Abstract
Shifting gaze requires precise coordination of eye and head movements. It is clear that the superior colliculus (SC) is involved with saccadic gaze shifts. Here we investigate its role in controlling both eye and head movements during gaze shifts. Gaze shifts of the same amplitude can be evoked from different SC sites by controlled electrical microstimulation. To describe how the SC coordinates the eye and the head, we compare the characteristics of these amplitude-matched gaze shifts evoked from different SC sites. We show that matched amplitude gaze shifts elicited from progressively more caudal sites are progressively slower and associated with a greater head contribution. Stimulation at more caudal SC sites decreased the peak velocity of the eye but not of the head, suggesting that the lower peak gaze velocity for the caudal sites is due to the increased contribution of the slower-moving head. Eye–head coordination across the SC motor map is also indicated by the relative latencies of the eye and head movements. For some amplitudes of gaze shift, rostral stimulation evoked eye movement before head movement, whereas this reversed with caudal stimulation, which caused the head to move before the eyes. These results show that gaze shifts of similar amplitude evoked from different SC sites are produced with different kinematics and coordination of eye and head movements. In other words, gaze shifts evoked from different SC sites follow different amplitude–velocity curves, with different eye–head contributions. These findings shed light on mechanisms used by the central nervous system to translate a high-level motor representation (a desired gaze displacement on the SC map) into motor commands appropriate for the involved body segments (the eye and the head).
In natural conditions, an efficient motor response often consists of the well-coordinated mobilization of several effectors. There is still little mechanistic understanding of how the brain readily achieves such precise coordination of multiple body segments. A good example of such a coordination is the saccadic orientation of gaze towards a visual target of interest which is composed of combined eye and head movements. Commands for these movements arise principally from the superior colliculus (SC) (Sparks, 1999), a neural structure located at the roof of the mesencephalon. It has been shown that the SC contains a motor map encoding a ‘desired gaze displacement’ command (Munoz et al. 1991; Paré et al. 1994; Freedman et al. 1996; Freedman & Sparks, 1997a; Guillaume & Pélisson, 2001a). It is postulated that the gaze displacement command which arises from the SC is decomposed into motor commands for eye and head components in structures located downstream from the SC (Galiana & Guitton, 1992; Goossens & Van Opstal, 1997; Freedman, 2001; Corneil et al. 2002b). The purpose of the present study is to get further insight into mechanisms underlying this decomposition through a careful analysis of saccadic gaze shifts evoked by electrical stimulation of the SC.
Kinematics and eye–head coordination of natural gaze shifts
The kinematics (the velocity profiles) and the eye–head coordination (the metrics of eye and head movements and the eye–head delay) of the saccadic gaze displacement directed at a visual target are now well characterized (cat: Guitton et al. 1984, 1990; Goffart et al. 1998; monkey: Tomlinson & Bahra, 1986a; Phillips et al. 1995; Freedman & Sparks, 1997b, 2000; human: Barnes, 1979; Fuller, 1992; Stahl, 1999). Concerning the kinematics, a saturating function describes the relationship between gaze peak velocity and amplitude, sometimes with a decline for the largest amplitudes. There is a linear relationship between the duration and the amplitude of gaze movement. These main sequence relationships can be modified by the stimulus modality: gaze peak velocity is greater for visual than for auditory targets (Goldring et al. 1996; Goossens & Van Opstal, 1997). Truncation of natural gaze shifts, initially planned for a much larger excursion, can also alter the main sequence (Corneil et al. 1999). Concerning the metrics, small-amplitude gaze shifts mainly consist of an eye saccade. As the gaze movement amplitude increases, the amplitude of the eye saccade increases until saturation, and the contribution of the head becomes more prominent. This eye–head coordination pattern varies quantitatively between species as a function of the possible range of eye movements in the orbit (oculomotor range), but its qualitative features remain constant across species. Several other factors modulate this basic eye–head coordination pattern. The position of the eyes in the orbit at the initiation of the gaze shifts is one of these factors. A deviation of the eye contralateral or ipsilateral to the impending gaze shift will be associated, respectively, with a decreased or an increased head contribution (Volle & Guitton, 1993; Fuller, 1996; Freedman & Sparks, 1997b; Gandhi & Sparks, 2001; Stahl, 1999, 2001). Another factor is the stimulus modality. The head contribution is larger in gaze shifts towards auditory targets than towards visual ones (Goossens & Van Opstal, 1997). The spatial and temporal predictability of target position (Barnes, 1979; Zangemeister & Stark, 1982) or the planning of future gaze shifts (Oommen et al. 2004) also influence the pattern of eye–head coordination. Finally, the adaptation to specific situations may also change eye–head coordination (Crawford & Guitton, 1997; Misslisch et al. 1998; Ceylan et al. 2000; Stahl, 2001; Constantin et al. 2004). All these studies clearly show that the kinematics and metrics of eye and head components can vary with context, but there is still little mechanistic understanding of how the brain implements such variability.
Gaze shifts evoked by electrical stimulation of the SC
Despite known limitations (possible involvement of fibres of passage and of retrogradely activated fibres), electrical microstimulation has been a fruitful approach to the study of the encoding of gaze amplitude in the SC. It has been demonstrated that the SC is organized as a gaze motor map: the size of gaze shifts is represented in an orderly manner by the position of the active locus in the deeper layers (Paré et al. 1994; Freedman et al. 1996; Guillaume & Pélisson, 2001a). These studies thus extented the previously proposed eye motor map (Robinson, 1972; Schiller & Stryker, 1972) to a gaze motor map. They also argued in favour of the classical scheme of an amplitude coding resting primarily on a place code (spatial location of collicular activity) (Stanford et al. 1996; Sparks & Gandhi, 2003). In our recent study (Guillaume & Pélisson, 2001a), we confirmed the existence of a topographic map for saccadic gaze shifts. Additionally we confirmed and extended to the head-unrestrained condition the strong effect of stimulation current intensity on the amplitude of evoked gaze shifts (Fig. 1A) (head restrained: Straschill & Rieger, 1973; Sparks & Mays, 1983; du Lac & Knudsen, 1990; Van Opstal et al. 1990; Salas et al. 1997; Herrero et al. 1998; head unrestrained: Paré et al. 1994; Freedman et al. 1996). Based on these findings, we concluded that the pattern of SC neuronal activity elicited by the stimulating electrode at different current strength (namely the size of the recruited neuronal population, see Yeomans, 1990 and Tehovnik, 1996) markedly interacts with the topographical encoding of gaze (motor map) within the SC deeper layers. Note that this conclusion holds for stimulation parameters that closely reproduce the natural activation of the SC during normal orienting behaviour. Indeed, the data reported in our previous study were mostly based on current intensities ranging from one to three times threshold (1 × T to 3 × T). As estimated after McIlwain (1982) and from the mean threshold intensity observed in our study (7.9 μA), the size of recruited population at 2 × T is about 1 mm in diameter (0.5 mm and 1.5 mm for 1 × T and 3 × T). This estimate at 2 × T closely matches the estimated size of neuronal population activated in relation to visually triggered saccades (Ottes et al. 1986; Kang & Lee, 2000).
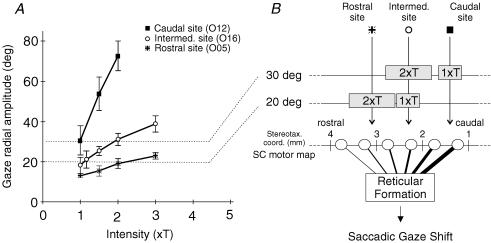
Figure 1. Rationale of the study.
A, Effect of current intensity on the metrics of gaze shifts evoked from a rostral (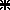 ), an intermediate (○) and a caudal (▪) collicular site. Gaze radial amplitude is plotted as a function of stimulation current intensity. This intensity is expressed relative to a threshold (T) defined as the intensity which evoked a gaze shift in >75% of stimulation trials. The frequency of stimulation was 300 pulse s−1 and its duration always outlasted the gaze shift duration in order to avoid movement truncation. Symbols represent means ±s.d.B, unidimentional representation of SC motor map and interpretation of the graph in A. The three collicular sites of graph A are positioned on the antero-posterior dimension of the SC (stereotaxic coordinates). Grey boxes correspond to the postulated size of collicular activations induced by the electrical stimulation. Open circles symbolize collicular output neurons. The projections of these neurons toward the reticular formation are symbolized by lines of increasing thickness to account for the known motor map organization (site-dependent encoding of gaze amplitude). The objective of the present study is to test whether gaze shifts of similar amplitude evoked from remote SC sites (e.g. 20 deg gaze shifts from ○ and
), an intermediate (○) and a caudal (▪) collicular site. Gaze radial amplitude is plotted as a function of stimulation current intensity. This intensity is expressed relative to a threshold (T) defined as the intensity which evoked a gaze shift in >75% of stimulation trials. The frequency of stimulation was 300 pulse s−1 and its duration always outlasted the gaze shift duration in order to avoid movement truncation. Symbols represent means ±s.d.B, unidimentional representation of SC motor map and interpretation of the graph in A. The three collicular sites of graph A are positioned on the antero-posterior dimension of the SC (stereotaxic coordinates). Grey boxes correspond to the postulated size of collicular activations induced by the electrical stimulation. Open circles symbolize collicular output neurons. The projections of these neurons toward the reticular formation are symbolized by lines of increasing thickness to account for the known motor map organization (site-dependent encoding of gaze amplitude). The objective of the present study is to test whether gaze shifts of similar amplitude evoked from remote SC sites (e.g. 20 deg gaze shifts from ○ and 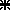 sites) have similar kinematics and eye–head coordination pattern.
sites) have similar kinematics and eye–head coordination pattern.
Kinematics and eye–head coordination of electrically evoked gaze shifts
Previous SC stimulation studies in head-unrestrained preparations have suggested that the elicited gaze shifts have roughly normal kinematics (velocity to amplitude relationship) and eye–head coordination characteristics (Roucoux et al. 1980; Munoz et al. 1991; Paré et al. 1994; Freedman et al. 1996; Klier et al. 2001). Paré et al. (1994) noted that the peak velocity of electrically evoked gaze shifts could be higher than that of natural ones, but this observation was made using high stimulation parameters (500–600 pulses s−1 and current intensity 2 × threshold). Nevertheless the kinematics and eye–head coordination of microstimulation-induced gaze shifts have never been studied in detail, and neither has their correlation to the position of the stimulation site in the SC.
In the present study, we took advantage of the interaction between the current intensity and the site of collicular electrical stimulation described above to determine whether the kinematics and eye–head coordination pattern depend on the site of stimulation in the SC. We compared the peak velocity and eye–head coordination of similar-amplitude gaze shifts evoked by the stimulation of remote SC sites (see Fig. 1B). We demonstrate for the first time that both the peak velocity and the pattern of eye–head coordination of similar-amplitude gaze shifts are continuously related to the stimulated locus in the SC map. These findings will be discussed in relation to recent gaze control models which attempt to account for the decomposition of the collicular ‘desired gaze displacement’ signal into separate motor commands for the eye and head components.
These results have been presented previously in abstract form (Pélisson & Guillaume, 2006).
Methods
This paper presents further analyses of data collected during the first of two experimental phases which were designed to study saccadic gaze shifts evoked by the electrical stimulation of the SC before (Phase I, Guillaume & Pélisson, 2001a) and during cerebellar fastigial nucleus inactivation (Phase II, Guillaume & Pélisson 2001b). The reader is refered to these publications for a more detailed description of the procedure.
Animal preparation
Two adult male cats were prepared under anaesthetic and aseptic conditions in accordance with the guidelines from the French Ministry of Agriculture (87/848) and from the European Community (86/609/EEC). The anaesthesia was induced and maintained by pentobarbital sodium (i.p. injection for induction: 30 mg kg−1; i.v. perfusion during surgery: 1–3 mg kg−1 h−1). Two coils were implanted, one on the eye sclera, and the second fixed to the skull, for the recording of gaze and head positions by the search-coil-in-magnetic-field technique (Robinson, 1963). A trephine hole was made in the skull, and a recording chamber was implanted over the SC to allow vertical access to both colliculi. Another hole was made and a chamber was implanted over the cerebellum for the purpose of our previously published study (Guillaume & Pélisson, 2001b). Finally, a U-shaped plastic piece was fixed to the skull with dental cement, allowing restraint of the animal's head during some experimental phases. During the first postoperative week the animals received i.m. injections of sodium amoxicillin antibiotic (Clamoxyl, 50 mg kg−1 (24 h)−1) and the eye on which the coil was sutured was cleaned twice daily with an eyewash. The wounds and the recording chambers were also cleaned daily (use of aseptic agents). After a supplementary recovery week, experimental sessions started. Each experimental session lasted approximately 3 h and a minimum interval of 2 days was observed between two consecutive sessions. At the end of the experiment, the animals were killed with an i.p. injection of 2.5 times the lethal dose of sodium pentobarbital.
Experimental setup and experimental paradigm
The animal lay in a hammock that gently restrained the body without constraint of natural movements of the head. The animal's head was situated at the centre of a 1 m coil frame (CNC Engineering). The cat faced a 19 deg-wide opaque screen situated in a frontoparallel plane at a distance of 41 cm. Prior to recording sessions, the animal was trained to orient its gaze towards a visual target which was suddenly presented to either side of the screen. The target, a spoon of food puree, was presented only when the animal quietly looked at a white plastic bolt located at the centre of the screen, and the animal was rewarded directly from the food target after correct orienting.
The experimental paradigm started with the lowering of an electrode into the SC deeper layers of the head-restrained animal. The electrode's entrance in the SC superficial layers was precisely identified by the visual activity recorded at this level. From this point, the electrode was further lowered by 1.8–2 mm. After verification that saccades could be elicited by low-current electrical stimulation (<30 μA), the animal's head was freed and recordings started. Electrical stimulation trials were randomly intermixed with visual trials (relative proportion about 50%). The electrical stimulation was manually triggered, while the animal awaited the visual target presentation and looked at the central bolt. Note that no intense fixation activity was required during this stimulation phase, because the reward was not directly contingent upon correct fixation but delivered only later after gaze orientation toward the target. The target was presented about 1 s after the end of the microstimulation-evoked response, and unpredictably to the left or right edge of the screen. Several experimental sessions with only visual trials were also performed. In those sessions, different sizes of opaque screen (7, 15, 19, 27 and 35 deg wide) were used to elicit natural gaze shifts of different amplitudes (see Goffart & Pélisson, 1998).
Electrical stimulation
A total of 20 stimulation sites have been tested: 8 in cat O and 12 in cat L (one site per experimental session). Stimulation sites were sampled by aiming our electrode penetrations across a wide range of the SC motor map along the horizontal meridian representation, in order to focus on nearly horizontally directed gaze shifts. The range of stereotaxic coordinates was A1.2 to A3.9 mm for the antero-posterior axis and 2–3.5 mm for the medio-lateral axis. Stimulation trains (0.5 ms cathodal pulses) of 300 ms duration and 300 pulse s−1 pulse frequency were first used to determine the threshold current intensity (T) defined as the intensity which evoked a gaze shift in more than 75% of stimulation trials (mean T = 8.0 ± 5.4 μA, n = 20 sites). Then, several current intensities which were multiples of T were tested at this pulse frequency of 300 pulse s−1 (up to 6 × T). For each current intensity a minimum of 20 stimulation trials were performed with some variability in this number (in general more trials for intensities close to the threshold). This corresponded to a total of 100–150 stimulation trials for each site. The range of absolute currents used was from 3.5 to 90 μA. Train duration was generally 300 ms, but was sometimes shortened when the primary gaze shift was large, in order to avoid the triggering of secondary movements which would reach mechanical limits. In all cases train duration was kept long enough not to truncate the primary gaze shift. This was allowed by online monitoring of the gaze position trace on a computer screen, together with a trace corresponding to the current passing through the electrode. Thus it was possible to check that evoked gaze saccades were completed before the end of the stimulation train. Offline analyses confirmed that most gaze shifts were indeed not truncated, and the few exceptions were excluded from further processing.
Data collection and analysis
Search coils signals were linearized and scaled online by a computer program, providing four signals proportional to the horizontal and vertical positions of gaze (eye-in-space) and head. These signals were recorded to disk at a sampling rate of 500 Hz (DataWave Software, Longmont, USA). Offline analysis was performed by software developed in the laboratory. Gaze and head signals were digitally filtered (FIR filter, 70 Hz cutoff frequency) and differentiated. Eye position was obtained by subtracting the head position from the gaze position signals. The onset and termination of gaze shifts and of head movements were automatically detected based on a velocity criterion (30 deg s−1) and, in a second time, checked and corrected manually if necessary. These corrections were needed when a post-saccadic gaze drift with a velocity exceeding the criterion level occurred (mostly for caudal SC sites). In these cases, the termination of the gaze saccade was defined as the sharp transition between the gaze shift deceleration phase and the phase of constant, low-level, drift velocity (see Guillaume & Pélisson, 2001a).
We selected for analysis only gaze shifts initiated from the central ±5 deg horizontal position range (eliminating 28.7% of the responses). Several parameters were extracted and analysed by a spreadsheet program (Statistica from StatSoft): amplitude of horizontal and vertical components of gaze shift, gaze radial amplitude (= √(horizontal amplitude2 + vertical amplitude2)), gaze shift direction (= arcsin (vertical amplitude/radial amplitude)), and gaze horizontal peak velocity. The horizontal head contribution to the gaze shift (or concurrent head displacement) was defined as the horizontal displacement of the head that occurred between the onset and the termination of the gaze shift. We also measured gaze and head latencies as the time interval between the onset of stimulation and the initiation of the eye saccade or of the head movement, respectively. Finally the eye–head delay was obtained by subtracting eye onset time from head onset time (positive or negative values corresponding to eye lead or lag, respectively).
The locations of stimulation sites along the SC antero-posterior axis were expressed as the mean horizontal amplitude of gaze shifts evoked by a standard electrical stimulation at a 2 × T current intensity (see Guillaume & Pélisson, 2001a).
Results
After the data selection based on gaze initial position was performed, the mean values (n = 20 sites) for initial horizontal position were −0.02 ± 1.4 (gaze), 0.94 ± 3.28 (eye) and −0.96 ± 3.52 (head) and those for initial vertical position were 1.6 ± 2.44 (gaze), 1.21 ± 3.21 (eye) and 0.39 ± 2.37 (head). The mean characteristics, after this selection, of gaze shifts evoked by the electrical stimulation of each of the 20 sites at a current intensity of 2 × T are given in Table 1. The range of horizontal amplitude was 7.0–76.8 deg. The direction of evoked gaze shifts was very close to the horizontal meridian (mean = −2.5 ± 15.8 deg, n = 20), resulting from our sampling strategy (see methods).
Table 1.
Characteristics of gaze shifts evoked by electrical stimulation with a current intensity of 2 × T, shown for all SC sites in both cats
| Site | Horizontal amplitude (deg) | Radial amplitude amp. (deg) | Direction (deg) |
|---|---|---|---|
| O05 | 17.2 ± 2.6 | 19.1 ± 2.4 | 25.8 ± 8.7 |
| O01 | −18.5 ± 4.8 | 19.6 ± 5.1 | −19.3 ± 2.9 |
| O14 | 20.5 ± 3.3 | 21.1 ± 3.3 | 13.7 ± 9.1 |
| O16 | −30.9 ± 3.4 | 31.1 ± 3.2 | 6.5 ± 2.4 |
| O13 | −38.6 ± 8.5 | 42.0 ± 8.3 | −23.2 ± 8.0 |
| O18 | 48.8 ± 8.1 | 50.4 ± 7.6 | −14.5 ± 5.9 |
| O12 | −72.4 ± 7.3 | 72.7 ± 7.4 | 5.2 ± 3.3 |
| O06 | −76.8 ± 5.5 | 77.3 ± 5.4 | −6.5 ± 2.9 |
| L05* | 7.0 | 7.2 | 13.5 |
| L12 | −19.2 ± 0.8 | 19.8 ± 1.2 | 14.1 ± 6.2 |
| L07* | −23.5 | 27.2 | −30.2 |
| L20 | 24.1 ± 4.1 | 24.1 ± 4.2 | 0.0 ± 3.1 |
| L04* | −26.0 | 26.3 | −8.7 |
| L19 | 39.3 ± 3.5 | 41.2 ± 3.7 | −17.5 ± 3.9 |
| L06 | −41.2 ± 9.1 | 42.5 ± 8.9 | −14.2 ± 6.9 |
| L21* | 42.4 | 42.7 | 6.8 |
| L14* | 44.0 | 47.0 | 20.6 |
| L02 | 44.2 ± 6.0 | 44.5 ± 6.1 | 6.7 ± 4.0 |
| L01 | −51.0 ± 5.7 | 53.2 ± 5.9 | −16.5 ± 5.0 |
| L13 | 54.2 ± 7.3 | 55.4 ± 7.5 | −11.9 ± 3.0 |
Values are means ±s.d. except for the sites marked with an asterisk for which the intensity of 2 ×T was not tested. In theses cases, values are linear interpolation from the two closest intensity values.
General observations
As stated in the Introduction, the amplitude of gaze shifts evoked by electrical stimulation of the SC deeper layers depended both on the locus of stimulation along the SC antero-posterior axis and on the stimulation current intensity. This can be seen in Fig. 1 which illustrates the amplitude of gaze shifts evoked from three different collicular sites as a function of stimulation intensity (Cat O). As a consequence of this interaction, gaze shifts of similar amplitude could be evoked from largely separated collicular regions. For example, 20 deg gaze shifts were elicited either by application of a 2 ×T intensity stimulation at a rostral site (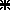 ) or by a 1 × T intensity stimulation applied to a site located ∼0.8 mm more caudally (○). Thus, as schematized on the right side of Fig. 1, the small weight of the rostral SC in gaze amplitude encoding relative to that of more caudal sites (gaze motor map) can be compensated for by the increase of the size of the activated neuronal population. In the rest of this paper, we examine the kinematics and eye–head coordination properties of gaze shifts of comparable amplitude but evoked from separate SC regions.
) or by a 1 × T intensity stimulation applied to a site located ∼0.8 mm more caudally (○). Thus, as schematized on the right side of Fig. 1, the small weight of the rostral SC in gaze amplitude encoding relative to that of more caudal sites (gaze motor map) can be compensated for by the increase of the size of the activated neuronal population. In the rest of this paper, we examine the kinematics and eye–head coordination properties of gaze shifts of comparable amplitude but evoked from separate SC regions.
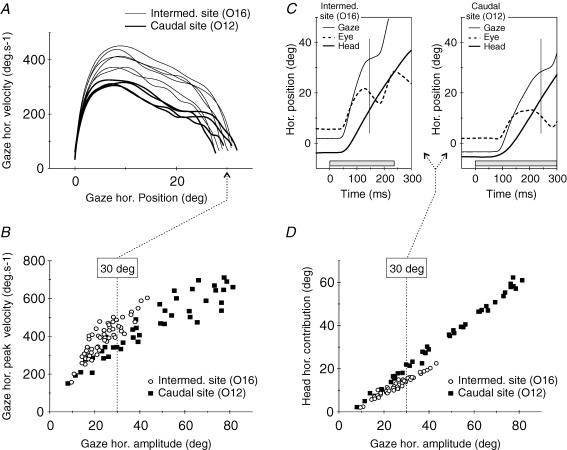
Figure 2 illustrates the data obtained for an intermediate site (O16) and a caudal site (O12) of the SC in cat O. The phase plane plots (Fig. 2A) compare the velocity profiles of individual gaze shifts. Although the amplitude of these two classes of gaze responses is similar (around 30 deg), movements evoked by stimulation of the caudal region (O12) were slower than those evoked from the more rostral site (O16). Figure 2B confirms this difference by showing for each site the horizontal main sequence relationship obtained by pooling together the movements evoked at different current intensities. Although the range of tested intensities was smaller for the caudal site (1 × T to 2 × T) than for the more rostral site (1 × T to 3 × T), the corresponding range of gaze shift amplitudes was larger, denoting a stronger sensitivity to current intensity (see Fig. 1). Note that for comparable amplitudes, gaze horizontal peak velocity was always lower for the caudal site. Panels C and D present the eye–head coordination pattern of the same gaze shifts. The two individual 30 deg gaze shifts plotted in Fig. 2C again illustrate that the gaze shift evoked from the more caudal site was slower and lasted longer than that evoked from the other site. This plot also indicates that the head contribution to the gaze shift (or concurrent head displacement, i.e. head displacement that occurred between the onset and the termination of the saccadic gaze shift) evoked from the more caudal site was much larger, and the eye saccade was accordingly smaller, than for the other site. Figure 2D shows the relationship between head contribution and gaze shift amplitude for all responses elicited from these two sites (same data as in Fig. 2B). The clear separation between the two data sets indicates that gaze shifts evoked by stimulation of the intermediate SC site were composed of a smaller head component than gaze shifts evoked by stimulation of the caudal site.
Figure 2. Comparison of the kinematics and eye–head coordination pattern of gaze shifts evoked by the stimulation of two different collicular sites in cat O.
Mean gaze radial amplitudes at 2 ×T were 31.1 deg and 72.7 deg for sites O16 and O12, respectively. A, velocity versus position phase plane plots of the horizontal component of individuals' gaze shifts. Although their horizontal amplitude was similar (around 30 deg), gaze shifts evoked from sites O16 and O12 using different current intensities differed in their kinematics. B, horizontal main sequence relationship for all gaze shifts evoked from these two collicular sites (all current intensities pooled together: from 1 ×T to 3 ×T and from 1 ×T to 2 ×T for O16 and O12, respectively). Note that, for a similar horizontal amplitude, the horizontal peak velocity was systematically lower for O12 than for O16. C, difference in the eye-head coordination pattern of two individual 30 deg gaze shifts evoked from sites O16 and O12 using different current intensities. Grey boxes show stimulation duration and vertical lines indicate the end of the saccadic gaze shifts. D, relationship between head horizontal contribution (concurrent head displacement) and gaze horizontal amplitude for all gaze shifts evoked from the two collicular sites, indicating a systematically higher head contribution for site O12.
Quantitative analysis
Linear regression analyses were performed to quantitatively describe these peak velocity and head contribution modifications. The general principle of these analyses is first to classify all gaze shifts, i.e. evoked by the stimulation of all SC sites, according to their amplitude into six bins of 5 deg ([10–15[, [15–20[, [20–25[, [25–30[, [30–35[, [35–40[deg, the reversed square bracket ‘[’ indicates that the high limit is excluded from the bin) and then to study, for each bin, the relationship between the parameter of interest (gaze peak velocity, head contribution, eye or head peak velocity, eye–head delay, eye or head latency) and the position of the stimulated site on the SC motor map. Note that a bin thus comprised movements evoked from different SC loci and with different stimulation intensities (e.g. for the [15–20[deg bin, a rostral stimulation at 3 × T and a caudal one at 1 × T).
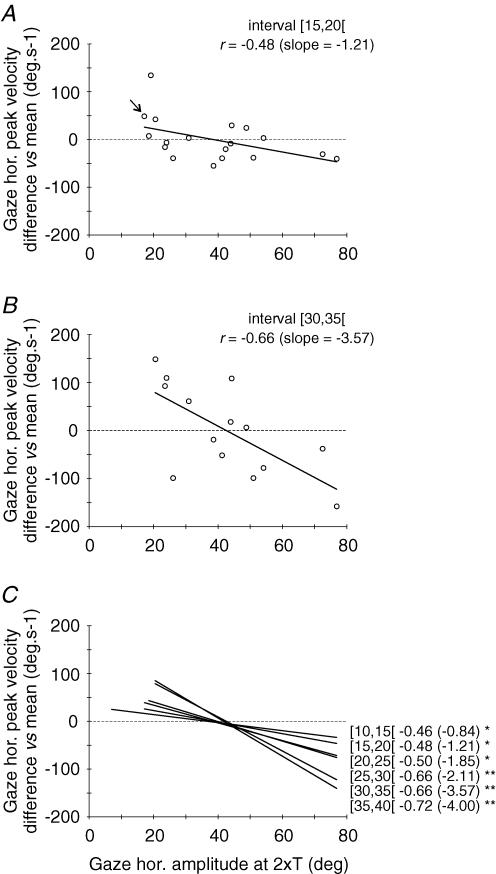
In order to merge data from the two cats, a normalization procedure was first conducted separately for each cat as follows. For each amplitude bin, the mean value of the studied parameter (e.g. gaze peak velocity) across all SC stimulation sites in each cat was calculated. This grand mean was then subtracted from the individual values obtained for each stimulation site. Let us consider for example the [15–20[bin in cat O. For the rostral site O05 (gaze horizontal displacement evoked at 2 × T = 17.2 deg), the mean gaze peak velocity is 335.7 deg s−1. For the same bin and same cat, the grand mean peak velocity calculated across all sites is 287.4 deg s−1 (these grand mean values are reported for both cats in Table 2). Thus the normalization for this [15–20[bin and this SC map position (17.2 deg at 2 × T) consisted of calculating the difference between these two values of gaze peak velocity (335.7–287.4 = 48.3 deg s−1, see Fig. 3A, arrow). All normalized values computed in this way from the two cats were then merged for the subsequent analyses described below.
Table 2.
Results of regression analyses, using site position as predictor, for the differences in all considered parameters with respect to mean values (see text for details)
| Amplitude bins (deg) | Number of sites | Gaze peak velocity | Head contribution | Eye–head delay |
|---|---|---|---|---|
| [10–15[ | 8(O), 12(L) | −0.46 (−0.84)* | 0.57 (+0.05)** | −0.47 (−0.62)** |
| [15–20[ | 8(O), 10(L) | −0.48 (−1.21)* | 0.57 (+0.07)* | −0.44 (−0.45) ns |
| [20–25[ | 8(O), 10(L) | −0.50 (−1.85)* | 0.69 (+0.10)** | −0.53 (−0.51)** |
| [25–30[ | 7(O), 8(L) | −0.66 (−2.11)** | 0.34 (+0.06) ns | −0.44 (−0.34) ns |
| [30–35[ | 6(O), 8(L) | −0.66 (−3.57)** | 0.56 (+0.11)*; | 0.01 (+0.01) ns |
| [35–40[ | 6(O), 8(L) | −0.72 (−4.00)** | 0.55 (+0.13)* | −0.39 (−0.20) ns |
| Amplitude bins (deg) | Eye peak velocity | Head peak velocity | Gaze latency | Head latency |
|---|---|---|---|---|
| [10–15[ | −0.56 (−1.16)* | −0.02 (−0.02) ns | 0.45 (+0.84)* | 0.22 (+0.23) ns |
| [15–20[ | −0.60 (−1.53)** | 0.29 (+0.46) ns | 0.31 (+0.54) ns | 0.07 (+0.08) ns |
| [20–25[ | −0.59 (−1.96)** | −0.11 (−0.20) ns | 0.67 (+0.95)** | 0.59 (+0.43)** |
| [25–30[ | −0.65 (−1.98)** | −0.33 (−0.48) ns | 0.52 (+0.67)* | 0.54 (+0.32)* |
| [30–35[ | −0.71 (−3.53)** | −0.22 (−0.47) ns | 0.60 (+1.31)* | 0.61 (+1.32)* |
| [35–40[ | −0.69 (−3.16)** | −0.40 (−0.99) ns | 0.66 (+0.76)** | 0.75 (+0.54)** |
| Amplitude bins (deg) | Mean gaze peak velocity all sites, Cat O (deg s−1) | Mean gaze peak velocity all sites, Cat L (deg s−1) | Mean head contribution all sites, Cat O (deg) | Mean head contribution all sites, Cat L (deg) |
|---|---|---|---|---|
| [10–15[ | 221.2 | 239.2 | 5.2 | 6.1 |
| [15–20[ | 287.4 | 311.4 | 8.8 | 11.1 |
| [20–25[ | 348.9 | 380.6 | 12.8 | 15.3 |
| [25–30[ | 367.4 | 402.9 | 15.9 | 21.2 |
| [30–35[ | 387.5 | 463.1 | 20.6 | 24.9 |
| [35–40[ | 460.9 | 512.9 | 25.0 | 29.5 |
Values of the top two parts are correlation coefficients r and slopes of the regression line (in parentheses). Statistical significance of analyses is indicated:
P < 0.05
P < 0.01
=P > 0.05
Also indicated (bottom parts) are grand mean values pooled over all SC sites for each cat, of gaze peak velocity and of head contribution for each 5 deg interval of gaze shift amplitude.
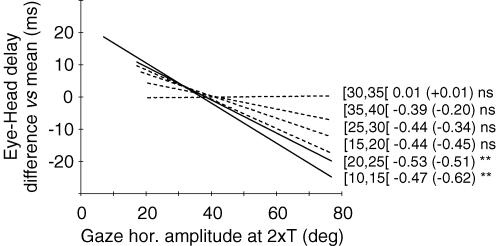
Figure 3. Effect of SC site position on gaze peak velocity for similar-amplitude gaze shifts.
Gaze shifts, pooled over all tested sites, were sorted in six different intervals (5 deg bin width) of horizontal amplitude within a 10–40 deg range. After binning, the difference between the mean value computed for each site and the grand mean computed for the 5 deg interval (across all sites of the corresponding cat) is plotted as a function of site position (expressed as the mean gaze amplitude at 2 ×T). Positive and negative values thus indicate sites for which the stimulation evoked faster or slower gaze shifts than the mean, respectively. See text for a more detailed description of this normalization procedure. Panels A and B show relationships obtained for the [15–20[and the [30–35[amplitude intervals, respectively. The arrow in A shows the site which is taken as an example in the text. C summarizes the data by superimposing the linear regression functions obtained for all six amplitude intervals. Labels near each regression line identify amplitude intervals with correlation coefficients r and slopes (in parentheses). All relationships are statistically significant: *P < 0.05; **P < 0.01 (see also Table 2).
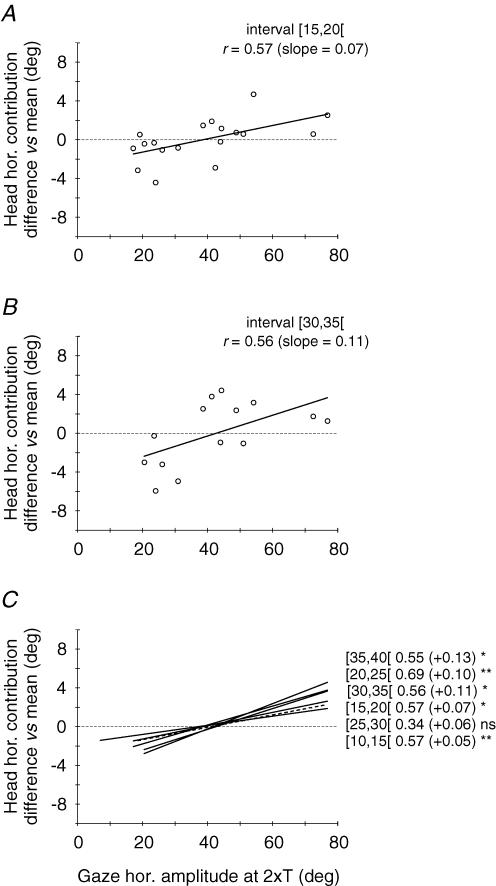
For each of the six amplitude bins, these normalized values obtained from the two cats were plotted as a function of the site position in the SC map (i.e. mean gaze horizontal amplitude at 2 × T, see Methods). Finally, a linear regression analysis was performed on these plots. Figures 3 and 4 show the results for gaze peak velocity and head contribution, respectively. Examples of the relationships obtained for the [15–20[deg and [30–35[deg bins are shown in panels A and B of each figure. The linear regression analysis showed that both gaze peak velocity and head contribution were significantly related to site position. Panels C summarize for peak velocity (Fig. 3) and head contribution (Fig. 4) the regression lines computed for each class of gaze shift amplitude. Overall, amplitude-matched gaze shifts evoked from different SC sites showed a decreasing peak velocity and an increasing head contribution as the stimulation electrode was moved caudally on the SC map. Parameters of each regression analysis are given in Table 2.
Figure 4. Effect of SC site position on eye–head coordination pattern for similar-amplitude gaze shifts.
The difference in head horizontal contribution with respect to the grand mean is plotted as a function of the site position for 5 deg intervals of gaze shift amplitude. Same format as in Fig. 3. Continuous and dashed lines correspond to statistically significant and non-significant relationships, respectively. Statistical significance of analyses is indicated: *P < 0.05; **P < 0.01; ns =P > 0.05 (see also Table 2).
These two opposite relationships relative to the site of SC stimulation predict that gaze peak velocity and head contribution are negatively related. To test this hypothesis, we performed, separately for each cat, a correlation analysis for each 5 deg width amplitude bin. We found a negative correlation between gaze peak velocity and head contribution in four out of six bins for cat L (the two non-significant correlations were observed for [15–20[and [20–25[bins, regression line slopes: 2.68 and 0.03) and in four out of six bins for cat O (the two non-significant correlations were observed for [10–15[and [15–20[bins, regression line slopes: 0.09 and −1.89). Thus, for most gaze amplitudes, a decrease of gaze peak velocity was significantly correlated with an increase of head contribution.
To better understand the effect of site position on gaze peak velocity, we performed the same regression analysis as that reported above, separately for eye peak velocity and head peak velocity. The eye peak velocity versus site position regression was significant for all six amplitude bins, with a mean slope of −2.22 ± 0.93 deg s−1 deg−1. In contrast, none of the head peak velocity regressions were significant, and the mean slope was only −0.28 ± 0.49 deg s−1 deg−1. Thus, as the electrode moved caudally on the SC map, the eye velocity decreased but the head velocity remained nearly constant. This is consistent with the observation noted above of an increased relative head contribution as a function of stimulation distance from the SC rostral pole.
One alternative explanation for the observed increase of head contribution as gaze shifts were evoked from increasingly caudal SC stimulation sites is that the delay between eye and head movement onsets (eye–head delay) varied in a systematic way such that the head would increasingly lead the eyes. Data plotted in Fig. 5 indeed show that in some cases eye–head delay tends to decrease, i.e. head movement onset tends to lead the eyes, when the SC stimulation site is located more caudally. A statistically significant negative relationship between eye–head delay and the antero-posterior position of the stimulated site was found for only two out of six gaze amplitude bins; a similar, although not statistically significant, trend was observed for three other cases, and no tendency was observed for the remaining case. This global, but weak, tendency of eye–head delay to decrease with the SC stimulation site is related to a stronger increase of eye latency as a function of SC stimulation locus (mean slope = 0.85 ± 0.27 ms deg−1) than the corresponding increase of head latency (mean slope = 0.49 ± 0.44 ms deg−1).
Figure 5. Summary of the effect of SC site position on eye–head delay for similar-amplitude gaze shifts.
The difference in eye–head delay with respect to the grand mean is plotted as a function of the site position for 5 deg intervals of gaze shift amplitude. Linear regression functions obtained for all six amplitude intervals are superimposed. Same format as in Fig. 3C. Continuous and dashed lines correspond to statistically significant and non-significant relationships, respectively. Statistical significance of analyses is indicated: **P < 0.01; ns =P > 0.05 (see also Table 2).
Tables 2 and 3 summarize the results presented so far. Statistically significant results (P < 0.05) are indicated (asterisks) for regression analyses using site position as predictor (gaze peak velocity, head contribution, eye–head delay, eye and head peak velocity, eye and head latency) and for correlation analysis (peak velocity versus head contribution). Table 2 shows that regressions for gaze peak velocity were statistically significant for the six amplitude bins. Regressions for head contribution were statistically significant in five cases. These significant modifications of head contribution as a function of site position were accompanied by significant eye–head delay modifications in only two cases.
Table 3.
Results of correlation analyses, performed separately for each cat, between gaze peak velocity and head contribution
| Amplitude bins (deg) | Cat O | Cat L |
|---|---|---|
| [10–15[ | 0.00 (+0.09) ns | −0.27 (−5.97)* |
| [15–20[ | −0.09 (−1.89) ns | 0.11 (2.68) ns |
| [20–25[ | −0.49 (−12.24)*** | 0.00 (0.03) ns |
| [25–30[ | −0.60 (−11.04)*** | −0.44 (−9.35)** |
| [30–35[ | −0.69 (−12.86)** | −0.41 (−8.97)* |
| [35–40[ | −0.57 (−10.03)** | −0.54 (−12.71)*** |
Values are correlation coefficients r and slopes of the regression line (in parentheses). Statistical significance of analyses is indicated:
P < 0.05
P < 0.01
P < 0.001.
=P > 0.05.
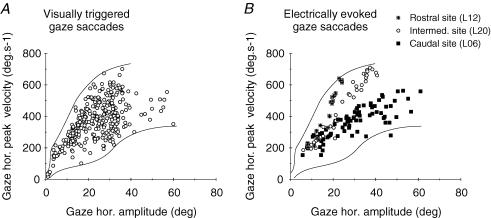
Finally we compared the kinematics (peak velocity) of gaze shifts evoked by electrical stimulation of the SC to that of natural gaze shifts triggered by visual targets. Figure 6 shows for cat L the relationship between peak velocity and amplitude (main sequence) of the horizontal component of visually triggered gaze saccades (Fig. 6A) and of gaze saccades evoked by the stimulation of three different collicular sites (Fig. 6B). For Fig. 6A, only gaze saccades with a direction between −30 and +30 deg have been included to avoid any variation in horizontal component peak velocity due to component stretching. As previously shown, the peak velocity of electrically evoked gaze shifts is higher for a rostral collicular site (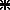 ) than for a caudal site (▪). Values for an intermediate site (○) fall in between. When considered separately for each collicular site, the main sequence relationships of electrically evoked gaze shifts are much less variable than the main sequence of visually triggered gaze shifts. Nevertheless, when taken together, data from these three different sites occupy the whole space delimited by the outlines of the relationship for visually triggered gaze shifts.
) than for a caudal site (▪). Values for an intermediate site (○) fall in between. When considered separately for each collicular site, the main sequence relationships of electrically evoked gaze shifts are much less variable than the main sequence of visually triggered gaze shifts. Nevertheless, when taken together, data from these three different sites occupy the whole space delimited by the outlines of the relationship for visually triggered gaze shifts.
Figure 6. Kinematics of natural gaze shifts triggered by visual stimulation and of gaze shifts evoked by electrical stimulation of the SC.
A, horizontal main sequence relationship for visually triggered gaze shifts in cat L. Outlines of the scatter plot have been made by hand. B, horizontal main sequence relationship for gaze shifts evoked by the electrical stimulation of a rostral (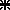 ), an intermediate (○) and a caudal (▪) collicular site (all current intensities pooled together). Outlines of the relationship shown in A have been superimposed. Note the differences in gaze horizontal peak velocity between the three different SC sites and the overlap between the two data sets formed by the electrically evoked and visually triggered gaze shifts. See text for further details.
), an intermediate (○) and a caudal (▪) collicular site (all current intensities pooled together). Outlines of the relationship shown in A have been superimposed. Note the differences in gaze horizontal peak velocity between the three different SC sites and the overlap between the two data sets formed by the electrically evoked and visually triggered gaze shifts. See text for further details.
Discussion
In this study, we compared the kinematics and eye–head coordination of gaze shifts evoked by electrical stimulation of largely separated SC sites. Gaze shifts of similar amplitude were elicited from different SC loci by varying the stimulation current intensity. This approach builds upon our previous demonstration that the locus and the intensity of an electrical stimulation applied to the SC deeper layers are two parameters that interact in the specification of the amplitude of evoked gaze shifts (see Fig. 1 and Guillaume & Pélisson, 2001a). The latter parameter alters the radius of the field of excitation (Yeomans, 1990) and therefore affects the size of the recruited SC neuronal population.
This procedure of varying both stimulation current intensity and site position allowed us to demonstrate that both the speed and eye–head coordination of amplitude-matched gaze shifts depended in a orderly fashion on the stimulated site position in the SC map. Indeed, gaze shifts evoked by the stimulation of an anterior or intermediate site were systematically faster than amplitude-matched gaze shifts evoked by the stimulation of more caudal sites. In addition, the latter were associated with a systematically larger relative head contribution. In other words, gaze shifts evoked by the electrical stimulation of different SC sites are not on the same amplitude/velocity curves (Figs 2B and 6B) and amplitude/head contribution lines (Fig. 2D). Finally, these peak velocity and head contribution modifications were in some cases associated with a change in eye–head temporal coupling (Fig. 5).
To our knowledge, this study is the first to demonstrate that both gaze kinematics and eye–head coordination vary in an orderly fashion with the position of the activated neuronal population in the motor map of the SC. In a previous study in the monkey, Freedman et al. (1996) had already shown that similar-amplitude gaze shifts evoked by stimulation of different sites could have different kinematics (their Figs 8A and B) but, unfortunately, they did not compare the relative contributions of the eye and head to these gaze shifts. Note further that these data cannot be directly compared to ours since Freedman et al. manipulated the amplitude of gaze shifts by varying the duration of stimulation train (hence truncating gaze shifts) rather than its intensity.
In the following, we first evaluate the possible contribution of non-specific factors and comment about the electrical stimulation intensities used in this study, and then propose possible interpretations of the present findings concerning the role of the SC in eye–head coordination.
Contribution of non-specific factors?
It could be suggested that the effects of SC stimulation locus on the kinematic properties and eye–head coordination pattern observed in the present study are not directly related to the collicular encoding of coordinated eye-head gaze shifts, but rather to factors like the position of gaze, or the relative positions of the eye and head, at the beginning of the evoked gaze shift (see Fuller, 1992; Stahl, 1999). However, the contribution of these factors is unlikely in our study because we selected for analysis only gaze shifts initiated from a central horizontal position (±5 deg). As a consequence, the initial gaze position was close to zero on average. In addition, initial horizontal and vertical positions of the eye in the orbit and of the head with respect to the body were also on average near zero. Another issue is whether the initial state of visual fixation when the collicular microstimulation was applied (cats looking at the centre of the opaque screen) may have interfered with the results. This is a reasonable possibility, given that visual fixation can delay the triggering of both visually and electrically elicited gaze shifts and can reduce the amplitude of the latter (Paré et al. 1994). However, as noted by Paré et al. (1994), the effects on electrically elicited gaze shifts are strongest when the stimulation pulse frequency is less than 300 pulse s−1 and are very inconsistent at 300 pulse s−1 (significant effects observed only in one out of the three sites tested at 300 pulse s−1, see their Table 1). Thus the 300 pulse s−1 pulse frequency used in our study is not likely to favour a major effect of visual fixation. In addition, the fixation spot in our study was only an arbitrary reference spatial location where the food target was never presented and thus was not likely to require a level of animal's attention high enough to strongly engage the fixation system (see Methods). Nevertheless we cannot definitively reject any contribution of visual fixation in our study, in particular for gaze shifts evoked with low current intensities in caudal sites. In these cases, by competing with the effect of the electrical stimulation (through lateral inhibitory interactions between different parts of the motor map), the effect of fixation could be seen as equivalent to the effect of reducing the intensity of electrical stimulation. The use of low current intensities is addressed in the next paragraph.
Issues related to SC stimulation intensity
To obtain similar amplitude-gaze shifts from the stimulation of different collicular zones, several current intensities have been tested for each site. Particularly, low current intensities have been used to obtain small-amplitude gaze saccades from SC caudal zones. The fact that these gaze saccades were slower than matched-amplitude ones evoked by the stimulation of more rostral zones certainly results in part from such current intensity variations. However, this is not the only explanation, because this velocity reduction did not correspond to a global slowing of both eye and head components, as would be predicted by a pure current intensity effect, but rather, resulted from a specific reduction of the eye velocity only (see Table 2). Thus for similar-amplitude gaze saccades, this selective reduction of eye, but not head, velocity resulted both in a reduction of the gaze velocity and in an increase of the relative head contribution. We propose in the next section two possible, non-mutually exclusive, neural mechanisms that could account for this specific pattern of results.
Another issue related to the deliberate use of different current intensities between SC sites is that, particularly for caudal sites, the neural activation levels may not have reached the hypothesized ‘natural’ level of activation. Concerning this point, several comments upon current intensities can be made. First, only movements obtained with an intensity equal to or larger than the behaviourally defined threshold (T) are included in the analysis. Note that even for caudal sites, such intensities were already high and values higher than 2 × T could not be used because the amplitude of evoked gaze shifts already reached 60–80 deg (see Guillaume & Pélisson, 2001a). Second, Fig. 6 shows that the peak velocity of electrically evoked gaze shifts was in the same range as natural gaze shifts. Third, as shown in Fig. 6B, even when using relatively high current intensities (1.5 × T up to 2 × T) to elicit 30–40 deg gaze shifts from the caudal site (L6), gaze peak velocities still do not reach the values obtained from the more rostral site (L20). This last observation suggests that the site dependency of gaze velocity found in our study is not simply related to a problem of too weak activation.
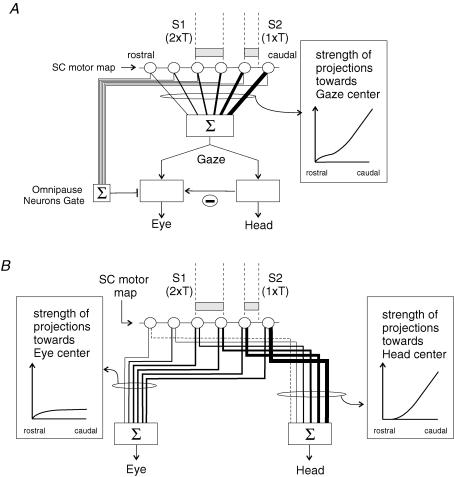
Two possible explanations
In most models, the decomposition of a desired gaze displacement signal into eye and head motor commands is supposed to take place in neural centres located downstream from the SC (Galiana & Guitton, 1992; Goossens & Van Opstal, 1997; Freedman, 2001). At first sight, to the extent that eye and head initial positions do not vary as in the present study, these models predict that gaze shifts of a given amplitude but evoked from separate SC loci would have similar kinematics and eye–head coordination. Indeed, because the decomposition occurs downstream of the SC, a constant pattern of eye–head coordination is expected as long as the SC stimulation yields the same ‘desired gaze displacement’ signal, regardless of the locus of stimulation in the motor map. However, this prediction may not be valid if, by way of the omnipause neurons, eye and head premotor centres are temporally gated independently (Goossens & Van Opstal, 1997; Freedman, 2001; Corneil et al. 2002b). In this case, both the temporal coupling between eye and head (eye–head delay) and head contribution could vary as a function of SC stimulation site. Along this line, we propose a first possible explanation of our findings (Fig. 7A), based on the proposal of two separate initiation mechanisms for eye and head displacements (Goossens & Van Opstal, 1997; Corneil et al. 2002b). We found that the modification of eye–head coordination pattern when more caudal SC sites were stimulated was sometimes associated with a significant decrease in eye–head delay (i.e. an increased head lead), resulting from a significant increase in the latency of the eye component relative to stimulation onset. This early (relative to the eye) head initiation is reminiscent of ‘early head movements’ that we could elicit in a previous work by a visual target presentation or by a low-intensity electrical stimulation of the SC (Pélisson et al. 2001). When a low-intensity stimulation is applied in the caudal SC, the low neuronal activation would require more time to open the eye gate (omnipause neurons) as compared to when a stronger stimulation is applied more rostrally (irrespective of the fact that these two stimulations give the same gaze shift amplitude). In contrast, the gaze command would drive the head pathway with a nearly constant delay, unrelated to the SC stimulation site and intensity. Thus, for caudal stimulation the head velocity reached at the time of eye initiation would be higher than for more rostral stimulation. This would ‘mechanically’ result in a larger contribution of the head relative to that of the eye for these gaze shifts, as compared to matched-amplitude gaze shifts resulting from the same desired gaze displacement signal but evoked from more rostral sites. Nevertheless, this does not explain the decrease of eye peak velocity as more caudal sites are considered. Thus, to account for this observation, we propose that this direct effect of head lead on head contribution can be reinforced by different interactions between head and eye velocity signals taking place downstream from the SC. First, although the vestibulo-ocular reflex (VOR) gain has been shown to be reduced during saccadic gaze shifts, it is still debated whether or not the VOR is completely switched off during saccades (Roucoux et al. 1980; Tomlinson & Bahra, 1986b; Laurutis & Robinson, 1986; Guitton & Volle, 1987; Lefèvre et al. 1992; Tabak et al. 1996; Pélisson et al. 1988; Roy & Cullen, 1998, 2002; Cullen et al. 2004). Thus, it cannot yet be excluded that some residual VOR reduces the eye movement component and hence participates in the observed increase of head contribution. Second, the head command could partly inhibit the eye command through a direct neural interaction between premotor pathways, as proposed by Freedman (2001). These two indirect effects could both contribute to the observed negative relationship between eye peak velocity and distance of the stimulated site from the rostral SC pole.
Figure 7. Two different schemes proposed to account for the current findings.
Open circles symbolize the collicular output neurons forming a unidimensional gaze motor map. Lines of different thickness leaving the collicular map represent projections of different strength. The two electrical stimulations (S1: 2 ×T and S2: 1 ×T) applied at two SC loci (grey boxes correspond to the postulated size of collicular activations induced by the electrical stimulation) are assumed to elicit gaze shifts of the same amplitude (e.g. 30 deg). A, this scheme (i) assumes that desired gaze displacement (Gaze), resulting from the weighted sum of collicular activity (according to gradient of projection strength shown on the right), is decomposed into eye and head components (Eye, Head) in a downstream structure from the SC; (ii) incorporates the gating mechanism of the oculomotor system (omnipause neurons, Gate) and a neural inhibition of the eye pathway by the head pathway (−). B, this scheme postulates that the transformation of the ‘desired gaze displacement’ expressed by SC neurons is achieved through diverging projections of collicular neurons to eye and head premotor centres. The specific gradients of the strength of these connections (boxes on each side of the scheme) determine the collicular locus-dependent pattern of eye–head coordination observed experimentally. See text for further details.
This first explanation is however, not able to account for all of our data. Indeed, for some amplitude bins, even if the variation of head contribution as a function of SC site location reached a statistically significant level, the modification of eye-head delay did not (3 bins) or was even completely absent (one bin, slope = 0.01). Thus, for these cases, an increasing head lead could not be invoked to explain the increased head contribution. In addition, even when significant, it is difficult to predict quantitatively whether the outcomes of such modifications of eye–head delay are compatible with the observed changes of head coordination.
We therefore propose a second possible explanation of our results (Fig. 7B), which is based on a specific organization of collicular projections to eye and head centres. Note that this second explanation and the first one are not mutually exclusive. Rather this specific organization of collicular projections could come into play on the top of the separate initiation mechanisms for eye and head movements and of the potential interactions between eye and head centres. Nevertheless, for the sake of simplicity, Fig. 7B presents only the main principle of the scheme, i.e. the specific organization of collicular projections. Anatomical studies have shown that SC neurons could contact the premotor structures involved in eye and head movements, either directly through two axonal branches or indirectly through reticular relay neurons which themselves contact both eye and head premotor structures (in the cat: Grantyn & Grantyn, 1982; Grantyn et al. 1987; Grantyn & Berthoz, 1987; see Isa & Sasaki, 2002 for a review; in the monkey: Cowie et al. 1994; Cowie & Robinson, 1994; Robinson et al. 1994; Scudder et al. 1996a, b; Corneil et al. 2002a, b). The site dependency of eye–head coordination observed in the present study suggests that these collicular (direct and indirect) projections obey different rostro-caudal gradients of connection strength for the eye and the head premotor centres. Concerning the projections to oculomotor centres, the most anterior part of the SC map would be characterized by a steeply increasing strength when moving caudally. This could be due to an increase in the number of efferent neurons (Olivier et al. 1991; Grantyn et al. 2002) and/or of synaptic buttons carried by each neuron (Moschovakis et al. 1998). For the rest (caudalmost two-thirds or so) of the SC map, the projections strength would remain constant, in agreement with experimental data regarding the density of output neurons (Olivier et al. 1991; Grantyn et al. 2002) (note that the number of synaptic buttons and the weight of synapses have not been adequately tested in this part of the SC). Note also that this postulated rostro-caudal pattern of connection strength could contribute to the saturation of desired eye displacement proposed in earlier models (Guitton & Volle, 1987; Guitton et al. 1990; Phillips et al. 1995; Goossens & Van Opstal, 1997). Concerning now the collicular coding of head movement, the most anterior part of the map would be very little concerned with head movement and projections to the cephalomotor centres would originate mainly from intermediate and caudal sites of the SC map with a steep gradient of synaptic strength (see recent electrophysiological evidence by Corneil et al. 2002a, b). Hence, the stimulation of a rostral site would lead to a gaze shift with a negligible head contribution because it would activate a neuronal SC population projecting nearly exclusively to the eye premotor centre. Stimulation of an intermediate site would activate a neuronal population that has equal access to eye and head premotor centres, leading to a gaze shift with balanced eye and head contributions. Note that less current will be required to evoke a gaze shift of the same amplitude as that evoked from more rostral sites, because the mean connection strength of SC output neurons has increased from the rostral to the intermediate site. For the same reason, stimulation of a caudal site will require an even lower current level, to yield the same-amplitude gaze shift, recruiting thus even fewer neurons. Importantly however, because the differential connection strength of SC output neurons to eye and head centres differs strongly from that in the rostral and intermediate sites, the eye–head coordination pattern will again markedly differ. Namely, by recruiting fewer caudal output neurons, each of which having a similar effect on preoculomotor centres as intermediate output neurons, the smaller current intensity would result in a reduced eye drive relative to that for more rostrally elicited gaze shifts, in agreement with the observed negative regression between eye peak velocity and site location. For the head contribution instead, the reduced number of activated neurons in the caudal SC would be compensated for by the marked increase of their projection strength to head motor centres, leading to a globally constant head drive, in agreement with the absence of significant regression between head peak velocity and site location. To recapitulate, considering gaze shifts of constant amplitude, the decreased eye drive and the constant head drive when moving caudally on the SC motor map combine to explain the observed decrease of gaze peak velocity and increase of relative head contribution.
Conclusion
The present study demonstrates that the sharing between eye and head components of gaze shifts evoked by SC electrical stimulation depends in an orderly fashion on the stimulation site in the SC motor map. Indeed the test of several current intensities for each SC site allows us to evoke a large range of gaze shift amplitude. Gaze amplitude/velocity and gaze amplitude/head contribution relationships were not the same for the different collicular sites. A first interpretation of these findings, based on separate eye and head movement initiation mechanisms, is compatible with models postulating that gaze decomposition into eye and head components occurs downstream from the SC. However, to account for the observed cases in which head contribution was modified without any eye–head delay change, we proposed another explanation based on specific patterns of SC projections to eye- and head-related centres. Enriched with separate initiation mechanisms for eye and head components, this more general interpretation can account for the whole data set of the present study. These findings make it possible to approach mechanisms used by the central nervous system to translate a high-level motor representation that is effector independent in control signals appropriate for the involved body segments. They illustrate how brainstem mechanisms might translate a desired saccadic gaze displacement in commands for the generation of coordinated eye and head displacements.
Acknowledgments
We acknowledge Marie-Line Loyalle for taking care of the animals. This research was supported by Institut National de la Santé et de la Recherche Médicale U534. Alain Guillaume was supported by a fellowship from the Fondation pour la Recherche Médicale.
References
- Barnes GR. Head-eye coordination in normals and in patients with vestibular disorders. Adv Otorhinolaryngol. 1979;25:197–201. doi: 10.1159/000402942. [DOI] [PubMed] [Google Scholar]
- Ceylan M, Henriques DY, Tweed DB, Crawford JD. Task-dependent constraints in motor control: pinhole goggles make the head move like an eye. J Neurosci. 2000;20:2719–2730. doi: 10.1523/JNEUROSCI.20-07-02719.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantin AG, Wang H, Crawford JD. Role of superior colliculus in adaptive eye-head coordination during gaze shifts. J Neurophysiol. 2004;92:2168–2184. doi: 10.1152/jn.00103.2004. [DOI] [PubMed] [Google Scholar]
- Corneil BD, Hing CA, Bautista DV, Munoz DP. Human eye-head gaze shifts in a distractor task. I. Truncated gaze shifts. J Neurophysiol. 1999;82:1390–1405. doi: 10.1152/jn.1999.82.3.1390. [DOI] [PubMed] [Google Scholar]
- Corneil BD, Olivier E, Munoz DP. Neck muscle responses to stimulation of monkey superior colliculus. I. Topography and manipulation of stimulation parameters. J Neurophysiol. 2002a;88:1980–1999. doi: 10.1152/jn.2002.88.4.1980. [DOI] [PubMed] [Google Scholar]
- Corneil BD, Olivier E, Munoz DP. Neck muscle responses to stimulation of monkey superior colliculus. II. Gaze shift initiation and volitional head movements. J Neurophysiol. 2002b;88:2000–2018. doi: 10.1152/jn.2002.88.4.2000. [DOI] [PubMed] [Google Scholar]
- Cowie RJ, Robinson DL. Subcortical contributions to head movements in macaques. I. Contrasting effects of electrical stimulation of a medial pontomedullary region and the superior colliculus. J Neurophysiol. 1994;72:2648–2664. doi: 10.1152/jn.1994.72.6.2648. [DOI] [PubMed] [Google Scholar]
- Cowie RJ, Smith MK, Robinson DL. Subcortical contributions to head movements in macaques. II. Connections of a medial pontomedullary head-movement region. J Neurophysiol. 1994;72:2665–2682. doi: 10.1152/jn.1994.72.6.2665. [DOI] [PubMed] [Google Scholar]
- Crawford JD, Guitton D. Primate head-free saccade generator implements a desired (post-VOR) eye position command by anticipating intended head motion. J Neurophysiol. 1997;78:2811–2816. doi: 10.1152/jn.1997.78.5.2811. [DOI] [PubMed] [Google Scholar]
- Cullen KE, Huterer M, Braidwood DA, Sylvestre PA. Time course of vestibuloocular reflex suppression during gaze shifts. J Neurophysiol. 2004;92:3408–3422. doi: 10.1152/jn.01156.2003. [DOI] [PubMed] [Google Scholar]
- du Lac S, Knudsen EI. Neural maps of head movement vector and speed in the optic tectum of the barn owl. J Neurophysiol. 1990;63:131–146. doi: 10.1152/jn.1990.63.1.131. [DOI] [PubMed] [Google Scholar]
- Freedman EG. Interactions between eye and head control signals can account for movement kinematics. Biol Cybern. 2001;84:453–462. doi: 10.1007/PL00007989. [DOI] [PubMed] [Google Scholar]
- Freedman EG, Sparks DL. Activity of cells in the deeper layers of the superior colliculus of the rhesus monkey: evidence for a gaze displacement command. J Neurophysiol. 1997a;78:1669–1690. doi: 10.1152/jn.1997.78.3.1669. [DOI] [PubMed] [Google Scholar]
- Freedman EG, Sparks DL. Eye-head coordination during head-unrestrained gaze shifts in rhesus monkeys. J Neurophysiol. 1997b;77:2328–2348. doi: 10.1152/jn.1997.77.5.2328. [DOI] [PubMed] [Google Scholar]
- Freedman EG, Sparks DL. Coordination of the eyes and head: movement kinematics. Exp Brain Res. 2000;131:22–32. doi: 10.1007/s002219900296. [DOI] [PubMed] [Google Scholar]
- Freedman EG, Stanford TR, Sparks DL. Combined eye-head gaze shifts produced by electrical stimulation of the superior colliculus in rhesus monkeys. J Neurophysiol. 1996;76:927–952. doi: 10.1152/jn.1996.76.2.927. [DOI] [PubMed] [Google Scholar]
- Fuller JH. Head movement propensity. Exp Brain Res. 1992;92:152–164. doi: 10.1007/BF00230391. [DOI] [PubMed] [Google Scholar]
- Fuller JH. Eye position and target amplitude effects on human visual saccadic latencies. Exp Brain Res. 1996;109:457–466. doi: 10.1007/BF00229630. [DOI] [PubMed] [Google Scholar]
- Galiana HL, Guitton D. Central organization and modeling of eye-head coordination during orienting gaze shifts. Ann N Y Acad Sci. 1992;656:452–471. doi: 10.1111/j.1749-6632.1992.tb25228.x. [DOI] [PubMed] [Google Scholar]
- Gandhi NJ, Sparks DL. Experimental control of eye and head positions prior to head-unrestrained gaze shifts in monkey. Vision Res. 2001;41:3243–3254. doi: 10.1016/s0042-6989(01)00054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffart L, Pélisson D. Orienting gaze shifts during muscimol inactivation of caudal fastigial nucleus in the cat. I. Gaze dysmetria. J Neurophysiol. 1998;79:1942–1958. doi: 10.1152/jn.1998.79.4.1942. [DOI] [PubMed] [Google Scholar]
- Goffart L, Pélisson D, Guillaume A. Orienting gaze shifts during muscimol inactivation of caudal fastigial nucleus in the cat. II. Dynamics and eye-head coupling. J Neurophysiol. 1998;79:1959–1976. doi: 10.1152/jn.1998.79.4.1959. [DOI] [PubMed] [Google Scholar]
- Goldring JE, Dorris MC, Corneil BD, Ballantyne PA, Munoz DP. Combined eye-head gaze shifts to visual and auditory targets in humans. Exp Brain Res. 1996;111:68–78. doi: 10.1007/BF00229557. [DOI] [PubMed] [Google Scholar]
- Goossens HH, Van Opstal AJ. Human eye-head coordination in two dimensions under different sensorimotor conditions. Exp Brain Res. 1997;114:542–560. doi: 10.1007/pl00005663. [DOI] [PubMed] [Google Scholar]
- Grantyn A, Berthoz A. Reticulo-spinal neurons participating in the control of synergic eye and head movements during orienting in the cat. I. Behavioral properties. Exp Brain Res. 1987;66:339–354. doi: 10.1007/BF00243309. [DOI] [PubMed] [Google Scholar]
- Grantyn A, Brandi AM, Dubayle D, Graf W, Ugolini G, Hadjidimitrakis K, Moschovakis A. Density gradients of trans-synaptically labeled collicular neurons after injections of rabies virus in the lateral rectus muscle of the rhesus monkey. J Comp Neurol. 2002;451:346–361. doi: 10.1002/cne.10353. [DOI] [PubMed] [Google Scholar]
- Grantyn A, Grantyn R. Axonal patterns and sites of termination of cat superior colliculus neurons projecting in the tecto-bulbo-spinal tract. Exp Brain Res. 1982;46:243–256. doi: 10.1007/BF00237182. [DOI] [PubMed] [Google Scholar]
- Grantyn A, Ong-Meang Jacques V, Berthoz A. Reticulo-spinal neurons participating in the control of synergic eye and head movements during orienting in the cat. II. Morphological properties as revealed by intra-axonal injections of horseradish peroxidase. Exp Brain Res. 1987;66:355–377. doi: 10.1007/BF00243310. [DOI] [PubMed] [Google Scholar]
- Guillaume A, Pélisson D. Gaze shifts evoked by electrical stimulation of the superior colliculus in the head-unrestrained cat. I. Effect of the locus and of the parameters of stimulation. Eur J Neurosci. 2001a;14:1331–1344. doi: 10.1046/j.0953-816x.2001.01744.x. [DOI] [PubMed] [Google Scholar]
- Guillaume A, Pélisson D. Gaze shifts evoked by electrical stimulation of the superior colliculus in the head-unrestrained cat. II. Effect of muscimol inactivation of the caudal fastigial nucleus. Eur J Neurosci. 2001b;14:1345–1359. doi: 10.1046/j.0953-816x.2001.01739.x. [DOI] [PubMed] [Google Scholar]
- Guitton D, Douglas RM, Volle M. Eye-head coordination in cats. J Neurophysiol. 1984;52:1030–1050. doi: 10.1152/jn.1984.52.6.1030. [DOI] [PubMed] [Google Scholar]
- Guitton D, Munoz DP, Galiana HL. Gaze control in the cat: studies and modeling of the coupling between orienting eye and head movements in different behavioral tasks. J Neurophysiol. 1990;64:509–531. doi: 10.1152/jn.1990.64.2.509. [DOI] [PubMed] [Google Scholar]
- Guitton D, Volle M. Gaze control in humans: eye-head coordination during orienting movements to targets within and beyond the oculomotor range. J Neurophysiol. 1987;58:427–459. doi: 10.1152/jn.1987.58.3.427. [DOI] [PubMed] [Google Scholar]
- Herrero L, Rodriguez F, Salas C, Torres B. Tail and eye movements evoked by electrical microstimulation of the optic tectum in goldfish. Exp Brain Res. 1998;120:291–305. doi: 10.1007/s002210050403. [DOI] [PubMed] [Google Scholar]
- Isa T, Sasaki S. Brainstem control of head movements during orienting; organization of the premotor circuits. Prog Neurobiol. 2002;66:205–241. doi: 10.1016/s0301-0082(02)00006-0. [DOI] [PubMed] [Google Scholar]
- Kang I, Lee C. Properties of saccade-related neurons in the cat superior colliculus: patterns of movement fields and discharge timing. Exp Brain Res. 2000;131:149–164. doi: 10.1007/s002219900265. [DOI] [PubMed] [Google Scholar]
- Klier EM, Wang H, Crawford JD. The superior colliculus encodes gaze commands in retinal coordinates. Nat Neurosci. 2001;4:627–632. doi: 10.1038/88450. [DOI] [PubMed] [Google Scholar]
- Laurutis VP, Robinson DA. The vestibulo-ocular reflex during human saccadic eye movements. J Physiol. 1986;373:209–233. doi: 10.1113/jphysiol.1986.sp016043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefèvre P, Bottemanne I, Roucoux A. Experimental study and modeling of vestibulo-ocular reflex modulation during large shifts of gaze in humans. Exp Brain Res. 1992;91:496–508. doi: 10.1007/BF00227846. [DOI] [PubMed] [Google Scholar]
- McIlwain JT. Lateral spread of neural excitation during microstimulation in intermediate gray layer of cat's superior colliculus. J Neurophysiol. 1982;47:167–178. doi: 10.1152/jn.1982.47.2.167. [DOI] [PubMed] [Google Scholar]
- Misslisch H, Tweed D, Vilis T. Neural constraints on eye motion in human eye-head saccades. J Neurophysiol. 1998;79:859–869. doi: 10.1152/jn.1998.79.2.859. [DOI] [PubMed] [Google Scholar]
- Moschovakis AK, Dalezios Y, Petit J, Grantyn A. New mechanism that accounts for position sensitivity of saccades evoked in response to stimulation of superior colliculus. J Neurophysiol. 1998;80:3373–3379. doi: 10.1152/jn.1998.80.6.3373. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Guitton D, Pelisson D. Control of orienting gaze shifts by the tectoreticulospinal system in the head-free cat. III. Spatiotemporal characteristics of phasic motor discharges. J Neurophysiol. 1991;66:1642–1666. doi: 10.1152/jn.1991.66.5.1642. [DOI] [PubMed] [Google Scholar]
- Olivier E, Chat M, Grantyn A. Rostrocaudal and lateromedial density distributions of superior colliculus neurons projecting in the predorsal bundle and to the spinal cord: a retrograde HRP study in the cat. Exp Brain Res. 1991;87:268–282. doi: 10.1007/BF00231844. [DOI] [PubMed] [Google Scholar]
- Oommen BS, Smith RM, Stahl JS. The influence of future gaze orientation upon eye-head coupling during saccades. Exp Brain Res. 2004;155:9–18. doi: 10.1007/s00221-003-1694-z. [DOI] [PubMed] [Google Scholar]
- Ottes FP, Van Gisbergen JA, Eggermont JJ. Visuomotor fields of the superior colliculus: a quantitative model. Vision Res. 1986;26:857–873. doi: 10.1016/0042-6989(86)90144-6. [DOI] [PubMed] [Google Scholar]
- Paré M, Crommelinck M, Guitton D. Gaze shifts evoked by stimulation of the superior colliculus in the head-free cat conform to the motor map but also depend on stimulus strength and fixation activity. Exp Brain Res. 1994;101:123–139. doi: 10.1007/BF00243222. [DOI] [PubMed] [Google Scholar]
- Pélisson D, Goffart L, Guillaume A, Catz N, Raboyeau G. Early head movements elicited by visual stimuli or collicular electrical stimulation in the cat. Vision Res. 2001;41:3283–3294. doi: 10.1016/s0042-6989(01)00224-3. [DOI] [PubMed] [Google Scholar]
- Pélisson D, Guillaume A. Differences in speed and head contribution for amplitude-matched gaze shifts evoked from different superior colliculus loci in the cat.16th Neural Control of Movement meeting, 2–7 May, Key Biscane (Florida) [Google Scholar]
- Pélisson D, Prablanc C, Urquizar C. Vestibuloocular reflex inhibition and gaze saccade control characteristics during eye-head orientation in humans. J Neurophysiol. 1988;59:997–1013. doi: 10.1152/jn.1988.59.3.997. [DOI] [PubMed] [Google Scholar]
- Phillips JO, Ling L, Fuchs AF, Siebold C, Plorde JJ. Rapid horizontal gaze movement in the monkey. J Neurophysiol. 1995;73:1632–1652. doi: 10.1152/jn.1995.73.4.1632. [DOI] [PubMed] [Google Scholar]
- Robinson DA. A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans Biomed Eng. 1963;10:137–145. doi: 10.1109/tbmel.1963.4322822. [DOI] [PubMed] [Google Scholar]
- Robinson DA. Eye movements evoked by collicular stimulation in the alert monkey. Vision Res. 1972;12:1795–1808. doi: 10.1016/0042-6989(72)90070-3. [DOI] [PubMed] [Google Scholar]
- Robinson FR, Phillips JO, Fuchs AF. Coordination of gaze shifts in primates: brainstem inputs to neck and extraocular motoneuron pools. J Comp Neurol. 1994;346:43–62. doi: 10.1002/cne.903460104. [DOI] [PubMed] [Google Scholar]
- Roucoux A, Guitton D, Crommelinck M. Stimulation of the superior colliculus in the alert cat. II. Eye and head movements evoked when the head is unrestrained. Exp Brain Res. 1980;39:75–85. doi: 10.1007/BF00237071. [DOI] [PubMed] [Google Scholar]
- Roy JE, Cullen KE. A neural correlate for vestibuloocular reflex suppression during voluntary eye-head gaze shifts. Nat Neurosci. 1998;1:404–410. doi: 10.1038/1619. [DOI] [PubMed] [Google Scholar]
- Roy JE, Cullen KE. Vestibuloocular reflex signal modulation during voluntary and passive head movements. J Neurophysiol. 2002;87:2337–2357. doi: 10.1152/jn.2002.87.5.2337. [DOI] [PubMed] [Google Scholar]
- Salas C, Herrero L, Rodriguez F, Torres B. Tectal codification of eye movements in goldfish studied by electrical microstimulation. Neuroscience. 1997;78:271–288. doi: 10.1016/s0306-4522(97)83048-5. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Stryker M. Single-unit recording and stimulation in superior colliculus of the alert rhesus monkey. J Neurophysiol. 1972;35:915–924. doi: 10.1152/jn.1972.35.6.915. [DOI] [PubMed] [Google Scholar]
- Scudder CA, Moschovakis AK, Karabelas AB, Highstein SM. Anatomy and physiology of saccadic long-lead burst neurons recorded in the alert squirrel monkey. I. Descending projections from the mesencephalon. J Neurophysiol. 1996a;76:332–352. doi: 10.1152/jn.1996.76.1.332. [DOI] [PubMed] [Google Scholar]
- Scudder CA, Moschovakis AK, Karabelas AB, Highstein SM. Anatomy and physiology of saccadic long-lead burst neurons recorded in the alert squirrel monkey. II. Pontine neurons. J Neurophysiol. 1996b;76:353–370. doi: 10.1152/jn.1996.76.1.353. [DOI] [PubMed] [Google Scholar]
- Sparks DL. Conceptual issues related to the role of the superior colliculus in the control of gaze. Curr Opin Neurobiol. 1999;9:698–707. doi: 10.1016/s0959-4388(99)00039-2. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Gandhi NJ. Single cell signals: an oculomotor perspective. Prog Brain Res. 2003;142:35–53. doi: 10.1016/S0079-6123(03)42005-0. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Mays LE. Spatial localization of saccade targets. I. Compensation for stimulation-induced perturbations in eye position. J Neurophysiol. 1983;49:45–63. doi: 10.1152/jn.1983.49.1.45. [DOI] [PubMed] [Google Scholar]
- Stahl JS. Amplitude of human head movements associated with horizontal saccades. Exp Brain Res. 1999;126:41–54. doi: 10.1007/s002210050715. [DOI] [PubMed] [Google Scholar]
- Stahl JS. Adaptive plasticity of head movement propensity. Exp Brain Res. 2001;139:201–208. doi: 10.1007/s002210100749. [DOI] [PubMed] [Google Scholar]
- Stanford TR, Freedman EG, Sparks DL. Site and parameters of microstimulation: evidence for independent effects on the properties of saccades evoked from the primate superior colliculus. J Neurophysiol. 1996;76:3360–3381. doi: 10.1152/jn.1996.76.5.3360. [DOI] [PubMed] [Google Scholar]
- Straschill M, Rieger P. Eye movements evoked by focal stimulation of the cat's superior colliculus. Brain Res. 1973;59:211–227. doi: 10.1016/0006-8993(73)90262-x. [DOI] [PubMed] [Google Scholar]
- Tabak S, Smeets JB, Collewijn H. Modulation of the human vestibuloocular reflex during saccades: probing by high-frequency oscillation and torque pulses of the head. J Neurophysiol. 1996;76:3249–3263. doi: 10.1152/jn.1996.76.5.3249. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ. Electrical stimulation of neural tissue to evoke behavioral responses. J Neurosci Meth. 1996;65:1–17. doi: 10.1016/0165-0270(95)00131-x. [DOI] [PubMed] [Google Scholar]
- Tomlinson RD, Bahra PS. Combined eye-head gaze shifts in the primate. I. Metrics. J Neurophysiol. 1986a;56:1542–1557. doi: 10.1152/jn.1986.56.6.1542. [DOI] [PubMed] [Google Scholar]
- Tomlinson RD, Bahra PS. Combined eye-head gaze shifts in the primate. II. Interactions between saccades and the vestibuloocular reflex. J Neurophysiol. 1986b;56:1558–1570. doi: 10.1152/jn.1986.56.6.1558. [DOI] [PubMed] [Google Scholar]
- Van Opstal AJ, Van Gisbergen JA, Smit AC. Comparison of saccades evoked by visual stimulation and collicular electrical stimulation in the alert monkey. Exp Brain Res. 1990;79:299–312. doi: 10.1007/BF00608239. [DOI] [PubMed] [Google Scholar]
- Volle M, Guitton D. Human gaze shifts in which head and eyes are not initially aligned. Exp Brain Res. 1993;94:463–470. doi: 10.1007/BF00230204. [DOI] [PubMed] [Google Scholar]
- Yeomans JS. Principles of Brain Stimulation. New York: Oxford University Press; 1990. [Google Scholar]
- Zangemeister WH, Stark L. Types of gaze movement: variable interactions of eye and head movements. Exp Neurol. 1982;77:563–577. doi: 10.1016/0014-4886(82)90228-x. [DOI] [PubMed] [Google Scholar]