Abstract
Aberrant GABAergic inhibition in spinal dorsal horn may underlie some forms of neuropathic pain. Potential, but yet unexplored, mechanisms include reduced excitability, abnormal discharge patterns or altered synaptic input of spinal GABAergic neurons. To test these hypotheses, we quantitatively compared active and passive membrane properties, firing patterns in response to depolarizing current steps and synaptic input of GABAergic neurons in spinal dorsal horn lamina II of neuropathic and of control animals. Transgenic mice were used which expressed enhanced green fluorescent protein (EGFP) controlled by the GAD67 promoter, thereby labelling one-third of all spinal GABAergic neurons. In all neuropathic mice included in this study, chronic constriction injury of one sciatic nerve led to tactile allodynia and thermal hyperalgesia. Control mice were sham-operated. Membrane excitability of GABAergic neurons from neuropathic or sham-treated animals was indistinguishable. The most frequent firing patterns observed in neuropathic and sham-operated animals were the initial burst (neuropathic: 46%, sham-treated: 42%), the gap (neuropathic: 31%, sham-treated: 29%) and the tonic firing pattern (neuropathic: 16%, sham-treated: 24%). The synaptic input from dorsal root afferents was similar in neuropathic and in control animals. Thus, a reduced membrane excitability, altered firing patterns or changes in synaptic input of this group of GABAergic neurons in lamina II of the spinal cord dorsal horn are unlikely causes for neuropathic pain.
Injury of sensory nerves may cause severe forms of hyperalgesia (enhanced responsiveness to noxious stimuli) and allodynia (pain evoked by normally non painful stimuli), two characteristic features of neuropathic pain.
Several lines of evidence support the idea that impaired inhibition of sensory information in spinal dorsal horn contributes to neuropathic pain. γ-Aminobutyric acid (GABA) is the main inhibitory neurotransmitter in the nervous system, including lamina II of spinal dorsal horn, where nociceptive nerve fibres terminate. After peripheral nerve injury, there is a loss of GABAA receptor-mediated postsynaptic inhibition in the superficial dorsal horn (Moore et al. 2002). Intrathecal application of the GABAA receptor antagonist bicuculline produces behavioural signs of tactile allodynia in the rat (Yaksh, 1989; Malan et al. 2002) and enhanced reflex activity (Sivilotti & Woolf, 1994).
After nerve injury GABA immunoreactivity is decreased in the spinal dorsal horn (Ibuki et al. 1997; Eaton et al. 1998). On the other hand, there is no specific loss of GABAergic neurons in the rat spinal dorsal horn after chronic constriction injury (Bennett & Xie, 1988) of the sciatic nerve (Polgár et al. 2003; Polgár et al. 2004) and in the spared nerve injury model (Malmberg & Basbaum, 1998; Decosterd & Woolf, 2000; Polgár et al. 2005), suggesting that down-regulation of GABA synthesis rather than cell death accounts for reduced GABA immunoreactivity.
Taken together, these studies suggest that spinal GABAergic inhibition is abnormal in neuropathic pain states, e.g. by insufficient activity of GABAergic neurons or by a diminished effect of GABA on spinal dorsal horn neurons. Up to now, no studies have evaluated the functional properties of GABAergic neurons in neuropathic animals.
We tested the hypothesis that nerve injury causes changes in the physiological properties of spinal lamina II GABAergic neurons. A reduced excitability, changes of firing patterns (e.g. from tonic to single spike firing pattern) or changed primary afferent input, all could result in an impaired GABAergic inhibition.
Until recently, it was technically very demanding to record from identified spinal GABAergic neurons (see for example Jonas et al. 1998). To study a representative subgroup of spinal lamina II GABAergic neurons, we took advantage of recently generated transgenic GIN mice (GFP-expressing inhibitory neurons; Oliva et al. 2000), in which one-third of all GABAergic neurons in the spinal dorsal horn are labelled (Heinke et al. 2004) to compare active and passive membrane properties of GABAergic neurons in normal and in neuropathic animals.
Methods
Animals
All procedures used were in accordance with European Communities Council directives (86/609/EEC) and were approved by the Austrian Federal Ministry for Education, Science and Culture. Homozygous transgenic mice expressing EGFP controlled by the GAD67 promoter were obtained from The Jackson Laboratory (Bar Harbor, ME, USA; strain name: FVB-TgN(GadGFP)45704Swn) and interbred at a local facility.
Nerve ligation
Male adult mice (26–32 g body weight) were deeply anaesthetized with isoflurane (1.2–1.5 vol%) and the sciatic nerve was exposed unilaterally at the mid-thigh level. Proximal to the trifurcation, about 8 mm of nerve was freed of adhering tissue and three ligatures (7–0 prolene) were tied around it with about 1 mm spacing. The ligatures were tied until they elicited a twitch in the hind limb. The constriction of the sciatic nerve reduced blood flow without arresting it. The incision was then closed in two layers. Sham-treated mice whose nerve was exposed without ligation were used as control animals.
Behaviour
All behavioural tests were conducted between 08.00 h and 18.00 h. The behavioural tests were performed on each hindpaw on two consecutive days before nerve ligation and every second day from day 1 onward after surgery. In every case tests were performed until the ninth day after the operation. The electrophysiological recordings were conducted on day 10 or 11 after nerve ligation. Mechanical thresholds were assessed with calibrated von Frey monofilaments with incremental stiffness (Stoelting, WoodDale, IL, USA) using the up-and-down method of Dixon (1965) at regular intervals. Mice were placed in individual round plastic boxes (internal diameter 8 cm) on a mesh metal floor. Testing was initiated with the 0.6 g hair. If the mouse responded to touching the footpad with this hair for 3 s by brisk withdrawal of the respective hindpaw, the response was considered positive. In the absence of a paw withdrawal response, a thicker hair corresponding to a stronger stimulus was chosen. In case of a positive response, the next weaker stimulus was applied. Each hair was presented perpendicular to the paw. The procedure was repeated until six responses (either positive or negative) were recorded. A 50% threshold in grams, which indicated the force of von Frey hair at which an animal reacts in 50% of the presentations, was calculated using the method from Chaplan et al. (1994). 50% threshold in grams = [10(Xf + k)/10000]; where Xf is the value (in log units) of the final von Frey hair used; k is the tabular value (kDa; Appendix from Chaplan et al. 1994) for the pattern of positive/negative responses; and δ is the mean difference between stimuli (log units).
Thermal nociceptive thresholds were determined according to Hargreaves et al. (1988). Responses to thermal stimuli were tested with a Plantar Analgesia Instrument (Ugo Basile, Italy). The animals were placed in a plastic cage on a glass floor. A mobile radiant heat source located underneath the glass was focused onto one hind paw. The paw withdrawal latency was recorded by a digital timer. A significant reduction in paw withdrawal latency compared to normal baseline was interpreted as thermal hyperalgesia.
Slice preparation and single cell recording
Ten or 11 days after the surgery, the lumbar spinal cord was removed from CCI-operated and sham-operated mice under deep ether anaesthesia. The isolated spinal cord was then transferred into preoxygenated incubation solution consisting of (mm): NaCl 95, KCl 1.8, KH2PO4 1.2, CaCl2 0.5, MgSO4 7, NaHCO3 26, glucose 15, sucrose 50, oxygenated with 95% O2, 5% CO2; pH 7.4, measured osmolarity 310–320 mosmol l−1. After removal of the dura mater, all ventral and dorsal roots, except the left dorsal L4–L6 roots were cut. Transverse slices (L4–L6) were cut on a microslicer (DTK-1000, Dosaka, Kyoto, Japan) to a thickness of 500–600 μm. The slices were stored in oxygenated incubation solution at room temperature.
A single slice was then transferred to the recording chamber where it was superfused with oxygenated recording solution at 3 ml min−1. The recording solution was identical to the incubation solution except for (mm): NaCl 127, CaCl2 2.4, MgSO4 1.3 and sucrose 0. Experiments were performed at room temperature (20–24°C).
Dorsal horn neurons were visualized with Dodt-infrared optics (Dodt et al. 1999). Lamina II was identified as a translucent band across the dorsal horn. EGFP-expressing neurons in lamina II were detected by epifluorescence and were recorded in the whole cell patch-clamp configuration with glass pipettes (3–6 MΩ) filled with internal solution (mm): potassium gluconate 120, KCl 20, MgCl2 2, Na2ATP 2, NaGTP 0.5, Hepes 20, EGTA 0.5, pH 7.28 with KOH, measured osmolarity 300 mosmol l−1) as described elsewhere (Heinke et al. 2004). Voltage-clamp and current-clamp recordings were made using a patch-clamp and a multiclamp amplifier (Axopatch 200B and Axopatch 700B) and the pCLAMP 9 acquisition software (Molecular Devices, Union City, CA, USA). Results obtained with the patch-clamp amplifier were compared with the results obtained from the multiclamp amplifier. Signals were low-pass filtered at 2–10 kHz, amplified 5-fold, sampled at 5–10 kHz and analysed offline using pCLAMP 9. Serial resistance was usually between 10 and 30 MΩ. No correction for the liquid junction potential was made. At the end of the experiment, the distance of the recorded neuron from the ventral border of the white matter overlying lamina I was measured. The borders of lamina II were set from 20 to 100 μm from the border of the overlying white matter.
Passive membrane properties
The resting membrane potential was measured immediately after establishing the whole-cell configuration. Only neurons that had a resting membrane potential more negative than −50 mV were studied further. Membrane resistance and capacitance were calculated from the reaction to 100 ms-long hyperpolarizing voltage steps from −70 to −80 mV. The responses to 20 such voltage steps were averaged and the membrane resistance was then calculated from the difference in steady-state current at the two voltages. The total membrane capacitance was calculated from the area under the capacitive transient, corresponding to the charge moved by the voltage steps.
Firing patterns and active membrane properties
Firing patterns were determined in response to depolarizing current injections of 1 s duration. Firing patterns were routinely elicited from different holding potentials (one from between −50 and −65 mV, one from between −65 and −80 mV and one from a holding potential more negative than −80 mV) to detect voltage dependence of the firing patterns. The action potential width was determined at the base of the first action potential evoked by depolarizing current injected from a holding potential around −70 mV. The action potential height was determined from the same point. The action potential threshold was measured by means of a voltage step protocol. Holding potential was −80 mV and increasing voltage injections (in 2 mV steps) were used to determine the threshold of the fast Na+ current. This method takes into account the facilitating or the inhibiting effect of other voltage-dependent membrane currents (like the A-current) on the action potential generation.
Primary afferent stimulation
The dorsal root was stimulated through a suction electrode with a constant current stimulator (A320, WPI, Sarasota, FL, USA) at 0.1 ms pulse width. Excitatory postsynaptic currents were classified according to their latency and threshold to be Aδ- or C-fibre-evoked as previously described (Ruscheweyh & Sandkühler, 2002). Constant latencies and absence of failures during 10 Hz stimulation (Aδ-fibres) or 1 Hz stimulation (for C-fibres) were used as criteria for apparently monosynaptic transmission.
Statistical analysis
All values are means ± standard error of mean (s.e.m.). Two-way analysis of variance (ANOVA) (for behavioural tests), t test, χ2 test and Mann–Whitney rank sum test were used for statistical comparison. ANOVA was followed by a Mann–Whitney test corrected by the Bonferroni adjustment.
Results
Behaviour
All of the mice that had undergone CCI and that were included in this study displayed alterations in their posture, holding the affected paw in an everted position with the toes plantar flexed, and tending to avoid bearing weight on it. Sham-operated animals had normal posture and gait. Animals were tested at 2 day intervals following surgery.
Thermal hyperalgesia
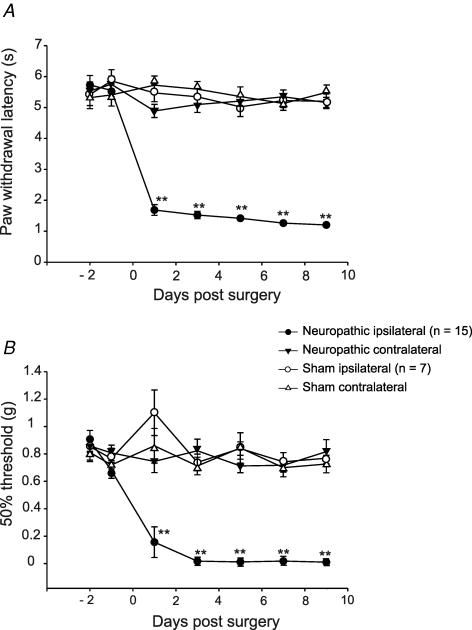
All CCI-operated animals (n = 15) exhibited significantly shortened withdrawal latencies in response to a thermal stimulus on the side of nerve injury (CCI ipsilateral) on day 1 postoperatively (reduced from 5.6 ± 0.2 s preoperatively to 1.7 ± 0.4 s; P < 0.001; 2-way ANOVA; as compared to the contralateral side (4.9 ± 0.2 s; CCI contralateral) and to sham-treated animals ipsilateral (5.5 ± 0.3 s) and contralateral sides (5.7 ± 0.3 s)). Latencies remained reduced throughout the observation period of 10 days after surgery (Fig. 1A). There were no significant differences between withdrawal latencies of the hind paw contralateral to the operation and ipsi- and contralateral hindpaws of sham-operated animals (n = 7) at any point in time (P > 0.05; 2-way ANOVA).
Figure 1. Time course of thermal hyperalgesia (A) and mechanical allodynia (B) after chronic constriction injury.
Withdrawal thresholds to thermal (A) or mechanical (B) stimulation in CCI (n = 15) and sham-operated (n = 7) animals. Results for both ipsilateral and contralateral hindpaws are shown for each group. Tests were carried out on days 2 and 1 before operation and on days 1, 3, 5, 7 and 9 after operation. Thermal hyperalgesia is indicated by a significant reduction in the withdrawal latency, mechanical allodynia by a significant reduction of the 50% withdrawal thresholds. Data are expressed as means ±s.e.m. (*P < 0.05; **P < 0.01, 2-way ANOVA).
Mechanical allodynia
Animals with a CCI of one sciatic nerve developed hypersensitivity to innocuous mechanical von Frey hair stimulation 24 h after the surgery. Mechanical allodynia persisted undiminished for at least 9 days (Fig. 1B). The force required to elicit paw withdrawal dropped from a mean baseline value of 0.9 ± 0.07 g – tested on two consecutive days before the operation – to 0.02 ± 0.07 g 1 week after surgery (P < 0.001; 2-way ANOVA; as compared to the contralateral site and to sham-treated animals ipsi- and contralateral sides). Hindpaw withdrawal thresholds contralateral to the surgery were assessed over the whole period and did not change significantly in mechanical sensitivity from presurgical baseline or sham-operated levels (P > 0.05; 2-way ANOVA). Withdrawal latencies in the sham operated animals (ipsi- and contralateral to the operated side) remained unchanged from preoperative values (0.9 ± 0.08 g).
Physiological properties of GABAergic neurons in neuropathic animals
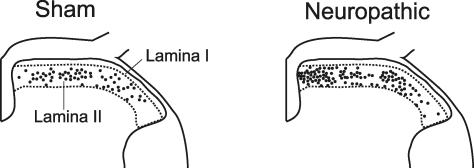
Whole cell patch-clamp recordings were obtained from a total of 169 lamina II GABAergic neurons, 110 of which were from 15 neuropathic animals and 59 neurons were from seven sham-operated mice. Transverse L4 to L6 spinal cord slices were acutely prepared and used to investigate membrane and discharge properties and synaptic input from primary afferent fibres. Care was taken to record from neurons throughout the whole dorsoventral and mediolateral expansion of lamina II (Fig. 2). The distances of the recording sites from the overlying white matter were similar for GABAergic neurons in neuropathic and in sham-treated animals (71 ± 2 μm (n = 110) and 65 ± 3 μm (n = 59); P > 0.05; t test).
Figure 2. Recording sites in lumbar spinal cord segments L4–L6 of sham-operated and neuropathic animals.
Recording sites were located through a microscope at ×100 magnification and documented on a standard histological section through the L4 segment. Each dot in the sketch illustrates one recording site of one neuron.
Membrane properties
The active and passive membrane properties of GABAergic cells in lamina II of the spinal dorsal horn of neuropathic and control animals are summarized in Table 1. Cell capacitance of GABAergic neurons recorded in neuropathic and in sham-treated mice did not differ, confirming that similar groups of neurons, with respect to cell size, were tested. In addition, there was also no difference in membrane resistance, a parameter for the ion channel conductances in neurons from neuropathic and sham-treated animals. The resting membrane potential and the action potential threshold were similar in both groups, reflecting likewise excitable membranes. The action potential shape was recorded with a multiclamp amplifier in bridge mode. There was no significant difference in the action potential width and height between the CCI-operated and the sham-operated animals. In comparison to the multiclamp amplifier, action potentials were slightly but not significantly narrower and larger when measured with the patch-clamp amplifier in current-clamp fast mode (2.4 ± 0.1 versus 2.5 ± 0.1 ms; 76 ± 2 versus 73 ± 2 mV).
Table 1.
Passive and active membrane properties of spinal lamina II GABAergic neurons of neuropathic and sham-operated animals
| Neuropathic (n = 110) | Sham (n = 59) | |
|---|---|---|
| Resting membrane potential (RMP; mV) | −62 ± 1 | −60 ± 1 |
| Membrane resistance (MΩ) | 1002 ± 46 | 932 ± 76 |
| Cell capacitance (pF) | 41 ± 1.4 | 45 ± 1.9 |
| Action potential threshold (mV) | −35 ± 1 | −35 ± 1 |
| Action potential threshold – RMP (mV) | 27 ± 1 | 25 ± 1 |
| Action potential width from base (ms) | 2.8 ± 0.1 (51) | 2.8 ± 0.1 (25) |
| Action potential height from base (mV) | 65 ± 2 (51) | 64 ± 2 (25) |
Voltage-clamp measurements were made with a patch-clamp amplifier, current-clamp measurements were made with a multiclamp amplifier in bridge mode. Statistical significance was assessed by one-way ANOVA that yielded P > 0.05 for every row. Numbers of observations for neuropathic and sham-operated animals account for all parameters tested if not otherwise stated (in parentheses)
Firing patterns
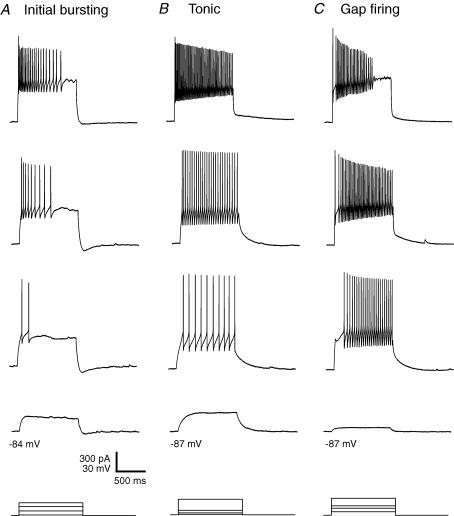
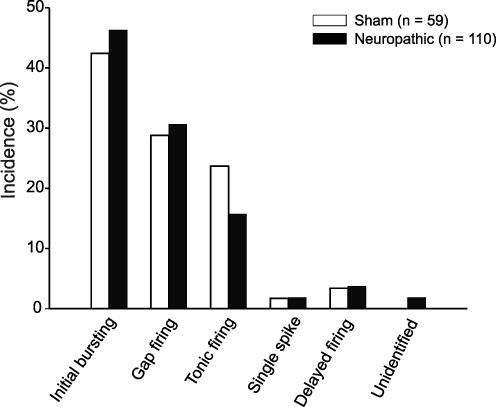
Firing patterns were recorded with a multiclamp amplifier in bridge mode and a patch-clamp amplifier, respectively. As no difference in the incidence of the firing patterns was detected, data were pooled. The initial burst, tonic and gap firing patterns were most frequent in GABAergic lamina II neurons from both neuropathic and sham-treated animals (Figs 3 and 4). Initial bursting neurons (sham-treated: 42%; neuropathic: 46%; P > 0.05; Fig. 4) discharged only at the beginning of the current pulse (Fig. 3A). With stronger current injections, the initial burst firing pattern converts into a tonic firing pattern with a strong frequency adaptation (Ruscheweyh et al. 2004). Tonically discharging neurons (sham-treated: 24%; neuropathic: 16%; P > 0.05; Fig. 4) fired action potentials throughout the duration of the depolarizing current pulse with only some degree of frequency adaptation (Fig. 3B). Neurons with a gap firing pattern (sham-treated: 29%; neuropathic: 31%; P > 0.05; Fig. 4) showed a long first interspike interval, followed by tonic firing (Fig. 3C). This firing pattern could be evoked only from holding potentials more negative than −75 mV. A voltage-dependent potassium current (A-current) exhibiting a slow kinetic underlies the gap firing pattern (Ruscheweyh et al. 2004). This A-current could invariably be observed in voltage-clamp recordings (data not shown). These A-currents reduce the excitability of neurons (Banks et al. 1996).
Figure 3. Representative firing patterns of spinal lamina II GABAergic neurons in neuropathic and sham-operated animals.
Firing patterns were obtained in response to depolarizing current injected from hyperpolarized holding potentials. Representative examples are shown. A, initial burst firing pattern. B, tonic firing pattern. C, gap firing pattern. Bottom traces: injected currents, superimposed.
Figure 4. Distribution of firing patterns among spinal lamina II GABAergic neurons in neuropathic and sham-operated animals.
Percentages of neurons showing the respective firing pattern are given. The group ‘Unidentified’ consists of two neurons that could not be classified into any of the groups according to the classification in lamina II of the rat (Ruscheweyh & Sandkühler, 2002).
The delayed firing pattern, showing a delay between the onset of the current pulse and the first action potential, was observed infrequently. A voltage-dependent, rapidly activating and inactivating A-current is responsible for the delayed firing pattern (Ruscheweyh & Sandkühler, 2002). Only one neuron (2%) from sham-operated animals and two neurons (2%) from the CCI-operated animals exhibited a single spike firing pattern responding with a single action potential to the current pulse.
We next compared membrane properties of GABAergic cells grouped by their firing patterns of neuropathic and sham-operated animals. Results are summarized in Table 2. Membrane resistance was higher (P = 0.02; t test) and cell capacitance lower (P = 0.002; t test) in gap firing neurons of neuropathic animals when compared to sham-treated animals.
Table 2.
Passive and active membrane properties of GABAergic spinal lamina II neurons of sham and neuropathic animals classified according to their firing properties
| Initial burst firing neurons | Gap firing neurons | Tonic firing neurons | ||||
|---|---|---|---|---|---|---|
| Neuropathic (n = 53) | Sham (n = 26) | Neuropathic (n = 37) | Sham (n = 19) | Neuropathic (n = 18) | Sham (n = 12) | |
| Resting membrane potential (RMP; mV) | −60 ± 1 | −59 ± 1 | −62 ± 1 | −62 ± 2 | −63 ± 2 | −61 ± 2 |
| Membrane resistance (MΩ) | 808 ± 57 | 816 ± 91 | 1268 ± 81 | 1020 ± 129* | 1023 ± 105 | 1090 ± 230 |
| Cell capacitance (pF) | 47 ± 2 | 47 ± 3 | 33 ± 1 | 47 ± 4* | 39 ± 3 | 38 ± 2 |
| Action potential threshold (mV) | −35 ± 1 | −34 ± 1 | −33 ± 1 | −35 ± 1 | −38 ± 1 | −36 ± 1 |
| Action potential threshold – RMP (mV) | 25 ± 1 | 23 ± 2 | 28 ± 2 | 26 ± 2 | 25 ± 2 | 25 ± 2 |
| Action potential width from base (ms) | 3 ± 0.1 (27) | 3 ± 0.1 (13) | 2.5 ± 0.1 (13) | 2.8 ± 0.1 (4) | 2.3 ± 0.3 (13) | 2.3 ± 0.1 (5) |
| Action potential height from base (mV) | 64 ± 2 (27) | 61 ± 3 (13) | 67 ± 2 (13) | 71 ± 3 (4) | 70 ± 6 (13) | 71 ± 2 (5) |
Statistical significance was assessed by the t test (sham-treated compared to neuropathic)
P < 0.05; Numbers of observations for neuropathic and sham-operated animals account for all parameters tested if not otherwise stated (in parentheses)
Input from primary afferent fibres
In 41 neurons we measured the excitatory postsynaptic currents evoked by electrical stimulation of the dorsal root at C-fibre strength. The results for 23 neurons from neuropathic and 18 neurons from sham-operated mice are summarized in Table 3. Altogether, in neuropathic animals the proportion of GABAergic neurons, which received monosynaptic input from primary afferents, was smaller than in sham-operated animals. The slow conduction velocities (1.8–5 m s−1) and the high thresholds of A-fibres (30 μA to 0.18 mA) also in neuropathic mice suggests that they were not Aβ-fibres. There was no significant difference in the distribution of primary afferent input among neurons from neuropathic and control animals.
Table 3.
Primary afferent input to spinal lamina II GABAergic neurons of neuropathic and sham-operated animals
| Type of primary afferent input | Neuropathic (n = 23) | Sham (n = 18) |
|---|---|---|
| Aδ-fibre monosynaptic | 22% (5) | 33% (6) |
| Aδ-fibre polysynaptic | 35% (8) | 39% (7) |
| C-fibre monosynaptic | 9% (2) | 17% (3) |
| C-fibre polysynaptic | 43% (10) | 28% (5) |
| Convergent Aδ- and C-fibre | 9% (2) | 17% (3) |
| Total monosynaptic | 31% (7) | 50% (9) |
| Total polysynaptic | 78% (18) | 67% (12) |
n, number of neurons with input from primary afferents. Percentages add up to more than 100% because individual neurons could receive both Aδ- and C-fibre input. There was no significant difference in any of the parameters between neuropathic and sham-operated animals (P > 0.05; χ2-test).
Discussion
Spinal disinhibition is a proposed mechanism underlying neuropathic pain (Sugimoto et al. 1989; Sugimoto et al. 1990; Woolf & Mannion, 1999). However, it is not known whether disinhibition is primarily due to impaired function of GABAergic neurons or a reduced efficacy of GABA on nociceptive neurons. So far, no studies have evaluated the functional properties of GABAergic neurons in neuropathic animals.
Here, we tested the hypothesis that nerve injury alters excitability or discharge properties of spinal GABAergic neurons thereby reducing GABAergic inhibition of spinal nociception. We quantitatively compared active and passive membrane properties of identified spinal dorsal horn lamina II GABAergic neurons in neuropathic and in sham-operated mice.
Membrane properties
All of the key membrane properties tested here (cell capacitance, membrane resistance, RMP, AP threshold, AP width, AP height) were very similar in sham-treated and CCI-operated animals. Unidentified lamina I and lamina II neurons from rats that have undergone peripheral nerve injury (sciatic nerve transection (SNT), spared nerve injury (SNI) or CCI) also do not show any significant difference in the resting membrane potential compared to naïve animals (Moore et al. 2002; Coull et al. 2003).
Patch-clamp amplifiers may distort action potential waveforms (Magistretti et al. 1996). The action potential shape, as reported in Table 1 was therefore recorded with a multiclamp amplifier. When measured with the patch-clamp amplifier, action potential width was narrower and action potential height was larger.
Firing patterns
In the present study, three types of firing patterns occurred frequently among spinal dorsal horn neurons of neuropathic and sham operated animals, reflecting differences in the input–output functions of the neurons. The most frequent firing pattern to occur was the initial burst firing pattern. Initial bursting neurons may act as novelty detectors because they discharge only at the beginning of a depolarization. They also encode the strength of the stimulus. Initial burst firing neurons have been observed in the superficial dorsal horn of the rat (Thomson et al. 1989; Jo et al. 1998; Ruscheweyh & Sandkühler, 2002) and hamster (Grudt & Perl, 2002). Gap and tonic firing patterns were the second and the third most frequent firing patterns in spinal lamina II GABAergic neurons in animals that had undergone chronic constriction injury and sham animals. Gap firing patterns show a long first interspike interval, followed by tonic firing. A slow A-current, which is only activated from holding potentials more negative than −75 mV, is responsible for the gap firing pattern (Ruscheweyh & Sandkühler, 2002). Tonic firing neurons encode both, the intensity and the duration of a stimulus. Tonically discharging dorsal horn neurons have been previously described (Thomson et al. 1989; Lopez-Garcia & King, 1994; Hochman et al. 1997; Jo et al. 1998; Ruscheweyh & Sandkühler, 2002).
A small group of GABAergic neurons labelled by GFP expressed from a prion promoter discharge tonically in 100% of the cases, while unidentified neurons fire transiently in the same study (Hantman et al. 2004) similar to the initial burst firing pattern described here. In that study firing patterns were apparently elicited from the RMP, which was around −48 mV. From this membrane potential it is unlikely to detect a gap or an initial burst firing pattern. In vivo whole cell current-clamp recordings of unidentified superficial dorsal horn neurons of naïve animals revealed tonic, initial burst, delayed and single spike firing patterns in similar proportions (Graham et al. 2004). No predictive relationship could be discovered between spinal dorsal horn neuronal responses to current injections and functionally relevant stimulation (brush, pinch) so far (Graham et al. 2004).
Primary afferent input
We measured the primary afferent input of lamina II GABAergic neurons to test the hypothesis that peripheral nerve injury leads to an altered afferent drive. GABAergic neurons in neuropathic and in sham-operated animals had similar input from Aδ- and C-fibre afferents, not supporting the notion that modification of synaptic input from primary afferents to GABAergic neurons in lamina II would contribute to neuropathic pain symptoms. According to the ‘gate control theory’, GABAergic neurons can be activated by Aβ-fibre stimulation and inhibit nociceptive fibres presynaptically via primary afferent depolarization (Melzack & Wall, 1965; Calvillo et al. 1982) and/or spinal nociceptive neurons postsynaptically. Here, we found no clear evidence for Aβ-fibre input to GABAergic neurons in lamina II.
There is an equal decrease in the number of A and C fibres distal to the lesion in CCI operated animals, preserving a constant ratio between the two axonal types. The behavioural changes following CCI are probably due to the pathological alterations in myelinated and in non-myelinated axons (Gabay & Tal, 2004).
The conduction velocities for Aβ, Aδ and C fibres from naïve animals and animals that have undergone peripheral nerve injury (SNI, CCI and SNT) are similar, and while the response thresholds for Aβ and Aδ fibres do not change, the activation threshold of C fibres is reduced after SNI (Kohno et al. 2003). Unidentified spinal dorsal horn neurons of naïve animals receive mostly (69%) polysynaptic Aβ/Aδ or monosynaptic Aδ input, while unidentified spinal dorsal horn neurons from CCI and SNT (but not SNI) animals predominantly exhibit Aβ fibre-mediated EPSCs (Okamoto et al. 2001; Kohno et al. 2003).
Subgroups of spinal GABAergic neurons
We have studied spinal lamina II neurons, which express EGFP under the control of the promoter for GAD67 (Oliva et al. 2000; Heinke et al. 2004). One-third of all spinal dorsal horn GABAergic neurons are labelled with this method (Heinke et al. 2004). Thus, it cannot be excluded that other GABAergic neurons in lamina II not studied here could have had changed their properties in neuropathic animals. The group of EGFP-labelled spinal GABAergic neurons displays, however, morphological and neurochemical properties (Heinke et al. 2004) which are similar to the group of all lamina II GABAergic neurons. Thus, GABAergic neurons labelled by expression of EGFP from the GAD67 promoter might be a representative sample of GABAergic neurons in the spinal dorsal horn lamina II. In contrast, the subgroup of GABAergic neurons labelled by the expression of GFP from a prion promoter (Hantman et al. 2004) is a morphologically and electrophysiologically distinct group of GABAergic neurons, namely tonic central cells (Hantman et al. 2004; Ramón y Cajal, 1909).
Here, we have characterized GABAergic neurons in spinal lamina II where most of the nociceptive Aδ- and C-fibres terminate. Thus, we have not addressed if GABAergic neurons in other laminae of the spinal dorsal horn might have changed their physiological properties following CCI of the sciatic nerve.
Different neuropathic pain models
Nerve injury models are not equivalent in terms of altered processing in the spinal dorsal horn. For example, in both, spared nerve injured and CCI rats, but not sciatic nerve transected rats, there is a loss of GABA-mediated IPSCs in lamina II neurons (Moore et al. 2002). Furthermore, there is an increase in Aβ-fibre evoked EPSCs in lamina II neurons from animals that have undergone SNT and CCI, but not SNI (Kohno et al. 2003). Whether membrane properties of GABAergic lamina II neurons might be altered in other neuropathic pain models than the ones used in this study awaits further investigation.
Alternative mechanisms underlying impaired GABAergic inhibition
We report that excitability and discharge properties of GABAergic neurons remained unchanged in neuropathic animals. GABA release could still be altered by presynaptic mechanisms. For example, release probability from vesicles could be impaired, or vesicle content could be reduced, e.g. if the GABA transporter (VGAT) (McIntire et al. 1997; Chaudhry et al. 1998) would have a reduced activity. Furthermore, GABAergic inhibition could also be impaired by several postsynaptic mechanisms.
Recently, a new mechanism of abnormal GABAergic action in neuropathic animals has been proposed: a reduction in the expression of potassium–chloride cotransporter KCC2, leading to an unphysiologically high intracellular Cl− concentration. Upon activation of GABAA receptors the shifted anion reversal potential then leads to a Cl− efflux and therefore depolarization, rather than to a Cl− influx and hyperpolarization (Coull et al. 2003). Signalling of ATP-stimulated microglia through BDNF also shifts the anion reversal potential in spinal lamina I neurons (Coull et al. 2005). However, GABA receptor agonists decrease pain-like behaviour after nerve injury (Malan et al. 2002), indicating that the overall GABAergic effect, also in the case of neuropathic pain states, in the spinal cord is antinociception not pain.
Another potential mechanism resulting in reduced spinal inhibition is apoptosis of inhibitory spinal dorsal horn neurons. Recently published data have shown that apoptosis after peripheral nerve injury only occurs if there is synaptic input from primary afferent fibres (Scholz et al. 2005). Axotomy alone is insufficient to provoke a loss of dorsal horn neurons (Coggeshall et al. 2001). Apoptosis of GABAergic neurons or down-regulation of spinal GABA synthesis after peripheral nerve injury could account for loss of GABA immunoreactivity in the spinal dorsal horn after nerve injury (Castro-Lopes et al. 1993; Castro-Lopes et al. 1995; Ibuki et al. 1997; Eaton et al. 1998). Quantitative studies on the proportions of neurons in lamina I–III two weeks after nerve ligation in the CCI model found no evidence for a selective loss of GABAergic neurons (Polgár et al. 2003; Polgár et al. 2004).
In conclusion, impaired GABAergic inhibition in neuropathic pain states may have a variety of cellular and molecular causes. Available evidence does not suggest that reduced excitability or altered firing patterns of GABAergic neurons in spinal cord lamina II would be one of them.
Acknowledgments
This work was supported by grant #P19367 from the Austrian Science Fund (FWF) to J.S. We thank Lydia Biko for technical assistance.
References
- Banks MI, Haberly LB, Jackson MB. Layer-specific properties of the transient K current (IA) in piriform cortex. J Neurosci. 1996;16:3862–3876. doi: 10.1523/JNEUROSCI.16-12-03862.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Calvillo O, Madrid J, Rudomin P. Presynaptic depolarization of unmyelinated primary afferent fibers in the spinal cord of the cat. Neuroscience. 1982;7:1389–1409. doi: 10.1016/0306-4522(82)90252-4. [DOI] [PubMed] [Google Scholar]
- Castro-Lopes JM, Malcangio M, Pan BH, Bowery NG. Complex changes of GABAA and GABAB receptor binding in the spinal cord dorsal horn following peripheral inflammation or neurectomy. Brain Res. 1995;679:289–297. doi: 10.1016/0006-8993(95)00262-o. [DOI] [PubMed] [Google Scholar]
- Castro-Lopes JM, Tavares I, Coimbra A. GABA decreases in the spinal cord dorsal horn after peripheral neurectomy. Brain Res. 1993;620:287–291. doi: 10.1016/0006-8993(93)90167-l. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Meth. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, Reimer RJ, Bellocchio EE, Danbolt NC, Osen KK, Edwards RH, Storm-Mathisen J. The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. J Neurosci. 1998;18:9733–9750. doi: 10.1523/JNEUROSCI.18-23-09733.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggeshall RE, Lekan HA, White FA, Woolf CJ. A-fiber sensory input induces neuronal cell death in the dorsal horn of the adult rat spinal cord. J Comp Neurol. 2001;435:276–282. doi: 10.1002/cne.1029. [DOI] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A, De Koninck P, De Koninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424:938–942. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- Dixon WJ. The up-and-down method for small samples. J Am Statist Assoc. 1965;60:967–978. [Google Scholar]
- Dodt H-U, Eder M, Frick A, Zieglgänsberger W. Precisely localized LTD in the neocortex revealed by infrared-guided laser stimulation. Science. 1999;286:110–113. doi: 10.1126/science.286.5437.110. [DOI] [PubMed] [Google Scholar]
- Eaton MJ, Plunkett JA, Karmally S, Martinez MA, Montanez K. Changes in GAD- and GABA-immunoreactivity in the spinal dorsal horn after peripheral nerve injury and promotion of recovery by lumbar transplant of immortalized serotonergic precursors. J Chem Neuroanat. 1998;16:57–72. doi: 10.1016/s0891-0618(98)00062-3. [DOI] [PubMed] [Google Scholar]
- Gabay E, Tal M. Pain behavior and nerve electrophysiology in the CCI model of neuropathic pain. Pain. 2004;110:354–360. doi: 10.1016/j.pain.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Graham BA, Brichta AM, Callister RJ. In vivo responses of mouse superficial dorsal horn neurones to both current injection and peripheral cutaneous stimulation. J Physiol. 2004;561:749–763. doi: 10.1113/jphysiol.2004.072645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grudt TJ, Perl ER. Correlations between neuronal morphology and electrophysiological features in the rodent superficial dorsal horn. J Physiol. 2002;540:189–207. doi: 10.1113/jphysiol.2001.012890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantman AW, van den Pol AN, Perl ER. Morphological and physiological features of a set of spinal substantia gelatinosa neurons defined by green fluorescent protein expression. J Neurosci. 2004;24:836–842. doi: 10.1523/JNEUROSCI.4221-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Heinke B, Ruscheweyh R, Forsthuber L, Wunderbaldinger G, Sandkühler J. Physiological, neurochemical and morphological properties of a subgroup of GABAergic spinal lamina II neurones identified by expression of green fluorescent protein in mice. J Physiol. 2004;560:249–266. doi: 10.1113/jphysiol.2004.070540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochman S, Garraway SM, Pockett S. Membrane properties of deep dorsal horn neurons from neonatal rat spinal cord in vitro. Brain Res. 1997;767:214–219. doi: 10.1016/s0006-8993(97)00578-7. [DOI] [PubMed] [Google Scholar]
- Ibuki T, Hama AT, Wang XT, Pappas GD, Sagen J. Loss of GABA-immunoreactivity in the spinal dorsal horn of rats with peripheral nerve injury and promotion of recovery by adrenal medullary grafts. Neuroscience. 1997;76:845–858. doi: 10.1016/s0306-4522(96)00341-7. [DOI] [PubMed] [Google Scholar]
- Jo YH, Stoeckel ME, Schlichter R. Electrophysiological properties of cultured neonatal rat dorsal horn neurons containing GABA and met-enkephalin-like immunoreactivity. J Neurophysiol. 1998;79:1583–1586. doi: 10.1152/jn.1998.79.3.1583. [DOI] [PubMed] [Google Scholar]
- Jonas P, Bischofberger J, Sandkühler J. Corelease of two fast neurotransmitters at a central synapse. Science. 1998;281:419–424. doi: 10.1126/science.281.5375.419. [DOI] [PubMed] [Google Scholar]
- Kohno T, Moore KA, Baba H, Woolf CJ. Peripheral nerve injury alters excitatory synaptic transmission in lamina II of the rat dorsal horn. J Physiol. 2003;548:131–138. doi: 10.1113/jphysiol.2002.036186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Garcia JA, King AE. Membrane properties of physiologically classified rat dorsal horn neurons in vitro: correlation with cutaneous sensory afferent input. Eur J Neurosci. 1994;6:998–1007. doi: 10.1111/j.1460-9568.1994.tb00594.x. [DOI] [PubMed] [Google Scholar]
- Magistretti J, Mantegazza M, Guatteo E, Wanke E. Action potentials recorded with patch-clamp amplifiers: are they genuine? Trends Neurosci. 1996;19:530–534. doi: 10.1016/s0166-2236(96)40004-2. [DOI] [PubMed] [Google Scholar]
- Malan TP, Mata HP, Porreca F. Spinal GABAA and GABAB receptor pharmacology in a rat model of neuropathic pain. Anesthesiology. 2002;96:1161–1167. doi: 10.1097/00000542-200205000-00020. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Basbaum AI. Partial sciatic nerve injury in the mouse as a model of neuropathic pain: behavioral and neuroanatomical correlates. Pain. 1998;76:215–222. doi: 10.1016/s0304-3959(98)00045-1. [DOI] [PubMed] [Google Scholar]
- McIntire SL, Reimer RJ, Schuske K, Edwards RH, Jorgensen EM. Identification and characterization of the vesicular GABA transporter. Nature. 1997;389:870–876. doi: 10.1038/39908. [DOI] [PubMed] [Google Scholar]
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, Woolf CJ. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci. 2002;22:6724–6731. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Baba H, Goldstein PA, Higashi H, Shimoji K, Yoshimura M. Reorganization of sensory pathways in the rat spinal dorsal horn following peripheral nerve injury. J Physiol. 2001;532:241–250. doi: 10.1111/j.1469-7793.2001.0241g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva AA, Jiang M, Lam T, Smith KL, Swann JW. Novel hippocampal interneuronal subtypes identified using transgenic mice that express green fluorescent protein in GABAergic interneurons. J Neurosci. 2000;20:3354–3368. doi: 10.1523/JNEUROSCI.20-09-03354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polgár E, Gray S, Riddell JS, Todd AJ. Lack of evidence for significant neuronal loss in laminae I–III of the spinal dorsal horn of the rat in the chronic constriction injury model. Pain. 2004;111:144–150. doi: 10.1016/j.pain.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Polgár E, Hughes DI, Arham AZ, Todd AJ. Loss of neurons from laminas I–III of the spinal dorsal horn is not required for development of tactile allodynia in the spared nerve injury model of neuropathic pain. J Neurosci. 2005;25:6658–6666. doi: 10.1523/JNEUROSCI.1490-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polgár E, Hughes DI, Riddell JS, Maxwell DJ, Puskár Z, Todd AJ. Selective loss of spinal GABAergic or glycinergic neurons is not necessary for development of thermal hyperalgesia in the chronic constriction injury model of neuropathic pain. Pain. 2003;104:229–239. doi: 10.1016/s0304-3959(03)00011-3. [DOI] [PubMed] [Google Scholar]
- Ramón y Cajal S. Histologie Du Système Nerveux de L'homme et Des Vertébrés. Paris: Maloine; 1909. [Google Scholar]
- Ruscheweyh R, Ikeda H, Heinke B, Sandkühler J. Distinctive membrane and discharge properties of rat spinal lamina I projection neurones in vitro. J Physiol. 2004;555:527–543. doi: 10.1113/jphysiol.2003.054049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscheweyh R, Sandkühler J. Lamina-specific membrane and discharge properties of rat spinal dorsal horn neurones in vitro. J Physiol. 2002;541:231–244. doi: 10.1113/jphysiol.2002.017756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J, Broom DC, Youn DH, Mills CD, Kohno T, Suter MR, Moore KA, Decosterd I, Coggeshall RE, Woolf CJ. Blocking caspase activity prevents transsynaptic neuronal apoptosis and the loss of inhibition in lamina II of the dorsal horn after peripheral nerve injury. J Neurosci. 2005;25:7317–7323. doi: 10.1523/JNEUROSCI.1526-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivilotti L, Woolf CJ. The contribution of GABAA and glycine receptors to central sensitization: Disinhibition and touch-evoked allodynia in the spinal cord. J Neurophysiol. 1994;72:169–179. doi: 10.1152/jn.1994.72.1.169. [DOI] [PubMed] [Google Scholar]
- Sugimoto T, Bennett GJ, Kajander KC. Strychnine-enhanced transsynaptic degeneration of dorsal horn neurons in rats with an experimental painful peripheral neuropathy. Neurosci Lett. 1989;98:139–143. doi: 10.1016/0304-3940(89)90499-0. [DOI] [PubMed] [Google Scholar]
- Sugimoto T, Bennett GJ, Kajander KC. Transsynaptic degeneration in the superficial dorsal horn after sciatic nerve injury: effects of a chronic constriction injury, transection, and strychnine. Pain. 1990;42:205–213. doi: 10.1016/0304-3959(90)91164-E. [DOI] [PubMed] [Google Scholar]
- Thomson AM, West DC, Headley PM. Membrane characteristics and synaptic responsiveness of superficial dorsal horn neurons in a slice preparation of adult rat spinal cord. Eur J Neurosci. 1989;1:479–488. doi: 10.1111/j.1460-9568.1989.tb00354.x. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–1964. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- Yaksh TL. Behavioral and autonomic correlates of the tactile evoked allodynia produced by spinal glycine inhibition: effects of modulatory receptor systems and excitatory amino acid antagonists. Pain. 1989;37:111–123. doi: 10.1016/0304-3959(89)90160-7. [DOI] [PubMed] [Google Scholar]