Abstract
5′AMP-activated protein kinase (AMPK) is a key regulator of cellular metabolism and is regulated in muscle during exercise. We have previously established that only three of 12 possible AMPK α/β/γ-heterotrimers are present in human skeletal muscle. Previous studies describe discrepancies between total AMPK activity and regulation of its target acetyl-CoA-carboxylase (ACC)β. Also, exercise training decreases expression of the regulatory γ3 AMPK subunit and attenuates α2 AMPK activity during exercise. We hypothesize that these observations reflect a differential regulation of the AMPK heterotrimers. We provide evidence here that only the α2/β2/γ3 subunit is phosphorylated and activated during high-intensity exercise in vivo. The activity associated with the remaining two AMPK heterotrimers, α1/β2/γ1 and α2/β2/γ1, is either unchanged (20 min, 80% maximal oxygen uptake (V˙O2,peak)) or decreased (30 or 120 s sprint-exercise). The differential activity of the heterotrimers leads to a total α-AMPK activity, that is decreased (30 s trial), unchanged (120 s trial) and increased (20 min trial). AMPK activity associated with the α2/β2/γ3 heterotrimer was strongly correlated to γ3-associated α-Thr-172 AMPK phosphorylation (r2 = 0.84, P < 0.001) and to ACCβ Ser-221 phosphorylation (r2 = 0.65, P < 0.001). These data single out the α2/β2/γ3 heterotrimer as an important actor in exercise-regulated AMPK signalling in human skeletal muscle, probably mediating phosphorylation of ACCβ.
5′AMP-activated protein kinase (AMPK) is activated in response to numerous cellular stresses including metabolic poisons (e.g. arsenite, azide, dinitrophenol), pharmacological agents (e.g. metformin, thiazolidinediones) and pathological stressors (e.g. glucose deprivation, ischaemia and hyperosmolarity) (Kahn et al. 2005). Exercise is another well-established physiological stimulus for AMPK activation in skeletal muscle. During exercise the AMPK system is activated in an intensity- and time-related manner in both rodent and human skeletal muscle (Winder & Hardie, 1996; Rasmussen & Winder, 1997; Chen et al. 2000, 2003; Fujii et al. 2000; Wojtaszewski et al. 2000). In general, studies in human muscle have reported that α2- rather than α1-associated activity is more prone to be increased during exercise in vivo (Chen et al. 2000; Fujii et al. 2000; Wojtaszewski et al. 2000; McConell et al. 2005).
Causal links between AMPK and cellular biological effects in skeletal muscle have been reported. Thus, AMPK seems to regulate several aspects of carbohydrate and fat metabolism either directly by interfering with key enzymes, or indirectly via regulation of gene transcription in resting muscle (Kahn et al. 2005). It is currently hypothesized that some of the beneficial effects of exercise on insulin action, and prevention of type 2 diabetes, are mediated by AMPK (Fisher et al. 2002; Iglesias et al. 2002), a premise that is supported by the observation that the antidiabetic drug metformin also acts through AMPK (Zhou et al. 2001; Musi et al. 2002).
AMPK is a member of the Snf1/AMPK serine/threonine protein kinase family, and exists as heterotrimeric complexes comprising a catalytic α subunit and regulatory β and γ subunits, each of which is expressed as two or more isoforms encoded by distinct genes (Hardie et al. 2003). The catalytic activity of the α subunits (α1 and α2) is increased after phosphorylation at the Thr-172 residue within the activation loop (Hawley et al. 1996), by upstream kinases (e.g. LKB1 and CaMKKα/β)(Hawley et al. 2003; Woods et al. 2003; Hawley et al. 2005; Hurley et al. 2005; Sakamoto et al. 2005). The β subunit of AMPK (β1 and β2) contains two conserved domains, one required for assembly of the α/β/γ heterotrimer, and a glycogen-binding domain that may target AMPK to glycogen particles (Hudson et al. 2003; Polekhina et al. 2003). The γ subunit (γ1, γ2 and γ3) contains two ‘Bateman domains’ (Bateman, 1997; Adams et al. 2004) that bind the regulatory nucleotides AMP and ATP. Binding of AMP activates the kinase allosterically and promotes the AMPK α-Thr-172 phosphorylation by upstream kinases as well as by inhibiting dephosphorylation by phosphatases (Hardie et al. 1999; Adams et al. 2004).
Although we have been able to detect all seven subunit isoforms of AMPK in human skeletal muscle (Frosig et al. 2004; Wojtaszewski et al. 2005), only three (α1/β2/γ1, α2/β2/γ1 and α2/β2/γ3) of the 12 possible AMPK heterotrimeric complexes are present to a significant extent, and a marked difference in abundance among these is apparent (α2/β2/γ1 >> α2/β2/γ3 ≥ α1/β2/γ1) (Wojtaszewski et al. 2005). Until now, AMPK signalling in skeletal muscle in response to exercise has only been reported either as total AMPK activity, total α-Thr-172 phosphorylation, or as the activity associated with either of the two catalytic α isoforms, α1 and α2.
As mentioned, differential regulation between the two catalytic α subunits has been observed during conditions in which global AMPK activity is increased (Wojtaszewski et al. 2002; Nielsen et al. 2003). Disassociation between total AMPK activity and regulation of its downstream target acetyl-CoA-carboxylase (ACC)β has also been reported (Wojtaszewski et al. 2002). In addition, different AMPK heterotrimeric complexes display different sensitivities to allosteric activation by AMP in vitro (Salt et al. 1998). Thus, it is likely that also between the two different α2 AMPK heterotrimers (γ1 versus γ3) differential regulation occurs in response to various stimuli. In fact, exercise training attenuates exercise-induced AMPK α2 activity in skeletal muscle (Nielsen et al. 2003; Yu et al. 2003; McConell et al. 2005) and reduces the expression of AMPK γ3 mRNA and protein, whereas it increases expression of α1, β2 and γ1 (Langfort et al. 2003; Nielsen et al. 2003; Frosig et al. 2004; Wojtaszewski et al. 2005). Extending on these observations, we hypothesize that exercise in human skeletal muscle primarily activates the α2/β2/γ3 AMPK heterotrimer rather than α1/β2/γ1 and α2/β2/γ1, even though α2/β2/γ3 only accounts for one-fifth of all heterotrimers.
In the present study, AMPK signalling in skeletal muscle was investigated in vivo during different exercise modalities in humans. We report that the three AMPK heterotrimers present in human skeletal muscle undergo differential regulation during exercise, an observation that is highly relevant in elucidating the physiological role of AMPK in skeletal muscle and in the exploration of muscular AMPK as a potential drug target in diseases associated with insulin resistance.
Methods
Thirty healthy young men (age 27 ± 1 years, body weight 79 ± 2 kg, body mass index (BMI) 24 ± 0 kg m−2) gave their written informed consent to participate in this study, which was approved by the Copenhagen Ethics Committee (no. KF01277313) and was in agreement with the Declaration of Helsinki II.
One to two weeks prior to the experimental day the maximal oxygen uptake was determined during incremental cycling on an ergometer (V˙O2,peak 52 ± 1 ml min−1 kg−1). Subjects with a V˙O2,peak below 40 or above 60 ml min−1 kg−1 were excluded from this study. Subjects were randomly assigned to one of three exercise interventions, and they all performed one test trial prior to the experiment. The subjects were instructed not to perform moderate-heavy physical activity the day before the experiment. On the experimental day, the subjects arrived at the laboratory in the morning 3 h after a light breakfast, with the use of minimal physical effort. After 45 min of rest, a needle biopsy from the vastus lateralis muscle was obtained under local anaesthesia (2–3 ml of 2% lidocaine). The subject then performed bicycle exercise in accordance to one of the following three protocols:
(1) Eleven subjects performed 20 min of bicycling at 80%V˙O2,peak (77 ± 3%V˙O2,peak, work rate = 222 ± 8 W, total work performed 266 ± 9 kJ).
(2) Nine subjects performed a 120 s bicycle test at a work rate (376 ± 18 W) corresponding to 110% of peak work rate, which was defined as the highest work intensity maintained for a whole minute during the incremental V˙O2,peaktest. Within the first 30 s, the subject increased the pedal frequency to the range of 100–120 min−1 before resistance was applied to the bike. Exercise duration was slightly variable (115 ± 4 s) due to the onset of fatigue at somewhat different time points (total work performed 43 ± 2 kJ). When occurring, fatigue quickly developed into a state where pedal frequency dropped markedly, at which time the test was terminated.
(3) Ten subjects performed a 30 s ‘all out’ sprint exercise trial. Without resistance on the bike, the subject increased the pedal frequency to ∼140 min−1 and after 10 s a workload corresponding to 7.5 N (kg body weight)−1 was applied. On average the test lasted 30.5 ± 0.5 s, and in this period the average work rate was 658 ± 26 W (total work performed 21 ± 1 kJ).
Independent of exercise protocol, the subject was placed in the supine position immediately after exercise, and a second biopsy was obtained from the vastus lateralis muscle. One insertion was made in each leg, and the pre- and post-exercise biopsies were randomly taken in the dominant and the non-dominant leg. The biopsies were frozen in liquid nitrogen within 15 s after the termination of the exercise. The biopsies were stored at −80°C.
Muscle lysate preparation
Homogenates and lysates were prepared from 70 mg (w/w) muscle, that was freeze-dried, dissected free of visible fat, blood and connective tissue and homogenized in 50 mm Hepes (pH 7.5), 10% glycerol, 20 mm Na-pyrophosphate, 150 mm NaCl, 1% NP-40, 20 mm β-glycerophosphate, 10 mm NaF, 2 mm PMSF, 1 mm EDTA, 1 mm EGTA, 10 μg ml−1 aprotenin, 10 μg ml−1 leupeptin, 2 mm Na3VO4, 3 mm benzamidine. Homogenates rotated end over end at 4°C for one hour. Lysates were prepared from the homogenates by centrifuging 25 min at 17 500 g and 4°C. Total homogenate and lysate protein content were analysed by the bicinchoninic acid method (Pierce Biotechnology, Inc., Rockford, IL, USA). Unless stated specifically, all chemicals were of analytic grade from Sigma-Aldrich (Denmark).
Muscle glycogen
Muscle glycogen content was measured in muscle homogenates (150 μg of protein) as glycosyl units after acid hydrolysis determined by a fluorometric method (Lowry & Passonneau, 1972).
Muscle lactate, adenosine triphosphate (ATP), creatine (Cr), and phosphocreatine (PCr)
Freeze-dried muscle biopsy specimens were extracted with perchloric acid, neutralized, and analysed for lactate, ATP, Cr and PCr as previously described (Lowry & Passonneau, 1972). The estimations of free concentrations of ADP and AMP were based on the near-equilibrium nature of the creatine phosphokinase and adenylate kinase reactions, respectively. Free ADP was estimated from the measured ATP, Cr and PCr contents, and the H+ concentration was estimated using the measured muscle lactate content according to the formula presented by Mannion et al. (1993) for dry muscle. The equilibrium constant (Kobs) value employed for creatine phosphokinase was 1.66 × 109m−1 (Lawson & Veech, 1979). Free AMP was estimated from the measured ATP and the estimated free ADP using a Kobs for adenylate kinase of 1.05 (Lawson & Veech, 1979).
SDS-PAGE and Western blotting
Muscle lysate proteins were separated using 10% Tris-HCl gels (Biorad, Denmark), and transferred (semidry) to PVDF-membranes (Immobilion Tranfer Membrane, Millipore A/S, Denmark). After blocking (Tris-buffered saline + 0.05% Tween-20 (TBST) + 2% skimmed milk), the membranes were incubated with primary antibodies (TBST + 2% skimmed milk) followed by incubation in horseradish peroxidase-conjugated secondary antibody (TBST + 2% skimmed milk) (DAKO, Denmark). Following detection and quantification using a CCD-image sensor and 1D software (Kodak Image Station, 2000MM, Kodak, Denmark), the protein content was expressed in arbitrary units relative to a human skeletal muscle control sample. By loading a control sample in different amounts it was ensured that the quantification was within the linear response range for each particular protein probed for.
Antibodies used for AMPK subunit isoform detection
The primary antibodies used for detection of the AMPK subunit isoforms α1, α2, β1, β2, γ1 and γ3 were as previously described (Wojtaszewski et al. 2005). Phosphorylation of AMPK α subunits (Thr-172) and acetyl-CoA-carboxylase-β (ACCβ) (Ser-221) was detected using phospho-specific antibodies (Cell Signalling Technology Inc., MA, USA and Upstate Biotechnology, MA, USA, respectively).
Detection of the AMPK heterotrimeric composition
The subunit isoforms (α1, α2, γ3 and α-Thr-172) was immunoprecipitated (IP) from 400 μg of muscle lysate using specific antibodies and sepharose-coupled G-protein overnight at 4°C in IP-buffer (50 mm NaCl, 1% Triton X-100, 50 mm NaF, 5 mm Na-pyrophosphate, 20 mm Tris-base (pH 7.5), 500 μm PMSF, 2 mm DTT, 4 μg ml−1 leupeptin, 50 μg ml−1 soybean trypsin inhibitor, 6 mm benzamidine and 250 mm sucrose). Samples of the IP, the post-IP lysate and the pre-IP lysate were prepared with Laemmli buffer and boiled for 3 min at 96°C, and analysed by SDS-PAGE and Western blotting using each of the seven (α1, α2, β1, β2, γ1, γ3 and α-Thr-172) antibodies recognizing the various AMPK subunits and the phosphorylated α subunits. The precipitation efficiency and the degree of co-immunoprecipitation were evaluated by comparing the signal in the pre- to that of the post-IP.
AMPK activity
Isoform-specific AMPK activity was measured on IPs from 200 μg of muscle lysate protein as described above. After an overnight incubation at 4°C, the IP was washed once in ip-buffer, once in 480 mm Hepes (pH 7.0) and 240 mm NaCl, and twice in 240 mm Hepes (pH 7.0) and 120 mm NaCl leaving 10 μl of buffer with the Sepharose after the last wash. The reaction ran for 30 min at 30°C in a total volume of 30 μl containing 833 μm DTT, 200 μm AMP, 100 μm AMARA-peptide, 5 mm MgCl2, 200 μm ATP and 2 μCi of [γ-32P]-ATP. The reaction was stopped by spotting 25 μl onto a piece of P81 filter paper, which was then washed for four times 15 min in 1% phosphoric acid. The dried filter paper was analysed for activity using liquid scintillation counting.
The α2/β2/γ1 activity was analysed by immunodepleting lysates for α2/β2/γ3 heterotrimers by an overnight γ3 IP, followed by yet another overnight α2 IP on which the α2/β2/γ1 activity was measured. Neither one nor two overnight incubations at 4°C had any influence of the phosphorylation state of α-AMPK subunits (data not shown). Also the activity associated with each of the three complexes was unaffected by either one or two overnight IPs compared to a 4 h IP (data not shown).
Statistical analyses
Results are presented as mean ± s.e.m. Statistical evaluation was performed where appropriate by paired Student's t test or by one- or two-way ANOVA for repeated measurements using the Tukey's post hoc test. Differences between groups were considered statistically significant for P < 0.05.
Results
The heterotrimeric composition using co-immunoprecipitation (co-IP) analyses extend and confirm our previous findings of the presence of only three heterotrimers in human skeletal muscle; α1/β2/γ1, α2/β2/γ1 and α2/β2/γ3. The present analyses were performed on biopsies from 11 subjects, allowing for interpersonal variation compared to the earlier study using a pool of biopsy material from different subjects (Wojtaszewski et al. 2005). Because the β1 isoform was not co-immunoprecipitated with either of the two α isoforms (data not shown) and all α1 and α2 co-immunoprecipitated with β2 (Wojtaszewski et al. 2005), the relative contribution to total AMPK heterotrimers of either of the two α isoforms can be estimated by comparing the amount of β2 co-immunoprecipitated with α1 and α2. Doing so, the present study reveals that complexes containing the α1/β2 isoforms contribute with the minority (15 ± 9%, n = 11) of all complexes (Fig. 1A and B), whereas the α2/β2 isoforms contribute with the majority (99 ± 1%, n = 11). Acknowledging the limitation of using the multi-step IP procedure and the semiquantitative nature of the Western blotting technique, in particular when comparing very strong to very low signals (as in the case of the α2/β2 analysis (Fig. 1B)), we consider that the contribution of the α2/β2 complexes is overestimated rather than that of the α1/β2 complexes being underestimated.
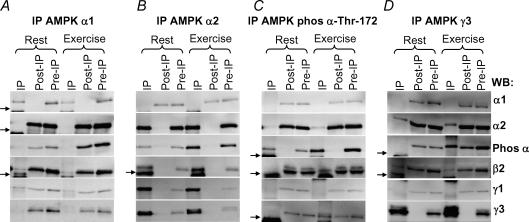
Figure 1. AMPK heterotrimer composition and phosphorylation in human skeletal muscle.
Lysates were prepared from biopsies taken in the vastus lateralis muscle before and after 20 min of exercise at 80%V˙O2,peak (n = 11). From 400 μg of lysate AMPK α1 (A), α2 (B), phospho α-Thr-172 (C) or γ3 (D) was immunoprecipitated (ip). The figure shows representative blots of the IP, post-IP and pre-IP (lysate) in the rested and exercised state. One-eighth of the IP corresponding to 50 μg was loaded together with 20 μg of the post- and pre-IPs. The blotted membranes were analysed with anti-α1, -α2, -phospho α-Thr-172, -β2, -γ1 and -γ3 as indicated to the far right. The small arrow indicates IgG light and heavy chains on the blots.
No measurable γ3 was associated with α1 (Fig. 1A and D). Comparing the amount of α2 co-immunoprecipitated with γ3, it was evident that 17 ± 4% (n = 11) of α2/β2 was associated with γ3 (Fig. 1D). Based on our previous observation that γ1, but not γ2, co-immunoprecipitated with α1, α2 or β2 (Wojtaszewski et al. 2005), we anticipate that the remaining trimeric complexes are α1/β2/γ1 and α2/β2/γ1. However, the anti-γ1 antibodies available do not fully immunoprecipitate all γ1 protein, making measurements of the relative amount of α1/β2/γ1 and α2/β2/γ1 complexes difficult. Together with our previous results (Wojtaszewski et al. 2005) indicating a minor existence of α1/β2 and α2/β2 dimers, the approximate distribution of the three AMPK heterotrimers can however, be estimated to be ∼15% α1/β2/γ1, ∼65% α2/β2/γ1 and ∼20% α2/β2/γ3.
Comparing muscle biopsies obtained before and after exercise (20 min, 80%V˙O2,peak), no differences were apparent in the subunit isoform expression or the heterotrimeric subunit composition when analysed by co-IP using the anti-α1, α2 and γ3 antibodies (Fig. 1A, B and D).
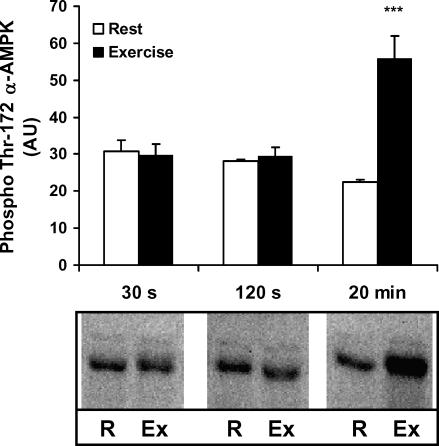
Limited to only three major heterotrimers, we aimed to evaluate the activation pattern among these during exercise. First we investigated the Thr-172 phosphorylation at α AMPK (p-AMPK) using a phospho-specific antibody (no signal observed using this antibody after phosphatase treatment of lysate (data not shown)). All phosphorylated α subunits were precipitated with the AMPK α-Thr-172 antibody (Fig. 1C). Interestingly, the changes associated with exercise (20 min, 80%V˙O2,peak) were largely confined to α2 (Fig. 1B and C) rather than to α1 complexes (Fig. 1A and C). Surprisingly, only ∼10% of all α2 protein was phosphorylated during exercise (Fig. 1C). When measured by direct blotting, the increase compared to rest in p-AMPK with exercise corresponded to 251 ± 28% (n = 11), i.e. the increase (exercise minus rest) corresponded to 55 ± 5% (n = 11) of the phosphorylation seen during exercise (Fig. 2). In agreement, in the exercised state 59 ± 6% (n = 11) of all phosphorylated α subunits was associated with γ3 (Fig. 1D). Because p-AMPK associated with γ3 at rest was hardly detectable (Fig. 1D), and because the changes in total p-AMPK corresponded to the changes in p-AMPK associated with γ3 (55% versus 59%), these data suggest that the γ3-containing heterotrimers are retaining the majority of the Thr-172 α-AMPK phosphorylation during this type of exercise. In line with this, no apparent difference in the γ1-associated p-AMPK was observed between rest and exercise (Fig. 1C). These data also suggest that in resting muscle most or all of the phosphorylated AMPK heterotrimers must be α1/β2/γ1 and α2/β2/γ1. Finally, the phosphorylated γ3 heterotrimers in the exercised state accounted only for 32 ± 5% (n = 11) of all the γ3 heterotrimers, still leaving the majority of these unphosphorylated and available for activation (Fig. 1C).
Figure 2. Thr-172 α-AMPK phosphorylation.
Lysates are from the vastus lateralis muscle before and after exercise for 30 s (n = 10), 120 s (n = 9) and 20 min (n = 11), respectively; 25 μg of lysate were run on SDS-PAGE and blotted for phosphorylation of Thr-172 α-AMPK. Results are means ±s.e.m., ***P < 0.001, difference between rest and exercise.
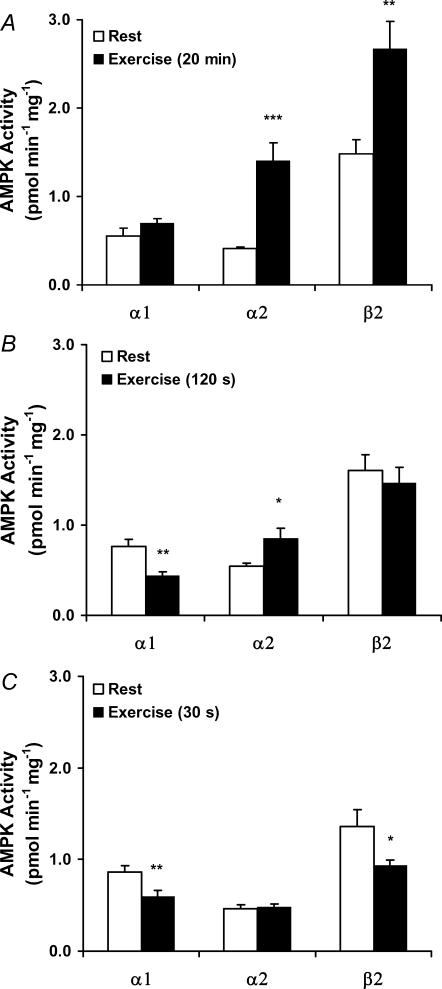
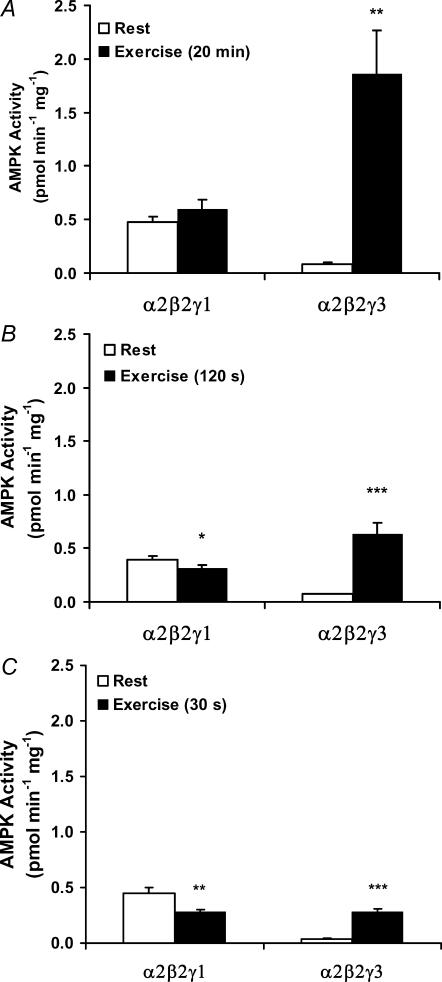
The present data on p-AMPK strongly indicate a differential regulation of the α2-associated activity depending on the γ isoform present in the complex; at least during activation of the AMPK system by a high-intensity exercise regime. To confirm this, activity assays were performed using IP of either α1, α2 or β2, in principle representing the trimers α1/β2/γ1, α2/β2/γ1+ α2/β2/γ3 and α1/β2/γ1 + α2/β2/γ1 + α2/β2/γ3, respectively. In agreement with the increased total p-AMPK (Fig. 2), total AMPK activity (as measured by β2 IP) increased (Fig. 3A). This was fully accounted for by the increase in α2 AMPK activity as the α1 AMPK activity remained unchanged by exercise (Fig. 3A). To fully decipher which of the two α2 complexes are regulated under these conditions, we performed analysis using first IP of γ3 (representing the α2/β2/γ3 heterotrimer). From the post-γ3 IP supernatant, α2 was subsequently immunopurified (representing the α2/β2/γ1 heterotrimer). This γ3 immunodepletion procedure was necessary to measure the α2/β2/γ1 activity as we have not been able to immunoprecipitate γ1 completely from human muscle. The activity associated with α2/β2/γ3 increased in response to exercise, and in fact the absolute increase was largely similar to that seen in total α2-associated activity (i.e. α2/β2/γ1 + α2/β2/γ3) (Fig. 3A), suggesting that only the activity of the α2/β2/γ3 heterotrimer was increased (Fig. 4A). This was substantiated by the observation that the α2-associated activity remaining after γ3 immunodepletion (i.e. α2/β2/γ1 activity) was not regulated by exercise (Fig. 4A). Thus, of the three AMPK trimers, only the α2/β2/γ3 was phosphorylated and activated by this exercise intervention (20 min, 80%V˙O2,peak).
Figure 3. AMPK subunit-associated activity in response to exercise.
Lysates are from the vastus lateralis muscle before and after exercise for A, 20 min (n = 11); B, 120 s (n = 9) and C, 30 s (n = 10). AMPK α1 (α1/β2/γ1), α2 (α2/β2/γ1 + α2/β2/γ3) or β2 α1/β2/γ1 + α2/β2/γ1 + α2/β2/γ3) were immunoprecipitated from 200 μg of lysate, and activity against the AMARA peptide was measured in the presence of 200 μm AMP. Results are means ±s.e.m., *P < 0.05, **P < 0.01 and ***P < 0.001, significant differences between rest and exercise.
Figure 4. AMPK subunit-associated activity in response to exercise.
Lysates are from the vastus lateralis muscle before and after exercise for A, 20 min (n = 11); B, 120 s (n = 9) and C, 30 s (n = 10). AMPK γ3 (α2/β2/γ3) was immunoprecipitated from 200 μg of lysate, and activity against the AMARA peptide was measured in the presence of 200 μm AMP. The α2/β2/γ1-associated activity was determined by IP of α2 after immunodepleting the lysate for γ3. Results are means ±s.e.m., *P < 0.05, **P < 0.01 and ***P < 0.001, significant differences between rest and exercise.
We were then intrigued to investigate the AMPK regulation during more severe exercise during which cellular energy status is more markedly disturbed. Previously is has been shown by Chen et al. (2000) that a 30 s bicycle sprint activates both AMPK α1 and α2, whereas more moderate exercise normally only activates α2 (Fujii et al. 2000; Wojtaszewski et al. 2000, 2003; Nielsen et al. 2003), as seen in the 20 min trial. Thus, subjects performed an exercise regime leading to exhaustion within either 120 or 30 s. In accordance with the marked differences in exercise intensity (222 ± 8, 376 ± 18 and 658 ± 26 W for the 20 min, 120 s and 30 s trial, respectively), the rate of glycogen degradation was markedly different (8 ± 1, 49 ± 8 and 172 ± 22 mmol (kg dry weight)−1 min−1 for the 20 min, 120 s and 30 s trial, respectively). Compared to the 20 min protocol, the two high-intensity exercise regimes lead to significantly higher accumulation of muscle lactate and a higher PCr degradation (Table 1). No significant differences were observed between the two short-term high-intensity exercise regimes regarding lactate accumulation, glycogen degradation or changes in PCr and ATP concentrations (Table 1).
Table 1.
Parameters of cellular energy status
| 30 s (n = 10) | 120 s (n = 9) | 20 min (n = 11) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Rest | Exercise | Delta | Rest | Exercise | Delta | Rest | Exercise | Delta | |
| Lactate, mmol (kg dry weight)−1 | 7 ± 2 | 89 ± 6***††† | 82 ± 6††† | 5 ± 1 | 86 ± 3***††† | 81 ± 3††† | 5 ± 1 | 21 ± 3*** | 16 ± 3 |
| Cr, mmol (kg dry weight)−1 | 48 ± 4 | 103 ± 5***††† | 55 ± 3††† | 59 ± 7 | 110 ± 6***††† | 51 ± 6††† | 43 ± 4 | 62 ± 7*** | 19 ± 6 |
| PCr, mmol (kg dry weight)−1 | 81 ± 5 | 41 ± 3***††† | −41 ± 5†† | 90 ± 7 | 36 ± 4***††† | −54 ± 9††† | 81 ± 5 | 76 ± 6 | 5 ± 9 |
| PCr/(Cr + PCr)(× 100) | 63 ± 1 | 29 ± 0***††† | −34 ± 1††† | 61 ± 1 | 24 ± 2***††† | −36 ± 3††† | 66 ± 1 | 56 ± 4** | − 10 ± 4 |
| ATP, mmol (kg dry weight)−1 | 25 ± 1 | 21 ± 1 | 5 ± 1 | 26 ± 1 | 20 ± 1 | 6 ± 2 | 26 ± 2 | 26 ± 2 | − 1 ± 3 |
| AMPfree, μmol (kg dry weight)−1 | 0.8 ± 0.1 | 1.4 ± 0.2‡‡‡ | 0.7 ± 0.2 | 1.1 ± 0.2 | 2.6 ± 0.7‡‡‡ | 1.5 ± 0.6 | 0.7 ± 0.1 | 1.5 ± 0.5‡‡‡ | 0.9 ± 0.5 |
| AMPfree/ATP (×103) | 32 ± 2 | 60 ± 8‡‡‡ | 27 ± 8 | 41 ± 5 | 129 ± 35‡‡‡ | 88 ± 35 | 27 ± 3 | 70 ± 28‡‡‡ | 43 ± 27 |
| Glycogen, mmol (kg dry weight)−1 | 436 ± 45 | 350 ± 39*** | −86 ± 11††† | 425 ± 24 | 340 ± 28*** | − 98 ± 15†† | 454 ± 30 | 282 ± 27*** | −157 ± 13 |
Values are mean ±s.e.m. Cr, creatine; PCr, phosphocreatine; AMPfree, free AMP calculated as described in Methods. Delta: exercise minus rest value, calculated as mean of the individual differences. Significantly different from Rest
(P < 0.05
P < 0.01
P < 0.001)
Significantly different from 20 min
(P < 0.01
P < 0.001)
Significant main effect of exercise
(P < 0.001).
In response to both high-intensity exercise protocols, there was, surprisingly, no detectable increase in p-AMPK (Fig. 2). In line with this, the total AMPK activity (β2 IP) was unchanged (120 s trial) or slightly decreased (30 s trial) (Fig. 3B and C). These outcomes were again the result of highly differential regulation of the activity associated with the α1 and α2 complexes. Thus, in the 120 s trial, α1-associated activity decreased significantly, whereas α2 activity increased significantly. The magnitude of the changes was approximately equal, giving rise to the unchanged total AMPK activity (Fig. 3B). In the 30 s trial, α1-associated activity also decreased significantly, whereas the α2 activity was unchanged. In agreement, total AMPK activity slightly decreased in this trial (Fig. 3C). Between the two α2 complexes, only the α2/β2/γ3 activity increased in response to exercise in both trials, whereas a minor but significant decrease in α2/β2/γ1 activity was observed (Fig. 4B and C). Thus, even during short-term high-intensity exercise, only the α2/β2/γ3 was activated.
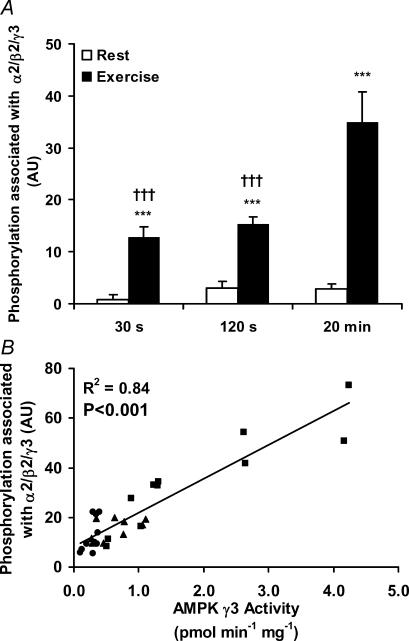
We measured the amount of phosphorylated α subunits associated with γ3 in all three exercise trials (Fig. 5A). The analyses reveal a similar pattern of regulation as for the γ3-associated AMPK activity (Fig. 4A–C). In line with this, a strong significant correlation was seen between these two measures (r2 = 0.84, P < 0.001), suggesting that differences in γ3 activity between trials indeed were related to changes in phosphorylated α subunits associated with γ3.
Figure 5. AMPK phosphorylation in relation to AMPK γ3-associated activity.
Lysates are from the vastus lateralis muscle before and after exercise for 30 s (n = 10), 120 s (n = 9) and 20 min (n = 11), respectively. A, 25 μg of lysate were run on SDS-PAGE and blotted for Thr-172 phosphorylation of α-AMPK. These signal intensities were multiplied with the amount of Thr-172 phosphorylated α-AMPK co-immunoprecipitated with γ3 to get the level of phosphorylation associated with the γ3 trimers. B, relationship between the γ3-associated level of Thr-172 α-AMPK phosphorylation and the AMPK activity of α2/β2/γ3. •= 30 s trial (n = 10); ▴= 120 s trial (n = 9) and ▪= 20 min trial (n = 11). A significant coefficient was found with linear regression analysis (r2 = 0.84, P < 0.001). Results are means ±s.e.m., ***P < 0.001, difference between rest and exercise, †††P < 0.001, significantly different from the 20 min trial.
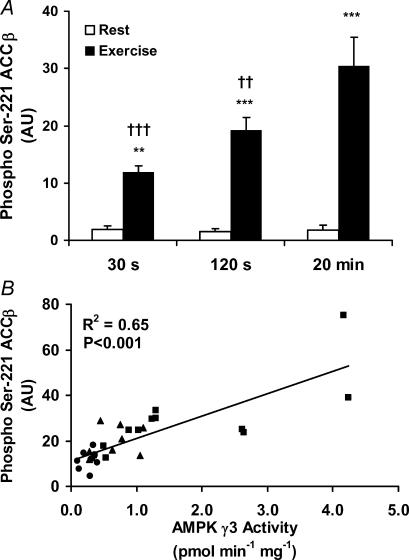
ACCβ is a target for AMPK in muscle. As expected, during exercise in all three trials Ser-221 phosphorylation of ACCβ increased (Fig. 6A). Like the γ3-associated AMPK activity, the changes in ACCβ phosphorylation were regulated in a workload- and/or time-dependent manner, and a strong and significant correlation was observed between these measures (r2 = 0.65, P < 0.001)(Fig. 6B). Although this does not prove a causal relationship, it indicates that phosphorylation of ACCβ may be a good endogenous predictor of α2/β2/γ3, but not of total AMPK activity during exercise.
Figure 6. ACCβ phosphorylation in relation to AMPK γ3-associated activity.
Lysates are from the vastus lateralis muscle before and after exercise for 30 s (n = 10), 120 s (n = 9) and 20 min (n = 11), respectively. A, 25 μg of lysate were run on SDS-PAGE and blotted for phospho Ser-221 ACCβ. B, relationship between the level of ACCβ phosphorylation and the AMPK activity of α2/β2/γ3. •= 30 s (n = 10), ▴= 120 s (n = 9) and ▪= 20 min (n = 11). A significant coefficient was found with linear regression analysis (r2 = 0.65, P < 0.001). Results are means ±s.e.m., ***P < 0.001, difference between rest and exercise, †P < 0.05 and †††P < 0.001, significantly different from the 20 min trial.
Discussion
The three AMPK heterotrimeric complexes present in human skeletal muscle display differential regulation during exercise. Previous studies have only divided the heterotrimers into two groups, the α1- and α2-containing heterotrimers. Doing so, it has been shown that AMPK α2 activity is activated during many exercise regimes with different work intensities and lengths (Chen et al. 2000, 2003; Fujii et al. 2000; Wojtaszewski et al. 2000, 2003; Nielsen et al. 2003; McConell et al. 2005; Dreyer et al. 2006). It seems that α2 is activated in a workload-dependent manner, and only at exercise intensities above 50%V˙O2,peak (Fujii et al. 2000; Wojtaszewski et al. 2000; Chen et al. 2003), or if low-intensity exercise continues to exhaustion (Wojtaszewski et al. 2002). Activation of α1 is somewhat more controversial, as several studies have been unable to show α1 activation (Fujii et al. 2000; Wojtaszewski et al. 2000, 2003; Nielsen et al. 2003; Yu et al. 2003; Akerstrom et al. 2006), whereas others have shown an activation at various work intensities (Chen et al. 2000, 2003; McConell et al. 2005; Lee-Young et al. 2006).
To get a better understanding of the activation of AMPK, we have analysed the specific heterotrimers separately. Our data predict a unique role of the α2/β2/γ3 heterotrimer in human skeletal muscle, as only this heterotrimer is phosphorylated and activated during the three high-intensity exercise regimes investigated. The α2/β2/γ3 heterotrimer only constitutes one-fifth of all AMPK heterotrimers, and only one-third of these heterotrimers contain phosphorylated α2 protein, indicating a large pool of spare AMPK signalling within the cell even during high-intensity exercise. Accordingly, only a small fraction (10%) of the total α2 protein pool is phosphorylated during exercise. AMPK can be activated through various mechanisms and elicits a number of different events in the cell depending on the stress situation. Our data support the idea that the whole pool of AMPK heterotrimers in the cell is divided into different subsets reacting to various stimuli and acting on various targets. Part of this diversity seems to be related to expression of different heterotrimers which create a basis for different AMP sensitivities but which may also localize the heterotrimers at different cellular locations. The question remains as to whether an exercise regime different from the three investigated, or other stimuli of AMPK may lead to another activation pattern of the three AMPK heterotrimers.
Total AMPK activity and total α-AMPK phosphorylation was regulated differently during the three exercise regimes, and did not covary with the γ3-associated AMPK activity. Surprisingly, this was due to a different regulation of the activity associated with γ1. Thus, in the 30 and 120 s trials, the increase in α2/β2/γ3 activity was counterbalanced by decreases in α1/β2/γ1 and α2/β2/γ1 activities. In contrast, the α1/β2/γ1 and α2/β2/γ1 activities were largely unchanged in the 20 min 80%V˙O2,peak exercise trial, resulting in a coordinated regulation of the total AMPK activity/phosphorylation and the α2/β2/γ3 activity/phosphorylation. It is interesting that both γ1 heterotrimers respond similarly in all three exercise trials regardless of α isoform in the complex. It is evident that α1 and α2 have different intrinsic activities (Michell et al. 1996), but our observations show that regulation of their activity is highly dependent on the γ isoform in the complex. This isoform-specific regulation of the heterotrimers suggests that the action of the upstream kinases or phosphatases is highly specific. The nature and regulation of the upstream kinase in human skeletal muscle is largely unknown, but a minor increase in activity of an unidentified AMPKK enzyme with exercise has been reported in human skeletal muscle (Chen et al. 2003). Recently, both CaMKKα/β and LKB1 have been identified as upstream kinases (Woods et al. 2003; Hurley et al. 2005; Hawley et al. 2003, 2005). Whereas the role for CaMKKα/β in muscle is unknown, LKB1 seems to be an important upstream kinase for AMPK during contractions, although LKB1 activity per se is not regulated during contractile activity in rodent muscle (Sakamoto et al. 2004; Taylor et al. 2004). Thus, the highly selective activation of the α2/β2/γ3 heterotrimer could be depending on colocalization of this complex with the upstream regulators as has been seen for other kinases in the same kinase family (Hook & Means, 2001). Since AMP binding facilitates the phosphorylation of liver AMPK by LKB1, another explanatory scenario is that such an effect is particular favourable for LKB1 acting on the α2/β2/γ3 heterotrimer in human skeletal muscle.
Surprisingly, we were not able to reproduce the findings by Chen and coworkers of an increase in α1-associated AMPK activity during either of the two high-intensity exercise regimes of which one, the 30 s trial, was similar to the one used by Chen et al. (2000). A range of methodological differences may explain this discrepancy, e.g. the metabolic fitness of the subjects studied and perhaps the different antibodies used. In this regard our co-IP experiments suggest a high degree of α-isoform specificity of the two antibodies used in the present study. Furthermore, the total AMPK activity pulled down by these two antibodies adds up to the total activity measured in the β2 IP, indicating consistency within the activity data. Still, although we and others have not previously reported significant increases in α1-associated AMPK activity, the present data do not exclude that such activation may take place during different exercise regimes or in a different group of subjects.
Although, α2/β2/γ3 phosphorylation/activation may be related to total work performed in the present study, neither the rate of energy turnover, rate of glycogen degradation nor parameters of cellular energy balance (PCr, AMPfree or the PCr/(PCr + Cr) and AMPfree/ATP ratios) associates positively with α2/β2/γ3 phosphorylation/activity. This could indicate that the degree of phosphorylation/activation at any given moment is dependent on multiple signals that may have cumulative effects over time.
One immediate and important observation in this study is that although p-AMPK may reflect the total AMPK activity within a sample, such measurements may cover highly different regulation among the heterotrimers. In fact, this may bring some explanation to previous observations that regulation of AMPK (measured as p-AMPK or α-AMPK-associated activity) is not in accordance with regulation of ACCβ (Ser-221 phosphorylation) (Wojtaszewski et al. 2002, 2003; Roepstorff et al. 2005). For future reference, interpretation of such data should be done with caution. Interestingly, under the conditions applied here, ACCβ phosphorylation is correlated to, and may be considered a good marker of, α2/β2/γ3 activity in human skeletal muscle. Also this may imply that the α2/β2/γ3 heterotrimer regulates ACCβ phosphorylation during exercise and thus may be an actor in regulating fatty acid oxidation. It might seem contradictory to start regulating fatty acid oxidation in the high-intensity exercise regimens that require anaerobic energy turnover, primarily via glycogenolysis. However, even though fatty acid oxidation decreases with exercise intensity, there is an increase compared to rest even at high exercise intensities (Romijn et al. 1993; Dean et al. 2000). In addition, fatty acid oxidation is important in the post-exercise recovery period where restoration of the glycogen storage is highly prioritized. Phosphorylation of ACCβ may therefore increase the capacity for free fatty acid (FFA) oxidation if the muscle is continuously being used for work (although at a lower intensity) or for an optimal post-exercise recovery sparing carbohydrate oxidation.
In the present study we have performed analyses of the AMPK system in an in vivo setting using IP on whole muscle tissue. We do acknowledge that this method has some limitations. The use of tissue preparation opens up the possibility that some of the changes observed may have occurred in cell types other than muscle fibres present within the tissue. Also, we cannot exclude that the antibodies used for immunoprecipitation may, to a small extent, influence the activity measured in vitro and, although the results from Western blotting do indicate total immunoprecipitation efficiency, this may vary to a small extent between antibodies, giving rise to some variability.
Northern blot analyses of human mRNA reveal that the γ3 isoform has a highly tissue-specific expression, and is solely found in skeletal muscle (Cheung et al. 2000; Milan et al. 2000). In human vastus lateralis muscle, the α2/β2/γ3 heterotrimer only accounts for approximately one-fifth of all AMPK heterotrimers when measured in co-IP experiments (Wojtaszewski et al. 2005 and current study). This is further supported by the present observation that the α2/β2/γ3 heterotrimer contributes very little to total AMPK activity in resting muscle, similarly to observations made in rat muscle (Cheung et al. 2000; Durante et al. 2002). Interestingly, in human skeletal muscle exercise training has been shown to reduce mRNA and γ3 protein expression (Nielsen et al. 2003; Frosig et al. 2004; Wojtaszewski et al. 2005). As hypothesized, the present finding, that only γ3 heterotrimers are activated in response to exercise, is in accordance with the observation of attenuated AMPK activation during acute exercise after a period of exercise training, even when exercise is performed at the same relative intensity (Nielsen et al. 2003; Yu et al. 2003; Frosig et al. 2004; McConell et al. 2005).
The regulatory γ3 protein has received special interest, as naturally occurring mutations result in pronounced pathological alterations that may be linked with changes in glycogen metabolism (Milan et al. 2000; Barnes et al. 2004). In muscle lacking catalytic or regulatory AMPK isoforms, glycogen metabolism is also affected (Mu et al. 2003; Barnes et al. 2004; Jorgensen et al. 2004). In addition, AMPK activity in muscle is influenced by glucose and/or glycogen availability (Derave et al. 2000; Wojtaszewski et al. 2003; Jorgensen et al. 2004). Thus, although we do not fully understand the mechanisms, there seems to be a delicate association between AMPK and many aspects of glucose/glycogen metabolism in skeletal muscle. Whether the selective activation of α2/β2/γ3 during exercise is caused by alteration in glucose/glycogen metabolism or is a regulator of such metabolic processes remains to be seen in future studies.
AMPK in both liver and muscle is hypothesized to be a potential target for pharmacological treatment of diseases associated with insulin resistance, e.g. type 2 diabetes mellitus (Kahn et al. 2005). Pharmacological AMPK activation in resting muscle improves insulin action similarly to prior exercise or exercise training (Fisher et al. 2002; Iglesias et al. 2002). Thus, the present findings do not only relate to exercise, because drug refinement specifically targeting the γ3 complex may be a suitable way of improving drug-action specificity and perhaps decreasing adverse effects, since γ3 is only expressed in skeletal muscle.
Acknowledgments
Professor Erik A. Richter is acknowledged for taking part in the exercise experiments and for taking the muscle biopsies. Camilla Aunsholm Madsen, Dyval Steinman, Richard Evering, Kim Sjøberg and Bruno Bisiani are acknowledged for their helpful contribution recruiting and testing the subjects. We are grateful for the kind donation of the α1 and α2 AMPK antibodies from Professor DG Hardie and the anti-β1 antibody from Dr Margit Malhapuu (Arexis, Sweden). This study was supported by the Commission of the European Union (Contract No LSHM-CT-2004-005272 EXGENESIS), the Danish Medical Research Council, the Novo Nordisk Foundation, the Danish Diabetes Association and the Copenhagen Muscle Research Centre. J.F.P.W. was supported by a Hallas Møller fellowship from the Novo Nordisk Foundation.
References
- Adams J, Chen ZP, Van Denderen BJW, Morton CJ, Parker MW, Witters LA, Stapleton D, Kemp BE. Intrasteric control of AMPK via the γ1 subunit AMP allosteric regulatory site. Protein Sci. 2004;13:155–165. doi: 10.1110/ps.03340004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerstrom TC, Birk JB, Klein DK, Erikstrup C, Plomgaard P, Pedersen BK, Wojtaszewski J. Oral glucose ingestion attenuates exercise-induced activation of 5′-AMP-activated protein kinase in human skeletal muscle. Biochem Biophys Res Commun. 2006;342:949–955. doi: 10.1016/j.bbrc.2006.02.057. [DOI] [PubMed] [Google Scholar]
- Barnes BR, Marklund S, Steiler TL, Walter M, Hjalm G, Amarger V, Mahlapuu M, Leng Y, Johansson C, Galuska D, Lindgren K, Abrink M, Stapleton D, Zierath JR, Andersson L. The AMPK-gamma 3 isoform has a key role for carbohydrate and lipid metabolism in glycolytic skeletal muscle. J Biol Chem. 2004;279:38441–38447. doi: 10.1074/jbc.M405533200. [DOI] [PubMed] [Google Scholar]
- Bateman A. The structure of a domain common to archaebacteria and the homocystinuria disease protein. Trends Biochem Sci. 1997;22:12–13. doi: 10.1016/s0968-0004(96)30046-7. [DOI] [PubMed] [Google Scholar]
- Chen ZP, McConell GK, Michell BJ, Snow RJ, Canny BJ, Kemp BE. AMPK signaling in contracting human skeletal muscle: acetyl-CoA carboxylase and NO synthase phosphorylation. Am J Physiol Endocrinol Metab. 2000;279:E1202–E1206. doi: 10.1152/ajpendo.2000.279.5.E1202. [DOI] [PubMed] [Google Scholar]
- Chen ZP, Stephens TJ, Murthy S, Canny BJ, Hargreaves M, Witters LA, Kemp BE, McConell GK. Effect of exercise intensity on skeletal muscle AMPK signaling in humans. Diabetes. 2003;52:2205–2212. doi: 10.2337/diabetes.52.9.2205. [DOI] [PubMed] [Google Scholar]
- Cheung CF, Salt IP, Davies A, Hardie DG, Carling D. Characterization of AMP-activated protein kinase gamma subunit isoforms and their role in AMP binding. Biochem J. 2000;346:659–669. [PMC free article] [PubMed] [Google Scholar]
- Dean D, Daugaard JR, Young ME, Saha A, Vavvas D, Asp S, Kiens B, Kim KH, Witters L, Richter EA, Ruderman N. Exercise diminishes the activity of acetyl-CoA carboxylase in human muscle. Diabetes. 2000;49:1295–1300. doi: 10.2337/diabetes.49.8.1295. [DOI] [PubMed] [Google Scholar]
- Derave W, Ai H, Ihlemann J, Witters LA, Kristiansen S, Richter EA, Ploug T. Dissociation of AMP-activated protein kinase activation and glucose transport in contracting slow-twitch muscle. Diabetes. 2000;49:1281–1287. doi: 10.2337/diabetes.49.8.1281. [DOI] [PubMed] [Google Scholar]
- Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol. 2006;576:613–624. doi: 10.1113/jphysiol.2006.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durante PE, Mustard KJ, Park SH, Winder WW, Hardie DG. Effects of endurance training on activity and expression of AMP-activated protein kinase isoforms in rat muscles. Am J Physiol Endocrinol Metab. 2002;283:E178–E186. doi: 10.1152/ajpendo.00404.2001. [DOI] [PubMed] [Google Scholar]
- Fisher JS, Gao J, Han DH, Holloszy JO, Nolte LA. Activation of AMP kinase enhances sensitivity of muscle glucose transport to insulin. Am J Physiol Endocrinol Metab. 2002;282:E18–E23. doi: 10.1152/ajpendo.2002.282.1.E18. [DOI] [PubMed] [Google Scholar]
- Frosig C, Jorgensen SB, Hardie DG, Richter EA, Wojtaszewski JF. 5′-AMP-activated protein kinase activity and protein expression are regulated by endurance training in human skeletal muscle. Am J Physiol Endocrinol Metab. 2004;286:E411–E417. doi: 10.1152/ajpendo.00317.2003. [DOI] [PubMed] [Google Scholar]
- Fujii N, Hayashi T, Hirshman MF, Smith TJ, Habinowski SA, Kaijser L, Mu J, Ljungqvist O, Birnbaum MJ, Witters LA, Thorell A, Goodyear LJ. Exercise induces isoform specific increase in 5′AMP-activated protein kinase activity in human skeletal muscle. Biochem Biophys Res Commun. 2000;273:1150–1155. doi: 10.1006/bbrc.2000.3073. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Salt IP, Hawley SA, Davies SP. AMP-activated protein kinase: an ultrasensitive system for monitoring cellular energy charge. Biochem J. 1999;338:717–722. [PMC free article] [PubMed] [Google Scholar]
- Hardie DG, Scott JW, Pan DA, Hudson ER. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 2003;546:113–120. doi: 10.1016/s0014-5793(03)00560-x. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRADα/β and MO25α/β are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem. 1996;271:27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-β is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Hook SS, Means AR. Ca2+/CaM-dependent kinases: From activation to function. Annu Rev Pharmacol Toxicol. 2001;41:471–505. doi: 10.1146/annurev.pharmtox.41.1.471. [DOI] [PubMed] [Google Scholar]
- Hudson ER, Pan DA, James J, Lucocq JM, Hawley SA, Green KA, Baba O, Terashima T, Hardie DG. A novel domain in AMP-activated protein kinase causes glycogen storage bodies similar to those seen in hereditary cardiac arrhythmias. Curr Biol. 2003;13:861–866. doi: 10.1016/s0960-9822(03)00249-5. [DOI] [PubMed] [Google Scholar]
- Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem. 2005;280:29060–29066. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- Iglesias MA, Ye JM, Frangioudakis G, Saha AK, Tomas E, Ruderman NB, Cooney GJ, Kraegen EW. AICAR administration causes an apparent enhancement of muscle and liver insulin action in insulin-resistant high-fat-fed rats. Diabetes. 2002;51:2886–2894. doi: 10.2337/diabetes.51.10.2886. [DOI] [PubMed] [Google Scholar]
- Jorgensen SB, Nielsen JN, Birk JB, Olsen GS, Viollet B, Andreelli F, Schjerling P, Vaulont S, Hardie DG, Hansen BF, Richter EA, Wojtaszewski JF. The α2-5′AMP-activated protein kinase is a site 2 glycogen synthase kinase in skeletal muscle and is responsive to glucose loading. Diabetes. 2004;53:3074–3081. doi: 10.2337/diabetes.53.12.3074. [DOI] [PubMed] [Google Scholar]
- Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Langfort J, Viese M, Ploug T, Dela F. Time course of GLUT4 and AMPK protein expression in human skeletal muscle during one month of physical training. Scand J Med Sports. 2003;13:169–174. doi: 10.1034/j.1600-0838.2003.20120.x. [DOI] [PubMed] [Google Scholar]
- Lawson JW, Veech RL. Effects of pH and free Mg2+ on the Keq of the creatine kinase reaction and other phosphate hydrolyses and phosphate transfer reactions. J Biol Chem. 1979;254:6528–6537. [PubMed] [Google Scholar]
- Lee-Young RS, Palmer MJ, Linden KC, Leplastrier K, Canny BJ, Hargreaves M, Wadley GD, Kemp BE, McConell GK. Carbohydrate ingestion does not alter skeletal muscle AMPK signaling during exercise in humans. Am J Physiol Endocrinol Metab. 2006;291:E566–E573. doi: 10.1152/ajpendo.00023.2006. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Passonneau JV. A Flexible System of Enzymatic Analysis. London: Academic Press, Inc.; 1972. pp. 1–291. [Google Scholar]
- Mannion AF, Jakeman PM, Willan PL. Determination of human skeletal muscle buffer value by homogenate technique: methods of measurement. J Appl Physiol. 1993;75:1412–1418. doi: 10.1152/jappl.1993.75.3.1412. [DOI] [PubMed] [Google Scholar]
- McConell GK, Lee-Young RS, Chen ZP, Stepto NK, Huynh NN, Stephens TJ, Canny BJ, Kemp BE. Short-term exercise training in humans reduces AMPK signalling during prolonged exercise independent of muscle glycogen. J Physiol. 2005;568:665–676. doi: 10.1113/jphysiol.2005.089839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell BJ, Stapleton D, Mitchelhill KI, House CM, Katsis F, Witters LA, Kemp BE. Isoform-specific purification and substrate specificity of the 5′-AMP-activated protein kinase. J Biol Chem. 1996;271:28445–28450. doi: 10.1074/jbc.271.45.28445. [DOI] [PubMed] [Google Scholar]
- Milan D, Jeon JT, Looft C, Amarger V, Robic A, Thelander M, Rogel-Gaillard C, Paul S, Iannuccelli N, Rask L, Ronne H, Lundstrom K, Reinsch N, Gellin J, Kalm E, Roy PL, Chardon P, Andersson L. A mutation in PRKAG3 associated with excess glycogen content in pig skeletal muscle. Science. 2000;288:1248–1251. doi: 10.1126/science.288.5469.1248. [DOI] [PubMed] [Google Scholar]
- Mu J, Barton ER, Birnbaum MJ. Selective suppression of AMP-activated protein kinase in skeletal muscle: update on ‘lazy mice’. Biochem Soc Trans. 2003;31:236–241. doi: 10.1042/bst0310236. [DOI] [PubMed] [Google Scholar]
- Musi N, Hirshman MF, Nygren J, Svanfeldt M, Bavenholm P, Rooyackers O, Zhou G, Williamson JM, Ljunqvist O, Efendic S, Moller DE, Thorell A, Goodyear LJ. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002;51:2074–2081. doi: 10.2337/diabetes.51.7.2074. [DOI] [PubMed] [Google Scholar]
- Nielsen JN, Mustard KJ, Graham DA, Yu H, MacDonald CS, Pilegaard H, Goodyear LJ, Hardie DG, Richter EA, Wojtaszewski JF. 5′-AMP-activated protein kinase activity and subunit expression in exercise-trained human skeletal muscle. J Appl Physiol. 2003;94:631–641. doi: 10.1152/japplphysiol.00642.2002. [DOI] [PubMed] [Google Scholar]
- Polekhina G, Gupta A, Michell BJ, van Denderen B, Murthy S, Feil SC, Jennings IG, Campbell DJ, Witters LA, Parker MW, Kemp BE, Stapleton D. AMPK β subunit targets metabolic stress sensing to glycogen. Curr Biol. 2003;13:867–871. doi: 10.1016/s0960-9822(03)00292-6. [DOI] [PubMed] [Google Scholar]
- Rasmussen BB, Winder WW. Effect of exercise intensity on skeletal muscle malonyl-CoA and acetyl-CoA carboxylase. J Appl Physiol. 1997;83:1104–1109. doi: 10.1152/jappl.1997.83.4.1104. [DOI] [PubMed] [Google Scholar]
- Roepstorff C, Halberg N, Hillig T, Saha AK, Ruderman NB, Wojtaszewski JF, Richter EA, Kiens B. Malonyl-CoA and carnitine in regulation of fat oxidation in human skeletal muscle during exercise. Am J Physiol Endocrinol Metab. 2005;288:E133–E142. doi: 10.1152/ajpendo.00379.2004. [DOI] [PubMed] [Google Scholar]
- Romijn JA, Coyle EF, Sidossis LS, Gastaldelli A, Horowitz JF, Endert E, Wolfe RR. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol Endocrinol Metab. 1993;265:E380–E391. doi: 10.1152/ajpendo.1993.265.3.E380. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Goransson O, Hardie DG, Alessi DR. Activity of LKB1 and AMPK-related kinases in skeletal muscle: Effects of contraction, phenformin, and AICAR. Am J Physiol Endocrinol Metab. 2004;287:E310–E317. doi: 10.1152/ajpendo.00074.2004. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, McCarthy A, Smith D, Green KA, Grahame HD, Ashworth A, Alessi DR. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J. 2005;24:1810–1820. doi: 10.1038/sj.emboj.7600667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt I, Celler JW, Hawley SA, Prescott A, Woods A, Carling D, Hardie DG. AMP-activated protein kinase: greater AMP dependence, and preferential nuclear localization, of complexes containing the α2 isoform. Biochem J. 1998;334:177–187. doi: 10.1042/bj3340177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor EB, Hurst D, Greenwood LJ, Lamb JD, Cline TD, Sudweeks SN, Winder WW. Endurance training increases LKB1 and MO25 protein but not AMP-activated protein kinase kinase activity in skeletal muscle. Am J Physiol Endocrinol Metab. 2004;287:E1082–E1089. doi: 10.1152/ajpendo.00179.2004. [DOI] [PubMed] [Google Scholar]
- Winder WW, Hardie DG. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol Endocrinol Metab. 1996;270:E299–E304. doi: 10.1152/ajpendo.1996.270.2.E299. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JFP, Birk JB, Frosig C, Holten M, Pilegaard H, Dela F. 5′AMP activated protein kinase expression in human skeletal muscle: effects of strength training and type 2 diabetes. J Physiol. 2005;564:563–573. doi: 10.1113/jphysiol.2005.082669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtaszewski JFP, Macdonald C, Nielsen JN, Hellsten Y, Hardie DG, Kemp BE, Kiens B, Richter EA. Regulation of AMPK actvity and sustrate utilization in exercising human skeletal muscle. Am J Physiol Endocrinol Metab. 2003;17:E813–E822. doi: 10.1152/ajpendo.00436.2002. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, Mourtzakis M, Hillig T, Saltin B, Pilegaard H. Dissociation of AMPK activity and ACCβ phosphorylation in human muscle during prolonged exercise. Biochem Biophys Res Commun. 2002;298:309–316. doi: 10.1016/s0006-291x(02)02465-8. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JFP, Nielsen P, Hansen BF, Richter EA, Kiens B. Isoform-specific and exercise intensity-dependent activation of 5′AMP-activated protein kinase in human skeletal muscle. J Physiol. 2000;528:221–226. doi: 10.1111/j.1469-7793.2000.t01-1-00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Vertommen D, Neumann D, Turk R, Bayliss J, Schlattner U, Wallimann T, Carling D, Rider MH. Identification of phosphorylation sites in AMP-activated protein kinase (AMPK) for upstream AMPK kinases and study of their roles by site-directed mutagenesis. J Biol Chem. 2003;278:28434–28442. doi: 10.1074/jbc.M303946200. [DOI] [PubMed] [Google Scholar]
- Yu M, Stepto NK, Chibalin AV, Fryer LG, Carling D, Krook A, Hawley JA, Zierath JR. Metabolic and mitogenic signal transduction in human skeletal muscle after intense cycling exercise. J Physiol. 2003;546:327–335. doi: 10.1113/jphysiol.2002.034223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]