Abstract
Previously, we found heightened expression of inhibitory neurochemicals and depressed expression of excitatory neurochemicals with a sudden drop in metabolic activity around postnatal day (P) 12 in rat brainstem respiratory nuclei, suggesting that this period is a critical window during which respiratory control or regulation may be distinctly different. To test this hypothesis, the hypoxic ventilatory responses (HVR) to 10% oxygen were tested in rats every day from P0 to P21. Our data indicate that (1) during normoxia (N), breathing frequency (f) increased with age, peaking at P13, followed by a gradual decline, whereas both tidal volume (VT) and minute ventilation (V˙E) significantly increased in the second postnatal week, followed by a progressive increase in VT and a relative plateau in V˙E; (2) during 5 min of hypoxia (H), V˙E exhibited a biphasic response from P3 onward. Significantly, the ratio of V˙E(H) to V˙E(N) was generally > 1 during development, except for P13–16, when it was < 1 after the first 1–2 min, with the lowest value at P13; (3) the H : N ratio for f, VT and V˙E during the first 30 s and the last minute of hypoxia all showed a distinct dip at P13, after which the VT and V˙E values rose again, while the f values declined through P21; and (4) the H : N ratios for f, VT and V˙E averaged over 5 min of hypoxia all exhibited a sudden fall at P13. The f ratio remained low thereafter, while those for VT and V˙E increased again with age until P21. Thus, hypoxic ventilatory response is influenced by both f and VT before P13, but predominantly by VT after P13. The striking changes in normoxic ventilation as well as HVR at or around P13, together with our previous neurochemical and metabolic data, strongly suggests that the end of the second postnatal week is a critical period of development for brainstem respiratory nuclei in the rat.
The regulation of ventilation is vital to survival. An important aspect of this adjustment is the response to hypoxia, or hypoxic ventilatory response (HVR), which undergoes developmental regulation via various mechanisms (Berquin et al. 2000; Waters & Gozal, 2003; Simakajornboon & Kuptanon, 2005). Mammalian HVR to acute hypoxia is known to be biphasic, consisting of an initial increase in ventilation followed by a later ventilatory suppression that is termed hypoxic ventilatory depression (HVD) or hypoxic ventilatory roll-off (Vizek et al. 1987; Powell et al. 1998). HVR is a complex process in which several excitatory, inhibitory and modulatory components are involved. The early HVR may be mediated by glutamate and its N-methyl-d-aspartate (NMDA) receptors (Kazemi & Hoop, 1991; Ohtake et al. 1998; Richter et al. 1999) via the carotid body–nucleus tractus solitarii (NTS) pathway and perhaps other central pathways (Mizusawa et al. 1994; Ohtake et al. 2000). HVD, in general, was postulated to be attributable to the central depressant effect of hypoxia (Vizek et al. 1987), but peripheral (arterial chemoreceptor) mechanism may not be excluded. One possibility is that HVD may act by depressing synaptic pathways activated by arterial chemoreceptors (Powell et al. 1998). Several neurotransmitters and neuromodulators, such as γ-aminobutyric acid (GABA) (Kneussl et al. 1986; Taveira da Silva et al. 1987; Kazemi & Hoop, 1991; Richter et al. 1999), serotonin (5-HT) (Di Pasquale et al. 1992; Richter et al. 1999), adenosine (Neylon & Marshall, 1991; Elnazir et al. 1996; Richter et al. 1999), and platelet-derived growth factor (PDGF-β) (Gozal et al. 2000; Simakajornboon & Kuptanon, 2005) may play important roles in HVD. In addition, hypoxic hypometabolism (Mortola, 1999) may contribute to HVD, and nitric oxide has been implicated in both excitatory and inhibitory components of the HVR (Gozal et al. 1997). Gasping activity during hypoxia is reportedly mediated by 5-HT2A receptors (Tryba et al. 2006).
HVD differs between the neonate and the adult. In neonatal mammals exposed to sustained hypoxia, the minute ventilation (V˙E) gradually decreases to levels near or below those observed in normoxia (baseline) (Eden & Hanson, 1987; Mortola & Rezzonico, 1988; Fung et al. 1996; Cohen et al. 1997; Martin et al. 1998), whereas in adult mammals, HVD displays a milder ventilatory decline to levels that remain higher than the baseline (Easton et al. 1986; Vizek et al. 1987; Gershan et al. 1994; Maxova & Vizek, 2001). However, these studies concentrated only on a selected number of time points; for example, in two studies (Eden & Hanson, 1987; Fung et al. 1996), two relatively close time series covered P1, P3, P5, P7, P14, and P1, P3, P5, P8, P13, respectively. Thus, to date, developmental changes in HVR have not been characterized on a day-to-day basis. It is also not known if there is a developmental window when HVR is at its lowest.
Previously, we conducted detailed, day-to-day studies of various brainstem respiratory nuclei of the rat and found that neurotransmitters and receptors underwent distinct developmental changes. Significantly, at or around postnatal day (P) 12, the expression of the excitatory neurotransmitter glutamate and its NMDA receptors precipitously dropped, while the expression of inhibitory neurotransmitter GABA, GABAB receptors, and glycine receptors rose sharply, concomitant with a sudden fall in cytochrome oxidase (CO) activity, a sensitive indicator of metabolic capacity and neuronal activity (Wong-Riley, 1989; Liu & Wong-Riley, 2002, 2003, 2005; Wong-Riley & Liu, 2005). We hypothesized that at and around P12 is a critical period in the postnatal development of the rat respiratory control network, during which there may be a transient dominance of inhibitory over excitatory neurotransmission, rendering the animal less capable of overcoming exogenous respiratory stresses. The current study was undertaken to test our hypothesis that the ventilatory control system, and the HVR in particular, is significantly altered during the presumed critical period. Our results indicate that, indeed, respiratory development is highly dynamic and that major changers in the HVR can occur over a 1–2 day period at the end of the second postnatal week. Thus, dynamic changes in neurochemical and metabolic development have a major behavioural impact on respiration.
Methods
Animals
All experiments and animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health), and all protocols were approved by the Medical College of Wisconsin Animal Care and Use Committee.
A total of 96 Sprague-Dawley rats (Harlan, Indianapolis, IN, USA) from eight litters were used in this study. The litter size was 10–15 pups. Each pup was studied every fifth day, first under room air and then when subjected to 5 min of hypoxia (10% O2 + 90% N2). The days were staggered such that every single day between P0 (day of birth) and P21 was studied. Approximately 12–17 animals from seven different litters were studied for each postnatal day from P0 to P21. In addition, six animals from a single litter were studied under normoxia daily from P0 to P21 to serve as a reference for the normoxic data.
Whole body plethysmography under normoxia and hypoxia
Physiological studies were conducted in an airtight barometric 1.27 l plethysmographic chamber for the measurement of respiratory frequency (f) and tidal volume (VT) and the calculation of minute ventilation (V˙E). For P0–P10 animals, a doughnut-shaped Styrofoam block (0.324 l in volume) was placed in the upper part of the chamber to reduce the ‘effective volume’ of the plethysmographic chamber and enhance the pressure signal. This modification allowed us to perform our studies without substantially altering the environment or the characteristics of the measuring system. Pressure changes were monitored with a pressure transducer, while temperature (T) and relative humidity (RH) were monitored by another transducer connected to a RH/T Transmitter (model HX93AV-RP1, Omega Engineering Inc., Stamford, CT, USA). Pressure, RH and T signals were computer recorded via a data acquisition device (model DI-158 U, DATAQ Instruments, Akron, OH, USA) connected to the transducers described above. Data were calculated using the formulae of Drorbaugh & Fenn (1955). The system was calibrated by applying 0.1 ml of air into the plethysmographic chamber 10 times before and after each animal's recording. These applications were done via a syringe driven by a weight to produce stable 0.1 ml injections at a speed of ∼200 min−1, comparable to the mean frequency of respiration between P0 and P21. Rectal temperatures of each animal were measured before and after each recording with a 30-gauge microprocessor thermometer (model HH21, Omega Engineering Inc.). At each age between P0 and P21, each animal was studied for 5 min while breathing room air (normoxia) and then subjected to 5 min of 10% O2 + 90% N2 (hypoxia). Approximately 4.5 l (∼3 times the chamber volume) of the hypoxic mixture was flushed through the chamber in 30 s, during which a small vent hole (2 mm in diameter) in the chamber was maintained at an open state from a few seconds before to a few seconds after the flushing period to prevent substantial pressure build-up, and pressure in the chamber equalized to that of room air before the chamber was sealed and recordings began. The plethysmograph was set on a heating pad, which maintained the floor of the chamber above ambient temperature. Radiant cooling from the walls and lid of the plethysmograph prevented internal air temperatures from rising excessively. Internal air temperature was continuously monitored, and did not change more than 0.5°C during any single experiment (the mean for the entire study was 25.9 ± 0.66°C). Body contact of the rat pups with the warmed bottom of the plethysmograph helped maintain the pups' temperature at or above 34°C. This was confirmed by the measurement of body temperature before and after each session. Starting at ∼30 min before each experiment, each animal was warmed by a heating pad to maintain the rectal temperature at or above 34°C before being placed on a soft pad inside the chamber.
Statistical analyses
Ventilation in normoxia and hypoxia was continuously monitored for 5 min each. The normoxic values represent the mean of the 5 min, while the hypoxic values were grouped either into 30 s bins during the 5 min or expressed as the mean of 5 min. Values that were greater than 2 standard deviations above or below the mean were deleted. Statistical analyses were done using one-way ANOVA (to control for the type I comparison-wise error rate) and Tukey's Studentized range test (between successive age groups, e.g. P2 versus P3 and P3 versus P4, to control for the type I experiment-wise error rate). Significance was set at P < 0.01 for one-way ANOVA and P < 0.05 for Tukey's test.
Results
Postnatal development of ventilatory response during normoxia
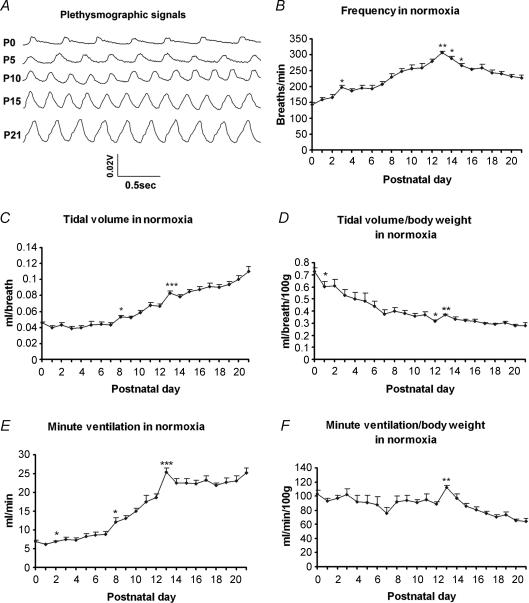
Postnatal developmental trends of respiratory frequency (f), tidal volume (VT), and minute ventilation (V˙E) during normoxia are shown in Fig. 1. Representative raw plethysmographic signals at various ages are presented in Fig. 1A. Respiratory frequencies underwent a steady increase with age, peaking at postnatal day (P) 13 (P < 0.01), then decreased significantly at P14 (P < 0.05) and P15 (P < 0.05), followed by a statistically insignificant decline until P21, the oldest age group studied (Fig. 1B). Absolute tidal volume in normoxia before adjustment to body weight displayed a plateau during the first postnatal week, a significant increase during the second postnatal week, especially between P7 and P8 (P < 0.05) and between P12 and P13 (P < 0.001), followed by a gradual increase in the third week that did not reach statistical significance between successive days, but was significantly higher at P21 than at P13 or P14 (P < 0.001 for both) (Fig. 1C). When VT was normalized to body weight, it exhibited a steady decrease with age, with a small but significant fall at P1 (P < 0.05) and P12 (P < 0.05), a significant increase at P13 (P < 0.01), followed by a relative plateau until P21 (Fig. 1D). The V˙E response under normoxia shared a trend similar to that of VT, with a slight rise during the first postnatal week (reaching significance from P1 to P2; P < 0.05), a dramatic increase during the second week, with a small peak at P8 (P < 0.05) and a more prominent peak at P13 (P < 0.001), followed by a fall at P14 and a relative plateau until P21 (Fig. 1E). When V˙E was normalized to body weight, it presented a fluctuating pattern from P0 through P12, with a slight dip at P7 and P12, a significant increase at P13 (P < 0.01), followed by a gradual decrease until P21 (Fig. 1F).
Figure 1. Postnatal development of ventilatory response during normoxia.
Representative raw plethysmographic signals at P0, P5, P10, P15 and P21 are shown (A). The developmental trends of frequency (f) (B), tidal volume (VT) (C), and minute ventilation (V˙E) (E) during normoxia all increased significantly during the 2nd postnatal week, peaking at P13 (P < 0.001), after which F values fell steadily until P21 (the last postnatal day tested), while VT and V˙E values initially fell at P14 but rose again until P21. When VT was normalized to body weight (D), its trend decreased with age, with a significant fall from P0 to P1, and from P11 to P12 (P < 0.05 for both), and a significant increase at P13 (P < 0.01) followed by a relative plateau until a small rise at P21. The developmental trend of V˙E normalized to body weight (F) showed minor fluctuations from P0 to P12. There was a significant rise in V˙E at P13 (P < 0.01), followed by a gradual decrease thereafter. ANOVA within each parameter showed significant differences among ages (P < 0.01). Statistical comparisons (Tukey's Studentized range test) between successive age groups: *P < 0.05, **P < 0.01, ***P < 0.001 (significance between one age group and its adjacent younger age group).
The above data were virtually identical to those of six animals tested only for normoxia from P0 to P21 (data not shown).
After P21, the absolute VT values exhibited significant and progressive increases at P28, P35 and P42 (data not shown). However, when adjusted to body weight, VT values declined with age. Likewise, the absolute V˙E values increased dramatically at P28, P35 and P42, but when adjusted to body weight, the values showed significant decline due to decreases in VT as well as in frequency (data not shown).
Postnatal development of ventilatory response during hypoxia
Frequency (f) changes with age during a 5 min exposure to hypoxia
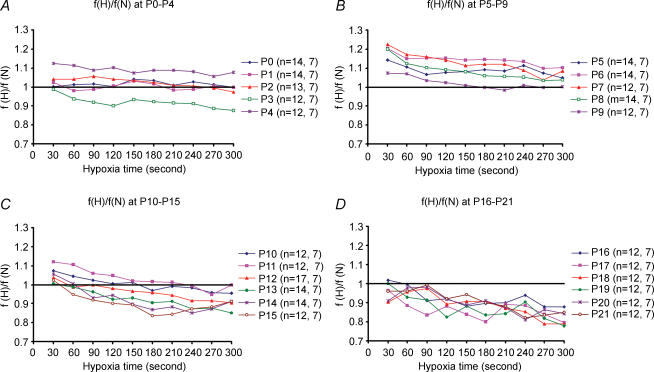
Changes in frequency (f) every 30 s during a 5 min exposure to hypoxia (inspired O2 fraction: 0.1) were tested every day from P0 to P21, with individual rats exposed to hypoxia every fifth day only. Results were expressed as a ratio of frequency during hypoxia (f(H)) versus mean frequency during normoxia (f(N)), with the normoxic value at each age set at 1 (Fig. 2). Data are presented in groups for ages P0–4 (Fig. 2A), P5–9 (Fig. 2B), P10–15 (Fig. 2C), and P16–21 (Fig. 2D). From P0 through P11, f responses were generally above or close to the baseline (normoxia), with the exception of P3, which was below the baseline after the first 30 s. Starting from P12 through P21, f responses to hypoxia were lower than that of the baseline, except between 0.5 min and 1 min when it was close to the baseline. For most of these ages, f-values during the first 30 s to 1 min were the highest, followed by a decline, with some fluctuations and sometimes a further fall after 4 or 4.5 min of hypoxia.
Figure 2. Frequency (f) changes with age during a 5 min exposure to hypoxia.
Changes in f values every 30 s during a 5 min exposure to hypoxia (H) (inspired O2 fraction: 0.1) as compared to normoxia (i.e. f(H) : f(N) ratio; with f(N) values = 1) are depicted for ages P0–4 (A), P5–9 (B), P10–15 (C) and P16–21 (D). The number of animals (n), followed by the number of litters at each day tested are indicated in brackets. Note that the values were typically higher during the first 30 s to 1 min (except for P0) than the rest of the hypoxic period and that between P0 and P11, the ratios were generally higher than 1 (except for P3). Starting at P12, the ratios after the first 0.5–1 min fell significantly below 1 and remained low through P21.
Tidal volume (VT) changes with age during a 5 min exposure to hypoxia
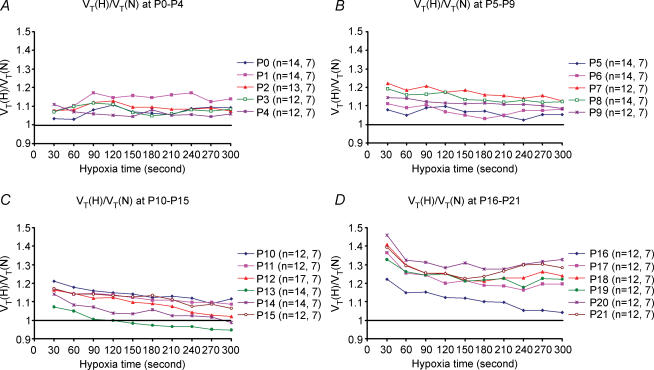
Changes in tidal volume (VT) were also analysed every 30 s during 5 min of exposure to hypoxia. Results were expressed as a ratio of VT during hypoxia (VT(H)) versus VT during normoxia (VT(N)), with the values of normoxic response set at 1. Data are plotted for various age groups (Fig. 3A–D). From P0 through P11, VT responses to hypoxia were generally above the baseline (normoxia), and the trends were relatively parallel to the baseline, with only minor fluctuations. From P12 to P16, VT responses attained the highest values during the first 30 s, followed by a gradual decrease, with the lowest values at the end of a 5 min exposure to hypoxia. Notably, at P13, VT response was above the baseline during the first minute of hypoxia, virtually at the baseline during the second minute, but fell below the baseline during the last 3 min of hypoxia (Fig. 3C). P13 was the only time during development when the VT(H)/VT(N) ratio fell below the baseline. From P14 to P16, the trends were all above the baseline, shifting successively to higher values with age. From P17 through P21, VT responses showed the highest value during the first 30 s, followed by a distinct fall in the next 30 s, then minor fluctuations and a final small increase during the last 1 or 2 min of a 5 min hypoxic exposure.
Figure 3. Tidal volume (VT) changes with age during a 5 min exposure to hypoxia.
Changes in VT values every 30 s during a 5 min exposure to hypoxia (H) (10% O2) as compared to normoxia (i.e. VT(H) : VT(N) ratio; with VT(N) values being 1) are depicted for ages P0–4 (A), P5–9 (B), P10–15 (C) and P16–21 (D). The number of animals (n), followed by the number of litters at each day tested are indicated in brackets. Note that starting at P4, the values were generally higher for the first 30 s and lower for the rest of the hypoxic period. Significantly, the only age when the ratio fell below 1 was at P13 (C).
Changes in minute ventilation (V˙E) with age during a 5 min exposure to hypoxia
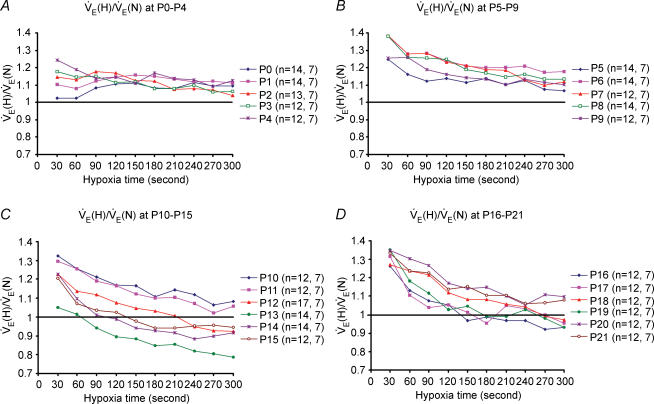
Changes in minute ventilation (V˙E) were calculated every 30 s during 5 min of exposure to hypoxia, and results were expressed as a ratio of V˙E during hypoxia (V˙E(H)) versusV˙E during normoxia (V˙E(N)), with the values of normoxic response set at 1. Data are presented for daily age groups (Fig. 4A–D). At P0–3, V˙E responses to hypoxia were generally above and relatively parallel to the baseline (normoxia), except for the first 30 s in P0 animals, during which the V˙E value was at baseline. From P4 through P11, V˙E responses to hypoxia were above the baseline for the entire 5 min exposure to hypoxia, with the highest value during the first 30 s (which was sustained for the next 30 s at P9) followed by a gradual decline. From P12 to P19, V˙E responses to hypoxia were highest during the first 30 s, followed by a decrease with time. Significantly, the lowest ventilatory response to hypoxia occurred at P13, when only the first minute was close to the baseline and the subsequent 4 min were substantially below the baseline (Fig. 4C). The trends of V˙E responses to hypoxia started to shift successively upwards from P14 to P19, with values of the last 1.5–3 min still below the baseline for P14–16. At P20 and P21, the trends became more similar to those of P10 and P11, with all V˙E values above the baseline during the entire 5 min exposure to hypoxia.
Figure 4. Changes in minute ventilation (V˙E) with age during a 5 min exposure to hypoxia.
Changes in V˙E values every 30 s during a 5 min exposure to hypoxia (H) (10% O2) as compared to normoxia (i.e. V˙E(H): V˙E(N) ratio; with V˙E(N) values being 1) are depicted for ages P0–4 (A), P5–9 (B), P10–15 (C) and P16–21 (D). The number of animals (n), followed by the number of litters at each day tested are indicated in brackets. Note that from P3 onwards the values were higher during the first 30 s than the rest of the hypoxic period, indicating a biphasic response. The late phase is essentially a plateau with minor fluctuations from P0 to P9, but from P10 to P21, the late phase shows a gradual decline with time during the 5 min period. Notably, the ratios were above 1 for most of the ages except for P13–P16, when they fell below 1 after the first 1–2 min of hypoxic exposure. The value was the lowest at P13, but steadily increased thereafter from P14 to P21.
Early and late ventilatory hypoxic response (HVR)
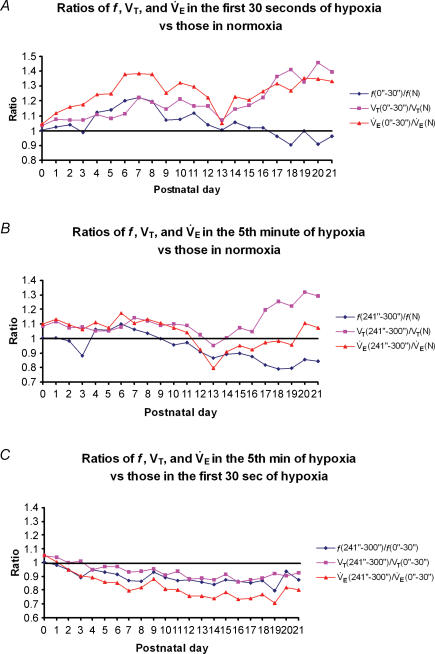
To investigate the biphasic HVR further, we chose values from the first 30 s and the last 1 min of a 5 min exposure to hypoxia as representative of early and late phase response, respectively. The f, VT and V˙E values are shown in Fig. 5. In the early phase (first 30 s), the ratio of f, VT and V˙E values during the first 30 s versus those of normoxia shared a similar trend from P0 through P13 (Fig. 5A), with a relative bell-shape curve peaking at around P7 and sharply declined at P13 (P < 0.05 for VT and V˙E at P13). From P14 through P21, VT and V˙E increased generally with age, while the values of f declined with age, falling below the baseline from P17 to P21.
Figure 5. Changes in the early and late ventilatory hypoxic responses (HVR) with age.
Changes in the ratio of frequency (f), tidal volume (VT), and minute ventilation (V˙E) in the first 30 s (A) or the last minute (B) of response to 5 min of hypoxia versus those in normoxia showed similar bell-shaped trends from P0 to P12 (except for a significant fall of the f ratio at P3, P < 0.05), with the peak at around P6–P7. At P12, the trends shifted downward, followed by a significant drop at P13. From P14 onwards, values for VT and V˙E increased with age, while that of f decreased with age for both the first 30 s and the last minute of HVR. C, the ratios of f, VT and V˙E in the fifth minute versus those in the first 30 s of HVR all decreased gradually with age, with only minor fluctuations in between. The values for all three parameters during the fifth minute are significantly lower than those in the first 30 s from P6 to P21 (P < 0.05–0.001).
During the late phase, the ratio of f, VT and V˙E values during the last minute of a 5 min response versus those of normoxia showed a developmental trend roughly parallel to that of the baseline (normoxia) from P0 to P11, the main exception being a precipitous fall in the frequency ratio at P3 (P < 0.05) (Fig. 5B). At P12 and P13, all three ratios were distinctly reduced below 1 (P < 0.05 for V˙E), with the lowest VT and V˙E values for the entire first three postnatal weeks occurring at P13. From P14 to P21, VT increased with age, while V˙E values remained below the baseline through P19, rising slightly above the baseline at P20 and P21. Frequency ratios, on the other hand, remained below the baseline from P10 through P21, with slight undulations during the third postnatal week.
In comparing the early and the late responses (Fig. 5C), the ratio of f, VT and V˙E during the fifth minute versus those of the first 30 s all showed a gradual decline with age, falling below a ratio of 1 starting at P2–P4 through P21. The values for all three parameters during the fifth minute are significantly lower than those in the first 30 s from P6 to P21 (P < 0.05–0.001).
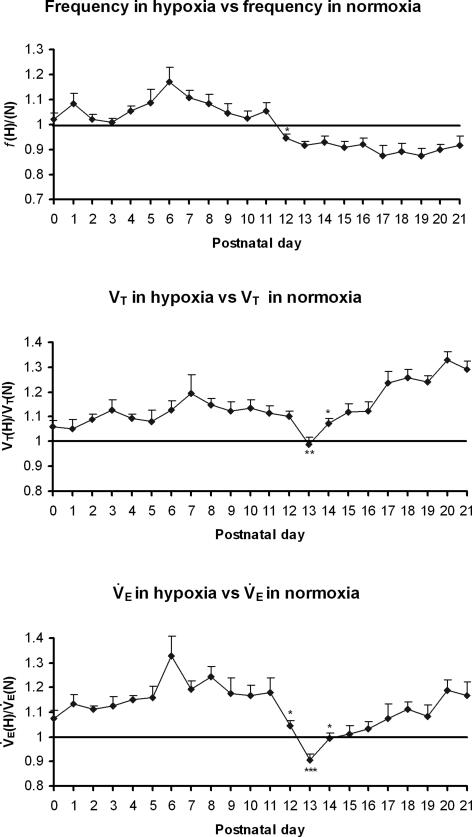
Developmental changes in the mean values of frequency, tidal volume, and minute ventilation during the entire 5 min exposure to hypoxia
The mean values of f, VT and V˙E during the entire 5 min of exposure to hypoxia (H) were plotted against normoxic (N) values for each of the postnatal days from P0 to P21 (Fig. 6). From P0 to P13, H : N ratios for f, VT and V˙E shared a similar developmental trend, with an initial slight increase with age (except for a fall in f at P2 and P3), peaking at P6 (f and V˙E) or P7 (VT), followed by a decline and a plateau until a dramatic fall in f ratios at P12 (P < 0.05) and in VT ratios at P13 (P < 0.01), resulting in a striking decrease in V˙E ratios at P12 (P < 0.05) and a further significant drop below the baseline at P13 (P < 0.001). From P14 to P21, f ratios assumed a plateau that was lower than the baseline, whereas ratios for VT and V˙E shared similar trend, significantly increasing at P14 (P < 0.05) and thereafter.
Figure 6. Developmental changes in the mean values of frequency, tidal volume and minute ventilation during the entire 5 min exposure to hypoxia.
Changes in the mean values of frequency (f) (A), tidal volume (VT) (B), and minute ventilation (V˙E) (C) averaged over 5 min of hypoxic ventilatory response versus those in normoxia indicate that their values were all above 1 from P0 to P12, with a peak at P6 (for f and V˙E) or P7 (for VT). At P12, the f and V˙E ratios dropped precipitously (P < 0.05 for both), and at P13, the ratios for VT and VE were significantly lower than those at P12 (P < 0.01−0.001) with the VT and V˙E ratios reaching their lowest on that day, and V˙E ratio significantly below 1. After P13, VT and V˙E ratios both increased with age (especially at P14, P < 0.05) while f ratios remained below 1 through P21. ANOVA of each parameter indicated significant differences among ages (P < 0.01). Statistical comparisons (Tukey's Studentized range test) between successive age groups: *P < 0.05; **P < 0.01; ***P < 0.001.
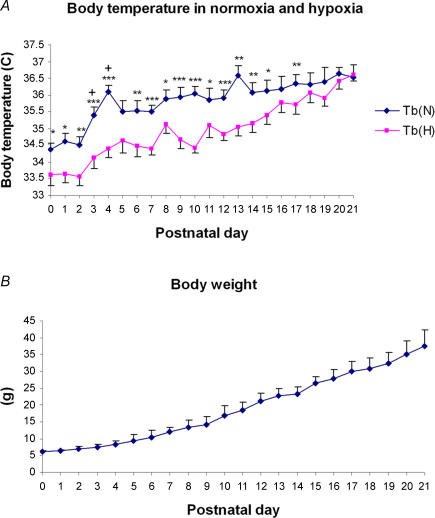
Changes in body temperature and body weight with age
Changes in body temperatures (Tb) with age during normoxia and hypoxia are plotted in Fig. 7A. Under normoxic conditions, Tb was lowest at P0–P2, increasing significantly at P3 and P4, still rising until another peak occurred at P13, followed by a fall at P14 and a gradual increase with age until P21. After 5 min of exposure to hypoxia, Tb fell ∼1–2°C at P0–13, but remained close to the baseline (Tb in normoxia) thereafter, with the two trends intersecting at P21. The body weight of animals exhibited a steady increase with age (Fig. 7B).
Figure 7. Changes in body temperature (Tb) and body weight with age.
A, under normoxic conditions, Tb was lowest at P0–P2, increased significantly between P3 and P4, then gradually increased until another peak at P13, followed by a small decline at P14 and a gradual increase with age until P21. After 5 min of exposure to hypoxia, Tb fell 1–2°C from P0–15, but was close to the baseline (Tb in normoxia) thereafter, and the two trends intersected at P21. Statistical comparisons (Student's t test) between Tb in normoxia and hypoxia: *P < 0.05; **P < 0.01; ***P < 0.001. B, the body weight of animals increased steadily with age.
Discussion
The present comprehensive, daily monitoring of ventilation during normoxia and hypoxia throughout the first three postnatal weeks has uncovered surprisingly dynamic developmental and functional changes not previously reported. These perturbations occur over a 1–2 day period at the end of the second postnatal week, corresponding temporally with distinct neurochemical and metabolic changes in brainstem respiratory nuclei reported previously (reviewed in Wong-Riley & Liu, 2005). Thus, neurochemical development has a major impact on the respiratory behaviour of the animal during a critical window of respiratory maturation. Our major findings are: (1) during normoxia (N), breathing frequency (f) increased with age, peaked at P13, and then gradually declined; both tidal volume (VT) and minute ventilation (V˙E) significantly increased in the second postnatal week, followed by a progressive increase in VT and a relative plateau in V˙E; (2) during 5 min of hypoxia (H) (FIO2 = 0.10), V˙E exhibited a biphasic response from P3 onward, with an initial heightened response followed by a gradual decline. Significantly, the ratio of V˙E(H) to V˙E(N) was generally > 1 during development, except for P13–16, when it was < 1 after the first 1–2 min, with the lowest value at P13; (3) the H : N ratio for f, VT and V˙E during the first 30 s and the last minute of hypoxia all showed a distinct dip at P13, after which the VT and V˙E values rose again, while the f values declined through P21; and (4) the H : N ratios for f, VT and V˙E averaged over 5 min of hypoxia all exhibited a sudden fall at P13. The f ratio remained low thereafter, while those for VT and V˙E increased again with age until P21. Thus, hypoxic ventilatory response is influenced by both f and VT before P13, but mainly by VT after P13.
Development of respiratory response to normoxia
Compared to published data, our normoxic V˙E values were in the mid range, being ∼30% lower than some reports (Eden & Hanson, 1987; Cameron et al. 2000) but ∼40% or 74% higher than others (Mortola et al. 1986; Thomas et al. 2000). These differences may result from varying experimental conditions, including animal strains, equipment, body temperature, plethymographic chamber temperature and relative humidity. We chose to average the normoxic values over a 5 min period rather than hand-select the ‘best value’, because hypoxic responses were monitored over a 5 min period and cross-comparisons between the two conditions would be more comparable and objective.
The relatively small size of the neonate signifies a greater surface area-to-body mass ratio, requiring a higher metabolic rate per unit body weight than the older animals to compensate for greater heat loss (Mortola, 1984). Thus, during normoxia, neonatal rat pups had relatively high VT and V˙E values when adjusted to body weight. During the first postnatal week, the absolute tidal volume in response to normoxia changed very little, while the VT adjusted to body weight exhibited a steady decline with age. During the second week, both f and absolute VT sharply increased, resulting in an increase in V˙E that peaked at P13. This corresponds to the development of the lungs, in which the rapid outgrowth of secondary septa is largely completed by the end of the second postnatal week and the surface area for gas exchange is increasing (Burri, 1974; Burri et al. 1974; Blanco, 1995). When adjusted to body weight, VT still showed a small but significant increase at P13, while V˙E reached its highest value on that day, most likely reflecting the highest peak attained for f at P13. From P14 onward and during the third postnatal week, f values steadily declined, while those of absolute VT increased, denoting the attainment of more mature, deeper and slower breaths. At the same time, there is an enormous thinning of the alveolar septa in the lung (Burri, 1974), indicating a tremendous increase in the efficiency of gas exchange. Thus, the alveolar ventilation is actually increasing even though the minute ventilation stays relatively constant. The developmental pattern of f, with rise in the first two weeks and fall in the third postnatal week was also evident in the data of Huang et al. (2004), although they reported only six time points.
The reason for increased baseline ventilation at and around P13 is not entirely clear at this time. Many factors are expected to be involved, such as the maturational process of neurotransmission, imbalance of excitatory versus inhibitory synapses, conversion of neonatal to mature forms of receptor subunit composition, hypothalamic and other supra-brainstem influences, maturational process of the gas exchange mechanism in the lung, adjustment of body temperature, and maturational process of locomotion and sleep (see below). The end of the second postnatal week is a period of multiple developmental changes that may impinge upon respiratory behaviour of the animal. However, even though the baseline ventilation is increased, the animals are less capable of responding to hypoxia.
Development of respiratory response to hypoxia
Since neonatal hypoxic exposure could alter hypoxic ventilatory responses (HVR) in later life (Bavis et al. 2004), the hypoxic challenge in the present study was presented to individual rat pups for only 5 min every fifth day. Responses to normoxia were essentially normal during the period studied, indicating that there was no detectable lasting effect of the hypoxic regimen, at least not for the first three postnatal weeks.
In agreement with published reports (Mortola, 1984, 1999; Easton et al. 1986; Eden & Hanson, 1987; Gershan et al. 1994; Moss, 2002), the HVR is a biphasic response. This was clearly the case from P3 onward, with responses during the first 30 s to 1 min being higher than the rest of the 5 min period. The initial heightened response is thought to be mediated primarily by the carotid body, while the subsequent reduced response is termed hypoxic ventilatory depression (HVD) and is attributed mainly to a combination of central and peripheral mechanisms (reviewed in Bissonnette, 2000). Of special significance is our finding that this biphasic response underwent developmental changes during the first three postnatal weeks. From P0 to P11, the ratio of V˙E in hypoxia to that in normoxia throughout the 5 min period was > 1, indicating that the system was able to respond adequately to hypoxia. However, between P12 and P16, much of the late phase of HVR was < 1, suggesting that the HVD was much stronger, as the basic ventilatory drive during normoxia is high during this time. This reduction was due mainly to decreased frequency response, but on P13, when HVR was at its lowest, the decrease was caused by a distinct suppression of both f and VT. P13 was the only time during development when the VT(H) : VT(N) ratio fell below 1. Between P17 and P21, HVR returned to a level above the baseline, indicating that, once again, the system regained a better capability to cope with hypoxia. This renewed ability was due primarily to increasing tidal volume, despite decreasing frequency of respiration, with age.
The most consistent and significant finding in the present study was that, regardless of the way the data were analysed (normoxia; every 30 s for 5 min of hypoxia; first 30 s of HVR; 5th min of HVR; and HVR averaged over 5 min), the period around P13 (P12–P15) stood out as developmentally distinct. Both f and V˙E had reached their highest peak at P13 under normoxia, and HVR (analysed in four different ways) was lowest at P13, with values at P12–P15 being lower than the rest of the three postnatal weeks. The validity of these findings is strengthened by the relatively large number of animals examined each day of the first three postnatal weeks. Thus, the end of the second postnatal week is distinctly marked as a time of substantially reduced ability of the rat pups to respond to hypoxia.
Another developmental perturbation occurred at P3, the only time during the 1st postnatal week when the breathing frequency in response to hypoxia fell below baseline (Fig. 2A). However, HVR (V˙E) may be marginally adequate at this time because tidal volume was above the baseline level. Interestingly, a consistent plateau in cytochrome oxidase activity, which reflects neuronal activity, occurred in the pre-Bötzinger complex at P3–4 (Liu & Wong-Riley, 2001; Liu & Wong-Riley, 2002).
Correlation with neurochemical and metabolic development
The HVR is highly dependent upon modulation of glutamatergic and the GABAergic transmission systems (Gozal et al. 2000). A significant reduction in HVR around P13 may indicate an intrinsic imbalance between the two systems. We found depressed expression of glutamate and NMDA receptor and heightened expression of GABA, GABAB, and glycine receptors, with a sudden and drastic drop in cytochrome oxidase activity in several brainstem respiratory nuclei at P12 (Liu & Wong-Riley, 2002, 2003, 2005; Wong-Riley & Liu, 2005). Such a transient imbalance between excitatory and inhibitory drives may render the respiratory control network less capable of responding to external stressors. At the same time, there is an apparent switch in the predominant expression of GABAA receptor subunits from a neonatal (α3) to an adult (α1) form (Liu & Wong-Riley, 2004, 2006; Wong-Riley & Liu, 2005), consistent with a developmental switch in GABA transmission from a neonatal depolarizing to a more mature hyperpolarizing mode in a number of brain regions (Ben-Ari et al. 1989; Ritter & Zhang, 2000; Gao & van den Pol, 2001). This may contribute further to the instability of the transmission system because a small change in GABA-mediated inhibition is known to profoundly alter neuronal excitability (Mody et al. 1994). Simultaneous changes in other neurochemicals and receptor subunit types may also contribute to developmental perturbations, but their roles remain to be explored.
The apparent one-day discrepancy between drastically reduced expression of excitatory neurochemicals plus reduced metabolic activity at P12 reported previously (Liu & Wong-Riley, 2002, 2003, 2005; Wong-Riley & Liu, 2005) and the marked decrease in HVR at P13 found in the present study may represent a 24-h lag in physiological function affected by neurochemical and metabolic changes. Alternatively, the non-correspondence may reflect the somewhat slower maturation of the present group of animals, even though the strain is the same (Sprague-Dawley). Indeed, eye opening in the present group was 1–2 days later, and preliminary analysis indicates that the fall in cytochrome oxidase activity occurred at P13 and not at P12 as observed previously. However, these differences need to be verified in detail. Suffice it to say that functional and neurochemical/metabolic perturbations occur within a very narrow time window of development.
Developmental changes in body temperature
The present study also indicated that the increase in body temperature with age did not follow a smooth path, but exhibited two distinct peaks, one at P4 and another one at P13 (Fig. 7A). Notably, breathing frequency in normoxia was significantly increased from P2 to P3 and peaks at P13 (Fig. 1B). Increased core temperature could signal a change in hypothalamic regulation and increased thyroid hormone levels raising the basal metabolic rate or an uncoupling of electron transport and oxidative phosphorylation (Dussault & Labrie, 1975; Lanks et al. 1986). If the heat generation is at the expense of ATP generation, it could result in decreased energy supply for neuronal activity. Exposure to hypoxia lowers the body temperature by 1–2°C during the first two postnatal weeks. This may be due to hypoxia-induced decrease in metabolic rate, especially the component of V˙O2 related to thermogenesis (Mortola & Frappell, 2000). By the third postnatal week, with the acquisition of a thick coat of fur, increased body mass and reduced frequency of respiration, the animals were able to maintain their body temperature fairly well during 5 min of hypoxia.
Significance of the end of the second postnatal week in rats: is there a critical period?
The overriding insight gained from the present and our previous studies is that the end of the second postnatal week is a highly plastic narrow window of respiratory development. This time window may be regarded as the ‘critical period’ previously described as a period ‘devoted to structural and/or functional shaping of the neural system subserving respiratory control’ (Carroll, 2003). Neurochemically, there is an imbalance between excitatory and inhibitory systems (reviewed in Wong-Riley & Liu, 2005), and physiologically, the response to hypoxia is at its weakest (the present study). The response to hypercapnia is also immature at this stage (unpublished observations of H. Forster's group). This window also marks other bodily changes, such as the opening of eyelids, the opening of the external auditory canal, the onset of non-REM sleep, the onset of the power-law distribution of wake bout distribution, the thickening of fur, a switch from polyneuronal to mononeuronal innervation of muscle fibres, the pruning of synapses onto Purkinje cells of the cerebellum, and a change from crawling to walking (Jouvet-Mounier et al. 1970; Brown et al. 1976; Crepel et al. 1976; Hoath, 1986; Petrosini et al. 1990; Blumberg et al. 2005). Other neurochemical and hormonal changes may also contribute to dynamic homeostatic regulation at this time. If such a critical period exists in humans (weeks or months in humans instead of days in rats), and if respiratory insults are introduced at this time to a vulnerable infant, especially during sleep when the respiratory control system is further suppressed (Olson & Simon, 1996; Moss, 2002), it is possible that catastrophic events, such as Sudden Infant Death Syndrome, may result.
Acknowledgments
This study was supported by a grant from the Children's Hospital and Health System Foundation, Milwaukee, Wisconsin. We greatly appreciate Drs H. Forster and A. Tryba for critically reading the paper, Dr R. Franciosi for support through the years, and Dr H. Meng for lending an able hand.
References
- Bavis RW, Olson EB, Jr, Vidruk EH, Fuller DD, Mitchell GS. Developmental plasticity of the hypoxic ventilatory response in rats induced by neonatal hypoxia. J Physiol. 2004;557:645–660. doi: 10.1113/jphysiol.2004.061408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurons. J Physiol. 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berquin P, Cayetanot F, Gros F, Larnicol N. Postnatal changes in Fos-like immunoreactivity evoked by hypoxia in the rat brainstem and hypothalamus. Brain Res. 2000;877:149–159. doi: 10.1016/s0006-8993(00)02632-9. [DOI] [PubMed] [Google Scholar]
- Bissonnette JM. Mechanisms regulating hypoxic respiratory depression during fetal and postnatal life. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1391–R1400. doi: 10.1152/ajpregu.2000.278.6.R1391. [DOI] [PubMed] [Google Scholar]
- Blanco LN. Mechanisms for the generation of gas-exchange surface area in rat lung. Am J Physiol Lung Cell Mol Physiol. 1995;269:L698–L708. doi: 10.1152/ajplung.1995.269.5.L698. [DOI] [PubMed] [Google Scholar]
- Blumberg MS, Seelke AMH, Lowen SB, Karlsson KA. Dynamics of sleep-wake cyclicity in developing rats. Proc Natl Acad Sci U S A. 2005;102:14860–14864. doi: 10.1073/pnas.0506340102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC, Jansen JKS, Van Essen D. Polyneuronal innervation of skeletal muscle in new-born rats and its elimination during maturation. J Physiol. 1976;261:387–422. doi: 10.1113/jphysiol.1976.sp011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burri PH. The postnatal growth of the rat lung. 3. Morphology. Anat Rec. 1974;180:77–98. doi: 10.1002/ar.1091800109. [DOI] [PubMed] [Google Scholar]
- Burri PH, Dbaly J, Weibel ER. The postnatal growth of the rat lung. I. Morphometry. Anat Rec. 1974;178:711–730. doi: 10.1002/ar.1091780405. [DOI] [PubMed] [Google Scholar]
- Cameron YL, Merazzi D, Mortola JP. Variability of the breathing pattern in newborn rats: effects of ambient temperature in normoxia or hypoxia. Pediatr Res. 2000;47:813–818. doi: 10.1203/00006450-200006000-00022. [DOI] [PubMed] [Google Scholar]
- Carrol JL. Developmental plasticity in respiratory control. J Appl Physiol. 2003;94:375–389. doi: 10.1152/japplphysiol.00809.2002. [DOI] [PubMed] [Google Scholar]
- Cohen G, Malcolm G, Henderson-Smart D. Ventilatory response of the newborn infant to mild hypoxia. Pediatr Pulmonol. 1997;24:163–172. doi: 10.1002/(sici)1099-0496(199709)24:3<163::aid-ppul1>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Crepel F, Mariani J, Delhaye-Bouchaud N. Evidence for a multiple innervation of Purkinje cells by climbing fibers in the immature rat cerebellum. J Neurobiol. 1976;7:567–578. doi: 10.1002/neu.480070609. [DOI] [PubMed] [Google Scholar]
- Di Pasquale E, Morin D, Monteau R, Hilaire G. Serotonergic modulation of the respiratory rhythm generator at birth: an in vitro study in the rat. Neurosci Lett. 1992;143:91–95. doi: 10.1016/0304-3940(92)90240-8. [DOI] [PubMed] [Google Scholar]
- Drorbaugh JE, Fenn WO. A barometric method for measuring ventilation in newborn infants. Pediatrics. 1955;16:81–87. [PubMed] [Google Scholar]
- Dussault JH, Labrie F. Development of the hypothalamic-pituitary-thyroid axis in the neonatal rat. Endocrinology. 1975;97:1321–1324. doi: 10.1210/endo-97-5-1321. [DOI] [PubMed] [Google Scholar]
- Easton PA, Slykerman LJ, Anthonisen NR. Ventilatory response to sustained hypoxia in normal adults. J Appl Physiol. 1986;61:906–911. doi: 10.1152/jappl.1986.61.3.906. [DOI] [PubMed] [Google Scholar]
- Eden GJ, Hanson MA. Maturation of the respiratory response to acute hypoxia in the newborn rat. J Physiol. 1987;392:1–9. doi: 10.1113/jphysiol.1987.sp016765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elnazir B, Marshall JM, Kumar P. Postnatal development of the pattern of respiratory and cardiovascular response to systemic hypoxia in the piglet: the roles of adenosine. J Physiol. 1996;492:573–585. doi: 10.1113/jphysiol.1996.sp021330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung ML, Wang W, Darnall RA, St John WM. Characterization of ventilatory responses to hypoxia in neonatal rats. Respir Physiol. 1996;103:57–66. doi: 10.1016/0034-5687(95)00077-1. [DOI] [PubMed] [Google Scholar]
- Gao XB, van den Pol AN. GABA, not glutamate, a primary transmitter driving action potentials in developing hypothalamic neurons. J Neurophysiol. 2001;85:425–434. doi: 10.1152/jn.2001.85.1.425. [DOI] [PubMed] [Google Scholar]
- Gershan WM, Forster HV, Lowry TF, Korducki MJ, Forster AL, Forster MA, Ohtake PJ, Aaron EA, Garber AK. Effect of metabolic rate on ventilatory roll-off during hypoxia. J Appl Physiol. 1994;76:2310–2314. doi: 10.1152/jappl.1994.76.6.2310. [DOI] [PubMed] [Google Scholar]
- Gozal D, Gozal E, Torres JE, Gozal YM, Nuckton TJ, Hornby PJ. Nitric oxide modulates ventilatory responses to hypoxia in the developing rat. Am J Respir Crit Care Med. 1997;155:1755–1762. doi: 10.1164/ajrccm.155.5.9154888. [DOI] [PubMed] [Google Scholar]
- Gozal D, Simakajornboon N, Czapla MA, Xue YD, Gozal E, Vlasic V, Lasky JA, Liu JY. Brainstem activation of platelet-derived growth factor-β receptor modulates the late phase of the hypoxic ventilatory response. J Neurochem. 2000;74:310–319. doi: 10.1046/j.1471-4159.2000.0740310.x. [DOI] [PubMed] [Google Scholar]
- Hoath SB. Treatment of the neonatal rat with epidermal growth factor: differences in time and organ response. Pediatr Res. 1986;20:468–472. doi: 10.1203/00006450-198605000-00017. [DOI] [PubMed] [Google Scholar]
- Huang Y-H, Brown AR, Costy-Bennett S, Luo Z, Fregosi RF. Influence of prenatal nicotine exposure on postnatal development of breathing pattern. Resp Physiol Neurobiol. 2004;143:1–8. doi: 10.1016/j.resp.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Jouvet-Mounier D, Astic L, Lacote D. Ontogenesis of the states of sleep in rat, cat, and guinea pig during the first postnatal month. Dev Psychobiol. 1970;2:216–239. doi: 10.1002/dev.420020407. [DOI] [PubMed] [Google Scholar]
- Kazemi H, Hoop B. Glutamic acid and gamma-aminobutyric acid neurotransmitters in central control of breathing. J Appl Physiol. 1991;70:1–7. doi: 10.1152/jappl.1991.70.1.1. [DOI] [PubMed] [Google Scholar]
- Kneussl MP, Pappagianopoulos P, Hoop B, Kazemi H. Reversible depression of ventilation and cardiovascular function by ventriculocisternal perfusion with γ-aminobutyric acid in dogs. Am Rev Respir Dis. 1986;133:1024–1028. doi: 10.1164/arrd.1986.133.6.1024. [DOI] [PubMed] [Google Scholar]
- Lanks KW, Hitti IF, Chin NW. Substrate utilization for lactate and energy production by heat-shocked L929 cells. J Cell Physiol. 1986;127:451–456. doi: 10.1002/jcp.1041270315. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MTT. Postnatal expression of neurotransmitters, receptors, and cytochrome oxidase in the rat pre-Botzinger complex. J Appl Physiol. 2002;92:923–934. doi: 10.1152/japplphysiol.00977.2001. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MTT. Postnatal changes in cytochrome oxidase expressions in brain stem nuclei of rats: implications for sensitive periods. J Appl Physiol. 2003;95:2285–2291. doi: 10.1152/japplphysiol.00638.2003. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MTT. Developmental changes in the expression of GABAA receptor subunits α1, α2, and α3 in the rat pre-Bötzinger complex. J Appl Physiol. 2004;96:1825–1831. doi: 10.1152/japplphysiol.01264.2003. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MTT. Postnatal developmental expressions of neurotransmitters and receptors in various brain stem nuclei of rats. J Appl Physiol. 2005;98:1442–1457. doi: 10.1152/japplphysiol.01301.2004. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MT. Developmental changes in the expression of GABAA receptor subunits α1, α2, and α3 in brain stem nuclei of rats. Brain Res. 2006;1098:129–138. doi: 10.1016/j.brainres.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Liu YY, Wong-Riley MTT. Developmental study of cytochrome oxidase activity in the brain stem respiratory nuclei of postnatal rats. J Appl Physiol. 2001;90:685–694. doi: 10.1152/jappl.2001.90.2.685. [DOI] [PubMed] [Google Scholar]
- Liu XB, Murray KD, Jones EG. Switching of NMDA receptor 2A and 2B subunits at thalamic and cortical synapses during early postnatal development. J Neurosci. 2004;24:8885–8895. doi: 10.1523/JNEUROSCI.2476-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RJ, DiFiore JM, Jana L, Davis RL, Miller MJ, Coles SK, Dick TE. Persistence of the biphasic ventilatory response to hypoxia in preterm infants. J Pediatr. 1998;132:960–964. doi: 10.1016/s0022-3476(98)70391-9. [DOI] [PubMed] [Google Scholar]
- Maxova H, Vizek M. Biphasic ventilatory response to hypoxia in unanesthetized rats. Physiol Res. 2001;50:91–96. [PubMed] [Google Scholar]
- Mizusawa A, Ogawa H, Kikuchi Y, Hida W, Kurosawa H, Okabe S, Takishima T, Shirato K. In vivo release of glutamate in nucleus tractus solitarii of the rat during hypoxia. J Physiol. 1994;478:55–66. doi: 10.1113/jphysiol.1994.sp020229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody I, De Koninck Y, Otis TS, Soltesz I. Bridging the cleft at GABA synapses in the brain. Trends Neurosci. 1994;17:517–525. doi: 10.1016/0166-2236(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Mortola JP. Breathing pattern in newborns. J Appl Physiol. 1984;56:1533–1540. doi: 10.1152/jappl.1984.56.6.1533. [DOI] [PubMed] [Google Scholar]
- Mortola JP. How newborn mammals cope with hypoxia. Respir Physiol. 1999;116:95–103. doi: 10.1016/s0034-5687(99)00038-9. [DOI] [PubMed] [Google Scholar]
- Mortola JP, Frappell PB. Ventilatory responses to changes in temperature in mammals and other vertebrates. Annu Rev Physiol. 2000;62:847–874. doi: 10.1146/annurev.physiol.62.1.847. [DOI] [PubMed] [Google Scholar]
- Mortola JP, Morgan CA, Virgona V. Respiratory adaptation to chronic hypoxia in newborn rats. J Appl Physiol. 1986;61:1329–1336. doi: 10.1152/jappl.1986.61.4.1329. [DOI] [PubMed] [Google Scholar]
- Mortola JP, Rezzonico R. Metabolic and ventilatory rates in newborn kittens during acute hypoxia. Respir Physiol. 1988;73:55–67. doi: 10.1016/0034-5687(88)90127-2. [DOI] [PubMed] [Google Scholar]
- Moss IR. Maturation of respiratory control in the behaving mammal. Respir Physiol Neurobiol. 2002;132:131–144. doi: 10.1016/s1569-9048(02)00070-8. [DOI] [PubMed] [Google Scholar]
- Neylon M, Marshall JM. The role of adenosine in the respiratory and cardiovascular response to systemic hypoxia in the rat. J Physiol. 1991;440:529–545. doi: 10.1113/jphysiol.1991.sp018723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtake PJ, Simakajornboon N, Fehniger MD, Xue YD, Gozal D. N-Methyl-D-aspartate receptor expression in the nucleus tractus solitarii and maturation of hypoxic ventilatory response in the rat. Am J Respir Crit Care Med. 2000;162:1140–1147. doi: 10.1164/ajrccm.162.3.9903094. [DOI] [PubMed] [Google Scholar]
- Ohtake PJ, Torres JE, Gozal YM, Graff GR, Gozal D. NMDA receptors mediate peripheral chemoreceptor afferent input in the conscious rat. J Appl Physiol. 1998;84:853–861. doi: 10.1152/jappl.1998.84.3.853. [DOI] [PubMed] [Google Scholar]
- Olson EJ, Simon PM. Sleep-wake cycles and the management of respiratory failure. Curr Opin Pulm Med. 1996;2:500–506. [PubMed] [Google Scholar]
- Petrosini L, Molinari M, Gremoli T. Hemicerebellectomy and motor behaviour in rats. I. Development of motor function after neonatal lesion. Exp Brain Res. 1990;82:472–482. doi: 10.1007/BF00228789. [DOI] [PubMed] [Google Scholar]
- Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol. 1998;112:123–134. doi: 10.1016/s0034-5687(98)00026-7. [DOI] [PubMed] [Google Scholar]
- Richter DW, Schmidt-Garcon P, Pierrefiche O, Bischoff AM, Lalley PM. Neurotransmitters and neuromodulators controlling the hypoxic respiratory response in anaesthetized cats. J Physiol. 1999;514:567–578. doi: 10.1111/j.1469-7793.1999.567ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter B, Zhang W. Early postnatal maturation of GABAA inhibition in the brainstem respiratory rhythm-generating network of mouse. Eur J Neurosci. 2000;12:2975–2984. doi: 10.1046/j.1460-9568.2000.00152.x. [DOI] [PubMed] [Google Scholar]
- Simakajornboon N, Kuptanon T. Maturational changes in neuromodulation of central pathways underlying hypoxic ventilatory response. Respir Physiol Neurobiol. 2005;149:273–286. doi: 10.1016/j.resp.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Taveira da Silva AM, Hartley B, Hamosh P, Quest JA, Gillis RA. Respiratory depressant effects of GABA α- and β-receptor agonists in the cat. J Appl Physiol. 1987;62:2264–2272. doi: 10.1152/jappl.1987.62.6.2264. [DOI] [PubMed] [Google Scholar]
- Thomas AJ, Erokwu BO, Yamamoto BK, Ernsberger P, Bishara O, Strohl KP. Alterations in respiratory behavior, brain neurochemistry and receptor density induced by pharmacologic suppression of sleep in the neonatal period. Brain Res Dev Brain Res. 2000;120:181–189. doi: 10.1016/s0165-3806(00)00008-0. [DOI] [PubMed] [Google Scholar]
- Tryba AK, Pena F, Ramirez JM. Gasping activity in vitro: a rhythm dependent on 5-HT2A receptors. J Neurosci. 2006;26:2623–2634. doi: 10.1523/JNEUROSCI.4186-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizek M, Pickett CK, Weil JV. Biphasic ventilatory response of adult cats to sustained hypoxia has central origin. J Appl Physiol. 1987;63:1658–1664. doi: 10.1152/jappl.1987.63.4.1658. [DOI] [PubMed] [Google Scholar]
- Waters KA, Gozal D. Responses to hypoxia during early development. Respir Physiol Neurobiol. 2003;136:115–129. doi: 10.1016/s1569-9048(03)00076-4. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MTT. Cytochrome oxidase: an endogenous metabolic marker for neuronal activity. Trends Neurosci. 1989;12:94–101. doi: 10.1016/0166-2236(89)90165-3. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MTT, Liu Q. Neurochemical development of brain stem nuclei involved in the control of respiration. Respir Physiol Neurobiol. 2005;149:83–98. doi: 10.1016/j.resp.2005.01.011. [DOI] [PubMed] [Google Scholar]