Abstract
Lifestyle interventions including exercise programmes are cornerstones in the prevention of obesity-related diabetes. In this study, we demonstrate that a single bout of exercise inhibits high-fat diet-induced insulin resistance. Diet-induced obesity (DIO) increased the expression and activity of the protein tyrosine phosphatase 1B (PTP1B) and attenuated insulin signalling in gastrocnemius muscle of rats, a phenomenon which was reversed by a single session of exercise. In addition, DIO was observed to lead to serine phosphorylation of insulin receptor substrate 1 (IRS-1), which was also reversed by exercise in muscle in parallel with a reduction in c-Jun N-terminal kinase (JNK) activity. Thus, acute exercise increased the insulin sensitivity during high-fat feeding in obese rats. Overall, these results provide new insights into the mechanism by which exercise restores insulin sensitivity.
Insulin resistance of skeletal muscle glucose transport is a key defect in the development of impaired glucose tolerance and type 2 diabetes. It is well established that chronic exercise can have beneficial effects on insulin action in insulin-resistant states (Henriksen, 2002). It is important to note that improvements in glucose tolerance can be observed in people with mild type 2 diabetes mellitus after acute exercise (Azevedo et al. 1995; Kennedy et al. 1999). The molecular mechanism for enhanced glucose uptake with chronic exercise may be partly related to increased expression and activity of key proteins known to regulate glucose metabolism in skeletal muscle (Hjeltnes et al. 1998; Chibalin et al. 2000; Zierath, 2002).
The action of insulin is mediated by receptor binding at the surface of insulin-sensitive tissue (Czech & Corvera, 1999). The insulin receptor (IR) is a protein with endogenous tyrosine kinase activity that, following activation by insulin, undergoes rapid autophophorylation and subsequently phophorylates intracelular protein substrates, such as insulin receptor substrate 1 and 2 (IRS-1 and IRS-2) (Cheatham & Kahn, 1995). Phosphorylation of IRS-1 and IRS-2 tyrosine residues induces activation of phosphatidylinositol 3-kinase (PI3-K) by binding the p85 subunit and activating the catalytic p110 subunit (White & Kahn, 1994). Activation of a serine/threonine kinase Akt occurs downstream from PI3-K. Once phosphorylated, Akt contributes to various biological processes including regulation of glucose uptake (Virkamaki et al. 1999).
Dephosphorylation of IR and IRS-1 or serine phosphorylation of IR substrates are the main mechanisms that suppress the insulin pathway (Ventre et al. 1997; Greene et al. 2003). Protein tyrosine phosphatases (PTPs) are important regulators of tyrosine phosphorylation-dependent signalling events and may represent novel targets for therapeutic intervention in a variety of human diseases (Tonks, 2003). Several PTPs, including PTPα, PTPɛ, CD45, SHP2, LAR and PTP1B, have been implicated as negative regulators of insulin signalling (Asante-Appiah & Kennedy, 2003). PTP1B is a major PTP implicated in the regulation of insulin action, including in the insulin-resistant state (Seely et al. 1996; Elchebly et al. 1999). c-Jun N-terminal kinase (JNK) is a member of the mitogen-activated protein (MAP) kinase family (Weston et al. 2002) and can be activated by tumour necrosis factor α (TNFα) (Hirosumi et al. 2002) and interleukin 1β (IL 1β) (Major & Wolf, 2001). In addition, JNK might serve as a feedback inhibitor during insulin stimulation (Lee et al. 2003). Three JNK isoforms have been described, JNK1, 2 and 3 (Ip & Davis, 1998), of which JNK1 is most involved in the pathophysiology of obesity and insulin resistance (Hirosumi et al. 2002). JNK activation induces inhibitory serine 307 (Ser307) phosphorylation of IRS-1, (Aguirre et al. 2000; Lee et al. 2003). Ser307 is located next to the PTB domain in IRS-1 and its phosphorylation inhibits the interaction of the PTB domain with the phosphorylated NPEY motif in the activated insulin receptor, causing insulin resistance (Aguirre et al. 2002). Previous studies suggest that, in addition to JNK, IκB kinase beta (IKKβ) phoshorylation also increases serine phosphorylation of IRS-1. Thus, the IKK complex appears to be another candidate that plays a key role in the phosphorylation of IRS-1 and in the regulation of insulin sensitivity.
As much of the molecular basis underlying the beneficial effects of exercise in the insulin-resistant state remains unclear, the current study was designed to investigate the effects of a single bout of exercise on PTP1B activity and IRS-1 serine phosphorylation associated with insulin resistance induced by DIO.
Methods
Experimental animals and diet
Male Wistar rats from the University of Campinas Central Animal Breeding Center were used in the experiments. All experiments were approved by the Ethics Committee of the State University of Campinas (UNICAMP).
The 4-week-old Wistar rats were divided into three groups, control rats fed standard rodent chow (Table 1), obese rats fed on an obesity-inducing diet for 3 months (DIO) (Table 1) and DIO rats submitted to a single bout of exercise (DIO + EXE).
Table 1.
Components of rat diet and rat chow
| Ingredients | Standard chow (g) | High-fat diet (g) |
|---|---|---|
| Casein | 202 | 200 |
| Sucrose | 100 | 100 |
| Cornstarch | 397 | 115.5 |
| Dextrinated starch | 130.5 | 132 |
| Lard | — | 312 |
| Soybean oil | 70 | 40 |
| Cellulose | 50 | 50 |
| Mineral mix American Institute of Nutrition (AIN)-93 | 35 | 35 |
| Vitamin mix AIN-93 | 10 | 10 |
| l-cystine | 3 | 3 |
| Choline | 2.5 | 2.5 |
Exercise protocol
Rats were acclimated to 10 min swimming for 2 days. The animals swam for two 3 h bouts separated by a 45 min rest period and the water temperature was maintained at ∼34°C. This exercise protocol was adaptated from a published procedure (Chibalin et al. 2000). After the last bout of exercise, animals were fed ad libitum and food was withdrawn 6 h before the tissue extraction. The rats were anaesthetized with intraperitoneal injection of sodium thiopental (40 mg (kg body weight)−1) 8 and 16 h after the exercise protocol. Following the experimental procedures, the rats were killed under anaesthesia (200 mg kg−1 thiopental) following the recommendations of the NIH.
Insulin tolerance test, serum insulin quantification and glycogen formation
The rats were given an insulin tolerance test (ITT; 1.5 IU insulin (kg body weight)−1) 16 h after the exercise protocol. Briefly, 1.5 IU kg−1 human recombinant insulin (Humulin R) from Eli Lilly (Indianapolis, IN, USA) was infused intraperitoneally to anaesthetized rats, the blood samples were collected at 0, 5, 10, 15, 20, 25 and 30 min from the tail for serum glucose determination. The rate constant for plasma glucose disappearance (Kitt) was calculated using the formula 0.693/biological half life (t1/2). The plasma glucose (t1/2) was calculated from the slope of last square analysis of the plasma glucose concentration during the linear phase of decline (Bonora et al. 1989). Plasma glucose level was determined using a glucose meter (Advantage, Boehringer Mannheim, USA). Plasma was separated by centrifugation (1100 g) for 15 min at 4°C and stored at −80°C until assayed. Radioimmunoassay was employed to measure serum insulin level, according to a previous description (Scott et al. 1981). Glycogen content in gastrocnemius muscle fragments was measured, according to a previously described method (Pimenta et al. 1989).
Hyperinsulinaemic–euglycaemic clamp procedures
HPLC-purified 2-deoxy-d-[1-14C]glucose (2-[14C]DG) was obtained from Amersham Biosciences Group (UK). The Harvard apparatus (model 11) and Harvard compact infusion pumps (model 975) were obtained from South Natick, MA, USA.
After 6 h of fasting, animals were anaesthetized intraperitoneally and catheters were then inserted into the left jugular vein (for tracer infusions) and carotid artery (for blood sampling), as previously described (Prada et al. 2000). Experiments were started when glycaemia had returned to stable levels, 30 min after the end of the surgical procedure. A 120 min hyperinsulinaemic–euglycaemic clamp procedure was conducted in anaesthetized catheterized rats, as shown previously (Prada et al. 2000, 2005), with continuous infusion of human insulin at a rate of 3.6 mU (kg body wt) −1 min−1 to raise the plasma insulin concentration to approximately 800–900 pmol l−1. Blood samples (20 μl) were collected at 5 min intervals for the immediate measurement of plasma glucose concentrations, and 10% unlabelled glucose was infused at variable rates to maintain plasma glucose at fasting levels. To estimate insulin-stimulated glucose transport and metabolism in skeletal muscle, (2-[14C]DG) was administered as a bolus (10 μCi) 45 min before the end of the clamp procedure. All infusions were performed using Harvard infusion pumps. At the end of the clamp procedure, animals were killed by an intravenous injection of ketamin and diazepam. Within 2 min, both portions of gastrocnemius from hindlimbs were removed. Each tissue, once exposed, was dissected out within 2 s, weighed, frozen with liquid N2 and stored at −80°C for later analysis.
Analytical procedures for hyperinsulinemic-euglycemic clamping
Plasma glucose was measured using a glucometer (Advantage, Boehringer Mannheim, USA). The whole blood glucose uptake was obtained from averaged rates of the last 30 min of 10% unlabelled glucose infusion during clamp procedures. Glucose transport activity in skeletal muscle was calculated from the tissue 2-deoxy-d-glucose (2DG) profile, as described before (Ferre et al. 1985; McGuinness & Mari, 1997; Prada et al. 2005).
Protein analysis by immunoblotting
As soon as anaesthesia was assured by the loss of pedal and corneal reflexes, the abdominal cavity was opened, the cava vein exposed, and 0.2 ml normal saline or insulin (10−9m) injected. At 90 s after insulin injection, both portions of gastrocnemius were ablated, pooled, minced coarsely and homogenized immediately in extraction buffer containing (mm): Tris 100 (pH 7.4), sodium pyrophosphate 100, sodium fluoride 100, EDTA 10, sodium vanadate 10 and phenylmethylsulfonyl fluoride (PMSF) 2, and 0.1 mg aprotinin ml−1 and 1% Triton-X 100 at 4°C with a Polytron PTA 20S generator (Brinkmann Instruments model PT 10/35) operated at maximum speed for 30 s. The extracts were centrifuged at 15 000 r.p.m. (9000 g) and 4°C in a Beckman 70.1 Ti rotor (Palo Alto, CA, USA) for 45 min to remove insoluble material, and the supernatants of these tissues were used for protein quantification using the Bradford method (Bradford, 1976).
Proteins were denaturated by boiling in Laemmli (Laemmli, 1970) sample buffer containing 100 mm DTT, run on SDS-PAGE, transferred to nitrocellulose membranes, which were blocked, probed and developed as previously described (Saad et al. 1997). The β subunit of the IR (IRβ), IRS-1 and IRS-2 were immunoprecipitated from rat muscle with or without previous insulin infusion. Antibodies used for immunoblotting were anti-phosphotyrosine, anti-IR, anti-IRS-1, anti-IRS-2, anti-PTP1B, anti-PI3-K, antiphosphoserine-IRS-1307 (Upstate Biotechnology, NY, USA), antiphospho-Akt (Cell Signalling Technology, MA, USA), anti-Akt, anti-JNK, antiphospho-JNK, antiphospho-c-jun, anti-IκBα and anti-SOCS3 (Santa Cruz Biotechnology Inc., CA, USA). Blots were exposed to preflashed Kodak XAR film with Cronex Lightning Plus intensifying screens at −80°C for 12–48 h. Band intensities were quantified by optical desitometry (Scion Image software, ScionCorp, Frederick, MD, USA) of the developed autoradiographs.
Protein tyrosine phosphatase activity assay
The gastrocnemius muscles were removed and homogenized in the solubilization buffer containing (mm): Tris 20 (pH 7.6), EDTA 5, PMSF 2, EGTA 1 and NaCl 130, and 0.1 mg aprotinin ml−1 and 1% Triton X-100. The lysates were centrifuged (15 000 g for 25 min at 4°C) and the supernatants were collected for immunoprecipitation, as previously described. Immunoprecipitates were washed in PTP assay buffer containing (mm): Hepes 100 (pH 7.6), EDTA 2, DTT 1 and NaCl 150, and 0.5 mg ml−1 bovine serum albumin. The pp60c-src C-terminal phosphoregulatory peptide (TSTEPQpYQPGENL; Biomol) was added to a final concentration of 200 μm in a total reaction volume of 60 μl in a PTP assay buffer for the immunoprecipitation. The reaction was then allowed to proceed for 1 h at 30°C. At the end of the reaction, 40 μl aliquots were placed into a 96-well plate, 100 μl Biomol Green reagent (Biomol) was added, and absorbance was measured at 630 nm (Taghibiglou et al. 2002).
Statistical analysis
Where appropriate, the results were expressed as the means ± s.e.m. Differences between the control group and DIO and between DIO and DIO + EXE were evaluated using one-way analysis of variance (ANOVA). When the ANOVA indicated significance, a Bonferroni post hoc test was performed.
Results
Physiological and metabolic parameters
Table 2 shows comparative data regarding control, DIO and DIO + EXE rats. Rats fed on the high-fat diet for 12 weeks had a greater body weight, epididymal fat and fasting serum insulin than age-matched controls. No significant variations were found in body weight, epididymal fat and fasting serum insulin in DIO + EXE compared to DIO rats. The fasting glucose concentration was similar between the groups; however, the decrease in the glucose disappearance rate (Kitt), induced by the high-fat diet, returned to the basal levels 16 h after acute exercise.
Table 2.
Characteristics of Wistar rats after 3 months on a high-fat diet (DIO), DIO rats submitted to acute exercise (DIO + EXE) and their age-matched controls
| Groups | Number of rats (n) | Body weight (g) | Epididimal fat (g) | Fasting insulin (ng ml−1) | Plasma glucose (mg dl−1) | Kitt (% min−1) |
|---|---|---|---|---|---|---|
| Control | 6 | 403.4 ± 21.0 | 5.95 ± 0.97 | 3.28 ± 0.15 | 73.7 ± 6.9 | 3.79 ± 0.2 |
| DIO | 8 | 544.7 ± 32.1* | 11.85 ± 1.47** | 7.97 ± 0.88* | 84.4 ± 7.3 | 1.90 ± 0.4** |
| DIO + EXE | 8 | 546.2 ± 25.2* | 12.1 ± 1.34** | 6.39 ± 1.9* | 82.1 ± 10.0 | 4.69 ± 0.6# |
P < 0.01
P < 0.001 versus control group
P < 0.001 versus DIO.
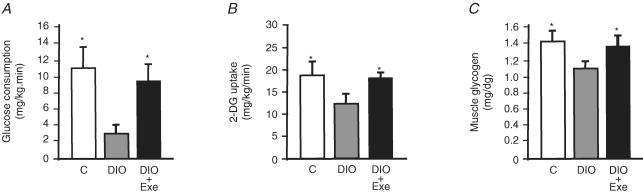
A hyperinsulinaemic–euglycaemic clamp procedure with tracer infusions was performed to examine the effects of acute exercise on the metabolism of glucose in skeletal muscle. The glucose infusion rate needed to clamp glycaemia at fasting levels in the presence of a constant infusion of insulin (3.6 mU (kg body weight)−1 min−1) was 4-fold lower in DIO rats than in controls and returned to control levels in DIO + EXE rats (Fig. 1A).
Figure 1. Effects of acute exercise on glucose uptake and glycogen content in control, DIO and DIO + EXE rats.
A, steady-state glucose infusion rates obtained from averaged rates of 90–120 min of 10% unlabelled glucose infusion during hyperinsulinaemic–euglycaemic clamp procedures in the control, DIO and DIO rats submitted to acute exercise. B, glucose transport in gastrocnemius muscle was evaluated by 2-deoxy-d-glucose uptake during the last 45 min of the hyperinsulinaemic–euglycaemic clamp studies. C, muscular glycogen content is expressed as mg (100 g tissue)−1. Bars represent means ±s.e.m. of n = 5 rats. *P < 0.05, versus DIO rats.
Using 2DG uptake analysis, the insulin-stimulated glucose uptake in skeletal muscle was quantified. As shown in Fig. 1B, DIO rats presented a significant reduction in glucose uptake in the skeletal muscle when compared to control group. In contrast, 16 h after the exercise protocol, insulin induced an increased glucose uptake of 33.8% in the muscle of DIO + EXE rats when compared to DIO rats. In addition, we evaluated the relative quantities of muscular glycogen in controls, DIO and DIO + EXE rats. The high-fat diet decreased glycogen levels in the gastrocnemius of DIO rats when compared to the control group, and returned to control levels 16 h after a single bout of exercise (Fig. 1C).
A single bout of exercise improves insulin signalling in the muscle of DIO rats
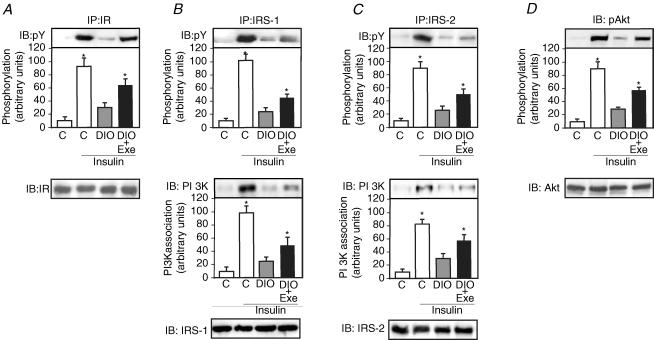
The effect of in vivo intravenous insulin infusion on IR tyrosine phosphorylation was examined in the gastrocnemius muscle of control, DIO and DIO + EXE rats. The muscles were immunoprecipitated with anti-IR antibody and then blotted with anti-phosphotyrosine antibody. Insulin induced an increase in IR tyrosine phosphorylation levels in muscle from control, DIO and DIO + EXE rats. In the control animals, insulin increased IR tyrosine phosphorylation by 9.6-fold, compared with 3.1-fold increases in the muscle of DIO rats, representing reductions in IR tyrosine phosphorylation of 4.0-fold. Insulin increased IR tyrosine phosphorylation by 6.6-fold in the muscle from DIO + EXE rats, representing an increase in IR tyrosine phosphorylation of 2.6-fold compared with DIO rats (Fig. 2A upper panel). There was no difference in basal levels of IR tyrosine phosphorylation between the three groups (data not shown). The protein expression of IR in the gastrocnemius muscle of control, DIO and DIO + EXE rats was quantified by immunoprecipitation and immunoblotting with anti-IR antibody. The IR protein levels were not different between the groups (Fig. 2A lower panel).
Figure 2. Insulin signalling in muscle of controls, DIO and DIO + EXE rats.
Muscle extracts from rats injected with saline or insulin were prepared as described in the Methods. A, tissue extracts were immunoprecipitated (IP) with anti-IRβ antibody and immunoblotting (IB) with anti-PY antibody (upper panel) or anti-IRβ antibody (lower panel). B and C, tissue extracts were also IP with anti-IRS-1 and anti-IRS-2 antibodies and IB with anti-PY antibody (upper panels), anti-PI3K antibodies (middle panels) or anti-IRS-1, anti-IRS-2 antibody (lower panel). D, muscle extracts were IB with anti-phospho Akt and anti-Akt antibody (upper and lower panel, respectively). The results of scanning densitometry were expressed as arbitrary units. Bars represent means ±s.e.m. of n = 6–8 rats. *P < 0.05, versus DIO rats.
IRS-1 tyrosine phosphorylation and IRS-1–PI-3 kinase association increased in control animals by 10.6- and 10.1-fold following insulin administration, respectively, compared with 2.5- and 2.6-fold increases in the muscle of DIO rats (representing reductions in IRS-1 tyrosine phosphorylation and IRS-1–PI3K association of 6.4- and 5.6-fold, respectively), and increases of 4.7- and 5.0-fold in the muscle of DIO + EXE rats (representing increases in IRS-1 tyrosine phosphorylation and IRS-1–PI3K association of 2.4- and 2.5-fold, respectively, compared with DIO rats) (Fig. 2B upper and middle panel). IRS-2 tyrosine phosphorylation and IRS-2–PI-3 kinase association increased in control animals by 9.2- and 8.5-fold following insulin administration, respectively, compared with 2.6- and 3.0-fold increases in the muscle of DIO rats (representing reductions in IRS-2 tyrosine phosphorylation and in IRS-2–PI3K association of 5.1- and 3.7-fold, respectively), and increased 5.1- and 5.9-fold in the muscle of DIO + EXE rats (representing increases in IRS-2 tyrosine phosphorylation and in IRS-2–PI3K association of 2.5 and 2.4-fold, respectively, compared with DIO rats) (Fig. 2C upper and middle panel). There were no differences in basal levels of IRS-1 and IRS-2 tyrosine phosphorylation between the three groups (data not shown). The protein expression of IRS-1 and IRS-2 in the gastrocnemius muscle from control, DIO and DIO + EXE rats were quantified by immunoprecipitation and immunoblotting with anti-IRS-1 or anti-IRS-2 antibodies. The IRS-1 and IRS-2 protein levels were not different between the groups (Fig. 2B and C lower panels). Finally, in gastrocnemius muscle from control rats, insulin increased Akt serine phosphorylation by 9.3-fold, compared with 2.8-fold increase in the muscle from DIO rats (representing reductions in Akt serine phosphorylation of 4.6-fold) and increases of 5.8-fold in the muscle of DIO + EXE rats (representing an increase in Akt serine phosphorylation of 2.6-fold compared with DIO rats) (Fig. 2D upper panel). There were no differences between the basal levels of Akt serine phosphorylation in the three groups (data not shown). The protein expression of Akt in the gastrocnemius muscle of control, DIO and DIO + EXE rats was quantified by immunoblotting with anti-Akt antibodies. The Akt protein levels were not different between the groups (Fig. 2D lower panel).
Acute exercise-mediated suppression of PTP1B activity in DIO rats
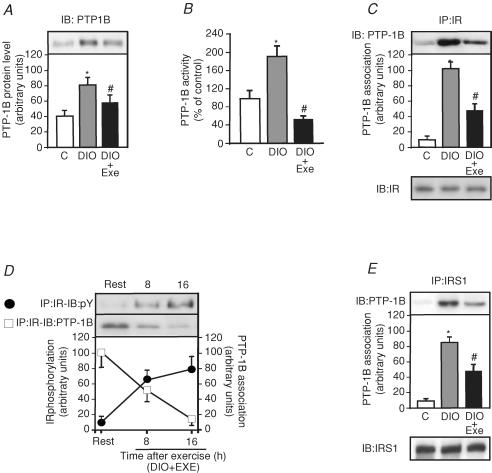
Obesity induced by diet increased the expression of PTP1B in DIO rats by 2.0-fold compared to control rats, a phenomenon that was reversed by acute exercise (Fig. 3A). Figure 3B shows that PTP1B activity increased in the muscle of DIO rats by 95% when compared to controls and acute exercise decreased PTP1B by 61% compared to DIO rats. To further explore the possibility that acute exercise mediated suppression of PTP1B activity in DIO rats, we observed that insulin induced IR tyrosine phosphorylation and IR/PTP1B interaction in muscle from DIO + EXE rats. The high-fat diet increased the IR/PTP1B association by 10.6-fold in the gastrocnemius muscle of DIO rats when compared with control rats and, in the muscle of DIO + EXE rats, IR/PTP1B association was decreased by 2.1-fold when compared with DIO rats (Fig. 3C upper panel). The IR protein levels were not different between the groups (Fig. 3C lower panel). As shown in Fig. 3D, insulin, in a time-dependent manner, induced increases in IR tyrosine phosphorylation in muscle from DIO rats after the exercise protocol, with a concomitant reduction of IR–PTP1B association. We also evaluated the IRS-1–PTP1B association in muscle from controls, DIO and DIO + EXE rats. The high-fat diet induced an increase in IRS-1–PTP1B association by 8.8-fold in gastrocnemius muscle of DIO rats when compared with control rats, and in the muscle of DIO + EXE rats IRS-1–PTP1B association was decreased by 1.7-fold when compared with DIO rats (Fig. 3E upper panel). The IRS-1 protein levels were not different between the groups (Fig. 3E lower panel).
Figure 3. Effect of acute exercise on PTP1B protein levels, activity and PTP1B association with IRβand IRS-1.
A, PTP1B protein level in DIO and DIO + EXE rats were compared with control group. B, PTP1B assay was performed as described in the Methods. C, tissue extracts were immunoprecipitated (IP) with anti-IRβ followed by immunoblotting (IB) with anti-PTP1B antibody or anti-IRβ antibody (upper and lower panels). D, insulin-stimulated IRβ phosphorylation (•) and the IRβ–PTP1B association (□) were determined using IP with anti-IRβ and IB with anti-PY antibody and IP with anti-IRβ followed by IB with anti-PTP1B antibody. E, IP with anti-IRS-1 followed by IB with anti-PTP1B antibody to evaluated the IRS-1–PTP1B association (upper panel). Muscle extracts were also IP with anti-IRS-1 and IB with anti-IRS-1 antibody (lower panels). The results of scanning densitometry were expressed as arbitrary units. Bars represent means ±s.e.m. of n = 6–8 rats. *P < 0.05, versus control and #P < 0.05, DIO + EXE versus DIO.
A single bout of exercise inhibits Ser307 phosphorylation of IRS-1, JNK activity and IκBα degradation in DIO rats
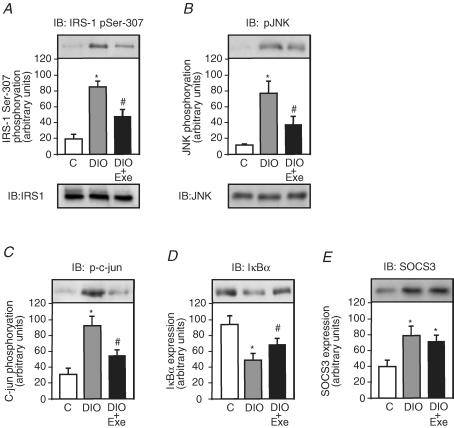
Among the serine residues that become phosphorylated in response to risk factors of insulin resistance, Ser307 has been studied extensively and Ser307 phosphorylation has become a molecular indicator of insulin resistance (Eldar-Finkelman & Krebs, 1997; Aguirre et al. 2002; Hirosumi et al. 2002; Lee et al. 2003); however, the effect of acute exercise on high-fat diet-induced IRS-1 serine phosphorylation has not been identified. To address this issue, we tested Ser307 phosphorylation in the gastrocnemius muscle of control, DIO and DIO + EXE rats. The muscles were blotted with anti-IRS-1 phosphoserine antibody. The high-fat diet increased IRS-1 serine phosphorylation levels in the muscle of DIO rats by 4.5-fold when compared with control rats. In the muscle of DIO + EXE rats, IRS-1 serine phosphorylation decreased by 1.7-fold when compared with DIO rats (Fig. 4A).
Figure 4. Effect of acute exercise on IRS-1 serine phosphorylation, JNK activity, IκBα degradation and IRS-1 and JNK protein levels in muscle of controls, DIO and DIO + EXE rats.
Tissue extracts were immunoblotted (IB) with anti-IRS-1307 phosphoserine antibody (A upper panel), anti IRS-1 antibody (A lower panel), anti-phospho JNK antibody (B upper panel), anti-JNK antibody (B lower panel), antiphospho-c-Jun antibody (C), anti-IκBα antibody (D) and anti-SOCS3 (E) in control, DIO and DIO + EXE rats. The results of scanning densitometry were expressed as arbitrary units. Bars represent means ±s.e.m. of n = 6–8 rats. *P < 0.05, versus control and #P < 0.05, DIO + EXE versus DIO.
JNK activation was determined by monitoring phosphorylation of JNK (Thr183 and Tyr185) and c-Jun (Ser63), which is a substrate of JNK. The high-fat diet induced an increase in JNK phosphorylation in the muscle of DIO rats by 7.2-fold when compared with control rats. In the muscle of DIO + EXE rats, JNK serine phosphorylation decreased by 2.0-fold when compared with DIO rats (Fig. 4B upper panel). The JNK protein levels were not different between the groups (Fig. 4B lower panel). Consistent with JNK activation, c-Jun phosphorylation was 3.1-fold higher in the muscle of DIO rats when compared with control rats. In the muscle of DIO rats submitted to acute exercise, c-Jun phosphorylation decreased by 1.7-fold when compared with DIO rats (Fig. 4C). Finally, we examined the IKK–NF-κB pathway, an important regulator of inflammation, in obesity- and inflammation-induced insulin resistance. The main function of the IKK complex is the activation of NF-κB through phosphorylation and degradation of IκBα (Hevener et al. 2003; Greten et al. 2004; Viatour et al. 2005). Thus, to assess NF-κB activation, we observed IκBα degradation in the muscle of control, DIO and DIO + EXE rats. The high-fat diet led to a decrease in IκBα expression levels in the muscle of DIO rats by 1.9-fold, compared with control rats. However, in the muscle of DIO + EXE rats, IκBα degradation was decreased by 1.4-fold when compared to DIO rats (Fig. 4D). The high-fat diet increased SOCS 3 expression in the muscle of DIO rats by 2.0-fold when compared to the control; however, acute exercise did not change the high-fat diet-induced modulation of SOCS 3 expression in this tissue (Fig. 4E).
Discussion
Impaired insulin action on whole-body glucose uptake is a hallmark feature of type 2 diabetes mellitus. Physical exercise has been linked to improved glucose homeostasis and enhanced insulin sensitivity immediately after an acute bout of exercise in humans (Devlin et al. 1987; Zierath, 1995) and rodents (Richter et al. 1982; Wallberg-Henriksson, 1987; Wallberg-Henriksson et al. 1988). In this study, we demonstrate that a single bout of exercise partially restored the insulin signalling in muscle of obese rats by different mechanisms. High-fat diet was observed to lead to an increase in the PTP1B protein level and in the activity and serine phosphorylation of IRS-1; it is interesting that acute exercise reversed these parameters in parallel with a reduction in JNK activity and IκBα degradation. However, the acute exercise had no effect on high-fat diet-induced SOCS3 expression.
Several mechanisms may be involved in the pathogenesis of insulin resistance in muscle. The ability of PTP1B to negatively regulate insulin receptor kinase has been established at the molecular level (Myers et al. 2001) and ablation of the PTP1B gene yields mice displaying characteristics which suggest that inhibition of PTP1B function may be an effective strategy for the treatment of diabetes and obesity (Elchebly et al. 1999). In accordance with this, our results show decreased activity and expression of PTP1B in DIO rats after a single bout of exercise. Furthermore, the reduction of PTP1B activity in rats submitted to acute exercise was accompanied by increased insulin sensitivity in skeletal muscle and correlates with increases in tyrosyl phosphorylation of IR, IRS-1 and IRS-2 and with reduction of IR–PTP1B and IRS-1–PTP1B association in skeletal muscle. In contrast to our results, it has been recently was reported that the amount of PTP1B associated with IR-β is not different in the muscle of normal rats at 5, 29 and 53 h after cessation of chronic voluntary exercise (Kump & Booth, 2005). These apparent contradictory results may be related to the protocol of exercise and changes in physiological and metabolic parameters in DIO rats.
Serine phosphorylation of IRS proteins is believed to be a major mechanism of suppression of IRS-1 and IRS-2 activity that contributes to insulin resistance (Saltiel & Olefsky, 1996; Saltiel & Kahn, 2001). Regulation of serine phosphorylation of IR, IRS-1 and IRS-2 proteins has been a focus of investigation in the search for the molecular mechanism of insulin resistance. Our results show a marked reduction in IRS-1 serine phosphorylation, 16 h after acute exercise in DIO rats in parallel with an increase in IR autophosphorylation. A previous study demonstrated that treatment of cultured murine adipocytes with TNF-α induces serine phosphorylation of IRS-1 and converts it into an inhibitor of the IR tyrosine kinase activity in vitro (Hotamisligil et al. 1996). The IRS-1-mediated inhibition of IR tyrosine kinase activity could occur by direct or indirect interactions between the IR and IRS-1 (Backer et al. 1993; O'Neill et al. 1994). Serine-phosphorylated IRS-1 might associate with the IR to block the autophosphorylation reaction; alternatively, serine-phosphorylated IRS-1 might act indirectly on the IR through an association with an inhibitor that acts on the IR in a stoichometric or catalytic fashion (Hotamisligil et al. 1996). Taken together, these data suggest that a high-fat diet mediates insulin resistance, at least in part, by inducing IRS-1 serine phosphorylation and decreasing IRS-1 and IRS-2 tyrosine phosphorylation and that this effect is inhibited by acute exercise. Studies suggest that overexpression of SOCS3 decreases insulin-induced IRS-1 and IRS-2 tyrosine phosphorylation levels, inducing insulin resistance (Ueki et al. 2004). However, this modulation of SOCS3 by DIO was not reversed by acute exercise. As the IR–IRS-1/2 pathway is involved in glucose uptake and glycogen synthesis in muscle, we suggest that acute exercise, by acting on this pathway, reverses insulin resistance of DIO animals.
Activation of inflammatory signalling, including of the IκB–NFκB pathway may also contribute to mediated the serine phosphorylation of IRS-1(Gao et al. 2002). However, few studies have examined the effect of acute exercise on the IκB–NFκB pathway. In rats, exercise activates IκB–NFκB signalling in muscle (Ji et al. 2004), and acute fatiguing exercise in humans reduces NFκB activity. Similar to a recent study showing that 8 weeks of aerobic exercise training reduced IκB–NFκB signalling in vastus lateralis muscle from subjects with type 2 diabetes (Sriwijitkamol et al. 2006), our results show that the high levels of IRS-1, phosphorylated at Ser307, in DIO rats correlated with the disappearance of IκBα. This finding is an indication of IKK activation and suggests that acute exercise is able to reduce IKK activation and restore the IκBα expression.
Recently, JNK has been linked to the regulation of insulin signalling by several studies (Aguirre et al. 2000, 2002; Rui et al. 2001; Hirosumi et al. 2002; Lee et al. 2003). It has been suggested that JNK contributes to insulin resistance by phosphorylating IRS-1 at Ser307, and this phosphorylation leads to inhibition of the IRS-1 function (Aguirre et al. 2000, 2002; Rui et al. 2001; Lee et al. 2003; Prattali et al. 2005). However, the effect of exercise on JNK activity remains unclear. Several studies suggest that the activity of JNK intracellular signalling cascade is increased following prolonged running exercise (Boppart et al. 2000; Thompson et al. 2003). In contrast, JNK phosphorylation was reduced after resistance exercise in old men (Williamson et al. 2003). In this study, we observed that a single bout of exercise inhibited DIO-induced JNK activity, and that this inhibition was accompanied by a reduction in IRS-1 serine phosphorylation at Ser307.
In accordance with the results of Oakes et al. (1997) we observed that a single bout of exercise completely normalized the insulin action in the diet-induced obese state; however, our data show only a partial amelioration of insulin signalling. Taken together, these data suggest that the complete normalization, by acute exercise, of the insulin action in obesity induced by diet may be caused by other factors. One possibility may be associated with the increase in other insulin-independent signalling pathways. It has been postulated that AMP kinase is a important mediator of acute exercise-induced glucose uptake in muscle (Sakamoto & Goodyear, 2002; Wojtaszewski et al. 2002; Krook et al. 2004). In addition, in human subjects with type 2 diabetes, where there is impaired insulin signalling in skeletal muscle, acute exercise results in normal activation of AMP kinase (Musi et al. 2001; Koistinen et al. 2003).
In summary, a single bout of exercise improves insulin sensitivity in DIO rats by reversing high-fat diet-induced decreases in insulin-stimulated IR, IRS-1 and IRS-2 tyrosine phosphorylation. The effect of acute exercise on insulin action is further supported by our findings that DIO + EXE rats show a reduction in PTP1B activity and IRS-1 serine phosphorylation, mechanisms by which a single session of exercise may protect against high-fat diet-induced insulin resistance. Overall, these results provide new insights into the mechanism by which physical activity restores insulin sensitivity.
Acknowledgments
The authors thank Mr Luiz Janeri, Jósimo Pinheiro and Márcio A da Cruz for technical assistance. This study was supported by grants from fundação de amparo à pesquisa do estado de São Paulo (FAPESP) and conselho nacional de Pesquisa (CNPq).
References
- Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307) J Biol Chem. 2000;275:9047–9054. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem. 2002;277:1531–1537. doi: 10.1074/jbc.M101521200. [DOI] [PubMed] [Google Scholar]
- Asante-Appiah E, Kennedy BP. Protein tyrosine phosphatases: the quest for negative regulators of insulin action. Am J Physiol Endocrinol Metab. 2003;284:E663–E670. doi: 10.1152/ajpendo.00462.2002. [DOI] [PubMed] [Google Scholar]
- Azevedo JL, Jr, Carey JO, Pories WJ, Morris PG, Dohm GL. Hypoxia stimulates glucose transport in insulin-resistant human skeletal muscle. Diabetes. 1995;44:695–698. doi: 10.2337/diab.44.6.695. [DOI] [PubMed] [Google Scholar]
- Backer JM, Myers MG, Jr, Sun XJ, Chin DJ, Shoelson SE, Miralpeix M, White MF. Association of IRS-1 with the insulin receptor and the phosphatidylinositol 3′-kinase. Formation of binary and ternary signaling complexes in intact cells. J Biol Chem. 1993;268:8204–8212. [PubMed] [Google Scholar]
- Bonora E, Moghetti P, Zancanaro C, Cigolini M, Querena M, Cacciatori V, Corgnati A, Muggeo M. Estimates of in vivo insulin action in man: comparison of insulin tolerance tests with euglycemic and hyperglycemic glucose clamp studies. J Clin Endocrinol Metab. 1989;68:374–378. doi: 10.1210/jcem-68-2-374. [DOI] [PubMed] [Google Scholar]
- Boppart MD, Asp S, Wojtaszewski JF, Fielding RA, Mohr T, Goodyear LJ. Marathon running transiently increases c-Jun NH2-terminal kinase and p38 activities in human skeletal muscle. J Physiol. 2000;526:663–669. doi: 10.1111/j.1469-7793.2000.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cheatham B, Kahn CR. Insulin action and the insulin signaling network. Endocr Rev. 1995;16:117–142. doi: 10.1210/edrv-16-2-117. [DOI] [PubMed] [Google Scholar]
- Chibalin AV, Yu M, Ryder JW, Song XM, Galuska D, Krook A, Wallberg-Henriksson H, Zierath JR. Exercise-induced changes in expression and activity of proteins involved in insulin signal transduction in skeletal muscle: differential effects on insulin-receptor substrates 1 and 2. Proc Natl Acad Sci U S A. 2000;97:38–43. doi: 10.1073/pnas.97.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech MP, Corvera S. Signaling mechanisms that regulate glucose transport. J Biol Chem. 1999;274:1865–1868. doi: 10.1074/jbc.274.4.1865. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Hirshman M, Horton ED, Horton ES. Enhanced peripheral and splanchnic insulin sensitivity in NIDDM men after single bout of exercise. Diabetes. 1987;36:434–439. doi: 10.2337/diab.36.4.434. [DOI] [PubMed] [Google Scholar]
- Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283:1544–1548. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- Eldar-Finkelman H, Krebs EG. Phosphorylation of insulin receptor substrate 1 by glycogen synthase kinase 3 impairs insulin action. Proc Natl Acad Sci U S A. 1997;94:9660–9664. doi: 10.1073/pnas.94.18.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre P, Leturque A, Burnol AF, Penicaud L, Girard J. A method to quantify glucose utilization in vivo in skeletal muscle and white adipose tissue of the anaesthetized rat. Biochem J. 1985;228:103–110. doi: 10.1042/bj2280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Hwang D, Bataille F, Lefevre M, York D, Quon MJ, Ye J. Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. J Biol Chem. 2002;277:48115–48121. doi: 10.1074/jbc.M209459200. [DOI] [PubMed] [Google Scholar]
- Greene MW, Sakaue H, Wang L, Alessi DR, Roth RA. Modulation of insulin-stimulated degradation of human insulin receptor substrate-1 by Serine 312 phosphorylation. J Biol Chem. 2003;278:8199–8211. doi: 10.1074/jbc.M209153200. [DOI] [PubMed] [Google Scholar]
- Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Henriksen EJ. Invited review: effects of acute exercise and exercise training on insulin resistance. J Appl Physiol. 2002;93:788–796. doi: 10.1152/japplphysiol.01219.2001. [DOI] [PubMed] [Google Scholar]
- Hevener AL, He W, Barak Y, Le J, Bandyopadhyay G, Olson P, Wilkes J, Evans RM, Olefsky J. Muscle-specific Pparg deletion causes insulin resistance. Nat Med. 2003;9:1491–1497. doi: 10.1038/nm956. [DOI] [PubMed] [Google Scholar]
- Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- Hjeltnes N, Galuska D, Bjornholm M, Aksnes AK, Lannem A, Zierath JR, Wallberg-Henriksson H. Exercise-induced overexpression of key regulatory proteins involved in glucose uptake and metabolism in tetraplegic persons: molecular mechanism for improved glucose homeostasis. FASEB J. 1998;12:1701–1712. doi: 10.1096/fasebj.12.15.1701. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- Ip YT, Davis RJ. Signal transduction by the c-Jun N-terminal kinase (JNK) – from inflammation to development. Curr Opin Cell Biol. 1998;10:205–219. doi: 10.1016/s0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- Ji LL, Gomez-Cabrera MC, Steinhafel N, Vina J. Acute exercise activates nuclear factor (NF)-kappaB signaling pathway in rat skeletal muscle. FASEB J. 2004;18:1499–1506. doi: 10.1096/fj.04-1846com. [DOI] [PubMed] [Google Scholar]
- Kennedy JW, Hirshman MF, Gervino EV, Ocel JV, Forse RA, Hoenig SJ, Aronson D, Goodyear LJ, Horton ES. Acute exercise induces GLUT4 translocation in skeletal muscle of normal human subjects and subjects with type 2 diabetes. Diabetes. 1999;48:1192–1197. doi: 10.2337/diabetes.48.5.1192. [DOI] [PubMed] [Google Scholar]
- Koistinen HA, Galuska D, Chibalin AV, Yang J, Zierath JR, Holman GD, Wallberg-Henriksson H. 5-amino-imidazole carboxamide riboside increases glucose transport and cell-surface GLUT4 content in skeletal muscle from subjects with type 2 diabetes. Diabetes. 2003;52:1066–1072. doi: 10.2337/diabetes.52.5.1066. [DOI] [PubMed] [Google Scholar]
- Krook A, Wallberg-Henriksson H, Zierath JR. Sending the signal: molecular mechanisms regulating glucose uptake. Med Sci Sports Exerc. 2004;36:1212–1217. doi: 10.1249/01.mss.0000132387.25853.3b. [DOI] [PubMed] [Google Scholar]
- Kump DS, Booth FW. Alterations in insulin receptor signalling in the rat epitrochlearis muscle upon cessation of voluntary exercise. J Physiol. 2005;562:829–838. doi: 10.1113/jphysiol.2004.073593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee YH, Giraud J, Davis RJ, White MF. c-Jun N-terminal kinase (JNK) mediates feedback inhibition of the insulin signaling cascade. J Biol Chem. 2003;278:2896–2902. doi: 10.1074/jbc.M208359200. [DOI] [PubMed] [Google Scholar]
- McGuinness OP, Mari A. Assessment of insulin action on glucose uptake and production during a euglycemic-hyperinsulinemic clamp in dog: a new kinetic analysis. Metabolism. 1997;46:1116–1127. doi: 10.1016/s0026-0495(97)90202-x. [DOI] [PubMed] [Google Scholar]
- Major CD, Wolf BA. Interleukin-1beta stimulation of c-Jun NH2-terminal kinase activity in insulin-secreting cells: evidence for cytoplasmic restriction. Diabetes. 2001;50:2721–2728. doi: 10.2337/diabetes.50.12.2721. [DOI] [PubMed] [Google Scholar]
- Musi N, Hayashi T, Fujii N, Hirshman MF, Witters LA, Goodyear LJ. AMP-activated protein kinase activity and glucose uptake in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2001;280:E677–E684. doi: 10.1152/ajpendo.2001.280.5.E677. [DOI] [PubMed] [Google Scholar]
- Myers MP, Andersen JN, Cheng A, Tremblay ML, Horvath CM, Parisien JP, Salmeen A, Barford D, Tonks NK. TYK2 and JAK2 are substrates of protein-tyrosine phosphatase 1B. J Biol Chem. 2001;276:47771–47774. doi: 10.1074/jbc.C100583200. [DOI] [PubMed] [Google Scholar]
- O'Neill TJ, Craparo A, Gustafson TA. Characterization of an interaction between insulin receptor substrate 1 and the insulin receptor by using the two-hybrid system. Mol Cell Biol. 1994;14:6433–6442. doi: 10.1128/mcb.14.10.6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes ND, Bell KS, Furler SM, Camilleri S, Saha AK, Ruderman NB, Chisholm DJ, Kraegen EW. Diet-induced muscle insulin resistance in rats is ameliorated by acute dietary lipid withdrawal or a single bout of exercise: parallel relationship between insulin stimulation of glucose uptake and suppression of long-chain fatty acyl-CoA. Diabetes. 1997;46:2022–2028. doi: 10.2337/diab.46.12.2022. [DOI] [PubMed] [Google Scholar]
- Pimenta WP, Saad MJ, Paccola GM, Piccinato CE, Foss MC. Effect of oral glucose on peripheral muscle fuel metabolism in fasted men. Braz J Med Biol Res. 1989;22:465–476. [PubMed] [Google Scholar]
- Prada P, Okamoto MM, Furukawa LN, Machado UF, Heimann JC, Dolnikoff MS. High- or low-salt diet from weaning to adulthood: effect on insulin sensitivity in Wistar rats. Hypertension. 2000;35:424–429. doi: 10.1161/01.hyp.35.1.424. [DOI] [PubMed] [Google Scholar]
- Prada P, Zecchin HG, Gasparetti AL, Torsoni MA, Ueno M, Hirata AE, Corezola Do Amaral ME, Hoer NF, Boschero AC, Saad MJ. Western diet modulates insulin signaling, c-Jun N-terminal kinase activity, and insulin receptor substrate-1ser307 phosphorylation in a tissue-specific fashion. Endocrinology. 2005;146:1576–1587. doi: 10.1210/en.2004-0767. [DOI] [PubMed] [Google Scholar]
- Prattali RR, Barreiro GC, Caliseo CT, Fugiwara FY, Ueno M, Prada PO, Velloso LA, Saad MJ, Carvalheira JB. Aspirin inhibits serine phosphorylation of insulin receptor substrate 1 in growth hormone treated animals. FEBS Lett. 2005;579:3152–3158. doi: 10.1016/j.febslet.2005.04.075. [DOI] [PubMed] [Google Scholar]
- Richter EA, Garetto LP, Goodman MN, Ruderman NB. Muscle glucose metabolism following exercise in the rat: increased sensitivity to insulin. J Clin Invest. 1982;69:785–793. doi: 10.1172/JCI110517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui L, Aguirre V, Kim JK, Shulman GI, Lee A, Corbould A, Dunaif A, White MF. Insulin/IGF-1 and TNF-alpha stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J Clin Invest. 2001;107:181–189. doi: 10.1172/JCI10934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad MJ, Maeda L, Brenelli SL, Carvalho CR, Paiva RS, Velloso LA. Defects in insulin signal transduction in liver and muscle of pregnant rats. Diabetologia. 1997;40:179–186. doi: 10.1007/s001250050660. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Goodyear LJ. Invited review: intracellular signaling in contracting skeletal muscle. J Appl Physiol. 2002;93:369–383. doi: 10.1152/japplphysiol.00167.2002. [DOI] [PubMed] [Google Scholar]
- Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- Saltiel AR, Olefsky JM. Thiazolidinediones in the treatment of insulin resistance and type II diabetes. Diabetes. 1996;45:1661–1669. doi: 10.2337/diab.45.12.1661. [DOI] [PubMed] [Google Scholar]
- Scott AM, Atwater I, Rojas E. A method for the simultaneous measurement of insulin release and B cell membrane potential in single mouse islets of Langerhans. Diabetologia. 1981;21:470–475. doi: 10.1007/BF00257788. [DOI] [PubMed] [Google Scholar]
- Seely BL, Staubs PA, Reichart DR, Berhanu P, Milarski KL, Saltiel AR, Kusari J, Olefsky JM. Protein tyrosine phosphatase 1B interacts with the activated insulin receptor. Diabetes. 1996;45:1379–1385. doi: 10.2337/diab.45.10.1379. [DOI] [PubMed] [Google Scholar]
- Sriwijitkamol A, Christ-Roberts C, Berria R, Eagan P, Pratipanawatr T, Defronzo RA, Mandarino LJ, Musi N. reduced skeletal muscle inhibitor of kappaB beta content is associated with insulin resistance in subjects with type 2 diabetes: reversal by exercise training. Diabetes. 2006;55:760–767. doi: 10.2337/diabetes.55.03.06.db05-0677. [DOI] [PubMed] [Google Scholar]
- Taghibiglou C, Rashid-Kolvear F, Van Iderstine SC, Le-Tien H, Fantus IG, Lewis GF, Adeli K. Hepatic very low density lipoprotein-ApoB overproduction is associated with attenuated hepatic insulin signaling and overexpression of protein-tyrosine phosphatase 1B in a fructose-fed hamster model of insulin resistance. J Biol Chem. 2002;277:793–803. doi: 10.1074/jbc.M106737200. [DOI] [PubMed] [Google Scholar]
- Thompson HS, Maynard EB, Morales ER, Scordilis SP. Exercise-induced HSP27, HSP70 and MAPK responses in human skeletal muscle. Acta Physiol Scand. 2003;178:61–72. doi: 10.1046/j.1365-201X.2003.01112.x. [DOI] [PubMed] [Google Scholar]
- Tonks NK. PTP1B: from the sidelines to the front lines! FEBS Lett. 2003;546:140–148. doi: 10.1016/s0014-5793(03)00603-3. [DOI] [PubMed] [Google Scholar]
- Ueki K, Kondo T, Kahn CR. Suppressor of cytokine signaling 1 (SOCS-1) and SOCS-3 cause insulin resistance through inhibition of tyrosine phosphorylation of insulin receptor substrate proteins by discrete mechanisms. Mol Cell Biol. 2004;24:5434–5446. doi: 10.1128/MCB.24.12.5434-5446.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventre J, Doebber T, Wu M, MacNaul K, Stevens K, Pasparakis M, Kollias G, Moller DE. Targeted disruption of the tumor necrosis factor-alpha gene: metabolic consequences in obese and nonobese mice. Diabetes. 1997;46:1526–1531. doi: 10.2337/diab.46.9.1526. [DOI] [PubMed] [Google Scholar]
- Viatour P, Merville MP, Bours V, Chariot A. Phosphorylation of NF-kappaB and IkappaB proteins: implications in cancer and inflammation. Trends Biochem Sci. 2005;30:43–52. doi: 10.1016/j.tibs.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Virkamaki A, Ueki K, Kahn CR. Protein–protein interaction in insulin signaling and the molecular mechanisms of insulin resistance. J Clin Invest. 1999;103:931–943. doi: 10.1172/JCI6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallberg-Henriksson H. Glucose transport into skeletal muscle. Influence of contractile activity, insulin, catecholamines and diabetes mellitus. Acta Physiol Scand Suppl. 1987;564:1–80. [PubMed] [Google Scholar]
- Wallberg-Henriksson H, Constable SH, Young DA, Holloszy JO. Glucose transport into rat skeletal muscle: interaction between exercise and insulin. J Appl Physiol. 1988;65:909–913. doi: 10.1152/jappl.1988.65.2.909. [DOI] [PubMed] [Google Scholar]
- Weston CR, Lambright DG, Davis RJ. Signal transduction. MAP kinase signaling specificity. Science. 2002;296:2345–2347. doi: 10.1126/science.1073344. [DOI] [PubMed] [Google Scholar]
- White MF, Kahn CR. The insulin signaling system. J Biol Chem. 1994;269:1–4. [PubMed] [Google Scholar]
- Williamson D, Gallagher P, Harber M, Hollon C, Trappe S. Mitogen-activated protein kinase (MAPK) pathway activation: effects of age and acute exercise on human skeletal muscle. J Physiol. 2003;547:977–987. doi: 10.1113/jphysiol.2002.036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtaszewski JF, Nielsen JN, Richter EA. Invited review: effect of acute exercise on insulin signaling and action in humans. J Appl Physiol. 2002;93:384–392. doi: 10.1152/japplphysiol.00043.2002. [DOI] [PubMed] [Google Scholar]
- Zierath JR. In vitro studies of human skeletal muscle: hormonal and metabolic regulation of glucose transport. Acta Physiol Scand Suppl. 1995;626:1–96. [PubMed] [Google Scholar]
- Zierath JR. Invited review: exercise training-induced changes in insulin signaling in skeletal muscle. J Appl Physiol. 2002;93:773–781. doi: 10.1152/japplphysiol.00126.2002. [DOI] [PubMed] [Google Scholar]