Abstract
Extrasynaptic GABAA receptors that are tonically activated by ambient GABA are important for controlling neuronal excitability. In hippocampal pyramidal neurons, the subunit composition of these extrasynaptic receptors may include α5βγ and/or α4βδ subunits. Our present studies reveal that a component of the tonic current in the hippocampus is highly sensitive to inhibition by Zn2+. This component is probably not mediated by either α5βγ or α4βδ receptors, but might be explained by the presence of αβ isoforms. Using patch-clamp recording from pyramidal neurons, a small tonic current measured in the absence of exogenous GABA exhibited both high and low sensitivity to Zn2+ inhibition (IC50 values, 1.89 and 223 μm, respectively). Using low nanomolar and micromolar GABA concentrations to replicate tonic currents, we identified two components that are mediated by benzodiazepine-sensitive and -insensitive receptors. The latter indicated that extrasynaptic GABAA receptors exist that are devoid of γ2 subunits. To distinguish whether the benzodiazepine-insensitive receptors were αβ or αβδ isoforms, we used single-channel recording. Expressing recombinant α1β3γ2, α5β3γ2, α4β3δ and α1β3 receptors in human embryonic kidney (HEK) or mouse fibroblast (Ltk) cells, revealed similar openings with high main conductances (∼25–28 pS) for γ2 or δ subunit-containing receptors whereas αβ receptors were characterized by a lower main conductance state (∼11 pS). Recording from pyramidal cell somata revealed a similar range of channel conductances, indicative of a mixture of GABAA receptors in the extrasynaptic membrane. The lowest conductance state (∼11 pS) was the most sensitive to Zn2+ inhibition in accord with the presence of αβ receptors. This receptor type is estimated to account for up to 10% of all extrasynaptic GABAA receptors on hippocampal pyramidal neurons.
The distribution of specific GABAA receptor isoforms at synaptic and extrasynaptic locations will profoundly influence neuronal excitability. Currently, the archetypal synaptic GABAA receptor is likely to be composed of αβγ subunits (Moss & Smart, 2001; Farrant & Nusser, 2005), whereas extrasynaptic GABAA receptors will not only comprise these subtypes (Thomas et al. 2005), but also αβδ subtypes (Farrant & Nusser, 2005; Mangan et al. 2005). In addition, some populations of extrasynaptic receptors will also contain specific α isoforms, such as α4, α5 and α6 (Semyanov et al. 2004; Caraiscos et al. 2004; Farrant & Nusser, 2005). Thus expressing such a diverse spectrum of receptor subunits will confer quite distinctive pharmacological and physiological profiles on the extrasynaptic GABAA receptors. With regard to their physiology, low concentrations of ambient GABA can activate extrasynaptic receptors causing a small, but persistent, Cl− current (Isaacson, 2000; Mody, 2001), which results in tonic inhibition thus enabling neuronal excitability to be regulated (Mitchell & Silver, 2003).
Previous studies of cerebellar (Brickley et al. 1999) and hippocampal neurons (Yeung et al. 2003) indicate that some of the GABAA receptors mediating tonic inhibition have a high sensitivity to GABA. Variations in GABA potency have been reported to depend on the α subunit present in recombinant αβγ receptors, with a relative order, based on GABA EC50 values determined from dose–response curves, of: α6 > α1 > α2 > α4 > α5 ≈ α3 (Knoflach et al. 1996; Böhme et al. 2004; Feng & Macdonald, 2004). However, as α6 subunit-containing receptors are found exclusively in cerebellar granule cells (Fritschy & Brünig, 2003) and the dorsal cochlear nucleus (Sieghart & Sperk, 2002), they cannot account for the high GABA sensitivity associated with tonic inhibition in hippocampal pyramidal cells. Alternatively, the high GABA potency might indicate the presence of δ subunit-containing receptors. Recombinant α4β3δ receptors are highly sensitive to GABA (Brown et al. 2002) and α4βδ isoforms have been proposed as extrasynaptic receptors on hippocampal pyramidal cells (Mangan et al. 2005). Furthermore, a comparison of GABA potency on recombinant α1β2/3γ2 and α1β2/3 receptors, revealed that receptors lacking γ2 subunits are at least 5-fold more sensitive to GABA (Verdoorn et al. 1990; Sigel et al. 1990; Fisher & Macdonald, 1997; Amato et al. 1999), raising the possibility that αβ receptors may also contribute to the extrasynaptic receptor population. However, generally it is thought that αβ receptors are unlikely to exist in neurons; however, immunocytochemistry (Sieghart & Sperk, 2002) and single-channel (Brickley et al. 1999) studies have both provided some evidence to the contrary. As the γ2 subunit is important in mediating the clustering of GABAA receptors near to the scaffold protein gephyrin at inhibitory synapses (Moss & Smart, 2001; Luscher & Keller, 2004), it appears that αβ GABAA receptors are unlikely to reside in significant numbers at synapses.
The aim of this study was to investigate whether αβ subunit GABAA receptors are expressed on the surface of hippocampal pyramidal cells. By using a combination of pharmacological and electrophysiological approaches, we show that δ and γ subunit-lacking αβ GABAA receptors are likely to be expressed in low numbers in the extrasynaptic membranes of pyramidal neurons where they can contribute to the level of tonic inhibition.
Methods
Culturing of primary hippocampal neurons
Hippocampal neurons were cultured from embryonic day (E) 18 Wistar rat fetuses. Pooled hippocampi (n = 6), were incubated for 15 min in 1 mg ml−1 trypsin in Hank's balanced salt solution (HBSS) at 37°C (95% air–5% CO2), followed by three 5 min washes in HBSS. Neurons were dissociated by mechanical trituration (three times) using polished Pasteur pipettes in minimum essential medium (MEM) with Earle's salts (Invitrogen) supplemented with 10% fetal calf serum (FCS), 0.06% (w/v) d-glucose and 50 units ml−1 penicillin-G and 50 μg ml−1 streptomycin. The final cell suspension was centrifuged for 10 min at 100 g. The cells were resuspended in supplemented MEM, prior to seeding onto poly-l-lysine-coated coverslips and incubation at 37°C (95% air–5%CO2). After 3–5 h of incubation, the medium was replaced by Neurobasal media (Invitrogen) supplemented with 2% FCS, 0.36% w/v d-glucose, 115 units ml−1 penicillin-G and 115 μg ml−1 streptomycin, 0.5 mm glutamine and 0.02 arbitrary units (50-fold dilution) of the additive, B-27 (Invitrogen). Neurons were used for electrophysiological recordings after 7–10 days in vitro.
Cell lines and expression of recombinant GABAA receptors
Human embryonic kidney (HEK) cells were cultured as previously described (Wooltorton et al. 1997). HEK cells were plated onto poly-l-lysine-coated glass coverslips and transfected using a calcium phosphate protocol. cDNAs for the selected combination of human α1/5, β2/3 and γ2 GABAA receptor subunits (Hadingham et al. 1993a, b) and enhanced green fluorescent protein (EGFP) were present in equal amounts (1 μg of each per culture dish). The DNA solutions were mixed with 340 mm CaCl2 before the precipitate was formed by gentle addition of an equal volume of a double-strength HBSS containing (mm): NaCl 280, Na2HPO4 2.8, Hepes 50; pH 7.2 to the DNA–CaCl2 solution. The DNA–calcium phosphate suspension was carefully added to the seeded HEK cells with the transfection proceeding overnight while incubating at 37°C. Cells were used for electrophysiological recording 18–72 h after transfection.
To examine the properties of α4β3δ GABAA receptors, we promoted the stable expression of this receptor in Ltk cells (Brown et al. 2002). The cells were maintained in DMEM supplemented with 4.5 mg ml−1 glucose, 4 mml-glutamine, 0.11 mg ml−1 sodium pyruvate, 10% FCS, 1 mg ml−1 geneticin and 0.2 mg ml−1 zeocin. After seeding the cells onto glass coverslips (same method as for HEK cells), the expression of the GABAA receptors was induced overnight in supplemented DMEM plus 0.5 μm dexamethasone. Electrophysiological recordings were performed within 48 h after the induction of receptor expression.
Patch-clamp electrophysiology
Whole-cell and single-channel GABA currents were recorded from hippocampal pyramidal neurons, transfected HEK cells or Ltk cells using an Axopatch 200B patch-clamp amplifier. Patch electrodes (4–6 MΩ for whole cell and 9–16 MΩ for single channels) were filled with an internal solution containing (mm): CsCl 120, MgCl21, EGTA 11, tetraethylammonium hydroxide 33, Hepes 10, CaCl2 1 and ATP 2; pH adjusted to 7.1 with HCl (approximately 8 mm). The cells were constantly perfused with a Krebs solution containing (mm): NaCl 140, KCl 4.7, MgCl2 1.2, CaCl2 2.52, glucose 11 and Hepes 5; pH 7.4. In whole-cell recordings, the Krebs solution was supplemented with 0.5 μm tetrodotoxin (TTX), 20 μmd-amino-5-phosphonopentanoic acid (AP5) and 10 μm 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), to block voltage-activated Na+ channels and NMDA and non-NMDA receptor-mediated EPSCs, respectively. Membrane currents were filtered at 2.3–2.5 kHz (single-channel currents) or 5 kHz (whole-cell currents; −3 dB, 8-pole Bessel, 48 dB octave−1), and digitized using a Digidata 1320A (Axon Instruments) prior to being recorded directly onto a Dell PC Pentium IV, using Clamplex 8.2 (whole-cell recording). For single-channel experiments, currents were recorded onto a DTR-1201 digital tape-recorder prior to off-line A/D conversion and final analysis with the single channel analysis suite: SCAN and EKDIST (http://www.ucl.ac.uk/Pharmacology/dcpr95.html). Any change exceeding 10% in the membrane conductance and/or series resistance resulted in the termination of the recording.
Analysis of whole-cell current data from hippocampal pyramidal cells
The amplitudes of GABA induced membrane currents (I) were determined at a holding potential of −70 mV. The GABA concentration–response relationships were determined by normalizing the GABA currents to the response induced by a maximum saturating concentration of GABA in control Krebs solution (Imax) and subsequently fitted with the Hill equation:
where EC50 represents the concentration of the agonist ([A]) inducing 50% of the maximal current evoked by a saturating concentration of the agonist and n is the Hill coefficient.
For quantifying the suppression of the tonic GABA current, the shifts in the baseline current were normalized to the maximum change usually achieved with 1 mm Zn2+ or 30 μm bicuculline. All-point histograms were constructed for the tonic current taking data samples before and during drug application (10 or 20 s) and fitted with a single Gaussian distribution function of the form:
The fit was constrained symmetrically around the peak frequency to avoid any bias caused by the presence of miniature IPSCs (mIPSCs; these are depicted in the histogram as the ‘shoulder’). A defines the amplitude and C is a constant defining the pedestal of the histogram. This function provided the Gaussian mean baseline current (μ) and standard deviation (σ). Paired t test analysis was used to compare the effects of tricine, bicuculline and Zn2+ on tonic and phasic currents.
To determine the potency of Zn2+ where the inhibition–concentration relationship for the mean baseline current was monophasic, the data were fitted to the equation:
where the IC50 is the antagonist concentration (B) eliciting half-maximal inhibition of the tonic current. For those inhibition–concentration relationships which were clearly biphasic, the data were fitted to the equation:
where a and b represent the relative proportions of each individual component described by IC50′ and IC50′′, respectively.
Analysis of single-channel records
Single GABA channel currents were recorded from excised outside-out membrane patches held at −70 mV. Patches showing channel current stacking, which indicated multiple channels in a patch, were only included in the analysis if the number of multiple channel openings never exceeded 2% of all detected openings (Macdonald et al. 1989; Smart, 1992). Further evaluation of channel numbers in each patch was performed as previously reported (Mortensen et al. 2004). Single-channel records were initially filtered at 10 kHz prior to storage on DAT. Records were then digitized at 20 kHz ensuring that additional filtering (∼2.5 kHz, 36 dB octave−1) did not suppress the amplitude of very brief openings. This was important because the precise determination of various single-channel amplitude levels was critical for identifying the presence of particular GABAA receptor assemblies. Channel openings and closures were idealized using time course fitting using SCAN. SCAN automatically corrects for any baseline current drift that may occur. For the analysis in EKDIST, only openings longer than twice the rise time of the filter were considered. A minimum time-resolution was usually set at 100 μs for both open and shut times. The amplitude distributions were then fitted with multiple Gaussian components that defined the mean current levels, their standard deviations and the total areas of all components, by using a non-linear least-squares routine. The single-channel conductances were calculated from the mean current levels determined from the Gaussian curve fits, and the difference between the patch holding potential and GABA response reversal potential.
Drugs and solutions
Drugs and solutions were rapidly applied to the HEK cells using a modified Y-tube positioned approximately 300 μm from the recorded cell (Wooltorton et al. 1997). The 10–90% solution exchange times of the application system were within 18–25 ms as measured in open-tip recordings. All drugs were dissolved in the Krebs solution.
Results
GABA potencies on pyramidal neurons and recombinant αβγ GABAA receptors
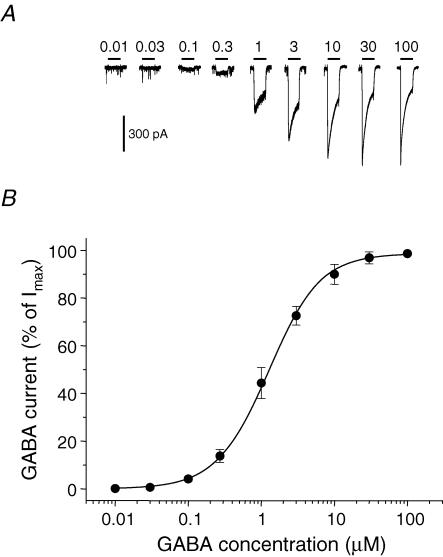
An EC50 value for GABA, determined for a single neuron, will reflect the combined sensitivities to GABA of all the different GABAA receptors contributing to its response (synaptic and extrasynaptic), weighted according to the relative amounts of each isoform present on the cell surface. Whole-cell GABA concentration–response curves, generated using data obtained from hippocampal pyramidal neurons (Fig. 1A), revealed a mean GABA EC50 of 1.24 ± 0.04 μm (slope, 1.20 ± 0.04, n = 10 cells; Fig. 1B). This is a relatively low value when compared to GABA EC50 values obtained from recombinant αβγ receptors, which normally range from 3 to 17 μm (Fisher & Macdonald, 1997; Mortensen et al. 2004; Böhme et al. 2004; Feng & Macdonald, 2004; Caraiscos et al. 2004). Although the reported variation in the EC50 will partly depend on the speed of GABA application, this higher sensitivity of pyramidal neurons to GABA could reflect the presence of mixed GABAA receptor populations with some possessing a higher affinity for GABA. Extrasynaptic GABAA receptors that underpin tonic inhibition have already been proposed to have a higher sensitivity to GABA than their synaptic counterparts (Stell & Mody, 2002). In this regard, previous studies have suggested that extrasynaptic GABAA receptors in pyramidal neurons could be composed of α5βγ (although these are not particularly sensitive to GABA compared to α1 subunit-containing receptors; Caraiscos et al. 2004), as well as the higher sensitivity α4βδ receptors (Brown et al. 2002; Mangan et al. 2005). Furthermore, in the cerebellum, higher affinity αβ isoforms have also been proposed as extrasynaptic receptors (Brickley et al. 1999).
Figure 1. GABA sensitivity of hippocampal pyramidal neurons.
A, membrane currents recorded from a single pyramidal neuron voltage clamped at −70 mV. The currents were activated by range of GABA concentrations applied for 4 s (at time shown). The occasional downward deflections represent miniature IPSCs. B, GABA concentration–response curve for peak GABA-activated currents. Responses have been normalized to the response induced by a saturating concentration of GABA. The data were accrued from cultured hippocampal pyramidal neurons after 10–14 days in vitro (DIV). Each data point represents mean ±s.e.m. (n = 10). The data are fitted with the Hill equation.
Modulation of the tonic conductance in cultured hippocampal neurons
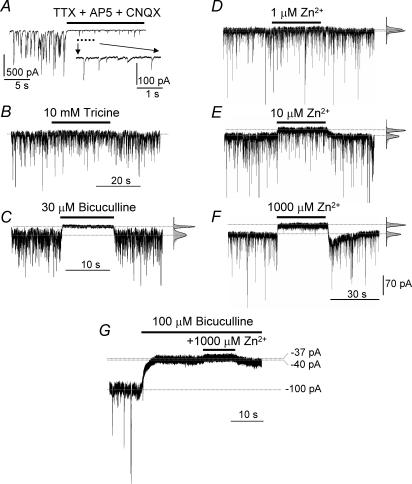
Both the phasic and tonic GABA current components were isolated in whole-cell patch-clamp recordings from hippocampal pyramidal neurons in the presence of TTX, AP5 and CNQX (see Methods; Fig. 2A). We used Zn2+ as a pharmacological tool to separate GABAA receptors lacking γ subunits (αβ and αβδ) from those containing γ subunits (αβγ). This cation readily discriminates between αβ and α4βδ receptors with their high sensitivities to inhibition (IC50, 88 nm and 6–16 μm, respectively) from the less sensitive αβγ GABAA receptors (IC50, 300 μm; Krishek et al. 1998; Hosie et al. 2003). Benzodiazepines were also used to distinguish between αβ/α4βδ and αβγ receptors as these ligands will not modulate GABAA receptors that lack the γ subunit (Pritchett et al. 1989; Sigel & Buhr, 1997; Klausberger et al. 2001). To first check that the ambient background levels of Zn2+ were not persistently occluding the activity of highly Zn2+-sensitive extrasynaptic GABAA receptor isoforms, a high concentration (10 mm) of the Zn2+ chelator tricine was applied. This had only a minor effect (< 5% increase in holding current) in a few cells (3/14) while no effect was observed on the mean holding current in the majority of cells (11/14; Fig. 2B, P > 0.05). In addition, 10 mm tricine neither changed the mean mIPSC frequency (4.4 ± 1.4 Hz, P > 0.05) nor the mean amplitude (33 ± 7 pA, P > 0.05). This indicated that a very low ambient Zn2+ concentrations was likely to be present during perfusion with our Krebs solution and from previous titration studies, this concentration is likely to be less than 90 nm (Hosie et al. 2003). Therefore, any suppression by Zn2+ of high affinity GABAA receptor populations in the extrasynaptic compartment was assumed to be minimal (Fig. 2B). The GABAergic nature of the phasic and tonic currents was established using the competitive GABA antagonist bicuculline. At 30 μm, this antagonist completely blocked the phasic GABAA receptor mIPSCs and the tonic inhibition (Fig. 2C). The application of Zn2+ also inhibited both the tonic and the phasic inhibitory GABAA receptor currents, but the tonic current was relatively more sensitive to inhibition than the phasic current at lower Zn2+ concentrations. The mean tonic current of −107 ± 12 pA appeared unaffected by 1 μm Zn2+ (−101 ± 0.6 pA). However, it was significantly reduced to −89 ± 2 pA in the presence of 10 μm Zn2+, and maximally reduced to −65 ± 5 pA in the presence of 1 mm Zn2+ (P < 0.05), leaving only the residual holding current. The standard deviation of the tonic noise (6.2 ± 1.3 pA) was unaffected by 1 μm Zn2+ (5.9 ± 0.2 pA). However, it was reduced to 4.2 ± 0.4 pA in the presence of 10 μm Zn2+, and to 3.9 ± 0.3 pA in 1 mm Zn2+ (Fig. 2D–F, n = 9, P < 0.05). These data predicted the IC50 for inhibition by Zn2+ of the tonic current to be approximately 5–30 μm. With regard to the phasic current, the mean mIPSC amplitude of 48 ± 3 pA was unaffected by 1 μm Zn2+, decreased to 40 ± 0.2 pA in the presence of 10 μm Zn2+, and to 17 ± 1.4 pA in 1 mm Zn2+ (Fig. 2D–F, P < 0.05). These data predicted an IC50 of Zn2+ for inhibition of the phasic current of approximately 300 μm. Taken together, these results implied that the GABAA receptor component involved in tonic inhibition possessed a relatively high sensitivity to Zn2+.
Figure 2. Functional profile of phasic and tonic inhibitory membrane currents from pyramidal neurons.
A, spontaneous and miniature synaptic currents recorded from a cultured hippocampal pyramidal neuron held at −70 mV before and after the application of 0.5 μm TTX, 20 μmd-amino-5-phosphonopentanoic acid (AP5) and 10 μm 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) to the Krebs solution. A selected portion of the record is shown below at high time resolution. GABA-mediated miniature IPSCs (mIPSCs) and the tonic (baseline) current were recorded in the presence of TTX, AP5 and CNQX, before and after recovery from the application of 10 mm tricine (B), 30 μm bicuculline (C), 1 μm Zn2+ (D), 10 μm Zn2+ (E) and 1 mm Zn2+ (F). GABAA receptors are almost solely responsible for the tonic current, as 1 mm Zn2+ only has a negligible inhibitory effect after GABAA receptors have been completely blocked with 100 μm bicuculline (G). The tonic current before (lower) and after (upper) ligand exposure are shown as dotted lines. The Gaussian curves depict the mean current and standard deviation of the noise neglecting the distortion caused by the mIPSCs (see Methods).
High concentrations of Zn2+ are known to affect the release of neurotransmitters and to modulate the function of voltage-activated ion channels (Xie & Smart, 1991; Xie et al. 1994; Smart et al. 1994; Harrison & Gibbons, 1994). To ensure that the observed effects of Zn2+ on the holding current in our study derived mainly from the inhibition of GABAA receptors, we first blocked these receptors with a supersaturating concentration (100 μm) of bicuculline (Ueno et al. 1997) and then co-applied our highest concentration of Zn2+ (1 mm). Under these conditions, only a small additional outward current was observed (3.5 ± 1.1% change of holding current, P > 0.05) suggesting that bicuculline and Zn2+ were both mainly targeting GABAA receptors (Fig. 2G). The very small additional inhibition by Zn2+ may reflect inhibition of a non-GABAergic conductance. The block resulting from the higher concentrations of Zn2+ agreed with with the level of block caused by the GABAA receptor antagonist picrotoxin (10 μm), which was also assumed to reflect a complete block of the tonic current (data not shown).
A rebound current response was often observed after the application of only higher concentrations of Zn2+ (Fig. 2F). This might be explained by the inhibited or shut GABAA receptors rapidly entering one or more open channel states directly after Zn2+ unbinding. Alternatively, it could simply reflect the ‘collection’ of many GABAA receptors in one or more Zn2+-blocked states (Gingrich & Burkat, 1998) and, following the removal of Zn2+, the tonic current transiently over-recovers following the reactivation of the ‘collected’ GABAA receptors before resuming the steady-state tonic current level. Many studies have indicated that the inhibition caused by Zn2+ is seemingly not dependent on the open/shut state(s) of the GABA receptor but largely a function of the subunit composition (Legendre & Westbrook, 1991; Smart, 1992; Berger et al. 1998). Generally, for αβ receptors, Zn2+ inhibition is non-competitive and largely independent of the liganded state of the receptor (Smart & Constanti, 1990; Draguhn et al. 1990). However, for αβγ isoforms, Zn2+ inhibition is dependent on the state of the receptor whereby ligand-exposed GABAA receptors are preferentially blocked (Gingrich & Burkat, 1998). This type of block was best described by using the generic mechanism of mixed inhibition (Smart & Constanti, 1986; Gingrich & Burkat, 1998). Notably, the major difference in Zn2+ sensitivity between αβ and αβγ receptors is maintained in all the above studies, ranging from 50-fold (Gingrich & Burkat, 1998) to 3000-fold (Hosie et al. 2003), which is why we used Zn2+ as a tool to detect the presence of αβ isoforms.
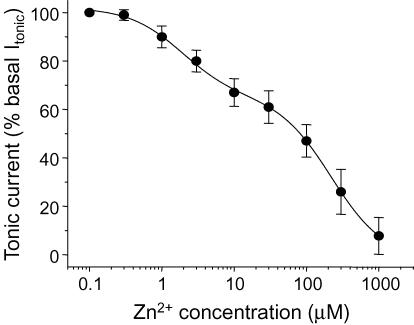
Analysis of the Zn2+ inhibition–concentration relationship for the tonic current revealed a clear biphasic curve (Fig. 3), indicative of a mixed population of highly Zn2+-sensitive (IC50′, 1.89 ± 0.3 μm, n = 8) and less Zn2+-sensitive GABAA receptors (IC50′′, 223 ± 7 μmn = 8) being involved in tonic inhibition on hippocampal pyramidal neurons. The highly Zn2+-sensitive receptors represented the smallest component (35 ± 5% of the total) compared to the low sensitivity receptors (65 ± 6%).
Figure 3. Zn2+ concentration–response curve for the inhibition of the tonic current in pyramidal neurons.
The tonic current prior to application of Zn2+ was defined as 100% and used to normalize the level of inhibition. Each data point represents mean ±s.e.m. (n = 8). The biphasic curve fit was achieved using the inhibition function (see Methods). This yielded two IC50 values: 1.89 ± 0.3 μm (35 ± 5% of the population) and 223 ± 7 μm (65 ± 6%).
Recruitment of different GABAA receptor populations using low ambient GABA concentrations
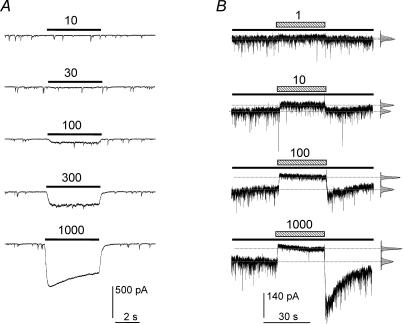
Although dissociated hippocampal cultures exhibited some degree of tonic GABAA receptor activation, the level of inhibition by Zn2+ appeared variable. This variation was assumed to reflect differences in the ambient concentration of GABA. In order to compensate and thus ensure stable levels of tonic inhibition, low concentrations of GABA were added to the external solution (Fig. 4A). After titration, 10 nm GABA was found to have little effect on the level of tonic inhibition, whereas 100 nm GABA was an appropriate compensating concentration because it consistently increased the level of tonic inhibition without affecting the frequency, amplitude or the decay of mIPSCs (data not shown). By contrast, the higher concentrations of 300–1000 nm significantly activated the cell surface GABAA receptors and caused inhibition of the mIPSC amplitudes (Fig. 4A).
Figure 4. Titration of exogenous GABA concentrations required to reproduce the tonic current in pyramidal neurons.
A, application of low GABA concentrations (10, 30, 100, 300 and 1000 nm) and their effect on the tonic and phasic currents in the presence of 0.5 μm TTX, 20 μmd-amino-5-phosphonopentanoic acid (AP5) and 10 μm 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX). B, in the presence of a background 100 nm concentration of GABA (continuous lines), the tonic and phasic currents were exposed to 1, 10, 100 and 1000 μm Zn2+ (hatched bars). The dotted lines reveal the shifts in the tonic current.
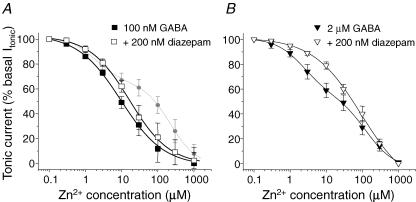
In the presence of 100 nm ambient GABA, Zn2+ displayed consistent inhibition of the tonic baseline current (Fig. 4B). This resulted in a monophasic Zn2+ inhibition curve with an IC50 of 9.5 ± 1.1 μm (Fig. 5A, n = 9) indicating that the tonic GABA concentration was sufficient to activate highly Zn2+ sensitive GABAA receptors, possibly those receptors that would be lacking a γ2 subunit. Nevertheless, if a significant component of this tonic current was also mediated by αβγ receptors (i.e. activated by 100 nm GABA), then the Zn2+ inhibition curve should be laterally displaced to higher concentrations of Zn2+ if the activity of such receptors was increased, because they would become the dominant component of the inhibition curve and they exhibit lower sensitivity to Zn2+ inhibition. However, the application of 200 nm diazepam did not significantly shift the Zn2+ inhibition curve in the presence of 100 nm GABA as would have been expected if many αβγ receptors had been active (IC50, 12.6 ± 1.2 μm; Fig. 5A).
Figure 5. Two components characterize the Zn2+ inhibition of the tonic current induced by GABA application.
Zn2+ inhibition concentration–response curves for the tonic current of cultured pyramidal neurons induced by 100 nm GABA (A) or 2 μm GABA (B), in the absence (filled symbols) or presence (open symbols) of 200 nm diazepam. The currents are normalized to the control tonic current in the absence of Zn2+. The dotted line in (A) represents the biphasic Zn2+ inhibition curve taken from Fig. 3 for comparison. Each data point represents mean ±s.e.m. (n = 8–9).
Increasing the ambient GABA concentration to 2 μm resulted in a biphasic Zn2+ inhibition curve (IC50 values, 2.4 ± 0.5 μm, 43%; and 130 ± 25 μm, 57%; Fig. 5B), suggesting that at least two GABAA receptor populations were active. In the presence of 200 nm diazepam, the component with low Zn2+ sensitivity became dominant resulting in a monophasic inhibition curve. This indicates that at this higher GABA concentration, a component of the GABA tonic current was presumably supported by γ2 subunit-containing GABAA receptors (IC50, 94 ± 14 μm; Fig. 5B). Although the pharmacological analyses with Zn2+ and benzodiazepines indicated the likelihood of at least two populations of receptors expressed with and without the γ2 subunit, single-channel recording was used to provide corroborating evidence. This was important because identifying αβ from αβδ GABAA receptors by Zn2+ sensitivity alone is quite difficult given that Zn2+ potency differs by only 30-fold between these two receptor isoforms.
Single-channel conductance levels of recombinant αβ, αβγ and αβδ GABAA receptors
Previous studies using light and electron microscopic immunofluorescence and immunogold methods have previously reported the presence of α1–5, β1–3, γ2 and δ subunits in hippocampal neurons (Fritschy et al. 1998; Pirker et al. 2000; Brünig et al. 2002; Fritschy & Brünig, 2003). By monitoring Zn2+ inhibition in these cells, our results indicated a prevalence of γ2 subunit-containing GABAA receptors; however, these receptors are unlikely to account for the differential sensitivity to Zn2+ given that such receptors containing α1/2/3 subunits are considered the dominant synaptic and perisynaptic forms of the GABAA receptor (Craig et al. 1994; Somogyi et al. 1996) and α5 subunit-containing receptors are likely to be the dominant type in extrasynaptic compartments (Caraiscos et al. 2004). The pharmacological data implied that some extrasynaptic GABAA receptors must lack the γ2 subunit. If this is so, then these receptors may be detectable using single-channel recording to reveal their unique properties (Moss et al. 1990; Smart, 1992; Angelotti & Macdonald, 1993).
In order to obtain precise identifiable profiles for some of the GABAA receptors likely to be expressed in the synaptic and extrasynaptic membranes of hippocampal pyramidal neurons, we first analysed single GABA channel currents recorded from the following four recombinant GABAA receptors: α1β3γ2 (Fig. 6Aa), α5β3γ2 (Fig. 6Ba), α4β3δ (Fig. 6Ca) and α1β3 (Fig. 6Da). Our primary focus was on the single-channel conductance levels that could be used to characterize each receptor. To define these levels clearly, we applied near maximal concentrations of GABA (selected from dose–response curves: αβγ, 100 μm; α4β3δ, 30 μm; αβ, 10 μm) to activate bursts and clusters of channel openings that could be identified as the activations of single ion channels. This is a particularly useful diagnostic indicator for identifying αβ receptors given their lower conductance state compared to αβγ and αβδ subunit-containing receptors (Moss et al. 1990; Angelotti et al. 1993; Fisher & Macdonald, 1997).
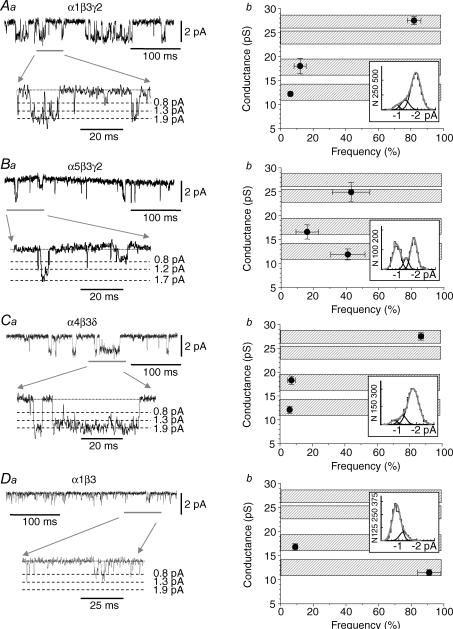
Figure 6. Single-channel conductances associated with recombinant GABAA receptors.
The lefthand column (Aa–Da) shows GABA-activated single channel currents recorded from outside-out patches taken from HEK (Aa, Ba and Da) or Ltk (Ca) cells expressing α1β3γ2 (Aa; 100 μm GABA), α5β3γ2 (Ba; 100 μm GABA), α4β3δ (Ca; 30 μm GABA) and α1β3 (Da; 10 μm GABA). Selected portions of the single-channel current recordings (grey line) are shown at high time resolution (lower traces) together with the associated zero current level (dotted line) and various current amplitude levels (dashed lines). The righthand column shows the frequencies of the various conductance levels obtained by analysis of the single channel records for α1β3γ2 receptors (Ab), α5β3γ2 (Bb), α4β3δ (Cb) and α1β3 receptors (Db). Data points are means ±s.e.m. from n = 4–8 patches. The hatched bars indicate the approximate range of conductance levels that are common to all four receptors. The insets show the frequency distributions for the various conductance levels.
Three very similar channel conductance levels were identified for α1β3γ2, α5β3γ2 and α4β3δ receptors, designated as high, medium and low, with values of 25–28, 17–19 and 12–13 pS, respectively (Fig. 6Ab, Bb and Cb). The frequencies of openings to these conductance levels were quite similar for α1β3γ2 and α4β3δ receptors. Openings to the high conductance state (27–28 pS) dominated the frequency distributions for both α1β3γ2 and α4β3δ receptors (82 ± 4% and 87 ± 2%, respectively, Fig. 6Ab and Cb). By contrast, for α5β3γ2 receptors, both high (24.9 ± 2.0 pS) and low (11.9 ± 1.1 pS) conductances appeared with equal frequency (43 ± 12% and 41 ± 11%, Fig. 6Bb). For α1β3 receptors, although channel openings with high conductance (25–28 pS) were absent as previously reported, two conductance states could still be discerned which were comparable to the medium and low conductance states of the αβγ and α4βδ receptors. For the α1β3 receptors, channel openings to the medium conductance level (16.8 ± 0.7 pS) were quite infrequent (9 ± 1.4%; Fig. 6Db) with the majority (91 ± 7%) of openings occurring to the low conductance level (11.5 ± 0.6 pS). As a result of the overlapping conductance levels between the αβ and αβγ and α4βδ receptors, the possiblity cannot be excluded that the openings to the lower conductance levels observed for α1β3γ2, α5β3γ2 and α4β3δ receptors do not reflect incompletely assembled γ or δ subunit-lacking GABAA receptors (see below).
Single-channel conductance levels for GABAA receptors in hippocampal neurons
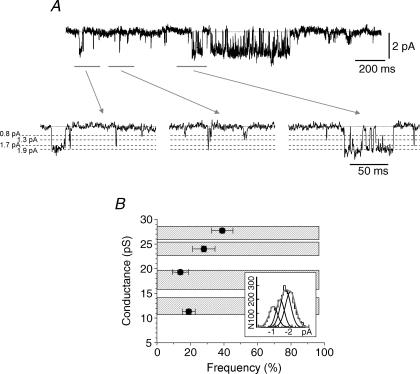
We used the single-channel conductance profiles for the selected recombinant GABAA receptors to facilitate our interpretation of GABA single-channel conductance levels in hippocampal neurons. Using outside-out patches, the application of 10 μm GABA revealed channel openings to four different conductance levels (Fig. 7A and B). We used 10 μm GABA as this exceeds the EC50 for activation of GABAA receptors with both high and low sensitivity to GABA. Two quite close high conductance levels at 24 ± 0.6 (28 ± 7%) and 27.7 ± 0.5 pS (39 ± 6%) were identified. These conductances are very similar to those measured for the recombinant α1β3γ2, α4β3δ and α5β3γ2 receptors (27.5 pS for α1β3γ2/α4β3δ and 24.9 pS for α5β3γ2), indicating that the native GABAA receptor population probably contains mixtures of α1βγ, α4βδ and α5βγ receptors. The lower frequencies of openings to the higher conductance levels in neurons, compared with those observed for recombinant α1/5β3γ2 and α4β3δ receptors, is another indication of the expected heterogeneity in the GABAA receptor population in pyramidal cells. Although channel openings to higher conductance levels are believed to mainly originate from α1/α5βγ and α4βδ receptors, we cannot exclude the possibility that some openings may stem from other γ subunit-containing GABAA receptors (e.g. α2–4βγ receptors). Two further conductance states were also identified: a medium conductance level (19.3 ± 0.5 pS, 14 ± 5%) and a low conductance level (11.3 ± 0.5 pS, 19 ± 3.5%) that correspond to the medium and low conductance states measured with the selected recombinant GABAA receptors (Fig. 7B). The latter conductance state could be indicative of the low conductance states of αβγ or αβδ receptors, but equally it might also represent the existence of αβ receptor isoforms in neurons, especially when considering the relative frequency of openings to this low conductance level.
Figure 7. Multiple single GABA channel conductances are present in hippocampal pyramidal neurons.
A, single-channel currents activated by applying 10 μm GABA to an outside-out patch taken from a hippocampal pyramidal neuron. Three segments (grey lines) are shown at high time resolution (lower panel) and the relevant conductance levels are shown by dashed lines. B, relationship between the four conductance levels and their relative frequencies are shown. All the data points are mean ±s.e.m. from n = 9 patches. Hatched bars indicate the conductance level ranges found in the analyses of the recombinant GABAA receptors taken from Fig. 6 for comparison.
Identification of a Zn2+-sensitive GABA channel conductance level in hippocampal pyramidal neurons
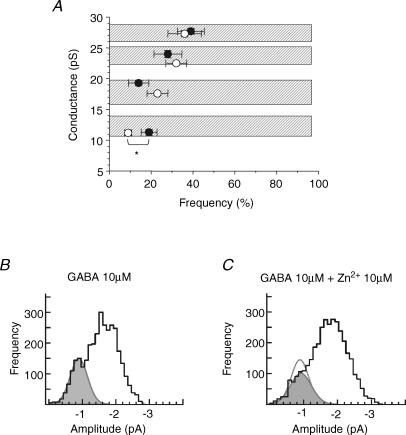
To identify any αβ subunit-containing GABAA receptors in pyramidal neurons, single GABA-activated channel currents were evoked by 10 μm GABA prior to the addition of 10 μm Zn2+. This concentration of Zn2+ is predicted to inhibit the activity of virtually all αβ receptors (by > 98%; IC50, 88 nm; Hosie et al. 2003) and substantially reduce αβδ receptor activity (by approximately 37–65%; IC50, 6–16 μm; Krishek et al. 1998; Nagaya & Macdonald, 2001; Brown et al. 2002) without causing any significant antagonism at αβγ GABAA receptors (< 3%, IC50, 300 μm). Under these conditions, single-channel current analyses revealed that only the frequency of occurrence of the low conductance level (11–12 pS; Fig. 8A) in hippocampal neurons was significantly reduced by 10 μm Zn2+ (from 19 ± 3.5% to 9 ± 1.7%; Fig. 8B and C, n = 9, P < 0.05). The incomplete reduction in activity of this conductance state by Zn2+ most probably reflects the presence of similar-sized low conductance states originating from the less Zn2+-sensitive αβγ and α4βδ receptors, as demonstrated previously with recombinant GABAA receptors.
Figure 8. Zn2+ inhibition of the GABA channel low conductance level in pyramidal neurons.
A, relationship between the openings of the GABA channel to various conductance levels, following activation by 10 μm GABA and their relative frequency before (•, taken from Fig. 7B) and then after the co-application of GABA with 10 μm Zn2+ (○). Data were recorded from nine outside-out patches at −70 mV. The asterisk indicates that only the lowest conductance state was significantly inhibited by Zn2+ (P < 0.05). B and C, GABA single-channel current amplitude histograms compiled following activation of the channels by 10 μm GABA in the absence (B) and presence (C) of 10 μm Zn2+. A single Gaussian (grey filled area) has been fitted to both distributions to highlight the low conductance level (∼0.8 pA; 11–12 pS). The area of this Gaussian decreases from 19% in control (B) to 9% in the presence of Zn2+ (C). The control Gaussian is superimposed on C as a dotted line.
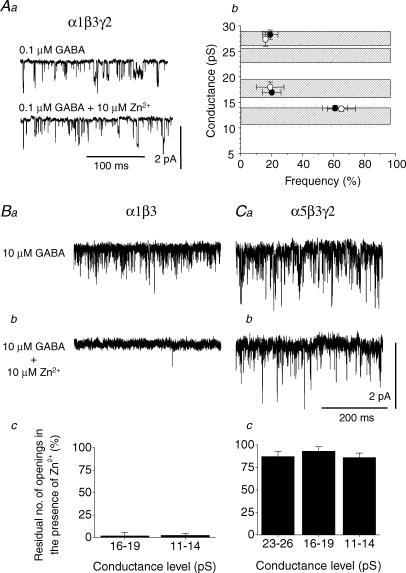
To control for the presence of low conductance openings which originate from αβγ receptors, the α1β3γ2 receptor subunit combination was expressed in HEK cells. Outside-out patches revealed three conductance states in the presence of 0.1 μm GABA as observed previously; however, the frequency of the low conductance state was relatively high compared to the frequencies of the medium and high conductance states (Fig. 9Aa). The application of 10 μm Zn2+ did not affect these low conductance states (Fig. 9Aa and Ab) indicating that the sensitivity to Zn2+ is determined by the receptor isoform as expected (Draguhn et al. 1990; Smart et al. 1991; Hosie et al. 2003), rather than by the conductance level.
Figure 9. Zn2+ inhibition of GABA channel conductance states for α1β3γ2, α1β3 and α5β3γ2 GABAA receptors expressed in HEK cells.
Aa, single GABA channel currents activated by 0.1 μm GABA for α1β3γ2 receptors in the absence or presence of 10 μm Zn2+. Ab, relationship between the openings of the α1β3γ2 GABA channel to various conductance levels and their relative frequency before (•) and after co-application of 0.1 μm GABA and 10 μm Zn2+ (○;n = 7 patches). The concentration of GABA was titrated to 0.1 μm to promote the opening frequency of GABA channels to the lowest conductance state. Ba and b, single-channel openings by α1β3 GABAA receptors induced by 10 μm GABA in the absence (a) and presence (b) of 10 μm Zn2+. Bc, bargraph of the number of residual α1β3 channel openings to the medium and low conductance levels in the presence of Zn2+ (n = 3). Ca, single-channel openings for α5β3γ2 GABAA receptors induced by 10 μm GABA in the absence (a) and presence (b) of 10 μm Zn2+. Cc, bargraph of GABA channel openings for α5β3γ2 receptors in the presence of Zn2+ (n = 4). All currents are recorded from outside-out patches at −70 mV.
Another factor that may confound our identification of those receptors that underlie the Zn2+-sensitive, low-conductance states is that α5β3γ2 receptors are reported to have a higher sensitivity to Zn2+ than other γ2 subunit-containing receptors (IC50, 20 μm; Burgard et al. 1996). To address this point we compared the Zn2+ sensitivity of single-channel openings, induced by 10 μm GABA, from recombinant α1β3 and α5β3γ2 receptors (Fig. 9B and C). Single-channel openings by α1β3 receptors were almost completely blocked by 10 μm Zn2+ (Fig. 9Ba and Bb) with only a residual level of 2–3% of openings observed during Zn2+ application (Fig. 9Bc). By contrast, single-channel openings for α5β3γ2, exhibited only very low sensitivity to 10 μm Zn2+ (Fig. 9Ca and Cb), where 86–93% of openings remained during Zn2+ application (Fig. 9Cc). An analysis of the open times for α5β3γ2 receptors in the absence and presence of Zn2+ was performed to address whether Zn2+ had affected the open state kinetics. The dwell times and their areas resolved in the presence of 10 μm GABA (τ1, 0.38 ± 0.05 ms, 91 ± 9%; τ2, 3.41 ± 0.16 ms, 9 ± 4%) were not statistically different from those resolved in the presence of 10 μm GABA plus 10 μm Zn2+ (τ1, 0.43 ± 0.02 ms, 92 ± 7%; τ2, 3.55 ± 0.23 ms, 8 ± 3%; n = 4).
These results suggest that in hippocampal neurons, the low conductance states that remain in the presence of Zn2+ (Fig. 8) probably reflect the activation of α5βγ and α4βδ receptors. The frequency of openings to the low conductance state for a ‘pure’ population of recombinant α4βδ receptors is only around 5% of the total openings (Fig. 6Cb). Given that Zn2+ will inhibit such openings for α4βδ receptors by approximately 50%, the largest inhibition of low conductance states that could be expected from these receptors alone would only be approximately 2.5%. Similarly, the relatively frequent openings to the low conductance state for a ‘pure’ recombinant α5β3γ2 receptor population (∼40%, Fig. 6Bb) will be inhibited by Zn2+ by approximately 10% yielding a maximal inhibition of only 4%. Of course, the proportions of low conductance openings from α5βγ and αβδ receptors in the mixed populations of extrasynaptic GABAA receptors on hippocampal pyramidal cells that remain in the presence of Zn2+ are likely to be considerably lower than in the pure recombinant receptor populations. Thus, allowing for their relative sensitivities to Zn2+ inhibition, the α4 and α5 subunit-containing receptors cannot account for the 10% inhibition in the low conductance states observed in Fig. 8. Based on these considerations, we suggest that up to 10% of extrasynaptic GABAA receptors on hippocampal pyramidal cells are likely to be formed from the highly Zn2+-sensitive αβ isoform.
Discussion
A growing body of evidence suggests that a continuous activation of extrasynaptic GABAA receptors by low basal concentrations of GABA results in a tonic inhibition of neurons (Mody, 2001; Semyanov et al. 2004; Farrant & Nusser, 2005). Several factors will influence the extent to which extrasynaptic GABAA receptors are tonically active. These include the GABAA receptor isoforms that are present and their affinities for GABA, the basal GABA concentration around the extrasynaptic domains resulting from spillover from nearby GABAergic synapses, and the activity of GABA transporters that will tightly regulate basal GABA concentrations.
The innate level of tonic inhibition in our cultured hippocampal neurons is in close agreement with previous findings using similar preparations (Bai et al. 2001; Caraiscos et al. 2004), but variations in the level of inhibition are also evident. Although Bai et al. (2001) reproduced their results in brain slices, others have had to pretreat their slices with vigabatrin, an inhibitor of GABA transaminase, to achieve a resolvable inhibition of the tonic current with gabazine (Overstreet & Westbrook, 2001). Semyanov et al. (2003) in another brain slice study, reported a tonic current in stratum radiatum interneurons, but no tonic current in pyramidal cells. However, by also using hippocampal slices, Stell & Mody (2002) observed a tonic current in CA1 pyramidal neurons. It is quite probable that the different levels of tonic inhibition reflect varying ambient GABA concentrations in different preparations under different experimental conditions. It was for this reason that we choose to normalize the GABA concentration in our cultures to provide a consistent level of tonic inhibition.
Estimates of ambient GABA concentrations from in vivo microdialysis range from tens of nanomolar to a few micromolar (Lerma et al. 1986; Tossman et al. 1986; Xi et al. 2003); however, it is likely that this method will fail to accurately detect variations in GABA concentrations near inhibitory synapses. In our study, the small and variable tonic current in hippocampal neurons, which could be inhibited by bicuculline and Zn2+ in the absence of exogenous GABA, suggested that very low GABA concentrations were in close proximity to the neurons. This was supported by the induction of small current responses to low (30–50 nm) GABA concentrations which indicated that the ambient GABA concentration was probably lower than 30 nm. However, this estimate is a mean value and probably endogenous GABA concentrations have a highly non-uniform distribution between the synaptic and extrasynaptic zones (see below).
A variation in the subunit composition of extrasynaptic GABAA receptors may also affect the tonic current. Some receptors may include the α5 subunit particularly as it is prominently expressed in the hippocampus (Sieghart, 1995; Sur et al. 1998;1999; Pirker et al. 2000; Brünig et al. 2002) and shows a diffuse extrasynaptic distribution (Brünig et al. 2002; Crestani et al. 2002), and this would agree with recent evidence of the importance of α5βγ receptors in tonic inhibition of pyramidal neurons (Caraiscos et al. 2004). Similarly, other studies have also shown that α4βδ GABAA receptors may be important extrasynaptic receptors on pyramidal neurons (Mangan et al. 2005). However, this does not exclude the possibility that other GABAA receptors contribute to tonic inhibition in these cells. Our GABA concentration–response relationships for pyramidal neurons indicated a higher sensitivity to GABA than would be expected if only αβγ receptors were present extrasynaptically. Of course, whole-cell applications of GABA will unavoidably activate both synaptic and extrasynaptic receptors, but even so the reported EC50 values for GABA activating recombinant α1β2/3γ2 receptors (3–17 μm) (Fisher & Macdonald, 1997; Mortensen et al. 2004; Böhme et al. 2004; Feng & Macdonald, 2004; Caraiscos et al. 2004) and α5β3γ2 receptors (11–19 μm) (Böhme et al. 2004; Caraiscos et al. 2004) are not easily reconciled with values obtained for native hippocampal receptors. Therefore, other GABAA receptors that exhibit higher sensitivities to GABA, such as α1β1/3 (EC50, 1.0–2.7 μm (Angelotti et al. 1993; Fisher & Macdonald, 1997; Amato et al. 1999; Wilkins & Smart, 2002; Hosie et al. 2003) and α4βδ receptors (EC50, 0.5 μm) (Mangan et al. 2005), probably complement the extrasynaptic receptor population. Our proposition that not only α4βδ receptors but also αβ receptors are partly responsible for the tonic inhibition in these neurons was based initially on the sensitivity of the tonic current to Zn2+ inhibition. The Zn2+ inhibition curves obtained with sufficient GABA to differentially activate αβ/α4βδ and αβγ receptors, displayed two components with high and low sensitivity to Zn2+. These components correlated well with the different sensitivities to Zn2+ inhibition of recombinant αβ/α4βδ and αβγ receptors, demonstrating that this ion is a useful tool to identify and separate GABAA receptor populations that differ in their incorporation of the γ2 subunit.
The biphasic inhibition curve determined from our cultured neurons (in the absence of co-applied agonist) could be explained by the selective activation of different GABAA receptor populations by endogenous GABA. We propose that higher concentrations of endogenously released GABA (much higher than 30 nm) are likely to activate synaptic (most probably αβγ) receptors with some spillover, and consequent dilution to lower concentrations, into the perisynaptic zone, where αβγ and other GABAA receptor isoforms (e.g. αβ and αβδ) may reside. However, endogenous GABA reaching the extensive extrasynaptic zone is predicted to be so dilute (< 30 nm) that most extrasynaptic GABAA receptors (including αβ and αβδ receptors) would not be activated. This non-uniform GABA concentration gradient from the inhibitory synapses to the perisynaptic zones would be effectively abolished by applying a uniform low exogenous GABA concentration (100 nm), which increased the tonic current by mostly activating the more GABA-sensitive, extrasynaptic receptors (e.g. αβ/α4βδ receptors) to such an extent that αβγ receptor activation (from synaptic and perisynaptic regions) was no longer resolved in the Zn2+ inhibition experiments. The resulting monophasic Zn2+ inhibition curve was also unaffected by diazepam, as expected if the majority of activated GABAA receptors lacked the γ2 subunit. When the ambient GABA concentration was further increased to low micromolar levels, both extrasynaptic αβ/α4βδ and αβγ receptors were activated, resulting once more in a biphasic Zn2+ inhibition curve. Under these conditions, diazepam potentiated the activation of the γ2 subunit-containing receptors to such a degree that they dominated the GABA response thereby transforming the Zn2+ inhibition relationship into another monophasic curve. However, despite separating αβ/αβδ from αβγ receptors in the extrasynaptic compartment based on their differential sensitivities to Zn2+ inhibition, using this criterion alone to separate αβ from αβδ receptors was not definitive. For this reason we relied on the acquisition of single-channel conductance ‘fingerprints’ to propose the existence of αβ receptors on hippocampal neurons.
Single-channel currents for recombinant γ2 or δ subunit-containing receptors such as α1β3γ2, α5β3γ2 and α4β3δ, were characterized by three to four similar conductance levels which were dominated by the higher conductance states (25–28 pS). By contrast, recombinant α1β3 receptors shared only the two lower conductance levels with γ2 or δ subunit-containing receptors, with the low conductance 11.5 pS state dominating. These data corresponded well with previous reports for the main conductance levels for α1β1/3γ2S/l (27.1–32 pS) and α1β1/3 (11–15.3 pS) receptors (Verdoorn et al. 1990; Angelotti & Macdonald, 1993; Fisher & Macdonald, 1997). For α1β3δ receptors, a single-channel conductance state of 26.7 pS has been reported (Fisher & Macdonald, 1997). Our single-channel recordings from pyramidal neurons revealed two high conductance states in the 25–28 pS range which were comparable to values for recombinant α1β3γ2/α4β3δ and α5β3γ2 receptors. In addition, two additional low conductance levels (∼19 and ∼11 pS) were observed in pyramidal neurons. It was significant that the lowest level, which corresponded closely with the main conductance level for recombinant αβ receptors, was relatively abundant (19% of all openings).
Further evidence of extrasynaptic αβ receptors came from the sensitivity to Zn2+ inhibition of the low (11 pS) conductance state. However, not all GABAA receptors exhibiting this low conductance level can be simply classified as αβ because Yeung et al. (2003) reported an increase in channel open probability by midazolam of all the three conductance levels that they resolved (including the 11 pS state) indicating the presence of γ2 subunits in these receptors. The most likely reason for this is that αβ, αβδ and αβγ receptors can all induce openings to the lowest conductance state, although in our study these states were quite infrequent for αβδ and αβγ receptors. With regard to their pharmacology, Zn2+ will inhibit the low conductance states arising from αβ receptor activation, whereas midazolam will potentiate openings to similar conductance states that are induced by αβγ receptors. Although α5β3γ2 receptors are reported to have a higher sensitivity to Zn2+ inhibition compared to other αβγ receptors, their IC50 is reported to be 20 μm, which is within the sensitivity range exhibited by αβδ receptors (Burgard et al. 1996) and much lower than that observed with αβ receptors. It is interesting that we did not observe such a high sensitivity to Zn2+ in our single-channel experiments on α5β3γ2 receptors.
The number of αβ receptors present in extrasynaptic membranes is probably quite low. From the single-channel conductance distributions the relative proportion can be estimated to be up to 10%. Their relatively rapid desensitization kinetics (Krampfl et al. 2000) might reduce their activity in the continued presence of GABA which could argue against a role in tonic inhibition. However, our single-channel results clearly show continued activity of αβ receptors, even when applying exogenous GABA concentrations much higher than the ambient levels of GABA we believe exist around neurons. This clearly indicates that αβ receptors do have the potential to support tonic inhibition in vivo. The existence of extrasynaptic αβ receptors in cerebellar granule neurons has previously been postulated based on the observation of low conductance single-channel currents (Brickley et al. 1999), giving rise to the prospect that αβ receptors may be more widely distributed on central neurons than previously assumed. Immunocytochemical data also support the notion of αβ receptors in the CNS. It is worth noting that up to 50% of α4 receptors in the forebrain are purported not to associate with γ1–3 subunits or δ subunits (Bencsits et al. 1999). In addition, the δ subunit knockout mouse revealed cerebellar GABAA receptors that lacked γ1–3 subunits (Tretter et al. 2001). Lastly, although under somewhat unusual conditions, the γ2 knockout mouse displayed GABAA receptors with low single-channel conductances in accord with αβ assemblies, suggesting that native neurons can indeed support the expression of such GABAA receptors (Gunther et al. 1995). The presence of αβ receptors in the extrasynaptic membrane will offer neurons the capability of detecting low concentrations of ambient GABA while being highly sensitive to Zn2+ inhibition. Their low conductance and generally short mean open time will provide only limited opportunity for charge transfer across the cell membrane and thus a modest contribution to tonic inhibition. Nevertheless, such receptors will add further diversity to those GABAA receptors that underpin tonic inhibition.
Acknowledgments
This work was supported by the MRC and Wellcome Trust. We thank Philip Thomas for his comments on the manuscript.
References
- Amato A, Connolly CN, Moss SJ, Smart TG. Modulation of neuronal and recombinant GABAA receptors by redox reagents. J Physiol. 1999;517:35–50. doi: 10.1111/j.1469-7793.1999.0035z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelotti TP, Macdonald RL. Assembly of GABAA receptor subunits: α1β1 and α1β1γ2S subunits produce unique ion channels with dissimilar single-channel properties. J Neurosci. 1993;13:1429–1440. doi: 10.1523/JNEUROSCI.13-04-01429.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelotti TP, Uhler MD, Macdonald RL. Assembly of GABAA receptor subunits: analysis of transient single-cell expression utilizing a fluorescent substrate/marker gene technique. J Neurosci. 1993;13:1418–1428. doi: 10.1523/JNEUROSCI.13-04-01418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai D, Zhu G, Pennefather P, Jackson MF, MacDonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by γ-aminobutyric acidA receptors in hippocampal neurons. Mol Pharmacol. 2001;59:814–824. doi: 10.1124/mol.59.4.814. [DOI] [PubMed] [Google Scholar]
- Bencsits E, Ebert V, Tretter V, Sieghart W. A significant part of native γ-aminobutyric acidA receptors containing α4 subunits do not contain γ or δ subunits. J Biol Chem. 1999;274:19613–19616. doi: 10.1074/jbc.274.28.19613. [DOI] [PubMed] [Google Scholar]
- Berger T, Schwarz C, Kraushaar U, Monyer H. Dentate gyrus basket cell GABAA receptors are blocked by Zn2+ via changes in their desensitization kinetics: an in situ patch-clamp and single-cell PCR study. J Neurosci. 1998;18:2437–2448. doi: 10.1523/JNEUROSCI.18-07-02437.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhme I, Rabe H, Lüddens H. Four amino acids in the α subunits determine the γ-aminobutyric acid sensitivities of GABAA receptor subtypes. J Biol Chem. 2004;279:35193–35200. doi: 10.1074/jbc.M405653200. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Single-channel properties of synaptic and extrasynaptic GABAA receptors suggest differential targeting of receptor subtypes. J Neurosci. 1999;19:2960–2973. doi: 10.1523/JNEUROSCI.19-08-02960.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human α4β3δ GABAA receptors. Br J Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brünig I, Scotti E, Sidler C, Fritschy JM. Intact sorting, targeting, and clustering of γ-aminobutyric acid A receptor subtypes in hippocampal neurons in vitro. J Comp Neurol. 2002;443:43–55. doi: 10.1002/cne.10102. [DOI] [PubMed] [Google Scholar]
- Burgard EC, Tietz EI, Neelands TR, Macdonald RL. Properties of recombinant γ-aminobutyric acidA receptor isoforms containing the α5 subunit subtype. Mol Pharmacol. 1996;50:119–127. [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You T, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, MacDonald JF, Orser BA. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by α5 subunit-containing γ-aminobutyric acid type A receptors. Proc Natl Acad Sci U S A. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AM, Blackstone CD, Huganir RL, Banker G. Selective clustering of glutamate and γ-aminobutyric acid receptors opposite terminals releasing the corresponding neurotransmitters. Proc Natl Acad Sci U S A. 1994;91:12373–12377. doi: 10.1073/pnas.91.26.12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Bluthmann H, Möhler H, Rudolph U. Trace fear conditioning involves hippocampal α5 GABAA receptors. Proc Natl Acad Sci U S A. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draguhn A, Verdorn TA, Ewert M, Seeburg PH, Sakmann B. Functional and molecular distinction between recombinant rat GABAA receptor subtypes by Zn2+ Neuron. 1990;5:781–788. doi: 10.1016/0896-6273(90)90337-f. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Feng HJ, Macdonald RL. Multiple actions of propofol on αβγ and αβδ GABAA receptors. Mol Pharmacol. 2004;66:1517–1524. doi: 10.1124/mol.104.003426. [DOI] [PubMed] [Google Scholar]
- Fisher JL, Macdonald RL. Single channel properties of recombinant GABAA receptors containing γ2 or δ subtypes expressed with α1 and β3 subtypes in mouse L929 cells. J Physiol. 1997;505:283–297. doi: 10.1111/j.1469-7793.1997.283bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM, Brünig I. Formation and plasticity of GABAergic synapses: physiological mechanisms and pathophysiological implications. Pharmacol Ther. 2003;98:299–323. doi: 10.1016/s0163-7258(03)00037-8. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Johnson DK, Möhler H, Rudolph U. Independent assembly and subcellular targeting of GABAA-receptor subtypes demonstrated in mouse hippocampal and olfactory neurons in vivo. Neurosci Lett. 1998;249:99–102. doi: 10.1016/s0304-3940(98)00397-8. [DOI] [PubMed] [Google Scholar]
- Gingrich KJ, Burkat PM. Zn2+ inhibition of recombinant GABAA receptors: an allosteric, state-dependent mechanism determined by the γ-subunit. J Physiol. 1998;506:609–625. doi: 10.1111/j.1469-7793.1998.609bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther U, Benson J, Benke D, Fritschy JM, Reyes G, Knoflach F, et al. Benzodiazepine-insensitive mice generated by targeted disruption of the γ2 subunit gene of γ-aminobutyric acid type A receptors. Proc Natl Acad Sci U S A. 1995;92:7749–7753. doi: 10.1073/pnas.92.17.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadingham KL, Wingrove P, Le Bourdelles B, Palmer KJ, Ragan CI, Whiting PJ. Cloning of cDNA sequences encoding human α2 and α3 γ-aminobutyric acidA receptor subunits and characterization of the benzodiazepine pharmacology of recombinant α1-, α2-, α3-, and α5-containing human γ-aminobutyric acidA receptors. Mol Pharmacol. 1993a;43:970–975. [PubMed] [Google Scholar]
- Hadingham KL, Wingrove PB, Wafford KA, Bain C, Kemp JA, Palmer KJ, Wilson AW, Wilcox AS, Sikela JM, Ragan CI. Role of the β subunit in determining the pharmacology of human γ- aminobutyric acid type A receptors. Mol Pharmacol. 1993b;44:1211–1218. [PubMed] [Google Scholar]
- Harrison NL, Gibbons SJ. Zn2+: an endogenous modulator of ligand- and voltage-gated ion channels. Neuropharmacology. 1994;33:935–952. doi: 10.1016/0028-3908(94)90152-x. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Dunne EL, Harvey RJ, Smart TG. Zinc-mediated inhibition of GABAA receptors: descrete binding sites underlie subtype specificity. Nat Neurosci. 2003;6:362–369. doi: 10.1038/nn1030. [DOI] [PubMed] [Google Scholar]
- Isaacson JS. Spillover in the spotlight. Curr Biol. 2000;10:R475–R477. doi: 10.1016/s0960-9822(00)00551-0. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Sarto I, Ehya N, Fuchs K, Furtmüller R, Mayer B, Huck S, Sieghart W. Alternate use of distinct intersubunit contacts controls GABAA receptor assembly and stoichiometry. J Neurosci. 2001;21:9124–9133. doi: 10.1523/JNEUROSCI.21-23-09124.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoflach F, Benke D, Wang Y, Scheurer L, Lüddens H, Hamilton BJ, Carter DB, Möhler H, Benson JA. Pharmacological modulation of the diazepam-insensitive recombinant γ-aminobutyric acidA receptors α4β2γ2 and α6β2γ2. Mol Pharmacol. 1996;50:1253–1261. [PubMed] [Google Scholar]
- Krampfl K, Bufler J, Lepier A, Dudel J, Adelsberger H. Desensitization characteristics of rat recombinant GABAA receptors consisting of α1β2γ2S and α1β2 subunits expressed in HEK293 cells. Neurosci Lett. 2000;278:21–24. doi: 10.1016/s0304-3940(99)00888-5. [DOI] [PubMed] [Google Scholar]
- Krishek BJ, Moss SJ, Smart TG. Interaction of H+ and Zn2+ on recombinant and native rat neuronal GABAA receptors. J Physiol. 1998;507:639–652. doi: 10.1111/j.1469-7793.1998.639bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre P, Westbrook GL. Noncompetitive inhibition of γ-aminobutyric acidA channels by Zn. Mol Pharmacol. 1991;39:267–274. [PubMed] [Google Scholar]
- Lerma J, Herranz AS, Herreras O, Abraira V, Martin del Rio R. In vivo determination of extracellular concentration of amino acids in the rat hippocampus. A method based on brain dialysis and computerized analysis. Brain Res. 1986;384:145–155. doi: 10.1016/0006-8993(86)91230-8. [DOI] [PubMed] [Google Scholar]
- Luscher B, Keller CA. Regulation of GABAA receptor trafficking, channel activity, and functional plasticity of inhibitory synapses. Pharmacol Ther. 2004;102:195–221. doi: 10.1016/j.pharmthera.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Rogers CJ, Twyman RE. Kinetic properties of the GABAA receptor main conductance state of mouse spinal cord neurones in culture. J Physiol. 1989;410:479–499. doi: 10.1113/jphysiol.1989.sp017545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan PS, Sun C, Carpenter M, Goodkin HP, Sieghart W, Kapur J. Cultured hippocampal pyramidal neurons express two kinds of GABAA receptors. Mol Pharmacol. 2005;67:775–788. doi: 10.1124/mol.104.007385. [DOI] [PubMed] [Google Scholar]
- Mitchell SJ, Silver RA. Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron. 2003;38:433–445. doi: 10.1016/s0896-6273(03)00200-9. [DOI] [PubMed] [Google Scholar]
- Mody I. Distinguishing between GABAA receptors responsible for tonic and phasic conductances. Neurochem Res. 2001;26:907–913. doi: 10.1023/a:1012376215967. [DOI] [PubMed] [Google Scholar]
- Mortensen M, Kristiansen U, Ebert B, Frølund B, Krogsgaard-Larsen P, Smart TG. Activation of single heteromeric GABAA receptor ion channels by full and partial agonists. J Physiol. 2004;557:389–413. doi: 10.1113/jphysiol.2003.054734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss SJ, Smart TG. Constructing inhibitory synapses. Nat Rev Neurosci. 2001;2:240–250. doi: 10.1038/35067500. [DOI] [PubMed] [Google Scholar]
- Moss SJ, Smart TG, Porter NM, Nayeem N, Devine J, Stephenson FA, Macdonald RL, Barnard EA. Cloned GABA receptors are maintained in a stable cell line: allosteric and channel properties. Eur J Pharmacol. 1990;189:77–88. doi: 10.1016/0922-4106(90)90232-m. [DOI] [PubMed] [Google Scholar]
- Nagaya N, Macdonald RL. Two γ2L subunit domains confer low Zn2+ sensitivity to ternary GABAA receptors. J Physiol. 2001;532:17–30. doi: 10.1111/j.1469-7793.2001.0017g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet LS, Westbrook GL. Paradoxical reduction of synaptic inhibition by vigabatrin. J Neurophysiol. 2001;86:596–603. doi: 10.1152/jn.2001.86.2.596. [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Pritchett DB, Sontheimer H, Shivers BD, Ymer S, Kettenmann H, Schofield PR, Seeburg PH. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989;338:582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat Neurosci. 2003;6:484–490. doi: 10.1038/nn1043. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Sieghart W. Structure and pharmacology of gamma-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- Sieghart W, Sperk G. Subunit composition, distribution and function of GABAA receptor subtypes. Curr Top Med Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- Sigel E, Baur R, Trube G, Mohler H, Malherbe P. The effect of subunit composition of rat brain GABAA receptors on channel function. Neuron. 1990;5:703–711. doi: 10.1016/0896-6273(90)90224-4. [DOI] [PubMed] [Google Scholar]
- Sigel E, Buhr A. The benzodiazepine binding site of GABAA receptors. Trends Pharmacol Sci. 1997;18:425–429. doi: 10.1016/s0165-6147(97)01118-8. [DOI] [PubMed] [Google Scholar]
- Smart TG. A novel modulatory binding site for zinc on the GABAA receptor complex in cultured rat neurones. J Physiol. 1992;447:587–625. doi: 10.1113/jphysiol.1992.sp019020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart TG, Constanti A. Studies on the mechanism of action of picrotoxinin and other convulsants at the crustacean muscle GABA receptor. Proc R Soc Lond B Biol Sci. 1986;227:191–216. doi: 10.1098/rspb.1986.0019. [DOI] [PubMed] [Google Scholar]
- Smart TG, Constanti A. Differential effects of zinc on the vertebrate GABAA receptor complex. Br J Pharmacol. 1990;99:643–654. doi: 10.1111/j.1476-5381.1990.tb12984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart TG, Moss SJ, Xie X, Huganir RL. GABAA receptors are differentially sensitive to zinc: dependence on subunit composition. Br J Pharmacol. 1991;103:1837–1839. doi: 10.1111/j.1476-5381.1991.tb12337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart TG, Xie X, Krishek BJ. Modulation of inhibitory and excitatory amino acid receptor ion channels by zinc. Prog Neurobiol. 1994;42:393–341. doi: 10.1016/0301-0082(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Fritschy JM, Benke D, Roberts JD, Sieghart W. The γ2 subunit of the GABAA receptor is concentrated in synaptic junctions containing the α1 and β2/3 subunits in hippocampus, cerebellum and globus pallidus. Neuropharmacology. 1996;35:1425–1444. doi: 10.1016/s0028-3908(96)00086-x. [DOI] [PubMed] [Google Scholar]
- Stell BM, Mody I. Receptors with different affinities mediate phasic and tonic GABAA conductances in hippocampal neurons. J Neurosci. 2002;22:RC223. doi: 10.1523/JNEUROSCI.22-10-j0003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur C, Fresu L, Howell O, McKernan RM, Atack JR. Autoradiographic localization of α5 subunit-containing GABAA receptors in rat brain. Brain Res. 1999;822:265–270. doi: 10.1016/s0006-8993(99)01152-x. [DOI] [PubMed] [Google Scholar]
- Sur C, Quirk K, Dewar D, Atack J, McKernan R. Rat and human hippocampal α5 subunit-containing γ-aminobutyric acidA receptors have α5β3γ2 pharmacological characteristics. Mol Pharmacol. 1998;54:928–933. doi: 10.1124/mol.54.5.928. [DOI] [PubMed] [Google Scholar]
- Thomas P, Mortensen M, Hosie AM, Smart TG. Dynamic mobility of functional GABAA receptors at inhibitory synapses. Nat Neurosci. 2005;8:889–897. doi: 10.1038/nn1483. [DOI] [PubMed] [Google Scholar]
- Tossman U, Jonsson G, Ungerstedt U. Regional distribution and extracellular levels of amino acids in rat central nervous system. Acta Physiol Scand. 1986;127:533–545. doi: 10.1111/j.1748-1716.1986.tb07938.x. [DOI] [PubMed] [Google Scholar]
- Tretter V, Hauer B, Nusser Z, Mihalek RM, Hoger H, Homanics GE, Somogyi P, Sieghart W. Targeted disruption of the GABAA receptor δ subunit gene leads to an up-regulation of γ2 subunit-containing receptors in cerebellar granule cells. J Biol Chem. 2001;276:10532–10538. doi: 10.1074/jbc.M011054200. [DOI] [PubMed] [Google Scholar]
- Ueno S, Bracamontes J, Zorumski C, Weiss DS, Steinbach JH. Bicuculline and gabazine are allosteric inhibitors of channel opening of the GABAA receptor. J Neurosci. 1997;17:625–635. doi: 10.1523/JNEUROSCI.17-02-00625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdoorn TA, Draguhn A, Ymer S, Seeburg PH, Sakmann B. Functional properties of recombinant rat GABAA receptors depend upon subunit composition. Neuron. 1990;4:919–928. doi: 10.1016/0896-6273(90)90145-6. [DOI] [PubMed] [Google Scholar]
- Wilkins ME, Smart TG. Redox modulation of GABAA receptors obscured by Zn2+ complexation. Neuropharmacology. 2002;43:938–944. doi: 10.1016/s0028-3908(02)00238-1. [DOI] [PubMed] [Google Scholar]
- Wooltorton JR, Moss SJ, Smart TG. Pharmacological and physiological characterization of murine homomeric β3 GABAA receptors. Eur J Neurosci. 1997;9:2225–2235. doi: 10.1111/j.1460-9568.1997.tb01641.x. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Ramamoorthy S, Shen H, Lake R, Samuvel DJ, Kalivas PW. GABA transmission in the nucleus accumbens is altered after withdrawal from repeated cocaine. J Neurosci. 2003;23:3498–3505. doi: 10.1523/JNEUROSCI.23-08-03498.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Hider RC, Smart TG. Modulation of GABA-mediated synaptic transmission by endogenous zinc in the immature rat hippocampus in vitro. J Physiol. 1994;478:75–86. doi: 10.1113/jphysiol.1994.sp020231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie XM, Smart TG. A physiological role for endogenous zinc in rat hippocampal synaptic neurotransmission. Nature. 1991;349:521–524. doi: 10.1038/349521a0. [DOI] [PubMed] [Google Scholar]
- Yeung JY, Canning KJ, Zhu G, Pennefather P, MacDonald JF, Orser BA. Tonically activated GABAA receptors in hippocampal neurons are high-affinity, low-conductance sensors for extracellular GABA. Mol Pharmacol. 2003;63:2–8. doi: 10.1124/mol.63.1.2. [DOI] [PubMed] [Google Scholar]