Abstract
The vacuolar-type H+-ATPase (V-ATPase) in the plasma membrane of a variety of cells serves as an acid-secreting pathway, and its activity is closely related to cellular functions. Massive proton secretion often leads to electrolyte disturbances in the vicinity of the cell and may in turn affect the activity of the V-ATPase. We characterized, for the first time, the proton currents mediated by plasmalemmal V-ATPase in murine osteoclast-like cells and investigated its activity over a wide range of pH gradients across the membrane (ΔpH = extracellular pH – intracellular pH). The V-ATPase currents were identified as outward H+ currents and were dependent on ATP and sensitive to the inhibitors bafilomycin A1 and N,N′-dicyclohexylcarbodiimide. Although H+ was transported uphill, the electrochemical gradient for H+ affected the current. The currents were increased by elevating ΔpH and depolarization, and were reduced by lowering ΔpH and hyperpolarization. Elevation of extracellular Ca2+ (5–40 mm) diminished the currents in a dose-dependent manner and made the voltage dependence more marked. Extracellular Mg2+ mimicked the inhibition. With 40 mm Ca2+, the currents decreased to < 40% at 0 mV and to < 10% at about −80 mV. Increases in the intracellular Ca2+ (0.5–5 μm) did not affect the current. The data suggest that acid secretion through the plasmalemmal V-ATPase is regulated by a combination of the pH gradient, the membrane potential and the extracellular divalent cations. In osteoclasts, the activity-dependent accumulation of acids and Ca2+ in the closed extracellular compartment might serve as negative feedback signals for regulating the V-ATPase.
The regulation of pH balance in the intracellular and extracellular environments is one of most important and ubiquitous mechanisms underlying homeostasis. The vacuolar-type H+-ATPase (V-ATPase) is an electrogenic H+ pump that is widely distributed in living organisms. V-ATPases acidify lysozomes, produce a proton-motive force and regulate the functions of organelles (Kawanishi-Nishi et al. 2003). It is now recognized that V-ATPases are present in the plasma membrane of various animal cells, including epithelial cells, kidney cells, phagocytes and osteoclasts (Nelson & Harvey, 1999). In these cells, the V-ATPase is an essential proton-secreting pathway and also generates a driving force for membrane transport. The activity is closely linked to the specialized functions of these cells, such as regulation of acid–base balance in kidney cells, killing of pathogens in phagocytes and bone resorption in osteoclasts.
The uphill H+ transport by the V-ATPase uses energy produced by ATP hydrolysis. The activity of the V-ATPase can be regulated in various ways, for instance, dissociation/assembly of the constitutive subunits (Wagner et al. 2004), interaction with the actin cytoskeleton (Lee et al. 1999; Breton et al. 2000; Holliday et al. 2000) and exocytotic fusion of vesicles bearing the enzyme (Nanda et al. 1996). As a consequence of the diversity of the regulatory mechanisms, the ability of the pump to transport H+ could vary in response to changes in cellular conditions. We also must remember that the V-ATPase would often face extreme ionic environments as a result of its own activity. For example, if protons are secreted into a closed extracellular compartment, the plasmalemmal V-ATPase may be exposed to strongly acidic conditions.
For osteoclasts, the large multinuclear cells responsible for bone resorption, the ambient ionic environment is more complicated. The plasma membrane facing the bone surface (ruffled membrane) is rich in V-ATPase (Rousselle & Heymann, 2002). The pump secretes the protons generated via carbonic anhydrase II into the resorption pit isolated from the extracellular fluid, and demineralizes the calcified bone tissue. Therefore, the V-ATPase might be exposed not only to an excess of H+ but also to high concentrations of Ca2+ liberated from bone. The inhibitory role of elevated extracellular Ca2+ in osteoclast function is well understood; high extracellular Ca2+ inhibits bone resorption by direct action on osteoclasts, such as reorganization of actin cytoskeleton, inhibition of releasing enzymes (Miyauchi et al. 1990: Zaidi et al. 1993) and apoptosis (Lorget et al. 2000). There is, however, very little information available concerning effects of Ca2+ on the action of the plasmalemmal V-ATPase.
Our focus for this study was to investigate the activity of the plasmalemmal V-ATPase in response to perturbations of the ion environments, especially of H+ and Ca2+. Proton currents through the native V-ATPase at the plasma membrane of mammalian cells have not yet been characterized. Here we used whole-cell clamp recordings to identify the pump currents in mammalian osteoclast-like cells expressing plasmalemmal V-ATPases. This electrophysiological method permits the control of intracellular/extracellular ionic conditions and membrane voltages over wide ranges and therefore the evaluation of the V-ATPase activity in real time. A preliminary account of part of this study has been reported (Sakai et al. 2006).
Methods
Cells
A mouse macrophage cell line (RAW264 cells; Riken Cell Bank, Tsukuba, Japan) that differentiates into osteoclast-like multinucleated cells was maintained in Dulbecco's modified Eagle's medium (DMEM) containing 100 U ml−1 penicillin, 0.1 mg ml−1 streptomycin, 0.25 ng ml−1 amphotericin B and 10% fetal calf serum (FCS) at 37°C in a 95% air–5% CO2 atmosphere. Treatment with 50–75 ng ml−1 of the soluble form of the receptor activator of nuclear factor κB (NF-κB) ligand (sRANKL; PeproTech EC, London, UK) in α-minimum essential medium (α-MEM) with 10% FCS induced differentiation of RAW264 cells into multinucleated cells. Osteoclast-like cells, identified using phase-contrast microscopy and tartrate-resistant acid phosphatase (TRAP) activity, appeared within 4 days and were maintained for 10–12 days.
Electrophysiological recordings
Whole-cell recordings were made as described elsewhere (Shibata et al. 1997). Hydrogen currents were recorded from differentiated cells containing four to 10 nuclei. The pipette solutions contained (mm): (1) 120 Mes, 65 NMDG-aspartate, 3 MgCl2 and 1 BAPTA (pH 5.5); (2) 120 Mes, 35 NMDG-aspartate, 3 MgCl2 and 1 BAPTA (pH 6.5); and (3) 100 Hepes, 75 NMDG-aspartate, 3 MgCl2 and 1 EGTA (pH 7.3). Na2ATP (0–10 mm) was added immediately before use. The extracellular solutions contained (mm): (1) 100 Hepes, 75 NMDG-aspartate, 1 CaCl2 and 1 MgCl2 (pH 7.3); (2) 100 Hepes, 75 NMDG-aspartate, 1 CaCl2 and 1 MgCl2 (pH 6.7); and (3) 120 Mes, 65 NMDG-aspartate, 1 CaCl2 and 1 MgCl2 (pH 5.5). The pH was adjusted using CsOH. Ten millimolar glucose, 0.1% bovine serum albumin and 50 μm 4,4′-diisothiocyanato-2,2′-stilbenesulphonate (DIDS), a blocker for Cl−–anion transport, were added to the bath solution. Most of the currents recorded under these conditions are carried by protons (Mori et al. 2002, 2003). Two hundred micromolar ZnCl2 was also added to block the voltage-gated proton (H+) channel that is expressed in osteoclasts (Nordström et al. 1995; Mori et al. 2002, 2003). The high-Ca2+ (5–40 mm) or high-Mg2+ (10–40 mm) extracellular solutions were prepared by replacing NMDG-aspartate with CaCl2 or MgCl2. Pipette solutions containing 0.5–5 μm Ca2+ were made with mixtures of Ca2+ and BAPTA (Sakai et al. 1999). The osmolarity of all solutions was 280–290 mosmol l−1. The reference electrode was a Ag–AgCl wire connected to the bath solution through a Ringer–agar bridge. The pipette resistances were 5–15 MΩ. The cell capacitance was 151 ± 7 pF (n = 165). Current signals were recorded with an amplifier (Axopatch 200A; Axon Instruments, Union City, CA, USA), digitized at 4 kHz with an analog-to-digital converter (Digidata 1200, Axon Instruments) and analysed using pCLAMP software (Axon Instruments). Voltage steps or voltage ramps (200 mV s−1) were applied at a holding potential of −80 mV every 10–20 s.
Cells were exposed to inhibitors, acids or high Ca2+ by perfusing 10 ml of the bath solutions containing these substances at about 0.1 ml s−1 (volume of the recording chamber, 2 ml).
Data analyses
All experiments were conducted at room temperature (22–25°C). Data are expressed as means ± s.e.m. Leak currents were not subtracted in each recording, since the V-ATPase current was small and showed linear I–V relationships. To exclude leak and unidentified currents, we analysed the blocker-sensitive difference currents. The statistical significances (P < 0.05) were evaluated using Student's t unpaired test.
Materials
Mes and BAPTA were purchased from Dojindo Laboratories (Kumamoto, Japan), and bafilomycin A1 from WAKO Chemicals (Osaka, Japan). All other chemicals were obtained from Sigma (St Louis, MO, USA) unless otherwise specified. A concentrated stock solution of Na2ATP was prepared in 1 m Tris-Cl and stored in a freezer. Bafilomycin A1 and DIDS were dissolved in DMSO, and N,N′-dicyclohexylcarbodiimide (DCCD) in dichloromethane. The final concentrations were < 0.1% for DMSO and dichloromethane. These levels of the solvents did not affect the results.
Results
Proton currents mediated by the plasmalemmal V-ATPase
To identify the H+ currents transported by the plasmalemmal V-ATPase, whole-cell clamp recordings were made on osteoclast-like cells in solutions without the major ions, Na+, K+ and Cl−, and to which were added DIDS (50 μm), an inhibitor of Cl−–anion transport, and 200 μm Zn2+, a blocker of the H+ channel. The pH gradient across the membrane (ΔpH = pHo− pHi) was set at 1.8 (pHo/pHi= 7.3/5.5), to increase the H+ efflux. More than 85% of the cells tested (n = 26) developed outward H+ currents at 0 mV during intracellular dialysis with the 5 mm ATP-containing solution (Fig. 1A, ▪). In contrast, in cells dialysed with the ATP-free solution, the currents in all of the cells (n = 17) were decreased (Fig. 1A, □). The shift with 1.25 mm ATP was slight (Fig. 1A, ^).
Figure 1. ATP-dependent H+ currents in osteoclast-like cells.
Whole-cell currents were recorded with a pHo/pHi of 7.3/5.5. A, shift in the current amplitudes (ΔH+ current) at 0 mV developed following the rupture of the patch membrane (time 0). The data were obtained from 3 different cells intracellularly dialysed with 5 mm ATP (▪, 115 pF), 1.25 mm ATP (^, 84 pF) or ATP-free solution (□, 137 pF). The curves are single exponential fits for the data (time constant, τ= 240, 129 and 96 s). B, the dose–response relationship of ΔH+ current densities for ATP at a pHo/pHi of 7.3/5.5. Data (means ± s.e.m.) were obtained from the steady-state current amplitudes (0 mV) normalized to the cell capacitances, after intracellular dialysis for 10–20 min with different concentrations of ATP (▪): −0.070 ± 0.007 pA pF−1 (n = 17) for no ATP; 0.014 ± 0.045 pA pF−1 (n = 9) for 1.25 mm; 0.039 ± 0.032 pA pF−1 (n = 8) for 2.5 mm; 0.094 ± 0.021 pA pF−1 (n = 36) for 5 mm; and 0.164 ± 0.043 pA pF−1 (n = 9) for 10 mm ATP. The open square represents the data obtained from cells pretreated with 200 nm bafilomycin A1 (Bafilo; 0.006 ± 0.010 pA pF−1, n = 6; *P < 0.05 compared with the data for 5 mM in the absence of bafilomycin). C, the dose–response relationship of ATP-dependent ΔH+ current densities at a pHo/pHi of 7.3/6.5: −0.026 ±0.010 pA pF−1 (n = 7) for no ATP; 0.027 ± 0.025 pA pF−1 (n = 19) for 1.25 mm; and 0.071 ± 0.043 pA pF−1 (n = 10) for 5 mM.
The shift of the H+ current measured at the steady state was increased by ATP in a dose-dependent manner (Fig. 1B). The current shift was reduced to nearly zero (Fig. 1B, □) in the presence of 200 nm bafilomycin A1, a selective blocker of V-ATPases. Therefore, the major part of the ATP-dependent H+ currents seemed to be mediated by the V-ATPase in the plasma membrane. Similar ATP-dependent shift of the H+ current was observed under more physiological pHi (pHo/pHi= 7.3/6.5; Fig. 1C). Later experiments were conducted on the currents recorded in the presence of 5 mm ATP.
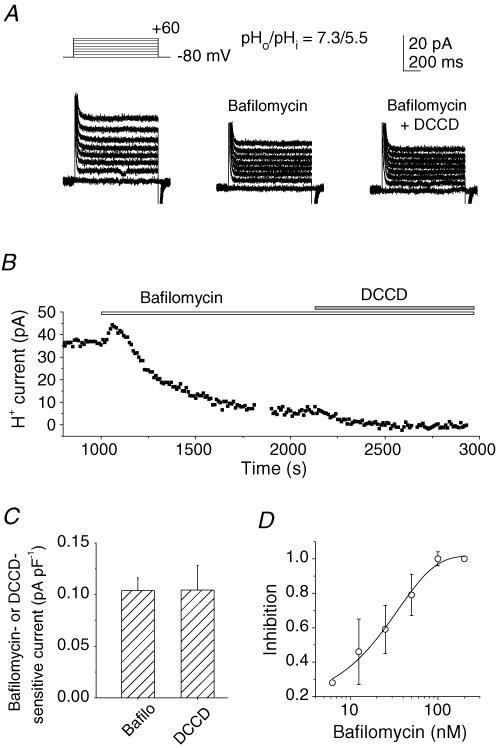
Bafilomycin A1 (200 nm) diminished the H+ currents at different potentials (Fig. 2A, left and middle panels). The H+ currents at 0 mV were decreased slowly, and the steady state was reached within 5–15 min (Fig. 2B). The subsequent addition of another blocker for V-ATPases, N,N′-dicyclohexylcarbodiimide (DCCD; 100 μm), decreased the current more in half of the cells tested (n = 14), but this further reduction was only slight (by ∼5% of the bafilomycin-sensitive current). Consequently there was no significant difference between the bafilomycin-sensitive and the DCCD-sensitive currents, normalized to the cell capacitance, among different cells (Fig. 2C). Amiloride (0.2 mm)-sensitive currents were negligible in the present experimental conditions (data not shown). Bafilomycin A1 inhibited the H+ current in a dose-dependent manner (Fig. 2D). A transient increase in the current often appeared immediately after the onset of perfusion (Fig. 2B). The mechanism has yet to be identified, but was unlikely to result from actions of bafilomycin A1 because it did not depend on the dose and was seen with perfusion of other solutions (Figs 3A, 4A and 5A). Inhibition of the H+ efflux by 200 nm bafilomycin A1 or 100 μm DCCD was accompanied by a depolarization of 10–60 mV, implying that the activity of the plasmalemmal V-ATPase contributes to the regulation of the membrane potential. We hereafter investigated the features of V-ATPase-mediated H+ currents from the bafilomycin (200 nm)-sensitive or DCCD (100 μm)-sensitive components.
Figure 2. V-ATPase currents in osteoclast-like cells.
A, whole-cell currents in a cell before (left panel) and 10 min after application of 200 nm bafilomycin A1 (middle panel) and with addition of DCCD (100 μm; right panel). Leak currents were not subtracted. Capacitive currents at the onset and the end of the depolarization pulses are truncated. B, a representative time course for changes in the H+ currents at 0 mV during subsequent application of bafilomycin A1 (200 nm) and DCCD (100 μm). The abscissa represents the time after rupture of the patch membrane. Bafilomycin A1 was added after the currents reached a steady state. A and B were obtained from different cells. C, bafilomycin A1 (200 nm, n = 25) and DCCD (100 μm, n = 11)-sensitive currents. The data are the current densities at 0 mV obtained from different cells: 0.104 ± 0.012 (n = 25) and 0.104 ± 0.024 pA pF−1 (n = 11), respectively. D, dose–response relationship of inhibition of V-ATPase currents by bafilomycin A1 (6.25–200 nm). The inhibition at different concentrations was normalized to the value at 200 nm in each cell and averaged (n = 2–5). In A–D, the pipette contained 5 mm ATP; pHo/pHi was 7.3/5.5. Data in C and D are means ± s.e.m.
Figure 3. The pH dependence of the V-ATPase currents.
A, time courses for changes in the H+ currents at 0 mV after rupture of the patch membrane and subsequent application of acidic solutions (pHo= 7.3, 6.7 and 5.5) and bafilomycin A1 (200 nm). pHi was 5.5. B, the V-ATPase current densities at 0 mV at different combinations of pHo and pHi: 0.104 ± 0.012 pA pF−1 (n = 25) for 7.3/5.5; 0.050 ± 0.008 pA pF−1 (n = 8) for 5.5/5.5; 0.047 ± 0.019 pA pF−1 (n = 7) for 7.3/7.3; and 0.027 ± 0.015 pA pF−1 (n = 8) for 5.5/7.3. Data are compared to those at a pHo/pHi of 7.3/5.5 (left column): *P < 0.05; **P < 0.005; N.S., not significant. C, current–voltage (I–V) relationships for the V-ATPase current at three values of ΔpH: 1.8 (▪, n = 6), 0 (▵ for 7.3/7.3, n = 4; ▴ for 5.5/5.5, n = 4) and −1.8 (□, n = 5). The pipette contained 5 mm ATP.
Figure 4. Effects of extracellular Ca2+ on the V-ATPase current.
A, time courses of the changes in the H+ currents at 0 mV in the presence of 10–40 mm Ca2+ and subsequent application of 200 nm bafilomycin A1 (upper panel) and the recovery following washing of 40 mm Ca2+ (lower panel). The abscissa shows the time after rupture of the patch membrane. B, Ca2+-sensitive currents. The current densities (at 0 mV) were 0.025 ± 0.006 pA pF−1 (n = 7) for 10 mm Ca2+ and 0.058 ± 0.017 pA pF−1 (n = 8) for 40 mm Ca2+. In cells treated with 200 nm bafilomycin A1 (+ Bafilo), Ca2+-sensitive currents were reduced significantly, to 0.009 ± 0.012 pA pF−1 (n = 5) *P < 0.05. C, bafilomycin A1 (200 nm)-sensitive currents in the presence of different concentrations of extracellular Ca2+: 0.104 ± 0.012 pA pF−1 (n = 25) for 1 mm; 0.068 ± 0.011 pA pF−1 (n = 4) for 5 mm; 0.055 ± 0.019 pA pF−1 (n = 5) for 10 mm; and 0.036 ± 0.008 pA pF−1 (n = 8) for 40 mm Ca2+. pHo/pHi was 7.3/5.5. The pipette contained 5 mm ATP. **P < 0.01 compared with the data at 1 mM Ca2+.
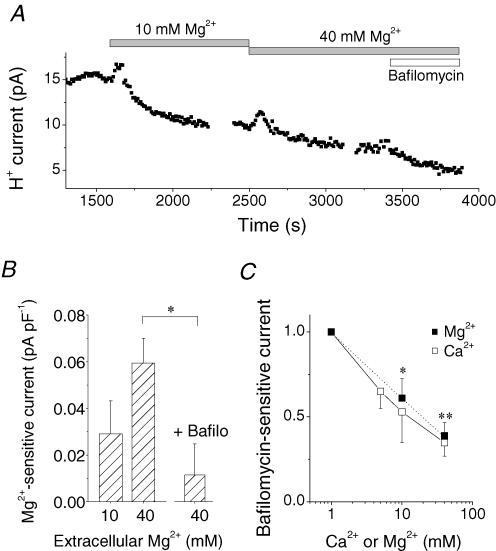
Figure 5. Effects of extracellular Mg2+ on the V-ATPase current.
A, a time course of the changes in the H+ currents at 0 mV in the presence of 10–40 mm Mg2+ and subsequent application of 200 nm bafilomycin A1. The abscissa shows the time after rupture of the patch membrane. B, Mg2+-sensitive currents. The current densities (at 0 mV) were 0.038 ± 0.015 pA pF−1 (n = 7) for 10 mm Mg2+ and 0.070 ± 0.018 pA pF−1 (n = 5) for 40 mm Mg2+. Pretreatment with 200 nm bafilomycin A1 (+ Bafilo) significantly decreased the 40 mm Mg2+-sensitive currents, to 0.014 ± 0.017 pA pF−1 (n = 5). C, bafilomycin A1 (200 nm)-sensitive currents in the presence of different concentrations of extracellular Mg2+ (▪) and Ca2+ (□). The data are normalized to the values at 1 mm and averaged: for Mg2+, 0.61 ± 0.11 (n = 10) at 10 mm and 0.39 ± 0.08 (n = 8) at 40 mm; for Ca2+, 0.65 ± 0.10 (n = 4) at 5 mm, 0.53 ± 0.18 (n = 5) at 10 mm and 0.35 ± 0.08 at 40 mm. pHo/pHi was 7.3/5.5. The pipette contained 5 mm ATP. *P < 0.05. **P < 0.05. In C, * and ** denote P values for 10–40 mm Mg2+ compared to the control (1 mm Mg2+).
pH dependence and voltage dependence of the V-ATPase activity
At a membrane potential of 0 mV, the H+ current was diminished by decreasing pHo from 7.3 to 6.7 and then to 5.5 (Fig. 3A). The pHi was kept constant at 5.5 throughout the experiment. Addition of 200 nm bafilomycin A1 decreased the current further, implying that the V-ATPase current was still present even in the very acidic environment. In measurements of the current density at 0 mV with different values of ΔpH and with variable combinations of pHo and pHi values (Fig. 3B), the current amplitudes varied greatly among cells, but were reduced by decreasing ΔpH. There were no significant differences in the values with symmetric values of pH (ΔpH, 0) with pHo/pHi ratios of 5.5/5.5 and 7.3/7.3. Although the difference between pHo/pHi ratios of 7.3/7.3 and 5.5/7.3 was not significant, lowering pHo from 7.3 to 5.5 always decreased the current in single cells with a pHi of 7.3 (n = 3). Therefore, extracellular acidification is likely to decrease the H+ efflux over a wide range of values of pHi (5.5–7.3). It should be noted that the outward H+ currents remained even when the ΔpH was reversed (ΔpH, −1.8; pHo/pHi= 5.5/7.3). Thus, the V-ATPase could carry out uphill H+ transport against this large negative ΔpH.
The current–voltage (I–V) relationship shows the voltage dependence of the V-ATPase activity (Fig. 3C). The currents were increased by depolarization and decreased by hyperpolarization. With a ΔpH of 1.8, there were sizable outward currents even at −100 mV, near to the equilibrium potential for H+ (EH; −104 mV; ▪ in Fig. 3C). When ΔpH was decreased to 0 (▵ and in Fig. 3C) or to −1.8 (□ in Fig. 3C), the currents were greatly reduced over the entire voltage range, but the voltage dependence was conserved. The currents with ΔpH = 0 were still outward at about −80 mV, which was greatly negative to EH (0 mV). When the ΔpH was reversed, that is, when the extracellular space was more acidic than the cytosol (ΔpH, −1.8), depolarization to approximately −40 mV was required to extrude H+ against this large pH gradient; the currents were negligible below −40 mV in five of seven cells tested. Proton secretion mediated by the V-ATPase was thus dependent on both pH and voltage. Extracellular acidification and hyperpolarization seem to diminish the pump currents. Therefore, depolarization might play a crucial role in maintaining efficient H+ efflux when the cells are exposed to strong acids (pHo≪ pHi).
Extracellular Ca2+ inhibits V-ATPase currents
Calcium is an important signalling ion which regulates a variety of cellular functions. Intriguingly, increases in the extracellular Ca2+ concentration from 1 to 10–40 mm decreased the H+ currents (Fig. 4A, upper panel). When cells were exposed to 40 mm Ca2+ for 10–15 min, the current recovered to 79 ± 15% (n = 7) of the control level within 30 min after the washout (Fig. 4A, lower panel). Recovery was less (29 ± 14%, n = 7) after longer (> 25 min) exposure to 40 mm Ca2+.
The current (at 0 mV) inhibited by 10 and 40 mm Ca2+ is summarized in Fig. 4B(pHo/pHi= 7.3/5.5). The Ca2+-sensitive components were significantly smaller in the cells pretreated with 200 nm bafilomycin A1 (right column in Fig. 4B), indicating that Ca2+ is likely to inhibit the V-ATPase current. The Ca2+-induced inhibition was also observed under more physiological values of pHi (0.049 ± 0.020 pA pF−1 for pHo/pHi= 7.3/6.5, n = 4). The inhibition of the H+ current by 40 mm Ca2+ depolarized the cells by 20 ± 4 mV (n = 7). We examined the V-ATPase activity in the presence of high Ca2+ (5–40 mm). Although the amount of inhibition varied widely among cells, the bafilomycin A1-sensitive current was decreased by elevating the extracellular Ca2+ in a dose-dependent manner (Fig. 4C). Forty millimolar Ca2+ reduced the current amplitude to < 40% of the control value at 0 mV.
The inhibitory action of Ca2+ was mimicked by Mg2+. A rise in extracellular Mg2+ from 1 to 10–40 mm decreased the H+ currents (Fig. 5A and B). Pretreatment with 200 nm bafilomycin A1 significantly reduced the Mg2+-sensitve currents (Fig. 5B, right column). Relative amplitude of the bafilomycin A1-sensitive current was plotted against the concentration of extracellular Mg2+ (Fig. 5C, ▪). Calcium (Fig. 5C, □) seemed to be slightly more potent than Mg2+, but the difference was not significant.
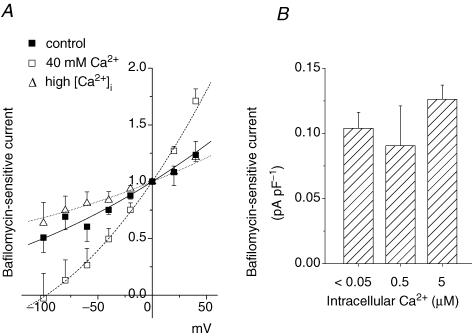
In the presence of 40 mm Ca2+, the voltage dependence of the bafilomycin-sensitive currents with a ΔpH of 1.8 was intensified (□ in Fig. 6A) compared to the control (1 mm Ca2+; ▪ in Fig. 6A). At −80 mV, the current amplitude was < 15% of that at 0 mV. Therefore, H+ secretion through the V-ATPase was greatly suppressed by hyperpolarization, probably to less than 10% of the control values, when the cell was exposed to high Ca2+.
Figure 6. Effects of extracellular and intracellular Ca2+ on the I–V relationship of the V-ATPase current.
The pipette contained 5 mm ATP. pHo/pHi was 7.3/5.5. A, I–V curves for relative bafilomycin A1 (200 nm)-sensitive currents recorded with various combinations of extracellular and intracellular Ca2+ concentrations, respectively, in 6 cells: 1 mm and < 0.05 μm (▪); 40 mm and < 0.05 μm (□); and 1 mm and 0.5–5 μm (▵). Data in each cell were normalized to the values at 0 mV and averaged. B, the bafilomycin-sensitive current densities at 0 mV with different intracellular Ca2+ levels: 0.104 ± 0.012 pA pF−1 (n = 25) for < 0.05 μm; 0.091 ± 0.031 pA pF−1 (n = 6) for 0.5 μm; and 0.126 ± 0.011 pA pF−1 (n = 4) for 5 μm Ca2+. The extracellular Ca2+ was 1 mm. Data are means ± s.e.m.
By contrast, an increase in intracellular Ca2+ (0.5–5 μm) did not seem to perturb the V-ATPase current very much. The current (at 0 mV) was unchanged over the wide range of intracellular Ca2+ levels (Fig. 6B), although the slope of the I–V curve was a little less steep than the control (Fig. 6A, ▵).
Discussion
The V-ATPase is an electrogenic H+ pump which combines enzymatic and electrogenic actions. The present study revealed, for the first time, fundamental electrophysiological properties of the plasmalemmal V-ATPase in mammalian cells, and how its functioning is affected by the ambient pH, the membrane potential and Ca2+ concentrations. These are crucial signals for regulating cellular functions.
The V-ATPase currents at the plasma membrane
It is not easy to separate the tiny H+ currents mediated by the plasmalemmal V-ATPase from the much larger currents through ion channels located in the plasma membrane. The osteoclast-like cells used in this study express sufficient plasmalemmal V-ATPase current to be analysed. Omission of major ions (Na+, K+ and Cl−) and addition of DIDS eliminated most of the currents through other ion channels. In addition, Zn2+ inhibited the H+ channels significantly over the voltage range examined. Under these experimental conditions, we could identify the plasmalemmal V-ATPase current consistently. The V-ATPase current was characterized by several specific features, such as ATP dependence, sensitivity to bafilomycin A1 (Crider et al. 1994; Zhang et al. 1994; Bowman & Bowman, 2002) and DCCD (Arai et al. 1987; Sze et al. 1992; Mattsson & Keeling, 1996), insensitivity to amiloride, outward uphill transport against large negative electrochemical gradients and a contribution to hyperpolarization. The enzyme activity was detected over a wide range of pHi values (5.5–7.3). Here we analysed the blocker-sensitive difference current, to exclude contamination with other currents, if any.
The current density was ∼0.1 pA pF−1 at 0 mV with 5 mm ATP at a ΔpH of 1.8. Remember that this represents the average value over the whole cell surface. V-ATPases are recruited by exocytotic fusion of vesicles bearing the enzyme (Nanda et al. 1996; Nelson & Harvey, 1999), often to specific regions of the plasma membrane while the cells fulfil their specialized functions. In osteoclasts carrying out bone resorption, the V-ATPase is exceptionally rich at the ruffled membrane (Arai et al. 1987; Laitala-Leinonen et al. 1996). Therefore, the efficacy of H+ secretion may be significantly higher in the hot spot. It should also be noted that the current amplitude is likely to be increased at physiological temperatures (∼37°C), probably by more than twofold (Kuno, M, preliminary observation).
Effects of the electrochemical gradient of H+ on the V-ATPase current
The availability of energy is, of course, an essential requirement for pump action. The intracellular ATP level would affect the V-ATPase activity. The cytosolic ATP concentration may vary from cell to cell and may change in single cells under different conditions. In the presence of 5 mm ATP, the V-ATPase could secrete H+ at potentials greatly negative to EH. Current reversal (H+ influx) was not apparent over the wide rage of voltages (−100 to +40 mV) and ΔpH (−1.8 to 1.8) studied.
The V-ATPase can transport H+ uphill, but the electrochemical gradient across the plasma membrane could modulate the H+ efflux. Depolarization facilitates the secretion of H+ and, in contrast, hyperpolarization suppresses it. This voltage dependence was conserved over a wide range of ΔpH values. Also, the H+ efflux was potentiated by increasing ΔpH and reduced by lowering ΔpH. The acute effects of pHo and pHi were almost reciprocal. That is, there was no apparent difference in the effects of intracellular alkalinization and extracellular acidification, and vice versa. Extracellular acidification decreased the H+ secretion at pHi values of both 5.5 and 7.3. Massive H+ secretion would in turn lead to decreases in ΔpH and hyperpolarization. In cells expressing plasmalemmal V-ATPases, the ΔpH and the membrane potential could be common self-regulatory signals to ensure the homeostasis of the pH around cells having this powerful H+-secreting pathway.
If sufficient ATP is available, acids could be secreted even at the resting potential at physiological values of pHi (∼7.3). However, a certain depolarization may be required to maintain H+ secretion when the extracellular space is highly acidic.
Inhibitory effects of extracellular divalent cations on the activity of V-ATPase
The present data show that a rise in extracelluar Ca2+ inhibited the V-ATPase current. Extracellular Mg2+ mimicked the inhibition. The mechanisms underlying the inhibition of the V-ATPase activity by divalent cations remain unresolved. The effect of surface potential on the H+ transfer across the membrane is not sufficient to explain the phenomenon, since 40 mm Ca2+ or Mg2+ further reduced H+ current compared with 10 mm of each. In addition, although the inhibition was reversible, prolonged exposure to high Ca2+ seemed to impair or delay the recovery. At this moment, there is little information available for interaction between divalent cations and the V-ATPase molecule per se. Otherwise, the inhibition of the V-ATPase activity might be secondary to a variety of cell responses triggered by high concentrations of divalent cations.
Whether this extracellular divalent cation-induced inhibition is common among many cell types or specific to osteoclasts remains to be resolved. An increase in the extracellular Ca2+ is known to inhibit bone resorption by direct action on osteoclasts. It is widely recognized that osteoclasts and their precursors possess Ca2+-sensing mechanisms and that polyvalent cations serve as the agonists (Brown & Brown, 2001). For example, both Ca2+ and Mg2+, at 10–15 mm, inhibit bone resorption and enzyme release (Zaidi et al. 1991). The Ca2+-sensitive response in osteoclasts is accompanied by a rise in the intracellular Ca2+ to ≥ 200–300 nm by a release of Ca2+ from the internal stores and/or Ca2+ influx through the plasma membrane (Malgaroli et al. 1989; Miyauchi et al. 1990; Zaidi et al. 1993; Grano et al. 1994; Bennett et al. 2001). Magnesium, however, does not mobilize intracellular Ca2+ stores (Malgaroli et al. 1989). Our present data showed that levels of intracellular Ca2+ up to 5 μm did not inhibit the V-ATPase, implying that the divalent cation-induced inhibition is not mediated by an increase in cytosolic free Ca2+.
A rise in external Ca2+ or polyvalent cations is also known to affect transmembrane ion transport in osteoclasts, such as activation of Cl− channels (Fujita et al. 1996; Shibata et al. 1997; Sakai et al. 1999; Sakuta et al. 2002) and inhibition of K+ channels (Arkett et al. 1994; Hammerland et al. 1994; Yamashita et al. 1994; Shibata et al. 1997). The Ca2+- or Mg2+-induced inhibition of the V-ATPase was shown in the absence of K+ and Cl−, implying that disturbances of these ion channels are not essential for this inhibition.
Osteoclasts may produce a remarkable reorganization of the cytoskeleton in high Ca2+; the responses include retraction of lammelipodia, decrease in podosomes and de-adhesion (Miyauchi et al. 1990). Some subunits of the membrane sector of the V-ATPase are known to bind to actin microfilaments (Breton et al. 2000; Holliday et al. 2000; Vitavska et al. 2003). Perturbations of the interaction between the V-ATPase and the cytoskeleton may lead to a decrease in the activity of the plasmalemmal V-ATPase. Further studies are needed to elucidate the inhibitory mechanisms.
Roles of extracellular Ca2+ and acid in osteoclasts
The concentration of either Ca2+ or Mg2+ in tissue is generally maintained within a narrow range. Therefore, the high levels of divalent cations in these experiments (10–40 mm) may be unusual except for specialized cells like osteoclasts. In osteoclasts, the accumulation of Ca2+ in the closed extracellular compartment (resorption pit) is an additional outcome of their own V-ATPase activity. The Ca2+ concentration during the resorption process may rise up to 40 mm (Silver et al. 1988). It was noted that the voltage dependence of the H+ currents was potentiated by Ca2+; the inhibitory effect was more marked when cells were hyperpolarized. Consequently, elevating Ca2+ from 1 to 40 mm reduced the H+ efflux at 0 mV to < 40% and at −80 mV to < 10%. Thus, both extracellular H+ and Ca2+ may work as negative feedback signals to control the bone-resorbing function of osteoclasts.
The V-ATPases at both the plasma membrane and the intracellular vesicles may share basic H+-translocating properties and at least part of the regulatory mechanisms. The present study suggests that whole-cell clamp recordings of the plasmalemmal V-ATPase in osteoclast-like cells can be a promising strategy to resolve actions of the V-ATPases under variable cell conditions.
Acknowledgments
We would like to thank Dr Charles Edwards for critically reading the manuscript, M. Okina for technical assistance and Y. Nagatomo for secretarial assistance. This work was supported by a Grant-in-Aid for Scientific Research from The Ministry of Education, Science and Culture, Japan.
References
- Arai H, Berne M, Forgac M. Inhibition of the coated vesicle proton pump and labeling of a 17,000 dalton polypeptide by DCCD. J Biol Chem. 1987;262:11006–11011. [PubMed] [Google Scholar]
- Arkett SA, Dixon SJ, Sims SM. Effects of extracelllar calcium and protons on osteoclast potassium currents. J Memb Biol. 1994;140:163–171. doi: 10.1007/BF00232904. [DOI] [PubMed] [Google Scholar]
- Bennett BD, Alvarez U, Hruska KA. Receptor-operated osteoclast calcium sensing. Endocrinology. 2001;142:1968–1974. doi: 10.1210/endo.142.5.8125. [DOI] [PubMed] [Google Scholar]
- Bowman BJ, Bowman EJ. Mutations in subunit c of the vacuolar ATPase confer resistance to bafilomycin and identify a conserved antibiotic binding site. J Biol Chem. 2002;277:3965–3972. doi: 10.1074/jbc.M109756200. [DOI] [PubMed] [Google Scholar]
- Breton S, Wiederhold T, Marshansky V, Nsumu NN, Ramesh V, Brown D. The B1 subunit of the H+ ATPase is a PDZ domain-binding protein: colocalization with the NHE-RF in renal B-intercalated cells. J Biol Chem. 2000;275:18219–18224. doi: 10.1074/jbc.M909857199. [DOI] [PubMed] [Google Scholar]
- Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev. 2001;81:239–297. doi: 10.1152/physrev.2001.81.1.239. [DOI] [PubMed] [Google Scholar]
- Crider BP, Xie XS, Stone DK. Bafilomycin inhibits proton flow through the H+ channel of vacuolar proton pumps. J Biol Chem. 1994;269:17379–17381. [PubMed] [Google Scholar]
- Fujita H, Matsumoto T, Kawashima H, Ogata E, Fujita T, Yamashita Y. Activation of Cl− channels by extracellular Ca2+ in freshly isolated rabbit osteoclasts. J Cell Physiol. 1996;169:217–225. doi: 10.1002/(SICI)1097-4652(199610)169:1<217::AID-JCP22>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Grano M, Faccio R, Colucci S, Paniccia R, Baldini N, Zallone AZ, Teti A. Extracellular Ca2+ sensing is modulated by pH in human osteoclast-like cells in vitro. Am J Physiol. 1994;267:C961–C968. doi: 10.1152/ajpcell.1994.267.4.C961. [DOI] [PubMed] [Google Scholar]
- Hammerland LG, Parihar AS, Nemeth EF, Sanguinetti MC. Voltage-activated potassium currents of rabbit osteoclasts: effects of extracellular Ca2+ Am J Physiol. 1994;267:C1103–C1111. doi: 10.1152/ajpcell.1994.267.4.C1103. [DOI] [PubMed] [Google Scholar]
- Holliday LS, Lu M, Lee BS, Nelson RD, Solivan S, Zhang L, Gluck SL. The amino-terminal domain of the B subunit of vacuolar H+-ATPase contains a filamentous actin binding site. J Biol Chem. 2000;275:32331–32337. doi: 10.1074/jbc.M004795200. [DOI] [PubMed] [Google Scholar]
- Kawanishi-Nishi S, Nishi T, Forgac M. Proton translocation driven by ATP hydrolysis in V-ATPases. FEBS Lett. 2003;545:76–85. doi: 10.1016/s0014-5793(03)00396-x. [DOI] [PubMed] [Google Scholar]
- Laitala-Leinonen T, Howell ML, Dean GE, Väänänen K. Resorption-cycle-dependent polarization of mRNAs for different subunits of V-ATPase in bone-resorbing osteoclasts. Mol Biol Cell. 1996;7:129–142. doi: 10.1091/mbc.7.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BS, Gluck SL, Holliday LS. Interaction between vacuolar H+-ATPase and microfilaments during osteoclast activation. J Biol Chem. 1999;274:29164–29171. doi: 10.1074/jbc.274.41.29164. [DOI] [PubMed] [Google Scholar]
- Lorget F, Kamel S, Mentaverri R, Wattel A, Naassila M, Maamer M, Brazier M. High extracellular calcium concentrations directly stimulate osteoclast apoptosis. Biochem Biophys Res Commun. 2000;268:899–903. doi: 10.1006/bbrc.2000.2229. [DOI] [PubMed] [Google Scholar]
- Malgaroli A, Meldolesi J, Zallone AZ, Teti A. Control of cytosolic free calcium in rat and chicken osteoclasts: the role of extracellular calcium and calcitonin. J Biol Chem. 1989;264:14342–14347. [PubMed] [Google Scholar]
- Mattsson JP, Keeling DJ. [3H]Bafilomycin as a probe for the transmembrane proton channel of the osteoclast vacuolar H+-ATPase. Biochem Biophys Acta. 1996;1280:98–106. doi: 10.1016/0005-2736(95)00285-5. [DOI] [PubMed] [Google Scholar]
- Miyauchi A, Hruska KA, Greenfield EM, Duncan R, Alvalez J, Barattolo R, Colucci S, Zambonin-Zallone A, Teitelbaum SL. Osteoclast cytosolic calcium, regulated by voltage-gated calcium channels and extracellular calcium, controls podosome assembly and bone resorption. J Cell Biol. 1990;111:2543–2552. doi: 10.1083/jcb.111.6.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H, Sakai H, Morihata H, Kawawaki J, Amano H, Yamano T, Kuno M. Regulatory mechanisms and physiological relevance of a voltage-gated H+ channel in murine osteoclasts: phorbol myristate acetate induces cell acidosis and the channel activation. J Bone Miner Res. 2003;18:2069–2076. doi: 10.1359/jbmr.2003.18.11.2069. [DOI] [PubMed] [Google Scholar]
- Mori H, Sakai H, Morihata H, Kawawaki J, Yamano T, Kuno M. A voltage-gated H+ channel is a powerful mechanism for pH homeostasis in murine osteoclasts. Kobe J Med Sci. 2002;48:87–96. [PubMed] [Google Scholar]
- Nanda A, Brumell JH, Nordoström T, Kjeldsen L, Sengeløv H, Borregaard N, Rotstein OD, Grinstein S. Activation of proton pumping in human neutrophils occurs by exocytosis of vesicles bearing vacuolar-type H+-ATPases. J Biol Chem. 1996;271:15963–15970. doi: 10.1074/jbc.271.27.15963. [DOI] [PubMed] [Google Scholar]
- Nelson N, Harvey WR. Vacuolar and plasma membrane proton-adenosinetriphosphatases. Physiol Rev. 1999;79:361–385. doi: 10.1152/physrev.1999.79.2.361. [DOI] [PubMed] [Google Scholar]
- Nordström T, Rotstein OD, Romanek R, Asotra S, Heersche JN, Manolson MF, Brisseau GF, Grinstein S. Regulation of cytoplasmic pH in osteoclasts. Contribution of proton pumps and a proton-selective conductance. J Biol Chem. 1995;270:2203–2212. doi: 10.1074/jbc.270.5.2203. [DOI] [PubMed] [Google Scholar]
- Rousselle A-V, Heymann D. Osteoclastic acidification pathways during bone resorption. Bone. 2002;30:533–540. doi: 10.1016/s8756-3282(02)00672-5. [DOI] [PubMed] [Google Scholar]
- Sakai H, Kawawaki J, Moriura Y, Mori H, Morihata H, Kuno M. Proton currents through the plasmalemmal vacuolar-type H+-ATPase in osteoclast. 50th Annual Meeting for the Biophysical Society 2006. Biophysical J. 2006:283a. Abstract. [Google Scholar]
- Sakai H, Nakamura F, Kuno M. Synergetic activation of outwardly rectifying Cl− currents by hypotonic stress and external Ca2+ in murine osteoclasts. J Physiol. 1999;515:157–168. doi: 10.1111/j.1469-7793.1999.157ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuta K, Sakai H, Mori H, Morihata H, Kuno M. Na+-dependence of extracellular calcium-sensing mechanisms leading to activation of an outwardly rectifying Cl− channel in murine osteoclasts. Bone. 2002;31:374–380. doi: 10.1016/s8756-3282(02)00838-4. [DOI] [PubMed] [Google Scholar]
- Shibata T, Sakai H, Nakamura F, Shioi A, Kuno M. Differential effect of high extracellular Ca2+ on K+ and Cl− conductances in murine osteoclasts. J Memb Biol. 1997;158:59–67. doi: 10.1007/s002329900243. [DOI] [PubMed] [Google Scholar]
- Silver IA, Murrills RJ, Etherington DJ. Microelectrode studies on the acidic microenvironment beneath adherent macrophages and osteoclasts. Exp Cell Res. 1988;175:266–276. doi: 10.1016/0014-4827(88)90191-7. [DOI] [PubMed] [Google Scholar]
- Sze H, Ward JM, Lai S. Vacuolar H+-translocating ATPases from plants: structure, function and isoforms. J Bioenerg Biomembr. 1992;24:371–381. doi: 10.1007/BF00762530. [DOI] [PubMed] [Google Scholar]
- Vitavska O, Wieczorek H, Merzendorfer H. A novel role for subunit C in mediating binding of the H+-V-ATPase to the actin cytoskeleton. J Biol Chem. 2003;278:18499–18505. doi: 10.1074/jbc.M212844200. [DOI] [PubMed] [Google Scholar]
- Wagner CA, Finberg KE, Breton S, Marshansky V, Brown D, Geibel JP. Renal vacuolar H+-ATPase. Physiol Rev. 2004;84:1263–1314. doi: 10.1152/physrev.00045.2003. [DOI] [PubMed] [Google Scholar]
- Yamashita N, Ishii T, Ogata E, Matumoto T. Inhibition of inwardly rectifying K+ currents by external Ca2+ ions in freshly isolated rabbit osteoclasts. J Physiol. 1994;480:217–224. doi: 10.1113/jphysiol.1994.sp020354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi M, Alam ASMT, Huang CL-H, Pazianas M, Bax CMR, Bax BL, Moonga BS, Bevis PJR, Shankar VS. Extracellular Ca2+ sensing by the osteoclast. Cell Calcium. 1993;14:271–277. doi: 10.1016/0143-4160(93)90048-b. [DOI] [PubMed] [Google Scholar]
- Zaidi M, Kerby J, Huang CL, Alam T, Rathod H, Chambers TJ, Moonga BS. Divalent cations mimic the inhibitory effect of extracellular ionized calcium on bone resorption by isolated rat osteoclasts: further evidence for a ‘calcium receptor’. J Cell Physiol. 1991;149:422–427. doi: 10.1002/jcp.1041490310. [DOI] [PubMed] [Google Scholar]
- Zhang J, Feng Y, Forgac M. Proton conduction and bafilomycin binding by the Vo domain of the coated vesicle V-ATPase. J Biol Chem. 1994;269:23518–23523. [PubMed] [Google Scholar]