Abstract
Intermuscular coherence analysis can be used to assess the common drive to muscles. Coherence in the β-frequency band (15–35 Hz) is thought to arise from common cortical sources. Intermuscular coherence analysis is a potentially attractive tool for the investigation of motor cortical excitability changes because it is non-invasive and can be done relatively quickly. We carried out this study to test the hypothesis that intermuscular coherence analysis was able to detect cortical excitability changes in healthy subjects following transcranial direct current stimulation (tDCS). tDCS has been shown to increase (anodal stimulation) or decrease (cathodal stimulation) the size of the muscle potential evoked by TMS. We found that anodal tDCS caused an increase in motor evoked potential (MEP) size that was paralleled by an increase in β-band intermuscular coherence. Similarly, the reduction in MEP size produced by cathodal tDCS was paralleled by a reduction in β-band intermuscular coherence, while sham stimulation did not result in any change in either MEP amplitude or β-band intermuscular coherence. The similar pattern of change observed for MEP and intermuscular coherence may indicate similar mechanisms of action, although this cannot be assumed without further investigation. These changes do suggest that at least some of the action of tDCS is on cortical networks, and that combined tDCS and intermuscular coherence analysis may be useful in the diagnosis of pathologies affecting motor cortical excitability.
Intermuscular coherence analysis has been shown to be capable of reflecting motor cortical function, and is a potentially appealing option for the investigation of motor control. It is a measure of the similarity between a pair of signals in the frequency domain (Challis & Kitney, 1991). As the frequency domain equivalent of cross correlation, it can be used to infer information concerning the neural drive behind a movement (Nielsen et al. 2002; Norton et al. 2004). Intermuscular coherence analysis can provide information about common drive during a movement and it has a number of practical advantages. First, the subject only needs to produce a brief contraction and the procedure is therefore relatively quick. Second, the EMG equipment needed is commonly available. Third, as a correlational measure it is not influenced by the amplitude of the time domain signal or the power in the frequency domain. This means that it can be used in patients who are only able to produce a weak or brief muscle contraction. Lastly, it has been shown to be a reliable measure with high test–retest reproducibility (Pohja et al. 2005). Coherence between muscles is generally found in two frequency bands, α (5–15 Hz) and β (15–35 Hz). On the basis of animal studies and clinical studies that have utilized EEG/EMG coherence, the β-band is believed to be cortical in origin (Mima & Hallet, 1999; Gerloff et al. 2006).
Motor cortical functions are affected in many neurological diseases. A better understanding of the underlying mechanisms responsible for the functional derangement could have potentially important therapeutic implications. Transcranial magnetic stimulation (TMS) has proven to be a robust tool in providing mechanistic insight into motor cortical functions (Chen, 2000). Transcranial direct current stimulation (tDCS) of the motor cortex has been shown to produce lasting excitability changes in corticospinal pathways (Nitsche & Paulus, 2000). In healthy subjects, stimulation with the cathode over the motor cortex (cathodal stimulation) decreases the size of the motor evoked potential (MEP) from TMS and anodal stimulation increases the MEP size (Nitsche & Paulus, 2000). Using pharmacological manipulations and electrophysiological techniques, it has been shown that modulation of corticospinal excitability during tDCS is dependent on the level of membrane polarization, while the after-effects of tDCS are primarily due to modifications of intracortical synaptic activity (Liebetanz et al. 2002; Nitsche et al. 2005).
Because methods that can be feasibly used in human subjects to investigate these changes are limited, the primary objective of this study was to test the hypothesis that intermuscular coherence analysis was able to detect motor cortical excitability changes in healthy subjects following tDCS. While previous studies have used perturbations to examine the effect of tDCS, in this study we were able to examine its effect on the normal control of a movement and on cortico-cortical networks responsible for movement generation (Nielsen, 2002). We also wanted to compare the patterns of change in TMS and intermuscular coherence analysis following tDCS. The similarities and differences may be instructive in pointing towards possible mechanisms that can be systematically investigated in future studies.
Methods
All experiments were carried out with local ethical committee approval, in accordance with the Declaration of Helsinki and the informed consent of the subjects. Ten healthy subjects (five female, age 27.4 ± 9.2 years; one left-handed) participated in this study, with no adverse effects reported. All experiments were carried out on the right hand and left motor cortex.
TMS
TMS was delivered through a Magstim 200 stimulator (Magstim Company, Winchester, MA, USA) with a figure-of-eight coil (outer diameter 70 mm). The first dorsal interosseous (FDI) and extensor digitorum communis (EDC) muscles were chosen for recording because they are far enough apart that cross-talk is not an issue, yet close enough that their cortical representation allows simultaneous stimulation of both muscle groups by the TMS coil. Surface (Ag/AgCl) EMG electrodes measuring 2.5 × 1 cm were placed in a monopolar configuration over the FDI and EDC muscles. MEP data were recorded from the FDI muscle in all 10 subjects, while MEP data for the EDC muscle were also recorded in 5 of the 10 subjects. The TMS coil was placed tangentially to the scalp with the handle directed posterolaterally at an angle of ∼45 deg from the midline. The position that evoked a consistent twitch in the FDI and EDC muscles was marked on the scalp (‘hotspot’). Stimulation intensity was adjusted to evoke a consistent MEP of approximately 0.8–1.0 mV. To compensate for inherent variability in MEPs recorded at rest, 15 MEPs were recorded and averaged in each test epoch. For each tDCS session, the subject's hotspot and required TMS intensity were determined. MEPs were recorded with the subject at rest, verified by audio and visual inspection of the raw EMG trace. MEP size was measured from the peak-to-peak amplitude using custom written scripts in Matlab (Mathworks, Nattick, MT, USA) and Microsoft Excel (Microsoft, Redmond, WA, USA). Signals were amplified (1 mV per division for MEP recording, and 200 μV per division for coherence recording) (Advantage EMG machine; Neuroscan, Inc., VA, USA) and collected on a computer running Axoscope through a Digidata 1200 data acquisition board (Axon Instruments, Molecular Devices Corporation, Sunnyvale, CA, USA) and band-pass filtered offline (3–300 Hz for coherence analysis, and 5–500 Hz for MEP). Data were sampled at 1 kHz and stored for offline analysis.
Coherence assessment
Coherence was calculated from 30 s records of wrist extension and finger abduction against mild resistance applied by the experimenter. Subjects were given verbal encouragement and visual feedback via an oscilloscope of the raw EMG trace from both muscle groups to maintain a consistent level of contraction throughout each trial. Coherence and phase relationships were calculated using scripts based on those developed by Halliday et al. (1995) (http://www.neurospec.org). Segments of length 512 were used giving a frequency resolution of ∼1.9 Hz (1000 Hz/512 segments). A 95% confidence limit was calculated for the coherence spectra using equations developed by Amjad et al. (1997). Coherence within a test was only considered significant if the phase relationship between the muscles was consistent, as described in previous publications (Hamm & McCurdy, 1995; Farmer et al. 1997; Halliday & Rosenberg, 1999; Mima & Hallet, 1999).
tDCS
tDCS was applied using a custom-built constant current stimulator (Type CX-6650; Rolf Schneider Electronics, Gleichen, Germany). Following rigorous skin preparation to reduce impedance, saline-soaked sponge electrodes (5 × 7 cm) were positioned over the motor cortex and contralateral orbit (Nitsche & Paulus, 2000), using the hotspot identified with TMS as the centre of the cortical electrode. Stimulation was applied for 10 min at a current of 1 mA. Sham stimulation was applied by turning on the stimulator for 10 s and then turning it off, while leaving the electrodes in place. Most subjects experienced a mild tingling sensation at the site of electrode contact that was independent of polarity and usually subsided over a period of a few seconds.
Experimental procedure
Baseline TMS values were established followed by baseline values for coherence. Each subject then underwent tDCS with the polarity randomized and known only to the experimenter. TMS was repeated immediately, and 5 and 10 min following stimulation, with coherence measured directly after TMS at each time interval. Each subject received all three stimulation conditions (sham, cathodal and anodal) with a minimum of 24 h elapsing between each experiment. The TMS stimulation intensity for each subject remained consistent across all experiments (maximal coefficient of variation <9.5%).
Statistical analysis
All analysis was performed offline. Statistical analysis was performed using SPSS 12.0, Sigmaplot 8 (SPSS, Chicago, IL, USA) and Matlab. Area of coherence was computed by calculating the area under the curve and above the 95% confidence limit. The area of coherence and mean level of significant coherence was limited to the β frequency band (15–35 Hz) (Mima & Hallet, 1999) for assessment of corticomuscular drive. We also examined the area of coherence and the mean level of significant coherence in the α-band (5–15 Hz). Paired t tests were used to compare changes in MEP amplitude and area of coherence following tDCS to baseline. Analysis of variance (ANOVA) was used to analyse the root mean square (r.m.s.) amplitude of the EMG signals recorded under different tDCS conditions. Lastly, Spearman's rank coefficient was used to analyse correlation between MEP amplitude and area of β-band coherence changes following tDCS.
Results
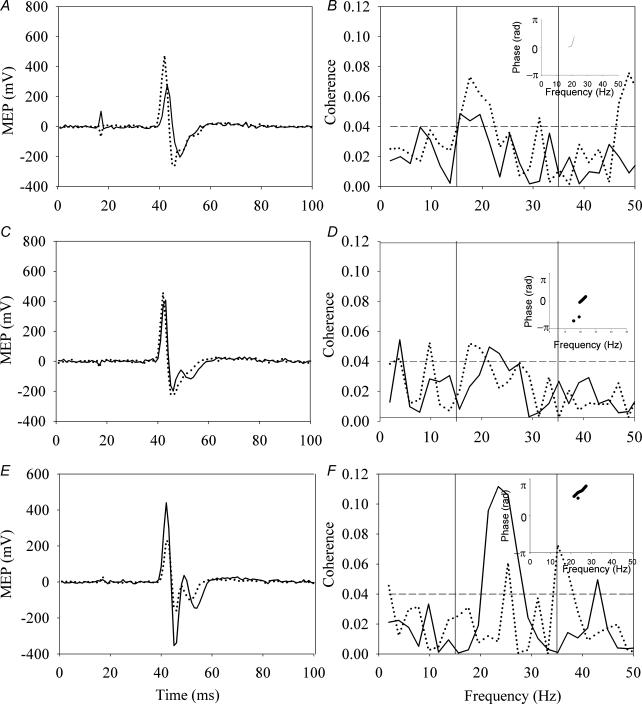
In keeping with previous studies, we found an increase in the MEP amplitude following 10 min of weak anodal stimulation and a decrease following cathodal stimulation. No change was seen with sham stimulation. Figure 1 illustrates typical results from each of the tDCS polarities and sham stimulation. The top panel illustrates the results obtained with anodal stimulation, the middle panel shows the effect of sham stimulation, while the cathodal stimulation results are illustrated in the bottom panel. The left-hand column shows the change in MEP amplitude for the FDI muscle, the middle column shows the change in MEP amplitude for the EDC muscle, and the right-hand column illustrates the change in area of coherence. There were no significant differences in the baseline measurements for MEP amplitude or coherence in either of the stimulation polarities or the sham condition (P > 0.4, paired t test). Also shown (insets of plots in right column) are the phase plots for the regions of statistically significant coherence. These indicate a stable phase relationship over the region in which the coherence is calculated.
Figure 1. Typical results in each of the three stimulation conditions.
The left panel illustrates the motor evoked potential (MEP) response in the first dorsal interosseous (FDI) muscle, while the middle panel illustrates the MEP response in the extensor digitorum communis (EDC) muscle before (continuous line) and immediately after (dashed line) 10 min of transcranial direct current stimulation (tDCS), average of 15 responses. The right panel illustrates the area of coherence between the FDI and EDC during a sustained contraction before and after tDCS. The top row illustrates the results obtained with anodal stimulation (A, B and C), the middle with sham stimulation (D, E and F), and the bottom with cathodal stimulation (G, H and I). The phase plots (insets in C, F and I) show that there is a consistent phase relationship between the two muscles in the frequency range in which the coherence is found, indicating that the coherence assessment is valid.
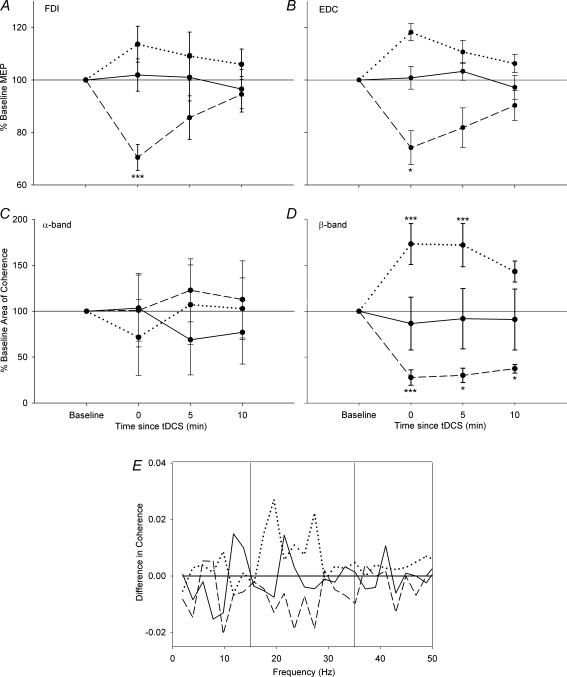
Comparisons for each subject were performed between the pre-stimulation records and those obtained immediately, and 5 and 10 min after stimulation. Pooled results from all subjects are shown in Fig. 2. The progression of MEP changes over time for the FDI and EDC muscles for each of the three stimulation conditions are shown in plots Fig. 2A and B, respectively, while plots Fig. 2C and D show changes in the area of coherence for the α- and β-bands, respectively. Following anodal stimulation, there was an increase in the MEP amplitude (to 114% of baseline for FDI and 118% for EDC, P < 0.07, paired t test) and area of β-band coherence (to 118% of baseline P < 0.005, paired t test). In contrast, cathodal stimulation resulted in significant decreases in both MEP amplitude (to 70% of baseline for FDI and 74% for EDC, P < 0.05, paired t test) and area of β-band coherence (to 83% of baseline, P < 0.005, paired t test), while sham stimulation resulted in no significant changes (to 104% of baseline for β-band area of coherence, 102 and 101% for FDI and EDC MEP amplitudes, respectively, P > 0.05 in all cases, paired t test). In all stimulation conditions, no significant change in α-band coherence was observed (to 78, 101 and 103% for anodal, cathodal and sham stimulation, respectively, P > 0.2 in all cases, paired t test). Details of the statistical analyses are reported in Table 1. The change in coherence profile is shown in Fig. 2E. This was done by subtracting the significant coherence (above 95% confidence limit) immediately after stimulation from that observed before stimulation and averaging across all subjects (Riddle & Baker, 2005). A value of zero indicates no change between pre- and post-stimulation conditions, while positive numbers indicate an increase in coherence at that frequency. Sham stimulation did not lead to a significant change, and the values varied around zero. In contrast, following anodal stimulation there was a consistent increase in the β-band region, and after cathodal stimulation there was a consistent decrease across all β-band frequencies.
Figure 2. Group results illustrating changes in MEP and coherence.
Changes in MEP peak-to-peak amplitude for the FDI (A) and EDC (B) muscles are represented in the top row, while changes in the area of coherence for the α- (C) and β-band (D) are in the middle row. The bottom row displays the significant subtracted coherence (E) (Riddle & Baker, 2005) for each of the three stimulation conditions. The group results show the same trend as the typical results in Fig. 1. The continuous line shows the effect of sham stimulation, the dotted line shows the effect of anodal stimulation, and the dashed line shows the effect of cathodal tDCS. The MEP amplitude increased significantly, as did the area of β-band coherence, after anodal tDCS, and it decreased with cathodal tDCS, whilst no change was observed with sham stimulation. No significant change was observed in the α-band. E, change in coherence across all subjects and stimulation conditions. Anodal stimulation gave rise to a broad increase in coherence in the β-band, cathodal stimulation led to a broad decrease in coherence in the β-band, and sham stimulation did not produce any trend in the results. Error bars represent the variation between subjects. *P < 0.05, **P < 0.01, ***P < 0.005.
Table 1.
Results of paired t tests for changes in MEP amplitude and area of coherence following tDCS
| Parameter | Stimulation condition | Time point | t value | P value | d.f. |
|---|---|---|---|---|---|
| FDI MEP amplitude | Anodal | 0 | −2.101 | 0.065 | 9 |
| 5 | −0.706 | 0.498 | |||
| 10 | −0.90 | 0.930 | |||
| Sham | 0 | −1.698 | 0.124 | ||
| 5 | −0.044 | 0.966 | |||
| 10 | −0.844 | 0.421 | |||
| Cathodal | 0 | 4.035 | 0.003* | ||
| 5 | 1.522 | 0.162 | |||
| 10 | −0.277 | 0.788 | |||
| EDC MEP amplitude | Anodal | 0 | −2.772 | 0.050 | 4 |
| 5 | −0.789 | 0.474 | |||
| 10 | −0.629 | 0.564 | |||
| Sham | 0 | −0.975 | 0.385 | ||
| 5 | 0.088 | 0.934 | |||
| 10 | −0.066 | 0.951 | |||
| Cathodal | 0 | 4.358 | 0.012* | ||
| 5 | 2.194 | 0.093 | |||
| 10 | 0.900 | 0.419 | |||
| Area of β-band coherence | Anodal | 0 | 7.521 | 0.000* | 9 |
| 5 | 6.423 | 0.002* | |||
| 10 | 2.185 | 0.068 | |||
| Sham | 0 | −0.527 | 0.611 | ||
| 5 | −0.807 | 0.441 | |||
| 10 | −0.366 | 0.724 | |||
| Cathodal | 0 | 8.428 | 0.000* | ||
| 5 | 2.486 | 0.035* | |||
| 10 | 2.853 | 0.019* |
MEP, motor evoked potential; tDCS, transcranial direct current stimulation. FDI, first dorsal interosseous; EDC, extensor digitorum communis. Time point refers to the number of minutes after tDCS.
Statistically significant difference (P < 0.05).
Degrees of freedom (d.f.) differ for the FDI and EDC MEP amplitude tests because the MEP in the EDC muscle was recorded from five subjects only.
Although, as a correlational measure, coherence is insensitive to the amplitude of either the raw EMG signal or the power in the β-band, changes in the strength of contraction may reflect changes in the underlying control of the contraction. To confirm that the contraction strength was similar in all conditions we measured the r.m.s. amplitude of the signal and the power in the β-band. We found that the r.m.s. amplitude of the EMG signal was not statistically different between recorded trials for each subject (0.34 versus 0.32 versus 0.36 mV, d.f. = 39, F = 1.02, P > 0.6, ANOVA) and the power in the β-spectrum remained constant (79 versus 78 versus 76 dB, d.f. = 39, F = 1.10, P > 0.5, ANOVA).
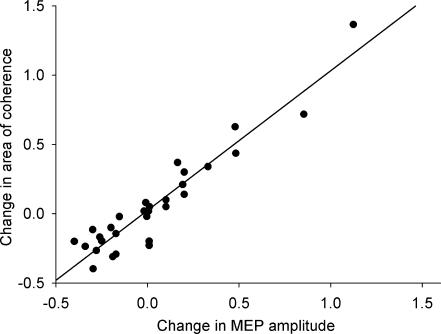
Since the coherence analysis computes the common drive to both muscles, we averaged the MEP change to the two muscles and correlated that with the change in area of β-band coherence, Fig. 3. This was significant at the t = 0 and t = 5 time points. The significant relationship between changes in MEP amplitude and area of β-band coherence (P < 0.05, r = 0.94, Spearman's rank coefficient) indicate that both of these measures respond similarly to tDCS.
Figure 3. Correlation between MEP and coherence changes.
Intermuscular coherence computes the common drive to the two muscles. The change in the average MEP amplitude from the FDI and EDC muscles was plotted against the change in area of β-band coherence. There is a significant relationship at t = 0 between these two measures (P < 0.05, r = 0.94, Spearman's rank coefficient). The fitted regression line goes through 0,0, indicating that when there is no change in one measure there is no change in the other measure.
Discussion
In this study, we demonstrated that increases in MEP size resulting from anodal tDCS of the motor cortex were paralleled by increases in intermuscular coherence in the β-frequency band. Likewise, there was a decrease in intermuscular coherence associated with cathodal tDCS, and a concomitant decrease in the size of the MEP. In contrast, sham tDCS had no effect on the MEP size or intermuscular coherence. The changes were similar in terms of amplitude and duration. These findings support our hypothesis that intermuscular coherence analysis is able to detect motor cortical excitability changes after tDCS. The magnitude and duration of change in MEP size following tDCS that we found were slightly less than those reported by Nitsche & Paulus (2001) and Nitsche et al. (2003). It is difficult to speculate possible reasons for the discrepancy but the state of the subject's relaxation and the target muscle used could have potentially contributed to the differences.
Origin of β-band coherence
In both animal and human studies, coherence between motor cortex and muscles is found in the β-band (Farmer et al. 1993; Pohja et al. 2002; Semmler, 2002). Intermuscular coherence analysis has revealed the presence of both α- and β-band coherence in humans; however, studies in both non-human primates and humans have found that only the β-band is cortical in origin (Mima & Hallet, 1999; Kilner et al. 2000). In patients with anatomically complete spinal cord lesions, recordings from muscles below the lesions during muscle spasms (i.e. with no cortex attached to the muscles) have found no β-band coherence (Norton et al. 2003). Furthermore, in patients with incomplete spinal cord injury, β-band coherence was found to correlate with volitional muscle strength, and was a predictor for functional recovery after a treadmill training programme, providing further evidence for the cortical origin of β-band intermuscular coherence (Norton & Gorassini, 2006). In the present study, there were no significant changes in the α-band coherence, suggesting there is likely to be a cortical origin for the observed plasticity.
Coherence as a clinical measure
Coherence is likely to be a clinically relevant measure that has functional correlates. Indeed, in patients with impaired motor control such as those with cortical stroke (Farmer et al. 1993; Gerloff et al. 2006) or Parkinson's disease (Marsden et al. 2000), the amount of synchrony between motor units in the β-frequency band is reduced. In contrast, EEG/EMG coherence in the β-band was significantly increased following visuo-motor skill training sessions (Perez et al. 2006). Anodal stimulation has been used to enhance functional recovery in stroke patients when applied prior to a functional hand training session (Hummel et al. 2005; Hummel & Cohen, 2005). This may help to facilitate rate of force development during rapid contractions that is important during activities involving multiple muscle groups and in promoting skilled muscle synergies (Semmler et al. 2004).
Mechanistic insights
The changes in coherence associated with tDCS of the motor cortex suggest that short-term plasticity within corticomotor networks was induced by the stimulation protocol. A previous study has shown, indirectly, that the supplementary motor area is involved in the effects of tDCS (Baudewig et al. 2001). In support of the cortical nature of the effect, intracortical inhibition elicited by paired-pulse TMS, a method of assessing cortical networks, has also been shown to be affected by tDCS (Nitsche et al. 2005). The bidirectional change observed for β-band coherence and the lack of such a change in the α-band after tDCS provides further evidence for the cortical origin of the effect. Our present results have further demonstrated that corticocortical networks are affected by tDCS. More direct evidence that the primary motor cortex (M1) is responsible for coherent corticomuscular oscillations came from a recent study by Gerloff et al. (2006). In that study, patients with congenital lesions resulting in dissociation of M1 and primary somatosensory cortex (S1) were studied. While the corresponding S1 remained in the lesioned hemisphere contralateral to the paretic hand, the corresponding M1 was in the ipsilateral hemisphere. Interestingly, in those subjects, β-band coherence was only found in the hemisphere ipsilateral to the paretic hand, indicating that the corticomuscular coherence originated primarily in M1 rather than S1.
To date, studies that have evaluated the mechanisms of coherence remain sparse. Using carbamazepine, a sodium channel blocker, Riddle et al. (2004) showed that coherence in the β-band was enhanced. The same medication was also used to modulate the after effects of tDCS (Liebetanz et al. 2002). Using MEPs evoked by TMS as an outcome measure, it was shown that carbamazepine did not have any effect on cathodal stimulation, while it completely abolished the effects of anodal stimulation. In the same study, Liebetanz et al. demonstrated that glutaminergic pathways also played a role in the response to tDCS as dextromethorphan, an NMDA antagonist, abolished both anodal and cathodal tDCS after-effects completely. The contrasting findings from these two studies indicate that although we demonstrated comparable patterns of change for MEP amplitude and intermuscular coherence after tDCS, mechanistic similarity between these methods cannot be assumed without further experiments. Direct evidence can only be obtained via more specific manipulations, possibly by pharmacological and neurophysiological means.
Acknowledgments
We wish to thank all of our subjects in this study. We are grateful to David Cornish, Ashley Watson and James Lu for their assistance in the initial stages of the study. Funding was provided by the Canada Foundation for Innovation and the Alberta Heritage Fund for Medical Research. J.A.N. is supported by a National Institutes of Health grant to Monica Gorassini.
Supplementary material
The online version of this paper can be accessed at:
DOI: 10.1113/jphysiol.2006.116939
http://jp.physoc.org/cgi/content/full/jphysiol.2006.116939/DC1 and contains supplemental material consisting of a figure entitled: Individual results showing the change in area of βband coherence for each stimulation condition.
This material can also be found as part of the full-text HTML version available from http://www.blackwell-synergy.com
References
- Amjad AM, Halliday DM, Rosenberg JR, Conway BA. An extended difference of coherence test for comparing and combining several independent coherence estimates: theory and application to the study of motor units and physiological tremor. J Neurosci Methods. 1997;73:69–79. doi: 10.1016/s0165-0270(96)02214-5. [DOI] [PubMed] [Google Scholar]
- Baudewig J, Nitsche MA, Paulus W, Frahm J. Regional modulation of BOLD MRI responses to human sensorimotor activation by transcranial direct current stimulation. Magn Reson Med. 2001;45:196–201. doi: 10.1002/1522-2594(200102)45:2<196::aid-mrm1026>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Challis RE, Kitney RI. Biomedical signal processing. Part 3. The power spectrum and coherence function. Med Biol Eng Comput. 1991;29:225–241. doi: 10.1007/BF02446704. [DOI] [PubMed] [Google Scholar]
- Chen R. Studies of human motor physiology with transcranial magnetic stimulation. Muscle Nerve. 2000;S9:26–32. doi: 10.1002/1097-4598(2000)999:9<::aid-mus6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Farmer SF, Halliday DM, Conway BA, Stephens JA, Rosenberg JR. A review of recent applicationss of cross-correlation methodologies to human motor unit recording. J Neurosci Methods. 1997;74:175–187. doi: 10.1016/s0165-0270(97)02248-6. [DOI] [PubMed] [Google Scholar]
- Farmer SF, Swash M, Ingram DA, Stephens JA. Changes in motor unit synchronization following central nervous lesions in man. J Physiol. 1993;463:83–105. doi: 10.1113/jphysiol.1993.sp019585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerloff C, Braun C, Staudt M, Hegner YL, Dichgans J, Krageloh-Mann I. Coherent corticomuscular oscillations originate from primary motor cortex: evidence from patients with early brain lesions. Human Brain Mapping. 2006;27:789–798. doi: 10.1002/hbm.20220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday DM, Rosenberg JR. Time and frequency domain analysis of spike train and time series data. In: Windhorst U, Johansson R, editors. Modern Techniques in Neuroscience Research. Berlin: Springer Verlag Heidelberg; 1999. pp. 503–543. [Google Scholar]
- Halliday DM, Rosenberg JR, Amjad AM, Breeze P, Conway BA, Farmer SF. A framework for the analysis of mixed time series/point process data-theory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Prog Biophysics Mol Biol. 1995;64:237–278. doi: 10.1016/s0079-6107(96)00009-0. [DOI] [PubMed] [Google Scholar]
- Hamm TM, McCurdy ML. The use of coherence spectra to determine common synaptic inputs to motoneurone pools of the cat during fictive locomotion. In: Taylor A, Gladden MH, Durbaba R, editors. Alpha and Gamma Motor Systems. New York: Plenum Press; 1995. pp. 309–315. [Google Scholar]
- Hummel F, Celnik PA, Giraux P, Floel A, Wu WH, Gerloff C, Cohen LG. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128:490–499. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- Hummel F, Cohen LG. Improvement of motor function with noninvasive cortical stimulation in a patient with chronic stroke. Neurorehabil Neural Repair. 2005;19:14–19. doi: 10.1177/1545968304272698. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Baker SN, Salenius S, Hari R, Lemon R. Human cortical muscle coherence is directly related to specific motor parameters. J Neurosci. 2000;20:8838–8845. doi: 10.1523/JNEUROSCI.20-23-08838.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebetanz D, Nitsche MA, Tergau F, Paulus W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain. 2002;125:2238–2247. doi: 10.1093/brain/awf238. [DOI] [PubMed] [Google Scholar]
- Marsden JF, Ashby P, Limousin-Dowsey P, Rothwell JC, Brown P. Coherence between cerebellar thalamus, cortex and muscle in man: cerebellar thalamus interactions. Brain. 2000;123:1459–1470. doi: 10.1093/brain/123.7.1459. [DOI] [PubMed] [Google Scholar]
- Mima T, Hallet M. Corticomuscular coherence: a review. J Clin Neurophysiol. 1999;16:501–520. doi: 10.1097/00004691-199911000-00002. [DOI] [PubMed] [Google Scholar]
- Nielsen JB. Motoneuronal drive during human walking. Brain Res Rev. 2002;40:192–201. doi: 10.1016/s0165-0173(02)00201-1. [DOI] [PubMed] [Google Scholar]
- Nielsen JB, Tijssen MAJ, Hansen NL, Crone C, Petersen NT, Brown P, Van Dijk JG, Rothwell JC. Corticospinal transmission to leg motoneurones in human subjects with deficient glycinergic inhibition. J Physiol. 2002;544:631–640. doi: 10.1113/jphysiol.22.024091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Nitsche MS, Klein CC, Tergau F, Rothwell JC, Paulus W. Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clin Neurophysiol. 2003;114:600–604. doi: 10.1016/s1388-2457(02)00412-1. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899–1901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Seeber A, Frommann K, Klein CC, Rochford C, Nitsche MS, Fricke K, Liebetanz D, Lang N, Antal A, Paulus W, Tergau F. Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J Physiol. 2005;568:291–303. doi: 10.1113/jphysiol.2005.092429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton JA, Gorassini MA. Changes in cortically related intermuscular coherence accompanying improvements in locomotor skills in incomplete spinal cord injury. J Neurophysiol. 2006;95:2580–2590. doi: 10.1152/jn.01289.2005. [DOI] [PubMed] [Google Scholar]
- Norton JA, Wood DE, Day BL. Is the spinal cord the generator of 16-Hz orthostatic tremor? Neurology. 2004;62:632–634. doi: 10.1212/wnl.62.4.632. [DOI] [PubMed] [Google Scholar]
- Norton JA, Wood DE, Marsden JF, Day BL. Spinally generated electromyographic oscillations and spasms in a low-thoracic complete paraplegic. Mov Disord. 2003;18:101–106. doi: 10.1002/mds.10298. [DOI] [PubMed] [Google Scholar]
- Perez MA, Lundbye-Jensen J, Nielsen JB. Changes in corticospinal drive to spinal motoneurones following visuo-motor skill learning in humans. J Physiol. 2006;573:843–855. doi: 10.1113/jphysiol.2006.105361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohja M, Salenius S, Hari R. Cortico-muscular coupling in a human subject with mirror movements – a magnetoencephalographic study. Neurosci Lett. 2002;327:185–188. doi: 10.1016/s0304-3940(02)00426-3. [DOI] [PubMed] [Google Scholar]
- Pohja M, Salenius S, Hari R. Reproducibility of cortex-muscle coherence. Neuroimage. 2005;26:764–770. doi: 10.1016/j.neuroimage.2005.02.031. [DOI] [PubMed] [Google Scholar]
- Riddle CN, Baker SN. Manipulation of peripheral neural feedback loops alters human corticomuscular coherence. J Physiol. 2005;566:625–639. doi: 10.1113/jphysiol.2005.089607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle CN, Baker MR, Baker SN. The effect of carbamazepine on human corticomuscular coherence. Neuroimage. 2004;22:333–340. doi: 10.1016/j.neuroimage.2003.12.040. [DOI] [PubMed] [Google Scholar]
- Semmler JG. Motor unit synchonization and neuromuscular performance. Exerc Sport Sci Rev. 2002;30:8–14. doi: 10.1097/00003677-200201000-00003. [DOI] [PubMed] [Google Scholar]
- Semmler JG, Sale MV, Meyer FG, Nordstrom MA. Motor-unit coherence and its relation with synchrony are influenced by training. J Neurophysiol. 2004;92:3320–3331. doi: 10.1152/jn.00316.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.