Abstract
The ventral tegmental area (VTA) and in particular VTA dopamine (DA) neurons are postulated to play a central role in reward, motivation and drug addiction. However, most evidence implicating VTA DA neurons in these functions is based on indirect electrophysiological characterization, rather than cytochemical identification. These physiological criteria were first established in the substantia nigra pars compacta (SNc), but their validity in the VTA is uncertain. In the current study we found that while 88 ± 2% of SNc neurons labelled by the neuronal marker NeuN were co-labelled for the catecholamine enzyme tyrosine hydroxylase (TH), a much smaller percentage (55 ± 2%) of VTA neurons co-expressed TH. In addition, using in vitro whole-cell recordings we found that widely accepted physiological criteria for VTA DA neurons, including the hyperpolarization-activated inwardly rectifying non-specific cation current (Ih), spike duration, and inhibition by DA D2 receptor agonists, do not reliably predict the DA content of VTA neurons. We could not distinguish DA neurons from other VTA neurons by size, shape, input resistance, Ih size, or spontaneous firing rate. Although the absence of an Ih reliably predicted that a VTA neuron was non-dopaminergic, and Ih(−) neurons differ from Ih(+) neurons in firing rate, interspike interval (ISI) standard deviation, and ISI skew, no physiological property examined here is both sensitive and selective for DA neurons in the VTA. We conclude that reliable physiological criteria for VTA DA neuron identification have yet to be determined, and that the criteria currently being used are unreliable.
Ventral tegmental area (VTA) neurons are the source of dopamine (DA) projections to the limbic forebrain and have been implicated in attention, memory, reward and motivation (Chudasama & Robbins, 2004; Wise, 2004; Nicola et al. 2005). In vivo single unit electrophysiological recordings have demonstrated that VTA neurons respond to novel stimuli, unexpected rewards, and reward predictive sensory cues, and that they fire in a pattern consistent with the encoding of reward expectancy error (Schultz, 1998; Hyland et al. 2002; Dommett et al. 2005). The conclusion that neurons with these patterns of response are dopaminergic is critical to hypotheses about the role of dopamine signalling in motivation, reward and drug addiction.
Unfortunately, current criteria for VTA DA neuron identification are based largely on indirect and in some cases conflicting evidence. Furthermore, when these criteria are used, subsets of putative DA neurons have different patterns of response (e.g. Kiyatkin & Rebec, 2001; Hyland et al. 2002; Tobler et al. 2003). For example, in vivo studies in which DA neurons were identified during extracellular recordings by action potential waveform and firing pattern properties found both excitations and inhibitions induced by noxious stimuli (Mantz et al. 1989; Romo & Schultz, 1989). However, Ungless et al. (2004), using in vivo intracellular recordings, showed that only inhibitions occurred in cytochemically confirmed TH(+) neurons, therefore clarifying DA neuron function in this behavioural paradigm, and bringing into focus the need for improved criteria for in vivo identification of DA neurons.
The criteria for identifying midbrain DA neurons were originally developed from recordings in the substantia nigra pars compacta (SNc). In vivo and in vitro recordings were used to characterize SNc neurons by such properties as action potential shape, firing rate, firing pattern and pharmacology. Experiments involving either the catecholamine neuron-selective neurotoxin 6-hydroxydopamine (6-OHDA) or cytochemical methods indicated that SNc dopaminergic neurons have relatively long duration action potentials, low firing rates, slow conduction velocity, pacemaker firing in vitro or burst firing in vivo, and DA agonist-induced inhibitions (Guyenet & Aghajanian, 1978; Grace & Bunney, 1980, 1983; Grace & Onn, 1989). Conversely, SNc neurons with short duration action potentials, higher firing rates and phasic or burst firing were not dopaminergic (Grace & Onn, 1989). In in vitro slice preparations, SNc neurons that were not cytochemically identified but categorized as DA-containing according to the physiological properties listed above, were also shown to express a hyperpolarization-activated inwardly rectifying non-specific cation current (Ih), whereas putative non-DA neurons did not (Lacey et al. 1989).
In contrast to the SN, the VTA is a midbrain region without well-delineated cytoarchitectonic boundaries. It is medially and dorsally continuous with the SNc. Because of this anatomical proximity, and the roughly similar electrophysiological properties shared by the neurons of these two brain regions, it has typically been assumed that neuronal subgroups in these two regions can be distinguished by the same physiological criteria. In a few cases, with relatively small numbers of neurons, electrophysiologically characterized VTA neurons have been cytochemically assessed, most commonly by filling recorded neurons with a marker and subsequently processing the tissue for tyrosine hydroxylase (TH) immunoreactivity (Pickel et al. 1976, 1977). Using this direct approach, physiological properties assumed to be exclusive to DA neurons have also been found in non-DA VTA neurons (Johnson & North, 1992b; Cameron et al. 1997; Jones & Kauer, 1999; Margolis et al. 2003, 2006; but see Korotkova et al. 2003). Importantly, while the absence of an Ih in a VTA neuron is a reliable predictor that the cell is not DA-containing (Margolis et al. 2003, 2006; but see Jones & Kauer, 1999), the presence of an Ih does not reliably predict TH co-labelling (Cameron et al. 1997; Jones & Kauer, 1999; Margolis et al. 2003, 2006). Thus, although the numbers of neurons in these studies are small, they provide evidence that a significant number of Ih(+) neurons are not dopaminergic.
An alternative pharmacological approach to classifying neurons in the VTA has been used by some investigators. Johnson & North (1992b) identified two major classes of VTA neuron; putative DA cells termed principal neurons that are inhibited by DA agonists, and putative GABAergic interneurons, or secondary neurons, that are inhibited by μ-opioid receptor (MOP-R) agonists (Johnson & North, 1992b, a). However, despite the wide acceptance of this simple two-neuron model circuit (e.g. Self & Nestler, 1998; Spanagel & Weiss, 1999; Georges et al. 2006; Marinelli et al. 2006), even the original report presented evidence that these categorizations were imperfect. For example, 3 out of 8 (38%) principal neurons were reported to be TH(−) (Johnson & North, 1992b). Furthermore, subsequent studies demonstrated a third group of VTA neurons, termed tertiary cells, that are inhibited by both DA and MOP-R agonists. Approximately one-third of tertiary neurons are TH(+) (Cameron et al. 1997). Combining Ih detection with MOP-R sensitivity also did not yield an accurate classification scheme, as Ih(+) neurons that were inhibited by MOP-R agonists were found to include both TH(+) and TH(−) neurons (Margolis et al. 2003).
In an attempt to evaluate current methods and determine a more reliable approach for identifying dopaminergic neurons in vitro, we analysed a variety of structural, electrophysiological and pharmacological properties of cytochemically identified VTA neurons to determine whether there is a unique constellation of properties that unambiguously identify a VTA neuron as dopaminergic. We found that the properties generally used to identify DA neurons in the VTA, including action potential duration, inhibition by DA D2 receptor agonists, and the presence of an Ih, are found in a large proportion of TH(−) VTA neurons. We also explored potential alternative measures that might differentiate DA from non-DA neurons in the VTA. Based on these data and recent studies of identified VTA projection neurons (Margolis et al. 2006), we conclude that pharmacological and physiological characteristics more reliably correspond to neurotransmitter content in subsets of VTA neurons defined by projection target, and that this approach may more closely reflect the functional organization of the VTA.
Methods
Slice preparation and electrophysiology
All procedures conformed to NIH and Ernest Gallo Clinic and Research Center animal care policy standards.
Male Sprague-Dawley rats, 20–36 days old, were anaesthetized with isoflurane, decapitated, and the brains removed. Horizontal brain slices (150 μm thick) containing the VTA were prepared using a vibratome (Leica Instruments, Germany). Slices were submerged in Ringer solution containing (mm): 119 NaCl, 2.5 KCl, 1.3 MgSO4, 1.0 NaH2PO4, 2.5 CaCl2, 26.2 NaHCO3, and 11 glucose saturated with 95% O2–5% CO2 and allowed to equilibrate at 35°C for at least 1 h.
Individual slices were visualized under a Zeiss Axioskop with differential interference contrast optics and infrared illumination, using a Zeiss Axiocam MRm and Axiovision 4 software. Whole-cell patch clamp recordings were made at 31°C using 2.5–4 MΩ pipettes containing (mm): 123 potassium gluconate, 10 Hepes, 0.2 EGTA, 8 NaCl, 2 MgATP, and 0.3 Na3GTP (pH 7.2, osmolarity adjusted to 275). Biocytin (0.1%) was added to the internal solution in order to mark the recorded neuron for later cytochemical characterization.
Recordings were made using an Axopatch 1-D (Axon Instruments, Union City, CA, USA), filtered at 2 kHz and collected at 5 kHz using IGOR Pro (Wavemetrics, Lake Oswego, OR, USA). Liquid junction potentials were not corrected during current- or voltage-clamp recordings. Ih was measured by voltage clamping cells and stepping from −60 to −40, −50, −70, −80, −90, −100, −110 and −120 mV. Input resistance was monitored with hyperpolarizing pulses while holding the cell in current clamp mode (current set to 0 pA). Spontaneous activity was similarly recorded in current clamp mode.
Quinpirole was applied by bath perfusion. Stock solution was made and diluted in Ringer solution immediately prior to application. Quinpirole stock (1 mm) was diluted in H2O. Quinpirole, ATP and GTP were obtained from Sigma Chemical (St Louis, MO, USA).
Cytochemistry
For anatomical studies, Sprague-Dawley rats (34 days old) were perfused transcardially with saline followed by 4% paraformaldehyde. Their brains were removed and postfixed for 1 h in the same fixative and then stored at 4°C in PBS (pH 7.4) for 24 h. Horizontal brain slices (50 μm thick) containing the VTA were prepared using a vibratome. Every second slice was cytochemically processed. Slices were pre-blocked for 2 h at room temperature in PBS with 1% bovine serum albumin (BSA) and 5% normal goat serum and then agitated at 4°C for 48 h with a mouse anti-glutamic acid decarboxylase 67 kDa isoform monoclonal antibody (1 : 1000). Slices were rinsed with PBS and then incubated overnight at 4°C with Cy5 or FITC anti-mouse secondary antibody (1 : 50). Slices being processed for tyrosine hydroxylase (TH) were similarly pre-blocked for 2 h in PBS plus 0.3% (v/v) Tween, 0.2% BSA and 5% normal goat serum and then incubated at 4°C with a rabbit anti-tyrosine hydroxylase polyclonal antibody (1 : 100). The slices were then washed thoroughly in PBS with 0.3% Tween and 0.2% (w/v) BSA before being agitated overnight at 4°C with Cy5 or FITC anti-rabbit secondary antibody (1 : 100).
In a subset of tissue samples, NeuN staining commenced at the completion of the TH protocol above. Slices were pre-blocked for 2 h at room temperature in PBS with 0.3% Triton X, 0.2% BSA and 10% normal goat serum, rinsed, and incubated overnight at 4°C in mouse anti-neuronal nuclei monoclonal primary antibody (1 : 1000). Slices were thoroughly washed in PBS and agitated overnight at 4°C with Cy3 anti-mouse secondary antibody (1 : 100).
Slices used for electrophysiology were fixed immediately after recording in 4% formaldehyde for 2 h and then stored at 4°C in PBS. The slices were processed as described above for TH with the addition of FITC streptavidin (6.5 μl ml−1), Texas Red avidin (11.0 μl ml−1), or fluorescein (DTAF)-conjugated streptavidin (3.25 μl ml−1), to the secondary antibody incubation step.
In all cases, sections were mounted on slides using Bio-Rad Fluoroguard Antifade Reagent mounting media and visualized under a Zeiss Axioskop as previously described or under a Zeiss LSM 510 META microscope.
Unless otherwise stated, primary antibodies were obtained from Chemicon International (Temecula, CA, USA), secondary antibodies from Jackson ImmunoResearch Laboratories (West Grove, PA, USA), and all other reagents from Sigma Chemical.
Data analysis
In all non-electrophysiological analyses of cytochemically characterized neurons, cells were randomly sampled throughout the 3-dimensional extent of the VTA; the rostral border of the VTA was taken as the level of the caudal tip of the mammillary tract and the lateral border was the medial edge of the medial terminal nucleus of the accessory optic tract (MT). Tissue samples were considered dorsal to the VTA if the MT was no longer visible in recording slices, and if TH staining of cell bodies was not present medial to the SN in the tissue used for anatomical studies. Cell sizes and shapes were measured on the Zeiss Axioskop described above. NeuN-TH data were visually analysed from scans (400 μm × 400 μm) on the Zeiss LSM 510 META setup. Random scanning locations were selected using a random number generator. The criterion of no more than 5% of TH(+) neurons could be NeuN(−) was imposed to evaluate the quality of NeuN staining. Scans exceeding this constraint were rejected, and these had to account for fewer than 10% of the total scans per animal for the animal to be included in the study.
The reported firing rates are averages of the instantaneous firing rate over 10 min of continuous recording in stably firing neurons. Results are presented as mean ± s.e.m. where appropriate. The statistical significance of drug effects was tested using Student's unpaired t test, comparing the last 4 min of baseline to the last 4 min of drug application. The membrane potential reported here is the initial potential measured immediately after achieving whole-cell configuration. Ih magnitude was measured as the difference between the initial capacitative response to a voltage step from −60 to −120 mV and the final current during the same 200 ms step. Twelve neurons that were clearly Ih(+) were excluded from the Ih magnitude analysis due to noise artefacts. Neurons were considered Ih(−) if the slope of the I–V curve for hyperpolarizing steps from −60 to −90, −100, −110 and −120 mV was 0. In cases where the magnitude difference as described above was positive for two or more of these hyperpolarizing steps, additional steps to −130 and −140 mV were added to this slope analysis. The action potential height was measured as the difference between the action potential threshold and peak of the spike, and as the difference between the peak and the immediately following trough. All other analyses of spontaneous activity were done on 601 spikes under baseline conditions prior to drug application. Some constant portion of the positive skew in each of these experiments is accounted for by the negative current pulse given once every 10 s in order to monitor input resistance (Rinput).
Differences between Ih(+) TH(+), Ih(+) TH(−), and Ih(−) neuron populations were tested using a one-way ANOVA followed by Student-Neuman-Keuls multiple comparison procedure. P < 0.05 was required for significance.
Results
Anatomy and morphology
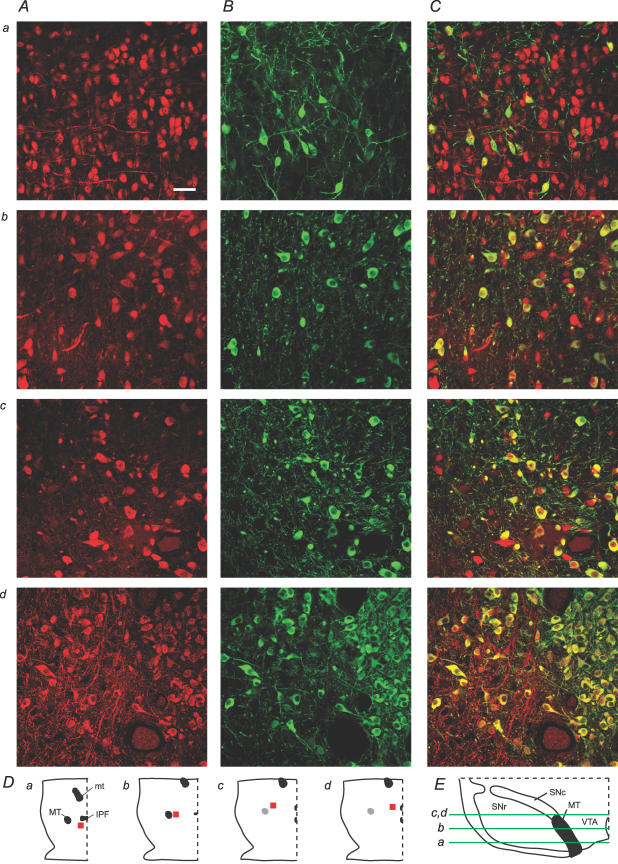
In order to evaluate how representative our sampling in electrophysiological recordings was of the overall VTA neuronal population, we cytochemically labelled horizontal sections containing the VTA for the neuron-specific nuclear protein NeuN and TH. These sections were taken from young rats corresponding in age to those we use for electrophysiology, and representative confocal images were collected in randomly selected areas throughout the dorsal–ventral (D–V), rostral–caudal (R–C) and medial–lateral (M–L) extents of the VTA of three rats. Overall, 55 ± 2% of NeuN-stained VTA neurons were co-labelled with TH (Fig. 1; n = 3 rats). The percentage TH(+) for individual scans ranged from 5% to 100%, suggesting great heterogeneity in different regions of the VTA. Co-labelling was also quantified in the SN of two of these rats, yielding a much higher percentage of NeuN-labelled neurons, 88 ± 2%, in the SNc (scan range: 74–94%) and a very low percentage, 8 ± 5%, in the SN pars reticulata (scan range: 0–16%).
Figure 1. Subregions of the VTA vary greatly in their neural density and DA neuron content.
A and B, NeuN (red) and TH (green) immunocytochemistry in various locations in the VTA (scale bar: 50 μm). C, overlays of NeuN and TH result in varied percentages of co-labelling (yellow) depending on VTA subregion. D, locations in the horizontal plane (red, not to scale). a, b, c and d correspond to those panels of the confocal scans in A, B and C. In each panel of D, right is lateral, left is midline, and top is rostral. Scans are from ventral (a), middle (b), and dorsal (c,d) sections. E, dorso-ventral location of the horizontal slices containing scans a–d. Medial is to the right, dorsal is up. MT, medial terminal nucleus of the accessory optic tract; mt, mammillary tract; IPF, interpeduncular fossa; SNc, substantia nigra pars compacta; SNr, substantia nigra pars reticulata.
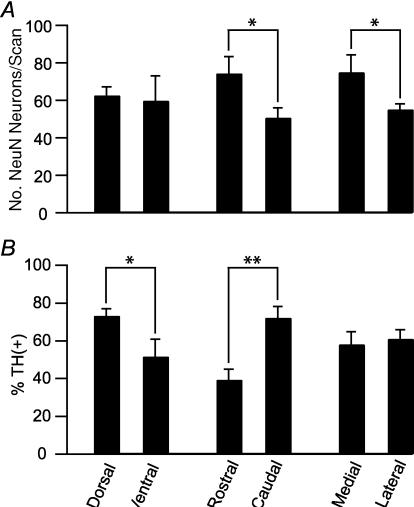
Neuron density and the proportion of TH(+) neurons were also evaluated in the three anatomical planes. Neuron density, as measured by the number of NeuN(+) neurons per scan, was greater rostral than caudal, and medial than lateral (Fig. 2A). The percentage of neurons that were TH(+) varied along the D–V and R–C axes. In the most ventral VTA (corresponding to −8.4 mm from the brain surface of the adult rat; Paxinos & Watson, 1998), 50 ± 10% of VTA neurons were TH(+) (n = 12 scans; Figs 1 (row a) and 2B). More dorsally (corresponding to −7.7 mm from the brain surface of the adult rat; Paxinos & Watson, 1998), there often were fewer neurons, but a high percentage were TH(+) (72 ± 4%; n = 19 scans; Figs 1 (rows c and d) and 2). The medial terminal nucleus of the accessory optic tract (MT) was used as the anatomical divide between rostral and caudal VTA (Fig. 1D). In rostral VTA, 39 ± 6% of neurons were TH(+) (n = 17 scans), while in caudal VTA, 72 ± 6% were TH(+) (n = 16 scans). No statistically significant differences were observed in TH content between the lateral and medial VTA (n = 18 medial scans, n = 26 lateral scans; Fig. 2B).
Figure 2. Dorsal and caudal VTA contain the greatest percentage of TH(+) neurons.
A, neurons are significantly more densely packed in the rostal than caudal (n = 17 and n = 16 scans, respectively) and medial compared to lateral (n = 18 and n = 26 scans, respectively) VTA. There was no difference between dorsal and ventral VTA neuron density (n = 19 and n = 12 scans, respectively). B, the percentage of TH(+) neurons is significantly greater in the dorsal and caudal VTA. ANOVA: *P < 0.05; **P < 0.0005.
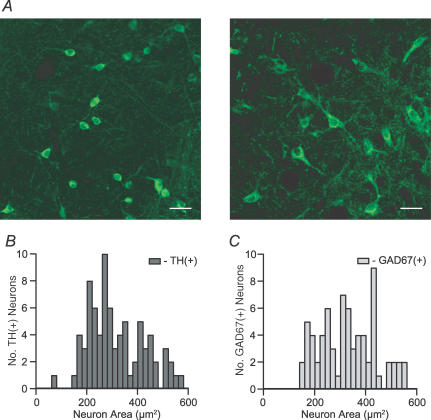
We examined the morphology of cytochemically identified VTA neurons. For comparison, we also labelled VTA tissue for the 67 kDa form of glutamate decarboxylase (GAD67), a marker for GABAergic neurons (Fig. 3A). GAD67(+) neurons were found throughout the VTA, with an overlapping distribution to that of TH(+) neurons. Any differences between TH(+), TH(−), and/or GAD67(+) cells would allow neuronal content identification in slice preparations prior to achieving electrical access to a cell. The sizes and shapes of TH(+) and GAD67(+) neurons sampled from throughout the D–V, R–C and M–L extents of the VTA were analysed. Neurons were classified as fusiform (elliptical body with exactly two obvious dendrites at opposite ends of the major axis), round, multipolar, or elliptical (similar to fusiform, but without a readily identifiable number of dendrites) (Table 1). There is a statistically significant difference between the distributions of these shapes according to TH and GAD67 content: most elliptical neurons (19/22) were TH(+). Round cells were twice as likely to be TH(+) compared to GAD67(+), whereas 63% of multipolar neurons were GAD67(+). However, none of these shapes was exclusive to one or the other type of cell.
Figure 3. Neuron size does not sort by neurotransmitter content in the VTA.
A, sample confocal images from GAD67 immunocytochemistry in horizontal brain slices containing the VTA show the various shapes and sizes of GABAergic neurons in the VTA (scale bar: 30 μm). B, cross-sectional areas of TH(+) and GAD67(+) cell bodies in the VTA have overlapping size distributions.
Table 1.
No soma shape is exclusive to dopaminergic neurons in the VTA
| Neuron shapes (P < 0.05) | ||||
|---|---|---|---|---|
| Fusiform | Round | Multipolar | Elliptical | |
| TH(+) | 19 (32%) | 12 (20%) | 10 (17%) | 19 (32%) |
| GAD67(+) | 20 (44%) | 5 (11%) | 17 (38%) | 3 (7%) |
We also tested the hypothesis that cell soma size could be used to delineate DA neurons. The cross-sectional area of neurons was measured in the tissue samples evaluated for cell shape. The distributions of TH(+) and GAD67(+) VTA neuron sizes were almost completely overlapping, and their means were not statistically different (P = 0.98; Fig. 3B and C).
Electrophysiological membrane properties
We made whole-cell patch clamp recordings in 115 neurons, filled them with biocytin, and followed the recordings with cytochemistry for TH to evaluate their DA content. Neurons were anatomically confirmed to be in the VTA, and TH content or lack thereof was only accepted as valid if the biocytin-filled neuron was in proximity to and in the same focal plane as other clearly TH-labelled neurons in the same tissue section. Only one neuron was recorded per slice, and no more than one biocytin-filled cell was ever detected in a slice.
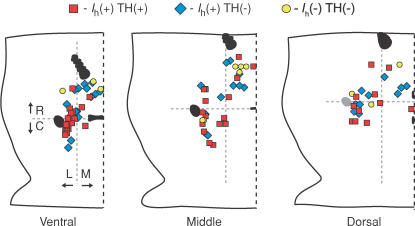
Among Ih(+) neurons, 54% (53/99) were confirmed TH(+) and all others in this sample were TH(−) yet found among unfilled TH(+) neurons. All 16 Ih(−) neurons recovered and processed for TH immunoreactivity were found to be TH(−). Figure 4 shows the locations of the recordings, delineated by TH content and Ih expression. About half of the Ih(−) neurons were recorded in experiments in which this type of neuron was specifically sought, so there was a conscious bias towards the medial, rostral VTA. All other neurons were selected at random locations throughout the slices. The anatomical distribution of percentage TH(+) was evaluated among recordings as it was in the NeuN anatomical experiment. Dorsal and ventral values in recordings were roughly equivalent (47% and 44%, respectively). In the R–C axis, the trend was similar in recordings to the cytochemistry; 30% of the recorded neurons anterior to the MT were TH(+), whereas 64% of those posterior to the MT were TH(+). The Ih(−) neurons biased the results along the M–L axis, leading to only 22% of medial cells being TH(+) in the physiologically characterized and labelled neurons. Laterally, 66% of neurons were TH(+), similar to the purely anatomical data described above.
Figure 4. Distribution of electrophysiological recordings in the VTA.
Whole-cell recordings were made in 150 μm horizontal brain sections containing the VTA. In each panel, right is midline, left is lateral, and top is rostral. Neurons were categorized here by their TH content evaluated by cytochemistry and the presence or absence of an Ih. The boundary between rostral and caudal VTA was taken as the middle of the MT, marked by the horizontal grey dashed line in each slice, which corresponded to −5.3, –5.5 and −5.5 mm from Bregma in the ventral, middle and dorsal VTA slices, respectively (Paxinos & Watson, 1998).
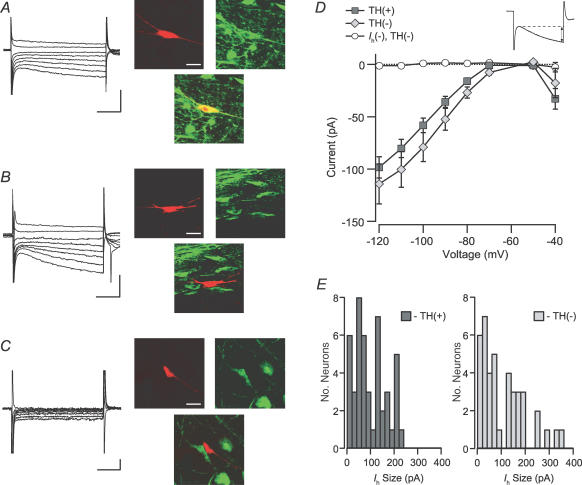
In whole-cell patch-clamp studies, when cytochemistry has not been used, the most common criterion for identifying putative DA neurons is the presence of a prominent Ih (e.g. Faleiro et al. 2004; Ye et al. 2004; Liu et al. 2005). We investigated here if the size of the Ih varies with DA content in 87 Ih(+) neurons (46/87 TH(+)). As stated above, VTA neurons that lacked an Ih were TH(−) (example in Fig. 5C). Among Ih(+) neurons, TH(+) (example in Fig. 5A) and TH(−) (example in Fig. 5B) neurons had similar distributions of Ih magnitudes (Fig. 5D and E). One noticeable difference, however, was that the neurons with the largest currents were TH(−).
Figure 5. Ih is similar between dopaminergic and non-dopaminergic neurons.
A, example Ih(+) neuron filled with biocytin (red; scale bar: 40 μm) during recording and immunocytochemically identified to be TH(+) (green, yellow; scale bars: 200 pA and 50 ms). B, example Ih(+) neuron that was TH(−) (scale bars: 200 pA and 50 ms). C, example Ih(−) neuron that was TH(−) (scale bars: 20 pA and 50 ms). D, the current induced by stepping the cells in voltage clamp to a variety of membrane potentials was not different between TH(+) and TH(−) Ih(+) neurons. Inset, the Ih was measured as the difference between the initial response to the step and the end of each 200 ms pulse. E, the magnitude of the Ih due to a step from −60 to −120 mV was not different between TH(+) and TH(−) Ih(+) neurons (n = 46 and n = 41, respectively). Ih(−) neurons were excluded from this analysis.
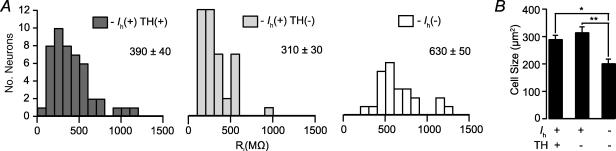
Rinput is also a measure that has frequently been reported to differ between putative DA and GABA VTA cells in vitro (Johnson & North, 1992b; Ye et al. 2004; Wang et al. 2005). However, one should note that this difference has not been observed in putative DA and GABA neurons in vitro either within the SNc (Lacey et al. 1989) or SNr (Nakanishi et al. 1987), or between the SNc and SNr (Yung et al. 1991). In this study we compared Rinput between Ih(+) TH(+), Ih(+) TH(−), and Ih(−) neurons. Since none of our 16 Ih(−) neurons were TH(+), data from 19 additional Ih(−) neurons, that were not cytochemically tested, were assumed to be TH(−) and included here and in the remaining analyses to increase the populations for this cell type. The Rinput of Ih(−) neurons was significantly greater than that of either group of Ih(+) neurons (Fig. 6A). However, there was no difference in mean Rinput between TH(+) and TH(−) groups of Ih(+) cells.
Figure 6. The input resistance of Ih(−) neurons is greater than that of Ih(+) neurons.
Input resistance was measured in current clamp mode with hyperpolarizing pulses. A, the input resistance of Ih(−) neurons is significantly greater than either TH(+) or TH(−) Ih(+) neurons, whose input resistances are similar (Ih(+) TH(+) versus Ih(−), P < 0.0001; Ih(+) TH(−) versus Ih(−), P < 0.000005; Ih(+) TH(+) versus Ih(+) TH(−), P = 0.58). B, the cross-sectional area of Ih(−) TH(−) neurons, as measured during recordings, was significantly smaller than those of either TH(+) or TH(−) Ih(+) neurons (n = 16, 28 and 28, respectively). *P < 0.001; **P < 0.0005.
Cell body size, which should correlate with Rinput, was also measured during electrophysiological recordings. Similar to Rinput, there was no difference in size between Ih(+) TH(+) and Ih(+) TH(−) neurons (Fig. 6B). However, Ih(−) neurons were significantly smaller than either TH(+) or TH(−) Ih(+) neurons (Fig. 6B). That Ih(−) neurons were smaller than either group of Ih(+) neurons corresponds with the difference in Rinput for these populations.
The initial membrane potential (Vmi) was also measured in both spontaneously firing and quiescent neurons immediately upon achieving whole-cell configuration. There was no difference between the Vmi values of Ih(+) TH(+), Ih(+) TH(−), and Ih(−) neurons (Table 2).
Table 2.
Among Ih(+) neurons, there are no differences between the basic membrane properties of DA and non-DA VTA neurons
| Vmi (mV) | AP threshold (mV) | AP height, threshold to peak (mV) | AP height, peak to trough (mV) | Fast afterhyperpolarization, magnitude from peak (mV) | Afterhyperpolarization, minimum (mV) | |
|---|---|---|---|---|---|---|
| Ih(+) TH(+) | −44 ± 1 | −24 ± 2 | 60 ± 3 | 89 ± 3 | 92 ± 5 | −53 ± 3 |
| n = 50 | n = 35 | n = 23 | n = 35 | |||
| Ih(+) TH(−) | −46 ± 1 | −28 ± 2 | 56 ± 3 | 81 ± 4 | 78 ± 6 | −53 ± 2 |
| n = 44 | n = 33 | n = 22 | n = 33 | |||
| Ih(−) | −45 ± 2 | −31.1 ± 0.9* | 57 ± 4 | 85 ± 5 | 89 ± 5 | −59 ± 2 |
| n = 16** | n = 26 | n = 21 | n = 26 | |||
| P = | 0.57 | 0.047 | 0.59 | 0.34 | 0.14 | 0.15 |
Ih(−) neurons differ from TH(+) neurons, P < 0.05.
Confirmed TH(−) neurons only are included in this measure. Vmi, initial membrane potential; AP, action potential..
Action potentials
Action potential (AP) shape and duration are consistently used in both in vitro and in vivo studies as a criterion for identifying putative DA neurons. We therefore measured a variety of AP characteristics. From current clamp recordings, the shapes of spontaneously occurring APs were compared among Ih(+) TH(+), Ih(+) TH(−), and Ih(−) VTA neurons (Table 2). The spike threshold was significantly more hyperpolarized in Ih(−) neurons than in either group of Ih(+) neurons (Ih(+) TH(+) versus Ih(−) and Ih(+) TH(−) versus Ih(−), P < 0.05). No differences were observed in the height of the APs as measured from the action potential threshold to the peak of the action potential, or as measured from the peak of the action potential to the maximum after-hyperpolarization (Ca2+-gated slow K+ current (IK(AHP))) (Johnston & Wu, 1995). A subset of neurons (Ih(+) TH(+): 23/35, Ih(+) TH(−): 22/33, Ih(−): 21/26) expressed a prominent fast hyperpolarization following the AP peak. This is probably a Ca2+-gated fast K+ current (IK(C)) (Johnston & Wu, 1995). The voltage difference between the AP peak and the minimum caused by the IK(C) was not different among the three groups. The peak negative voltage resulting from the IK(AHP) was also not different among these three types of VTA neurons.
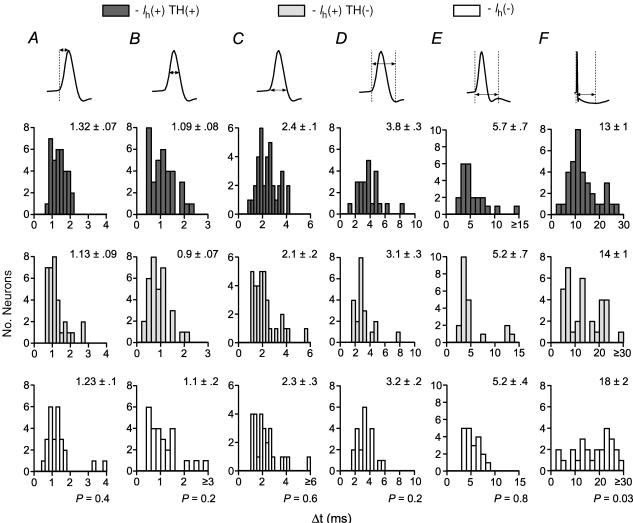
The AP duration was measured across six different intervals (Fig. 7). There was no difference among Ih(+) TH(+), Ih(+) TH(−), and Ih(−) neurons in the interval between AP threshold and peak (Fig. 7A), width at half-height (height measured between AP threshold and peak; Fig. 7B), or width at AP threshold (Fig. 7C). In neurons with a prominent IK(C), there was no difference among cell types in the AP duration measured between AP initiation and the IK(C) minimum (Fig. 7D) or between AP initiation and the maximum between IK(C) and IK(AHP) (Fig. 7E). However, Ih(−) neurons had a significantly longer duration interval between AP initiation and the voltage minimum due to the IK(AHP), although the span of these distributions were similar (Fig. 7F).
Figure 7. Action potential duration does not differ among TH(+) and TH(−) VTA neurons.
There were no differences among Ih(+) TH(+), Ih(+) TH(−), and Ih(−) neurons in action potential time to peak (A), duration at half-height (B), or width at base (C) (n = 35, 33 and 26, respectively). In neurons exhibiting a prominent Ca2+-gated fast K+ current, there was no difference among these types of cells in time to the fast K+ minimum (D) or time to the IK(AHP) (E) (n = 23, 22 and 21 for Ih(+) TH(+), Ih(+) TH(−), and Ih(−) neurons, respectively). F, the time to the peak (minimum) of the afterhyperpolarization was significantly longer in Ih(−) neurons than in either TH(+) or TH(−) Ih(+) neurons (Ih(+) TH(+) versus Ih(−) and Ih(+) TH(−) versus Ih(−), P < 0.05).
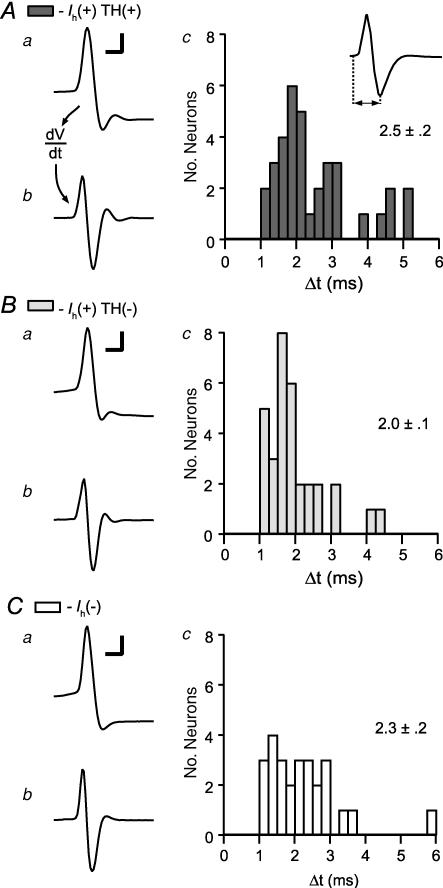
Recently, Ungless et al. (2004) suggested an AP duration measurement for use in identifying DA neurons in extracellular recordings: measuring from the AP initiation to the minimum of the first trough. Grace & Bunney (1983) showed that in the SNc, as in motoneurons (Freygang & Frank, 1959; Terzuolo & Araki, 1961), differentiating intracellularly recorded APs results in a waveform comparable to that recorded extracellularly in the same neuron. In order to compare our recordings with those of Ungless et al. we therefore differentiated the same APs that were analysed above (examples for each cell type in Fig. 8), and measured the resulting duration according to this new interval. AP durations measured in this manner were not different across Ih(+) TH(+), Ih(+) TH(−), and Ih(−) neurons (Fig. 8C; ANOVA: P = 0.13).
Figure 8. Differentiated whole-cell action potentials, a correlate of extracellular action potentials, are similar among VTA neurons.
From Ih(+) TH(+) (A), Ih(+) TH(−) (B) and Ih(−) (C) neurons: in each case example action potentials recorded in whole-cell configuration (I = 0; a) are shown (scale bars: 20 mV and 2 ms). The derivatives of these action potentials correspond to extracellularly recorded action potentials (b). c, the duration of these derived action potentials from initiation to the trough minimum is similar in all 3 types of neurons (n = 35, 32 and 26 for Ih(+) TH(+), Ih(+) TH(−), and Ih(−) neurons, respectively).
Spontaneous activity
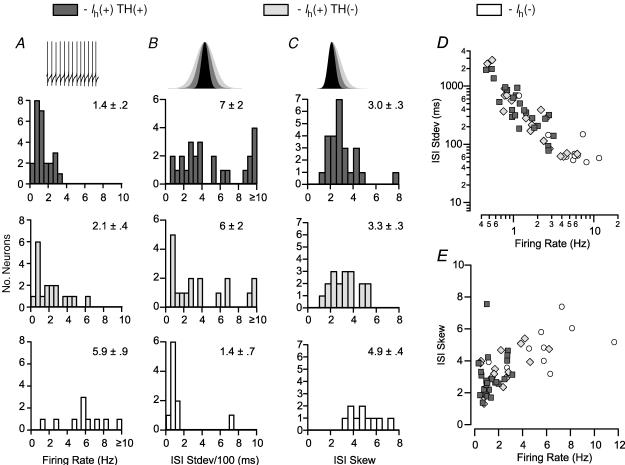
Of the 99 Ih(+) neurons in this study, 43 fired spontaneously at a stable rate for at least 10 min while being held in current clamp mode. Of these neurons, 58% were TH(+). We evaluated various properties of the firing patterns of these Ih(+) TH(+) and Ih(+) TH(−), as well as similarly spontaneously active Ih(−), neurons across a train of 601 APs (600 interspike intervals (ISIs)). The average firing rate of Ih(−) neurons was significantly faster than that of either group of Ih(+) neurons, whose firing rates were similar (Fig. 9A). The mean standard deviation of the ISI, a measure of the variability in firing, was not significantly different between these groups of neurons. However, examination of the distributions of this measure revealed that the ISI standard deviations of Ih(−) neurons were below 200 ms in every case except for one, while most Ih(+) TH(+) and Ih(+) TH(−) neurons have ISI standard deviations above this value (Fig. 9B). We also examined the skew of the distribution of ISIs in these spontaneously active neurons. A skew of 0 is indicative of a normal distribution and suggests that random noise can account for the variability in ISI. A positive skew indicates that there are more longer ISIs than would be predicted by a normal distribution. Ih(−) neurons had a significantly greater ISI skew than either group of Ih(+) neurons (Fig. 9C). There is an inverse relationship between ISI standard deviation and the firing rate among these neural groups (Fig. 9D). There were no differences in the coefficient of variations of these cell groups (Ih(+) TH(+): 0.58 ± 0.06, Ih(+) TH(−): 0.54 ± 0.08, Ih(−): 0.51 ± 0.08; ANOVA P = 0.8) Examining the relationship between ISI skew and the firing rate yielded further isolation of a subset of TH(−) neurons possessing both higher firing rates and greater ISI skews (Fig. 9E).
Figure 9. Firing pattern properties are different between Ih(+) dopaminergic and non-dopaminergic neurons.
A, stably spontaneously active Ih(−) neurons (n = 10) have a higher firing rate than either group of Ih(+) neurons (TH(+) n = 25; TH(−) n = 18; Ih(+) TH(+) versus Ih(−) and Ih(+) TH(−) versus Ih(−), P < 0.0005). B, in these neurons, the mean standard deviation of interspike intervals (ISIs) from a train of 601 consecutive action potentials is not different among cell types, although most Ih(−) neurons exhibit very small deviations (ANOVA: P = 0.13). C, the skew of the same sample of ISIs is significantly higher in Ih(−) neurons than in Ih(+) neurons (Ih(+) TH(+) versus Ih(−), P < 0.001; and Ih(+) TH(−) versus Ih(−), P < 0.005). D, there is an inverse relationship between firing rate and ISI standard deviation shared by the three cell types (note the exponential scales of axis and ordinate). E, there is a relationship between ISI skew and firing rate in spontaneously active VTA neurons.
Pharmacology
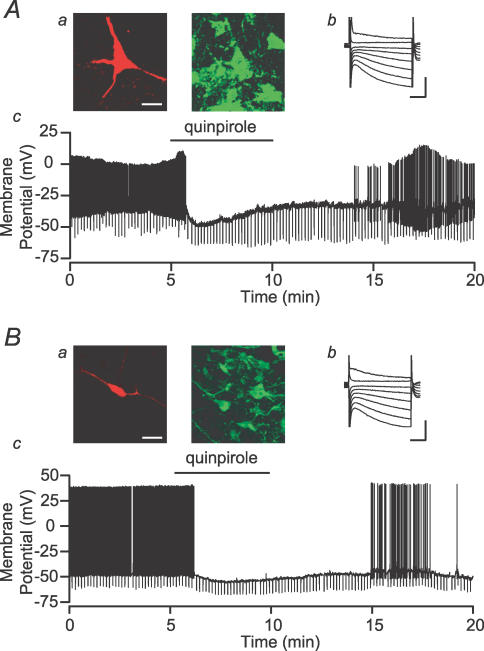
Johnson & North (1992b) defined VTA principal, putative dopaminergic, neurons as those that are hyperpolarized by DA D2 agonists. In many in vivo experiments (e.g. Georges et al. 2006; Morzorati & Marunde, 2006; Schwieler et al. 2006), inhibition by a DA D2 agonist has been used to identify putative DA neurons. We tested 33 Ih(+) neurons for their response to the DA D2 agonist quinpirole (1 μm). We found that both TH(+) and TH(−) neurons were inhibited or hyperpolarized by quinpirole (Fig. 10, Table 3). In fact, only 55% of those neurons that were inhibited by quinpirole were TH(+). Additionally, 20% of TH(+) neurons were insensitive to quinpirole (Table 3). Therefore, not only is this pharmacological effect not limited to DA neurons, it is not shared by all VTA DA neurons.
Figure 10. Both dopaminergic and non-dopaminergic VTA neurons are inhibited by dopamine Example neurons with biocytin fills (a; red; scale bar: 40 μm) and TH staining (a; green), Ih (b; scale bars: 200 pA and 50 ms), and response to bath application of the D2 agonist quinpirole (c; 1 μm).
A, an example TH(+) neuron is inhibited and hyperpolarized by quinpirole application. B, a sample TH(−) neuron is also inhibited and hyperpolarized by quinpirole.
Table 3.
Postsynaptic D2 inhibition does not correlate with TH content among Ih(+) VTA neurons (Fisher Exact Test, P = 0.3)
| D2 inhibited | No D2 effect | |
|---|---|---|
| TH(+) | 12 | 3 |
| TH(−) | 10 | 8 |
Discussion
Understanding the role of VTA neurons in reward, motivation and learning requires knowing how they respond to pharmacological agents, to salient sensory cues and to natural and drug rewards. Pharmacological manipulation of DA in the target nuclei of the VTA has implicated DA in these functions. In order to further investigate the relationship between these pharmacological actions and neural activity, many researchers have recorded from putative DA neurons in the VTA both in awake animals during learning and goal-directed behaviours and in in vitro slice preparations. In most cases, VTA neurons have been identified using indirect physiological and pharmacological criteria developed for neurons in the neighbouring SNc. However, in contrast to the SNc where nearly 90% of neurons are DA-containing, in the VTA only about 55% of neurons are dopaminergic. This figure makes it imperative to validate indirect criteria for distinguishing VTA DA neurons from neighbouring non-DA neurons. In this study, we systematically tested whether broadly accepted indirect criteria for identifying VTA DA neurons are valid by direct assessment of neurotransmitter content with cytochemistry in neurons electrophysiologically characterized by whole-cell recordings. Our results clearly indicate that such common criteria as Ih magnitude, action potential duration, Rinput and DA D2 receptor agonist inhibition do not provide consistent or unique markers to differentiate DA neurons from non-DA neurons in the VTA.
Technical issues
Possible technical limitations in our approach include insufficient intensity of labelling of DA neurons using TH cytochemistry, loss of TH antigenicity during recording, and experimenter bias in cell selection during recording. Cell damage is an unlikely source of large error since the single cell fills show a proportion of TH(+) cells similar to the randomly counted proportion of TH(+) cells among the total population assessed using the NeuN antibody. In these experiments we attempted to sample neurons throughout the VTA and without regard to size or shape. Only in the case of Ih(−) neurons was there any explicit bias on our part to achieve sufficient numbers of this cell type to be able to compare them to those of the other two neural groups analysed here. This accounts for less than 10% of the recordings included in these data. That the overall percentages of TH(+) neurons were similar for our concurrent cytochemistry for NeuN and TH and the cytochemistry following recordings suggests our sample of recorded neurons was representative of the population at large. The parallel difference along the R–C axis in these two data sets for TH labelling also suggests minimal additional bias on the part of the experimenter.
On the other hand, it is important to acknowledge the possibility that the percentage of TH(+) neurons we found using both approaches underestimates the actual percentage of DA neurons. Some TH(−) neurons could be dopaminergic yet express undetectable levels of TH in their somato-dendritic regions. For example, it has been suggested that TH is efficiently shipped to the terminals of some dopaminergic neurons, such that TH is not detectible at their cell bodies (Frain & Leviel, 1998). There is some evidence that all principal VTA neurons express TH mRNA from single cell RT-PCR experiments (Koyama et al. 2005); however, that work was done in 12- to 16-day-old rats, when the midbrain DA system is immature (Sato et al. 1991; Lieb et al. 1996; Wang et al. 2005), and the criteria used to identify principal neurons in that study are unclear. There are several reasons to think that an underestimate due to false negative cytochemical results would be very small. First, the percentage of NeuN(+) cells co-stained for TH was highly variable depending on the location in the VTA, and there were regions in which close to 100% of the neurons were co-labelled. Second, we found a much higher percentage of TH(+) neurons in the SNc than in the VTA, a finding very similar to previously published studies utilizing different methods to identify DA neurons (Swanson, 1982; Lacey et al. 1989). Third, GAD67(+) neurons are not sparse in the VTA, and there is evidence that TH and GAD are not colocalized in VTA neurons (Kosaka et al. 1987). It is unlikely that the technical inability to detect TH in dopaminergic neurons would covary with such unrelated groupings of VTA neurons. Further to this point, a high percentage of neurons that project to the nucleus accumbens (NAc), but not the medical prefrontal cortex (mPFC), are TH(+) (Swanson, 1982; Margolis et al. 2006), and, in previous work, we found that 100% of κ-opioid receptor (KOP-R) agonist-inhibited VTA neurons are TH(+) (Margolis et al. 2003). Another reason to doubt that TH would be present in the axon terminals but not the soma of VTA neurons is the evidence of significant somato-dendritic release of DA within the VTA (Kalivas & Duffy, 1991; Rice et al. 1997); it is unlikely that the cell bodies would not contain TH to support this function. Therefore, while we cannot rule out that some TH(−) neurons release DA at their terminals, the weight of current evidence supports the idea that a large percentage of VTA neurons are not dopaminergic.
Physiological markers used to identify VTA DA neurons
In whole-cell VTA experiments, the most widely used physiological marker for putative DA neurons is the presence of a pronounced Ih. Although all VTA DA neurons have a measurable Ih, as suggested by previous studies (Johnson & North, 1992b; Cameron et al. 1997; Margolis et al. 2003, 2006) and directly evaluated here, neither the presence of an Ih, nor the size of the Ih, predict TH content. In fact, the current data show that in the rat a VTA neuron with a very large Ih is less likely to be TH(+) than those with smaller currents. This finding is particularly important since it is often assumed that selecting VTA neurons by Ih size improves the likelihood that recordings are made in DA neurons. However, recent studies have shown that the Ih of NAc-projecting neurons is significantly smaller than that of mPFC- (Margolis et al. 2006) or amygdala- (Ford et al. 2006) projecting VTA neurons. Therefore, using a ‘large’ Ih as a criterion for choosing neurons to include in a study may actually diminish the likelihood that the recordings are made in dopaminergic, and NAc-projecting, neurons.
Action potential duration is another measure often used in vitro and in vivo to identify putative dopaminergic neurons. Different groups have suggested different measurement intervals for effectively delineating TH(+) from TH(−) neurons, with durations of putative DA neurons ranging from > 1.0 ms to 4–5 ms (Aghajanian & Bunney, 1973; Johnson & North, 1992b; Hyland et al. 2002). We examined a variety of AP characteristics here. In our data set, no measure of AP duration yielded a significant difference that correlated with TH staining. Only when measuring from AP initiation to the IK(AHP) minimum was any difference detectable among the three groups of neurons delineated here, and this difference was between Ih(−) neurons and both groups of Ih(+) neurons. Interestingly, in a previous study we found that AP duration does correlate with projection target; Ih(+) TH(+) neurons that project to the NAc have significantly longer duration APs than either Ih(+) TH(+) or Ih(−) neurons projecting to the mPFC (Margolis et al. 2006). However, the AP durations for Ih(+) and Ih(−) mPFC-projecting neurons are identical (Margolis et al. 2006). Therefore, while it is true that most VTA projections to the NAc are dopaminergic and NAc-projecting neurons have longer duration APs, there is no relationship between AP duration and DA content.
Recently, Ungless et al. (2004) suggested an AP duration measure and criterion for use as an indirect identifier for DA-containing VTA neurons using data collected during in vivo extracellular, juxtacellular recordings in the VTA, which accurately described 8/11 of the confirmed TH(+) neurons in that study. We investigated here whether we could observe a similar separation in vitro by differentiating the whole-cell AP, as it was previously shown that the differentiated intracellular waveform was equivalent to the extracellular AP in the midbrain (Freygang & Frank, 1959; Terzuolo & Araki, 1961; Grace & Bunney, 1983). We did not observe a difference between cell populations using this measure. Marinelli et al. (2006) recently pointed out that the filter settings used to establish this new criterion were very different from those typically used in extracellular recordings. Further, because the position of an extracellular electrode with respect to the cell soma will affect the duration of the voltage-gated Na+ current phase of the extracellularly recorded AP waveform (Gold et al. 2006), it is difficult to make further comparisons between this criterion and the APs presented here, where the electrode is in direct contact with the cell body. The criterion suggested by Ungless and colleagues at the very least does not have a whole-cell correlate, and may only apply to extracellular/juxtacellular recording. Therefore, care should be taken if this criterion is used in lieu of cytochemistry to identify VTA DA neurons in any other preparation.
Often in vivo studies identify putative dopaminergic neurons by testing for DA D2 autoreceptors with systemic administration of a DA agonist. However, Yim & Mogenson (1980) showed in vivo that subsets of both putative DA and non-DA neurons, identified by AP duration and firing pattern properties, were inhibited by DA. We confirm here that both TH(+) and TH(−) Ih(+) VTA neurons are inhibited by the DA D2 receptor agonist quinpirole. In addition, some TH(+) VTA neurons were not inhibited by quinpirole. Therefore, postsynaptic DA D2 receptors are neither exclusive to, nor expressed on, all DA neurons in the VTA.
The data presented here suggest that more accurate tools for identifying DA neurons may reside in firing pattern properties of VTA neurons. Firing rate, ISI standard deviation, and ISI skew each yielded population differences in this data set. Since in this slice preparation most inputs to these cells are severed, it is possible that these differences are intrinsic to these neurons and persist in vivo. It is important to note, however, that even in this preparation these measures are imperfect. They require the cell in question to be firing spontaneously and stably in the slice, which was the case in just less than half of the neurons in this study. Such properties could also be sensitive to experimental variables such as internal solution, flow rate and bath temperature. At the very least, these separations are possibly unique to slice preparations since it is well established that the firing patterns of SN dopaminergic neurons are very different in vitro and in vivo (Grace & Bunney, 1983; Grace & Onn, 1989), probably due to afferent input modulating the firing of these neurons in the intact brain.
Characterizing subgroups of non-DA VTA neurons
We confirm here that there is one population of TH(−) VTA neurons that is physiologically distinguishable from DA neurons. These neurons lack an Ih, and on average have smaller cell bodies, higher input resistances, and fire at faster rates than either group of Ih(+) neurons. However, their AP durations in this preparation are similar to those of Ih(+) neurons. On the other hand, their AP threshold voltage is more hyperpolarized than those of Ih(+) neurons. Neurons exhibiting this profile of characteristics probably represent a meaningful functional group. One prominent idea is that they are GABAergic inhibitory interneurons. This model arose from data showing that many μ-opioid receptor (MOP-R) agonist-inhibited VTA neurons are of this type, and putative DA neurons are disinhibited by MOP-R agonists (Johnson & North, 1992b, a). While some of these neurons may indeed be local interneurons, we recently showed that the projection from the VTA to the mPFC includes Ih(−) neurons (Margolis et al. 2006). Such projecting neurons could also contact other VTA neurons through local axon collaterals and thus play the same role as interneurons. There is additional evidence that a group of confirmed GABAergic neurons, dorsal to those described in the present study, fire at relatively high rates and are antidromically activated by stimulating the internal capsule (Steffensen et al. 1998). These neurons may be related to the Ih(−) neurons described here, although the data are inconclusive given the anatomical distinction between these GABAergic neurons and the observation that their action potential durations are briefer than those of the Ih(−) neurons described here. Therefore, while the neurotransmitter content of Ih(−) VTA neurons has not been confirmed, these neurons do account for at least part of the non-dopaminergic projection arising from the VTA.
Of greater relevance to the identification of putative VTA DA neurons, this study shows that there is a large population of non-DA neurons in the VTA that are not easily distinguished from DA neurons except by cytochemical techniques. The Ih(+) TH(−) neurons are similar to TH(+) VTA neurons in all properties measured in this study, including those properties usually used to identify DA neurons. These include Ih magnitude, AP duration, and inhibition by DA D2 receptor agonists. Some of these Ih(+) TH(−) neurons are inhibited by MOP-R agonists (Margolis et al. 2003). Furthermore, Ih(+) TH(−) neurons compose part of the VTA projections to both the mPFC and NAc (Margolis et al. 2006). The presence of this large population of non-DA VTA neurons presents a serious challenge to the use of the widely accepted electrophysiological or pharmacological criteria examined here. It also raises caveats about the common approach of analysing data from pooled VTA Ih(+) neurons. For example, if a pool of Ih(+) VTA neurons included both TH(+) and TH(−) cells and a manipulation produced significant but opposite effects on the two classes, the net effect might appear not statistically different from zero. This type of problem was reported when action potential duration was used to pool groups of VTA neurons (Kiyatkin & Rebec, 2001). Further, a large effect in TH(−) neurons pooled with no effect in TH(+) neurons could lead to the mistaken conclusion that an effect is seen in dopaminergic neurons.
Functional roles of non-DA VTA neurons
The NeuN data reported here show that about 45% of the neurons in the VTA are non-dopaminergic, and that non-dopaminergic neurons are intermixed with DA neurons through most of the VTA. However, we found large regional differences within the VTA. Since neurons in subregions of the VTA project to different rostral targets (Fallon & Moore, 1978; Fallon, 1988), and pharmacological manipulations of subregions can give rise to different behaviours (Ikemoto et al. 1998; Rodd-Henricks et al. 2000; Zangen et al. 2002), these anatomical and functional distinctions may be important to consider in future electrophysiological recordings. Previous studies have demonstrated that many of the non-DA neurons in the VTA are projecting neurons targeting brain regions including the NAc, mPFC, amygdala and hippocampus (Thierry et al. 1980; Swanson, 1982). At least some of these projections to the mPFC and NAc are GABAergic (Van Bockstaele & Pickel, 1995; Carr & Sesack, 2000a; Margolis et al. 2006). In fact, in the rat, non-dopaminergic neurons provide the majority of VTA efferents to a number of brain regions including the hippocampus and mPFC (Swanson, 1982).
The potential importance of such non-DA VTA neurons in reward mechanisms is highlighted by the work of van der Kooy and colleagues. They demonstrated that reward produced by intra-VTA injection of GABAA receptor antagonists in a place-conditioning lead to a DA-independent place preference, but GABAA receptor agonists produce a place preference that is dependent on DA (Laviolette & van der Kooy, 2001). Intra-VTA MOP-R agonist reward is also DA independent in MOP-R agonist naive animals, but becomes DA dependent after exposure of animals to a MOP-R agonist, and this switch involves a change in GABAA receptor signalling (Nader & van der Kooy, 1997; Laviolette et al. 2004). These experiments provide behavioural evidence for the relevance to opioid reward of non-dopaminergic VTA output, and the necessity to include such output in models of VTA function. Thus, identifying non-dopaminergic neurons in the VTA is important not simply to differentiate them from DA neurons, but because of the DA-independent contribution that these neurons appear to make to reinforcement and motivation.
Pharmacological probes and the relevance of projection target
Although our data show that inhibition by D2 agonists is not a reliable indicator of DA content among VTA neurons, other pharmacological tools may yet prove useful. We previously reported that a postsynaptic hyperpolarization in response to KOP-R agonist administration occurred exclusively in a subpopulation of dopaminergic, but never non-dopaminergic, neurons in the VTA (Margolis et al. 2003). This represents a specific pharmacological property that is limited in the VTA to DA neurons. Therefore, there is at least one pharmacological agent which can be used to identify a subset of VTA dopaminergic neurons. It remains to be determined if another type of receptor is exclusive to, and universally expressed on, VTA dopaminergic neurons, providing an accurate pharmacological identification tool.
In contrast to using physiological and pharmacological criteria to identify functional characteristics of VTA neurons, using projection target in conjunction with cytochemical identity may be the most heuristic approach to studying the function of VTA neurons. For instance, we found that KOP-R agonist-sensitive DA neurons project to the mPFC, but not the NAc (Margolis et al. 2006). Microdialysis studies have shown that other pharmacological agents in the VTA, such as orexin and endogenously released MOP-R ligands, differentially affect DA levels in the NAc and mPFC (Tanda & Di Chiara, 1998; Vittoz & Berridge, 2006). It is also clear from a variety of behavioural studies that DA plays different roles in each projection target; DA in the NAc is required for responding appropriately to reward predictive cues (Yun et al. 2004), whereas DA in the mPFC plays a role in working memory (Williams & Goldman-Rakic, 1995; Chudasama & Robbins, 2004). Further, for cocaine reinstatement, DA is required in the PFC but not in the NAc core (McFarland & Kalivas, 2001). The importance of input and projection target for determining the properties of VTA neurons is further illustrated by anatomical studies showing that DA neurons that receive input from the mPFC project back to the mPFC, but not to the NAc, while VTA GABAergic projections to the NAc, but not the mPFC, receive mPFC input (Carr & Sesack, 2000b). In this case, hypotheses about VTA neuron function must be formulated in terms of input sources, projection target and neurotransmitter content if they are to meaningfully address the contribution of the different subsets of VTA neurons to learning and goal-directed behaviour.
Conclusions
We examined in the VTA the markers that have been assumed to identify midbrain dopaminergic neurons, and compared these properties between cytochemically identified dopaminergic and non-dopaminergic VTA neurons. None of the generally accepted properties were associated exclusively, or even significantly, with confirmed dopaminergic neurons. Therefore, improved indirect methods of identifying VTA DA neurons need to be developed. Until a more accurate identification scheme is determined for VTA neurons, more care must be taken in attributing properties to putative dopaminergic VTA neurons when heterogeneous neural responses are observed in this brain region.
Acknowledgments
The authors would like to thank Junko Ishikawa and Brian Toy for their technical assistance. This study was sponsored by the Department of the Army, award number DAMD17-03-1-0059. The US Army Medical Research Acquisition Activity (820 Chandler Street, Fort Detrick, MD 21702-5014, USA) is the awarding and administering acquisition office. The content of the information does not necessarily reflect the position or the policy of the Government, and no official endorsement should be inferred. This research was also supported by funds provided by NIDA (award number DA-15686), and by the State of California for medical research on alcohol and substance abuse through the University of California, San Francisco.
References
- Aghajanian GK, Bunney BS. Central dopaminergic neurons – neurophysiological identification and responses to drugs. Life Sci. 1973;13:643–648. [Google Scholar]
- Cameron DL, Wessendorf MW, Williams JT. A subset of ventral tegmental area neurons is inhibited by dopamine, 5-hydroxytryptamine and opioids. Neuroscience. 1997;77:155–166. doi: 10.1016/s0306-4522(96)00444-7. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. GABA-containing neurons in the rat ventral tegmental area project to the prefrontal cortex. Synapse. 2000a;38:114–123. doi: 10.1002/1098-2396(200011)38:2<114::AID-SYN2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci. 2000b;20:3864–3873. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Psychopharmacological approaches to modulating attention in the five-choice serial reaction time task: implications for schizophrenia. Psychopharmacology (Berl) 2004;174:86–98. doi: 10.1007/s00213-004-1805-y. [DOI] [PubMed] [Google Scholar]
- Dommett E, Coizet V, Blaha CD, Martindale J, Lefebvre V, Walton N, Mayhew JE, Overton PG, Redgrave P. How visual stimuli activate dopaminergic neurons at short latency. Science. 2005;307:1476–1479. doi: 10.1126/science.1107026. [DOI] [PubMed] [Google Scholar]
- Faleiro LJ, Jones S, Kauer JA. Rapid synaptic plasticity of glutamatergic synapses on dopamine neurons in the ventral tegmental area in response to acute amphetamine injection. Neuropsychopharmacology. 2004;29:2115–2125. doi: 10.1038/sj.npp.1300495. [DOI] [PubMed] [Google Scholar]
- Fallon JH. Topographic organization of ascending dopaminergic projections. Ann N Y Acad Sci. 1988;537:1–9. doi: 10.1111/j.1749-6632.1988.tb42093.x. [DOI] [PubMed] [Google Scholar]
- Fallon JH, Moore RY. Catecholamine innervation of the basal forebrain. IV. Topography of the dopamine projection to the basal forebrain and neostriatum. J Comp Neurol. 1978;180:545–580. doi: 10.1002/cne.901800310. [DOI] [PubMed] [Google Scholar]
- Ford CP, Mark GP, Williams JT. Properties and opioid inhibition of mesolimbic dopamine neurons vary according to target location. J Neurosci. 2006;26:2788–2797. doi: 10.1523/JNEUROSCI.4331-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frain O, Leviel V. Mesencephalic THmRNA-reduced expression by blocking axonal transport with colchicine. Neuroreport. 1998;9:1529–1532. doi: 10.1097/00001756-199805110-00052. [DOI] [PubMed] [Google Scholar]
- Freygang WH, Jr, Frank K. Extracellular potentials from single spinal motoneurons. J Gen Physiol. 1959;42:749–760. doi: 10.1085/jgp.42.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Le Moine C, Aston-Jones G. No effect of morphine on ventral tegmental dopamine neurons during withdrawal. J Neurosci. 2006;26:5720–5726. doi: 10.1523/JNEUROSCI.5032-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold C, Henze DA, Koch C, Buzsaki G. On the origin of the extracellular action potential waveform: a modeling study. J Neurophysiol. 2006;95:3113–3128. doi: 10.1152/jn.00979.2005. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Nigral dopamine neurons: intracellular recording and identification with L-dopa injection and histofluorescence. Science. 1980;210:654–656. doi: 10.1126/science.7433992. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons – 1. Identification and characterization. Neuroscience. 1983;10:301–315. doi: 10.1016/0306-4522(83)90135-5. [DOI] [PubMed] [Google Scholar]
- Grace AA, Onn SP. Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J Neurosci. 1989;9:3463–3481. doi: 10.1523/JNEUROSCI.09-10-03463.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Aghajanian GK. Antidromic identification of dopaminergic and other output neurons of the rat substantia nigra. Brain Res. 1978;150:69–84. doi: 10.1016/0006-8993(78)90654-6. [DOI] [PubMed] [Google Scholar]
- Hyland BI, Reynolds JN, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience. 2002;114:475–492. doi: 10.1016/s0306-4522(02)00267-1. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Murphy JM, McBride WJ. Regional differences within the rat ventral tegmental area for muscimol self-infusions. Pharmacol Biochem Behav. 1998;61:87–92. doi: 10.1016/s0091-3057(98)00086-0. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992a;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol. 1992b;450:455–468. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D, Wu SM-S. Foundations of Cellular Neurophysiology. Cambridge: The MIT Press; 1995. [Google Scholar]
- Jones S, Kauer JA. Amphetamine depresses excitatory synaptic transmission via serotonin receptors in the ventral tegmental area. J Neurosci. 1999;19:9780–9787. doi: 10.1523/JNEUROSCI.19-22-09780.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. A comparison of axonal and somatodendritic dopamine release using in vivo dialysis. J Neurochem. 1991;56:961–967. doi: 10.1111/j.1471-4159.1991.tb02015.x. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Rebec GV. Impulse activity of ventral tegmental area neurons during heroin self-administration in rats. Neuroscience. 2001;102:565–580. doi: 10.1016/s0306-4522(00)00492-9. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci. 2003;23:7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka T, Kosaka K, Hataguchi Y, Nagatsu I, Wu JY, Ottersen OP, Storm-Mathisen J, Hama K. Catecholaminergic neurons containing GABA-like and/or glutamic acid decarboxylase-like immunoreactivities in various brain regions of the rat. Exp Brain Res. 1987;66:191–210. doi: 10.1007/BF00236215. [DOI] [PubMed] [Google Scholar]
- Koyama S, Kanemitsu Y, Weight FF. Spontaneous activity and properties of two types of principal neurons from the ventral tegmental area of rat. J Neurophysiol. 2005;93:3282–3293. doi: 10.1152/jn.00776.2004. [DOI] [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB, North RA. Two cell types in rat substantia nigra zona compacta distinguished by membrane properties and the actions of dopamine and opioids. J Neurosci. 1989;9:1233–1241. doi: 10.1523/JNEUROSCI.09-04-01233.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, Gallegos RA, Henriksen SJ, van der Kooy D. Opiate state controls bi-directional reward signaling via GABAA receptors in the ventral tegmental area. Nat Neurosci. 2004;7:160–169. doi: 10.1038/nn1182. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, van der Kooy D. GABAA receptors in the ventral tegmental area control bidirectional reward signalling between dopaminergic and non-dopaminergic neural motivational systems. Eur J Neurosci. 2001;13:1009–1015. doi: 10.1046/j.1460-9568.2001.01458.x. [DOI] [PubMed] [Google Scholar]
- Lieb K, Andersen C, Lazarov N, Zienecker R, Urban I, Reisert I, Pilgrim C. Pre- and postnatal development of dopaminergic neuron numbers in the male and female mouse midbrain. Brain Res Dev Brain Res. 1996;94:37–43. doi: 10.1016/0165-3806(96)00063-6. [DOI] [PubMed] [Google Scholar]
- Liu QS, Pu L, Poo MM. Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature. 2005;437:1027–1031. doi: 10.1038/nature04050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantz J, Thierry AM, Glowinski J. Effect of noxious tail pinch on the discharge rate of mesocortical and mesolimbic dopamine neurons: selective activation of the mesocortical system. Brain Res. 1989;476:377–381. doi: 10.1016/0006-8993(89)91263-8. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Hjelmstad GO, Bonci A, Fields HL. Kappa-opioid agonists directly inhibit midbrain dopaminergic neurons. J Neurosci. 2003;23:9981–9986. doi: 10.1523/JNEUROSCI.23-31-09981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Chefer VI, Shippenberg TS, Hjelmstad GO, Fields HL. Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proc Natl Acad Sci U S A. 2006;103:2938–2942. doi: 10.1073/pnas.0511159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli M, Rudick CN, Hu XT, White FJ. Excitability of dopamine neurons: modulation and physiological consequences. CNS Neurol Disord Drug Targets. 2006;5:79–97. doi: 10.2174/187152706784111542. [DOI] [PubMed] [Google Scholar]
- Morzorati SL, Marunde RL. Comparison of VTA dopamine neuron activity in lines of rats selectively bred to prefer or avoid alcohol. Alcohol Clin Exp Res. 2006;30:991–997. doi: 10.1111/j.1530-0277.2006.00113.x. [DOI] [PubMed] [Google Scholar]
- Nader K, van der Kooy D. Deprivation state switches the neurobiological substrates mediating opiate reward in the ventral tegmental area. J Neurosci. 1997;17:383–390. doi: 10.1523/JNEUROSCI.17-01-00383.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi H, Kita H, Kitai ST. Intracellular study of rat substantia nigra pars reticulata neurons in an in vitro slice preparation: electrical membrane properties and response characteristics to subthalamic stimulation. Brain Res. 1987;437:45–55. doi: 10.1016/0006-8993(87)91525-3. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Taha SA, Kim SW, Fields HL. Nucleus accumbens dopamine release is necessary and sufficient to promote the behavioral response to reward-predictive cues. Neuroscience. 2005;135:1025–1033. doi: 10.1016/j.neuroscience.2005.06.088. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1998. [Google Scholar]
- Pickel VM, Joh TH, Reis DJ. Monoamine-synthesizing enzymes in central dopaminergic, noradrenergic and serotonergic neurons. Immunocytochemical localization by light and electron microscopy. J Histochem Cytochem. 1976;24:792–806. doi: 10.1177/24.7.8567. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Joh TH, Reis DJ. Regional and ultrastructural localization of tyrosine hydroxylase by Immunocytochemistry in dopaminergic neurons of the mesolimbic and nigroneostriatal systems. Adv Biochem Psychopharmacol. 1977;16:321–329. [PubMed] [Google Scholar]
- Rice ME, Cragg SJ, Greenfield SA. Characteristics of electrically evoked somatodendritic dopamine release in substantia nigra and ventral tegmental area in vitro. J Neurophysiol. 1997;77:853–862. doi: 10.1152/jn.1997.77.2.853. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Crile RS, Murphy JM, McBride WJ. Regional heterogeneity for the intracranial self-administration of ethanol within the ventral tegmental area of female Wistar rats. Psychopharmacology (Berl) 2000;149:217–224. doi: 10.1007/s002139900347. [DOI] [PubMed] [Google Scholar]
- Romo R, Schultz W. Somatosensory input to dopamine neurones of the monkey midbrain: responses to pain pinch under anaesthesia and to active touch in behavioural context. Prog Brain Res. 1989;80:473–478. doi: 10.1016/s0079-6123(08)62245-1. discussion 465–466. [DOI] [PubMed] [Google Scholar]
- Sato M, Kiyama H, Yoshida S, Saika T, Tohyama M. Postnatal ontogeny of cells expressing prepro-neurotensin/neuromedin N mRNA in the rat forebrain and midbrain: a hybridization histochemical study involving isotope-labeled and enzyme-labeled probes. J Comp Neurol. 1991;310:300–315. doi: 10.1002/cne.903100303. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schwieler L, Erhardt S, Nilsson L, Linderholm K, Engberg G. Effects of COX-1 and COX-2 inhibitors on the firing of rat midbrain dopaminergic neurons – possible involvement of endogenous kynurenic acid. Synapse. 2006;59:290–298. doi: 10.1002/syn.20241. [DOI] [PubMed] [Google Scholar]
- Self DW, Nestler EJ. Relapse to drug-seeking: neural and molecular mechanisms. Drug Alcohol Depend. 1998;51:49–60. doi: 10.1016/s0376-8716(98)00065-9. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Weiss F. The dopamine hypothesis of reward: past and current status. Trends Neurosci. 1999;22:521–527. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- Steffensen SC, Svingos AL, Pickel VM, Henriksen SJ. Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. J Neurosci. 1998;18:8003–8015. doi: 10.1523/JNEUROSCI.18-19-08003.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Tanda G, Di Chiara G. A dopamine-μ1 opioid link in the rat ventral tegmentum shared by palatable food (Fonzies) and non-psychostimulant drugs of abuse. Eur J Neurosci. 1998;10:1179–1187. doi: 10.1046/j.1460-9568.1998.00135.x. [DOI] [PubMed] [Google Scholar]
- Terzuolo CA, Araki T. An analysis of intra- versus extracellular potential changes associated with activity of single spinal motoneurons. Ann N Y Acad Sci. 1961;94:547–558. doi: 10.1111/j.1749-6632.1961.tb35558.x. [DOI] [PubMed] [Google Scholar]
- Thierry AM, Deniau JM, Herve D, Chevalier G. Electrophysiological evidence for non-dopaminergic mesocortical and mesolimbic neurons in the rat. Brain Res. 1980;201:210–214. doi: 10.1016/0006-8993(80)90788-x. [DOI] [PubMed] [Google Scholar]
- Tobler PN, Dickinson A, Schultz W. Coding of predicted reward omission by dopamine neurons in a conditioned inhibition paradigm. J Neurosci. 2003;23:10402–10410. doi: 10.1523/JNEUROSCI.23-32-10402.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science. 2004;303:2040–2042. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Pickel VM. GABA-containing neurons in the ventral tegmental area project to the nucleus accumbens in rat brain. Brain Res. 1995;682:215–221. doi: 10.1016/0006-8993(95)00334-m. [DOI] [PubMed] [Google Scholar]
- Vittoz NM, Berridge CW. Hypocretin/orexin selectively increases dopamine efflux within the prefrontal cortex: involvement of the ventral tegmental area. Neuropsychopharmacology. 2006;31:384–395. doi: 10.1038/sj.npp.1300807. [DOI] [PubMed] [Google Scholar]
- Wang F, Xiao C, Ye JH. Taurine activates excitatory non-synaptic glycine receptors on dopamine neurones in ventral tegmental area of young rats. J Physiol. 2005;565:503–516. doi: 10.1113/jphysiol.2005.085423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Yim CY, Mogenson GJ. Electrophysiological studies of neurons in the ventral tegmental area of Tsai. Brain Res. 1980;181:301–313. doi: 10.1016/0006-8993(80)90614-9. [DOI] [PubMed] [Google Scholar]
- Ye JH, Wang F, Krnjevic K, Wang W, Xiong ZG, Zhang J. Presynaptic glycine receptors on GABAergic terminals facilitate discharge of dopaminergic neurons in ventral tegmental area. J Neurosci. 2004;24:8961–8974. doi: 10.1523/JNEUROSCI.2016-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun IA, Wakabayashi KT, Fields HL, Nicola SM. The ventral tegmental area is required for the behavioral and nucleus accumbens neuronal firing responses to incentive cues. J Neurosci. 2004;24:2923–2933. doi: 10.1523/JNEUROSCI.5282-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung WH, Hausser MA, Jack JJ. Electrophysiology of dopaminergic and non-dopaminergic neurones of the guinea-pig substantia nigra pars compacta in vitro. J Physiol. 1991;436:643–667. doi: 10.1113/jphysiol.1991.sp018571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangen A, Ikemoto S, Zadina JE, Wise RA. Rewarding and psychomotor stimulant effects of endomorphin-1: anteroposterior differences within the ventral tegmental area and lack of effect in nucleus accumbens. J Neurosci. 2002;22:7225–7233. doi: 10.1523/JNEUROSCI.22-16-07225.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]