Abstract
A phasic activation of small-conductance Ca2+-dependent K+ channels (SK channels) underlies spike-afterhyperpolarizations and spike-independent, transient hyperpolarizations in juvenile substantia nigra neurons. Outward current pulses that cause the spike-independent hyperpolarizations result from ryanodine receptor-mediated Ca2+ release from intracellular stores. To study the modulation of excitability by the outward current pulses, we recorded from GABAergic pars reticulata neurons of mice at postnatal days 12–16. We induced a prolongation of SK channel open states by 1-ethyl-2-benzimidazolinone (1-EBIO). In addition to a prolongation of spike-afterhyperpolarizations, 1-EBIO (200 μm) potentiated outward current pulses by increasing their duration. Neurons were manipulated by current injection to display continuous or discontinuous discharge. Despite the prolongation of the outward current pulses by 1-EBIO, continuous action potential discharge became more regular, although its frequency declined. Durations of silent periods (periods of >2× average interspike interval) increased. Caffeine (1 mm) further increased the duration of such silent periods. Caffeine, however, had no effect at short interspike intervals (<600 ms). Cyclopiazonic acid (10 μm) silenced discharge in 1-EBIO, but discharge reappeared with the depletion of Ca2+ stores. We conclude that the modulation of excitability by an activation of SK channels through ryanodine receptor-mediated release of Ca2+ critically depends on the frequency of discharge. Outward current pulses occur only if interspike intervals exceed the duration of spike-afterhyperpolarizations. In this instance, the phasic, spike-independent activation of SK channels supports pauses to interrupt autonomous discharge in juvenile GABAergic pars reticulata neurons.
The principal neurons of the substantia nigra (SN) are the GABAergic pars reticulata (SNr) neurons and the dopamine (DA) neurons in the pars compacta (SNc). The two types of neurons serve very different functions. GABAergic SNr neurons convey the final output signal of the basal ganglia system. DA neurons determine the dopamine supply for this system. In both types of neurons, rises in cytosolic Ca2+ activate small-conductance Ca2+-dependent K+ channels (SK channels). SK channels on the plasma membrane of DA neurons are formed by SK3 subunits (Wolfart et al. 2001; Sarpal et al. 2004), but by SK2 subunits in GABAergic SNr neurons (Stocker & Pedarzani, 2000).
SK channels are highly sensitive and fast Ca2+ sensors. They are ideally suited to couple intracellular Ca2+ levels to the membrane potential (for a review see Stocker, 2004). A local rise in cytosolic Ca2+ levels rapidly increases the open probability allowing for a more or less synchronous activation of SK channels. In juvenile GABAergic SNr neurons, tonic SK channel activity may be responsible for a small sustained hyperpolarization because the SK channel-selective blocker apamin slightly depolarizes these neurons (Yanovsky et al. 2005). A more phasic SK channel activity produces local outward current pulses that hyperpolarize the cell membrane potential independently of action potentials. These current pulses, which are generated by ryanodine receptor (RyR)-mediated Ca2+ release from intracellular stores, have been termed spontaneous miniature outward currents (SMOCs; Cui et al. 2004; Yanovsky et al. 2005). Here we will refer to them as outward current pulses (OCPs), because there is no proof that they are spontaneous or miniature currents. Ca2+ influx through voltage-dependent Ca2+ channels activates SK channels even more synchronously during the afterhyperpolarization (AHP) which follows each action potential (Shepard & Bunney, 1991; Ping & Shepard, 1996; Wolfart et al. 2001; Wolfart & Roeper, 2002; Atherton & Bevan, 2005; Yanovsky et al. 2005).
It is not entirely clear whether OCP-induced hyperpolarizations modulate the action potential discharge of developing SN neurons. Pharmacological depletion of Ca2+ stores did not affect the firing rate of DA neurons, suggesting that OCP-induced hyperpolarizations do not modulate the firing rate of these neurons (Seutin et al. 2000). Cui et al. (2004) suggested that the depletion of Ca2+ stores transformed the irregular firing pattern of postnatal DA neurons into an adult-like pacemaker pattern. When hyperpolarizing pulses were randomly injected into regularly firing neurons, irregular discharge was produced. However, RyR-mediated Ca2+ release from intracellular stores amplifies the AHP (Wolfart & Roeper, 2002). Therefore, a disadvantage of this experimental approach is that pharmacological depletion of intracellular stores reduces AHPs and, thereby, increases firing frequency. This problem can be avoided by studying GABAergic SNr neurons, as the AHP of these neurons is not amplified by RyR-mediated Ca2+ release from intracellular stores (Yanovsky et al. 2005).
1-Ethyl-2-benzimidazolinone (1-EBIO) is known to stabilize the binding of Ca2+ to SK channels, prolonging the time they are open (Pedarzani et al. 2001; Cingolani et al. 2002). Of course, 1-EBIO will potentiate any hyperpolarization that occurs due to an activation of SK channels. For GABAergic SNr neurons, we had to be aware of the fact that 1-EBIO would prolong OCPs and potentiate AHPs and, possibly, asynchronous openings of SK channels. By combining 1-EBIO applications with pharmacological manipulations to change Ca2+ release from intracellular stores in GABAergic SNr neurons, we expected to get a better insight into the functional role of OCP-induced hyperpolarizations.
We can conclude from our findings that OCP-induced hyperpolarizations play a role in a voltage range that is limited to membrane potentials slightly negative to spike threshold. In this range they have a dual role. They hyperpolarize the membrane, thereby prolonging periods without discharge, because they oppose inward currents that generate an autonomous discharge in GABAergic SNr neurons (Atherton & Bevan, 2005). At the same time, they restrain Na+ channel inactivation and, hence, a spike threshold increase that results from cells staying at rather positive membrane potentials for prolonged time periods.
Methods
Brain slice preparation
The method used for the preparation and maintenance of substantia nigra slices has been described in detail previously (Yanovsky et al. 2003, 2005). The number of animals used was kept to the minimum compatible with achieving statistically significant results. Experiments were approved by the animal welfare coordinator of our institution, according to article 1 of the German Animal Welfare Act 1998. In brief, C57BL/6 mice (12 days to 16 days old) were killed by rapid decapitation without anaesthesia in accordance with national and institutional guidelines, their brains removed and put into oxygenated cold solution containing (mm): NaCl 124, KCl 5, MgSO4 1.3, KH2PO4 1.25, CaCl2 2.5, NaHCO3 26, glucose 10, 6,7-dinitroquinoxaline-2,3-dione (DNQX, Axxora, Grünberg, Deutschland) 0.01; pH 7.4. Cross-sections were made through the forebrain at the level of the optic chiasm and through the rostral side of the cerebellum. Substantia nigra slices (250 μm thick) were obtained using a custom-built vibratome by cross-sectioning the block of brainstem extending from the pons to the hypothalamus in a caudal to rostral direction. Slices were allowed to equilibrate for at least 1 h at room temperature before commencement of recording.
Recording from visualized neurons
Slices were placed in a small (∼400 μl), submerged glass-bottomed recording chamber. Neurons were visualized by infrared video imaging (Newvicon C2400, Hamamatsu, Hamamatsu City, Japan) and differential interference optics (Stuart et al. 1993) using an upright compound microscope (Zeiss Axioscope FS, Zeiss, Oberkochen, Germany). Slices were superfused continuously at a rate of 2.0 ml min−1 with an oxygenated solution containing (mm): NaCl 127, KCl 2, MgSO4 1.3, KH2PO4 1.25, CaCl2 2.5, NaHCO3 26, and glucose 10, pH 7.35, saturated with 95% O2–5% CO2. Fast synaptic transmission was blocked by the AMPA receptor antagonist DNQX (10 μm), the NMDA receptor antagonist (E)-(±)-2-amino-4-methyl-5-phosphono-3-pentenoic acid (CGP37849, 1 μm, Biotrend (Tocris), Köln, Germany), the GABAA receptor antagonist 2-(3-carboxypropyl)-3-amino-6-(4-methoxyphenyl)pyridazinium bromide (SR-95531, gabazine, 1 μm, Sigma, Taufkirchen, Germany), and the glycine receptor antagonist, strychnine hydrochloride (1 μm, Sigma).
For gramicidin-perforated patch-clamp recording, (Akaike, 1996) pipettes (4.5–6.0 MΩ) were tip-filled with (mm): K-gluconate 145, KCl 5.0, Hepes 10 (pH 7.3), and back-filled with the same solution containing 50 μg ml−1 of gramicidin (Sigma). Gramicidin stock solution was prepared by dissolving it in DMSO (5 mg ml−1), and was then diluted to the final concentration with the pipette solution. Recordings were performed with a discontinuous single-electrode voltage-clamp amplifier (npi, Tamm, Germany) at room temperature (22–24°C). Non-dopaminergic (GABAergic) neurons were distinguished from DA neurons as previously described (Yanovsky et al. 2003, 2005). In brief, they were classified by their location inside the SN and by their electrical properties, which included hyperpolarization-activated inward current (Ih) and spike duration. To elicit Ih, a hyperpolarizing square pulse (–50 mV, holding potential –60 mV) of 1 s duration was applied to all cells, and the ratio of the instantaneous current measured at the end of the capacitive transient over the current measured at the end of the voltage command was calculated. We refer to SNc neurons with a pronounced Ih (ratio <0.6) and broad spikes (half-width >1.5 ms) and SNr neurons without or with small Ih (ratio > 0.6) and short duration spikes (half-width < 1 ms) as DA and GABAergic neurons, respectively (Lacey et al. 1987; Nakanishi et al. 1987; Grace & Onn, 1989; Yung et al. 1991; Häusser et al. 1995; Mercuri et al. 1995; Richards et al. 1997). SNr neurons with properties of DA neurons, and SNc neurons without properties of DA neurons were not considered in this study. For voltage clamp, switching frequency was 30 kHz. Experiments were started when series resistance was below 100 MΩ, which was determined from the steady-state responses to voltage commands. Experiments were discontinued as soon as the cells stopped discharging or required more than 30 pA of depolarizing current to maintain continuous discharge. Currents were filtered at 2 kHz with a four-pole Bessel filter, sampled at 5 kHz using pClamp9 software (Axon Instruments, Union City, CA, USA), and stored on a DAT recorder for off-line analysis.
Analysis was done using the following software: MiniAnalysis (Synaptosoft, Decatur, GA, USA), Igor Pro 3.01 (WaveMetrics Inc., Lake Oswego, OR, USA), and Prism4 (GraphPad, San Diego, CA, USA). In addition to the amplitude and frequency of OCPs, we measured the area enveloped by these composite events. The duration of action potentials, interspike intervals, coefficient of variation (CV = s.d./mean × 100%), firing rate, and characteristics of AHP in bridge or current-clamp mode were determined. Current traces were low-pass filtered (40–80 Hz) for display.
The effects on discharge of 1-EBIO, caffeine, and cyclopiazonic acid (CPA) were determined from the mean interval measured during 60–90 s in control and at maximal effect of drug application. For the quantification of effects on OCPs, we determined the frequency and the sum of the areas of OCPs recorded within 90 s before, during and after drug application. Because resting membrane potential cannot be determined for spontaneously active neurons, the threshold for spike generation (measured as a point in which dV/dt exceeded 10 mV ms−1 (from ∼0.5 mV ms−1)) was used as reference for measurements of AHP (25 measurements for every cell in control and at the maximal drug effect). Group data are presented as mean ± s.e.m. Student's t test or the Mann–Whitney non-parametric test was used, where appropriate, to determine statistical significance. Differences were considered statistically significant when P < 0.05.
Drugs and chemicals
Apamin, 1,3,7-trimethylxanthine (caffeine), and gramicidin, were obtained from Sigma. 4-(2-[7-Amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]-ethyl) phenol (ZM 241385), (6aR,11aS,11bR)-rel-2,6,6a,7,11a,11b-hexahydro-11-hydroxy-7,7-dimethyl-10-acetyl-9H-pyrrolo[1′,2′:2,3]isoindolo[4,5,6-cd]indol-9-one (cyclopiazonic acid (CPA)), 1H-pyrrole-2-carboxylic acid (ryanodine), 1-ethyl-2-benzimidazolinone (1-EBIO) and tetrodotoxin (TTX) were obtained from Tocris. All drugs were dissolved to their final concentration in the recording solution and bath applied for the times indicated with the description of the experiments. The adenosine A2A receptor antagonist ZM 241385 was always added when caffeine effects were studied. Stock solutions were prepared using H2O, recording solution or DMSO solution. In the case of DMSO, the final concentration never exceeded 0.2 μl DMSO solution/100 ml recording solution.
Results
Pharmacological properties of OCPs
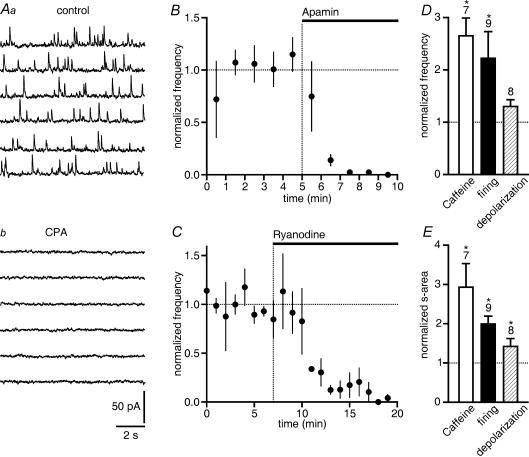
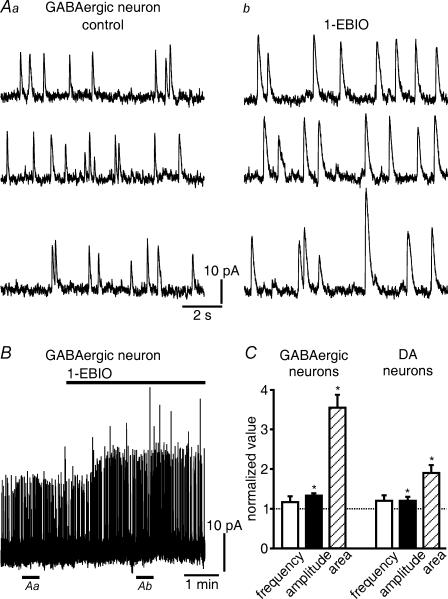
Neurons of the substantia nigra were classified as in our earlier studies (Yanovsky et al. 2003, 2005; see also Methods). OCP-induced hyperpolarizations (Seutin et al. 1998, 2000) and OCPs of developing DA neurons (Cui et al. 2004) have been previously described. Consistent with our previous report (Yanovsky et al. 2005), we observed OCPs in the majority (∼70%) of GABAergic SNr neurons (Vh –60 mV) from juvenile (P12–P16) mice. The aim of our first experiments was to confirm that randomly occurring OCPs, which we recorded in the presence of antagonists for glutamatergic, GABAergic and glycinergic synaptic transmission (Fig. 1A), represented phasic SK channel activations by RyR-mediated release of Ca2+ from intracellular stores. OCPs could be recorded even after TTX (0.3 μm) application to prevent spike generation. Superfusion with CPA (10 μm), which depletes intracellular Ca2+ stores by an inhibition of endoplasmic reticulum Ca2+-ATPases (Taylor & Broad, 1998), abolished OCPs within 3–5 min (Fig. 1A; n = 8). OCPs were prevented by the selective SK channel blocker apamin (0.3 μm; Fig. 1B). Ryanodine (10 μm), which blocks ryanodine receptors in a partially open state, first briefly potentiated OCPs but then completely eliminated them (Fig. 1C). Caffeine (1 mm), which increases the Ca2+ sensitivity of RyRs (Ehrlich et al. 1994), strongly enhanced OCP frequency (Fig. 1D). Because of the simultaneous prolongation of OCP duration, the probability for a superposition of OCPs became higher. For the sake of reliability, we counted superimposed OCPs as a single event, thus underestimating the frequency increase. We therefore also determined the sum of the area enveloped by all OCPs occurring during 90 s (Fig. 1E). After an action potential discharge lasting for 1–3 min, OCP frequency and the sum of OCP areas were also elevated (Fig. 1D and E). In addition, the summed OCP area increased when moderately shifting the holding potential to more positive values (from –60 to –55 mV; Fig. 1E). Taken together, these findings indicate that OCPs are generated by RyR-mediated, Ca2+-induced Ca2+ release from intracellular stores as has been shown previously for OCPs in DA neurons (Seutin et al. 2000; Cui et al. 2004). As would be expected for currents generated by an activation of SK channels, 1-EBIO (200 μm) increased the amplitude of OCPs (11.7 ± 0.9 pA in control versus 15.5 ± 1.2 pA in 1-EBIO for nine GABAergic SNr neurons; P < 0.0005; Fig. 2A and B), but had an even greater effect on the area enveloped by the OCPs (P < 0.0005; Fig. 2C). There was no increase in their frequency (about 0.4 Hz; Fig. 2C). For comparison, the changes were less pronounced in DA neurons (for amplitudes: 7.9 ± 0.6 pA in control versus 9.5 ± 1.3 pA in 1-EBIO; n = 6; P < 0.01; Fig. 2C) than in GABAergic SNr neurons. Potential reasons could be that SK channels in DA neurons are composed of a different subunit (Wolfart et al. 2001; Sarpal et al. 2004) and exhibit OCPs of longer duration (Yanovsky et al. 2005).
Figure 1. Randomly occurring outward current pulses (OCPs) are generated by RyR-mediated Ca2+ release from intracellular stores in GABAergic SNr neurons.
A, cyclopiazonic acid (CPA, 10 μm) abolished OCPs within minutes. Traces shown were recorded at a holding potential of –60 mV and sampled for one minute in control condition (Aa) and in CPA (Ab). Calibration in Ab applies also to Aa. B and C, summary graphs illustrating normalized OCP frequency over 1 min, before and following application of apamin (0.3 μm, n = 5) and ryanodine (10 μm, n = 3). B, apamin, which blocks small-conductance Ca2+-activated potassium channels, abolished OCPs. C, ryanodine (10 μm), which blocks ryanodine receptors, abolished OCPs (n = 3). D, frequency and E, sum of area (s-area) enveloped by OCPs were increased by caffeine (1 mm) and by action potential discharge recorded for 1–3 min. The summed OCP area was also enhanced upon depolarization by 5 mV (from –60 to –55 mV, E). The number of cells (D, E) and s.e.m. (B,C,D,E) are indicated in this and all other figures if appropriate. Asterisks (*P < 0.05) indicate a significant increase over baseline levels in this and all other figures (dashed line at 1.0 on y axis).
Figure 2. 1-EBIO potentiates OCPs.
A, the amplitudes of OCPs increased in 1-EBIO (200 μm). OCPs were recorded in control (Aa) and during 1-EBIO application (Ab). The periods for sampling these OCPs are indicated by horizontal lines underneath the trace in B. B, same cell as in A. Each upstroke is one OCP in this continuous recording. C, 1-EBIO markedly increased the area enveloped by a single OCP in GABAergic SNr neurons. There was little effect on amplitude, frequency was not changed. In DA neurons, which were recorded for comparison, the enlargement of the area was much less striking. OCPs occurring during 90 s were averaged, and the average in control normalized to 1 (n = 9 GABA cells and 6 DA cells, respectively). *P < 0.01.
Effects of 1-EBIO on action potential discharge
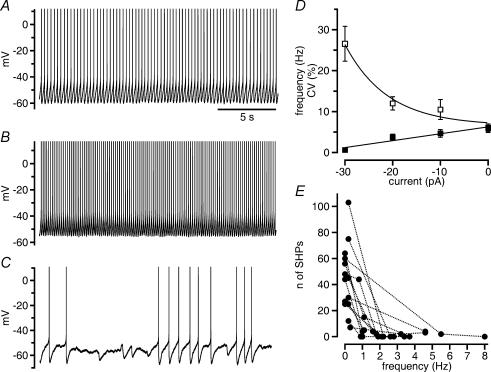
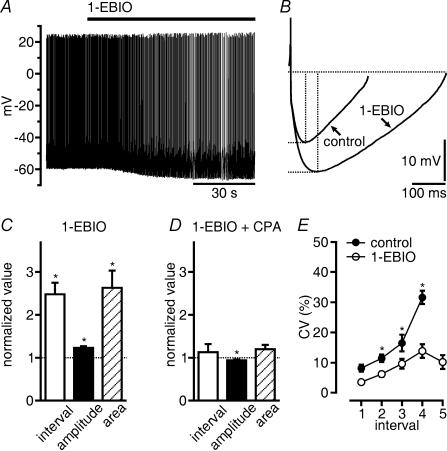
We next examined the possibility that, by prolonging OCPs, 1-EBIO could reduce the regularity of action potential discharge in GABAergic SNr neurons. Spontaneous discharge is a feature of GABAergic SNr neurons in slices (Atherton & Bevan, 2005; Yanovsky et al. 2005). About 80% of all GABAergic SNr neurons we recorded displayed continuous action potential discharge, the rate of which increased or decreased linearly over a range of 2–12 Hz upon the injection of depolarizing or hyperpolarizing direct current (< 30 pA), respectively (Fig. 3A–D). During the negative shift in the membrane potential, firing of GABAergic SNr neurons became increasingly irregular (Fig. 3C and D). Periods of varying durations occurred during which the cells did not generate action potentials (‘silent periods’; Richards et al. 1997; in our study, this was arbitrarily defined as an interruption of discharge for a time period >2× the average interspike interval), but displayed action potential-independent hyperpolarizations (Fig. 3C and E). Cells that displayed the latter discharge modality at rest underwent the opposite change upon the injection of depolarizing current (<30 pA). Cells that could not be driven to regular discharge with currents <30 pA were excluded from our study. Interspike intervals during continuous autonomous firing of GABAergic SNr neurons were strongly prolonged by 1-EBIO (from 350 ± 49 ms in control to 750 ± 92 ms in 1-EBIO; n = 21 cells; Fig. 4A and C). The change was consistent with changes induced by 1-EBIO in the AHP following each action potential. The AHP area markedly (by 163%) increased, whereas there was only a small increase in AHP amplitude (by 24%; amplitude in control 21 ± 1.2 mV; in 1-EBIO 26 ± 1.1 mV; P < 0.0001; n = 21 GABAergic SNr neurons; Fig. 4B and C).
Figure 3. OCP-induced hyperpolarizations occur during pauses interrupting discharge.
A–C, frequency and modality of spontaneous firing change with membrane polarization by current injection. Spontaneous discharge (A) increased in frequency upon injection of depolarizing direct current (10 pA, B) and decreased in frequency upon injection of the same amount of hyperpolarizing current (C). In the latter, the decrease in frequency was associated with the occurrence of pauses during which action potential-independent hyperpolarizations are visible. Time scale in A applies also to B and C, action potentials truncated. D, plot of the dependence of firing frequency (▪) and coefficient of variation (CV, □) on current injection (n = 12 cells). There was a linear decrease in firing rate with the injection of hyperpolarizing direct current. CV increased markedly at very low firing rate. The point for the injection of –30 pA comprises only four neurons, because the other eight neurons stopped firing. E, the occurrence of action potential-independent spontaneous hyperpolarizations (SHPs) decreased rapidly with an increase in firing frequency. Points connected by lines are from the same cells (n = 14).
Figure 4. 1-EBIO reduces discharge frequency and increases AHP area.
A, spontaneous firing of a GABA neuron is slowed down by 1-EBIO (200 μm). B, five AHPs in control and in 1-EBIO of the neuron shown in A were averaged and superimposed to illustrate the increase in amplitude and duration. C, summary of the increase in interspike interval, of the amplitude and area of AHPs of 21 GABAergic SNr neurons in 1-EBIO. Mean values of interspike intervals were calculated from intervals between spikes occurring during 90 s and normalized. Amplitude and duration of AHPs were measured as indicated by the dashed lines in B, and averages of 25 events were normalized. 1-EBIO potentiated AHP area more strongly than AHP amplitude. D, CPA (10 μm) applied in 1-EBIO slightly decreased AHP amplitude. CPA had no effect on interspike intervals of spontaneous discharge and AHP area (n = 13 GABAergic SNr neurons, display as in C). E, 1-EBIO increased the regularity of discharge of GABAergic SNr neurons at low spike frequency. Coefficients of variation (CV) were plotted against interspike intervals collected from 21 cells in which frequency of continuous discharge was changed by constant current injection. Each point represents an average of data obtained from 3 to 19 measurements falling into a bin of 200 ms. Continuous discharge only was considered for these neurons. Because this discharge pattern was preserved in 1-EBIO at lower frequencies than in control, not all intervals had a matching control. Numbers on the abscissa denote consecutive intervals of 200 ms in duration beginning at 300 ms. *P < 0.05.
In 1-EBIO, discharge became more regular although the frequency of discharge decreased. The increase in the CV that normally accompanies decreasing discharge rate was shifted to lower frequencies (Fig. 4E). Thus, in 1-EBIO GABAergic SNr neurons discharged regularly even at a discharge rate as low as 1 Hz (ratio CV/mean interspike interval of discharge during 90 s: 0.040 ± 0.003 ms−1 in control and 0.015 ± 0.001 ms−1 in 1-EBIO excluding silent periods; P < 0.0001; n = 23 cells). To further elucidate the relationship between discharge regularity, AHP duration and OCPs, we compared effects of 1-EBIO on continuous discharge of GABAergic SNr neurons that displayed OCPs under voltage clamp with those that did not. Spontaneous discharge occurred in these cells at frequencies between 2 and 5 Hz. With the slowing of discharge by 1-EBIO to between 1 and 2 Hz, discharge became more regular in the cells with OCPs (CV = 10.2 ± 1.3% in control; 7.2 ± 0.9% in 1-EBIO; P < 0.01; n = 8 cells), while discharge in cells without OCPs remained regular (CV = 8.1 ± 1.1% in control; 7.1 ± 1.4% in 1-EBIO; n = 10 cells). Thus, the prolongation of OCP duration did not disturb the regularity of continuous discharge.
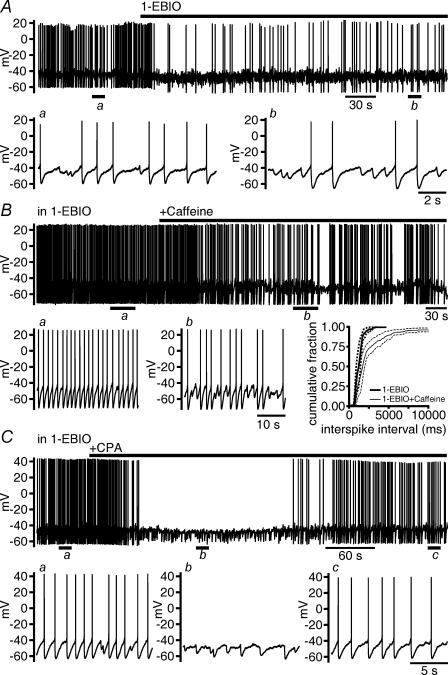
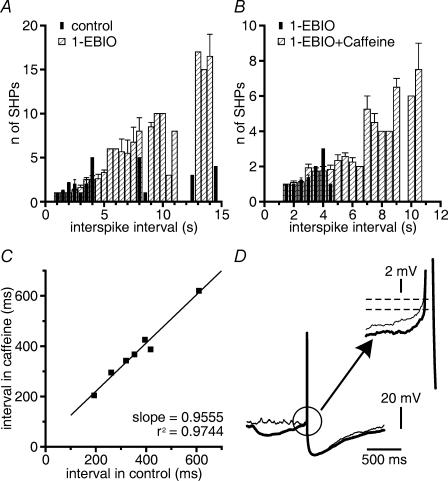
The prolongation of OCPs by 1-EBIO, however, was associated with an increase in the duration of silent periods that interrupted discharge (n = 9 cells; Fig. 5A). In cells firing action potentials, we observed action potential-independent hyperpolarizations that were probably generated by OCPs primarily during silent periods (Fig. 5Aa and Ab). By contrast, OCPs occurred randomly under voltage clamp. 1-EBIO did not significantly increase the frequency of OCPs (see above) under voltage clamp. To test whether there was an increase in the probability of occurrence of OCP-generated hyperpolarizations in 1-EBIO, we counted action potential-independent hyperpolarizations occurring between two consecutive spikes, while we changed the discharge rate by current injections. Sampling time was 90 s at each selected current injection, and the maximal interspike interval taken into consideration was 15 s (n = 5 cells tested in control and in 1-EBIO). In 1-EBIO we encountered long intervals between two consecutive spikes more often than in control. In control and in 1-EBIO (n = 5), action potential-independent hyperpolarizations could be observed only during pauses >1 s. An increase in the number of action potential-independent hyperpolarizations became obvious at frequencies of discharge <0.2 Hz (Fig. 6A). Thus, in 1-EBIO, the prolongation of silent periods coincided with the occurrence of a much larger number of action potential-independent hyperpolarizations of strongly enhanced duration.
Figure 5. Potentiation of OCPs is associated with prolonged silent periods interrupting discharge.
A, the prolongation of OCP-induced hyperpolarizations (displayed at an expanded time scale in a and b) by 1-EBIO (200 μm) was accompanied by a prolongation of silent periods. B, elevation of the frequency of OCP-induced hyperpolarizations (displayed at expanded time scale in a and b) by caffeine (1 mm) in 1-EBIO was accompanied by a prolongation of silent periods. Inset shows the cumulative histogram of interspike intervals for three cells. The histogram in caffeine is characterized by an increase in long interspike intervals. Average and standard errors (dashed lines) of data falling into a bin size of 10 ms (total number of intervals in 1-EBIO and 1-EBIO + caffeine, respectively: 61 and 125) were plotted. C, during the transient increase in the frequency of OCP-induced hyperpolarizations upon application of CPA (10 μm) in 1-EBIO, spike discharge ceased. Discharge restarted together with the later blockade of OCPs. OCP-induced hyperpolarizations and their deletion are displayed at higher time resolution in a, b and c.
Figure 6. OCPs occur during long interspike intervals.
A, the number of OCP-induced hyperpolarizations increases with the duration of interspike intervals. In the histogram the number of OCP-induced hyperpolarizations (SHPs) that occurred in 0.5 s bins was plotted for five cells in which the discharge frequency was varied by current injection. In 1-EBIO (200 μm) SHPs increased in number at interspike intervals >5 s. B, caffeine (1 mm, n = 3 cells) applied in 1-EBIO was able to increase the frequency of SHPs in spontaneously firing cells only at interspike intervals longer than 4 s. C, caffeine (1 mm) was unable to inhibit the discharge in control solution if interspike intervals were below 1 s (n = 7 cells). D, OCP-induced hyperpolarizations in 1-EBIO reduce spike threshold. The thresholds were measured for spikes triggered from baseline (thin line) or rising phase (thick line) of OCP-induced hyperpolarizations. For each condition 7–12 measurements were taken from each cell (P < 0.0005, n = 5 cells).
That the occurrence of action potential-independent hyperpolarizations depended on interspike interval could be further illustrated by applying caffeine which activates RyRs and, thereby, increases OCP frequency, but does not change AHPs of GABAergic SNr neurons (Yanovsky et al. 2005). In 1-EBIO, caffeine (1 mm; n = 3 cells; Fig. 5B) had a comparable inhibitory effect to caffeine application in control solution. The mean increase in interspike interval (mean interval 1.33 ± 0.22 s for 382 intervals in 1-EBIO and 5.25 ± 0.1 s for 103 intervals in 1-EBIO plus caffeine; P < 0.0001; Mann–Whitney test; n = 3 cells) resulted from prolonged silent periods, whereas short interspike intervals persisted as revealed by the analysis of the cumulative distribution of intervals (Fig. 5B inset). Caffeine elevated the number of action potential-independent hyperpolarizations in 1-EBIO only at frequencies of discharge <0.2 Hz (n = 3 cells; Fig. 6B). By contrast, at higher frequencies of discharge (2–5 Hz), caffeine had no effect on discharge (n = 7 cells; Fig. 6C).
To induce an effect opposite to the activation of RyRs by caffeine, we depleted Ca2+ stores in 1-EBIO using CPA. In 1-EBIO, CPA initially increased the frequency of action potential-independent hyperpolarizations (n = 8 cells), and the neurons eventually ceased to generate action potentials (n = 4 of 8 cells). The disappearance of action potential-independent hyperpolarizations with prolonged action of CPA was paralleled by a reappearance of discharge (Fig. 5C). In 1-EBIO, CPA (10 μm) decreased the AHP amplitude only slightly (from 24.8 ± 1.3 mV to 23.1 ± 1.2 mV; P < 0.01) and had no significant effect on AHP area (n = 13). Interspike intervals during trains of action potentials were not changed by CPA applied in 1-EBIO (n = 15 cells; Fig. 4D). The regularity of discharge in 1-EBIO was also not changed by CPA (ratio CV/mean interspike interval 0.020 ± 0.002 ms−1; n = 13 cells in CPA plus 1-EBIO versus 0.015 ± 0.001 ms−1; n = 23 cells in 1-EBIO for 90 s of discharge for each cell, silent periods excluded).
In mudpuppy cardiac neurons, OCPs generated by the activation of large-conductance, Ca2+-activated K+ channels reduce spike threshold (Parsons et al. 2002). We wondered whether OCPs generated by the activation of SK channels could have a similar effect. Indeed, action potential-independent hyperpolarizations in 1-EBIO had the capacity to reduce spike threshold (Fig. 6D). In each of five cells, the threshold of spike generation of spikes triggered from baseline was slightly more positive than the threshold of spikes triggered from the rising phase of action potential-independent hyperpolarizations (Fig. 6D). For each condition 7–12 measurements were taken from each cell. Threshold was –44 mV ± 1.4 mV if triggered from a flat baseline and –45 ± 1.4 mV if triggered from action potential-independent hyperpolarizations (P < 0.0005).
Discussion
Our main findings are as follows: the slowing of discharge of GABAergic SNr neurons by 1-EBIO (200 μm) is associated with an increase in AHP duration that fosters the regularity of discharge. The strong increase of OCP duration in 1-EBIO is accompanied by a prolongation of silent periods between periods of regular discharge. During silent periods, OCPs can restrain increases in spike threshold.
1-EBIO potentiated OCPs mainly by a prolongation of their duration. This increase in duration was much more pronounced in GABA than in DA neurons. In the absence of 1-EBIO, OCPs of DA neurons have a longer duration than those of GABAergic SNr neurons (Yanovsky et al. 2005). The difference may be related to the different channel composition. SK3 channels are expressed in DA neurons of SNc, SK2 channels are found in GABAergic SNr neurons (Stocker & Pedarzani, 2000). SK2 channels exhibit faster kinetics than SK3 channels (Glowatzki & Fuchs, 2000; Oliver et al. 2000).
1-EBIO stabilizes the interaction of SK channels and their Ca2+ sensor calmodulin, thereby increasing the open probability of SK channels (Xia et al. 1998; Oliver et al. 2000; Syme et al. 2000; Pedarzani et al. 2001). Very likely by the same mechanism, 1-EBIO increased the OCP and AHP duration in GABAergic SNr neurons, the latter being consistent with its effect in DA (Wolfart et al. 2001) and subthalamic neurons (Hallworth et al. 2003). It has been reported that OCPs may cause the discharge irregularity in postnatal DA neurons (Cui et al. 2004). Contrary to our expectation, the increase in OCP duration under voltage clamp was not associated with a more irregular continuous discharge in voltage recordings. In 1-EBIO, GABAergic SNr neurons discharged in a regular fashion, although discharge frequency was strongly reduced. The resulting discharge modality resembled the discharge of DA neurons.
1-EBIO elevates the Ca2+ sensitivity of SK channels. Mechanisms that determine the spatial and temporal pattern of rises in the intracellular Ca2+ level and, thereby, produce either tonic or phasic SK channel activity, are not altered. A longer open time of tonically active SK channels suffices to explain the slowing of discharge by 1-EBIO. The regularity of low-frequency discharge that is atypical for GABAergic SNr neurons can probably be explained by prolonged AHPs, analogous to the situation for DA neurons where SK channels determine the regularity of discharge (Wolfart et al. 2001). In DA neurons, the Ca2+ rise activating SK channels to generate the AHP is amplified by RyR-mediated Ca2+ release from intracellular stores that is induced by the Ca2+ influx through voltage-activated Ca2+ channels during an action potential. With respect to the AHPs of GABAergic SNr neurons, we found no amplification of the AHP by an additional activation of SK channels through Ca2+ release from intracellular stores (Yanovsky et al. 2005). The higher Ca2+ sensitivity of SK channels in 1-EBIO disclosed a small contribution of Ca2+ release from intracellular stores to the generation of the amplitude of the AHP in this study.
The regularity of low-frequency discharge despite 1-EBIO strongly potentiating OCPs excludes a major role for a phasic SK channel activation through RyR-mediated Ca2+ release during autonomous activity. The coupling of RyR openings to Ca2+ influx during the action potential, and the resulting occupancy of SK channels by Ca2+, favour the idea that OCPs do not occur until SK channels have closed again and membrane potential has returned close to resting potential at the end of the AHP. For a neuron in discharge mode therefore, the occurrence of OCPs is not random as it is under voltage clamp. Instead, the probability for OCPs to occur is a function of RyR activity on the one hand and AHP duration relative to interspike interval on the other hand. Several experimental observations lend support to this suggestion. Drugs like CPA that abolish OCPs do not increase continuous discharge rate (Seutin et al. 2000; Cui et al. 2004; Yanovsky et al. 2005). Caffeine has no inhibitory effect in neurons discharging regularly at relatively high rate, but has an inhibitory effect in control and in 1-EBIO if interspike intervals are long. An increase in the frequency of OCP-induced hyperpolarizations becomes visible only at interspike intervals that exceed the duration of AHPs. At low frequency of discharge, spikes of GABAergic SNr neurons occur in groups, and silent periods of varying durations are interspersed (Richards et al. 1997). Silent periods increased considerably upon application of 1-EBIO. The same effect could be achieved in 1-EBIO by increasing RyR activity with caffeine or by increasing cytosolic Ca2+ through the blockade of Ca2+ reuptake into stores by CPA. The latter effect vanished as soon as stores were depleted.
OCPs have been observed so far only in juvenile neurons. This may be indicative of a specific role of cytosolic Ca2+ in maturing neurons that may have a low Ca2+-buffering capacity. However, frequency of discharge needs to be considered as well. Because, in firing neurons, OCP-induced hyperpolarizations occur only towards the end of the AHP, a prolongation of interspike intervals increases the probability of occurrence of OCPs. A phasic activation of SK channels through RyR-mediated Ca2+ release could support the persistence of silent periods that may be caused by synaptic input. We noted a small but significant reduction in spike threshold of GABAergic SNr neurons by the long-lasting OCP-induced hyperpolarizations in 1-EBIO. In this capacity, OCPs can help to prevent spike inactivation during silent periods that hold membrane potential of GABAergic SNr neurons near –45 mV for extended time periods.
GABAergic SNr neurons, together with GABAergic neurons in the internal segment of the globus pallidus, provide a tonic inhibition to target structures, the motor nuclei in the thalamus and the superior colliculus. The tonic inhibition is interrupted by the action of inhibitory afferents from other basal ganglia nuclei. This ‘classical’ concept has been challenged recently. The GABAergic output neurons of the basal ganglia may exert not only an inhibitory, but also an excitatory effect (for a review see Graybiel, 2005). The discharge patterns of GABAergic SNr neurons have not been studied in such detail as has been done for DA neurons. GABAergic SNr neurons are much smaller in number and have been difficult to identify with blind recording techniques. GABA projection neurons in the SNr that have been recorded extracellularly in vivo have a continuous firing pattern that led to the suggestion of a frequency-coded output (Bunney et al. 1973; Wilson et al. 1977). A more recent study, however, describes a different pattern (Fedrowitz et al. 2003). In this study, the dominant discharge was termed grouped firing pattern and consisted of intermittent grouped discharges separated by periods of pauses. A rather linear current–frequency relationship predominated in vitro when the membrane potential was shifted in the depolarizing direction as reported in a preceding study (Richards et al. 1997). Only if the membrane potential was shifted to a negative direction, was the discharge interrupted by pauses of varying durations. A similar situation applies to DA neurons in vivo and in vitro. Whereas in vitro an extremely regular pacemaker activity prevails, bursting patterns predominate in vivo (Hyland et al. 2002). The activity in vivo probably reflects the control of autonomous activity by afferent inputs (Kitai et al. 1999). The phasic activation of SK channels by RyR-mediated Ca2+ release occurs in a voltage range near spike threshold. It occurs only in periods during which SK channel activation subsequent to action potential-dependent Ca2+ influx is not dominating. In addition to their capacity to inhibit discharge by hyperpolarizing the membrane, OCPs restrain spike inactivation through membrane hyperpolarization. In the absence of OCP-induced hyperpolarizations, inactivation of Na+ channels could occur during prolonged silent periods during which membrane potential is near spike threshold. In summary, we suggest that hyperpolarizations resulting from phasic SK channel activation by RyR-mediated Ca2+ release support the initiation of pauses in autonomous discharge. In this capacity the intrinsic mechanism may operate in conjunction with synaptic inputs, which have the main influence on discharge modality. However, more work is required to unravel a possible interaction between OCP-induced hyperpolarizations and synaptic inputs.
Acknowledgments
The authors thank Drs G. Drew and L. Godinho for critical discussions, C. Heuser for excellent technical assistance and A. Lewen for excellent editorial assistance. This study was supported by the Deutsche Forschungsgemeinschaft TP D9, Collaborative Research Center 488: Molecular and Cellular Bases of Neural Development, Heidelberg and MI 255/7-2.
References
- Akaike N. Gramicidin perforated patch recording and intracellular chloride activity in excitable cells. Prog Biophys Mol Biol. 1996;65:251–264. doi: 10.1016/s0079-6107(96)00013-2. [DOI] [PubMed] [Google Scholar]
- Atherton JF, Bevan MD. Ionic mechanisms underlying autonomous action potential generation in the somata and dendrites of GABAergic substantia nigra pars reticulata neurons in vitro. J Neurosci. 2005;25:8272–8281. doi: 10.1523/JNEUROSCI.1475-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunney BS, Walters JR, Roth RH, Aghajanian GK. Dopaminergic neurons: effect of antipsychotic drugs and amphetamine on single cell activity. J Pharmacol Exp Ther. 1973;185:560–571. [PubMed] [Google Scholar]
- Cingolani LA, Gymnopoulos M, Boccaccio A, Stocker M, Pedarzani P. Developmental regulation of small-conductance Ca2+-activated K+ channel expression and function in rat Purkinje neurons. J Neurosci. 2002;22:4456–4467. doi: 10.1523/JNEUROSCI.22-11-04456.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui G, Okamoto T, Morikawa H. Spontaneous opening of T-type Ca2+ channels contributes to the irregular firing of dopamine neurons in neonatal rats. J Neurosci. 2004;24:11079–11087. doi: 10.1523/JNEUROSCI.2713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich BE, Kaftan E, Bezprozvannaya S, Bezprozvanny I. The pharmacology of intracellular Ca2+-release channels. Trends Pharmacol Sci. 1994;15:145–149. doi: 10.1016/0165-6147(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Fedrowitz M, Lindemann S, Löscher W, Gernert M. Altered spontaneous discharge rate and pattern of basal ganglia output neurons in the circling (ci2) rat mutant. Neuroscience. 2003;118:867–878. doi: 10.1016/s0306-4522(02)00939-9. [DOI] [PubMed] [Google Scholar]
- Glowatzki E, Fuchs PA. Cholinergic synaptic inhibition of inner hair cells in the neonatal mammalian cochlea. Science. 2000;288:2366–2368. doi: 10.1126/science.288.5475.2366. [DOI] [PubMed] [Google Scholar]
- Grace AA, Onn S-P. Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J Neurosci. 1989;9:3463–3481. doi: 10.1523/JNEUROSCI.09-10-03463.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia: learning new tricks and loving it. Curr Opin Neurobiol. 2005;15:638–644. doi: 10.1016/j.conb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Hallworth NE, Wilson CJ, Bevan MD. Apamin-sensitive small conductance calcium-activated potassium channels, through their selective coupling to voltage-gated calcium channels, are critical determinants of the precision, pace, and pattern of action potential generation in rat subthalamic nucleus neurons in vitro. J Neurosci. 2003;23:7525–7542. doi: 10.1523/JNEUROSCI.23-20-07525.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häusser M, Stuart G, Racca C, Sakmann B. Axonal initiation and active dendritic propagation of action potentials in substantia nigra neurons. Neuron. 1995;15:637–647. doi: 10.1016/0896-6273(95)90152-3. [DOI] [PubMed] [Google Scholar]
- Hyland BI, Reynolds JNJ, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience. 2002;114:475–492. doi: 10.1016/s0306-4522(02)00267-1. [DOI] [PubMed] [Google Scholar]
- Kitai ST, Shepard PD, Callaway JC, Scroggs R. Afferent modulation of dopamine neuron firing patterns. Curr Opin Neurobiol. 1999;9:690–697. doi: 10.1016/s0959-4388(99)00040-9. [DOI] [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB, North RA. Dopamine acts on D2 receptors to increase potassium conductance in neurones of the rat substantia nigra zona compacta. J Physiol. 1987;392:397–416. doi: 10.1113/jphysiol.1987.sp016787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercuri NB, Bonci A, Calabresi P, Stefani A, Bernardi G. Properties of the hyperpolarization-activated cation current Ih in rat midbrain dopaminergic neurons. Eur J Neurosci. 1995;7:462–469. doi: 10.1111/j.1460-9568.1995.tb00342.x. [DOI] [PubMed] [Google Scholar]
- Nakanishi H, Kita H, Kitai ST. Intracellular study of rat substantia nigra pars reticulata neurons in an in vitro slice preparation: electrical membrane properties and response characteristics to subthalamic stimulation. Brain Res. 1987;437:45–55. doi: 10.1016/0006-8993(87)91525-3. [DOI] [PubMed] [Google Scholar]
- Oliver D, Klöcker N, Schuck J, Baukrowitz T, Ruppersberg JP, Fakler B. Gating of Ca2+-activated K+ channels controls fast inhibitory synaptic transmission at auditory outer hair cells. Neuron. 2000;26:595–601. doi: 10.1016/s0896-6273(00)81197-6. [DOI] [PubMed] [Google Scholar]
- Parsons RL, Barstow KL, Scornik FS. Spontaneous miniature hyperpolarizations affect threshold for action potential generation in mudpuppy cardiac neurons. J Neurophysiol. 2002;88:1119–1127. doi: 10.1152/jn.2002.88.3.1119. [DOI] [PubMed] [Google Scholar]
- Pedarzani P, Mosbacher J, Rivard A, Cingolani LA, Oliver D, Stocker M, Adelman JP, Fakler B. Control of electrical activity in central neurons by modulating the gating of small conductance Ca2+-activated K+ channels. J Biol Chem. 2001;276:9762–9769. doi: 10.1074/jbc.M010001200. [DOI] [PubMed] [Google Scholar]
- Ping HX, Shepard PD. Apamin-sensitive Ca2+-activated K+ channels regulate pacemaker activity in nigral dopamine neurons. Neuroreport. 1996;7:809–814. doi: 10.1097/00001756-199602290-00031. [DOI] [PubMed] [Google Scholar]
- Richards CD, Shiroyama T, Kitai ST. Electrophysiological and immunocytochemical characterization of GABA and dopamine neurons in the substantia nigra of the rat. Neuroscience. 1997;80:545–557. doi: 10.1016/s0306-4522(97)00093-6. [DOI] [PubMed] [Google Scholar]
- Sarpal D, Koenig JI, Adelman JP, Brady D, Clerkin Prendeville L, Shepard PD. Regional distribution of SK3 mRNA-containing neurons in the adult and adolescent rat ventral midbrain and their relationship to dopamine-containing cells. Synapse. 2004;53:104–113. doi: 10.1002/syn.20042. [DOI] [PubMed] [Google Scholar]
- Seutin V, Massotte L, Scuvée-Moreau J, Dresse A. Spontaneous apamin-sensitive hyperpolarizations in dopaminergic neurons of neonatal rats. J Neurophysiol. 1998;80:3361–3364. doi: 10.1152/jn.1998.80.6.3361. [DOI] [PubMed] [Google Scholar]
- Seutin V, Mkahli F, Massotte L, Dresse A. Calcium release from internal stores is required for the generation of spontaneous hyperpolarizations in dopaminergic neurons of neonatal rats. J Neurophysiol. 2000;83:192–197. doi: 10.1152/jn.2000.83.1.192. [DOI] [PubMed] [Google Scholar]
- Shepard PD, Bunney BS. Repetitive firing properties of putative dopamine-containing neurons in vitro: regulation by an apamin-sensitive Ca2+-activated K+ conductance. Exp Brain Res. 1991;86:141–150. doi: 10.1007/BF00231048. [DOI] [PubMed] [Google Scholar]
- Stocker M. Ca2+-activated K+ channels: molecular determinants and function of the SK family. Nat Rev Neurosci. 2004;5:758–770. doi: 10.1038/nrn1516. [DOI] [PubMed] [Google Scholar]
- Stocker M, Pedarzani P. Differential distribution of three Ca2+-activated K+ channel subunits, SK1, SK2, and SK3, in the adult rat central nervous system. Mol Cell Neurosci. 2000;15:476–493. doi: 10.1006/mcne.2000.0842. [DOI] [PubMed] [Google Scholar]
- Stuart GJ, Dodt H-U, Sakmann B. Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflugers Arch. 1993;423:511–518. doi: 10.1007/BF00374949. [DOI] [PubMed] [Google Scholar]
- Syme CA, Gerlach AC, Singh AK, Devor DC. Pharmacological activation of cloned intermediate- and small-conductance Ca2+-activated K+ channels. Am J Physiol Cell Physiol. 2000;278:C570–C581. doi: 10.1152/ajpcell.2000.278.3.C570. [DOI] [PubMed] [Google Scholar]
- Taylor CW, Broad LM. Pharmacological analysis of intracellular Ca2+ signalling: problems and pitfalls. Trends Pharmacol Sci. 1998;19:370–375. doi: 10.1016/s0165-6147(98)01243-7. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Young SJ, Groves PM. Statistical properties of neuronal spike trains in the substantia nigra: cell types and their interactions. Brain Res. 1977;136:243–260. doi: 10.1016/0006-8993(77)90801-0. [DOI] [PubMed] [Google Scholar]
- Wolfart J, Neuhoff H, Franz O, Roeper J. Differential expression of the small-conductance, calcium-activated potassium channel SK3 is critical for pacemaker control in dopaminergic midbrain neurons. J Neurosci. 2001;21:3443–3456. doi: 10.1523/JNEUROSCI.21-10-03443.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfart J, Roeper J. Selective coupling of T-type calcium channels to SK potassium channels prevents intrinsic bursting in dopaminergic midbrain neurons. J Neurosci. 2002;22:3404–3413. doi: 10.1523/JNEUROSCI.22-09-03404.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X-M, Fakler B, Rivard A, Wayman G, Johnson-Pais T, Keen JE, Ishii T, Hirschberg B, Bond CT, Lutsenko S, Maylie J, Adelman JP. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature. 1998;395:503–507. doi: 10.1038/26758. [DOI] [PubMed] [Google Scholar]
- Yanovsky Y, Mades S, Misgeld U. Retrograde signaling changes the venue of postsynaptic inhibition in rat substantia nigra. Neuroscience. 2003;122:317–328. doi: 10.1016/s0306-4522(03)00607-9. [DOI] [PubMed] [Google Scholar]
- Yanovsky Y, Zhang W, Misgeld U. Two pathways for the activation of small-conductance potassium channels in neurons of substantia nigra pars reticulata. Neuroscience. 2005;136:1027–1036. doi: 10.1016/j.neuroscience.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Yung WH, Häusser MA, Jack JJB. Electrophysiology of dopaminergic and non-dopaminergic neurones of the guinea-pig substantia nigra pars compacta in vitro. J Physiol. 1991;436:643–667. doi: 10.1113/jphysiol.1991.sp018571. [DOI] [PMC free article] [PubMed] [Google Scholar]