respectively).
Figure 6. Nicotine-induced changes in the frequency of spontaneous PSCs in stratum oriens, stratum pyramidale and stratum radiatum interneurons from WT, α7−/− and β2−/− mice.
Each column represents nicotine-induced changes of PSC frequency as a percentage of control (dashed line) in GABAergic interneurons localized on stratum oriens (SO), stratum pyramidale (SP) and stratum radiatum (SR) in WT, α7−/− and β2−/− mice. Note the lack of nicotine effect on spontaneous PSCs recorded from SR interneurons of α7−/− mice. *P < 0.05; **P < 0.01; n = 4–11.
The increase in frequency of spontaneous GABAergic PSCs induced by nicotine in β2−/− mice was 162 ± 21% (n = 6, P < 0.05), 120 ± 4% (n = 9, P < 0.05) and 181 ± 21% of control (n = 6, P < 0.05) in stratum oriens, stratum pyramidale and stratum radiatum, respectively. These effects were blocked by MLA (10 nm), indicating they were mediated by α7* receptors only (changes after nicotine: 105 ± 2%, n = 5, 99 ± 1%, n = 5, and 102 ± 2%, n = 6, respectively). In α7−/− mice, nicotine significantly (P < 0.05) enhanced the frequency of PSCs recorded from stratum oriens and stratum pyramidale interneurons (to 225 ± 32%, n = 6, and to 144 ± 10%, n = 5, of controls), respectively, but had no effect on stratum radiatum interneurons (to 98 ± 5%; n = 9, P > 0.05). The potentiating effects of nicotine on interneurons of stratum oriens and pyramidale were antagonized by DHβE 1 μm, indicating that they were mediated by β2* nAChRs (to 100 ± 4%, n = 6, and to 98 ± 7%, n = 4, of controls, respectively).
Finally, the average amplitude of GABAA-mediated PSCs was not changed by the application of nicotine. In stratum oriens, stratum pyramidale, and stratum radiatum interneurons, the average amplitude of GABAergic PSCs was 26.68 ± 4.63 pA (n = 6), 16.01 ± 2.57 pA (n = 11) and 14.44 ± 1.64 pA (n = 6) during control, and 33.90 ± 8.25, 16.35 ± 2.62 and 13.89 ± 1.97 pA after nicotine, respectively (P > 0.05). In α7−/− mice, it changed from 38.69 ± 6.64 pA (n = 6), 54.50 ± 8.71 pA (n = 5) and 54.86 ± 5.65 pA (n = 9) during control, to 46.91 ± 6.66, 41.92 ± 7.31 and 56.56 ± 5.25 pA after nicotine, respectively (P > 0.05). Also in β2−/− mice, nicotine did not change the average amplitude, from 24.73 ± 3.83 pA (n = 6), 36.37 ± 10.55 pA (n = 9), and 23.56 ± 2.88 pA (n = 6) during control, to 27.58 ± 3.80, 36.09 ± 9.90 and 21.39 ± 2.85 pA after nicotine, respectively (P > 0.05).
Interestingly, nicotine did not modify the frequency of miniature GABAergic events recorded in the presence of TTX (1 μm) from interneurons localized in stratum oriens (to 110 ± 24%, n = 5, and to 95 ± 2%, n = 5, for α7−/− and β2−/− mice, respectively; P > 0.05), in stratum pyramidale (to 99 ± 2%, n = 5, and to 97 ± 2%, n = 5, for α7−/− and β2−/− mice, respectively; P > 0.05) of α7−/− and β2−/− mice as well as in stratum radiatum of β2−/− mice (to 108 ± 3%; n = 6, P > 0.05; data not shown).
In summary, our results demonstrate that both preterminal α7* and β2* nAChRs modulate GABA release onto stratum oriens and stratum pyramidale interneurons, and that only preterminal α7* receptors modulate GABA release onto stratum radiatum interneurons.
Discussion
The present findings indicate that during the first week of postnatal life, nicotine, through α7* and β2* nAChRs, exerts a powerful regulatory action on network-driven oscillatory activity in the hippocampus. Moreover, the results demonstrate that in mice lacking the α7 nAChR subunit, nicotine fails to enhance interictal discharges obtained by blocking GABAA receptors with bicuculline. In agreement with these results, we found that nicotine-elicited regulation of glutamatergic signalling occurred via presynaptic α7* nAChRs, while nicotine-elicited modulation of GABAergic transmission needed the activation of both preterminal α7* and β2* nAChRs.
nAChRs contribute to GDP regulation
In a previous study from the neonatal rat hippocampus, it was reported that nicotine is able to increase the frequency of GDPs in a concentration-dependent manner. However, the nAChR subtypes involved in that effect were not identified (Maggi et al. 2001). In the present work, taking advantage of KO mice, we have clearly demonstrated that the potentiating effects of nicotine on GDPs are mediated by both α7 and β2-containing receptors. While in α7−/− mice, the potentiating effect of nicotine on GDPs was prevented by a low concentration of DHβE, which selectively blocks β2* nAChRs (Chavez-Noriega et al. 1997), in β2−/− mice it was antagonized by MLA, which selectively blocks β2* nAChRs. Since glutamatergic terminals projecting to pyramidal cells are controlled only by α7* nAChRs, in α7−/− mice the nicotine-elicited increase in GDP frequency could be attributed to (i) the enhancement of GABA release through the activation of β2* nAChRs present on interneurons, or (ii) the enhancement of glutamate release through the activation of β2* nAChRs present on glutamatergic nerve terminals innervating GABAergic interneurons. In both cases, the increased release of GABA would exert a powerful excitatory action on principal cells. However, it is likely that an increase of GABA release via the activation of β2* nAChRs present on interneurons projecting onto stratum oriens and stratum pyramidale interneurons accounted for nicotine-induced potentiation of GDPs, since nicotine failed to affect glutamatergic activity in interneurons.
Nicotine-elicited GDP modulation seen in β2−/− mice is mediated by α7* nAChRs, as assessed by its disappearance in the presence of MLA. Our results suggest that α7* nAChRs controlling GABA release onto stratum pyramidale, stratum oriens, stratum radiatum interneurons and pyramidal cells, as well as α7* nAChRs controlling glutamate release onto pyramidal cells, participate in this effect.
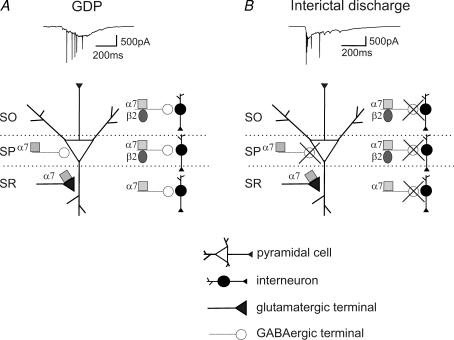
Regardless of the nAChRs involved in the potentiating effect of nicotine, the present experiments clearly indicate that, within the hippocampal network, different patterns are involved in the generation of GDPs and interictal discharges (Fig. 7A).
Figure 7. Schematic view of α7* and β2* nAChRs in the developing hippocampus.
A, GDP frequency can be modulated by (i) activation of presynaptic α7* nAChRs, controlling spontaneous glutamate release onto pyramidal cells; (ii) activation of preterminal α7* nAChRs, increasing spontaneous GABAergic PSC frequency in pyramidal cells and interneurons from stratum pyramidale, stratum radiatum and stratum oriens; and (iii) activation of preterminal β2* nAChRs, increasing spontaneous GABAergic PSC frequency in interneurons from stratum pyramidale and stratum oriens. B, in the presence of bicuculline, activation of presynaptic α7* nAChRs controlling spontaneous glutamate release onto pyramidal cells underpins the nicotine-elicited increase in interictal burst frequency. SO, stratum oriens; SP, stratum pyramidale; SR, stratum radiatum.
Activation of α7* but not β2* nAChRs increases the frequency of interictal bursts elicited by removing GABAA-mediated inhibition with bicuculline
At high concentrations, nicotine is known to cause seizures (Miner et al. 1984; Damaj et al. 1999). Although the mechanisms underlying the convulsant effects of nicotine have not been fully elucidated, this issue is important in view of the observation that several nAChR subunits are candidate genes for idiopathic epilepsies (Gotti & Clementi, 2004). By comparing different strains of mice, a positive correlation has been found between the sensitivity to nicotine-elicited seizures and the number of α bungarotoxin-binding sites in the hippocampus, indicating that α7* nAChRs are crucial for nicotine-elicited epileptiform discharges (Miner et al. 1984). Here we found that nicotine increased the frequency of interictal-like discharges caused by blocking GABAA receptors with bicuculline. In agreement with our results, it has recently been shown that nicotine increases epileptiform activity caused by bicuculline or DAP 4 in the hippocampus of adult rats (Roshan-Milani et al. 2003). However, unlike our results, nicotine-elicited increase in interictal bursts was dependent on the activation of both α7 and β2-containing receptors. Although we can not exclude the involvement of β2-containing receptors at late developmental stages, the observed differences can be attributed to the fact that, in order to block heteromeric nAChRs, Roshan-Milani et al. (2003) used high (10–30 μm) concentrations of DHβE, known to block not only β2* but also α7* nAChRs (Bertrand et al. 1992). Therefore, it is likely that presynaptic α7* nAChRs localized on recurrent glutamatergic collaterals, thought to be critical for generating bursting activity (Miles & Wong, 1987), facilitate glutamate release leading to an increase in the frequency of interictal discharges. This is in agreement with the increased sensitivity to nicotine-elicited seizures of mice expressing a gain-of-function mutated form of the α7 receptor (Broide et al. 2002). However, experiments have shown that nicotine-elicited seizures do not disappear in α7−/− mice (Franceschini et al. 2002), and that α3 and β4 nicotinic subunits, presumably located outside the hippocampus, are necessary for nicotine-elicited seizures (Salas et al. 2004). Moreover, mutations in the α4 and β2 nicotinic subunits have been shown to cause autosomal dominant nocturnal front lobe epilepsy (Steinlein et al. 1995; De Fusco et al. 2000). These mutations may facilitate the synchronization of spontaneous oscillations in thalamocortical circuits (Raggenbass & Bertrand, 2002). So far, a role of α7* nAChRs in human epilepsies remains elusive.
Our data suggest that, in mice, hippocampal presynaptic α7* nAChRs enhance glutamatergic activity and contribute to the modulation of bicuculline-induced interictal discharges during postnatal development (Fig. 7B).
Nicotine regulates glutamate release onto pyramidal cells via presynaptic α7* nAChRs
Although collaterals of the same axons may have different functional properties according to the targets they innervate (Scanziani et al. 1998), nicotine-elicited enhancement of glutamatergic transmission at Schaffer collateral–CA1 synapses was mediated, as in recurrent collaterals, by presynaptic α7* nAChRs, as suggested by the loss of this effect in α7−/− mice.
α7* nAChRs have been shown to be expressed on glutamatergic nerve terminals (Gray et al. 1996), on the soma of GABAergic interneurons (Alkondon et al. 1998) and on the dendrites of CA1 pyramidal cells (Ji et al. 2001; but see Khiroug et al. 2003). The present experiments were routinely performed in the presence of bicuculline, and therefore an indirect effect of nicotine via α7* nAChRs located on GABAergic interneurons could not have been detected. The possibility of a direct effect on the dendrites of CA1 principal cells is unlikely since we failed to detect any change in holding current or input conductance during nicotine application, suggesting that, if present, α7* receptors should be expressed at very low density on dendrites.
Nicotine does not affect the release of glutamate onto GABAergic interneurons
In contrast with the present data, previous results have demonstrated that nicotine can activate presynaptic/preterminal α3β4* receptors and increase the frequency of spontaneous EPSCs in stratum radiatum interneurons of juvenile rats (Alkondon & Albuquerque, 2002). This apparent discrepancy could be attributed to the different expression of α3β4* receptors in rats and mice or to the different experimental conditions used (neonatal versus juvenile animals). However, we cannot exclude the possibility that the concentration of nicotine (1 μm) used in the present study was too low to produce a statistically significant effect through α3β4* receptors, since the EC50 of nicotine for α3β4* receptors varies between 28 and 80 μm for rat and human receptors heterologously expressed (Chavez-Noriega et al. 1997; Xiao et al. 1998). Numerous studies, including the present one, have reported an effect of nicotine at low concentration mediated by presynaptic or preterminal α7* nAChRs, although the EC50 for mouse α7* nAChRs is also in the same order as for α3β4* receptors (38 ± 5 μm for mouse α7* nAChRs, Macor et al. 2001). This may be due to the fact that α7* receptors undergo fast concentration-dependent desensitization, whereas α3β4* receptors do not: as a consequence of the preferential desensitization of fully liganded α7* nAChRs, channels are open only within a limited concentration range, corresponding to what would produce a low fractional occupancy of the binding sites. The application of a low concentration of agonist can sustain that condition and maintain channel activation for several seconds during agonist application. Thus, the EC50 of α7* nAChRs calculated with net charge analysis is one to two orders of magnitude smaller than the ‘classical’ EC50 calculated with peak amplitude, the latter being biased by fast desensitization of the receptor (Papke & Porter Papke, 2002; Papke, 2005).
Nicotine regulates GABA release via preterminal α7* and β2* nAChRs
Our results are in agreement with previous studies showing that α7* and β2* receptors are present on interneurons (Frazier et al. 1998; Alkondon et al. 2000). Thus, in β2−/− mice, preterminal (axonal or somato-dendritic) α7* nAChRs mediate nicotine-induced potentiation of GABA release onto pyramidal cells and interneurons. Moreover, in α7−/− mice, preterminal β2* nAChRs modulate GABA release onto stratum oriens and stratum pyramidale interneurons, but not pyramidal cells or stratum radiatum interneurons. This is in contrast with previous data from adult rats suggesting that activation of β2* nAChR in stratum radiatum interneurons increases the frequency of spontaneous IPSCs (Alkondon & Albuquerque, 2001). Developmental differences in the expression of nAChRs or between different species may account for this apparent contradiction.
nAChRs expression during hippocampal development
In the rat hippocampus, mRNAs for the α7 and β2 subunits are present early during embryogenesis, but the expression patterns of α7* nAChR and β2* nAChRs differ. The density of [3H]epibatidine-binding sites, an indicator of heteromeric nAChR expression, remains stable (Tribollet et al. 2004) during postnatal development. Conversely, in the CA1 region of the hippocampus, both the expression of α7 mRNA and the density of [125I]-α-bungarotoxin-binding sites, an indicator of α7* nAChR expression, are particularly high during the first postnatal week and decrease subsequently (Shacka & Robinson, 1998; Tribollet et al. 2004). These data suggest that in the hippocampus, the balance between α7* and β2* nAChRs changes during postnatal development. This may lead to differences in nicotine-induced modulation of synaptic and network activity in the immature and in the adult hippocampus.
nAChRs and network oscillations during postnatal development
Synchronized oscillatory activity, such as GDPs, constitutes a peculiar feature of developing brain. GDPs have been recorded also in vivo in rat pups where they occur during immobility periods, sleep and feeding (Leinekugel et al. 2002). In this respect GDPs can be seen as a primordial form of synchrony between neurons, which precedes the development of the theta and gamma-rhythm.
Similar to GDPs, ‘waves’ of correlated activity have been found in several brain regions during postnatal development (review in O'Donovan, 1999). These waves are thought to be crucial for establishing neural connectivity. In the immature retina for example, blocking high-affinity nAChRs in the desensitized state with epibatidine leads to disruption of correlated network activity and to changes in the pattern of eye-specific connections from the retina to the lateral geniculate nucleus (Penn et al. 1998). α3−/− mice have altered retinal waves and β2−/− mice completely lack retinal waves, while both show delayed refinement of individual retinal ganglion cell dendrites (Bansal et al. 2000). In β2−/− mice, the pattern of retinogeniculate and retinocollicular projections, as well as the visual acuity, were found to be altered (Rossi et al. 2001). These studies demonstrate that nAChRs exert a crucial role in the epigenetic maturation of the visual system.
Interestingly, it has been recently reported that intrinsically bursting starburst cells underline the generation of retinal waves in perinatal rabbit retinas (Zheng et al. 2006). Nicotine, by shortening the duration of the afterhyperpolarization, increased the frequency of retinal waves. Therefore, the possibility exists that a similar mechanism is involved in nicotine-induced increase in frequency of GDPs, which at least in CA3 pyramidal cells can be triggered by intrinsic bursts (Sipila et al. 2005). However, unlike retinal waves, and in agreement with a previous report from the rat hippocampus (Cole & Nicoll, 1984), nicotine was unable to modify the afterhyperpolarization following brief trains of action potentials recorded from CA1 pyramidal cells in the immature hippocampus from WT mice in the presence of DNQX, bicuculline and d aminophosphonovalerate (data not shown).
In the developing hippocampus, GDPs are thought to be instructive in enhancing synaptic efficacy and in ‘unsilencing’ silent connections at emerging synapses (Kasyanov et al. 2004). They may contribute to the selective stabilization of neuronal circuits as in the visual cortex, but this remains to be demonstrated. Although activation of nAChRs powerfully regulates GDP frequency, the observation that GDPs are still present in α7 and β2 KO mice suggests that activation of α7* and β2* nAChRs is not essential for GDP generation.
In conclusion, our observations demonstrate that, in the developing hippocampus, a low concentration of nicotine modulates synaptic activity and network synchronization. The potency of these effects and the nAChR subtypes involved were not homogeneous and varied among different hippocampal layers, indicating that, during postnatal development, nAChRs participate in different ways in the fine regional tuning of immature hippocampal neurotransmission, which may play a crucial role in wiring the hippocampal circuitry.
Acknowledgments
This work was supported by funds from the Institut Pasteur, Collège de France, Centre National de la Recherche Scientifique, MILDT, ARC, Commission of the European communities to J.P.C., from Telethon (GGP030416B) to E.C., and from RFBR to V.S. C.L.M. was the recipient of a fellowship from the MENRT. We thank Drs L. Prado de Carvalho and P. Faure for their critical review and comments on the manuscript and Drs Marubio and Arthur Beaudet for the gift of α7 knock-out mice.
References
- Adams CE, Broide RS, Chen Y, Winzer-Serhan UH, Henderson TA, Leslie FM, Freedman R. Development of the alpha7 nicotinic cholinergic receptor in rat hippocampal formation. Brain Res Dev Brain Res. 2002;139:175–187. doi: 10.1016/s0165-3806(02)00547-3. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. Nicotinic acetylcholine receptor alpha7 and alpha4beta2 subtypes differentially control GABAergic input to CA1 neurons in rat hippocampus. J Neurophysiol. 2001;86:3043–3055. doi: 10.1152/jn.2001.86.6.3043. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. A non-alpha7 nicotinic acetylcholine receptor modulates excitatory input to hippocampal CA1 interneurons. J Neurophysiol. 2002;87:1651–1654. doi: 10.1152/jn.00708.2001. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. The nicotinic acetylcholine receptor subtypes and their function in the hippocampus and cerebral cortex. Prog Brain Res. 2004;145:109–120. doi: 10.1016/S0079-6123(03)45007-3. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Albuquerque EX. Alpha-bungarotoxin and methyllycaconitine-sensitive nicotinic receptors mediate fast synaptic transmission in interneurons of rat hippocampal slices. Brain Res. 1998;810:257–263. doi: 10.1016/s0006-8993(98)00880-4. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Almeida LE, Randall WR, Albuquerque EX. Nicotine at concentrations found in cigarette smokers activates and desensitizes nicotinic acetylcholine receptors in CA1 interneurons of rat hippocampus. Neuropharmacology. 2000;39:2726–2739. doi: 10.1016/s0028-3908(00)00156-8. [DOI] [PubMed] [Google Scholar]
- Aramakis VB, Hsieh CY, Leslie FM, Metherate R. A critical period for nicotine-induced disruption of synaptic development in rat auditory cortex. J Neurosci. 2000;20:6106–6116. doi: 10.1523/JNEUROSCI.20-16-06106.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal A, Singer JH, Hwang BJ, Xu W, Beaudet A, Feller MB. Mice lacking specific nicotinic acetylcholine receptor subunits exhibit dramatically altered spontaneous activity patterns and reveal a limited role for retinal waves in forming ON and OFF circuits in the inner retina. J Neurosci. 2000;20:7672–7681. doi: 10.1523/JNEUROSCI.20-20-07672.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Ben Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol. 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand D, Bertrand S, Ballivet M. Pharmacological properties of the homomeric alpha 7 receptor. Neurosci Lett. 1992;146:87–90. doi: 10.1016/0304-3940(92)90179-b. [DOI] [PubMed] [Google Scholar]
- Broide RS, Salas R, Ji D, Paylor R, Patrick JW, Dani JA, De Biasi M. Increased sensitivity to nicotine-induced seizures in mice expressing the L250T alpha 7 nicotinic acetylcholine receptor mutation. Mol Pharmacol. 2002;61:695–705. doi: 10.1124/mol.61.3.695. [DOI] [PubMed] [Google Scholar]
- Chang KT, Berg DK. Nicotinic acetylcholine receptors containing alpha7 subunits are required for reliable synaptic transmission in situ. J Neurosci. 1999;19:3701–3710. doi: 10.1523/JNEUROSCI.19-10-03701.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux JP, Edelstein SJ. Nicotinic Acetylcholine Receptors. New York: Odile Jacob; 2005. [Google Scholar]
- Chavez-Noriega LE, Crona JH, Washburn MS, Urrutia A, Elliott KJ, Johnson EC. Pharmacological characterization of recombinant human neuronal nicotinic acetylcholine receptors h alpha 2 beta 2, h alpha 2 beta 4, h alpha 3 beta 2, h alpha 3 beta 4, h alpha 4 beta 2, h alpha 4 beta 4 and h alpha 7 expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1997;280:346–356. [PubMed] [Google Scholar]
- Cherubini E, Gaiarsa JL, Ben Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 1991;14:515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Cole AE, Nicoll RA. The pharmacology of cholinergic excitatory responses in hippocampal pyramidal cells. Brain Res. 1984;305:283–290. doi: 10.1016/0006-8993(84)90434-7. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Glassco W, Dukat M, Martin BR. Pharmacological characterization of nicotine-induced seizures in mice. J Pharmacol Exp Ther. 1999;291:1284–1291. [PubMed] [Google Scholar]
- Dani JA, Heinemann S. Molecular and cellular aspects of nicotine abuse. Neuron. 1996;16:905–908. doi: 10.1016/s0896-6273(00)80112-9. [DOI] [PubMed] [Google Scholar]
- De Fusco M, Becchetti A, Patrignani A, Annesi G, Gambardella A, Quattrone A, Ballabio A, Wanke E, Casari G. The nicotinic receptor beta 2 subunit is mutant in nocturnal frontal lobe epilepsy. Nat Genet. 2000;26:275–276. doi: 10.1038/81566. [DOI] [PubMed] [Google Scholar]
- Ernst M, Moolchan ET, Robinson ML. Behavioral and neural consequences of prenatal exposure to nicotine. J Am Acad Child Adolesc Psychiatry. 2001;40:630–641. doi: 10.1097/00004583-200106000-00007. [DOI] [PubMed] [Google Scholar]
- Franceschini D, Paylor R, Broide R, Salas R, Bassetto L, Gotti C, De Biasi M. Absence of alpha7-containing neuronal nicotinic acetylcholine receptors does not prevent nicotine-induced seizures. Brain Res Mol Brain Res. 2002;98:29–40. doi: 10.1016/s0169-328x(01)00309-6. [DOI] [PubMed] [Google Scholar]
- Frazier CJ, Rollins YD, Breese CR, Leonard S, Freedman R, Dunwiddie TV. Acetylcholine activates an alpha-bungarotoxin-sensitive nicotinic current in rat hippocampal interneurons, but not pyramidal cells. J Neurosci. 1998;18:1187–1195. doi: 10.1523/JNEUROSCI.18-04-01187.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaschuk O, Linn J, Eilers J, Konnerth A. Large-scale oscillatory calcium waves in the immature cortex. Nat Neurosci. 2000;3:452–459. doi: 10.1038/74823. [DOI] [PubMed] [Google Scholar]
- Gotti C, Clementi F. Neuronal nicotinic receptors: from structure to pathology. Prog Neurobiol. 2004;74:363–396. doi: 10.1016/j.pneurobio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;383:713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- Gu X, Olson EC, Spitzer NC. Spontaneous neuronal calcium spikes and waves during early differentiation. J Neurosci. 1994;14:6325–6335. doi: 10.1523/JNEUROSCI.14-11-06325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D, Lape R, Dani JA. Timing and location of nicotinic activity enhances or depresses hippocampal synaptic plasticity. Neuron. 2001;31:131–141. doi: 10.1016/s0896-6273(01)00332-4. [DOI] [PubMed] [Google Scholar]
- Johns JM, Louis TM, Becker RF, Means LW. Behavioral effects of prenatal exposure to nicotine in guinea pigs. Neurobehav Toxicol Teratol. 1982;4:365–369. [PubMed] [Google Scholar]
- Kasa P. The cholinergic systems in brain and spinal cord. Prog Neurobiol. 1986;26:211–272. doi: 10.1016/0301-0082(86)90016-x. [DOI] [PubMed] [Google Scholar]
- Kasyanov AM, Safiulina VF, Voronin LL, Cherubini E. GABA-mediated giant depolarizing potentials as coincidence detectors for enhancing synaptic efficacy in the developing hippocampus. Proc Natl Acad Sci U S A. 2004;101:3967–3972. doi: 10.1073/pnas.0305974101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawa K. Acute synaptic modulation by nicotinic agonists in developing cerebellar Purkinje cells of the rat. J Physiol. 2002;538:87–102. doi: 10.1113/jphysiol.2001.012885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazipov R, Khalilov I, Tyzio R, Morozova E, Ben Ari Y, Holmes GL. Developmental changes in GABAergic actions and seizure susceptibility in the rat hippocampus. Eur J Neurosci. 2004;19:590–600. doi: 10.1111/j.0953-816x.2003.03152.x. [DOI] [PubMed] [Google Scholar]
- Khiroug L, Giniatullin R, Klein RC, Fayuk D, Yakel JL. Functional mapping and Ca2+ regulation of nicotinic acetylcholine receptor channels in rat hippocampal CA1 neurons. J Neurosci. 2003;23:9024–9031. doi: 10.1523/JNEUROSCI.23-27-09024.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinekugel X, Khazipov R, Cannon R, Hirase H, Ben Ari Y, Buzsaki G. Correlated bursts of activity in the neonatal hippocampus in vivo. Science. 2002;296:2049–2052. doi: 10.1126/science.1071111. [DOI] [PubMed] [Google Scholar]
- Lena C, Changeux JP, Mulle C. Evidence for ‘preterminal’ nicotinic receptors on GABAergic axons in the rat interpeduncular nucleus. J Neurosci. 1993;13:2680–2688. doi: 10.1523/JNEUROSCI.13-06-02680.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Briggs SJ, Christopher NC, Rose JE. Prenatal nicotine exposure and cognitive performance in rats. Neurotoxicol Teratol. 1993;15:251–260. doi: 10.1016/0892-0362(93)90006-a. [DOI] [PubMed] [Google Scholar]
- Linnet KM, Dalsgaard S, Obel C, Wisborg K, Henriksen TB, Rodriguez A, Kotimaa A, Moilanen I, Thomsen PH, Olsen J, Jarvelin MR. Maternal lifestyle factors in pregnancy risk of attention deficit hyperactivity disorder and associated behaviors: review of the current evidence. Am J Psychiatry. 2003;160:1028–1040. doi: 10.1176/appi.ajp.160.6.1028. [DOI] [PubMed] [Google Scholar]
- Macor JE, Gurley D, Lanthorn T, Loch J, Mack RA, Mullen G, Tran O, Wright N, Gordon JC. The 5 HT3 antagonist tropisetron (ICS 205–930) is a potent and selective alpha7 nicotinic receptor partial agonist. Bioorg Med Lett. 2001;11:319–321. doi: 10.1016/s0960-894x(00)00670-3. [DOI] [PubMed] [Google Scholar]
- Maggi L, Le Magueresse C, Changeux JP, Cherubini E. Nicotine activates immature ‘silent’ connections in the developing hippocampus. Proc Natl Acad Sci U S A. 2003;100:2059–2064. doi: 10.1073/pnas.0437947100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi L, Sher E, Cherubini E. Regulation of GABA release by nicotinic acetylcholine receptors in the neonatal rat hippocampus. J Physiol. 2001;536:89–100. doi: 10.1111/j.1469-7793.2001.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGehee DS. Nicotinic receptors and hippocampal synaptic plasticity…it's all in the timing. Trends Neurosci. 2002;25:171–172. doi: 10.1016/s0166-2236(00)02127-5. [DOI] [PubMed] [Google Scholar]
- Miles R, Wong RK. Latent synaptic pathways revealed after tetanic stimulation in the hippocampus. Nature. 1987;329:724–726. doi: 10.1038/329724a0. [DOI] [PubMed] [Google Scholar]
- Miner LL, Marks MJ, Collins AC. Classical genetic analysis of nicotine-induced seizures and nicotinic receptors. J Pharmacol Exp Ther. 1984;231:545–554. [PubMed] [Google Scholar]
- O'Donovan MJ. The origin of spontaneous activity in developing networks of the vertebrate nervous system. Curr Opin Neurobiol. 1999;9:94–104. doi: 10.1016/s0959-4388(99)80012-9. [DOI] [PubMed] [Google Scholar]
- Orr-Urtreger A, Goldner FM, Saeki M, Lorenzo I, Goldberg L, De Biasi M, Dani JA, Patrick JW, Beaudet AL. Mice deficient in the alpha7 neuronal nicotinic acetylcholine receptor lack alpha-bungarotoxin binding sites and hippocampal fast nicotinic currents. J Neurosci. 1997;17:9165–9171. doi: 10.1523/JNEUROSCI.17-23-09165.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL. Estimation of both the potency and efficacy of alpha7 nAChR agonists from single-concentration responses. Life Sci. 2005;78:2812–2819. doi: 10.1016/j.lfs.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Papke RL, Porter Papke JK. Comparative pharmacology of rat and human alpha7 nAChR conducted with net charge analysis. Br J Pharmacol. 2002;137:49–61. doi: 10.1038/sj.bjp.0704833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn AA, Riquelme PA, Feller MB, Shatz CJ. Competition in retinogeniculate patterning driven by spontaneous activity. Science. 1998;279:2108–2112. doi: 10.1126/science.279.5359.2108. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Lena C, Bessis A, Lallemand Y, Le Novere N, Vincent P, Pich EM, Brulet P, Changeux JP. Abnormal avoidance learning in mice lacking functional high-affinity nicotine receptor in the brain. Nature. 1995;374:65–67. doi: 10.1038/374065a0. [DOI] [PubMed] [Google Scholar]
- Raggenbass M, Bertrand D. Nicotinic receptors in circuit excitability and epilepsy. J Neurobiol. 2002;53:580–589. doi: 10.1002/neu.10152. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Levin ED. Cognitive effects of nicotine. Biol Psychiatry. 2001;49:258–267. doi: 10.1016/s0006-3223(00)01094-5. [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Roshan-Milani S, Ferrigan L, Khoshnood MJ, Davies CH, Cobb SR. Regulation of epileptiform activity in hippocampus by nicotinic acetylcholine receptor activation. Epilepsy Res. 2003;56:51–65. doi: 10.1016/j.eplepsyres.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Rossi FM, Pizzorusso T, Porciatti V, Marubio LM, Maffei L, Changeux JP. Requirement of the nicotinic acetylcholine receptor beta 2 subunit for the anatomical and functional development of the visual system. Proc Natl Acad Sci U S A. 2001;98:6453–6458. doi: 10.1073/pnas.101120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Cook KD, Bassetto L, De Biasi M. The alpha3 and beta4 nicotinic acetylcholine receptor subunits are necessary for nicotine-induced seizures and hypolocomotion in mice. Neuropharmacology. 2004;47:401–407. doi: 10.1016/j.neuropharm.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Scanziani M, Gahwiler BH, Charpak S. Target cell-specific modulation of transmitter release at terminals from a single axon. Proc Natl Acad Sci U S A. 1998;95:12004–12009. doi: 10.1073/pnas.95.20.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shacka JJ, Robinson SE. Postnatal developmental regulation of neuronal nicotinic receptor subunit alpha 7 and multiple alpha 4 and beta 2 mRNA species in the rat. Brain Res Dev Brain Res. 1998;109:67–75. doi: 10.1016/s0165-3806(98)00058-3. [DOI] [PubMed] [Google Scholar]
- Sipila ST, Huttu K, Soltesz I, Voipio J, Kaila K. Depolarizing GABA acts on intrinsically bursting pyramidal neurons to drive giant depolarizing potentials in the immature hippocampus. J Neurosci. 2005;25:5280–5289. doi: 10.1523/JNEUROSCI.0378-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinlein OK, Mulley JC, Propping P, Wallace RH, Phillips HA, Sutherland GR, Scheffer IE, Berkovic SF. A missense mutation in the neuronal nicotinic acetylcholine receptor alpha 4 subunit is associated with autosomal dominant nocturnal frontal lobe epilepsy. Nat Genet. 1995;11:201–203. doi: 10.1038/ng1095-201. [DOI] [PubMed] [Google Scholar]
- Tribollet E, Bertrand D, Marguerat A, Raggenbass M. Comparative distribution of nicotinic receptor subtypes during development, adulthood and aging: an autoradiographic study in the rat brain. Neuroscience. 2004;124:405–420. doi: 10.1016/j.neuroscience.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Wong RO, Chernjavsky A, Smith SJ, Shatz CJ. Early functional neural networks in the developing retina. Nature. 1995;374:716–718. doi: 10.1038/374716a0. [DOI] [PubMed] [Google Scholar]
- Wong RK, Traub RD. Synchronized burst discharge in disinhibited hippocampal slice. I. Initiation in CA2–CA3 region. J Neurophysiol. 1983;49:442–458. doi: 10.1152/jn.1983.49.2.442. [DOI] [PubMed] [Google Scholar]
- Wong RK, Traub RD, Miles R. Cellular basis of neuronal synchrony in epilepsy. Adv Neurol. 1986;44:583–592. [PubMed] [Google Scholar]
- Xiao Y, Meyer EL, Thompson JM, Surin A, Wroblewski J, Kellar KJ. Rat alpha3/beta4 subtype of neuronal nicotinic acetylcholine receptor stably expressed in a transfected cell line: pharmacology of ligand binding and function. Mol Pharmacol. 1998;54:322–333. doi: 10.1124/mol.54.2.322. [DOI] [PubMed] [Google Scholar]
- Zheng J, Lee S, Zhou ZJ. A transient network of intrinsically bursting starburst cells underlies the generation of retinal waves. Nat Neurosci. 2006;9:363–371. doi: 10.1038/nn1644. [DOI] [PubMed] [Google Scholar]
- Zoli M, Lena C, Picciotto MR, Changeux JP. Identification of four classes of brain nicotinic receptors using beta2 mutant mice. J Neurosci. 1998;18:4461–4472. doi: 10.1523/JNEUROSCI.18-12-04461.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]