Abstract
Prior studies investigating carotid baroreflex control of the cutaneous vasculature have yielded mixed findings. However, previously used methodological and analytical techniques may limit the ability to detect carotid baroreflex-mediated changes in cutaneous vascular conductance (CVC). The aim of this study was to test the hypothesis that dynamic carotid baroreceptor stimulation (i.e. 5 s trials) using neck pressure (NP, simulated carotid hypotension) and neck suction (NS, simulated carotid hypertension) will decrease and increase CVC, respectively, during normothermic and whole-body heating conditions in resting humans. Data were obtained from nine subjects (age, 31 ± 2 year). The ratio of forearm skin blood flux (laser-Doppler flowmetry) and arterial blood pressure (Finapres) was used as an index of CVC. Multiple 5 s trials of NP (+40Torr) and NS (−60Torr), as well as breath-hold/airflow control trials, were applied during end-expiratory breath-holds while subjects were normotheric and heat stressed (change in core temperature ∼0.75°C). CVC responses to each NP and NS trial were averaged into 1 s intervals during the following periods: 3 s prestimulus, 5 s during stimulus, and 5 s poststimulus. Peak CVC responses (3 s average) to NP and NS were compared to prestimulus values using paired t test. During normothermia, NP decreased CVC by 0.032 ± 0.007 arbitrary units (a.u.) mmHg−1; (P < 0.05); however, breath-hold/airflow control trials resulted in similar decreases in CVC. NS did not change CVC (Δ = 0.002 ± 0.005 a.u. mmHg−1; P = 0.63). During whole-body heating, NP decreased CVC (by 0.16 ± 0.04 a.u. mmHg−1; (P < 0.05), whereas NS increased CVC by 0.07 ± 0.03 a.u. mmHg−1; (P < 0.05). Furthermore, these changes were greater than, or directionally different from, the breath-hold/airflow control trials. These findings indicate that carotid baroreceptor stimulation elicits dynamic changes in CVC and that these changes are more apparent during whole-body heating.
In humans, whole-body heat stress redistributes upwards of 50% of cardiac output to the cutaneous vasculature (Rowell et al. 1969). In addition, orthostatic tolerance is reduced during whole-body heat stress under a variety of gravitational stressors (Lind et al. 1968; Allan & Crossley, 1972; Wilson et al. 2002). During the aforementioned heat stress condition, the cutaneous vasculature represents the primary contributing vascular bed to total peripheral resistance and would, therefore theoretically, be an important target for baroreflex control of arterial blood pressure. While the arterial baroreflex is the primary short-term regulator of arterial blood pressure, studies investigating arterial baroreflex control of the cutaneous vasculature during both normothermic and heat stress conditions have yielded mixed findings.
In 1970, Beiser et al. reported a decrease in cutaneous vascular resistance (the reciprocal of conductance) during normothermic conditions in response to loading the carotid baroreceptors using the neck chamber technique (Beiser et al. 1970). However, their use of strain gauge photoplethysmography, which does not discriminate between the blood flow responses in the skin and the underlying skeletal muscle, as well as the nature of the analysis used to determine baroreflex control of the cutaneous vasculature (i.e. subtraction of the response between a control forearm and an arm whose cutaneous vasculature was preconstricted using iontophoresis of adrenaline (epinephrine)) may have limited such interpretation. Furthermore, Crandall et al. (1996) did not observe changes in cutaneous vascular conductance (CVC) in response to carotid baroreceptor unloading using the neck chamber technique during both normothermic and whole-body heating conditions. Furthermore, findings by Wallin et al. (1975) and Wilson et al. (2001) have indicated an absence of isolated carotid baroreflex and simultaneous carotid and aortic baroreflex control of skin sympathetic nerve activity (sSNA), respectively. Wallin et al. (1975) measured the integrated sSNA response to direct, electrical stimulation of the carotid sinus nerve (simulated hypertension) during normothermia, and observed no reproducible changes in sSNA. However, Wallin et al. (1975) did not measure skin blood flow responses, and therefore interpretation of those findings as it pertains to carotid baroreflex control of the cutaneous vasculature remains limited. Wilson et al. (2001) reported that arterial baroreceptor stimulation with bolus sodium nitroprusside followed by bolus phenylephrine infusions (i.e. the modified Oxford technique), as well as sustained reductions in blood pressure with steady-state nitroprusside infusion, did not significantly change integrated sSNA. Due to the direct effect of the infused drugs on the cutaneous vasculture, Wilson et al. (2001) only examined the integrated neural response to this pharmacological perturbation, and thus did not assess arterial baroreflex control of the cutaneous vasculature (i.e. skin blood flow, or CVC). Furthermore, changes in CVC have not always been accompanied by changes in sSNA (Cui et al. 2004), further complicating the interpretation of such findings regarding arterial baroreflex control of the cutaneous vasculature. Finally, any potential dynamic response to carotid baroreceptor stimulation (i.e. of the order of seconds) was not assessed in the aforementioned study by Crandall et al. (1996). Despite the lack of a change in mean CVC in that study, it is possible that the carotid baroreceptor stimulation used by Crandall et al. (i.e. repeated 500 ms pulses) resulted in dynamic fluctuations in CVC that were perhaps masked due to averaging of the responses over a 1 min period, and/or the closed-loop nature of the stimulus (Crandall et al. 1996).
In the current investigation, we used the neck chamber technique to selectively load and unload the carotid baroreceptors to test the hypothesis that open-loop carotid baroreceptor stimulation with 5 s trials of neck pressure (NP) and neck suction (NS) will dynamically modulate CVC during normothermia and whole-body heating in resting humans.
Methods
Subjects
Nine subjects (4 men and 5 women) voluntarily participated in the present investigation (age, 31 ± 2 years; height, 166 ± 11 cm; weight, 69 ± 1 kg; mean ± s.e.m.). All procedures conformed to the standards set by the Declaration of Helsinki. Each subject signed an informed consent that was approved by the institutional review boards of the University of Texas South-western Medical Center and Presbyterian Hospital of Dallas. Prior to participation, all subjects were familiarized with the testing protocols. Subjects were healthy, non-smokers, free of known cardiovascular and respiratory diseases, and were not using prescription or over-the-counter medications. Subjects were advised to not consume alcohol for 24 h before any of the scheduled experiments. Subjects were also asked to refrain from the consumption of caffeinated beverages from 12 h before the scheduled experiments.
Instrumentation
Subjects were dressed in a tube-lined perfusion suit enabling the control of skin and core temperature (Tcore) via changing the temperature of the water perfusing the suit. Tcore was measured using a telemetry temperature pill swallowed by subjects at least 1 h before any data collection (normothermic Tcore data were identified prior to heat stress which was ∼2 h post-ingestion). Whole-body skin temperature (Tsk) was measured from the electrical average of six thermocouples (Taylor et al. 1989) fixed to the skin with porous adhesive tape. A separate thermocouple was fixed to the skin of the neck under the neck chamber to measure neck skin temperature (Tneck) during the baroreceptor perturbation trials. Non-invasive measures of arterial blood pressure were taken continuously using finger cuff photoplethysmography (Finapres, Ohmeda, Lousiville, CO, USA). Arterial blood pressure was also determined by auscultation of the brachial artery (SunTech, Meical Instruments, Raleigh, NC, USA). Diastolic blood pressures determined by auscultation were used to calibrate diastolic blood pressures determined by finger cuff photoplethysmography. This was done by determining the average diastolic blood pressure over the ∼30 s period during which the auscultation-derived diastolic pressures were collected, and correcting for the offset between the two measures (i.e. adjusting the finger diastolic pressures to the auscultation-derived pressures). Heart rate was collected from an electrocardiogram (ECG) signal (Agilent, Munich, Germany) interfaced with a cardiotachometer (1000 Hz sampling rate, CWE, Ardmore, PA, USA). Two laser Doppler flowmeter probes (Perimed, North Royalton, OH, USA) were placed on dorsal forearm skin midway between the wrist and the elbow. The values from these flow probes were averaged, with the resulting value used for the ensuing data and statistical analysis. Subjects were fitted with a malleable lead neck chamber covering the anterior two-thirds of the neck for baroreflex testing.
Experimental protocol
During normothermic testing, water at 34°C was perfused through the tube-lined suit for approximately 30 min before baroreceptor perturbations. Four trials of NP followed by four trials of NS were performed in randomized order for 16 total trials during normothermia (i.e. 4 NP, 4 NS, 4 NP, 4 NS, or 4 NS, 4 NP, 4 NS, 4 NP). A minimum of 45 s was allowed to pass between each trial to enable physiological variables to return to prestimulus values. Following approximately 30 min of baroreflex testing during normothermia, whole-body heating was performed by perfusing water at 44–48°C through the tube-lined suit. After an increase in Tcore of approximately 0.7°C was observed, the temperature of the water was decreased (42–44°C) to attenuate further increases in Tcore. After 5–10 min at these reduced water temperatures, baroreflex testing was repeated similar to normothermia. Under both thermal conditions, following baroreflex testing, neck chamber control trials were also performed to determine the effect of baroreceptor-independent stimulation associated with the application of the neck chamber technique and breath holding (see below).
Measurements and procedures
Testing was performed with subjects in the supine position. Cardiovascular variables were monitored and recorded on a computer equipped with customized software (Necsuc3) that collects and records data triggered from the R-wave of the ECG, as well as a second computer equipped with an online data acquisition program (Biopac, CA, USA). Carotid baroreflex control of mean arterial pressure (MAP) and CVC was assessed by applying single 5 s pulses of NP (+40Torr) and NS (−60Torr) as described by Potts et al. (1993). Briefly, NP and NS trials were performed during an approximately 15 s breath-hold at normal end-expiration, in order to minimize the respiratory modulation of HR and MAP (Eckberg et al. 1980). Peak responses for MAP were determined as the greatest change over a 3 s period in response to the application of NP or NS and these peak changes from repeated trials were averaged to provide a mean response for each subject. CVC was calculated using the following formula:
To determine the effect of the breath-hold, as well as changes in Tneck associated with the application of NP and NS, breath-hold/airflow control trials were also performed. During these control trials, the chamber was removed from the subject's neck and the subject held his/her breath for a similar duration, relative to the experimental trial, while the flow of air, normally used to generate chamber pressure (i.e. before the chamber was removed) was directed on the neck. The flow to the neck was adjusted in an effort to achieve similar changes in Tneck to those observed during actual trials of NP and NS. Care was taken to prevent air flow above the neck (i.e. facial region).
Data analysis
Steady-state haemodynamic and thermal variables were determined using 1 min averages during normothermic and heat-stress conditions. Responses to carotid baroreceptor stimulation were determined using two different analyses. For both analyses, data from each trial were averaged in 1 s intervals, beginning with 3 s prestimulus followed by 5 s during the carotid stimulation and 5 s following carotid stimulation (i.e. 13 s total per trial).
The traditional approach was used by determining the 3 s interval representing the largest change in CVC relative to the respective prestimulus value for each separate trial of NP and NS. The average of these peak responses was then determined for both NP and NS for each subject within each thermal condition.
A more conservative approach was performed by first averaging the second-by-second responses to all NP, as well as to all NS trials for each subject within each thermal condition. After all NP and NS trials were averaged, the 3 s interval of the averaged data with the largest change in CVC was then compared to the 3 s prestimulus period. This analysis resulted in slightly smaller average responses to NP and NS during both thermal conditions relative to the traditional approach. However, peak changes determined using this more conservative analysis minimized the influence of baroreflex-independent fluctuations in skin blood flow on the peak responses.
Breath-hold/airflow control trials were analysed using the more conservative approach (described above). Using the second-by-second analysis, peak CVC responses observed during NP and NS trials (conservative analysis) were then compared to breath-hold/airflow control trials at similar time points for each subject. Therefore, the comparison between the control trials and ‘actual’ trials was based on the time of the peak CVC response, as determined in relation to an individuals ‘conservative’ analysis. That is, if an individual's peak response to NP, as determined using conservative analysis, occurred during seconds 6 to 8, the comparison to the breath-hold/airflow control trials was made during those same time points. However, the same subject's peak response to NS may have occurred during seconds 5 to 7. Therefore, the comparison to the control trials was made during those time points. Breath-hold/airflow control trials were performed on eight subjects during normothermia and seven subjects during whole-body heating. The averaged peak change (3 s) in Tneck in response to NP and NS during normothermic conditions were ∼0.9°C and 0.4°C, respectively, while the breath-hold/airflow control trials applied during normothermic conditions changed Tneck by ∼0.9°C. The averaged peak change (3 s) in Tneck in response to NP and NS during whole-body heating were ∼1.5°C and 1.0°C, respectively, while the breath-hold/airflow control trials applied during whole-body heating changed Tneck by ∼1.6°C.
Statistical analysis
Comparisons of steady-state physiological variables between normothermia and whole-body heating were made using paired t tests. Comparisons of CVC in response to baroreflex testing were made within each thermal condition using paired t tests. Statistical significance was set at P < 0.05.
Results
Cardiovascular and thermal variables during normothermia and whole-body heating
Steady-state cardiovascular and thermal data during normothermia and whole-body heating are presented in Table 1. Skin blood flux increased from 18.7 ± 1.4 a.u. during normothermia to 66.8 ± 11.9 a.u. during whole-body heating (P < 0.05), resulting in an approximate four-fold increase in CVC (0.81 ± 0.14 a.u. mmHg−1) compared to normothermia (0.23 ± 0.02 a.u. mmHg−1; P < 0.05).
Table 1.
Cardiovascular and thermal variables
| Normothermia | Whole-body heating | |
|---|---|---|
| HR (beats min−1) | 60.9 ± 4.4 | 76.3 ± 5.3* |
| SBP (mmHg) | 114.2 ± 3.2 | 116.1 ± 4.3 |
| DBP (mmHg) | 67.8 ± 2.3 | 63.7 ± 1.9* |
| MAP (mmHg) | 83.3 ± 2.4 | 81.2 ± 2.5 |
| Tcore (°C) | 37.0 ± 0.1 | 37.8 ± 0.1* |
| Tsk (°C) | 34.7 ± 0.1 | 37.3 ± 0.2* |
| Skin blood flux (a.u.) | 18.7 ± 1.4 | 66.8 ± 11.9* |
| CVC (a.u. mmHg−1) | 0.23 ± 0.02 | 0.81 ± 0.14* |
HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; Tcore, core temperature; Tsk, skin temperature; CVC, cutaneous vascular conductance
significantly different from normothermia (P < 0.05)
Carotid baroreceptor stimulation during normothermia
Traditional analysis
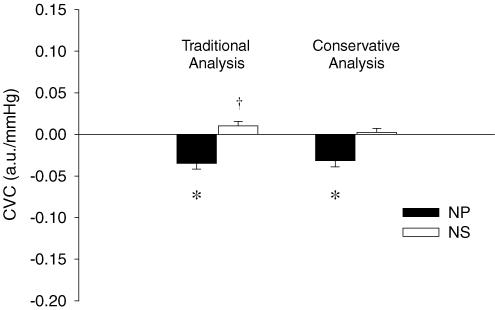
When the peak responses to each trial of NP (+40Torr) were determined for each subject, NP decreased CVC by 0.035 ± 0.007 a.u. mmHg−1 from baseline (P < 0.05) (see Fig. 1). This equated to a 16.9 ± 2.6% decrease in CVC from baseline. The peak responses to each separate trial of NS (−60Torr) tended to increase CVC 0.010 ± 0.005 a.u. mmHg−1 from baseline (P = 0.079), resulting in 5.1 ± 2.8% increase from baseline.
Figure 1. Carotid baroreflex-mediated changes in cutaneous vascular conductance (CVC) in response to neck pressure (NP, filled bars) and neck suction (NS, open bars) during normothermia.
Data are presented as a change from baseline (0.23 a.u. mmHg−1). Traditional analysis and conservative analysis (see Methods) of the same data are presented as means ±s.e.m.*Significantly different from prestimulus (P < 0.05). †Compared to prestimulus (P = 0.079). n = 9
Conservative analysis
When all trials to NP (+40Torr) within a subject were first averaged, the application of NP decreased CVC by 0.032 ± 0.007 a.u. mmHg−1 from baseline (P < 0.05). This equated to a 15.3 ± 2.9% reduction in CVC. However, using this analytical method for NS, CVC did not change (change (Δ) = 0.002 ± 0.005 a.u. mmHg−1) from baseline (P = 0.63; see Fig. 1).
Breath-hold/airflow control trial analysis
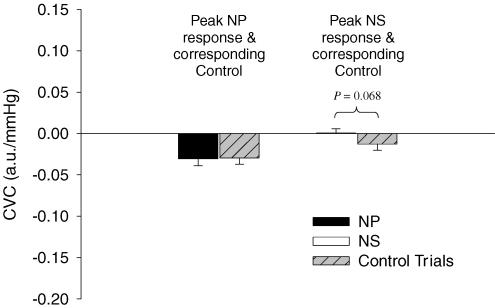
At the time of the peak CVC response during NP trials, the breath-hold/airflow control trial reduced CVC by 0.030 ± 0.007 a.u. mmHg−1 (P < 0.05) from prestimulus values. Peak decreases in CVC in response to actual NP trials from baseline (Δ = −0.031 ± 0.008 a.u. mmHg−1) were not different from changes in CVC observed during the breath-hold/airflow control trials (Δ = −0.030 ± 0.007 a.u. mmHg−1; P > 0.05).
At the time of the peak CVC response during NS trials, breath-hold/airflow control trials tended to decrease CVC by 0.013 ± 0.007 a.u. mmHg−1 (P = 0.11) from prestimulus values. The difference in the change in CVC between NS and breath-hold/airflow control trials (Δ = 0.013 ± 0.006 a.u. mmHg−1) tended to be different (P = 0.068), see Fig. 2.
Figure 2. Comparison of neck pressure (NP) and neck suction (NS) trials to breath-hold/airflow control trials (Control Trials) during normothermia.
Peak changes in cutaneous vascular conductance (CVC) in response to NP and NS were compared to the respective control trials from data obtained at the same time relative to the carotid stimulus. Data are presented as a change from baseline (0.23 a.u. mmHg−1). n = 8. Error bars are s.e.m.
Carotid baroreceptor stimulation during whole-body heating
Traditional analysis
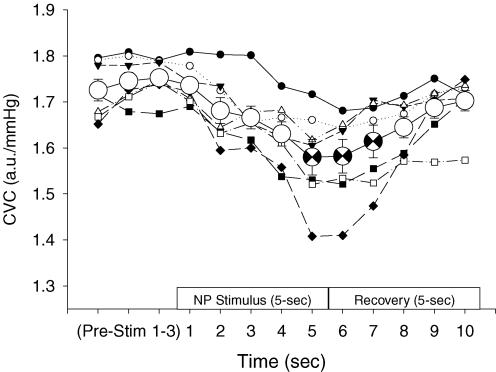
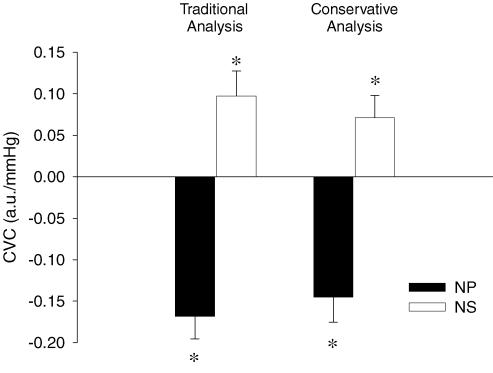
When peak responses to each separate trial of NP (+40Torr) were determined for each subject, NP decreased CVC by 0.168 ± 0.028 a.u. mmHg−1 from baseline (P < 0.05). This response equated to a 15.8 ± 3.4% reduction in CVC. Figure 3 depicts the CVC response to separate trials of NP, as well as the averaged response (see Conservative analysis below) in one subject. The peak responses to each separate trial of NS (−60Torr) increased CVC by 0.097 ± 0.030 a.u. mmHg−1 from baseline (P < 0.05). This response equated to a 9.0 ± 3.0% increase in CVC (see Fig. 4).
Figure 3. Representative data from one subject during whole-body heating in response to neck pressure (NP).
Cutaneous vascular conductance (CVC) responses to individual trials of NP are represented by the multiple data indicated with smaller symbols. Peak changes from these data were used for the traditional analysis. The averaged CVC response for these individual trials is indicated by the larger, open circle symbols (±s.e.m.). The averaged peak data are indicated by the partially filled, larger circles (occurring at seconds 5, 6 and 7); these are the data used for the conservative analysis. The x-axis indicates the 3 s during prestimulus (Pre-Stim 1–3), followed by the 5 s of the neck chamber stimulus and the 5 s of recovery.
Figure 4. Carotid baroreflex-mediated changes in cutaneous vascular conductance (CVC) in response to neck pressure (NP, filled bars) and neck suction (NS, open bars) during whole-body heating.
Data are presented as a change from baseline (0.81 a.u. mmHg−1). Traditional analysis and conservative analysis (see Methods) of the same data are presented as means ±s.e.m.*Significantly different from prestimulus (P < 0.05). n = 7
Conservative analysis
When all trials to NP (+40Torr) within a subject were first averaged before identifying the peak response, NP decreased CVC by 0.145 ± 0.030 a.u. mmHg−1 from baseline (P < 0.05). This response equated to a 13.7 ± 3.8% reduction in CVC. Using this analytical technique for NS, this perturbation increased CVC (Δ = 0.071 ± 0.027 a.u. mmHg−1 from baseline, P < 0.05). This response equated to a 6.7 ± 2.7% increase from baseline CVC (see Fig. 4).
Breath-hold/airflow control trial analysis
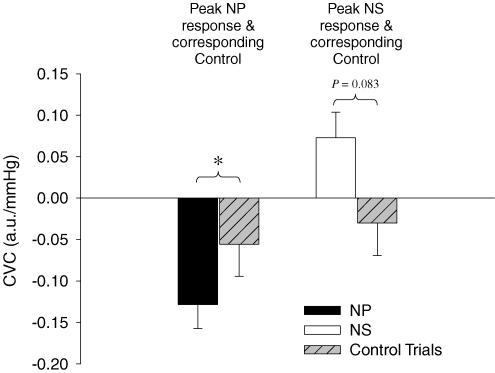
During whole-body heating, at the time of the peak CVC response during NP, CVC was not significantly changed during breath-hold/airflow control trials (Δ = −0.056 ± 0.039 a.u. mmHg−1, P = 0.20) compared to prestimulus values. Furthermore, the peak decrease in CVC in response to NP (Δ = −0.129 ± 0.029 a.u. mmHg−1) was greater than this non-significant change in CVC (Δ = −0.056 ± 0.039 a.u. mmHg−1) during the breath-hold/airflow control trial (P < 0.05), see Fig. 5.
Figure 5. Comparison of neck pressure (NP) and neck suction (NS) trial to breath-hold/airflow control trials (Control Trials) during whole-body heating.
Peak changes in cutaneous vascular conductance (CVC) in response to NP and NS were compared to the respective control trials from data obtained at the same time relative to the carotid stimulus. Data are presented as a change from baseline (0.81 a.u. mmHg−1). *Significantly different (P < 0.05). n = 7
At the time of the peak CVC response during the NS trials, CVC was likewise unchanged during breath-hold/airflow control trials (Δ = −0.030 ± 0.039 a.u. mmHg−1, P = 0.47) compared to prestimulus values. Peak increases in CVC to NS (Δ = 0.073 ± 0.031 a.u. mmHg−1) tended to be greater than decreases in CVC (Δ = −0.030 ± 0.039 a.u. mmHg−1; P = 0.083) during the breath-hold control trials (note the directional difference).
Discussion
The primary finding of this investigation is that carotid baroreceptor stimulation elicits dynamic changes in CVC during whole-body heating, despite previous contrary findings. In contrast, carotid baroreflex-mediated changes in CVC were less clear under normothermic conditions. The application of either NP or NS reduced Tneck during both thermal conditions, and because changes in Tneck may have contributed to the CVC response to the neck chamber stimulation, breath-hold/airflow control trials were also performed. These control trials resulted in slight reductions in CVC during both thermal conditions. While these reductions in CVC may partially account for changes in CVC observed during normothermia in response to NP, decreases in CVC in response to the breath-hold/airflow control trial were markedly smaller than that observed in response to NP during whole-body heating, as well as directionally different from NS. Taken together, these findings indicate that the carotid baroreflex exhibits an efferent limb governing the cutaneous vasculature, although this was only consistently observed while subjects were heat stressed.
Heat stress redistributes cardiac output to the cutaneous vasculature and it has been demonstrated that this redistribution can surpass 50% of the prevailing cardiac output (Rowell et al. 1969). Therefore, during heat stress, the cutaneous vasculature has the potential to become an important target for arterial blood pressure control. Despite this, prior evidence suggests that the carotid baroreflex does not control the cutaneous vasculature (Wallin et al. 1975; Crandall et al. 1996). This lack of control has been proposed as a potential rationale for the reduction in arterial baroreflex control of blood pressure observed during heat stress (Crandall, 2000), as well as the reduced orthostatic tolerance to a number of gravitational stressors (Lind et al. 1968; Allan & Crossley, 1972; Wilson et al. 2002) observed during heat stress.
In the current investigation, carotid baroreceptor unloading with NP decreased CVC while subjects were normothermic. However, it is important to note that the application of the breath-hold/airflow control trial resulted in similar decreases in CVC during normothermic conditions. Therefore, despite different responses between NP and NS observed during normothermia (i.e. decrease versus no change in CVC, respectively), the interpretation of such findings remains limited. During whole-body heating, NP decreased CVC by ∼14–16% (depending on the analysis used), while NS increased CVC by ∼7–9%. These changes were significantly different from those of breath-hold/airflow control trials, indicating carotid baroreflex modulation of the cutaneous vasculature. These findings are in contrast to the findings of Crandall et al. (1996), who evaluated carotid baroreflex control of the cutaneous vasculature during normothermic and heat stress conditions using 500 ms pulses of NP during each cardiac cycle over 3 min, and did not observe an effect of NP on CVC. However, the application of the baroreceptor stimulation, as well as the analysis of CVC, were markedly different between these two studies, and may explain the different interpretation of the respective findings. First, the baroreceptor stimulation in the current study was applied using 5 s trials separated by at least 45 s. This stimulation changed carotid transmural pressure for five consecutive seconds, and therefore increased mean carotid sinus pressure by a factor approximately equal to the chamber pressure achieved for each trial (i.e. mean estimated carotid sinus pressure is increased or decreased by the pressure attained in the collar) (Querry et al. 2001). However, 500 ms pulses, such as those used by Crandall et al. (1996), only briefly change carotid sinus pressure, primarily during the systolic phase of the cardiac cycle, and therefore did not alter mean carotid arterial pressure to the same extent as the present protocol. Another possible explanation for the discrepancy between these studies is that the role of the aortic baroreflex between the two studies was probably different. Potts et al. (1993) described the analysis of the 5 s stimulation of the carotid baroreceptors as being primarily a open-loop system (i.e. aortic baroreflex independent) due to the brevity of the resulting changes in arterial pressure after carotid stimulation. However, the resulting change in mean arterial pressure reported by Crandall et al. (1996) over the 3 min of NP application (i.e. a reported blood pressure increase of ∼10 mmHg) probably engaged the aortic baroreceptor population, which may have inhibited the effectiveness of carotid baroreceptor hypotension in altering CVC. However, this is purely speculative, as the relative role, or directional control of the cutaneous vasculature by the carotid versus aortic baroreflexes has not been examined.
Possibly more relevant to the difference between the findings of the current study and that of (Crandall et al. (1996) is the nature of the analysis of the responses. In the current study, the dynamic response (i.e. within seconds) to individual trials of carotid baroreceptor stimulation was assessed, while (Crandall et al. (1996) compared 1 min averages of CVC during NP to prestimulus baseline CVC. The latter technique (Crandall et al. 1996) clearly minimizes the ability to detect dynamic baroreflex-meditated fluctuations in CVC that may have occurred during NP. That is, the lack of change in mean CVC, averaged over 1 min, does not negate the notion that carotid baroreceptor stimulation is capable of causing dynamic oscillations in CVC.
Beiser et al. (1970) demonstrated a decrease in cutaneous vascular resistance of forearm skin in response to loading the carotid baroreceptors during normothermic conditions. Unlike the findings of Beiser et al. (1970), the application of NS did not increase CVC (i.e. decrease resistance) during normothermia in the current investigation. However, Beiser et al. (1970) used iontophoresis of adrenaline (epinephrine) to constrict the cutaneous vasculature of one arm prior to carotid baroreceptor stimulation, and compared changes in the ‘preconstricted arm’ to the other, non-treated arm. Also, Beiser et al. (1970) used 2 min trials of NS and strain gauge plethysmography of the forearm, compared to the 5 s NS stimuli and laser Doppler flowmetry indices of skin blood flow used in the current study. These methodological differences may explain differences in CVC responses to NS between these studies. Furthermore, it is possible that, in the current study, the normothermic condition (i.e. the water temperature used) resulted in relatively higher skin temperatures (albeit, only slightly) compared to those found by Beiser et al. (1970), and that this subtle elevation in skin temperature resulted in a withdrawal of sympathetic vasoconstrictor activity directed to the skin. Such an occurrence may have minimized the effectiveness of the hypertensive carotid baroreceptor challenge with NS in response to further withdrawal skin sympathetic activity. However, this hypothesis is purely speculative.
Studies investigating the arterial baroreflex control of sSNA are limited in number. In four subjects, electrical stimulation of the carotid sinus nerve performed by Wallin et al. (1975) did not reproducibly change sSNA. However, changes in skin blood flow were not measured by Wallin et al. (1975), and reliance on changes in integrated sSNA may limit the interpretation with respect to CVC responses. Furthermore, Wilson et al. (2001), observed no change in sSNA in response to pharmacologically induced baroreceptor stimulation. Similar to the work of Wallin et al. (1975), Wilson et al. (2001) also did not examine skin blood flow during baroreceptor stimulation due to the direct effect of the infused drugs on the cutaneous vasculature. The cutaneous vasculature is under direct neural control of both an active vasoconstrictor system and an active vasodilator system. It is therefore difficult to distinguish between concomitant changes in these two systems in the sSNA recording, as this may result in no net change in the integrated neural signal at a time when CVC is changing. Perhaps related to this, it has been demonstrated that changes in CVC are not always accompanied by changes in sSNA (Cui et al. 2004). This mismatch between sSNA and CVC may further complicate the interpretation of findings regarding arterial baroreflex control of CVC. While analysis of sSNA was not examined in the current study, the end-organ response (i.e. changes in skin blood flow and calculated CVC) was assessed during acute carotid baroreceptor stimulation.
The differential responses to NS between normothermia and whole-body heating in the current study (i.e. the absence during normothermia versus the presence during whole-body heating) may be related to steady-state CVC for the respective conditions. That is, when steady-state CVC is high, as during whole-body heating, changes in cutaneous vascular tone are more easily expressed, or identified. Another possible explanation for these differences is that the heat stress itself (i.e. elevated internal temperature, elevated skin temperature, etc) may directly influence the carotid baroreflex, or its associated neural pathways, ‘unveiling’ reflex control of the skin during a time when neural control of the skin becomes more important for arterial blood pressure regulation. Finally, given that during heat stress CVC is primarily controlled by an active vasodilator system, it may be that carotid baroreceptor control of CVC primarily occurs through modulation of the active vasodilator system whose activity is absent or greatly reduced during normothermia.
The influence of carotid baroreflex-mediated changes in CVC on mean arterial blood pressure is unclear and would depend on the thermal status of the individual. Due to the small fraction of cardiac output directed to the skin during normothermic conditions, carotid baroreflex-mediated changes in CVC will minimally contribute to total vascular conductance and, therefore, arterial blood pressure, in normothermic conditions. However, during heat stress conditions, cardiac output has been reported to more than double, with 100% of that increase going to the skin (Rowell et al. 1969). For example, if one assumes that cardiac output increases from ∼5 l min−1 to 11 l min−1 during a given heat stress at a blood pressure (gradient) of 85 mmHg, then total vascular conductance would be ∼129 ml min−1 mmHg−1. Assuming that ∼6.25 l min−1 is directed to the skin in this example, equivalent carotid baroreflex-mediated changes in CVC to those observed in the current investigation during whole-body heating (i.e. −15% to +10%, or ∼25% range), would correspond to ∼18 ml min−1 mmHg−1 (∼14% of total vascular conductance). This would represent an ∼11 mmHg range in which arterial blood pressure may be modulated by carotid baroreflex control of the cutaneous vasculature during whole-body heating.
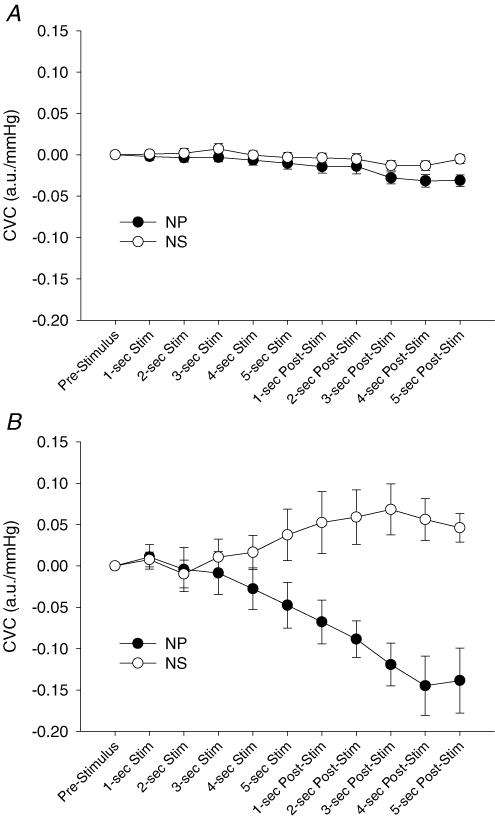
Both NP and NS reduced Tneck during normothermia and whole-body heating (see Methods). These control trials may help in discriminating between the role of true baroreflex-mediated changes in CVC and the role of potential Tneck changes, as well as the effect of the breath-hold itself. Although slight reductions in CVC were observed during these control trials in both thermal conditions, when the changes in CVC observed during the control trials were subtracted from the corresponding those of NP and NS trials, appropriate changes in CVC to these perturbations remained during whole-body heating. However, simple subtraction relies on the responses being additive (i.e. no interaction between baroreflex and potential breath-hold or Tsk responses), and therefore may not be the most appropriate analysis. Regardless, potential effects of the breath-hold and changes in Tneck are inadequate to describe different directional responses in CVC between NP and NS stimuli during whole-body heating (Fig. 6B).
Figure 6. Second-by-second group average responses for cutaneous vascular conductance (CVC) to neck pressure (NP) and neck suction (NS) during normothermia (A) and whole-body heating (B).
In summary, carotid baroreceptor unloading and loading, using the neck chamber technique, caused cutaneous vasoconstriction and vasodilation, respectively, during whole-body heating. These data strongly suggest that the carotid baroreflex exhibits an efferent limb governing the cutaneous vasculature, and therefore the cutaneous vasculature appears to be involved in moment-to-moment regulation of arterial blood pressure through the carotid baroreflex.
Acknowledgments
The authors thank all of the participants involved with the study. This work was supported by National Institutes of Health grants HL61388, HL67422 and HL082426–01A1.
References
- Allan JR, Crossley RJ. Effect of controlled elevation of body temperature on human tolerance to +G z acceleration. J Appl Physiol. 1972;33:418–420. doi: 10.1152/jappl.1972.33.4.418. [DOI] [PubMed] [Google Scholar]
- Beiser GD, Zelis R, Epstein SE, Mason DT, Braunwald E. The role of skin and muscle resistance vessels in reflexes mediated by the baroreceptor system. J Clin Invest. 1970;49:225–231. doi: 10.1172/JCI106231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall CG. Carotid baroreflex responsiveness in heat-stressed humans. Am J Physiol Heart Circ Physiol. 2000;279:H1955–H1962. doi: 10.1152/ajpheart.2000.279.4.H1955. [DOI] [PubMed] [Google Scholar]
- Crandall CG, Johnson JM, Kosiba WA, Kellogg DL., Jr Baroreceptor control of the cutaneous active vasodilator system. J Appl Physiol. 1996;81:2192–2198. doi: 10.1152/jappl.1996.81.5.2192. [DOI] [PubMed] [Google Scholar]
- Cui J, Wilson TE, Crandall CG. Orthostatic challenge does not alter skin sympathetic nerve activity in heat-stressed humans. Auton Neurosci. 2004;116:54–61. doi: 10.1016/j.autneu.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Eckberg DL, Kifle YT, Roberts VL. Phase relationship between normal human respiration and baroreflex responsiveness. J Physiol. 1980;304:489–502. doi: 10.1113/jphysiol.1980.sp013338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind AR, Leithead CS, McNicol GW. Cardiovascular changes during syncope induced by tilting men in the heat. J Appl Physiol. 1968;25:268–276. doi: 10.1152/jappl.1968.25.3.268. [DOI] [PubMed] [Google Scholar]
- Potts JT, Shi XR, Raven PB. Carotid baroreflex responsiveness during dynamic exercise in humans. Am J Physiol. 1993;265:H1928–H1938. doi: 10.1152/ajpheart.1993.265.6.H1928. [DOI] [PubMed] [Google Scholar]
- Querry RG, Smith SA, Stromstad M, Ide K, Secher NH, Raven PB. Anatomical and functional characteristics of carotid sinus stimulation in humans. Am J Physiol Heart Circ Physiol. 2001;280:H2390–H2398. doi: 10.1152/ajpheart.2001.280.5.H2390. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Brengelmann GL, Murray JA. Cardiovascular responses to sustained high skin temperature in resting man. J Appl Physiol. 1969;27:673–680. doi: 10.1152/jappl.1969.27.5.673. [DOI] [PubMed] [Google Scholar]
- Taylor WF, Johnson JM, Kosiba WA, Kwan CM. Cutaneous vascular responses to isometric handgrip exercise. J Appl Physiol. 1989;66:1586–1592. doi: 10.1152/jappl.1989.66.4.1586. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Sundlof G, Delius W. The effect of carotid sinus nerve stimulation on muscle and skin nerve sympathetic activity in man. Pflugers Arch. 1975;358:101–110. doi: 10.1007/BF00583921. [DOI] [PubMed] [Google Scholar]
- Wilson TE, Cui J, Crandall CG. Absence of arterial baroreflex modulation of skin sympathetic activity and sweat rate during whole-body heating in humans. J Physiol. 2001;536:615–623. doi: 10.1111/j.1469-7793.2001.0615c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TE, Cui J, Zhang R, Witkowski S, Crandall CG. Skin cooling maintains cerebral blood flow velocity and orthostatic tolerance during tilting in heated humans. J Appl Physiol. 2002;93:85–91. doi: 10.1152/japplphysiol.01043.2001. [DOI] [PubMed] [Google Scholar]