Abstract
To date, the neurotransmitter(s) and pathways involved in cutaneous active vasodilatation are not fully understood. The purpose of this study was to determine the potential involvement of neurokinin-1 (NK1) receptors to active vasodilatation. Our experimental model exploited our previous findings that repeated microdialysis infusions of substance P desensitize the NK1 receptors and that substance P-induced vasodilatation contains a substantial nitric oxide (NO) component. Eleven subjects were equipped with four microdialysis fibres on the ventral forearm. Site 1 served as a control and received a continuous infusion of Ringer solution. Site 2 received a continuous infusion of 10 mm l-NAME to inhibit NO synthase. Site 3 received a 10 μm dose of substance P to desensitize the NK1 receptors prior to whole-body heating. Site 4 received a 10 μm dose of substance P combined with 10 mm l-NAME. Red blood cell (RBC) flux was measured via laser-Doppler flowmetry, and cutaneous vascular conductance (CVC) was calculated as RBC flux/mean arterial pressure and normalized to maximal vasodilatation via 28 mm sodium nitroprusside. Substance P was infused for 15 min at 4 μl min−1 in sites 3 and 4, and skin blood flow was allowed to return to baseline (∼45–60 min). Subjects then underwent a period of whole-body heat stress to raise oral temperature 0.8–1.0°C above baseline. Pretreatment with substance P increased CVC to 48 ± 2% CVCmax, which was significantly greater than for sites pretreated with substance P combined with l-NAME (27 ± 2% CVCmax; P < 0.001). During whole-body heating, CVC in control sites increased to 69 ± 3% CVCmax. Sites pretreated with substance P (48 ± 3% CVCmax) were significantly reduced compared to control sites (P < 0.001). The CVC response to whole-body heat stress in l-NAME sites was significantly reduced (32 ± 3% CVCmax; P < 0.001) compared to both control sites and sites pretreated with substance P. The CVC response to whole-body heating was nearly abolished in sites pretreated with substance P combined with l-NAME (20 ± 2% CVCmax) and was significantly reduced compared to the other three sites (all P < 0.001). These data suggest NK1 receptors contribute to active vasodilatation and that combined NK1 receptor desensitization and NO synthase inhibition further diminishes active vasodilatation.
Active vasodilatation and sweating are the primary autonomic means by which humans defend against an increase in core temperature. The initial increase in skin blood flow attending an increase in core body temperature is via withdrawal of sympathetic adrenergic nerve activity, which approximately doubles resting skin blood flow (Grant & Holling, 1938; Roddie et al. 1957b). At a given core temperature threshold, vasodilator activity and sweating are initiated (Grant & Holling, 1938; Roddie et al. 1957a). The active vasodilator system has been shown to be under the control of sympathetic vasodilator nerves, as blocking or cutting the sympathetic nerves prevents the increase in skin blood flow during body heating (Grant & Holling, 1938; Grant & Pearson, 1938). Kellogg et al. (1995) were able to abolish both cutaneous vasodilatation and sweating in skin sites treated with botulinum toxin, thus providing evidence that these sympathetic nerves are cholinergic in nature.
Current theory regarding the mechanism(s) of cutaneous active vasodilatation suggests acetylcholine and an unknown neurotransmitter(s) are coreleased from sympathetic cholinergic nerves (Grant & Holling, 1938; Kellogg et al. 1995). Atropine, a muscarinic receptor antagonist, has been shown to eliminate the sweating response but not the increase in skin blood flow, suggesting acetylcholine mediates sweating while the neurotransmitter(s) mediates cutaneous vasodilatation (Fox & Hilton, 1958; Kellogg et al. 1995). However, the identity of the neurotransmitter(s) has yet to be determined.
Nitric oxide (NO) is a potent vasodilator and has been shown to contribute up to 40–50% to active vasodilatation (Kellogg et al. 1998, 2003; Shastry et al. 1998; 2000; Wilkins et al. 2003). Recent evidence from Bennett et al. 2003) suggests vasoactive intestinal polypeptide (VIP) contributes to cutaneous vasodilatation, as they observed an attenuated skin blood flow response to whole-body heating in the presence of the VIP analogue, VIP10–28. Our laboratory has demonstrated that VIP-mediated vasodilatation occurs predominantly through NO and H1 receptor activation components (Wilkins et al. 2004). Along these lines, we have recently demonstrated that active vasodilatation contains an H1 receptor activation component, and that a portion of the NO component can be explained by H1 receptor activation (Wong et al. 2004). However, our laboratory (Wilkins et al. 2005) was unable to confirm the findings of Bennett et al. (2004), and it has been shown that patients with cystic fibrosis, who have a reduced level of immunoreactive VIP nerve fibres in the skin, display a normal skin blood flow response to whole-body heating (Savage et al. 1990). Taken together, evidence of a role for VIP in active vasodilatation remains equivocal. Savage et al. (1990) also demonstrated normal immunoreactivity for substance P and calcitonin gene-related peptide (CGRP)-containing nerve fibres in cystic fibrosis patients, and suggested a possible role for substance P and/or CGRP in active vasodilatation. However, there have been no studies to date investigating a possible role for substance P in active vasodilatation.
Substance P binds with high affinity to the neurokinin-1 (NK1) receptor and has been shown to be located in nerves associated with blood vessels and mast cells in human skin (Hokfelt et al. 1980a, 1980b; Wallengren et al. 1987; Quartara & Maggi, 1997; Wallengren, 1997; Holzer, 1998). Substance P has been shown to degranulate cutaneous mast cells (Church et al. 1991) and substance P-induced vasodilatation has been shown to include an NO component (Klede et al. 2003; Wong et al. 2005). These studies suggest substance P may be a possible candidate as one of the unknown neurotransmitter(s) in cutaneous active vasodilatation.
Our laboratory (Wong et al. 2005) has recently provided evidence of NK1 receptor desensitization in the skin following a microdialysis infusion of substance P that lasts up to 3 h. In the present study, we exploited these findings to investigate the contribution of NK1 receptors, and, indirectly, a possible role for substance P in cutaneous active vasodilatation. We hypothesized: (1) the skin blood flow response to whole-body heating will be attenuated in skin sites pretreated with substance P prior to heat stress; and (2) there will be a further reduction in the skin blood flow response to whole-body heating in sites pretreated with substance P combined with an NO synthase inhibitor.
Methods
Subjects
Seven men (23 ± 1 years) and four women (22 ± 1 years) participated in this study. Prior to participation, each subject gave written informed consent as set forth by the Declaration of Helsinki. All protocols were approved by the Institutional Review Board of the University of Oregon. All subjects were healthy, normotensive, did not smoke, and did not have any history of diabetes or cardiovascular disease.
Instrumentation
All protocols were performed in a thermoneutral laboratory with the subjects in the supine position and the experimental arm at heart level. Subjects' electrocardiogram was continuously monitored, and blood pressure was measured via automated brachial auscultation every 5 min (CardioCap, Datex-Ohmeda, Tewksbury, MA, USA).
Subjects were instrumented with four microdialysis fibres (MD2000, Bioanalytical Systems, West Lafayette, IN, USA) on the ventral surface of the forearm. The microdialysis fibres had a 10 mm-long membrane with a 20 kDa molecular weight cut-off. To place the microdialysis fibres, a 25 gauge needle was inserted into the dermal layer of the skin in the absence of anaesthesia. The microdialysis fibre was then threaded through the lumen of the needle and the needle and microdialysis fibre were pulled through the skin. The membrane of the microdialysis fibre was left in the skin, and the needle was completely removed. During the trauma resolution period, each microdialysis fibre was perfused with lactated Ringer's solution (Abbott Laboratories, North Chicago, IL, USA) at a rate of 2 μl min−1 with a microinfusion pump (CMA/102, CMA Microdialysis, Stockholm, Sweden). The hyperaemia associated with placement of the microdialysis fibres was allowed to subside prior to beginning any experimental protocol (approximately 90–120 min). To obtain an index of skin blood flow, red blood cell (RBC) flux was monitored directly over each microdialysis membrane via laser-Doppler flowmetry (MoorLAB, Moor Instruments, Devon, UK).
All subjects wore a water-perfused suit to control whole-body temperature, which covered the entire body except the head, hands, feet, and experimental forearm. Subjects' oral temperature was used as an index of core temperature and was monitored for 5–10 min prior to, and for the duration of, the whole-body heating period. Thermoneutral water (33°C) was perfused through the suit during the trauma resolution period and baseline data collection period. During the whole-body heating period, subjects were covered with a water-impermeable rain suit to minimize evaporative heat loss, and hot water (50°C) was perfused through the suit to raise subjects' oral temperature 0.8–1.0°C above baseline.
Drugs
Substance P (Calbiochem, San Diego, CA, USA) was dissolved in sterile lactated Ringer solution to a final concentration of 10 μm. We have shown previously that this concentration of substance P results in a subsequent desensitization of the NK1 receptors for up to 3 h (Wong et al. 2005). A 10 mm solution of the l-arginine analogue, NG-nitro-l-arginine-methyl ester (l-NAME; Calbiochem, San Diego, CA, USA) dissolved in sterile lactated Ringer solution, was used to inhibit NO synthase. This concentration of l-NAME has been show to adequately inhibit NO in human skin (Kellogg et al. 1999; Minson et al. 2002). Maximal skin blood flow was achieved by perfusing each microdialysis fibre with a 28 mm solution of the endothelium-independent NO donor, sodium nitroprusside (SNP; Abbott Laboratories, North Chicago, IL, USA). This concentration of SNP has been shown previously to elicit maximal cutaneous vasodilatation in humans (Kellogg et al. 1999; Minson et al. 2001).
Protocol
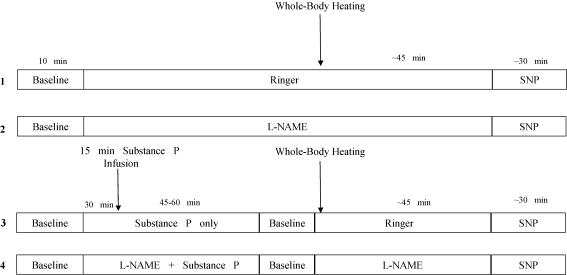
Figure 1 is a schematic diagram depicting the general experimental protocol. The microdialysis sites received the following treatments. Site 1 served as a control site and received Ringer solution only. Site 2 received 10 mml-NAME to inhibit NO synthase and was used to determine the independent contribution of NO to cutaneous active vasodilatation. Site 3 received a 10 μm dose of substance P in order to desensitize the NK1 receptors prior to the whole-body heating period, and was used to investigate the independent contributions of substance P and NK1 receptors to active vasodilatation. Site 4 received a 10 μm dose of substance P combined with 10 mml-NAME (final concentrations) in order to investigate the combined contributions of substance P and NO in cutaneous active vasodilatation.
Figure 1. Schematic drawing of the general experimental protocol.
Numbers on left refer to microdialysis sites. Site 1 served as a control. Site 2 served as an l-NAME ‘control’ and was used to determine the independent contribution of NO to active vasodilatation. Site 3 was used to determine the independent contribution of NK1 receptor activation in active vasodilatation. Site 4 was used to determine an interaction among NK1 receptors and NO in active vasodilatation.
Following the trauma-resolution period, baseline data were collected for 5–10 min. Site 2 was perfused with l-NAME for the duration of the protocol, and site 4 was perfused with l-NAME for at least 30 min. Following the 30 min l-NAME infusion in site 4, site 3 was perfused with substance P and site 4 was perfused with substance P combined with l-NAME. Both sites were infused for a period of 15 min at a rate of 4 μl min−1. The results from our previous investigation (Wong et al. 2005) indicated that this infusion period produced a consistent substance P-induced vasodilatation in the skin, and also rendered the NK1 receptors insensitive to a subsequent substance P infusion for up to 3 h. As such, delivery of substance P, monitoring of the substance P-induced vasodilatation, and the whole-body heating protocol were all carried out well under the 3 h time frame. Following the substance P infusion period, site 3 was perfused with Ringer solution and site 4 was perfused with l-NAME, both for the duration of the protocol, and the infusion pump was returned to a rate of 2 μl min−1.
The substance P-induced vasodilatation in sites 3 and 4 was allowed to return to baseline (∼45–60 min). After establishing a stable 5 min baseline, subjects underwent a period of whole-body heating. Subjects' oral temperature was raised 0.8–1.0°C (∼45 min) by pumping 50°C water through the water-perfused suit. Subjects' oral temperature was maintained at this level until a stable 10 min plateau in skin blood flow was achieved at each experimental site. At the end of the heating protocol, subjects were cooled by pumping thermoneutral water through the suit. Maximal cutaneous vasodilatation was achieved via SNP infusion at a rate of 4 μl min−1.
Data acquisition and analysis
Data were digitized and stored at 20 Hz on a personal computer and were analysed offline using signal-processing software (Windaq, Dataq Instruments, Akron, OH, USA). A stable 5 min period of skin blood flow was used for analysis of baseline, whole-body heating plateau, and maximal skin blood flow. Cutaneous vascular conductance (CVC) was calculated as RBC flux/mean arterial pressure and normalized to maximal values during 28 mm SNP infusion. Thus, data are presented as a percentage of maximal cutaneous vascular conductance (% CVCmax).
To determine the relative contribution of NK1 receptors, NO, and the combined contribution of NK1 receptors and NO, the plateau in skin blood flow achieved during whole-body heating was analysed using a one-way repeated measures ANOVA. The onset of vasodilatation in each experimental site was analysed with respect to the change in oral temperature (ΔTor; calculated as onset Tor − baseline Tor) required to elicit a significant increase in skin blood flow above baseline, and was compared across experimental sites using a one-way repeated measures ANOVA. A significant increase in skin blood flow above baseline was defined as an increase of 10–15 flux units (mV) for at least 10 s after the initiation of whole-body heating that continued to progressively increase. Thus, any increase in flux attributable to movement would have returned to baseline within 10 s and would not have progressively increased.
To ensure that the CVC responses to whole-body heating in sites pretreated with substance P and substance P plus l-NAME were not due to a non-specific effect of substance P on the ability of the blood vessel to vasodilate, raw maximal CVC values (calculated as RBC fluxmax/MAPmax) achieved during SNP infusion were compared with a one-way repeated measures ANOVA. For all ANOVAs, Tukey's multiple comparisons post hoc analysis was used to determine where significant differences occurred. A P value < 0.05 was considered statistically significant, and all values are presented as mean ± s.e.m.
Results
Substance P-induced vasodilatation
The initial baseline CVC averaged 9 ± 2% CVCmax. In substance P-only sites, CVC increased to 48 ± 3% CVCmax, which was significantly greater than both the initial and post-infusion baseline (both P < 0.001). The post-infusion baseline in substance P-only sites averaged 12 ± 2% CVCmax, which was not significantly different from the initial baseline. In substance P plus l-NAME sites, CVC increased to 27 ± 2% CVCmax, which was significantly attenuated compared to the vasodilatation elicited by substance P only (P < 0.01). The plateau in CVC in substance P plus l-NAME sites was significantly greater than both the initial and post-infusion baseline (both P < 0.001). The post-infusion baseline in substance P plus l-NAME sites averaged 8 ± 2% CVCmax, and was not significantly different from the initial baseline. The plateau in CVC to substance P infusion was similar to data reported in our previous investigation (Wong et al. 2005).
Whole-body heating CVC data
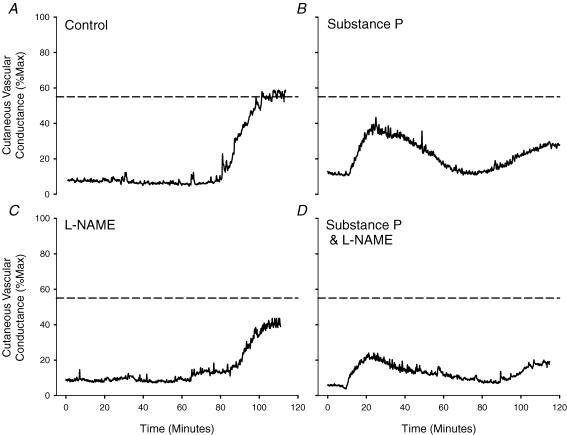
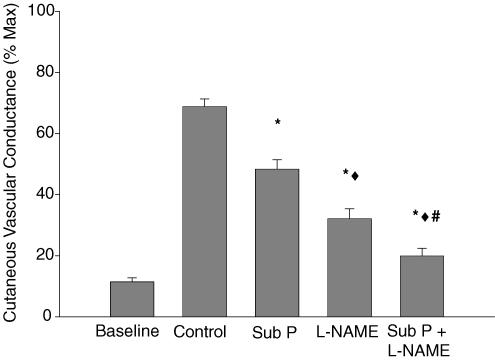
Figure 2 is a tracing from one subject depicting the skin blood flow response to whole-body heating in the four treatment sites. There was no significant difference in baseline CVC among treatment sites, thus, all baseline data have been grouped. Baseline CVC prior to whole-body heating averaged 10 ± 2% CVCmax. In control sites, CVC increased during whole-body heating to 69 ± 3% CVCmax. The CVC response to whole-body heating was significantly reduced in sites pretreated with substance P (48 ± 3% CVCmax; P < 0.001) compared to control sites. Sites that received l-NAME only (32 ± 3% CVCmax; P < 0.001) were significantly reduced compared to both control sites and sites pretreated with substance P. Furthermore, CVC in sites pretreated with substance P combined with l-NAME (20 ± 2% CVCmax; P < 0.001) were significantly reduced compared to the other three sites. The group mean data are summarized in Fig. 3.
Figure 2. Representative tracing of the skin blood flow response to whole-body heat stress.
A, control sites; B, substance P-only sites; C, l NAME; D, substance P +l NAME sites. Dashed line indicates CVC response to whole-body heating in control sites. Note the nearly abolished CVC response to whole-body heating in sites pretreated with substance P plus l NAME (D).
Figure 3. Group mean (±s.e.m) CVC at the plateau in skin blood flow during whole-body heating.
Sites pretreated with substance P and L l-NAME were significantly reduced during whole-body heating compared to control (*). l-NAME sites were significantly attenuated compared to substance P-only sites (♦). Additionally, sites pretreated with substance P plus l-NAME were significantly reduced compared to control (*), substance P only (♦), and l-NAME (#) sites during whole-body heating.
There was no significant difference in maximal raw CVC among treatment sites (Table 1). Raw maximal CVC averaged 3.0 ± 0.3 V (100 mmHg)−1 in control sites, 2.7 ± 0.3 V (100 mmHg)−1 in substance P treated sites, 2.9 ± 0.2 V (100 mmHg)−1 in L-NAME sites, and 3.1 ± 0.3 V 100 mmHg−1 in substance P plus l-NAME.
Table 1.
Maximal raw CVC values from each microdialysis site. There were no significant differences observed
| Raw maximal CVC values | |
|---|---|
| Control site | 3.0 ± 0.3 |
| Substance P site | 2.7 ± 0.3 |
| l-NAME site | 2.9 ± 0.2 |
| Substance P +l-NAME site | 3.1 ± 0.3 |
Values are mean ±s.e.m. (volts (100 mmHg)−1). CVC, cutaneous vascular conductance; l-NAME, NG-nitro-l-arginine methyl ester.
Onset of vasodilatation
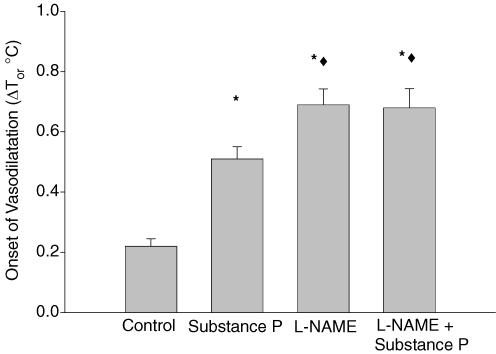
Prior to whole-body heating, mean baseline oral temperature was 36.3 ± 0.1°C. The onset of vasodilatation in control sites occurred with a ΔTor of 0.23 ± 0.02°C. Compared to control, the onset of vasodilatation occurred with a greater ΔTor in substance P-treated sites (0.51 ± 0.04°C; P < 0.001), l-NAME sites (0.70 ± 0.05°C; P < 0.001), and substance P plus l-NAME sites (0.69 ± 0.06°C; P < 0.001). Furthermore, l-NAME sites and substance P plus l-NAME sites were shifted to a higher ΔTor compared to substance P-treated sites (P < 0.01). There was no statistical difference between l-NAME and substance P plus l-NAME sites. These data are summarized in Fig. 4.
Figure 4. Group mean (±s.e.m) ΔTor (°C) required for the onset of vasodilatation.
The onset of active vasodilatation was shifted to a higher ΔTor in all three treatment sites compared to control sites (*). Additionally, the onset of active vasodilatation was shifted to a higher ΔTor in l-NAME and substance P plus l-NAME sites compared to substance P-only sites (♦).
Discussion
This is the first study to investigate the contribution of NK1 receptors in active vasodilatation. The major findings of this study are as follows: (1) NK1 receptor desensitization prior to whole-body heating significantly reduced the rise in skin blood flow during whole-body heating; and (2) combined NK1 receptor desensitization and NO synthase inhibition further diminished active vasodilation. Based on data from the present study, NK1 receptors directly mediate ∼35% of active vasodilatation, and NK1 receptor desensitization coupled with NO synthase inhibition accounted for greater than 80% of the skin blood flow response to whole-body heating (Fig. 3). The observation that sites pretreated with substance P combined with NO synthase inhibition unmasked a larger reduction in CVC than either site alone suggests that, while NK1 receptor activation may account for a portion of the NO component, the majority of the NO component is due to other sources. Data from this study also demonstrate that NK1 receptor activation is important in the initiation of the active vasodilator system, as evidenced by the delayed onset of active vasodilatation in sites pretreated with substance P (Fig. 4).
To date, the neurotransmitter(s) and specific pathways involved in cutaneous active vasodilatation are still unclear. Of the potential neurotransmitters, a role for vasoactive intestinal peptide (VIP) has received the most attention and has seemed the most likely. However, recent evidence from Bennett et al. (2004) provides the only data available in support of a role for VIP in cutaneous active vasodilatation, where the skin blood flow response to whole-body heating was attenuated by 30% in the presence of the VIP fragment, VIP10–28 (Bennett et al. 2004). Our laboratory (Wilkins et al. 2005) was unable to confirm the findings of Bennett et al. (2004), and it has been shown that patients with cystic fibrosis, who have a reduced level of immunoreactive VIP nerve fibres in the skin, display a normal skin blood flow response to whole-body heating (Savage et al. 1990). Importantly, immunoreactivity for substance P in these patients was normal. Taken together, these data leave open the possibility that substance P, which has the highest known affinity for the NK1 receptor (Quartara & Maggi, 1997), may be a candidate peptide involved in active vasodilatation. However, there is probably a complex interaction among the neuropeptides, local vasodilators, and intracellular pathways involved in active vasodilatation, and further, it is likely that these pathways have some degree of redundancy.
The present data raise the question as to the physiological role and mechanism of action of NK1 receptors in the cutaneous circulation. It is possible that NK1 receptor activation serves to increase the production of local vasodilator substances such as NO and, possibly, vasoactive prostanoids. It has been well established that NO contributes approximately 40–50% to active vasodilatation (Kellogg et al. 1998, 2003; Shastry et al. 1998, 2000), and NK1 receptor activation by substance P has been shown to include an NO component in the skin (Klede et al. 2003; Wong et al. 2005). Furthermore, activation of the NK1 receptor has been shown to work via the inositol triphosphate (IP3) second messenger system, which serves to increase the intracellular concentration of Ca2+ (Krause et al. 1990; Regoli et al. 1994; Mann et al. 1999). The production of NO via NO synthase is a Ca2+-dependent mechanism, and the production of prostaglandins has been shown to rely, at least in part, on an increase in intracellular Ca2+ and IP3 (Yousufzai et al. 1986; Marriott et al. 1991). However, data from the present study suggest NK1 receptor activation may only account for a portion of the NO component. This raises the question as to other potential source(s) of NO in active vasodilatation. Based on the available evidence, there are at least four possible additional sources of NO in the skin during active vasodilatation: (1) acetylcholine-induced NO production in the early stages of heating (Shibasaki et al. 2002); (2) H1 receptor-induced NO production (Wong et al. 2004); (3) VIP-mediated NO production (Wilkins et al. 2004); and (4) prostaglandin-induced NO production (McCord et al. 2006). However, the ability to clearly identify and quantify the sources of NO in active vasodilatation is confounded by the redundant nature of the pathways that can induce NO.
In addition to NO, there is considerable evidence in the literature demonstrating an interaction among substance P and prostaglandins, where substance P can induce prostaglandin release and vice versa (Marriott et al. 1991; Kopp et al. 1996; Kopp & Smith, 1993). In the context of cutaneous active vasodilatation, recent data from our laboratory have shown that a significant portion of cutaneous active vasodilatation can be explained by NO- and prostanoid-dependent mechanisms (McCord et al. 2006). Similar to the combined substance P plus l-NAME sites in the present investigation, McCord et al. (2006) demonstrated a profound reduction in active vasodilatation when NO synthase and cyclooxygenase were simultaneously inhibited. Thus, it is possible the observed prostanoid-dependent component of active vasodilatation is due to NK1 receptor activation. Alternatively, NO itself has been shown to increase the production of prostaglandins, demonstrating a link among NO and vasoactive prostanoids (Salvemini et al. 1993, 1995; Salvemini, 1997). These data suggest a potential link among NK1 receptor activation, NO, and vasoactive prostanoids in active vasodilatation, that has not been explored in the cutaneous circulation.
H1 receptor activation has also been shown to contribute to active vasodilatation (Wong et al. 2004). Although the contribution of histamine and/or H1 receptor activation to substance P-induced vasodilatation appears to be concentration dependent (Weidner et al. 2000; Wong et al. 2005), substance P has been shown to degranulate cutaneous mast cells, increase the concentration of histamine in the skin, and substance P-induced vasodilatation is attenuated in the presence of H1 receptor antagonists (Church et al. 1991; Petersen et al. 1994; Huttunen et al. 1996). Taken together, the vasoactive properties of substance P are consistent with currently known pathways of active vasodilatation.
Although data from the present study provide evidence to suggest a role for NK1 receptor activation in cutaneous active vasodilatation, we are faced with two limitations regarding the interpretation of the data. First, we cannot say for certain whether substance P is involved in active vasodilatation or if there is a non-specific interaction between an unknown vasodilator and the NK1 receptor. At this time, a role for substance P in active vasodilatation is speculative and based on the presupposition that substance P is the preferred ligand for the NK1 receptor in the skin. While substance P is the most likely candidate, as it has the highest known affinity for the NK1 receptor and a very low affinity for other tachykinin receptors (Quartara & Maggi, 1997), the converse relationship is not as well defined. That is, the NK1 receptor may be activated by other ligands bearing similar structure to substance P, such as neurokinin A (Quartara & Maggi, 1997).
Second, the data do not allow us to draw conclusions as to the source of substance P. The two most likely sources of substance P are: (1) vascular endothelial cells, and (2) cutaneous nerves. It has been shown that substance P-induced vasodilatation requires an intact endothelium, and substance P can be released from endothelial cells in response to an increase in blood flow (Ralevic et al. 1990; Jansen et al. 1991). Although reactive hyperaemia, a condition that significantly elevates cutaneous blood flow and presumably shear stress, has been shown to be mediated primarily by factors other than NO (Binggeli et al. 2003; Wong et al. 2003; Zhao et al. 2004), an endothelial source for substance P and NK1 receptor activation cannot be entirely ruled out. Inasmuch as the data from the present study suggest that a large portion of the NO component of active vasodilatation stems from non-NK1 receptor-mediated sources, it is entirely possible that an increase in cutaneous blood flow and shear stress results in an endothelial-derived source of substance P and subsequent NK1 receptor activation. A neural origin for substance P is another plausible source, as several investigators have shown that nerves in the dermal layer of the skin located near blood vessels contain substance P (Hokfelt et al. 1980a, 1980b; Wallengren et al. 1987; Wallengren, 1997; Holzer, 1998). Despite evidence that substance P is predominantly located on the afferent arm of sensory nerves involved in axon reflex-mediated vasodilatation, autonomic–sensory nerve interactions have been suggested (Weihe & Hartschuh, 1988).
The experimental model used in this study was based on our previous observation of NK1 receptor desensitization following microdialysis infusions of substance P (Wong et al. 2005). We chose to exploit these observations to investigate the contribution of NK1 receptors and, indirectly, substance P, in active vasodilatation in order to circumvent non-specific effects that can be associated with the use of traditional receptor antagonists. Initial pilot work in our laboratory using different NK1 receptor antagonists failed to attenuate substance P-induced vasodilatation. Increasing the concentration of the different NK1 receptor antagonists resulted in cutaneous vasodilatation and, at the highest concentrations used, caused maximal cutaneous vasodilatation. As such, we chose to use a novel, innovative experimental model to investigate the contribution of NK1 receptors in active vasodilatation.
We cannot completely rule out the possibility that infusion of substance P in skin sites prior to the whole-body heating period resulted in tachyphylaxis of the blood vessel and thus is responsible for the observed attenuated skin blood flow response to whole-body heating. However, this seems unlikely as there was no significant difference among treatment sites in the raw maximal CVC values achieved during SNP infusion, indicating sites receiving substance P responded in a similar manner to control and l-NAME-only sites (Table 1). These data suggest pretreatment with substance P did not render the vasculature insensitive to further stimuli and it was able to maximally vasodilate to a pharmacological agent known to elicit maximal vasodilatation in human skin (Kellogg et al. 1999; Minson et al. 2001). As such, we are confident the findings in this study are not simply due to tachyphylaxis. Alternatively, it is possible that the IP3 pathway was desensitized following substance P infusion, and that cutaneous active vasodilatation occurs via this pathway from a different mechanism than NK1 receptor activation. We are not able to exclude this as a possibility. As we used substance P prior to whole-body heating, and have previously demonstrated a desensitization consistent with NK1 receptor stimulation, we are confident that data from the present investigation are reflective of the NK1 receptor pathway in active vasodilatation and not due to non-specific interactions.
In conclusion, this is the first study to investigate the role of NK1 receptor activation and, indirectly, substance P, in cutaneous active vasodilatation and we have presented evidence that activation of NK1 receptors is involved in this response. Consistent with previous reports, the data further suggest that a small portion of the NO component may be attributable to NK1 receptor activation, while the majority of the NO component probably stems from other sources.
Acknowledgments
The authors would like to thank all of the subjects for their time and willingness to participate in this study. The authors thank Dr Ulla Kopp of The University of Iowa for her insightful comments and suggestions. This study was conducted by Brett J. Wong in partial fulfilment of the requirements for the degree of Doctor of Philosophy in the Department of Human Physiology at the University of Oregon. Funding for this study was provided in part by the Eugene Evonuk Foundation Fellowship in Environmental or Stress Physiology (Wong) and National Institutes of Health Grant HL-70928 (Minson).
References
- Bennett LA, Johnson JM, Stephens DP, Saad AR, Kellogg DL. Evidence for a role for vasoactive intestinal peptide in active vasodilatation in the cutaneous vasculature in humans. J Physiol. 2003;552:223–232. doi: 10.1113/jphysiol.2003.042135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binggeli C, Spieker LE, Corti R, Noll G. Statins enhance postischemic hyperemia in the skin circulation of hypercholesterolemic patients: a monitoring test of endothelial dysfunction for clinical practice? J Am Coll Cardiol. 2003;42:71–77. doi: 10.1016/s0735-1097(03)00505-9. [DOI] [PubMed] [Google Scholar]
- Church MK, Suhad E-L, Caulfield JP. Neuropeptide-induced secretion from human skin mast cells. Int Arch Allergy Appl Immunol. 1991;94:310–318. doi: 10.1159/000235393. [DOI] [PubMed] [Google Scholar]
- Fox RH, Hilton SM. Bradykinin formation in human skin as a factor in heat vasodilatation. J Physiol. 1958;142:219–232. doi: 10.1113/jphysiol.1958.sp006011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant RT, Holling HE. Further observations on the vascular responses of the human limb to body warming: evidence for sympathetic vasodilator nerves in the normal subject. Clin Sci. 1938;3:273–285. [Google Scholar]
- Grant RT, Pearson RSB. The blood circulation in the human limb; observations on the differences between the proximal and the distal parts and remarks on the regulation of core body temperature. Clin Sci. 1938;3:119–139. [Google Scholar]
- Hokfelt T, Johnsson O, Ljungdahl A, Lundberg JM, Schultzberg M. Peptidergic neurones. Nature. 1980a;284:515–521. doi: 10.1038/284515a0. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Lundberg JM, Schulzberg M, Johansson O, Ljungdahl A, Rehfeld J. Coexistence of peptides and putative transmitters in neurons. Adv Biochem Psychopharmacol. 1980b;22:1–23. [PubMed] [Google Scholar]
- Holzer P. Neurogenic vasodilatation and plasma leakage in the skin. Gen Pharmacol. 1998;30:5–11. doi: 10.1016/s0306-3623(97)00078-5. [DOI] [PubMed] [Google Scholar]
- Huttunen M, Harvima IT, Ackermann L, Harvima RJ, Naukkarinen A, Horsmanheimo M. Neuropeptide- and capsaicin-induced histamine release in skin monitored with the microdialysis technique. Acta Derm Venereol. 1996;76:205–209. doi: 10.2340/0001555576205209. [DOI] [PubMed] [Google Scholar]
- Jansen I, Alafaci C, McCulloch J, Uddman R, Edvinsson L. Tachykinins (substance P, neurokinin A, neuropeptide K, and neurokinin B) in the cerebral circulation: vasomotor responses in vitro and in situ. J Cereb Blood Flow Metab. 1991;11:567–575. doi: 10.1038/jcbfm.1991.105. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Crandall CG, Liu Y, Charkoudian N, Johnson JM. Nitric oxide and cutaneous active vasodilatation during heat stress in humans. J Appl Physiol. 1998;85:824–829. doi: 10.1152/jappl.1998.85.3.824. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Liu Y, Kosiba IF, O'Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol. 1999;86:1185–1190. doi: 10.1152/jappl.1999.86.4.1185. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Pergola PE, Piest KL, Kosiba WA, Crandall CG, Grossmann M, Johnson JM. Cutaneous active vasodilatation in humans is mediated by cholinergic nerve cotransmission. Circ Res. 1995;77:1222–1228. doi: 10.1161/01.res.77.6.1222. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Zhao JL, Friel C, Roman LJ. Nitric oxide concentration increases in the cutaneous interstitial space during heat stress in humans. J Appl Physiol. 2003;94:1971–1977. doi: 10.1152/japplphysiol.00826.2002. [DOI] [PubMed] [Google Scholar]
- Klede M, Clough G, Lischetzki G, Schmelz M. The effect of the nitric oxide synthase inhibitor N-nitro-l-argining-methyl ester on neuropeptide-induced vasodilatation and protein extravasation in human skin. J Vasc Res. 2003;40:105–114. doi: 10.1159/000070707. [DOI] [PubMed] [Google Scholar]
- Kopp UC, Farley DM, Smith LA. Renal sensory receptor activation causes prostaglandin-dependent release of substance P. Am J Physiol Regul Integr Comp Physiol. 1996;270:R720–R727. doi: 10.1152/ajpregu.1996.270.4.R720. [DOI] [PubMed] [Google Scholar]
- Kopp UC, Smith LA. Role of prostaglandins in renal sensory receptor activation by substance P and bradykinin. Am J Physiol Regul Integr Comp Physiol. 1993;265:R544–R551. doi: 10.1152/ajpregu.1993.265.3.R544. [DOI] [PubMed] [Google Scholar]
- Krause JE, Hershey AD, Dykema PE, Takeda Y. Molecular biological studies on the diversity of chemical signaling in tachykinin peptidergic neurons. Ann NY Acad Sci U S A. 1990;579:254–272. doi: 10.1111/j.1749-6632.1990.tb48367.x. [DOI] [PubMed] [Google Scholar]
- Mann PT, Southwell BR, Furness JB. Internalisation of the neurokinin 1 receptor in rat myenteric neurons. Neuroscience. 1999;91:353–362. doi: 10.1016/s0306-4522(98)00595-8. [DOI] [PubMed] [Google Scholar]
- Marriott DR, Wilkin GP, Wood JN. Substance P-induced release of prostaglandins from astrocytes: regional specialisation and correlation with phosphoinositol metabolism. J Neurochem. 1991;56:259–265. doi: 10.1111/j.1471-4159.1991.tb02590.x. [DOI] [PubMed] [Google Scholar]
- McCord GR, Cracowski J-L, Minson CT. Prostanoids contribute to cutaneous active vasodilation in humans. Am J Physiol Regul Integr Comp Physiol. 2006;291:R596–R602. doi: 10.1152/ajpregu.00710.2005. [DOI] [PubMed] [Google Scholar]
- Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol. 2001;91:1619–1626. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- Minson CT, Holowatz LA, Wong BJ, Kenney WL, Wilkins BW. Decreased nitric oxide- and axon reflex- mediated cutaneous vasodilatation with age during local heating. J Appl Physiol. 2002;93:1644–1649. doi: 10.1152/japplphysiol.00229.2002. [DOI] [PubMed] [Google Scholar]
- Petersen LJ, Poulsen LK, Søndergaard J, Skov PS. The use of cutaneous microdialysis to measure substance P-induced histamine release in human skin in vivo. J Allergy Clin Immunol. 1994;94:773–783. doi: 10.1016/0091-6749(94)90186-4. [DOI] [PubMed] [Google Scholar]
- Quartara L, Maggi CA. The tachykinin receptor. Part I: ligands and mechanisms of cellular activation. Neuropeptides. 1997;31:537–563. doi: 10.1016/s0143-4179(97)90001-9. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Milner P, Hudlická O, Kristek F, Burnstock G. Substance P is released from the endothelium of normal and capsaicin-treated rat hind-limb vasculature, in vivo, by increased flow. Circ Res. 1990;66:1178–1183. doi: 10.1161/01.res.66.5.1178. [DOI] [PubMed] [Google Scholar]
- Regoli D, Boudon A, Fauchere JL. Receptors and antagonists for substance P and related peptides. Pharmacol Rev. 1994;46:551–599. [PubMed] [Google Scholar]
- Roddie IC, Shepherd JT, Whelan RF. The contribution of constrictor and dilator nerves to the skin vasodilatation during body heating. J Physiol. 1957a;136:489–497. doi: 10.1113/jphysiol.1957.sp005775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roddie IC, Shepherd JT, Whelan RF. The vasomotor nerve supply to the skin and muscle of human forearm. Clin Sci. 1957b;16:67–74. [PubMed] [Google Scholar]
- Salvemini D. Regulation of cyclooxygenase enzymes by nitric oxide. Cell Mol Life Sci. 1997;53:576–582. doi: 10.1007/s000180050074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvemini D, Misko TP, Masferer JL, Seibert K, Currie MG, Needlman P. Nitric oxide activates cyclooxygenase enzymes. Pharmacology. 1993;90:7240–7244. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvemini D, Settle SL, Masferer JL, Seibert K, Currie MG, Needlman P. Regulation of prostaglandin production by nitric oxide; an in vivo analysis. Br J Pharmacol. 1995;114:1171–1178. doi: 10.1111/j.1476-5381.1995.tb13330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage MV, Brengelmann GL, Buchan AMJ, Freund PR. Cystic fibrosis, vasoactive intestinal polypeptide, active cutaneous vasodilation. J Appl Physiol. 1990;69:2149–2154. doi: 10.1152/jappl.1990.69.6.2149. [DOI] [PubMed] [Google Scholar]
- Shastry S, Dietz NM, Halliwill JR, Reed AS, Joyner MJ. Effects of nitric oxide synthase inhibition on cutaneous vasodilatation during body heating in humans. J Appl Physiol. 1998;85:830–834. doi: 10.1152/jappl.1998.85.3.830. [DOI] [PubMed] [Google Scholar]
- Shastry S, Minson CT, Wilson SA, Dietz NM, Joyner MJ. Effects of atropine and l-NAME on cutaneous blood flow during body heating in humans. J Appl Physiol. 2000;88:467–472. doi: 10.1152/jappl.2000.88.2.467. [DOI] [PubMed] [Google Scholar]
- Shibasaki M, Wilson TE, Cui J, Crandall CG. Acetylcholine release from cholinergic nerves contributes to cutaneous vasodilatation during heat stress. J Appl Physiol. 2002;93:1947–1952. doi: 10.1152/japplphysiol.00036.2002. [DOI] [PubMed] [Google Scholar]
- Wallengren J. Vasoactive peptides in the skin. J Investig Dermatol Symp Proc. 1997;2:49–55. doi: 10.1038/jidsymp.1997.11. [DOI] [PubMed] [Google Scholar]
- Wallengren J, Ekman R, Sundler F. Occurrence and distribution of neuropeptides in the human skin. Acta Derm Venereol. 1987;67:185–192. [PubMed] [Google Scholar]
- Weidner C, Klede M, Rukwied R, Lischetzki G, Neisius U, Skov PS, Petersen LJ, Schmelz M. Acute effects of substance P and calcitonin gene-related peptide in human skin – a microdialysis study. J Invest Dermatol. 2000;115:1015–1020. doi: 10.1046/j.1523-1747.2000.00142.x. [DOI] [PubMed] [Google Scholar]
- Weihe E, Hartschuh W. Multiple peptides in cutaneous nerves: regulators under physiological conditions and a pathogenetic role in skin disease? Semin Dermatol. 1988;7:284–300. [PubMed] [Google Scholar]
- Wilkins BW, Chung LH, Tublitz NJ, Wong BJ, Minson CT. Mechanisms of vasoactive intestinal peptide (VIP)-mediated vasodilation in human skin. J Appl Physiol. 2004;97:1291–1298. doi: 10.1152/japplphysiol.00366.2004. [DOI] [PubMed] [Google Scholar]
- Wilkins BW, Holowatz LA, Wong BJ, Minson CT. Nitric oxide is not permissive for cutaneous active vasodilatation in humans. J Physiol. 2003;548:963–969. doi: 10.1113/jphysiol.2002.035931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins BW, Wong BJ, Tublitz NJ, McCord GR, Minson CT. The vasoactive intestinal peptide fragment VIP10–28 and active vasodilation in human skin. J Appl Physiol. 2005;99:2294–2301. doi: 10.1152/japplphysiol.00500.2005. [DOI] [PubMed] [Google Scholar]
- Wong BJ, Tublitz NJ, Minson CT. Neurokinin-1 receptor desensitisation to consecutive microdialysis infusions of substance P in human skin. J Physiol. 2005;568:1047–1056. doi: 10.1113/jphysiol.2005.095372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong BJ, Wilkins BW, Holowatz LA, Minson CT. Nitric oxide synthase inhibition does not alter the reactive hyperemic response in the cutaneous circulation. J Appl Physiol. 2003;95:504–510. doi: 10.1152/japplphysiol.00254.2003. [DOI] [PubMed] [Google Scholar]
- Wong BJ, Wilkins BW, Minson CT. H1 but not H2 histamine receptor activation contributes to the rise in skin blood flow during whole body heating in humans. J Physiol. 2004;560:941–948. doi: 10.1113/jphysiol.2004.071779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousufzai SYK, Akhtar RA, Abdel-Latif AA. Effects of substance P on inositol triphosphate accumulation on contractile responses and on arachidonic acid release and prostaglandin biosynthesis in rabbit iris sphincter muscle. Exp Eye Res. 1986;43:215–226. doi: 10.1016/s0014-4835(86)80089-6. [DOI] [PubMed] [Google Scholar]
- Zhao JL, Pergola PE, Roman LJ, Kellogg DL., Jr Bioactive nitric oxide concentration does not increase during reactive hyperemia in human skin. J Appl Physiol. 2004;96:628–632. doi: 10.1152/japplphysiol.00639.2003. [DOI] [PubMed] [Google Scholar]