Abstract
The flowing blood generates shear stress at the endothelial cell surface. In endothelial cells, NAD(P)H oxidase complexes have been identified as major sources of superoxide anion (·O2−) formation. In this study, we analysed the effect of laminar shear stress on ·O2− formation by cytochrome c reduction assay and on NAD(P)H oxidase subunit expression by standard calibrated competitive reverse transcription-polymerase chain reaction and Western blot in human endothelial cells. Primary cultures of human umbilical vein endothelial cells were exposed to laminar shear stress in a cone-and-plate viscometer for up to 24 h. Short-term application of shear stress transiently induced ·O2− formation. This was inhibited by NAD(P)H oxidase inhibitor gp91ds-tat, but NAD(P)H oxidase subunit expression was unchanged. Long-term arterial laminar shear stress (30 dyne cm−2, 24 h) down-regulated ·O2− formation, and mRNA and protein expression of NAD(P)H oxidase subunits Nox2/gp91phox and p47phox. In parallel, endothelial NO formation and eNOS, but not Cu/Zn SOD, protein expression was increased. Down-regulation of ·O2− formation, gp91phox and p47phox expression by long-term laminar shear stress was blocked by l-NAME. NO donor DETA-NO down-regulates ·O2− formation, gp91phox and p47phox expression in static cultures. In conclusion, our data suggest a transient activation of ·O2− formation by short-term shear stress, followed by a down-regulation of endothelial NAD(P)H oxidase in response to long-term laminar shear stress. NO-mediated down-regulation by shear stress preferentially affects the gp91phox/p47phox-containing NAD(P)H oxidase complex. This mechanism might contribute to the regulation of endothelial NO/·O2− balance and the vasoprotective potential of physiological levels of laminar shear stress.
Oxidative stress and increased generation of reactive oxygen species like superoxide anions (·O2−) has been associated with endothelial dysfunction and clinical risk factors of atherosclerosis (Griendling & FitzGerald, 2003). Furthermore, superoxide anions can reduce NO availability by peroxynitrite (ONOO−) formation (Bachschmid et al. 2005). The major sources of endothelial superoxide anion generation are NAD(P)H oxidases (Gorlach et al. 2000). The endothelial NAD(P)H oxidase complex is composed of a membrane-bound flavocytochrome b558 consisting of gp91phox/Nox2 and p22phox and two cytosolic subunits (p47phox and p67phox) (Jones et al. 1996). The subunit gp91phox seems to be the limiting subunit of the NAD(P)H oxidase complex in endothelial cells (Rueckschloss et al. 2001).
Endothelial cells in vivo are constantly exposed to shear stress by the flowing blood. The impact of shear stress on endothelial superoxide anion formation is not clear. Oscillatory shear stress induced the generation of ·O2− in endothelial cells (De Keulenaer et al. 1998). This increased endothelial ·O2− formation in response to oscillatory shear stress involved a p47phox-containing NAD(P)H oxidase complex (Hwang et al. 2003a, b) and xanthine oxidase (McNally et al. 2003). Short-term application of pulsatile shear stress also increased ·O2− formation in bovine and murine endothelial cells (Go et al. 1999; Hwang et al. 2003b). However, the impact of long-term application of laminar shear stress on ·O2− formation in primary cultures of human endothelial cells has not been studied so far.
Increased endothelial NO synthase (eNOS) expression in response to long-term shear stress has been previously shown in bovine endothelial cells (Nishida et al. 1992). Short-term and long-term endothelial NO formation by shear stress can involve different mechanisms. Shear stress-induced NO production of an endothelium-intact arterial segment, as assessed by changes in the tone of a preconstricted endothelium-denuded detector ring, was biphasic and consisted of an initial transient Ca2+-dependent phase followed by a Ca2+-independent plateau phase (Ayajiki et al. 1996). While the first phase mainly represents a functional activation of eNOS, the second phase is accompanied by an up-regulation of eNOS expression. In addition, shear stress-dependent up-regulation of eNOS blocked activation of the caspase cascade in response to apoptosis-inducing exogenous oxygen radicals in endothelial cells (Dimmeler et al. 1999). Therefore, a major vasoprotective mechanism of shear stress could be the formation of NO (Fleming & Busse, 2003). The role of NO in the shear stress-dependent regulation of ·O2− formation is not known. In addition, regulation of NAD(P)H oxidase in response to shear stress is not well understood.
Therefore, we determined the ·O2− formation and expression of different NAD(P)H oxidase subunits in response to short-term and long-term laminar shear stress in human umbilical vein endothelial cells (HUVEC). Furthermore, the role of NO on NAD(P)H oxidase expression was analysed.
Methods
Cell culture and application of shear stress
Cell culture reagents and chemicals were purchased from Sigma-Aldrich, Munich, Germany, except when otherwise specified. Primary cultures of human umbilical vein endothelial cells (HUVEC) were isolated with collagenase II as previously described (Jaffe et al. 1973). The use of human umbilical vein endothelial cells for this study was approved (EK124082003) and followed the guidelines of the hospital's Ethical Committee at our University of Technology Dresden, Germany. In order to minimize variations of primary cultures, isolated endothelial cells were pooled and subsequently separated in medium M199 with 1.25 g l−1 sodium bicarbonate, 100 mg l−1l-glutamine (Life Technologies), supplemented with 10% fetal calf serum, 100 000 U l−1 penicillin, 100 mg l−1 streptomycin, 250 mg l−1 fungizone (Life Technologies) and 16.7 μg l−1 endothelial cell growth supplement (C. C. Pro, Neustadt, Germany). HUVEC were subjected to laminar shear stress in a cone-and-plate viscometer as described (Morawietz et al. 2000; Schubert et al. 2000). Laminar shear stress of 1–50 dyne cm−2 (0.1–5 N m−2) was applied in a humified environment with 5% CO2 at 37°C. Each sample was accompanied by a control from the same cell preparation incubated for the same period of time without application of shear stress. In order to keep the cell culture medium volume constant and to avoid a spill-over of the medium even at high rotational speed, for application of arterial levels of shear stress (15, 30, or 50 dyne cm−2) 5% dextran (Mr 71 400) was added to the cell culture medium to increase the viscosity of the medium 2.95-fold from 0.007 dyne cm−2 to 0.02065 dyne cm−2. In these experiments, each cell culture dish was accompanied by two controls from the same HUVEC preparation incubated under static conditions with cell culture medium supplemented with or without 5% dextran for 24 h. Dextran had no effect on mRNA expression in this study (not shown). The achievement of equal degrees of shear stress at lower rotational speed by using additional dextran has been shown to give equal results (Malek & Izumo, 1992) and did not affect cell viability, detachment or increased release of lactate dehydrogenase (LDH) into the medium as an indicator of cell integrity (Schubert et al. 2000).
In further studies, cells were incubated with the NO synthase inhibitor Nω-nitro-l-arginine methyl ester hydrochloride (l-NAME, 500 μmol l−1) or the PI3 kinase inhibitor Ly294002 (10 μmol l−1) during the application of shear stress (up to 24 h). In addition, static HUVEC were treated with the NO donor 2,2‘-(hydroxynitrosohydrazino)bis-ethanamine (DETA–NO) (500 μmol l−1) for up to 24 h.
Superoxide anion measurements
The amount of ·O2− was determined by cytochrome c assay. In order to quantify the ·O2−-related part, this reactive oxygen species was selectively measured by addition of SOD and catalase. Confluent HUVEC were subjected to laminar shear stress (30 dyne cm−2) for the indicated times (2, 8, 24 h) in medium M199 with 10% fetal calf serum without phenol red containing 5% dextran. Each experiment involved four dishes from the same primary HUVEC preparation (two dishes exposed to shear stress, two incubated in parallel for the same period of time without shear stress), and two dishes with the same amount of corresponding medium without cells (one treated like shear stress samples, one static control). Laminar shear stress was applied continuously for the same period of time to three of the six dishes (two with HUVEC, one control with medium). In two dishes with HUVEC (one with continuous exposure to laminar shear stress, and one as internal control without shear stress), superoxide dismutase (SOD: 40 μg ml−1, Sigma) and catalase (CAT: 380 U ml−1, ICN) were added 1.5 h before the measurement to the medium. After an additional 30 min, cytochrome c (40 μmol l−1) was added to all dishes. While HUVEC were continuously exposed to shear stress, every additional 30 min reduction of cytochrome c was determined spectrophotometrically at 550 nm in all dishes.
The NADPH oxidase-specific inhibitor gp91ds-tat prevents the intracellular activation of the NADPH oxidase complex by inhibiting p47phox association with gp91phox (Rey et al. 2001). The inhibitor gp91ds-tat (100 μmol l−1) was added 30 min before application of short-term shear stress (2 h, 30 dyne cm−2) and formation of reactive oxygen species was determined by cytochrome c assay. The samples were normalized to internal controls without or with shear stress containing a peptide with a scrambled gp91 sequence (scramb-tat) at the same concentration.
In the experiments using the NO donor DETA-NO, cells were treated with 500 μmol l−1 DETA-NO (Calbiochem) for 24 h and cytochrome c reduction assay performed as described. All experiments were normalized to the internal control with corresponding medium without cells in order to eliminate cell-independent changes of absorption. The generation of ·O2− was normalized to protein content of HUVEC determined by BCA Protein Assay (Pierce Biotechnology, Inc., Rockford, IL, USA).
Nitric oxide measurements
Nitrite, the major metabolite of NO in aqueous solution, release was measured using the Griess reaction (Osanai et al. 2000; Wessells et al. 2006). One hundred microlitres of endothelial cell supernatant were removed, and 100 μl Griess reagent containing sulfanilamide (50 μl of a 1% solution) and N-(1-naphthyl)ethylenediamine (50 μl of a 0.1% solution) was added to each tube for diazotization of sulfanilic acid by NO. After 5–10 min incubation at room temperature for full colour development, the nitrite release was measured as the increase in absorbance at 540 nm (Biowave UV/Vis Diode Array Spectrophotometer, Biochrom, Cambridge, UK) and compared with known concentrations of nitrite using a standard curve (0–50 μmol l−1). Absorbance was measured and nitrite release determined by use of linear regression analysis (y =ax+b, R2 > 0.99).
RNA isolation and RT-PCR
Total RNA from HUVEC was isolated using TRI Reagent. In the reverse transcriptase (RT) reaction, equal amounts of total RNA (500 ng) from HUVEC were incubated for 3 min at 70°C, and subsequently reverse transcribed into cDNA using random hexamer primers and SuperScript™ III reverse transcriptase (Invitrogen, Karlsruhe, Germany) for 1 h at 42°C. The mRNA expression of NAD(P)H oxidase subunits gp91phox, p22phox, p47phox and p67phox was quantified as previously described (Duerrschmidt et al. 2000; Rueckschloss et al. 2001). All PCR experiments were furthermore normalized to 18S rRNA RT-PCR reactions.
Protein isolation and Western blot analysis
Protein isolation and Western blot analysis was performed as described (Schubert et al. 2000) using antibodies specific for gp91phox (kindly provided by Mark T. Quinn, Montana State University, Bozeman, MT, USA) (Rueckschloss et al. 2002), p67phox, p47phox, eNOS (BD Transduction Laboratories, San Diego, CA, USA) and Cu/Zn SOD (Novacastra Laboratories Ltd, Newcastle upon Tyne, UK). The protein expression was detected with Western Lightning Chemiluminescence Reagent Plus (PerkinElmer Life Sciences, Boston, MA, USA) and quantified using AIDA Image Analyser software (Raytest, Berlin, Germany).
Statistics
Data are shown as means ±s.e.m. Statistical analysis was performed with the ANOVA procedure followed by Bonferroni's method (multiple comparison) or Student's t test (SigmaStat 3.11., Systat Software Inc., Richmond, CA, USA), as appropriate. A value of P < 0.05 was considered statistically significant.
Results
Regulation of superoxide anion and nitric oxide release by laminar shear stress
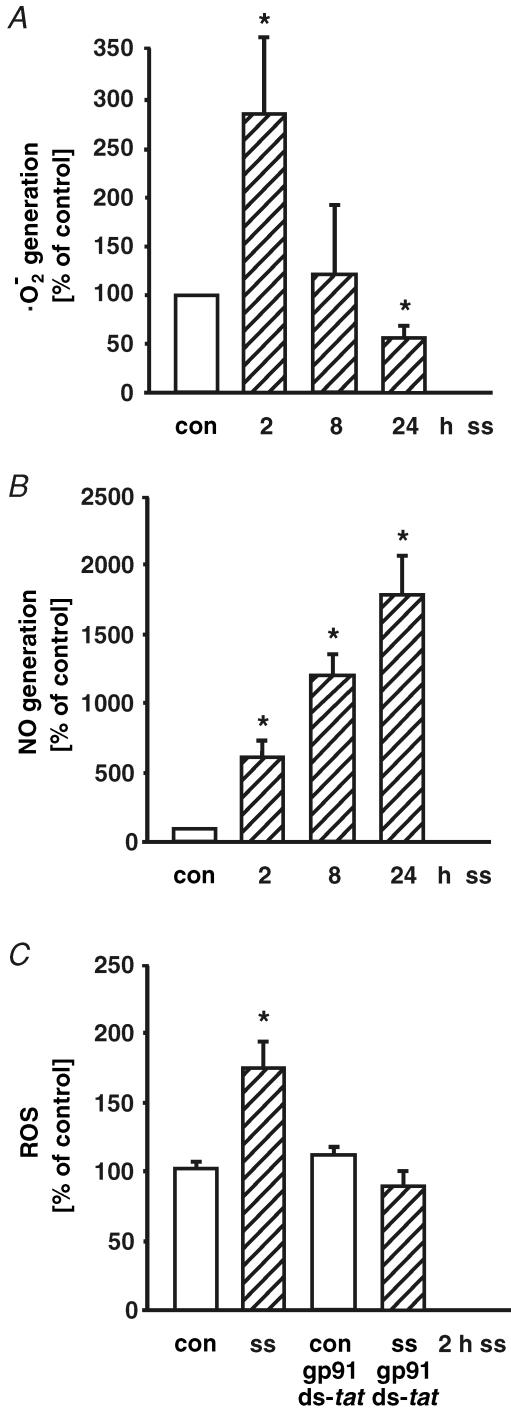
Primary cultures of human umbilical vein endothelial cells were exposed to laminar shear stress in a cone-and-plate viscometer for up to 24 h. The superoxide anion release during application of laminar shear stress was determined by cytochrome c reduction assay. As shown in Fig. 1A, short-term application of laminar shear stress (2 h, 30 dyne cm−2) transiently augmented ·O2− formation (to 7.5 ± 1.7 nmol (mg protein)−1, Fig. 1A). After 8 h of shear stress, ·O2− formation was comparable to control levels. However, long-term application of arterial levels of laminar shear stress decreased endothelial ·O2− formation (control: 4.0 ± 0.3 nmol (mg protein)−1; 24 h, 30 dyne cm−2: 2.1 ± 0.4 nmol (mg protein)−1, n = 8, P < 0.01, Fig. 1A). Superoxide anion formation was not significantly changed by application of long-term low laminar shear stress (1 dyne cm−2, 24 h) in human endothelial cells (81 ± 8% of control).
Figure 1. Time-dependent regulation of superoxide anion and nitric oxide generation by laminar shear stress in human endothelial cells.
Human endothelial cells were exposed to laminar shear stress (ss, 30 dyne cm−2) for 2, 8, or 24 h. Superoxide dismutase- and catalase-inhibited superoxide anion (·O2−) formation was determined by cytochrome c reduction assay (A). Release of nitrite, the major metabolite of NO in aqueous solution, was measured using the Griess reaction (B). Impact of NADPH oxidase inhibitor gp91ds-tat (100 μmol l−1) on formation of reactive oxygen species in response to short-term shear stress (ss, 2 h, 30 dyne cm−2) was determined by cytochrome c assay. Samples were normalized to internal controls without or with shear stress containing a peptide with a scrambled gp91 sequence (scramb-tat) at the same concentration (C). Values are given as means ±s.e.m. as a percentage of control; n ≥ 3 each; *P < 0.05 versus control.
Short-term application of high shear stress (2 h, 30 dyne cm−2) was sufficient to increase the NO formation (from 0.11 ± 0.02 μmol l−1 NO in control to 0.68 ± 0.09 μmol l−1 NO in HUVEC after shear stress). Long-term low (1 dyne cm−2, not shown) and high laminar shear stress (30 dyne cm−2, Fig. 1B) further increased NO generation in human endothelial cells.
The NADPH oxidase-specific inhibitor gp91ds-tat, normalized to an internal control with scrambled gp91 sequence (scramb-tat) at the same concentration, inhibited the augmented reactive oxygen species formation in response to short-term laminar shear stress (2 h, 30 dyne cm−2, Fig. 1C). These data suggest a crucial role of a gp91phox-containing NADPH oxidase complex in superoxide anion formation in response to laminar shear stress in human endothelial cells.
Down-regulation of NAD(P)H oxidase subunit expression by long-term laminar shear stress
In order to find a molecular source of this ·O2− formation in response to laminar shear stress, the expression of NAD(P)H oxidase subunits gp91phox, p22phox, p47phox and p67phox was determined.
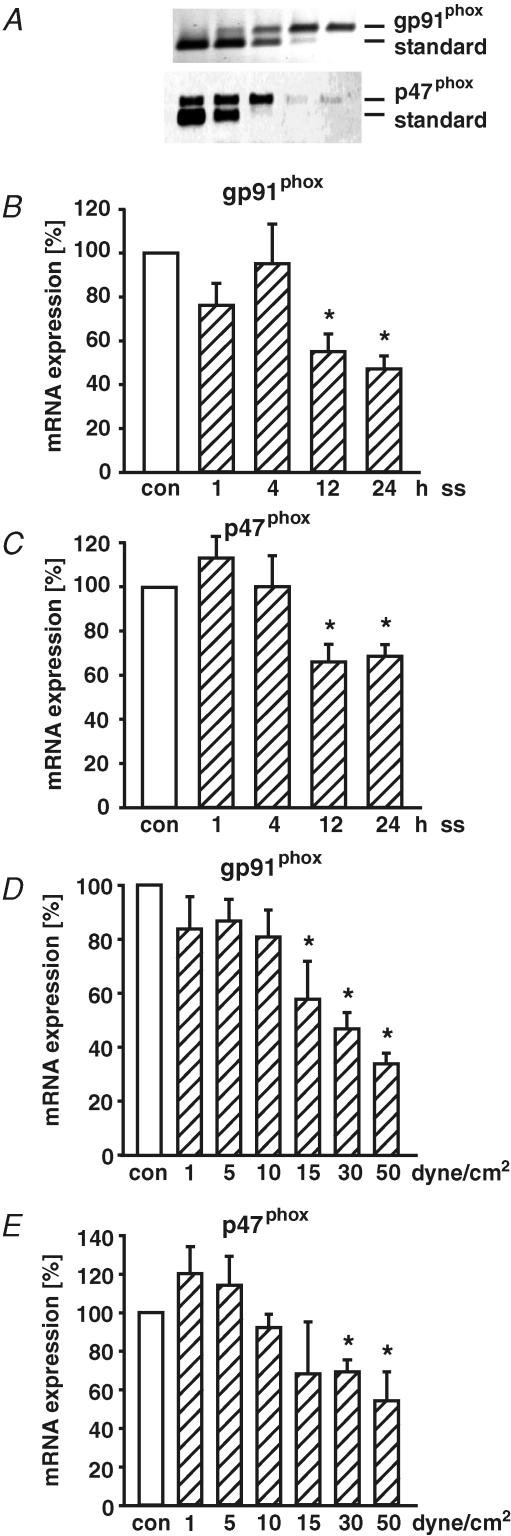
Application of shear stress (30 dyne cm−2) for 1 h did not increase the mRNA expression of the membrane-bound subunit gp91phox and the cytosolic subunit p47phox (Fig. 2). Furthermore, mRNA expression of p22phox (113 ± 12%) and p67phox (85 ± 13%) was not induced in response to 1 h of laminar shear stress as well.
Figure 2. Time- and dose-dependent regulation of NAD(P)H oxidase subunit mRNA expression in response to laminar shear stress.
The mRNA expression of NAD(P)H oxidase subunits gp91phox and p47phox was quantified by standard calibrated competitive reverse transcription-polymerase chain reaction (RT-PCR) (A) and normalized to 18S rRNA RT-PCR. The method compares amplification of an gp91phox and p47phox cDNA fragment from reverse transcribed total RNA of human endothelial cells (upper lane, longer fragment) versus different concentrations of an internally deleted and reverse transcribed cRNA standard (lower lane, shorter fragment) by PCR. Serial 1 : 3-dilution of appropriate cRNA standard was used. The PCR fragments were separated on agarose gels and stained with ethidium bromide. In time-course studies, human endothelial cells were exposed to laminar shear stress (ss) of 30 dyne cm−2 for 1, 4, 12 or 24 h and mRNA expression of NAD(P)H oxidase subunits gp91phox (B) and p47phox (C) was quantified. The dose-dependent regulation of gp91phox (D) and p47phox (E) was studied in HUVEC exposed to long-term (24 h) laminar shear stress (ss, 1, 5, 10, 15, 30, 50 dyne cm−2). Values are given as means ±s.e.m. as a percentage of control; n ≥ 5 each; *P < 0.05 versus control.
In contrast to the short-term effects, long-term application of high laminar shear stress (30 dyne cm−2) down-regulated gp91phox and p47phox mRNA expression in a time-dependent manner (Fig. 2B and C). This down-regulation of NAD(P)H oxidase subunits expression by long-term laminar shear stress was studied in more detail. Human endothelial cells were exposed to laminar shear stress of 1, 5, 10, 15, 30, or 50 dyne cm−2 for 24 h. The mRNA expression of NADPH oxidase subunits gp91phox and p47phox was down-regulated by long-term laminar shear stress in a dose-dependent manner (Fig. 2D and E). Because 30 dyne cm−2 is within the physiological range of arterial shear stress (Traub & Berk, 1998), all subsequent experiments studying high laminar shear stress were performed at this level.
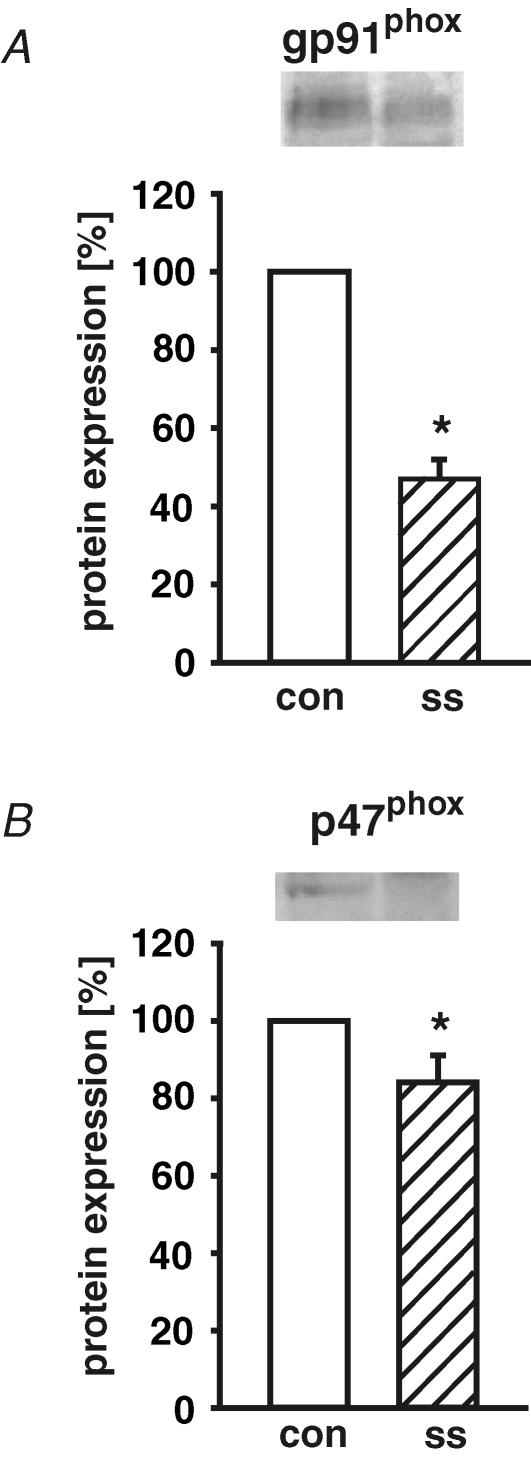
Next, the protein expression of NAD(P)H oxidase subunits in response to shear stress was analysed. The specificity of the antibodies was confirmed by Western blot analysis including protein preparations from leucocytes (for gp91phox and p47phox) as positive controls. After application of long-term high laminar shear stress (30 dyne cm−2, 24 h), protein expression of NAD(P)H oxidase subunits gp91phox and p47phox was down-regulated (Fig. 3). In addition, NAD(P)H oxidase subunit p67phox was even slightly induced by long-term laminar shear stress on the mRNA level (140 ± 14%versus control; n = 12; P < 0.05), but not significantly changed on the protein level. The p22phox mRNA expression was not regulated by different levels of long-term (24 h) laminar shear stress.
Figure 3. Down-regulation of NAD(P)H oxidase subunit protein expression by long-term arterial laminar shear stress.
Human endothelial cells were exposed to long-term (24 h) laminar shear stress (ss, 30 dyne cm−2). The protein expression of NAD(P)H oxidase subunits gp91phox (A) and p47phox (B) was determined by Western blot. Representative immunoblots for gp91phox and p47phox are shown above densitometric analyses. Values are given as means ±s.e.m. as a percentage of control without shear stress; n ≥ 5 each; *P < 0.05 versus control.
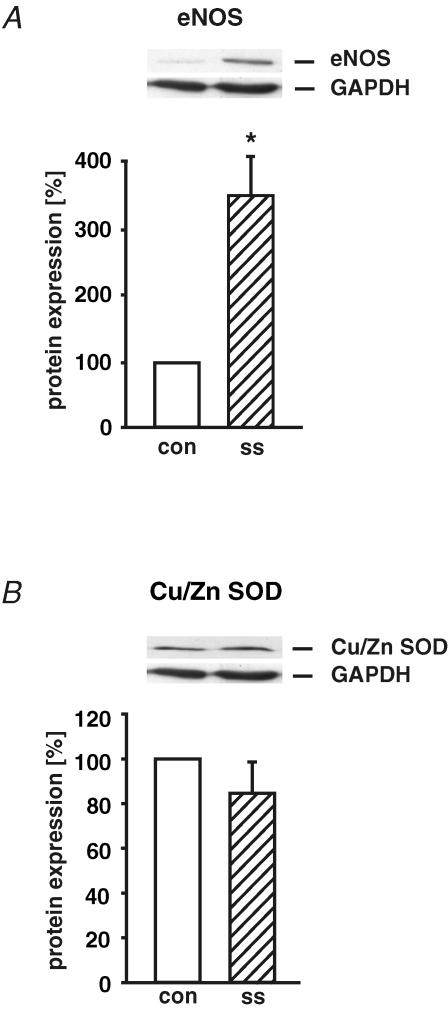
Up-regulation of eNOS protein expression by long-term arterial laminar shear stress
Human endothelial cells were exposed to long-term (24 h) arterial laminar shear stress (ss, 30 dyne cm−2) and the protein expression of eNOS and Cu/Zn SOD was determined by Western blot and normalized to GAPDH protein (as a percentage of control without shear stress). After application of laminar shear stress, eNOS protein expression was up-regulated 3.5-fold (Fig. 4A). In contrast, Cu/Zn SOD protein expression was not changed in response to high laminar shear stress (Fig. 4B).
Figure 4. Up-regulation of eNOS protein expression by long-term arterial laminar shear stress.
Human endothelial cells were exposed to long-term (24 h) laminar shear stress (ss, 30 dyne cm−2). The protein expression of eNOS (A) and Cu/Zn SOD (B) was determined by Western blot and normalized to GAPDH protein. Values are given as means ±s.e.m. as a percentage of control without shear stress; n = 4; *P < 0.05 versus control.
NO-dependent down-regulation of superoxide anion formation by long-term shear stress
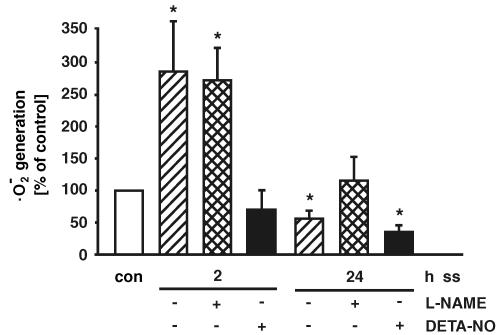
Because shear stress induced endothelial NO synthase and NO formation, we analysed the effect of the NOS inhibitor l-NAME on shear stress-dependent ·O2− formation in human endothelial cells. As shown in Fig. 5, l-NAME is not able to affect the shear stress-dependent induction of ·O2− generation after 2 h. l-NAME had no effect on ·O2− generation after 8 h as well. However, eNOS inhibition with l-NAME prevented the down-regulation of ·O2− formation after 24 h of high laminar shear stress (Fig. 5). To get further insight into the NO-dependent regulation of endothelial ·O2− formation, short- and long-term application of shear stress was repeated in the presence of the NO donor DETA-NO (500 μmol l−1). In this context it has to be considered that the amount of NO formation by DETA-NO exceeds the shear stress-dependent NO formation (immediately after addition of DETA-NO: 407 ± 40 μmol l−1 NO, 2 h: 279 ± 31 μmol l−1 NO, 24 h: 245 ± 31 μmol l−1 NO). Additional NO release by the NO donor DETA-NO prevented the increased ·O2− formation after 2 h of shear stress (Fig. 5). The ·O2− formation was down-regulated by a combination of long-term laminar shear stress and DETA-NO as well.
Figure 5. Impact of inhibition of endothelial NO synthase or additional NO formation on shear stress-dependent superoxide anion formation.
Human endothelial cells were exposed to short-term (2 h) or long-term (24 h) laminar shear stress (ss, 30 dyne cm−2) without or with the NO synthase inhibitor l-NAME (500 μmol l−1), or the NO donor DETA-NO (500 μmol l−1). Superoxide dismutase- and catalase-inhibited superoxide anion (·O2−) formation was determined by cytochrome c reduction assay. Values are given as means ±s.e.m. as a percentage of control; n ≥ 3 each; *P < 0.05 versus control.
Down-regulation of endothelial superoxide anion formation by NO donor
In order to further confirm the NO-dependent down-regulation of ·O2− generation, static cultures of human endothelial cells were exposed to DETA-NO (500 μmol l−1) and ·O2− formation measured. The NO donor reduced the endothelial superoxide anion formation to a similar extent to laminar shear stress. In additional experiments, human endothelial cells were incubated for 22.5 h with DETA-NO (500 μmol l−1), the culture medium containing the NO donor was removed and subsequently ·O2− generation was determined by SOD-specific cytochrome c assay (final measurement after 24 h). Even after removal of the compound, endothelial superoxide anion formation was down-regulated by the NO donor (control: 100 ± 25%, DETA-NO: 32 ± 12% of control, n = 4, P < 0.05). These data further support a NO-dependent down-regulation of superoxide anion formation.
Impact of NOS inhibition on shear stress-dependent down-regulation of NAD(P)H oxidase subunit expression
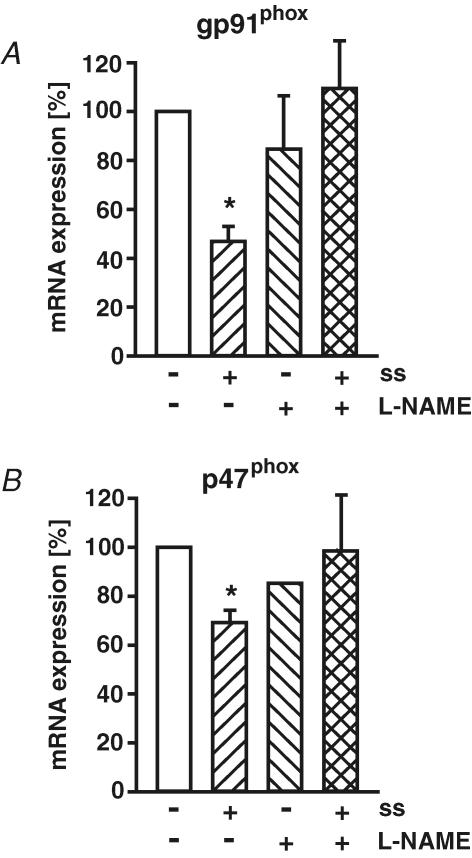
Next, we analysed the impact of NOS inhibition with l-NAME on the down-regulation of NAD(P)H oxidase subunit expression by high laminar shear stress. l-NAME itself had no effect on basal NAD(P)H oxidase expression. However, l-NAME prevented shear stress-dependent down-regulation of gp91phox and p47phox expression (Fig. 6). Inhibition of PI3 kinase by Ly294002 (10 μmol l−1) had no effect on basal gp91phox expression (92 ± 13% of control) and did not prevent down-regulation of gp91phox by long-term high laminar shear stress (30 dyne cm−2, 58 ± 8% of control, n = 4, P < 0.05).
Figure 6. Endothelial NO synthase inhibitor prevents down-regulation of specific NAD(P)H oxidase subunits by shear stress.
Human endothelial cells were exposed to long-term (24 h) laminar shear stress (ss, 30 dyne cm−2) without or with NO synthase inhibitor L-NAME (500 μmol l−1). The mRNA expression of NAD(P)H oxidase subunits gp91phox (A) and p47phox (B) was measured by standard calibrated competitive reverse transcription-polymerase chain reaction and normalized to 18S rRNA RT-PCR. Values are given as means ±s.e.m. as a percentage of control; n ≥ 5 each; *P < 0.05 versus control.
NO-dependent down-regulation of NAD(P)H oxidase subunit expression
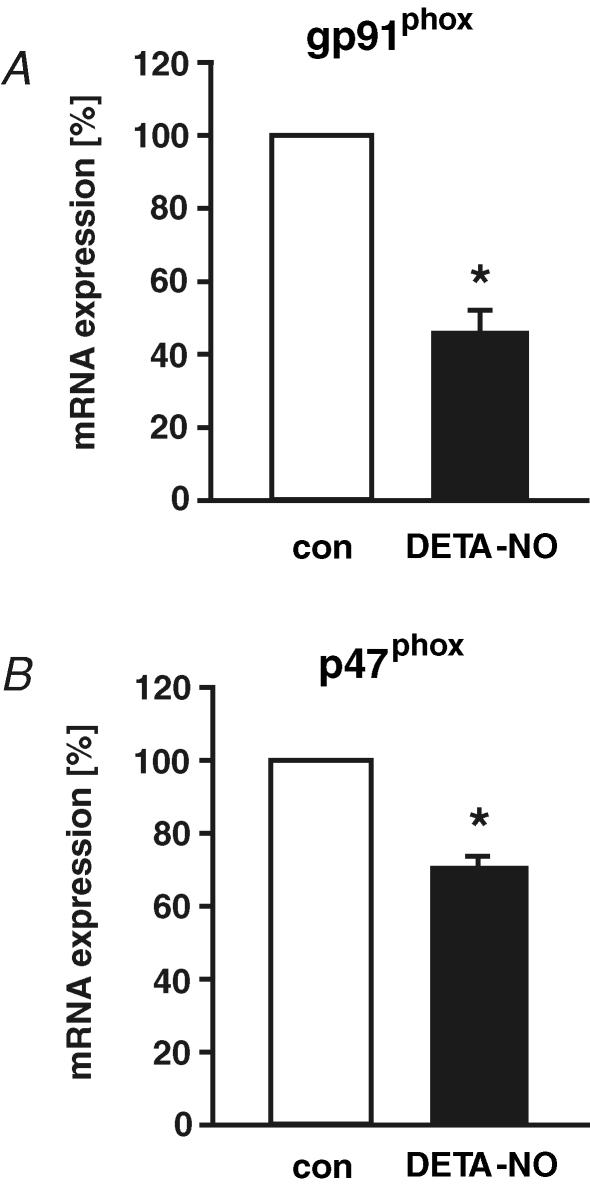
To further confirm the impact of NO on different NAD(P)H oxidase subunits, we analysed the effect of DETA-NO on NAD(P)H oxidase expression in static endothelial cells. In agreement with our findings using l-NAME, we found a NO-dependent down-regulation of NAD(P)H oxidase subunit gp91phox and p47phox expression in static endothelial cells (Fig. 7).
Figure 7. NO-dependent down-regulation of specific NAD(P)H oxidase subunit expression.
Human endothelial cells were exposed in static culture to NO donor DETA-NO (500 μmol l−1) for 24 h. The mRNA expression of NAD(P)H oxidase subunits gp91phox (A) and p47phox (B) was quantified by standard calibrated competitive reverse transcription-polymerase chain reaction and normalized to 18S rRNA RT-PCR. Values are given as means ±s.e.m. as a percentage of control; n ≥ 5 each; *P < 0.05 versus control.
Discussion
In this study, we show a short-term induction, but a NO-dependent down-regulation of superoxide anion formation during long-term exposure to laminar shear stress in primary cultures of human endothelial cells. The induction of superoxide anion formation by short-term shear stress in human endothelial cells in this study is in accordance with experiments using bovine aortic endothelial cells and a parallel-plate shear chamber (Go et al. 1999) or murine aortic endothelial cells (Hwang et al. 2003b). Oscillatory and low laminar shear stress (5 dyne cm−2) has been shown to increase intracellular ·O2− generation in a transient manner returning to baseline after 24 h in endothelial cells homogenized after application of shear stress (De Keulenaer et al. 1998). Oscillatory flow increased ·O2− generation after 2–4 h in bovine endothelial cells as well (Hwang et al. 2003a). In parallel, laminar shear stress increased NO levels after 2–24 h and endothelial eNOS protein expression. This finding is in agreement with previous studies (Nishida et al. 1992; Ayajiki et al. 1996; Tsao et al. 1996; Dimmeler et al. 1999; Davis et al. 2001). Additional mechanisms might involve post-trancriptional modifications of eNOS. The eNOS can be activated by phosphorylation of serine 1177 by protein kinase Akt/PKB in a redox-sensitive manner (Du et al. 2001). Furthermore, increased endothelial eNOS Ser-1177 phosphorylation by shear stress could involve products of vascular oxidative stress such as hydrogen peroxide (Kojda & Hambrecht, 2005). The down-regulation of superoxide anion formation by long-term laminar shear stress observed in this study might support this increased flow-dependent NO availability in human endothelial cells.
Several putative sources of endothelial superoxide anion formation have been suggested (Griendling et al. 2000; Liu et al. 2003). Growing evidence supports NAD(P)H oxidase complexes as major sources of superoxide anions in endothelial cells (Jones et al. 1996; Bayraktutan et al. 2000; Gorlach et al. 2000; Rueckschloss et al. 2001). Therefore, we focused in this study on the expression of the NAD(P)H oxidase subunits and the endothelial ·O2− formation in response to laminar shear stress.
The short-term induction of endothelial ·O2− formation by laminar shear stress might involve the gp91phox/p47phox-containing complex (Hwang et al. 2003b). Our data using the NADPH oxidase-specific inhibitor gp91ds-tat preventing the intracellular activation of the NADPH oxidase complex by inhibiting the association of p47phox with gp91phox (Rey et al. 2001) support this finding. Because we further did not observe an induction of NAD(P)H oxidase subunit expression by short-term shear stress, the increased ·O2− generation under these conditions is most probably mediated by an activation of NADPH oxidase complexes using preformed subunits. It cannot be excluded that other sources of reactive oxygen species are involved in this short-term response as well. However, the major focus of this study was the impact of long-term laminar shear stress on endothelial ·O2− formation.
The down-regulation of gp91phox mRNA and protein expression in response to long-term laminar shear stress is in the same order of magnitude as the shear stress-dependent down-regulation of ·O2− formation. This supports an essential role of gp91phox as the rate-limiting subunit of the NAD(P)H oxidase complex in human endothelial cells (Rueckschloss et al. 2001; Rueckschloss et al. 2002). Gp91phox shows a similar down-regulation on the mRNA and protein level to approximately 50% of control. The less pronounced down-regulation of p47phox protein expression in comparison to mRNA expression might reflect additional regulation on the post-transcriptional level including translation and protein stability. However, even minor changes in protein expression could result in more pronounced effects on the functional superoxide anion formation. In addition, p47phox is not a catalytic subunit and might therefore not directly be correlated with NAD(P)H oxidase activity.
NO synthase inhibitor l-NAME was not able to affect the shear stress-dependent induction of ·O2− generation after 2 h. The suggested functional activation of preformed superoxide anion generating complexes therefore most probably does not involve NO. Nevertheless, additional NO release by the NO donor DETA-NO seemed to cause scavenging of the increased ·O2− formation after 2 h of shear stress. In this context it has to be considered that the amount of NO formation by DETA-NO by far exceeds the shear stress-dependent NO formation. Thus, the down-regulation of ·O2− generation after 24 h DETA-NO could, beside the NO-mediated down-regulation of NAD(P)H oxidase subunit expression, involve some scavenging effects of ·O2− by NO. However, the NO-dependent down-regulation of endothelial superoxide anion formation even after previous removal of the NO donor supports an independent mechanism. Because l-NAME prevented the shear stress-dependent down-regulation of gp91phox and p47phox, this mechanism seems to involve an NO-dependent regulation of expression of subunits of the NAD(P)H oxidase complex. Interestingly, a slight increase of superoxide anion levels in samples with l-NAME compared to controls was observed which did not reach statistical significance. Under normal conditions, endothelial NO and ·O2− formation might be in an equilibrium including some NO-dependent reduction of superoxide anion levels by peroxynitrite formation. If the NO production is inhibited by l-NAME, this could result in slightly increased ·O2− levels (Munzel et al. 2000).
The down-regulation of endothelial ·O2− levels might involve changes in SOD expression as well. Several studies have previously determined the regulation of different SODs in response to laminar shear stress in endothelial cells (Inoue et al. 1996; De Keulenaer et al. 1998; Dimmeler et al. 1999; Chan et al. 2004). In our samples, laminar shear stress of 30 dyne cm−2 for 24 h increased protein expression of eNOS 3.5-fold, but the Cu/Zn SOD protein expression was not changed. Therefore, the decreased superoxide anion formation in response to laminar shear stress in our samples is not the result of an increased SOD expression.
In a recent study using a cranial window preparation in anaesthetized rats, increased intraluminal blood flow induced cerebral vasodilatation via activation of the NADPH oxidase by a PI3 kinase-dependent pathway (Paravicini et al. 2006). This is a very interesting finding suggesting even a transient vasodilatory effect of short-term released superoxide anions in response to rapid changes in blood flow in cerebral vessels. We did not observe an impact of PI3 kinase inhibition on down-regulation of gp91phox by long-term laminar shear stress. This further supports independent mechanisms of short-term and long-term effects of shear stress.
The in vivo relevance of the down-regulation of endothelial superoxide anion formation by long-term laminar shear stress observed in this study is supported by studies in porcine coronary arterioles (Sorop et al. 2003). Furthermore, cessation of flow in flow-adapted rat or mouse aorta increased generation of reactive oxygen species (Matsuzaki et al. 2005). In an interesting model of mouse arteriovenous fistula, creation of a shunt between right common carotid artery and jugular vein increased blood flow, shear stress, and subsequently a vascular NADPH oxidase complex comprising p47phox but not gp91phox (Castier et al. 2005). In this complex in vivo model, additional mechanisms in response to vessel dilatation-dependent increased mechanical stretch might be involved leading to activation of a Nox1/p47phox-containing NADPH oxidase complex in the adjacent vascular smooth muscle cells. In contrast, increased blood flow in mice subjected to voluntary training reduced vascular superoxide release, Nox1 and p47phox expression (Laufs et al. 2005). Finally, chronic exercise training of patients with coronary artery disease before coronary artery bypass grafting surgery increased flow and decreased generation of reactive oxygen species and expression of gp91phox in internal mammary arteries (Adams et al. 2005).
In conclusion, our data suggest a transient activation of ·O2− formation by short-term shear stress, but a down-regulation of endothelial ·O2− formation in response to long-term laminar shear stress. The NO-mediated down-regulation of ·O2− formation by long-term shear stress preferentially affects the gp91phox/p47phox-containing NAD(P)H oxidase complex. This mechanism might contribute to the regulation of endothelial NO/·O2− balance and the antiatherosclerotic and vasoprotective potential of laminar shear stress.
Acknowledgments
We would like to thank W. Goettsch for continuous support. This study was supported by the German Federal Ministry of Education and Research (BMBF) programme NBL3 of the University of Technology Dresden (C.S., PhD programme Metabolism and Endothelium; H.M., Professorship of Vascular Endothelium and Microcirculation), the Wilhelm Roux programme of the Martin Luther University Halle-Wittenberg (H.M., Project FKZ 2/23), and the Deutsche Forschungsgemeinschaft (H.M., Project SFB/TR C1).
References
- Adams V, Linke A, Krankel N, Erbs S, Gielen S, Mobius-Winkler S, Gummert JF, Mohr FW, Schuler G, Hambrecht R. Impact of regular physical activity on the NAD(P)H oxidase and angiotensin receptor system in patients with coronary artery disease. Circulation. 2005;111:555–562. doi: 10.1161/01.CIR.0000154560.88933.7E. [DOI] [PubMed] [Google Scholar]
- Ayajiki K, Kindermann M, Hecker M, Fleming I, Busse R. Intracellular pH and tyrosine phosphorylation but not calcium determine shear stress-induced nitric oxide production in native endothelial cells. Circ Res. 1996;78:750–758. doi: 10.1161/01.res.78.5.750. [DOI] [PubMed] [Google Scholar]
- Bachschmid M, Schildknecht S, Ullrich V. Redox regulation of vascular prostanoid synthesis by the nitric oxide-superoxide system. Biochem Biophys Res Commun. 2005;338:536–542. doi: 10.1016/j.bbrc.2005.08.157. [DOI] [PubMed] [Google Scholar]
- Bayraktutan U, Blayney L, Shah AM. Molecular characterization and localization of the NAD(P)H oxidase components gp91-phox and p22-phox in endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20:1903–1911. doi: 10.1161/01.atv.20.8.1903. [DOI] [PubMed] [Google Scholar]
- Castier Y, Brandes RP, Leseche G, Tedgui A, Lehoux S. p47phox-dependent NADPH oxidase regulates flow-induced vascular remodeling. Circ Res. 2005;97:533–540. doi: 10.1161/01.RES.0000181759.63239.21. [DOI] [PubMed] [Google Scholar]
- Chan BP, Reichert WM, Truskey GA. Synergistic effect of shear stress and streptavidin-biotin on the expression of endothelial vasodilator and cytoskeleton genes. Biotechnol Bioeng. 2004;88:750–758. doi: 10.1002/bit.20263. [DOI] [PubMed] [Google Scholar]
- Davis ME, Cai H, Drummond GR, Harrison DG. Shear stress regulates endothelial nitric oxide synthase expression through c-Src by divergent signaling pathways. Circ Res. 2001;89:1073–1080. doi: 10.1161/hh2301.100806. [DOI] [PubMed] [Google Scholar]
- De Keulenaer GW, Chappell DC, Ishizaka N, Nerem RM, Alexander RW, Griendling KK. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state: role of a superoxide-producing NADH oxidase. Circ Res. 1998;82:1094–1101. doi: 10.1161/01.res.82.10.1094. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Hermann C, Galle J, Zeiher AM. Upregulation of superoxide dismutase and nitric oxide synthase mediates the apoptosis-suppressive effects of shear stress on endothelial cells. Arterioscler Thromb Vasc Biol. 1999;19:656–664. doi: 10.1161/01.atv.19.3.656. [DOI] [PubMed] [Google Scholar]
- Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest. 2001;108:1341–1348. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerrschmidt N, Wippich N, Goettsch W, Broemme HJ, Morawietz H. Endothelin-1 induces NAD(P)H oxidase in human endothelial cells. Biochem Biophys Res Commun. 2000;269:713–717. doi: 10.1006/bbrc.2000.2354. [DOI] [PubMed] [Google Scholar]
- Fleming I, Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1–R12. doi: 10.1152/ajpregu.00323.2002. [DOI] [PubMed] [Google Scholar]
- Go YM, Patel RP, Maland MC, Park H, Beckman JS, Darley-Usmar VM, Jo H. Evidence for peroxynitrite as a signaling molecule in flow-dependent activation of c-Jun NH2-terminal kinase. Am J Physiol. 1999;277:H1647–H1653. doi: 10.1152/ajpheart.1999.277.4.H1647. [DOI] [PubMed] [Google Scholar]
- Gorlach A, Brandes RP, Nguyen K, Amidi M, Dehghani F, Busse R. A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ Res. 2000;87:26–32. doi: 10.1161/01.res.87.1.26. [DOI] [PubMed] [Google Scholar]
- Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: Part II: animal and human studies. Circulation. 2003;108:2034–2040. doi: 10.1161/01.CIR.0000093661.90582.c4. [DOI] [PubMed] [Google Scholar]
- Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- Hwang J, Ing MH, Salazar A, Lassegue B, Griendling K, Navab M, Sevanian A, Hsiai TK. Pulsatile versus oscillatory shear stress regulates NADPH oxidase subunit expression: implication for native LDL oxidation. Circ Res. 2003a;93:1225–1232. doi: 10.1161/01.RES.0000104087.29395.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J, Saha A, Boo YC, Sorescu GP, McNally JS, Holland SM, Dikalov S, Giddens DP, Griendling KK, Harrison DG, Jo H. Oscillatory shear stress stimulates endothelial production of O2− from p47phox-dependent NAD(P)H oxidases, leading to monocyte adhesion. J Biol Chem. 2003b;278:47291–47298. doi: 10.1074/jbc.M305150200. [DOI] [PubMed] [Google Scholar]
- Inoue N, Ramasamy S, Fukai T, Nerem RM, Harrison DG. Shear stress modulates expression of Cu/Zn superoxide dismutase in human aortic endothelial cells. Circ Res. 1996;79:32–37. doi: 10.1161/01.res.79.1.32. [DOI] [PubMed] [Google Scholar]
- Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SA, O'Donnell VB, Wood JD, Broughton JP, Hughes EJ, Jones OT. Expression of phagocyte NADPH oxidase components in human endothelial cells. Am J Physiol. 1996;271:H1626–H1634. doi: 10.1152/ajpheart.1996.271.4.H1626. [DOI] [PubMed] [Google Scholar]
- Kojda G, Hambrecht R. Molecular mechanisms of vascular adaptations to exercise. Physical activity as an effective antioxidant therapy? Cardiovasc Res. 2005;67:187–197. doi: 10.1016/j.cardiores.2005.04.032. [DOI] [PubMed] [Google Scholar]
- Laufs U, Wassmann S, Czech T, Munzel T, Eisenhauer M, Bohm M, Nickenig G. Physical inactivity increases oxidative stress, endothelial dysfunction, and atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:809–814. doi: 10.1161/01.ATV.0000158311.24443.af. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhao H, Li H, Kalyanaraman B, Nicolosi AC, Gutterman DD. Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ Res. 2003;93:573–580. doi: 10.1161/01.RES.0000091261.19387.AE. [DOI] [PubMed] [Google Scholar]
- Malek A, Izumo S. Physiological fluid shear stress causes downregulation of endothelin-1 mRNA in bovine aortic endothelium. Am J Physiol. 1992;263:C389–C396. doi: 10.1152/ajpcell.1992.263.2.C389. [DOI] [PubMed] [Google Scholar]
- Matsuzaki I, Chatterjee S, Debolt K, Manevich Y, Zhang Q, Fisher AB. Membrane depolarization and NADPH oxidase activation in aortic endothelium during ischemia reflect altered mechanotransduction. Am J Physiol Heart Circ Physiol. 2005;288:H336–H343. doi: 10.1152/ajpheart.00025.2004. [DOI] [PubMed] [Google Scholar]
- McNally JS, Davis ME, Giddens DP, Saha A, Hwang J, Dikalov S, Jo H, Harrison DG. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol Heart Circ Physiol. 2003;285:H2290–H2297. doi: 10.1152/ajpheart.00515.2003. [DOI] [PubMed] [Google Scholar]
- Morawietz H, Talanow R, Szibor M, Rueckschloss U, Schubert A, Bartling B, Darmer D, Holtz J. Regulation of the endothelin system by shear stress in human endothelial cells. J Physiol. 2000;525:761–770. doi: 10.1111/j.1469-7793.2000.00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munzel T, Li H, Mollnau H, Hink U, Matheis E, Hartmann M, Oelze M, Skatchkov M, Warnholtz A, Duncker L, Meinertz T, Forstermann U. Effects of long-term nitroglycerin treatment on endothelial nitric oxide synthase (NOS III) gene expression, NOS III-mediated superoxide production, and vascular NO bioavailability. Circ Res. 2000;86:e7–e12. doi: 10.1161/01.res.86.1.e7. [DOI] [PubMed] [Google Scholar]
- Nishida K, Harrison DG, Navas JP, Fisher AA, Dockery SP, Uematsu M, Nerem RM, Alexander RW, Murphy TJ. Molecular cloning and characterization of the constitutive bovine aortic endothelial cell nitric oxide synthase. J Clin Invest. 1992;90:2092–2096. doi: 10.1172/JCI116092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osanai T, Fujita N, Fujiwara N, Nakano T, Takahashi K, Guan W, Okumura K. Cross talk of shear-induced production of prostacyclin and nitric oxide in endothelial cells. Am J Physiol Heart Circ Physiol. 2000;278:H233–H238. doi: 10.1152/ajpheart.2000.278.1.H233. [DOI] [PubMed] [Google Scholar]
- Paravicini TM, Miller AA, Drummond GR, Sobey CG. Flow-induced cerebral vasodilatation in vivo involves activation of phosphatidylinositol-3 kinase, NADPH-oxidase, and nitric oxide synthase. J Cereb Blood Flow Metab. 2006;26:836–845. doi: 10.1038/sj.jcbfm.9600235. [DOI] [PubMed] [Google Scholar]
- Rey FE, Cifuentes ME, Kiarash A, Quinn MT, Pagano PJ. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O2− and systolic blood pressure in mice. Circ Res. 2001;89:408–414. doi: 10.1161/hh1701.096037. [DOI] [PubMed] [Google Scholar]
- Rueckschloss U, Galle J, Holtz J, Zerkowski HR, Morawietz H. Induction of NAD(P)H oxidase by oxidized low-density lipoprotein in human endothelial cells: antioxidative potential of hydroxymethylglutaryl coenzyme A reductase inhibitor therapy. Circulation. 2001;104:1767–1772. doi: 10.1161/hc4001.097056. [DOI] [PubMed] [Google Scholar]
- Rueckschloss U, Quinn MT, Holtz J, Morawietz H. Dose-dependent regulation of NAD(P)H oxidase expression by angiotensin II in human endothelial cells: protective effect of angiotensin II type 1 receptor blockade in patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2002;22:1845–1851. doi: 10.1161/01.atv.0000035392.38687.65. [DOI] [PubMed] [Google Scholar]
- Schubert A, Cattaruzza M, Hecker M, Darmer D, Holtz J, Morawietz H. Shear stress-dependent regulation of the human β-tubulin folding cofactor D gene. Circ Res. 2000;87:1188–1194. doi: 10.1161/01.res.87.12.1188. [DOI] [PubMed] [Google Scholar]
- Sorop O, Spaan JAE, Sweeney TE, VanBavel E. Effect of steady versus oscillatory flow on porcine coronary arterioles: involvement of NO and superoxide anion. Circ Res. 2003;92:1344–1351. doi: 10.1161/01.RES.0000078604.47063.2B. [DOI] [PubMed] [Google Scholar]
- Traub O, Berk BC. Laminar shear stress: mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler Thromb Vasc Biol. 1998;18:677–685. doi: 10.1161/01.atv.18.5.677. [DOI] [PubMed] [Google Scholar]
- Tsao PS, Buitrago R, Chan JR, Cooke JP. Fluid flow inhibits endothelial adhesiveness. Nitric oxide and transcriptional regulation of VCAM-1. Circulation. 1996;94:1682–1689. doi: 10.1161/01.cir.94.7.1682. [DOI] [PubMed] [Google Scholar]
- Wessells H, Teal TH, Engel K, Sullivan CJ, Gallis B, Tran KB, Chitaley K. Fluid shear stress-induced nitric oxide production in human cavernosal endothelial cells: inhibition by hyperglycaemia. BJU Int. 2006;97:1047–1052. doi: 10.1111/j.1464-410X.2006.06059.x. [DOI] [PubMed] [Google Scholar]