Abstract
Activity and calcium-dependent release of neurotransmitters from the somatodendritic compartment is an important signalling mechanism between neurones throughout the brain. NMDA receptors and vesicles filled with neurotransmitters occur in close proximity in many brain areas. It is unknown whether calcium influx through these receptors can trigger the release of somatodendritic vesicles directly, or whether postsynaptic action potential firing is necessary for release of these vesicles. Here we addressed this question by studying local release of serotonin (5-HT) from dorsal raphé nucleus (DRN) neurones. We performed capacitance measurements to monitor the secretion of vesicles in giant soma patches, in response to short depolarizations and action potential waveforms. Amperometric measurements confirmed that secreted vesicles contained 5-HT. Surprisingly, two-photon imaging of DRN neurones in slices revealed that dendritic calcium concentration changes in response to somatic firing were restricted to proximal dendritic areas. This implied that alternative calcium entry pathways may dominate the induction of vesicle secretion from distal dendrites. In line with this, transient NMDA receptor activation, in the absence of action potential firing, was sufficient to induce capacitance changes. By monitoring GABAergic transmission onto DRN 5-HT neurones in slices, we show that endogenous NMDA receptor activation, in the absence of postsynaptic firing, induced release of 5-HT, which in turn increased the frequency of GABAergic inputs through activation of 5-HT2 receptors. We propose here that calcium influx through NMDA receptors can directly induce postsynaptic 5-HT release from DRN neurones, which in turn may facilitate GABAergic input onto these cells.
Ever since the first indications that neuronal dendrites may secrete retrogradely acting neurotransmitters (Ralston, 1971), there has been a heated debate about the relevance of volume or paracrine transmission for neuropsychopharmacology and its underlying mechanisms. There are excellent reports on methods (Bunin & Wightman, 1998) that can be used to study paracrine actions of 5-hydroxytryptamine (5-HT; Bunin & Wightman, 1999) and dopamine (Jaffe et al. 1998), which are known to occur in various brain areas including thalamus (Munsch et al. 2003; Govindaiah & Cox, 2004) and substantia nigra (Nedergaard et al. 1988). In addition, there is some insight into somatodendritic secretion of neuropeptides in hypothalamus (Ludwig et al. 2002a; de Kock et al. 2003, 2004). However, we currently lack understanding of the mechanisms underlying somatodendritic secretion of monoamines, in particular 5-HT.
Volume transmission of 5-HT within the dorsal raphé nucleus (DRN) (Bunin & Wightman, 1998) may resemble volume transmission of neuropeptides in hypothalamus (Kombian et al. 1996; de Kock et al. 2003), since it acts relatively slowly, and affects the activity of the cell it is released from through activation of metabotropic receptors. DRN neurones release substantial amounts of 5-HT locally in the nucleus from extrasynaptic sites (Bunin & Wightman, 1998), acting both on dendritic 5-HT1A autoreceptors (Liu et al. 2005), and on 5-HT2 receptors (Liu et al. 2000) located on presynaptic GABAergic neurones. 5-HT-containing vesicles in DRN dendrites can be densely packed in small clusters, but are not necessarily associated with any form of synaptic membrane specialization (Chazal & Ralston, 1987). Electrical stimulation within the DRN leads to a rise of extracellular 5-HT at a rate typical of diffusion and with a transmitter half-life of around 200 ms. This implies that within the DRN, 5-HT molecules can diffuse extracellularly up to 20 μm away from the site of putative secretion (Bunin & Wightman, 1999), which is sufficient to reach neighbouring cells and synapses.
However, if 5-HT is secreted in a calcium-dependent manner through vesicle fusion, it is unclear what triggers 5-HT release in DRN cells. Are somatic action potentials, back-propagating into dendrites, involved, activating dendritic voltage-gated calcium channels (Hausser et al. 1995; Stuart et al. 1997), or are postsynaptic receptor-mediated increases in dendritic calcium sufficient for triggering 5-HT release (Munsch et al. 2003; Duguid & Smart, 2004; Govindaiah & Cox, 2004)? In the DRN, 5-HT neurones receive glutamatergic projections from the prefrontal cortex that activate both α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) and N-methyl-d-aspartate (NMDA) receptors (Celada et al. 2001) and induce dendritic calcium influx that could potentially directly trigger local release of 5-HT. A part of these projections most likely innervates the spiny dendrites of DRN neurons (Li et al. 2001). In addition, these glutamatergic synapses may occur in close proximity of 5-HT containing vesicles that are present in the postsynaptic compartments (Kapadia et al. 1985; Liposits et al. 1985; Chazal & Ralston, 1987). Hence, here we address the question whether NMDA receptor activation can induce local release of serotonin in the absence of postsynaptic firing. In addition, we investigated the physiological significance of NMDA receptor-induced 5-HT release for synaptic communication in DRN.
Methods
Slice procedure
Wistar rats (male, PN42-56; Harlan CPB, Zeist, the Netherlands) were used. All animal experimentation has been conducted in accordance with the Policies on the Use of Animals and Humans in Neuroscience Research (PHS policy) approved by the Society of Neuroscience, as well as by the Dutch Animal Ethical Committee, in agreement with European Law. Rats were killed by decapitation using a guillotine, without the use of anaesthetics. Coronal midbrain slices (400 μm) were prepared in ice-cold slice solution containing (mm): 3.5 KCl, 2.4 CaCl2, 1.3 MgSO4, 1.2 KH2PO4, 212.5 Sucrose, 26 NaHCO3, 10 d-glucose, carboxygenated in 5% CO2–95% O2. Slices were transferred to artificial cerebral spinal fluid (ACSF) containing (mm): 125 NaCl, 3 KCl, 1.2 NaH2PO4, 2.4 CaCl2, 1.3 MgSO4, 10 d-glucose, 25 NaHCO3, supplemented with 1 μm 6,7-dinitroquinoxaline-2,3-dione (DNQX; Alexis Biochemicals, Lausen, Switzerland) to prevent overexcitation by glutamatergic inputs. Slices were kept at 37°C for 1 h to recover and were subsequently stored at room temperature.
Nucleated patch recording
After the recovery phase, slices were transferred to the recording chamber, which was continuously perfused with ACSF, 1 μm DNQX and 10 μm bicuculline (Sigma-Aldrich, St Louis, USA) to block AMPA and GABAA receptors. Nucleated outside-out patches were pulled using 3–5 MΩ electrodes. Capacitance measurements using voltage steps or action potential command waveforms were made with intracellular medium consisting of (mm): 135 tetraethylammonium-acetate ((TEA)-acetate), 10 dipotassium phosphocreatine, 4 MgATP, 0.3 GTP (acid free), 0.1 EGTA, 10 Hepes, adjusted to pH 7.2 with TEA-OH. Voltage clamp recordings for amperometric recordings were made with intracellular medium containing (mm): 125 K-gluconate, 10 NaCl, 4.6 MgCl2, 4 K2ATP, 15 creatine phosphate, 20 U ml−1 phosphocreatine kinase, 0.1 EGTA, pH 7.3 (adjusted with KOH).
NMDA-induced capacitance changes were studied using intracellular medium containing (mm): 145 CsCl, 2 MgCl2, 0.1 EGTA, 10 Hepes, 2 MgATP, 0.1 GTP (acid free), pH 7.4 CsOH. During the latter experiments, extracellular medium consisted of 5 mm Ca2+ containing, Mg2+ free ACSF, supplemented with 10 μm glycine. The nucleated patches were positioned in front of a double-barrelled electrode attached to a piezo-element. Recordings were performed at 33°C.
Capacitance recordings
The membrane current in the nucleated patch configuration was monitored with an EPC8 amplifier (HEKA Elektronik, Lambrecht, Germany) and digitized with an ITC-18 computer interface (Instrutech, Port Washingon, NY, USA). Capacitance measurements were made using Pulse software (HEKA). The membrane capacitance, access conductance and membrane conductance were calculated according to the Lindau-Neher technique, implemented as the ‘sine plus DC’ feature of the Pulse lock-in module. A sine wave of 1 kHz, 40 mV peak-to-peak, was added to a holding potential of −70 mV. The reversal potential of the lock-in module was set to 0 mV. Membrane current was low-pass filtered at 3 kHz and sampled at 10 kHz. The membrane capacitance, access conductance and membrane conductance were calculated at 1 kHz.
Calcium currents evoked by depolarizations to induce capacitance changes were obtained in the presence of TTX (1 μm) and using a TEA-pipette medium that prevented outward K+ currents (see above). NMDA currents used to induce capacitance changes were obtained at −70 mV, at which there is no activation of voltage-gated currents (see above and Results). Capacitance changes were calculated as the difference between the average membrane capacitance during the 80 ms before depolarization or the NMDA application and the membrane capacitance during the first 10 ms of the sine wave segment after depolarization or NMDA application, respectively. The number of calcium ions that entered the cell during a pulse was determined (after subtraction of the leak current) as the integral of the calcium current:
where F is Faraday's constant (96 485 C mol−1) and NA is Avogadro's constant (6.022 × 1023 mol−1). Leak current was determined at a holding potential of −70 mV during a 2 ms interval between the first sine wave segment and the depolarization.
In addition to capacitance changes, we occasionally observed changes in membrane conductance. However, these changes had very different kinetics and did not induce measurable artifacts in the capacitance trace, which is in line with previous observations (Lindau & Neher, 1988). We conclude that cross-talk between capacitance and conductance traces under these conditions is minimal (see also de Kock et al. 2004).
Amperometric recording
Single-stranded insulated carbon fibres (diameter 6 μm, model CC-18, van den Hul, Oene, the Netherlands) were mounted in glass microcapillaries (GC150-10, Harvard Apparatus Ltd, Kent, UK). Gigaohm resistance (2–5 GΩ) to ground was achieved by insulating the microelectrode and carbon fibre with Sylgard. The tip of the carbon fibre was cut just before the experiment to ensure cleanliness and sensitivity of the exposed tip surface. Microelectrodes were filled with 1 m KCl and placed in close apposition to the cell surface. Amperometric currents were recorded with an EPC8 amplifier (HEKA Elektronik; electrode voltage set to +650 mV, sampled at 10 kHz and filtered at 3 kHz. Release was evoked by repetitive depolarization (25 pulses of 100 ms, 0.67 Hz) to 0 mV from a holding potential of −70 mV. Using this protocol and in particular using a 650 mV command voltage, we cannot exclude that also other substances than 5-HT (including for instance tryptophan) give an oxidation signal, but the primary aim of this set of experiments was to prove that the assumed vesicular nature of release, proposed on the basis of the capacitance recordings of nucleated outside-out patched, indeed could be confirmed using amperometry.
Two-photon calcium imaging
Large DRN neurones were targeted for whole-cell recordings and were filled through the recording pipette with Alexa 594 (120 μm) and Fluo4 (300 μm) (Molecular Probes, Eugene, OR, USA). After dye loading (15–30 min after break-in), cells were visualized using a Leica MP-RS two-photon laser-scanning microscope (Leica, Mannheim, Germany) with a ×63 objective and the Ti:Sapphire laser was tuned to 800 nm. Line-scans were taken at different locations on dendrites, and action potentials were evoked at the soma by current injection through the recording pipette. Z-stack image projections (at 1 μm intervals) to obtain an overview of cell morphology were made with Leica LCS software and ImageJ (NIH). Changes in fluorescence of fluo4 are expressed as Δgreen/red ratio (Sabatini & Regehr, 1995). Before stimulation, fluorescence was measured to obtain basal fluorescence (Fo). A region of line-scan outside of any indicator-filled region of interest (ROI) was used to measure background fluorescence (Fb). Relative fluorescence changes were calculated as follows:
where Ro is the baseline signal measured with Alexa594 and Rb is the background signal measured in this channel (Sabatini & Regehr, 1995).
Synaptic event recording
Slice procedure was as described above; thereafter slices were transferred to the recording chamber, which was initially perfused with tryptophan-loading solution, consisting of ACSF (2.4 mm Ca2+, 1.3 mm Mg2+), and supplemented with 10 μm glycine, 10 μm 6-cynano-7-nitroquinoxaline-2,3-dione (CNQX; Sigma-Aldrich) in order to block the AMPA receptor contribution, 10 μm fluoxetine to reduce reuptake of 5-HT, 0.1 μmN-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylc-yclo-hexanecarboxamide maleate salt (WAY-1000635; Sigma-Aldrich) to block postsynaptic 5-HT1A-receptors, 10 μm (2S,1′S,2′R)-2-(carboxy-cyclopropyl)glycine (L-CCG-III; Tocris Bioscience, Bristol, UK) to suppress reuptake of glutamate, 30 nm (RS)-α-cyclopropyl-4-phosphonophenylglycine (CPP; Tocris Bioscience) to block mGluR activity and 20–40 μml-tryptophan (Sigma-Aldrich), to boost the synthesis of 5-HT. After 15 min at 33°C, the recording chamber was perfused with similar ACSF except for the concentration of l-tryptophan, which was lowered to 1 μm. In current clamp recording we found that this was sufficient to silence firing of 5-HT neurones in the DRN (n = 6, not shown). Pipette medium contained (mm) 154 K-gluconate, 1 KCl, 0,1 EGTA, 10 Hepes, 10 glucose, 5 ATP, pH 7.4 (adjusted with KOH), 400 mosmol l−1. Recordings were performed at 33°C using an Axopatch 200B amplifier (Axon Instruments, Union City, CA, USA). Recording were analysed using Mini Analysis Program called Synaptosoft (version 6.0.3) by Justin Lee (Decatur, GA, USA), setting the threshold for detection at a minimal amplitude of 20 pA and a rise time of shorter than 1.5 ms and synaptic event half-width of more than 3 ms.
Results
Cellular recordings in DRN
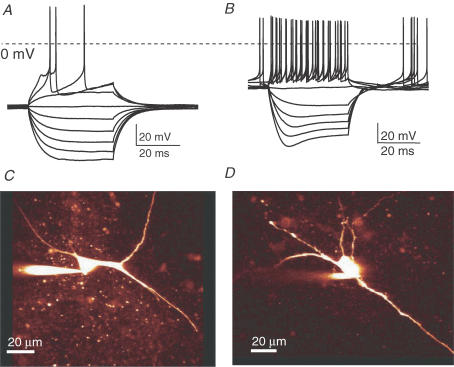
The DRN located on the ventral side of the periaquaductal grey contains a large cluster of 5-HT containing neurones in addition to non-5-HT neurones (Descarries et al. 1982). In whole-cell current-clamp recordings in the ventral portion of the DRN we distinguished between 5-HT containing neurones and non-5-HT neurones. In response to a series of current steps the putative 5-HT neurones produced membrane potential profiles (Fig. 1A) in line with those published previously (Li et al. 2001). In contrast, non-5-HT neurones displayed time-dependent depolarization (called a ‘sag’) in response to hyperpolarizing current pulses (Fig. 1B), in confirmation of previous observations (Li et al. 2001). The latter phenomenon is indicative of a hyperpolarization-activated cationic current (H current), not being expressed by 5-HT neurones. Moreover the morphology of both cell types was distinct (Fig. 1C and D). We thus selected neurones with large cell bodies and tested for the ‘absence’ of the H current. In our recordings the majority of DRN neurones (83%, 39 cells out of 47) showed a current clamp profile typical for 5-HT neurones (Fig. 1A). In a minority of the cells (13%, n = 6) the depolarizing ‘sag’ characteristic of non-5-HT neurones (Fig. 1B) was observed. Very few neurones had an intermediate profile and were excluded from further analysis (i.e. n = 2, not shown).
Figure 1. 5-HT neurones can reliably be selected from acute DRN slices.
A, current clamp profile of 5-HT neurone in acute brain slice of DRN from adult male animals (6–8 weeks, example trace). Long (400 ms) depolarizing and hyperpolarizing current pulses were injected into each neurone. B, current clamp profile of non-5-HT neurone in acute brain slice in DRN (example trace). Note the presence of H current, which is typical for non-5-HT neurones. C, confocal image of 5-HT neurone filled with Alexa 594. Note the magnocellular nature of the soma and dendrites. D, confocal image of a non-5-HT neurone.
Capacitance and amperometric recording in nucleated outside-out patches
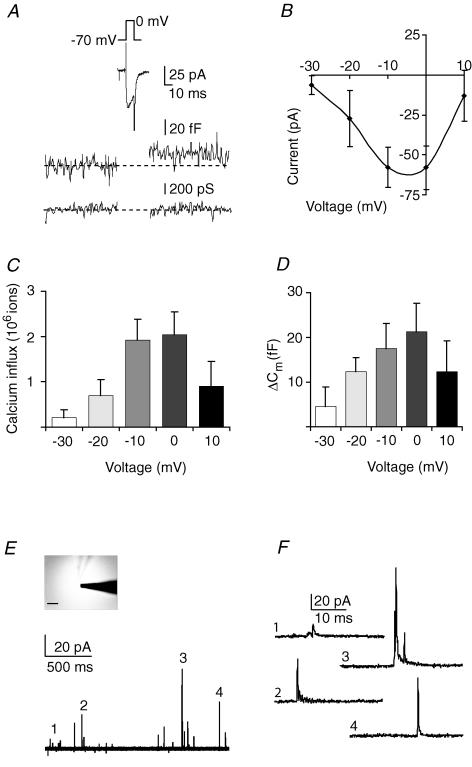
To test whether DRN neurones release vesicles from their somatodendritic compartment, we performed capacitance measurements. Due to morphological constraints, capacitance measurements can only be performed on spherical cells and not on neurones with extensive processes (Lindau & Neher, 1988). Hence, to directly study release from somatic compartments of putative 5-HT neurones, we needed to use nucleated outside-out membrane patches pulled from large cells from the ventral portion of acute DRN brain slices. This so-called somatic or ‘giant’ outside-out preparation is well-suited for studying ligand- and voltage-gated channels in combination with the fast application of agonists (Sather et al. 1992; Rozov et al. 1998; Bekkers, 2000; de Kock et al. 2004). We first wanted to test whether activation of voltage-gated calcium channels induces vesicular secretion in this preparation, to confirm earlier observations from our lab on another cell type (de Kock et al. 2004). In the presence of extracellular TTX, to block voltage-gated sodium current, and using a TEA-based intracellular solution that blocks outward potassium currents, short depolarizations were applied at different voltages while monitoring membrane capacitance (Fig. 2A). Calcium currents were preferentially activated at membrane potentials beyond −20 mV (Fig. 2A–C, n = 7 patches from N = 7 animals) leading to subsequent capacitance changes, proportional to the extent of calcium influx (Fig. 2D). These results indicate that calcium influx through somatic voltage-gated calcium channels can induce exocytosis in DRN neurones, indicative of non-synaptic vesicle release. Capacitance changes in these recordings ranged from 0 to 40 fF max, which would be the equivalent of 0–97 vesicles being secreted per trial, assuming that vesicles from the somatodendritic region of neurones in the CNS may produce a capacitance change of ∼0.412 ± 16 fF per vesicle (Klyachko & Jackson, 2002).
Figure 2. Voltage-dependent calcium channel activation induces exocytosis of 5-HT containing vesicles from somatic nucleated outside-out patches.
A, inward current, membrane capacitance and membrane conductance in nucleated patches from DRN from adult male animals (6–8 weeks, averaged trace, n = 7) during 10 ms depolarization to 0 mV. Calcium channels were preferably activated beyond −20 mV, which resulted in capacitance changes, indicative of exocytosis (averaged trace, N = 7). B, I–V relationship of inward current activated in nucleated patches from DRN neurones (n = 7). C, the integral of the calcium current was calculated to produce the absolute Ca2+ influx. D, capacitance changes in nucleated patches of DRN neurones are proportional to the amount of, and therefore most likely a consequence of, calcium influx (compare panel C and D). E, repetitive depolarization (0.1 Hz, 100 ms, 0 mV) of nucleated outside-out patch (see inset, carbon fibre approaching from right) of an identified 5-HT neurone induced amperometric spikes (overlay of 13 traces from one example recording, n = 10). F, individual amperometric spikes taken from same recording at an increased time resolution.
To test whether putative 5-HT neurones indeed secrete 5-HT, we additionally performed amperometric recordings (Angleson & Betz, 1997; Sutton et al. 2004) from the giant outside-out patches. Nucleated patches were pulled from identified 5-HT neurones (see Fig. 1) and positioned in contact with the surface of a carbon fibre (Fig. 2E inset). In this patch configuration, when vesicles fuse with the cell membrane to release their contents, the putative 5-HT content would produce an oxidation signal at the tip of the carbon fibre. Indeed, short depolarizations to activate voltage-gated calcium channels resulted in amperometric signals in identified 5-HT neurones (n = 10 from N = 3 animals; Fig. 2E). In the 5-HT cells, amperometric spikes, typical of vesicular 5-HT secretion, occurred (Fig. 2F). Note the limited area of the carbon fibre being attached to the surface of the giant outside out patch (i.e. less than 15% detection area, Fig. 2E inset), which may explain the relatively low probability of detecting 5-HT signal within recordings. In patches pulled from non-5-HT neurones (n = 5), or in patches from 5-HT neurones not being stimulated, no amperometric signals were found (n = 3, data not shown). In addition, in patches recorded with 1 mm EGTA instead of 100 μm EGTA in the pipette, also no amperometric signals could be obtained upon depolarizations (n = 8 patches from N = 3 animals, data not shown). Together these data imply that depolarization induced calcium-influx is capable of inducing 5-HT secretion from somatic outside out patches of the DRN neurones.
Single action potentials evoke somatodendritic release
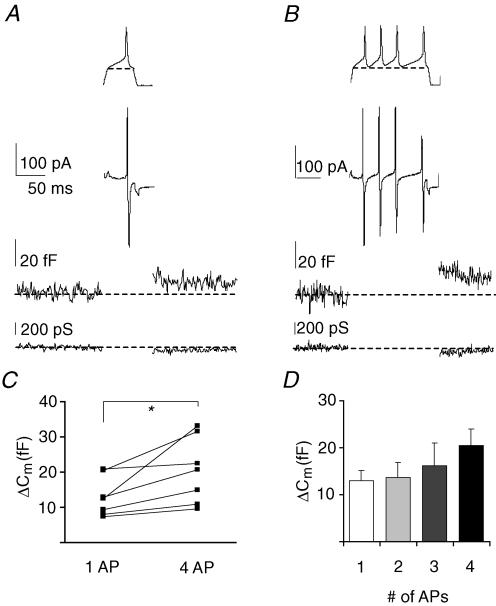
In vivo, somatic action potentials and backpropagating action potentials into the dendritic tree may account for the activation of voltage-gated calcium channels and subsequent somatodendritic release of 5-HT. Hence, waveforms of single action potentials, recorded from magnocellular neurones in previous experiments, were used in voltage clamp as stimulus templates to activate voltage-gated calcium channels in the nucleated patch recording to test whether these could induce changes in the membrane capacitance. In the presence of TTX (1 μm) and using the TEA-based pipette medium, both templates of single action potentials and trains of action potential templates induced inward currents in nucleated patches (Fig. 3A and B upper traces). In response to a single action potential, capacitance changes were evoked in 7 out of 7 nucleated patches. On average, a single action potential evoked capacitance changes of 13.03 ± 2.1 fF (Fig. 3A and C, N = 3 animals, n = 7 patches, n = 34 trials). We also applied a train of 2, 3 or 4 action potentials, which induced an increased trend of capacitance changes (of up to 20.4 ± 3.6 fF, Fig. 3B–D, paired t test, P < 0.05, n = 7 patches from N = 3 animals, n = 28 trials). This would imply that a maximum of ∼50 vesicles can be secreted per train of 4 action potentials.
Figure 3. Somatic vesicle release evoked by a single action potential.
A, inward current, membrane capacitance and membrane conductance during a single action potential in nucleated patches (averaged trace, n = 7). The voltage template is shown above the current trace (bracket line: voltage protocol was adjusted to start and end at −70 mV to perform capacitance recordings). B, analogous responses during a train of action potentials in nucleated patches (averaged trace, n = 7). The voltage template is shown above the current trace. C, pair wise comparison of capacitance changes between single and train of action potentials (paired t test, P < 0.05, paired data from individual experiments). D, capacitance changes are dependent on number of action potentials.
Single action potentials do not backpropagate into DRN dendrites
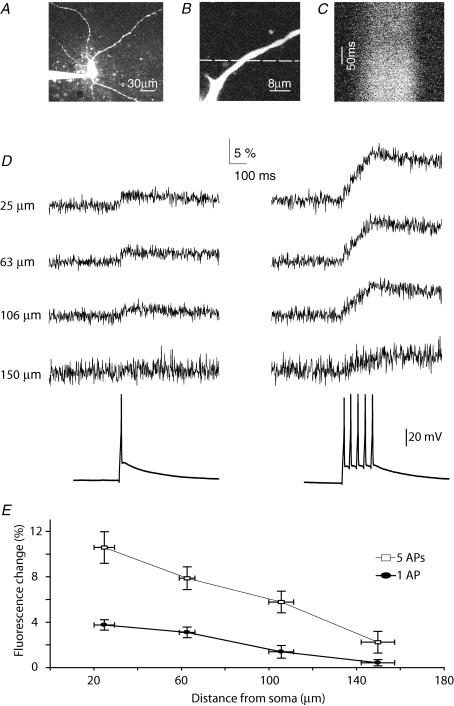
The nucleated outside out patches shown in Figs 2 and 3 consist predominantly of somatic membrane. To test if action potentials could also serve as the trigger for calcium-dependent release of 5-HT from distal dendritic compartments, we examined to what extent somatic action potential firing induced calcium concentration changes at different locations along the dendritic tree. To this end, putative 5-HT neurones were loaded with Alexa594 and calcium indicator Fluo4 through whole-cell recording pipettes (Fig. 4A). Line-scans (Fig. 4B) were taken at different dendritic locations and action potentials were induced by current injection at the soma. A single action potential resulted in a change in fluorescence of the calcium indicator dye in proximal regions of the dendrite (ΔG/R ratio; Fig. 4C–E, N = 2 animals, n = 8 patches), indicating that action potentials induced calcium influx in these regions. However, the fluorescence change rapidly decreased with distance from the soma (Fig. 4D and E). At locations ∼150 μm away from the soma, fluorescence changes hardly exceeded noise levels, suggesting that the somatic action potentials induced very little calcium influx at these locations (Fig. 4D and E). Inducing trains of five action potentials at 20 Hz induced much larger fluorescence changes, which were also well resolved at distal dendritic locations (150 μm; Fig. 4D and E). This suggests that in contrast to single action potentials, trains of action potentials do induce calcium influx at distal dendritic locations. These experiments indicate that in proximal dendritic regions up to 150 μm, single action potentials could serve as the trigger for 5-HT release, but that beyond 150 μm single action potentials will not induce sufficient amounts of calcium influx to trigger release.
Figure 4. Dendritic calcium influx induced by single somatic action potentials is restricted to proximal regions.
A, overview of a 5-HT neurone. B, magnified image of a dendritic region used for line-scans. Dashed line indicates location where line-scan was taken. C, line-scan image following dendritic fluorescence of Fluo4 over time. D, green fluorescence transients (Δgreen/red ratio) in response to either single somatic action potentials (left) or trains of 5 action potentials (right) at different dendritic locations. E, summary data (n = 8), showing that AP-induced changes in ΔG/R ratio decrease with distance from the soma. Note that at 150 μm distance from the soma, a single action potential induces almost no fluorescence change.
Somatodendritic release evoked by NMDA-mediated calcium influx
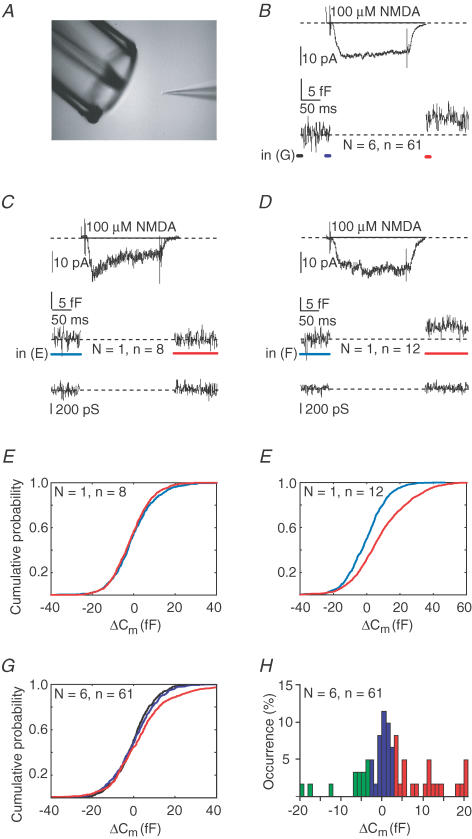
Since the backpropagating action potentials did not seem effective in generating a substantial calcium influx in dendrites more than 150 μm away from the soma, we hypothesized that an alternative source of calcium influx, such as the one through NMDA receptors, may contribute to exocytosis by 5-HT neurones. NMDA receptors are known to be both sodium and calcium permeable at a ratio of ∼85% : 15% (Burnashev et al. 1995), and are expressed by 5-HT neurones (Becquet et al. 1993). We studied NMDA-induced calcium influx and exocytosis by applying NMDA to nucleated patches (Fig. 5A) using Mg2+-free extracellular medium to allow NMDA receptor activation at very negative membrane potentials (nucleated patches were continuously voltage clamped at −70 mV to prevent voltage-gated calcium channel activation).
Figure 5. NMDAR activation induces exocytosis without action potential firing of postsynaptic compartment.
A, experimental set-up, with the nucleated patch positioned in front of a double-barrelled electrode attached to a piezo-element. NMDA (100 μm) is rapidly applied (200 ms) by repositioning of the double barrelled electrode. B, average NMDA-induced current and corresponding membrane capacitance during NMDA application recorded from adult (6–8 weeks, n = 6) male animals voltage-clamped at −70 mV (pooled data, 6 nucleated patches, total of 61 applications, mean ±s.d.: 10 ± 3 applications per patch). Note that during the full protocol, nucleated patches were continuously voltage clamped at −70 mV to prevent activation of voltage dependent calcium channels. C, average of NMDA-induced currents, capacitance changes and membrane conductance traces from one particular nucleated patch, which lacked NMDA induced capacitance changes (n = 8). D, same as C, but in this example on average, the NMDA applications resulted in increased membrane capacitance, being indicative of vesicle release. E, cumulative all-point histograms of the recording in C, showing the lack of capacitance changes when comparing the blue and red regions. F, cumulative all-point capacitance histograms of the blue and red regions of the recording shown in D, indicating exocytosis. G, cumulative all-point histograms of capacitance changes of all pooled recordings and analysing the black, blue and red regions of the average as shown in B. H, probability histogram of capacitance changes when pooling all trials from all recordings. In five out of six recordings, on average 40% of the trials gave capacitance changes > 3 fF. In addition, an average of 40%‘failures’ (i.e. changes between −3 and +3 fF) occurred, where in a minority of the trials negative events occurred, which may imply that in addition endocytosis may occur. As indicated in text, in one recording (shown in C and E) only failures were observed. Moreover, three very negative events, referred to in text, shown in H (i.e. < −10 fF) were all from one recording also showing failures and exocytotic events.
NMDA activated inward currents occurred in all nucleated patches tested (Fig. 5B, average NMDA current amplitude 23.4 ± 2.9 pA, n = 61 NMDA applications from n = 6 patches, N = 3 animals). Corresponding capacitance recordings showed that NMDA receptor activation induced capacitance changes (in five out of six recordings), which is indicative of vesicle release. In Fig. 5D and F, a representative experiment is shown in which NMDA application did result in significant increases in the capacitance of the patch. In this experiment and four other cells, positive capacitance changes occurred in a number of instances although failures occurred during each recording as well (Fig. 5H). The cell in which NMDA clearly induced an inward current but without capacitance changes is shown in Fig. 5C and E. These results show that activation of NMDA channels can induce exocytosis from the somatodendritic compartment of DRN neurones. The capacitance changes ranged from 3 to 20 fF, which would be the equivalent of ∼8–50 vesicles being released. In one particular recording, both positive and negative capacitance changes were observed (see Fig. 5H legend). Possibly such negative changes in capacitance could result from endocytosis, which may alter depending on the local calcium dynamics (see de Kock et al. 2004 for discussion on this topic).
Fast applications of NMDA in this patch clamp mode were difficult to combine with amperometric recordings, given the turbulence of medium surrounding the patch and the carbon fibre during pressure ejection from the application electrode. Therefore, we were unable to confirm the serotonergic content of released vesicle during NMDA application. However, in later experiments (Fig. 6) we provide additional evidence that 5-HT is released locally in response to NMDA receptor activation.
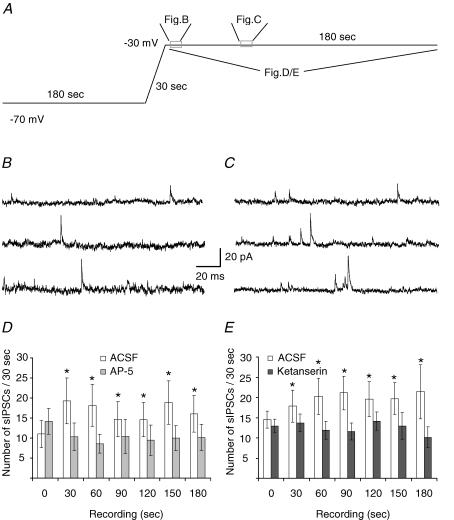
Figure 6. Postsynaptic NMDA receptor activation facilitates GABAergic transmission.
A, voltage protocol for NMDAR activation by endogenous glutamate. Experiments were performed upon preloading with tryptophan, and recorded in the presence of CNQX (1 μm), L-CGG-III (10 μm) and CPPG (30 nm) in addition fluoxetine (10 μm). B and C, example traces of IPSCs at start (B, shown by left box in A) and more toward the end (C, right box in A) at −30 mV (calibration 20 pA, 20 ms). D, at −30 mV the specific NMDA antagonist APV (50 μm) significantly suppressed the tonic increase in sIPSC frequency (n = 7, ANOVA followed by a post hoc Bonferroni multiple comparison test, *P < 0.05). E, at −30 mV the 5-HT2 antagonist ketanserin (1 μm) also significantly suppressed the tonic increase sIPSC frequency (n = 7, ANOVA followed by a post hoc Bonferroni multiple comparison test, *P < 0.05).
Physiological relevance of NMDA-induced dendritic signalling
Presynaptic GABA release in the DRN was previously shown to be modulated by 5-HT2 receptors (Liu et al. 2000). To test to what extent endogenous activation of NMDA receptors is capable of triggering retrograde 5-HT-mediated signalling via this pathway in the DRN slice preparation, whole-cell voltage-clamp recordings were made form ventral DRN neurones with a ‘5-HT’ current clamp profile (see Fig. 1A). In these experiments spontaneous GABAergic inhibitory postsynaptic currents (IPSCs) were recorded under asymmetrical chloride conditions. Activation of NMDA receptors was allowed, in the presence of a normal extracellular Mg2+ concentration. These recording conditions imply that inward synaptic currents are NMDAR dependent EPSCs whereas GABAAR mediated IPSCs are observed as outward events at depolarized potentials. For these experiments we only analysed the outward events. AMPA receptors were blocked by including CNQX in the bathing solution. In addition the DRN slices for these experiments were bathed in tryptophan, in order to boost the 5-HT synthesis, and in the presence of fluoxetine and L-CCG-III, in order to suppress reuptake of 5-HT and glutamate, respectively. In line with Liu et al. (2005), in current clamp recording we observed that tryptophan loading was sufficient to silence firing of 5-HT neurones in the DRN (n = 6, not shown). We have specifically tested that trypophane itself does not acutely induce a hyperpolarizing current (in voltage clamp, n = 6, not shown), and hence we rely on the idea that trypophane suffices as a precursor for 5-HT synthesis during the experiment. Finally, the mGluRs type II/III were blocked by CPPG to exclude heterosynaptic modulation.
The recorded cell was dialysed at −70 mV for at least 3 min. During this period, activation of NMDA receptors is unlikely to occur, due to the magnesium block. Then we slowly depolarized the membrane potential from −70 mV to −30 mV over 30 s to relieve magnesium block of the NMDA receptors; this is likely to also cause the inactivation of voltage-dependent calcium channels (Fig. 6A), as established in previous work (de Kock et al. 2004). Thus here we rely on the endogenous activation of NMDA receptors, amplified by glutamate reuptake blockade. Since the postsynaptic cell was under voltage clamp throughout the experiment, action potential firing was not occurring. Within 60 s after clamping the cell at −30 mV, the frequency of the outward detected GABAergic events increased to around 175% of the initial control level (Fig. 6B–D). During the subsequent 3 min period, the IPSC frequency remained to be increased (Fig. 6D and 53 ± 16%, N = 7 animals, n = 7 patches, for statistical details see legend). The IPSC amplitude was not affected in these recordings (n = 7, data not shown). The potentiation of IPSC frequency is most likely due to activation of 5-HT receptors on GABAergic neurones (see below).
In the presence of 50 μm of the NMDA antagonist d-APV, the observed increase in frequency of GABAergic IPSCs was suppressed (Fig. 6D, paired experimental design). This indicates that NMDARs are mostly responsible for the calcium influx and that no additional calcium influx, such as through voltage-gated calcium channels, occurred to mediate the release of retrogradely acting 5-HT. Similarly, the increase in sIPSC frequency was suppressed in recordings performed using a pipette medium containing 200 μm of BAPTA instead of 100 μm EGTA (ANOVA P < 0.05, n = 6 from N = 3 animals, not shown).
To find out whether the NMDAR-dependent facilitation of GABAergic transmission was mediated by the release of 5-HT from the postsynaptic neurone, we tested whether the potentiation of GABAergic transmission at −30 mV was sensitive to the 5-HT2 receptor antagonist ketanserin. Indeed, in the presence of 1 μm ketanserin, the increase of sIPSC frequency was also absent (Fig. 6E, n = 7 from N = 4 animals, paired experimental design, for statistical details see legend), showing that NMDAR activation induces release of 5-HT, which then acts presynaptically to stimulate GABAergic transmission. It should be noted that 5-HT is not likely to be secreted from neighbouring neurones other than the cell being recorded from, since the tryptophan loading of the neurones (see Methods) during these experiments brings the DRN neurones into a non-firing mode, conforming with previous work (Liu et al. 2005). Also, NMDA-induced secretion of 5-HT from neighbouring cells is not likely, since at resting membrane potential, due to the magnesium block, this process would be suppressed.
We conclude here that modulation of the GABA input may occur locally, being mediated at the level of the somatodendritic region, in the absence of postsynaptic firing. These data imply that calcium influx through NMDA channels may contribute to regulation of local secretion of 5-HT within the DRN.
Discussion
An unresolved question is whether activation of NMDA receptors is sufficient to induce somatodendritic secretion of neuroactive substances, without calcium influx through voltage-gated calcium channels. We have addressed this question specifically for secretion of 5-HT by dorsal raphé nucleus (DRN) neurones. We monitored vesicular secretion of 5-HT in nucleated outside-out patches of DRN neurones in response to short depolarization (10 ms) or fast (200 ms) application of NMDA. Influx of calcium through voltage-gated calcium channels triggered fusion of vesicles. However, two photon imaging of calcium-signalling in dendrites of 5-HT neurones in brain slices showed that calcium concentration changes induced by single somatic action potentials were limited to initial segments of dendrites within 150 μm from the soma. Even trains of action potentials induced only moderate changes in calcium levels at these sites. This implied that beyond 150 μm in distal dendrites of 5-HT neurones, other sources of calcium influx could dominate the induction of 5-HT vesicle fusion.
In nucleated outside-out patches, transient NMDA receptor activation also induced substantial amounts of vesicle fusion, in the absence of postsynaptic firing and without voltage-gated calcium channel activity. Therefore, glutamatergic inputs to DRN 5-HT neurones may induce local release of 5-HT directly. That this actually does occur was shown in recordings from 5-HT neurones in brain slices of DRN. These experiments showed that local secretion of 5-HT upon NMDA receptor activation, but in the absence of postsynaptic firing, increased GABA release from synaptic inputs to DRN 5-HT neurones.
Local release of 5-HT
Secretion of 5-HT in the DRN may occur in a very localized manner, possibly leading to heterosynaptic modulation between neighbouring synapses impinging on the same neurone, its dendrites or on nearby neurones. At the level of the somata of DRN neurones, simultaneous activity of voltage-gated calcium channels and NMDA receptors most likely leads to boosting of the local 5-HT secretion. In distal dendrites of DRN neurones, however, AMPA and NMDA receptor activation, in the absence of local voltage-gated calcium channel activity (see Fig. 4), is sufficient to induce release. Finally in the proximal dendrites, concurrent AMPA and NMDA receptor activation during back propagating action potentials may function as a coincidence detector, thereby facilitating 5-HT release when glutamatergic input is appropriately synchronized with DRN neurone firing. Therefore, NMDA receptor activation may be used to only increase 5-HT release at a few select synapses during different firing modes and thereby influence GABA release from a functionally distinct set of inputs.
What is the source of local 5-HT release? 5-HT containing vesicles are present in dendrites of DRN neurons (Kapadia et al. 1985; Liposits et al. 1985; Chazal & Ralston, 1987). Another source of local 5-HT could be axon collaterals from DRN neurones, although several studies report low incidence of these collaterals (Descarries et al. 1982; Blier et al. 1998). In addition, our amperometric measurements clearly indicate that serotonin can be released from non-synaptic specializations. We thus conclude that the source for local feedback by 5-HT is from somatic and most likely dendritic 5-HT containing vesicles.
NMDA-receptors on DRN neurones
Is there evidence that NMDA receptor-mediated release of 5-HT can occur in DRN in vivo that acting via modulation of GABAergic inputs to 5–HT neurones? Extracellular 5-HT in the DRN is controlled by intrinsic 5-HT receptor signalling mechanisms as well as afferent inputs (Adell et al. 2002). Both electrophysiological and neuroanatomical data show a tight innervation of DRN by axons coming from the prefrontal cortex (PFC) (Hajos et al. 1999; Celada et al. 2001; Hajos et al. 2003; Puig et al. 2005). At least part of the PFC axons project directly onto tryptophan hydroxylase-immunolabelled processes in the DRN (Jankowski & Sesack, 2004; Commons et al. 2005). The PFC has been shown to play a major role in activation of 5-HT1A receptor-induced inhibition of DRN neurones in vivo (Hajos et al. 1999; Stamford et al. 2000), which is most likely mediated by extrasynaptic secretion of 5-HT (Bunin & Wightman, 1998, 1999). In addition, 5-HT may act on 5-HT2 receptors of presynaptic GABA-containing neurones, thereby increasing inhibition of 5-HT neurones (Liu et al. 2000). NMDA receptors, in particular of the NR1/NR2D subtype, are expressed throughout the DRN (Tolle et al. 1993; Pallotta et al. 1998), both as synaptic and extrasynaptic receptors (Lozovaya et al. 2004). Hence their activation may contribute to induction of release of 5-HT from somata and dendrites, even before 5-HT neurones fire action potentials.
GABA-input to DRN neurones
GABA input occurs at the level of both the DRN somata and their dendrites (Mennini et al. 1986). Since 5-HT mediated effects on GABA inputs were previously shown to be sensitive to TTX (Liu et al. 2005), it has been suggested that 5-HT2 receptor are localized on GABAergic cell bodies rather than on their terminals impinging onto 5-HT DRN neurones. This idea together with fact that there may be non-synaptic release of 5-HT within the DRN, would imply that under physiological conditions, several DRN neurons being synchronized by multiple glutamate inputs including those of the PFC would be necessary and sufficient to induce secretion of 5-HT from the postsynaptic cell, which then would activate somatic 5-HT2 receptors on neighbouring GABAergic interneurones within the DRN.
Dendritic release of neurotransmitters
Dendritic vesicular secretion is a widespread phenomenon in the brain. Indeed, amperometric recordings in the substantia nigra showed that extrasynaptic or somatodendritic secretion of dopamine-containing vesicles (Jaffe et al. 1998) is sensitive to both glutamate application and electrical stimulation (Rice et al. 1997). Later studies also addressed to some extent the putative role of calcium influx through NMDA receptors in somatodendritic secretion of dopamine in the substantia nigra (Chen & Rice, 2002). In the olfactory system NMDA was reported to facilitate the secretion of extrasynaptic GABA (Chen et al. 2000; Halabisky et al. 2000) by increasing postsynaptic excitation and activating voltage-gated calcium channels (Isaacson, 2001). Although synaptic regulation of somatodendritic release may differ in different brain areas (Hoffman & Gerhardt, 1999; Chen & Rice, 2001), we have shown previously that in hypothalamic oxytocin neurones activation of NMDA receptors is sufficient to induce vesicle fusion, without the activation of voltage-gated calcium channels (de Kock et al. 2004). As we find in the present study on somatodendritic 5-HT release in DRN, dendritic secretion of oxytocin is also induced by calcium influx either through voltage-gated calcium channels during action potential firing or through NMDA receptors endogenously activated by glutamate (Kombian et al. 1997; de Kock et al. 2003). In addition, possibly oxytocin can be boosted by release of calcium from intracellular stores (Ludwig et al. 2002b).
The mechanism of direct stimulation of dendritic secretion by calcium influx through NMDA receptors may not be limited to the hypothalamus and DRN. Also in other brain areas extrasynaptic release of neurotransmitters may be triggered by NMDA receptor activation directly, or glutamate may depolarize neurones and induce action potential firing that then triggers vesicular secretion from dendrites by calcium influx through voltage-gated calcium channels. However, at present it is unknown whether in any of the other systems NMDA receptor-mediated dendritic release of neurotransmitters is involved in local heterosynaptic modulation of neighbouring synapses (de Kock et al. 2004), or whether more diffuse volume transmission-like processes affecting larger surrounding areas are involved (Zoli et al. 1999).
Heterosynaptic modulation
What are the distinct features of NMDA receptor-mediated somatodendritic secretion in the DRN? The somatodendritic secretion of 5-HT is rapid and transient in line with previous findings (Bunin & Wightman, 1999). It may occur in a very localized manner in the direct vicinity of active glutamate synapses, since somatic action potentials do not substantially increase calcium levels in distal dendrites (see Fig. 4). This mechanism of heterosynaptic modulation by which activity in one synapse releases substances from the postsynaptic neurone which then acts on a different nearby neurone, is distinct from previously described mechanisms of heterosynaptic modulation in which spill-over of neurotransmitter acts on presynaptic metabotropic receptors on neighbouring synapses (Grillner & Mercuri, 2002). The mechanism described here is also distinct from retrograde signalling by endocannabinoids, which are also released in a calcium-dependent manner, but need suprathreshold activation of voltage-gated calcium channels (Piomelli, 2003). Retrograde signalling through endocannabinoids is not likely to be localized and restricted to a particular subcellular compartment or subset of synapses, given that endocannabinoids are not released through vesicles. NMDA receptor-mediated release of 5-HT in the DRN is vesicular and can occur below the threshold of firing, which provides this cell system with an efficient pathway for localized heterosynaptic modulation. Subthreshold signalling between specific glutamate synapses and 5-HT release would enable single synaptic inputs from the PFC to regulate the excitability of dendritic regions of a single DRN 5-HT neurone.
Mechanisms underlying brain pathology
What is the relation between regulation of the 5-HT system at this very local level and the putative aetiology of neuropsychiatric disorders, in particular depression? Experimental work supports the idea that adaptive changes in the 5-HT system may occur after antidepressant treatments (Artigas et al. 1996a) and that there may be a substantial role for the PFC innervation of the DRN in the occurrence of depression (Celada et al. 2002). Moreover, an up-regulation in 5-HT1A autoreceptor density on DRN dendrites was shown to occur in human suicide victims with a known history of severe chronic depression (Stockmeier et al. 1998). In line with this, in animals, 5-HT1A receptors have been successfully targeted, in particular during the initial stages of drug therapy of chronic depression (Artigas et al. 1996b; Blier & Ward, 2003). In addition to changes in 5-HT autoreceptor density, putative changes in the expression of the 5-HT reuptake transporter in the DRN have been reported (Arango et al. 2001). This is interesting since the reuptake transporter may be the limiting factor in determining the distance over which extrasynaptic concentrations of 5-HT will diffuse (Bunin & Wightman, 1998, 1999). Since 5-HT1A autoreceptors are likely to be activated by the type of local 5-HT release described in this report and since the antidepressant serotonin's selective reuptake inhibitors (SSRIs) are likely to affect the concurrent rise in extracellular 5-HT concentration upon local release, perhaps the regulation of this local 5-HT release should now also be added to the list of potential therapeutic targets.
There is accumulating evidence implicating disturbances in glutamate metabolism and NMDA receptor expression and/or functioning in depression and suicidal behaviour (Paul et al. 1994; Skolnick et al. 1996; Paul & Skolnick, 2003). The new evidence on the local regulation of excitability of DRN neurones presented here may contribute to a better understanding of putative physiological or mechanistic epistase among the factors that control extracellular levels of 5-HT in the DRN, which in turn may be important in the aetiology of depression (Stoltenberg, 2005).
Acknowledgments
We thank Tessa Lodder for assistance during experiments, Trifarma (Milan, Italy) for donation of fluoxetine, Heidi de Wit for pilot studies and Matthijs Verhage for insightful discussion on earlier versions of this manuscript. The ENF department is supported by funding through NeuroBsik (http://www.mousephenomics.org), CNCR and NWO.
References
- Adell A, Celada P, Abellan MT, Artigas F. Origin and functional role of the extracellular serotonin in the midbrain raphe nuclei. Brain Res Brain Res Rev. 2002;39:154–180. doi: 10.1016/s0165-0173(02)00182-0. [DOI] [PubMed] [Google Scholar]
- Angleson JK, Betz WJ. Monitoring secretion in real time: capacitance, amperometry and fluorescence compared. Trends Neurosci. 1997;20:281–287. doi: 10.1016/s0166-2236(97)01083-7. [DOI] [PubMed] [Google Scholar]
- Arango V, Underwood MD, Boldrini M, Tamir H, Kassir SA, Hsiung S, Chen JJ, Mann JJ. Serotonin 1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology. 2001;25:892–903. doi: 10.1016/S0893-133X(01)00310-4. [DOI] [PubMed] [Google Scholar]
- Artigas F, Bel N, Casanovas JM, Romero L. Adaptative changes of the serotonergic system after antidepressant treatments. Adv Exp Med Biol. 1996a;398:51–59. doi: 10.1007/978-1-4613-0381-7_6. [DOI] [PubMed] [Google Scholar]
- Artigas F, Romero L, de Montigny C, Blier P. Acceleration of the effect of selected antidepressant drugs in major depression by 5-HT1A antagonists. Trends Neurosci. 1996b;19:378–383. doi: 10.1016/S0166-2236(96)10037-0. [DOI] [PubMed] [Google Scholar]
- Becquet D, Hery M, Deprez P, Faudon M, Fache MP, Giraud P, Hery F. N-methyl-D-aspartic acid/glycine interactions on the control of 5-hydroxytryptamine release in raphe primary cultures. J Neurochem. 1993;61:1692–1697. doi: 10.1111/j.1471-4159.1993.tb09805.x. [DOI] [PubMed] [Google Scholar]
- Bekkers JM. Properties of voltage-gated potassium currents in nucleated patches from large layer 5 cortical pyramidal neurons of the rat. J Physiol. 2000;525:593–609. doi: 10.1111/j.1469-7793.2000.t01-1-00593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blier P, Pineyro G, el Mansari M, Bergeron R, de Montigny C. Role of somatodendritic 5-HT autoreceptors in modulating 5-HT neurotransmission. Ann N Y Acad Sci. 1998;861:204–216. doi: 10.1111/j.1749-6632.1998.tb10192.x. [DOI] [PubMed] [Google Scholar]
- Blier P, Ward NM. Is there a role for 5-HT1A agonists in the treatment of depression? Biol Psychiatry. 2003;53:193–203. doi: 10.1016/s0006-3223(02)01643-8. [DOI] [PubMed] [Google Scholar]
- Bunin MA, Wightman RM. Quantitative evaluation of 5-hydroxytryptamine (serotonin) neuronal release and uptake: an investigation of extrasynaptic transmission. J Neurosci. 1998;18:4854–4860. doi: 10.1523/JNEUROSCI.18-13-04854.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunin MA, Wightman RM. Paracrine neurotransmission in the CNS: involvement of 5-HT. Trends Neurosci. 1999;22:377–382. doi: 10.1016/s0166-2236(99)01410-1. [DOI] [PubMed] [Google Scholar]
- Burnashev N, Zhou Z, Neher E, Sakmann B. Fractional calcium currents through recombinant GluR channels of the NMDA, AMPA and kainate receptor subtypes. J Physiol. 1995;485:403–418. doi: 10.1113/jphysiol.1995.sp020738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada P, Puig MV, Casanovas JM, Guillazo G, Artigas F. Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: Involvement of serotonin-1A, GABAA, and glutamate receptors. J Neurosci. 2001;21:9917–9929. doi: 10.1523/JNEUROSCI.21-24-09917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada P, Puig MV, Martin-Ruiz R, Casanovas JM, Artigas F. Control of the serotonergic system by the medial prefrontal cortex: potential role in the etiology of PTSD and depressive disorders. Neurotox Res. 2002;4:409–419. doi: 10.1080/10298420290030550. [DOI] [PubMed] [Google Scholar]
- Chazal G, Ralston HJ., 3rd Serotonin-containing structures in the nucleus raphe dorsalis of the cat: an ultrastructural analysis of dendrites, presynaptic dendrites, and axon terminals. J Comp Neurol. 1987;259:317–329. doi: 10.1002/cne.902590302. [DOI] [PubMed] [Google Scholar]
- Chen BT, Rice ME. Novel Ca2+ dependence and time course of somatodendritic dopamine release: substantia nigra versus striatum. J Neurosci. 2001;21:7841–7847. doi: 10.1523/JNEUROSCI.21-19-07841.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Rice ME. Synaptic regulation of somatodendritic dopamine release by glutamate and GABA differs between substantia nigra and ventral tegmental area. J Neurochem. 2002;81:158–169. doi: 10.1046/j.1471-4159.2002.00811.x. [DOI] [PubMed] [Google Scholar]
- Chen WR, Xiong W, Shepherd GM. Analysis of relations between NMDA receptors and GABA release at olfactory bulb reciprocal synapses. Neuron. 2000;25:625–633. doi: 10.1016/s0896-6273(00)81065-x. [DOI] [PubMed] [Google Scholar]
- Commons KG, Beck SG, Bey VW. Two populations of glutamatergic axons in the rat dorsal raphe nucleus defined by the vesicular glutamate transporters 1 and 2. Eur J Neurosci. 2005;21:1577–1586. doi: 10.1111/j.1460-9568.2005.03991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descarries L, Watkins KC, Garcia S, Beaudet A. The serotonin neurons in nucleus raphe dorsalis of adult rat: a light and electron microscope radioautographic study. J Comp Neurol. 1982;207:239–254. doi: 10.1002/cne.902070305. [DOI] [PubMed] [Google Scholar]
- Duguid IC, Smart TG. Retrograde activation of presynaptic NMDA receptors enhances GABA release at cerebellar interneuron-Purkinje cell synapses. Nat Neurosci. 2004;7:525–533. doi: 10.1038/nn1227. [DOI] [PubMed] [Google Scholar]
- Govindaiah, Cox CL. Synaptic activation of metabotropic glutamate receptors regulates dendritic outputs of thalamic interneurons. Neuron. 2004;41:611–623. doi: 10.1016/s0896-6273(04)00013-3. [DOI] [PubMed] [Google Scholar]
- Grillner P, Mercuri NB. Intrinsic membrane properties and synaptic inputs regulating the firing activity of the dopamine neurons. Behav Brain Res. 2002;130:149–169. doi: 10.1016/s0166-4328(01)00418-1. [DOI] [PubMed] [Google Scholar]
- Hajos M, Gartside SE, Varga V, Sharp T. In vivo inhibition of neuronal activity in the rat ventromedial prefrontal cortex by midbrain-raphe nuclei: role of 5-HT1A receptors. Neuropharmacology. 2003;45:72–81. doi: 10.1016/s0028-3908(03)00139-4. [DOI] [PubMed] [Google Scholar]
- Hajos M, Hajos-Korcsok E, Sharp T. Role of the medial prefrontal cortex in 5-HT1A receptor-induced inhibition of 5-HT neuronal activity in the rat. Br J Pharmacol. 1999;126:1741–1750. doi: 10.1038/sj.bjp.0702510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halabisky B, Friedman D, Radojicic M, Strowbridge BW. Calcium influx through NMDA receptors directly evokes GABA release in olfactory bulb granule cells. J Neurosci. 2000;20:5124–5134. doi: 10.1523/JNEUROSCI.20-13-05124.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausser M, Stuart G, Racca C, Sakmann B. Axonal initiation and active dendritic propagation of action potentials in substantia nigra neurons. Neuron. 1995;15:637–647. doi: 10.1016/0896-6273(95)90152-3. [DOI] [PubMed] [Google Scholar]
- Hoffman AF, Gerhardt GA. Differences in pharmacological properties of dopamine release between the substantia nigra and striatum: an in vivo electrochemical study. J Pharmacol Exp Ther. 1999;289:455–463. [PubMed] [Google Scholar]
- Isaacson JS. Mechanisms governing dendritic γ-aminobutyric acid (GABA) release in the rat olfactory bulb. Proc Natl Acad Sci U S A. 2001;98:337–342. doi: 10.1073/pnas.021445798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe EH, Marty A, Schulte A, Chow RH. Extrasynaptic vesicular transmitter release from the somata of substantia nigra neurons in rat midbrain slices. J Neurosci. 1998;18:3548–3553. doi: 10.1523/JNEUROSCI.18-10-03548.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski MP, Sesack SR. Prefrontal cortical projections to the rat dorsal raphe nucleus: ultrastructural features and associations with serotonin and γ-aminobutyric acid neurons. J Comp Neurol. 2004;468:518–529. doi: 10.1002/cne.10976. [DOI] [PubMed] [Google Scholar]
- Kapadia SE, de Lanerolle NC, LaMotte CC. Immunocytochemical and electron microscopic study of serotonin neuronal organization in the dorsal raphe nucleus of the monkey. Neuroscience. 1985;15:729–746. doi: 10.1016/0306-4522(85)90075-2. [DOI] [PubMed] [Google Scholar]
- Klyachko VA, Jackson MB. Capacitance steps and fusion pores of small and large-dense-core vesicles in nerve terminals. Nature. 2002;418:89–92. doi: 10.1038/nature00852. [DOI] [PubMed] [Google Scholar]
- de Kock CP, Burnashev N, Lodder JC, Mansvelder HD, Brussaard AB. NMDA receptors induce somatodendritic secretion in hypothalamic neurones of lactating female rats. J Physiol. 2004;561:53–64. doi: 10.1113/jphysiol.2004.069005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kock CP, Wierda KD, Bosman LW, Min R, Koksma JJ, Mansvelder HD, Verhage M, Brussaard AB. Somatodendritic secretion in oxytocin neurons is upregulated during the female reproductive cycle. J Neurosci. 2003;23:2726–2734. doi: 10.1523/JNEUROSCI.23-07-02726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kombian SB, Mouginot D, Pittman QJ. Dendritically released peptides act as retrograde modulators of afferent excitation in the supraoptic nucleus in vitro. Neuron. 1997;19:903–912. doi: 10.1016/s0896-6273(00)80971-x. [DOI] [PubMed] [Google Scholar]
- Kombian SB, Zidichouski JA, Pittman QJ. GABAB receptors presynaptically modulate excitatory synaptic transmission in the rat supraoptic nucleus in vitro. J Neurophysiol. 1996;76:1166–1179. doi: 10.1152/jn.1996.76.2.1166. [DOI] [PubMed] [Google Scholar]
- Li YQ, Li H, Kaneko T, Mizuno N. Morphological features and electrophysiological properties of serotonergic and non-serotonergic projection neurons in the dorsal raphe nucleus. An intracellular recording and labeling study in rat brain slices. Brain Res. 2001;900:110–118. doi: 10.1016/s0006-8993(01)02272-7. [DOI] [PubMed] [Google Scholar]
- Lindau M, Neher E. Patch-clamp techniques for time-resolved capacitance measurements in single cells. Pflugers Arch. 1988;411:137–146. doi: 10.1007/BF00582306. [DOI] [PubMed] [Google Scholar]
- Liposits Z, Gorcs T, Trombitas K. Ultrastructural analysis of central serotoninergic neurons immunolabeled by silver-gold-intensified diaminobenzidine chromogen. Completion of immunocytochemistry with X-ray microanalysis. J Histochem Cytochem. 1985;33:604–610. doi: 10.1177/33.6.3889144. [DOI] [PubMed] [Google Scholar]
- Liu R, Jolas T, Aghajanian G. Serotonin 5-HT2 receptors activate local GABA inhibitory inputs to serotonergic neurons of the dorsal raphe nucleus. Brain Res. 2000;873:34–45. doi: 10.1016/s0006-8993(00)02468-9. [DOI] [PubMed] [Google Scholar]
- Liu RJ, Lambe EK, Aghajanian GK. Somatodendritic autoreceptor regulation of serotonergic neurons: dependence on L-tryptophan and tryptophan hydroxylase-activating kinases. Eur J Neurosci. 2005;21:945–958. doi: 10.1111/j.1460-9568.2005.03930.x. [DOI] [PubMed] [Google Scholar]
- Lozovaya NA, Grebenyuk SE, Tsintsadze T, Feng B, Monaghan DT, Krishtal OA. Extrasynaptic NR2B and NR2D subunits of NMDA receptors shape ‘superslow’ afterburst EPSC in rat hippocampus. J Physiol. 2004;558:451–463. doi: 10.1113/jphysiol.2004.063792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig M, Sabatier N, Bull PM, Landgraf R, Dayanithi G, Leng G. Intracellular calcium stores regulate activity-dependent neuropeptide release from dendrites. Nature. 2002a;418:85–89. doi: 10.1038/nature00822. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Sabatier N, Dayanithi G, Russell JA, Leng G. The active role of dendrites in the regulation of magnocellular neurosecretory cell behavior. Prog Brain Res. 2002b;139:247–256. doi: 10.1016/s0079-6123(02)39021-6. [DOI] [PubMed] [Google Scholar]
- Mennini T, Gobbi M, Romandini S. Localization of GABAA and GABAB receptor subtypes on serotonergic neurons. Brain Res. 1986;371:372–375. doi: 10.1016/0006-8993(86)90378-1. [DOI] [PubMed] [Google Scholar]
- Munsch T, Freichel M, Flockerzi V, Pape HC. Contribution of transient receptor potential channels to the control of GABA release from dendrites. Proc Natl Acad Sci U S A. 2003;100:16065–16070. doi: 10.1073/pnas.2535311100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard S, Bolam JP, Greenfield SA. Facilitation of a dendritic calcium conductance by 5-hydroxytryptamine in the substantia nigra. Nature. 1988;333:174–177. doi: 10.1038/333174a0. [DOI] [PubMed] [Google Scholar]
- Pallotta M, Segieth J, Whitton PS. N-methyl-Daspartate receptors regulate 5-HT release in the raphe nuclei and frontal cortex of freely moving rats: differential role of 5-HT1A autoreceptors. Brain Res. 1998;783:173–178. doi: 10.1016/s0006-8993(97)01333-4. [DOI] [PubMed] [Google Scholar]
- Paul IA, Nowak G, Layer RT, Popik P, Skolnick P. Adaptation of the N-methyl-D-aspartate receptor complex following chronic antidepressant treatments. J Pharmacol Exp Ther. 1994;269:95–102. [PubMed] [Google Scholar]
- Paul IA, Skolnick P. Glutamate and depression: clinical and preclinical studies. Ann N Y Acad Sci. 2003;1003:250–272. doi: 10.1196/annals.1300.016. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Puig MV, Artigas F, Celada P. Modulation of the activity of pyramidal neurons in rat prefrontal cortex by raphe stimulation in vivo: involvement of serotonin and GABA. Cereb Cortex. 2005;15:1–14. doi: 10.1093/cercor/bhh104. [DOI] [PubMed] [Google Scholar]
- Ralston HJ., 3rd Evidence for presynaptic dendrites and a proposal for their mechanism of action. Nature. 1971;230:585–587. doi: 10.1038/230585a0. [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ, Greenfield SA. Characteristics of electrically evoked somatodendritic dopamine release in substantia nigra and ventral tegmental area in vitro. J Neurophysiol. 1997;77:853–862. doi: 10.1152/jn.1997.77.2.853. [DOI] [PubMed] [Google Scholar]
- Rozov A, Zilberter Y, Wollmuth LP, Burnashev N. Facilitation of currents through rat Ca2+-permeable AMPA receptor channels by activity-dependent relief from polyamine block. J Physiol. 1998;511:361–377. doi: 10.1111/j.1469-7793.1998.361bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini BL, Regehr WG. Detecting changes in calcium influx which contribute to synaptic modulation in mammalian brain slice. Neuropharmacology. 1995;34:1453–1467. doi: 10.1016/0028-3908(95)00129-t. [DOI] [PubMed] [Google Scholar]
- Sather W, Dieudonne S, MacDonald JF, Ascher P. Activation and desensitization of N-methyl-D-aspartate receptors in nucleated outside-out patches from mouse neurones. J Physiol. 1992;450:643–672. doi: 10.1113/jphysiol.1992.sp019148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnick P, Layer RT, Popik P, Nowak G, Paul IA, Trullas R. Adaptation of N-methyl-D-aspartate (NMDA) receptors following antidepressant treatment: implications for the pharmacotherapy of depression. Pharmacopsychiatry. 1996;29:23–26. doi: 10.1055/s-2007-979537. [DOI] [PubMed] [Google Scholar]
- Stamford JA, Davidson C, McLaughlin DP, Hopwood SE. Control of dorsal raphe 5-HT function by multiple 5-HT1 autoreceptors: parallel purposes or pointless plurality? Trends Neurosci. 2000;23:459–465. doi: 10.1016/s0166-2236(00)01631-3. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Shapiro LA, Dilley GE, Kolli TN, Friedman L, Rajkowska G. Increase in serotonin-1A autoreceptors in the midbrain of suicide victims with major depression—Postmortem evidence for decreased serotonin activity. J Neurosci. 1998;18:7394–7401. doi: 10.1523/JNEUROSCI.18-18-07394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltenberg SF. Epistasis among presynaptic serotonergic system components. Behav Genet. 2005;35:199–209. doi: 10.1007/s10519-004-1019-4. [DOI] [PubMed] [Google Scholar]
- Stuart G, Spruston N, Sakmann B, Hausser M. Action potential initiation and backpropagation in neurons of the mammalian CNS. Trends Neurosci. 1997;20:125–131. doi: 10.1016/s0166-2236(96)10075-8. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Wall NR, Aakalu GN, Schuman EM. Regulation of dendritic protein synthesis by miniature synaptic events. Science. 2004;304:1979–1983. doi: 10.1126/science.1096202. [DOI] [PubMed] [Google Scholar]
- Tolle TR, Berthele A, Zieglgansberger W, Seeburg PH, Wisden W. The differential expression of 16 NMDA and non-NMDA receptor subunits in the rat spinal cord and in periaqueductal gray. J Neurosci. 1993;13:5009–5028. doi: 10.1523/JNEUROSCI.13-12-05009.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoli M, Jansson A, Sykova E, Agnati LF, Fuxe K. Volume transmission in the CNS and its relevance for neuropsychopharmacology. Trends Pharmacol Sci. 1999;20:142–150. doi: 10.1016/s0165-6147(99)01343-7. [DOI] [PubMed] [Google Scholar]