Abstract
Vestibular compensation (the behavioural recovery that follows unilateral vestibular de-afferentation), is facilitated by histamine, and is associated with increased central histamine release and alterations in histamine H3 receptor expression in the vestibular nuclei. However, little is known of the effects of histamine on neurotransmission in the vestibular nuclei, and the mechanisms by which histamine may influence compensation are unclear. Here we examined the modulatory effects of histaminergic agents on the release of amino acid neurotransmitters in slices of the medial vestibular nucleus (MVN) prepared from normal and labyrinthectomised rats. The release of GABA, but not glutamate, glycine or aspartate, was robustly and reproducibly evoked by a high-K+ stimulus applied to normal MVN slices. Histamine inhibited the evoked release of GABA, both through a direct action on presynaptic H3 receptors (presumably located on GABAergic terminals), and through a novel, indirect pathway that involved the increased release of glycine by activation of postsynaptic H1/H2 receptors (presumably on glycinergic neurons). After unilateral labyrinthectomy (UL), the direct H3 receptor-mediated inhibition of GABA release was profoundly downregulated in both ipsi-lesional and contra-lesional MVNs. This effect appeared within 25 h post-UL and persisted for at least 3 weeks post-UL. In addition, at 25 h post-UL the indirect glycinergic pathway caused a marked suppression of GABA release in the contra-lesional but not ipsi-lesional MVN, which was overcome by strychnine. Stimulation of histamine H3 receptors at 25 h post-UL restored contra-lesional GABA release to normal, suggesting that acutely after UL H3 receptors may strongly modulate glycinergic and GABAergic neurotransmission in the MVN. These findings are the first to demonstrate the modulatory actions of the histaminergic system on neurotransmission in the vestibular nuclei, and the changes that occur during vestibular system plasticity. During vestibular compensation, histaminergic modulation of glycine and GABA release may contribute to the rebalancing of neural activity in the vestibular nuclei of the lesioned and intact sides.
Histaminergic drugs have long been used to treat disorders of the vestibular system in man (Fischer, 1991; Lacour & Sterkers, 2001), but the physiological mechanisms by which histamine modulates vestibular function remain largely unknown. Neurons in the medial vestibular nucleus (MVN) receive histaminergic afferents from the tuberomamillary nucleus of the posterior hypothalamus (Airaksinen & Panula, 1988; Steinbusch, 1991), and express postsynaptic H1 and H2 receptors as well as presynaptic H3 receptors (Airaksinen & Panula, 1988; Pollard et al. 1993; Pillot et al. 2002). Histaminergic agents modulate the activity of MVN neurons in vitro (Phelan et al. 1990; de Waele et al. 1992; Serafin et al. 1993; Wang & Dutia, 1995), and also modulate vestibular reflex function in vivo (Yabe et al. 1993). Intriguingly, imbalanced activity within the vestibular system itself can activate the central histaminergic system, as shown by increased histamine release in the hypothalamus in response to unilateral electrical or caloric stimulation of the labyrinth (Horii et al. 1993), and by increased expression of histidine decarboxylase mRNA in the tuberomamillary nuclei after unilateral vestibular neurectomy in cats (Tighilet et al. 2006).
Of particular interest is the role of histamine in facilitating neuronal and synaptic plasticity in the vestibular nuclei and related structures during ‘vestibular compensation’ (VC), the behavioural recovery that follows the loss of one vestibular labyrinth. Recent studies have suggested that after unilateral labyrinthectomy (UL), changes in the functional efficacy of GABA and glycine receptors on the deafferented MVN neurons, changes in their intrinsic membrane excitability, as well as a re-organization of synaptic connections within the vestibular nuclei of the two sides, are involved in VC (for reviews see Straka et al. 1994, Straka et al. 2005; Darlington et al. 2002). A number of behavioural studies have indicated that histamine facilitates VC, through mechanisms that are presently unknown (for review see Bergquist & Dutia, 2006). Thus betahistine, a H3 receptor antagonist and weak H1 receptor agonist (Van Cauwenberge & De Moor, 1997), or the more selective H3 receptor antagonist thioperamide, attenuate the initial symptoms of barrel-rolling after UL in the rat (Pan et al. 1998). In the cat, the rate of behavioural recovery after UL is accelerated by betahistine, albeit at high doses (Tighilet et al. 1995), as well as by thioperamide (Tighilet et al. 2006). These effects may be mediated in part by increased release of histamine within the vestibular nuclear complex, since H3 receptor antagonists increase histamine release in the brain (Arrang et al. 1983; review in Hancock, 2003; Leurs et al. 2005). The involvement of presynaptic H3 receptors is also indicated by the finding that H3 receptor mRNA expression (Lozada et al. 2004) and H3 receptor binding (Tighilet et al. 2006) change during vestibular compensation. Evidence for an increased release of histamine in the vestibular nuclei after UL has been provided by Tighilet & Lacour (1997), who demonstrated a depletion of histamine immunoreactivity in the vestibular nuclei during VC.
While H3 receptors were initially looked upon as autoreceptors, it has recently become evident that they also act as presynaptic heteroreceptors, inhibiting the release of other neurotransmitters in the brain (see Leurs et al. 2005 for review). H3 receptors display considerable heterogeneity, with functionally different splice variants (Wellendorph et al. 2002), making them potentially interesting and versatile candidates for presynaptic regulation of synaptic efficacy during brain plasticity. At present, however, there are no data on how histaminergic receptors regulate neurotransmitter release in the vestibular nuclei, so the physiological implications of the changes in MVN H3 receptor expression during VC are unknown. Here we have investigated the effect of histamine and histaminergic drugs on the release of neuroactive amino acids from slices of the MVN prepared from normal rats, and from animals that underwent VC for 25 h, 1 week and 3 weeks after UL, to elucidate the physiological actions of histamine on neurotransmitter release in the normal MVN, and the changes that occur during VC.
Methods
Drugs and chemicals
On the day of experiment drugs applied to the slices were dissolved in de-ionised water and diluted in filtered artificial cerebrospinal fluid (aCSF, consisting of (mm): 123 NaCl, 5 KCl, 2.4 CaCl2, 1.3 MgSO4, 26 NaHCO3, 1.2 KH2PO4, 10 d-glucose and 100 μm glutamine) or in a high-potassium modified aCSF where 54 mm NaCl was replaced with KCl resulting in a total K+ concentration of 60 mm. In experiments with zero calcium aCSF, CaCl2 was replaced with MgSO4. The following drugs were used: histamine, clobenpropit dihydrobromide, strychnine (Sigma Aldrich Ltd, Dorset, UK), and immepip dihydrobromide (Tocris Cookson Ltd, Bristol, UK). HPLC mobile phases were made from de-ionised filtered water, luminescence-quality ammonium acetate and HPLC-grade acetonitrile and methanol. The mobile phases were filtered and degassed through a 0.2 μm Anodisc membrane, and were used within a week of preparation.
Animals
Experimental design and procedures were carried out in compliance with the UK Animal (Scientific Procedures) Act 1986. Male Lister-hooded rats (150 g, 5–6 weeks of age, Charles River Ltd, UK) were housed 4–8 animals per cage under standard controlled environmental conditions.
Unilateral labyrinthectomy, UL
Animals were anaesthetised with halothane and injected with carprofen, 5 mg kg−1, s.c., for postsurgical analgesia. After local anaesthesia with 1% lidocaine hydrochloride, a left paramedian incision was made to expose the lamboidal ridge and the external ear canal. The external ear canal was opened just anterior to the exit point of the facial nerve. The tympanic membrane was opened at its caudal hemicircumference. A 0.7 mm drill was used to expose and fenestrate the most anterior part of the horizontal semicircular canal, by drilling into the caudal wall of the medial ear lateral and superior to the oval window close to the ceiling of the facial nerve canal. The opened horizontal canal was followed anteriorly and ventrally into the orifice of the vestibulum, where a 30 gauge needle was used to mechanically remove the epithelial lining and to aspirate all the contents. This was followed by instillation of 99% ethanol, which was aspirated before the bone was covered with fascia, and the skin was sutured.
Slice preparation
After decapitation under deep halothane anaesthesia, a block of brain tissue extending from the caudal half of the cerebrum to the first millimetres of the spinal cord was rapidly removed and placed in chilled aCSF continuously gassed with 95% O2–5% CO2, pH 7.4, and kept on ice. The tissue block was trimmed rostral and caudal to the cerebellum, and the cerebellum removed to expose the floor of the fourth ventricle. The brainstem was mounted ventral side down, and a 450 μm slice containing the two medial vestibular nuclei (MVNs) was made with a vibratome (World Precision Instruments Ltd, Hertfordshire, UK). The two MVN slices were separated along the midline and trimmed at their caudal, rostral and lateral borders. The MVNs were transferred to incubation chambers and superfused with 95% O2–5% CO2-gassed aCSF at room temperature for 30 min, and then at 32°C for 30 min before baseline sampling of the superfusate was started.
Superfusion experiments
The left and the right MVN were superfused at 32°C in separate chambers submerged in a reservoir of continuously oxygenated aCSF. A peristaltic pump (Minipump 3, Gilson Inc., Villiers le Bel, France) was used to pull aCSF through the chambers to a fraction collector (FC203B, Gilson Inc., Villiers le Bel, France) at a flow rate of 0.104 ml min−1. Fractions were collected every 5 min. Over the first 30 min, every second fraction was analysed to obtain the baseline outflow of amino acids from the slice. To change superfusion composition, the pump was stopped for a few seconds, during which time the chamber was manually transferred to a different reservoir containing the intended aCSF solution. The perfusion protocol included one or two short stimuli with histaminergic drugs given alone or together with high-K+ aCSF. The stimulus was given for 4 min, and care was taken to match the entire stimulus to one sample collected by the fraction collector. In the 15 min immediately following a stimulus, every 5 min fraction was taken for analysis. In some experiments the slices were pretreated with histamine antagonists, or Ca2+-free aCSF, which started 20 min before the short stimulus, and continued throughout the stimulus. A single high-K+ stimulus was always given before ending the experiment to confirm the viability of the slice. At the end of the experiment the wet tissue weight of each slice was determined. Slices that released less than 5 fmol GABA (mg wet tissue)−1 in response to the final high-K+ stimulus were not considered viable and were excluded from further analysis. This criterion gave an overall exclusion ratio of 13%.
HPLC analysis of amino acids
Amino acids were derivatized with o-phthaldialdehyde (OPA), separated and detected by HPLC followed by fluorescence detection. The equipment consisted of a cooled autosampler (AS-1559, Jasco Ltd, Great Dunmow, UK), a binary high-pressure gradient pump system (PU-2080, Jasco Ltd, Great Dunmow, UK), a column block heater (model 7971, Jones Chromatography Ltd, Mid Glamorgan, UK), and a fluorescence detector (FD-2020Plus, Jasco Ltd, Great Dunmow, UK) with excitation and emission wavelengths set at 340 nm and 455 nm, respectively. The signal from the detector was recorded and integrated with the Chrompass Chromatography software package (Jasco Inc, Japan).
The sample to be analysed (10 μl) was mixed with 4 μl of an OPA derivatisation reagent in an automated sequence before injection on the column. The OPA reagent was prepared fresh on the day of run by adding 3 μl of 2-mercapto-ethanol to 1 ml OPA reagent incomplete (both Sigma-Aldrich, UK) and was stored at 2°C for no more than 20 h. The derivatisation mixture was allowed to react in a separate vial for 1 min at 2°C and 12 μl of the resulting product was then injected on a reverse-phase Chromolith Speedrod column, 50 × 4.6 mm, coupled in series with a Chromolith Performance column, 100 × 4.6 mm (Merck KGaA, Germany). The OPA-2-mercapto-ethanol-amino acid derivates were then eluted in a linear gradient made up from two mobile phases, A and B, as described in Table 1. Mobile phase A contained 95% 50 mm ammonium acetate (adjusted to pH 5.5 with 50% acetic acid), 4% methanol and 1% acetonitrile. Mobile phase B contained 40% 50 mm ammonium acetate (pH 5.5), 20% methanol and 40% acetonitrile). With this protocol, aspartate (ASP), glutamate (GLU), glycine (GLY), taurine (TAU) and GABA were eluted within approximately 6 min. Except for GLY, which partly co-eluted with threonine, all measured amino acids were completely separated from other peaks both in a multiple amino acid standard (Sigma-Aldrich, UK) and in samples obtained from slice superfusates, rat brain tissue homogenates and microdialysates. The system was calibrated daily and monitored regularly with external standards at two levels containing 0.5 μm or 1 μm ASP, GLU, GLY, TAU and 0.05 μm or 0.1 μm GABA.
Table 1.
Gradient profile for eluting OPA-2-mercapto-ethanol-amino acid derivates
| Time (min) | Mobile phase A (%) | Mobile phase B (%) | Flow (ml min−1) |
|---|---|---|---|
| Pre-run | 100 | 0 | 2 |
| 0.1 | 75 | 25 | 2 |
| 6.0 | 33 | 67 | 2 |
| 6.1 | 0 | 100 | 4 |
| 7.6 | 0 | 100 | 4 |
| 7.7 | 100 | 0 | 4 |
| 8.5 | 100 | 0 | 4 |
A linear gradient going from 25% to 67% mobile phase B was run over 6 min. This was followed by a 100% B high-flow period to wash out late-eluting peaks, and the column was then conditioned with approximately four column volumes of mobile phase A. The pre-run period includes a derivatisation and injection procedure and adds 5 min to the total cycle time.
Calculations and statistics
The amino acid outflow (fmol mg−1) of each fraction was calculated by multiplying the measured concentration (fmol μl−1) with the corresponding superfusion volume (520 μl), and dividing by the wet tissue weight (mg). The mean baseline outflow rate was calculated as the mean of samples 2 and 3. When the initial response to a stimulus was positive in relation to baseline, the evoked outflow for each stimulus was calculated by determining the maximum area under the curve over the following 20 min after subtracting the baseline. When the initial response was negative relative to baseline, a negative area was calculated instead using the same algorithm. Statistical analysis was performed using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, California, USA). Group comparisions were made with one-way ANOVA. When appropriate, post hoc tests were performed using the Sidak–Holm step-down multiple comparisions procedure, which avoids type I errors while preserving statistical power (Ludbrook, 1998). The Sidak–Holm adjusted P values were calculated in spreadsheets using the formula
where i is the number of P values greater than or equal to the P value being adjusted.
Results
Characterisation of amino acid release from slices of the medial vestibular nucleus from normal animals
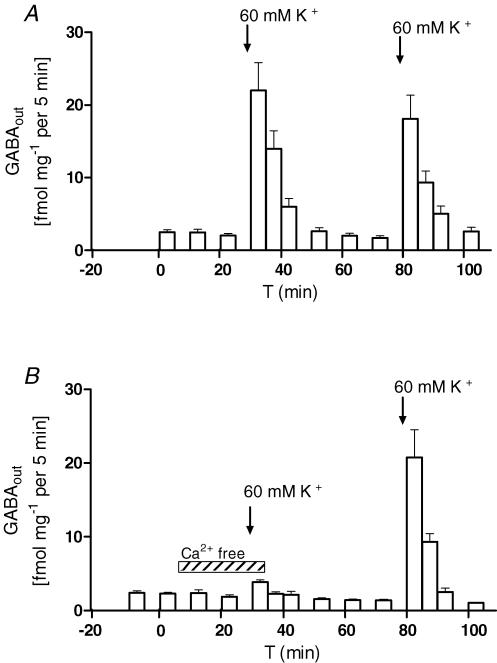
The release of amino acids from MVN slices prepared from normal animals was measured under baseline conditions and in response to a 4 min stimulus of high-K+ aCSF (see Methods). The baseline release of GABA, GLU, ASP and GLY was significantly decreased in Ca2+-free aCSF, while the baseline TAU outflow was not affected (Table 2). In response to the high-K+ stimulus, there was a large and repeatable increase in GABA release (Fig. 1A), which was inhibited by >90% in Ca2+-free aCSF (Fig. 1B, Table 2). By contrast, the K+ stimulus evoked small and insignificant increases in the outflow of TAU, and highly variable changes in the release of GLY, GLU and ASP, with either an increase or a decrease compared to baseline. As a consequence, the mean K+-evoked responses of GLY, GLU, and ASP were not significantly different from baseline (Table 2).
Table 2.
Baseline outflow and K+-evoked release of amino acids from MVN slices during normal or Ca2+-depleted conditions
| Baseline outflow Normal aCSF (fmol mg−1 min−1) (n = 45–53) | Ca2+-free aCSF (% change in baseline)b (n = 7) | K+-evoked release Normal aCSF (fmol mg−1) (n = 10) | Ca2+-free aCSF (fmol mg−1) (n = 7) | |
|---|---|---|---|---|
| GABA | 2.4 ± 0.1 | −30 ± 6** | 36 ± 5††† | 2.4 ± 0.6‡‡‡ |
| GLU | 46 ± 5 | −61 ± 11** | 17 ± 11 | 3.7 ± 4.7 |
| GLY | 82 ± 5 | −50 ± 4*** | 6 ± 20 | 13 ± 8 |
| ASP | 23 ± 2 | −55 ± 13* | −18 ± 11 | 1.6 ± 2.3 |
| TAU | 6.9 ± 0.6 | −8.6 ± 4.2 | 6.4 ± 3.1 | 7.8 ± 5.2 |
a Accumulated outflow over 20 min, baseline outflow subtracted. First stimulus, S1.
Within-slice comparison.
Significantly different from corresponding baseline P < 0.05, P < 0.01 and P < 0.001 rfespectively, one-way ANOVA (F = 19.4, d.f. = 9) followed by Sidak–Holm adjusted t tests.
Significantly different from 0.0 in one-sample t tests at level P < 0.001. P values adjusted for multiple comparison with the Sidak–Holm method.
Significantly different from K+-evoked outflow in normal aCSF at level P < 0.001, unpaired t test with Sidak–Holm adjusted P values.
Figure 1. K+-evoked GABA release is Ca2+ dependent.
A, GABA release (GABAout, mean ±s.e.m.) in normal aCSF, and in response to two 4 min stimuli with 60 mm K+ aCSF (arrows indicate start of stimuli), n = 10. B, calcium dependency of baseline and K+-evoked GABA overflow, n = 7. Calcium-depleted conditions are indicated by the hatched horizontal bar and the arrows indicate start of K+ stimuli.
Histamine inhibits K+-evoked GABA release through H3 receptor activation
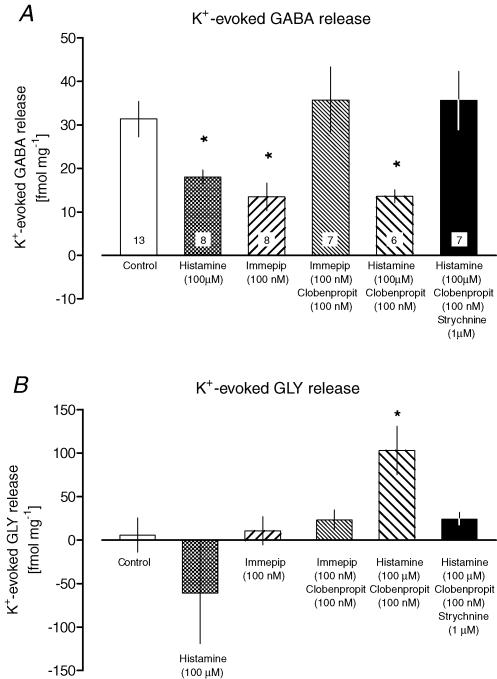
The effects of histamine on the K+-evoked GABA release were investigated by including histamine (100 μm) in the high-K+ stimulus pulse. In the presence of histamine, the mean K+-evoked GABA release was reduced to about half of that in controls (18.1 ± 1.6 fmol mg−1versus 31.3 ± 4.0 fmol mg−1, Fig. 2A). Histamine alone did not induce detectable changes in baseline outflow of any of the amino acids (n = 4; data not shown).
Figure 2. Histaminergic modulation of evoked GABA and glycine release.
A, histaminergic modulation of K+-evoked GABA release (accumulated release, fmol mg−1, mean ±s.e.m.). The agonists histamine and immepip were only present during the 4 min-long 60 mm K+ stimulus. The antagonists clobenpropit and strychnine were introduced 20 min before the K+ stimulus. One-way ANOVA (F = 4.85, d.f. = 5, P = 0.0013) was followed by Sidak–Holm adjusted t tests. *P < 0.05 compared to control. Each experiment was repeated in 6–13 slices, as indicated in the bars. B, histaminergic modulation of K+-evoked glycine (GLY) release. Bars show the GLY release in response to the 4 min-long 60 mm K+ stimulus, mean ±s.e.m., during treatment with histaminergic drugs and strychnine as described in A. One way ANOVA (F = 2.73, d.f. = 5, P = 0.0313) was followed by Sidak-Holm adjusted t tests. *P < 0.05 compared to control. Data obtained from the same slices as in A (n = 6–13, see A).
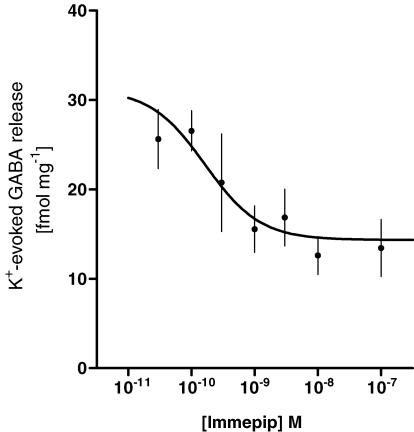
To determine whether the inhibition of evoked GABA release by histamine was due to its action on presynaptic H3 receptors, the selective H3 agonist immepip (0.03–100 nm) was included in the K+ stimulus. This resulted in a dose-dependent inhibition of K+-evoked GABA release as shown in Fig. 3. The IC50 of immepip, determined from this dose–response experiment, was 0.2 nm (95% confidence interval: 0.03–1 nm). The inhibition reached a plateau at approximately 50% of control release at immepip concentrations of 10–100 nm.
Figure 3. Dose-dependent inhibition of evoked GABA release by immepip.
The inhibitory effect of the H3 agonist immepip on K+-evoked GABA release was investigated in an accumulated dose–response, where increasing doses of immepip were presented in two successive K+ stimuli before confirming the viability of the slice with a third stimulus. Non-linear regression, sigmoidal dose–response with top restraint <31.2 fmol mg−1 (which was the mean response of stimulus 1 and 2 in control experiments). R2= 0.3328, n = 3–9 for each tested concentration.
The inhibitory effect of 100 nm immepip on K+-evoked GABA release was fully reversed by adding the selective H3-receptor reverse agonist clobenpropit (100 nm) to the K+ stimulus (Fig. 2A). When given alone, clobenpropit did not alter the K+-evoked GABA release as compared to control slices (n = 7; data not shown).
H1/H2 receptor activation also inhibits evoked GABA release
Since the maximum inhibition of K+-evoked GABA release obtained with immepip was similar to the inhibition seen with histamine, H3-receptor activation may explain most of the inhibitory effects of histamine. This hypothesis was investigated by pretreating MVN slices with clobenpropit before introducing the combined histamine and high-K+ stimulus. At the concentrations used here (100 nm), clobenpropit should inhibit H3 receptors selectively, leaving H1 and H2 receptors unaffected (Barnes et al. 1993). Unexpectedly, histamine reduced the evoked GABA release to about half of that in controls even after pretreatment with clobenpropit (Fig. 2A).
The H1/H2 receptor-mediated inhibition of evoked GABA release is strychnine sensitive
Pretreatment with 100 nm clobenpropit followed by the combined histamine and high-K+ stimulus was the only experimental condition that evoked a significant increase in GLY release (Fig. 2B). We investigated the effects of adding 1 μm strychnine to the clobenpropit pretreatment, to test the possibility that increased glycinergic transmission was involved in H1/H2 receptor-mediated inhibition of GABA release. The combined pretreatment completely abolished the inhibitory effect of histamine on evoked GABA release (Fig. 2A). Evoked GABA release was unaltered by pretreatment with strychnine alone (n = 7, data not shown).
Effects of unilateral and bilateral labyrinthectomy on amino acid release and on H3-mediated inhibition of GABA release
To determine if the baseline release of amino acids or the K+-evoked GABA release from the MVN is altered during vestibular compensation, we investigated MVN slices prepared from animals that had undergone either a bilateral labyrinthectomy 25 h earlier, or a unilateral (left) labyrinthectomy 25 h, 1 week or 3 weeks earlier. The left and right MVNs were superfused separately to reveal any lateral differences due to de-afferentation.
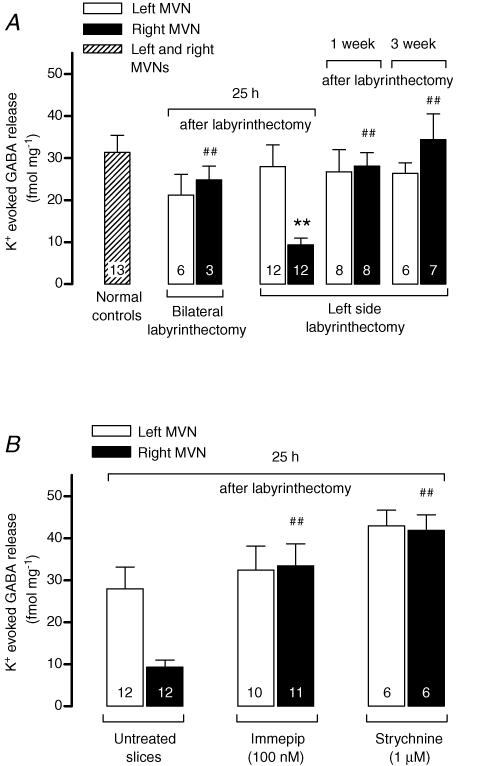
The baseline release of GABA was not significantly different from normal at any time after UL (Table 3). In MVN slices from bilaterally labyrinthectomised animals, the mean K+-evoked GABA release was lower than normal, but this difference did not reach significance (Fig. 4A). A marked asymmetry in evoked GABA release between the ipsi-lesional and contra-lesional MVNs was found in slices from 25 h post-UL animals, with the release from the contra-lesional MVN being significantly lower than normal (Fig. 4A). At later time-points (1 and 3 weeks post-UL), evoked GABA release in the contra-lesional MVN had recovered to the normal level, and there was no longer a significant difference in evoked GABA release from either the ipsi-or contra-lesional MVNs compared to that from normal slices (Fig. 4A).
Table 3.
Baseline release of amino acids (fmol mg−1 (fraction)−1) from MVN slices prepared from normal animals or from animals at different stages of compensation after vestibular de-afferentation
| Normal (n = 45–53) | BL, 25 h (n = 7) | UL, 25 h (n = 49) | UL, 1 week (n = 16) | UL, 3 weeks (n = 16) | |
|---|---|---|---|---|---|
| GABA | 2.4 ± 0.1 | 2.7 ± 0.2 | 2.8 ± 0.2 | 2.4 ± 0.2 | 2.4 ± 0.2 |
| GLU | 45.6 ± 4.9 | 20.3 ± 2.9 | 38.8 ± 2.5 | 28.3 ± 3.4 | 28.4 ± 3.5 |
| GLY | 81.6 ± 4.8 | 35.4 ± 1.8* | 63.4 ± 4.7 | 43.2 ± 5.1** | 36.6 ± 1.6** |
| ASP | 23.4 ± 2.2 | 8.6 ± 0.9 | 11.3 ± 0.9** | 10.3 ± 1.7* | 10.2 ± 2.3* |
| TAU | 6.9 ± 0.6 | 5.2 ± 0.5 | 9.5 ± 1.0 | 5.8 ± 0.7 | 4.0 ± 0.3 |
BL, bilateral labyrinthectomy; UL, unilateral labyrinthectomy. The baseline releases from the left and right side. MVNs were pooled since paired t tests revealed no differences between sides at any time point after UL or BL. Pooled data analysed by one-way ANOVA (P < 0.0001, F = 54.4, d.f. = 24) followed by Sidak–Holm adjusted t tests
*P < 0.05
P < 0.01 compared to baseline release in normal slices. Data from normal slices also displayed in Table 2.
Figure 4. K+-evoked GABA release (mean ±s.e.m) from vestibular slices of labyrinthectomised animals.
The number of slices included in each group is indicated inside the bars. The effects of labyrinthectomies (A) and of drug treatments of slices from 25 h post-UL animals (B) were analysed together by one-way ANOVA (P = 0.0003, F = 3.472, d.f. = 12) followed by Sidak–Holm corrected t tests. **P < 0.01 compared to normal controls, ##P < 0.01 compared to untreated contra-lesional slices prepared 25 h after UL. For clarity the untreated 25 h post-UL slices are shown in B as well as in A.
The baseline release of GLY was significantly lower than normal in slices from animals that received a bilateral labyrinthectomy 25 h earlier (Table 3). A similar trend was found in slices from UL animals, but the decrease in baseline GLY release was only significant after 1 and 3 weeks post-UL (Table 3). Baseline release of ASP was significantly lower than normal at all time points after UL, and a similar, although not significant, decrease in baseline ASP release was found after bilateral labyrinthectomy. The evoked release of GLY, GLU, ASP and TAU in response to the K+ stimulus was not altered at any time point post-labyrinthectomy (not shown).
Changes in H3 receptor-mediated effects on GABA release during vestibular compensation
In sharp contrast to its effects in the normal MVN, the H3 receptor agonist immepip (100 nm) did not inhibit K+-evoked GABA release from the ipsi-lesional MVN at any time point after UL. A trend towards a recovery of its normal inhibitory effects on ipsi-lesional GABA release was indicated by a gradual, although not significant, decrease in the evoked GABA response in the presence of immepip at 1 and 3 weeks post-UL (32.4 ± 5.8 fmol mg−1 at 25 h, 25.9 ± 4.0 fmol mg−1 at 1 week and 19.9 ± 4.1 fmol mg−1 at 3 weeks post UL; P = 0.215, one-way ANOVA F = 1.644 degrees of freedom (d.f.) = 2; n = 10, 8, and 8, respectively).
In the contra-lesional MVN, 100 nm immepip had a marked effect on K+-evoked GABA release at 25 h post-UL (Fig. 4B), but not at later time points (not shown). In the presence of immepip, the contra-lesional evoked GABA release was restored to a normal level, thereby abolishing the asymmetry in evoked GABA release between the ipsi- and contra-lesional MVNs that was characteristic at 25 h post-UL (Fig. 4B).
Since a glycinergic pathway is involved in the inhibition of evoked GABA release via H1/H2 receptors in normal MVN slices (above; Fig. 2), the effect of the glycine receptor antagonist strychnine (1 μm) on K+-evoked GABA release was also investigated with slices from 25 h post-UL animals (Fig. 4B). Strychnine treatment also restored K+-evoked GABA release from the 25 h post-UL contra-lesional MVN to a normal level, in a similar way to the effects of immepip (Fig. 4B).
Discussion
This study is the first to demonstrate that histamine has complex modulating effects on inhibitory neurotransmitter release in the MVN, and that histaminergic modulation of neurotransmitter release in the MVN changes during vestibular compensation after unilateral labyrinthectomy.
The origin of neuroactive amino acids released from normal slices of the MVN
The release of endogenous neuroactive amino acids from MVN slices has not previously been investigated. Our characterisation demonstrates that the basal release of GABA from MVN slices is two orders of magnitude smaller than that of GLU and GLY, and one order of magnitude smaller than that of ASP. High extracellular K+ evokes a robust and reproducible 10–20-fold increase in GABA release, which is almost completely calcium dependent. This indicates that the K+-evoked GABA release has neuronal origin, and suggests that GABAergic neurons and terminals have a low releasing activity in the unstimulated MVN slice. The rat MVN contains both GABAergic cell bodies and GABAergic terminals projecting from the cerebellum (Houser et al. 1984; Nomura et al. 1984; see also review by Barmack, 2003). In mice, approximately two-thirds of GABA immunoreactivity in the vestibular complex has been attributed to Purkinje cell terminals (Baurle et al. 1992). Such terminals will not be spontaneously active in the acutely prepared MVN slice, but will still be excitable, and probably make a substantial contribution to the evoked GABA release measured in these experiments. Similar to the baseline release of GABA, the baseline release of GLY, GLU and ASP was also calcium dependent. However, the K+-evoked response of those amino acids was highly variable between slices, precluding a systematic analysis of these neurotransmitters in the present study.
Histaminergic modulation of evoked GABA release in the normal MVN
Histamine inhibits evoked GABA release from the normal MVN in two different ways, as summarised in Fig. 5A–C. An H3-receptor mediated pathway was demonstrated by the potent and dose-dependent inhibitory action of the selective H3 agonist immepip on K+-evoked GABA release (Figs 3 and 5A and B). This was further corroborated by the finding that the reverse H3 agonist clobenpropit abolished the inhibitory effect of immepip (Fig. 2A). The potency of immepip (IC50: 0.03–1 nm), was at least three times higher than previously described in the striatum and substantia nigra (Garcia et al. 1997; Arias-Montano et al. 2001), but is in line with that observed for electrically evoked [3H]noradrenaline release from rat cortex (IC50: 0.6–1 nm, Alves-Rodrigues et al. 2001). The present findings should be seen in the light of previous reports of H3-receptor heterogeneity (Schwartz et al. 1991; Hancock, 2003; Leurs et al. 2005), and suggest that H3 receptors in the MVN are highly sensitive to activation in normal conditions.
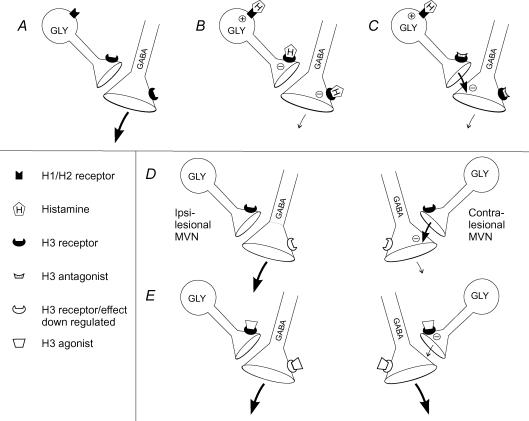
Figure 5. Proposed circuitry for histaminergic modulation of GABA release in the medial vestibular nucleus (MVN).
A, B and C, the inhibitory effects of histamine on GABA release from normal MVN slices. A, K+-evoked GABA release in untreated MVN slices. B, histamine inhibits GABA release directly by activation of H3 receptors on GABAergic terminals, but has a balanced effect on glycinergic neurons (GLY) due to activation of both H1/H2 and H3 receptors. C, in the presence of an H3 antagonist, histamine inhibits GABA release indirectly via H1/H2-mediated stimulation of GLY neurons. D and E, H3 receptor-mediated modulation of GABA release in the ipsi- and contra-lesional MVNs 25 h after unilateral labyrinthectomy. D, the suppression of contra-lesional GABA release together with normalisation of GABA release following treatment with strychnine (see Figure 4), indicates that glycinergic transmission is amplified in contra-lesional slices 25 h after unilateral labyrinthectomy. E, direct H3-mediated inhibition of GABA release is downmodulated, but the GABA release from the contra-lesional MVN is restored by H3-agonist treatment. The latter phenomenon can be mediated by inhibition of glycine release via activation of H3 receptors with normal sensitivity.
In addition to the direct action of histamine on H3 receptors, the present study revealed a novel indirect glycinergic pathway that inhibits GABA release in response to H1/H2 receptor activation. Histamine-induced inhibition of GABA release persisted after blockade of H3 receptors by clobenpropit, and this was the only experimental condition where GLY release was significantly increased in response to the high-K+ stimulus (Figs 2B and 5C). Increased glycinergic transmission was confirmed by the complete reversal of H1/H2 receptor mediated inhibition when slices were pretreated with the glycine receptor antagonist strychnine. We propose that excitatory H1/H2 receptors on glycinergic neurons can increase GLY release when activated in the presence of H3-receptor blockade, and that the increase in GLY release in turn inhibits GABA release (Fig. 5C). Glycinergic neurons in the MVN are known to be involved in the vestibulo-ocular reflex pathways, mediating the inhibitory projection to the ipsilateral abducens nucleus (Spencer et al. 1989), while the reciprocal vestibular commissural inhibitory system, which links the MVNs of the two sides, is predominantly GABAergic (Furuya & Koizumi, 1998). The present findings therefore demonstrate a novel possible route by which glycinergic neurons may also modulate the activity of the vestibular commissural system, by regulating the release of GABA within the MVN. The commissural inhibitory system plays a fundamentally important role in shaping the dynamics and response gain of MVN neurons to vestibular inputs. Histaminergic modulation of commissural GABA release may therefore be useful in situations where a high sensitivity to vestibular inputs is undesirable, for example in motion sickness when vestibular signals are in conflict with visual and proprioceptive information. As mentioned above, there is strong evidence for increased histamine release in such situations (Horii et al. 1993). In addition, histaminergic inhibition of GABA release at Purkinje cell synapses on MVN neurons might also regulate the gain of the cerebellar inhibitory input to the MVN, providing a further neuromodulatory control at the level of the brainstem to parallel the modulatory actions of histamine within the cerebellum itself (Zhu et al. 2006).
Changes in GABA neurotransmission in the MVN during vestibular compensation
Several previous studies have implicated changes in GABA receptor function as well as GABA expression and metabolism in the MVN during vestibular compensation. In cats subjected to UL, GABA immunoreactivity increases in neuronal varicosities in the ipsi-lesional MVN one week post-UL (Tighilet & Lacour, 2001). There is also an increase in mRNA levels for proteins mediating GABA synthesis and uptake (GAD65, GAD67 and GAT) in the ipsi-lesional vestibular nuclei 6–50 h after UL in rats (Horii et al. 2003). The responsiveness of MVN neurons to GABAergic compounds is markedly downregulated in the ipsi-lesional MVN compared to normal (Yamanaka et al. 2000; Johnston et al. 2001). However, this is not reflected in significant changes in expression of ipsi-lesional GABA receptor subunits (Eleore et al. 2005; Gliddon et al. 2005a; Zhang et al. 2005), suggesting that the changes in GABA receptor function may be due to post-translational modifications of the receptor subunits, or modulations of the receptor complex, rather than changes in expression levels.
In the present study, we did not detect any significant changes in either basal GABA release or K+-evoked GABA release in the ipsi-lesional MVN, at any time point after UL. However in the short term after UL there was a marked asymmetry in evoked GABA release between the ipsi- and contra-lesional MVNs, with GABA release from the contra-lesional MVN being significantly reduced at 25 h post-UL. This was due to a suppression of contra-lesional GABA release through the indirect glycinergic pathway, since the evoked GABA response was fully restored to normal by strychnine (Fig. 2). At later time points, the evoked release of GABA in both ipsi-lesional and contra-lesional MVN was not different from normal. The glycinergic suppression of contra-lesional GABA release at 25 h post-UL is therefore an early component of the response of the vestibular system to de-afferentation, which does not persist in the longer term (Guilding & Dutia, 2005).
It would thus appear that in the immediate aftermath of UL, GABAergic neurotransmission in both ipsi-lesional and contra-lesional MVN is attenuated: in the ipsi-lesional MVN by a reduced responsiveness of the MVN neurons to GABA (Yamanaka et al. 2000; Johnston et al. 2001), and in the contra-lesional MVN by a suppression of GABA release by endogenous GLY. It has been proposed that the downregulation of ipsi-lesional GABA receptors is a ‘compensatory’ process, since it will promote the recovery of the resting discharge of the de-afferented ipsi-lesional cells (Yamanaka et al. 2000; Straka et al. 2005). On this basis, the reduction in evoked contra-lesional GABA release is ‘anticompensatory’, in that the contra-lesional cells, which tend to be hyperactive after UL, will receive less GABAergic inhibition. The decrease in contra-lesional GABA release could, however, be counteracted by the increased glycinergic transmission, and because the contra-lesional MVN cells do not downregulate their GABA receptors (Yamanaka et al. 2000) or inhibitory GLY receptors (Vibert et al. 2000), they will continue to be responsive even to basal levels of these neurotransmitters in the initial period after UL. The present results imply an important role for glycine, in addition to GABA, in the MVN after UL, and further experiments are necessary to characterise the changes in glycinergic neurotransmission that may occur during compensation.
Histamine and vestibular compensation
While in the normal MVN the H3 agonist immepip caused the inhibition of evoked GABA release, this effect was markedly downregulated after UL. Immepip did not inhibit evoked GABA release at any time point post-UL in either the ipsi- or contra-lesional MVN. This lasting downregulation of H3 receptor effects could in part be explained by a change in H3-receptor subunit isoforms, as was recently indicated by changes in mRNA expression in the vestibular nuclei after UL (Lozada et al. 2004). Alternatively, an increase in constitutive receptor activity could account for a change in responsiveness, since agonistic effects of some H3 ligands are weaker and may even change to antagonism or reverse agonism when the constitutive activity increases (Gbahou et al. 2003). To the best of our knowledge, immepip has not been reported to act as a protean ligand in this manner, so at present the contribution of such a mechanism is not known.
In contrast to the sustained downregulation of its direct inhibitory effects on evoked GABA release after UL, immepip completely reversed the glycinergic suppression of GABA release in the contra-lesional MVN at 25 h post-UL, and removed the asymmetry in evoked GABA release between the ipsi- and contra-lesional MVN at this time point (Fig. 4). The effects of H3 receptor activation on glycinergic and GABAergic neurotransmission in the MVN therefore appear to be modulated differently after UL: the direct inhibition of GABA release by presynaptic H3 receptors is downregulated within 25 h post-UL and remains downregulated for more than 3 weeks post-UL, while in the short term after UL H3 receptors act to modulate the indirect, glycinergic pathway that suppresses GABA release in the contra-lesional MVN (Fig. 5D, E). The actions of histamine on MVN neurons during vestibular compensation are therefore also likely to differ at different times after UL. Assuming that the early asymmetry in evoked GABA release between the MVNs of the two sides is an undesirable consequence of UL, then histamine may facilitate behavioural recovery in the early stages of compensation by relieving the suppression of GABA release in the contra-lesional MVN and abolishing the asymmetry. At later stages of compensation, however, it is unlikely that histaminergic modulation of GABA release in the MVN may contribute to recovery, because the H3 receptor-mediated effects remain functionally downregulated in the long term, and the indirect glycinergic suppression of GABA release has subsided. Instead the main effects of histamine on MVN neurons are likely to be mediated by the excitatory postsynaptic H1/H2 receptors, as previously suggested (Wang & Dutia, 1995). The facilitation of vestibular compensation by H3 antagonists, which is typically seen after long-term treatments lasting several weeks (Tighilet et al. 1995; Pan et al. 1998; Tighilet et al. 2006), may furthermore involve the actions of histamine in other regions of the CNS that are implicated in the behavioural adaptation after labyrinthectomy, like the cerebellum and areas that regulate wakefulness and stress responses (for a discussion and review, see Bergquist & Dutia, 2006).
GABAergic transmission has important roles in vestibular physiology and is believed to play a role also in vestibular compensation (Gliddon et al. 2005b). This study demonstrates that GABA release in the MVN is strongly inhibited by histamine, both via presynaptic inhibitory H3 receptors and via H1/H2 receptors, which activate a glycinergic inhibitory pathway. Histamine stimulates MVN-neurons via H1/H2 activation (Phelan et al. 1990; de Waele et al. 1992; Wang & Dutia, 1995), and histaminergic inhibition of GABA release provides a parallel disinhibitory mechanism that may assist in tuning the efficacy of vestibular inhibitory commissural pathways, as well as cerebello-vestibular GABAergic projections. Histaminergic modulation of neurotransmitter release in the MVN may be important in vestibular synaptic plasticity and behavioural recovery after unilateral vestibular de-afferentation, and should be further explored.
Acknowledgments
Supported by the Wellcome Trust, project grant GR073041. F. Bergquist is the recipient of a postdoctoral fellowship from the Swedish Research Council (K 2005-98PK-15488-01A).
References
- Airaksinen MS, Panula P. The histaminergic system in the guinea pig central nervous system: an immunocytochemical mapping study using an antiserum against histamine. J Comp Neurol. 1988;273:163–186. doi: 10.1002/cne.902730204. [DOI] [PubMed] [Google Scholar]
- Alves-Rodrigues A, Lemstra S, Vollinga RC, Menge WM, Timmerman H, Leurs R. Pharmacological analysis of immepip and imetit homologues. Further evidence for histamine H3 receptor heterogeneity? Behav Brain Res. 2001;124:121–127. doi: 10.1016/s0166-4328(01)00224-8. [DOI] [PubMed] [Google Scholar]
- Arias-Montano JA, Floran B, Garcia M, Aceves J, Young JM. Histamine H3 receptor-mediated inhibition of depolarization-induced, dopamine D1 receptor-dependent release of [3H]-gamma-aminobutryic acid from rat striatal slices. Br J Pharmacol. 2001;133:165–171. doi: 10.1038/sj.bjp.0704053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrang JM, Garbarg M, Schwartz JC. Auto-inhibition of brain histamine release mediated by a novel class (H3) of histamine receptor. Nature. 1983;302:832–837. doi: 10.1038/302832a0. [DOI] [PubMed] [Google Scholar]
- Barmack NH. Central vestibular system: vestibular nuclei and posterior cerebellum. Brain Res Bull. 2003;60:511–541. doi: 10.1016/s0361-9230(03)00055-8. [DOI] [PubMed] [Google Scholar]
- Barnes JC, Brown JD, Clarke NP, Clapham J, Evans DJ, O'Shaughnessy CT. Pharmacological activity of VUF 9153, an isothiourea histamine H3 receptor antagonist. Eur J Pharmacol. 1993;250:147–152. doi: 10.1016/0014-2999(93)90632-r. [DOI] [PubMed] [Google Scholar]
- Baurle J, Grover BG, Grusser-Cornehls U. Plasticity of GABAergic terminals in Deiters' nucleus of weaver mutant and normal mice: a quantitative light microscopic study. Brain Res. 1992;591:305–318. doi: 10.1016/0006-8993(92)91712-n. [DOI] [PubMed] [Google Scholar]
- Bergquist F, Dutia MB. Central histaminergic modulation of vestibular function. Acta Physiol Sin. 2006;58:293–304. [PubMed] [Google Scholar]
- Darlington CL, Dutia MB, Smith PF. The contribution of the intrinsic excitability of vestibular nucleus neurons to recovery from vestibular damage. Eur J Neurosci. 2002;15:1719–1727. doi: 10.1046/j.1460-9568.2002.02024.x. [DOI] [PubMed] [Google Scholar]
- de Waele C, Serafin M, Khateb A, Vibert N, Yabe T, Arrang JM, Mulhethaler M, Vidal PP. An in vivo and in vitro study of the vestibular nuclei histaminergic receptors in the guinea pig. Ann N Y Acad Sci. 1992;656:550–565. doi: 10.1111/j.1749-6632.1992.tb25235.x. [DOI] [PubMed] [Google Scholar]
- Eleore L, Vassias I, Bernat I, Vidal PP, de Waele C. An in situ hybridization and immunofluorescence study of GABAA and GABAB receptors in the vestibular nuclei of the intact and unilaterally labyrinthectomized rat. Exp Brain Res. 2005;160:166–179. doi: 10.1007/s00221-004-1997-8. [DOI] [PubMed] [Google Scholar]
- Fischer AJ. Histamine in the treatment of vertigo. Acta Otolaryngol Suppl. 1991;479:24–28. doi: 10.3109/00016489109121145. [DOI] [PubMed] [Google Scholar]
- Furuya N, Koizumi T. Neurotransmitters of vestibular commissural inhibition in the cat. Acta Otolaryngol. 1998;118:64–69. doi: 10.1080/00016489850155143. [DOI] [PubMed] [Google Scholar]
- Garcia M, Floran B, Arias-Montano JA, Young JM, Aceves J. Histamine H3 receptor activation selectively inhibits dopamine D1 receptor-dependent [3H]GABA release from depolarization-stimulated slices of rat substantia nigra pars reticulata. Neuroscience. 1997;80:241–249. doi: 10.1016/s0306-4522(97)00100-0. [DOI] [PubMed] [Google Scholar]
- Gbahou F, Rouleau A, Morisset S, Parmentier R, Crochet S, Lin JS, Ligneau X, Tardivel-Lacombe J, Stark H, Schunack W, Ganellin CR, Schwartz JC, Arrang JM. Protean agonism at histamine H3 receptors in vitro and in vivo. Proc Natl Acad Sci U S A. 2003;100:11086–11091. doi: 10.1073/pnas.1932276100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliddon CM, Darlington CL, Smith PF. GABAA receptor subunit expression in the guinea pig vestibular nucleus complex during the development of vestibular compensation. Exp Brain Res. 2005a;166:71–77. doi: 10.1007/s00221-005-2344-4. [DOI] [PubMed] [Google Scholar]
- Gliddon CM, Darlington CL, Smith PF. GABAergic systems in the vestibular nucleus and their contribution to vestibular compensation. Prog Neurobiol. 2005b;75:53–81. doi: 10.1016/j.pneurobio.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Guilding C, Dutia MB. Early and late changes in vestibular neuronal excitability after deafferentation. Neuroreport. 2005;16:1415–1418. doi: 10.1097/01.wnr.0000176519.42218.a6. [DOI] [PubMed] [Google Scholar]
- Hancock AA. H3 receptor antagonists/inverse agonists as anti-obesity agents. Curr Opin Invest Drugs. 2003;4:1190–1197. [PubMed] [Google Scholar]
- Horii A, Kitahara T, Smith PF, Darlington CL, Masumura C, Kubo T. Effects of unilateral labyrinthectomy on GAD, GAT1 and GABA receptor gene expression in the rat vestibular nucleus. Neuroreport. 2003;14:2359–2363. doi: 10.1097/00001756-200312190-00014. [DOI] [PubMed] [Google Scholar]
- Horii A, Takeda N, Matsunaga T, Yamatodani A, Mochizuki T, Okakura-Mochizuki K, Wada H. Effect of unilateral vestibular stimulation on histamine release from the hypothalamus of rats in vivo. J Neurophysiol. 1993;70:1822–1826. doi: 10.1152/jn.1993.70.5.1822. [DOI] [PubMed] [Google Scholar]
- Houser CR, Barber RP, Vaughn JE. Immunocytochemical localization of glutamic acid decarboxylase in the dorsal lateral vestibular nucleus: evidence for an intrinsic and extrinsic GABAergic innervation. Neurosci Lett. 1984;47:213–220. doi: 10.1016/0304-3940(84)90516-0. [DOI] [PubMed] [Google Scholar]
- Johnston AR, Him A, Dutia MB. Differential regulation of GABAA and GABAB receptors during vestibular compensation. Neuroreport. 2001;12:597–600. doi: 10.1097/00001756-200103050-00033. [DOI] [PubMed] [Google Scholar]
- Lacour M, Sterkers O. Histamine and betahistine in the treatment of vertigo: elucidation of mechanisms of action. CNS Drugs. 2001;15:853–870. doi: 10.2165/00023210-200115110-00004. [DOI] [PubMed] [Google Scholar]
- Leurs R, Bakker RA, Timmerman H, de Esch IJ. The histamine H3 receptor: from gene cloning to H3 receptor drugs. Nat Rev Drug Discov. 2005;4:107–120. doi: 10.1038/nrd1631. [DOI] [PubMed] [Google Scholar]
- Lozada AF, Aarnisalo AA, Karlstedt K, Stark H, Panula P. Plasticity of histamine H3 receptor expression and binding in the vestibular nuclei after labyrinthectomy in rat. BMC Neurosci. 2004;5:32. doi: 10.1186/1471-2202-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludbrook J. Multiple comparison procedures updated. Clin Exp Pharmacol Physiol. 1998;25:1032–1037. doi: 10.1111/j.1440-1681.1998.tb02179.x. [DOI] [PubMed] [Google Scholar]
- Nomura I, Senba E, Kubo T, Shiraishi T, Matsunaga T, Tohyama M, Shiotani Y, Wu JY. Neuropeptides and gamma-aminobutyric acid in the vestibular nuclei of the rat: an immunohistochemical analysis. I. Distribution. Brain Res. 1984;311:109–118. doi: 10.1016/0006-8993(84)91403-3. [DOI] [PubMed] [Google Scholar]
- Pan JB, O'Neill AB, Hancock AA, Sullivan JP, Brioni JD. Histaminergic ligands attenuate barrel rotation in rats following unilateral labyrinthectomy. Meth Find Exp Clin Pharmacol. 1998;20:771–777. [PubMed] [Google Scholar]
- Phelan KD, Nakamura J, Gallagher JP. Histamine depolarizes rat medial vestibular nucleus neurons recorded intracellularly in vitro. Neurosci Lett. 1990;109:287–292. doi: 10.1016/0304-3940(90)90009-x. [DOI] [PubMed] [Google Scholar]
- Pillot C, Heron A, Cochois V, Tardivel-Lacombe J, Ligneau X, Schwartz JC, Arrang JM. A detailed mapping of the histamine H3 receptor and its gene transcripts in rat brain. Neuroscience. 2002;114:173–193. doi: 10.1016/s0306-4522(02)00135-5. [DOI] [PubMed] [Google Scholar]
- Pollard H, Moreau J, Arrang JM, Schwartz JC. A detailed autoradiographic mapping of histamine H3 receptors in rat brain areas. Neuroscience. 1993;52:169–189. doi: 10.1016/0306-4522(93)90191-h. [DOI] [PubMed] [Google Scholar]
- Schwartz JC, Arrang JM, Garbarg M, Pollard H, Ruat M. Histaminergic transmission in the mammalian brain. Physiol Rev. 1991;71:1–51. doi: 10.1152/physrev.1991.71.1.1. [DOI] [PubMed] [Google Scholar]
- Serafin M, Khateb A, Vibert N, Vidal PP, Muhlethaler M. Medial vestibular nucleus in the guinea-pig: histaminergic receptors. I. An in vitro study. Exp Brain Res. 1993;93:242–248. doi: 10.1007/BF00228391. [DOI] [PubMed] [Google Scholar]
- Spencer RF, Wenthold RJ, Baker R. Evidence for glycine as an inhibitory neurotransmitter of vestibular, reticular, and prepositus hypoglossi neurons that project to the cat abducens nucleus. J Neurosci. 1989;9:2718–2736. doi: 10.1523/JNEUROSCI.09-08-02718.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbusch HW. Distribution of histaminergic neurons and fibers in rat brain. Comparison with noradrenergic and serotonergic innervation of the vestibular system. Acta Otolaryngol Suppl. 1991;479:12–23. doi: 10.3109/00016489109121144. [DOI] [PubMed] [Google Scholar]
- Straka H, Kunkel AW, Dieringer N. Plasticity in vestibular and spinal circuits after hemilabyrinthectomy in the frog. Eur J Morphol. 1994;32:303–306. [PubMed] [Google Scholar]
- Straka H, Vibert N, Vidal PP, Moore LE, Dutia MB. Intrinsic membrane properties of vertebrate vestibular neurons: function, development and plasticity. Prog Neurobiol. 2005;76:349–392. doi: 10.1016/j.pneurobio.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Tighilet B, Lacour M. Histamine immunoreactivity changes in vestibular-lesioned and histaminergic-treated cats. Eur J Pharmacol. 1997;330:65–77. doi: 10.1016/s0014-2999(97)10124-8. [DOI] [PubMed] [Google Scholar]
- Tighilet B, Lacour M. Gamma amino butyric acid (GABA) immunoreactivity in the vestibular nuclei of normal and unilateral vestibular neurectomized cats. Eur J Neurosci. 2001;13:2255–2267. doi: 10.1046/j.0953-816x.2001.01622.x. [DOI] [PubMed] [Google Scholar]
- Tighilet B, Leonard J, Lacour M. Betahistine dihydrochloride treatment facilitates vestibular compensation in the cat. J Vestib Res. 1995;5:53–66. [PubMed] [Google Scholar]
- Tighilet B, Trottier S, Mourre C, Lacour M. Changes in the histaminergic system during vestibular compensation in the cat. J Physiol. 2006;573:723–739. doi: 10.1113/jphysiol.2006.107805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cauwenberge PB, De Moor SE. Physiopathology of H3-receptors and pharmacology of betahistine. Acta Otolaryngol Suppl. 1997;526:43–46. doi: 10.3109/00016489709124020. [DOI] [PubMed] [Google Scholar]
- Vibert N, Beraneck M, Bantikyan A, Vidal PP. Vestibular compensation modifies the sensitivity of vestibular neurones to inhibitory amino acids. Neuroreport. 2000;11:1921–1927. doi: 10.1097/00001756-200006260-00023. [DOI] [PubMed] [Google Scholar]
- Wang JJ, Dutia MB. Effects of histamine and betahistine on rat medial vestibular nucleus neurones: possible mechanism of action of anti-histaminergic drugs in vertigo and motion sickness. Exp Brain Res. 1995;105:18–24. doi: 10.1007/BF00242178. [DOI] [PubMed] [Google Scholar]
- Wellendorph P, Goodman MW, Burstein ES, Nash NR, Brann MR, Weiner DM. Molecular cloning and pharmacology of functionally distinct isoforms of the human histamine H3 receptor. Neuropharmacology. 2002;42:929–940. doi: 10.1016/s0028-3908(02)00041-2. [DOI] [PubMed] [Google Scholar]
- Yabe T, de Waele C, Serafin M, Vibert N, Arrang JM, Muhlethaler M, Vidal PP. Medial vestibular nucleus in the guinea-pig: histaminergic receptors. II. An in vivo study. Exp Brain Res. 1993;93:249–258. doi: 10.1007/BF00228392. [DOI] [PubMed] [Google Scholar]
- Yamanaka T, Him A, Cameron SA, Dutia MB. Rapid compensatory changes in GABA receptor efficacy in rat vestibular neurones after unilateral labyrinthectomy. J Physiol. 2000;523(Part 2):413–424. doi: 10.1111/j.1469-7793.2000.t01-1-00413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Ashton J, Horii A, Darlington CL, Smith PF. Immunocytochemical and stereological analysis of GABAB receptor subunit expression in the rat vestibular nucleus following unilateral vestibular deafferentation. Brain Res. 2005;1037:107–113. doi: 10.1016/j.brainres.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Zhu JN, Yung WH, Kwok-Chong Chow B, Chan YS, Wang JJ. The cerebellar-hypothalamic circuits: potential pathways underlying cerebellar involvement in somatic-visceral integration. Brain Res Brain Res Rev. 2006;52:93–106. doi: 10.1016/j.brainresrev.2006.01.003. [DOI] [PubMed] [Google Scholar]