Abstract
Administration of ghrelin, an endogenous ligand for the growth hormone secretagogue receptor 1a (GHSR 1a), induces potent stimulating effects on GH secretion and food intake. However, more than seven years after its discovery, the role of endogenous ghrelin remains elusive. Recently a second peptide, obestatin, also generated from proteolytic cleavage of preproghrelin has been identified. This peptide inhibits food intake and gastrointestinal motility but does not modify in vitro GH release from pituitary cells. In this study we have reinvestigated obestatin functions by measuring plasma ghrelin and obestatin levels in a period of spontaneous feeding in ad libitum fed and 24h-fasted mice. While fasting resulted in elevated ghrelin levels, obestatin levels were significantly reduced. Exogenous obestatin per se did not modify food intake in fasted and fed mice. However, it inhibited ghrelin orexigenic effect that were evident in fed mice only. The effects of obestatin on GH secretion were monitored in superfused pituitary explants and in freely moving rats. Obestatin was only effective in vivo to inhibit ghrelin stimulation of GH levels. Finally, the relationship between octanoylated ghrelin, obestatin and GH secretions was evaluated by iterative blood sampling every 20 minutes during 6 hours in freely moving adult male rats. The half-life of exogenous obestatin (10 μg iv) in plasma was about 22 minutes. Plasma obestatin levels exhibited an ultradian pulsatility with a frequency slightly lower than octanoylated ghrelin and GH. Ghrelin and obestatin levels were not strictly correlated.
In conclusion these results show that obestatin, like ghrelin, is secreted in a pulsatile manner and that in some conditions; obestatin can modulate exogenous ghrelin action. It remains to be determined whether obestatin modulates endogenous ghrelin actions.
Keywords: Animals; Eating; drug effects; Fasting; Growth Hormone; blood; secretion; Male; Mice; Mice, Inbred C57BL; Peptide Hormones; blood; pharmacology; physiology; Photoperiod; Rats; Rats, Sprague-Dawley
Keywords: Obestatin, Ghrelin, GH, food intake, mouse, rat
Introduction
Ghrelin was identified in 1999 as the endogenous ligand for the growth hormone secretagogue receptor 1a (GHSR 1a) (1). Soon after its discovery, in addition to its strong GH releasing activity (2, 3), ghrelin was found to increase food intake, downregulate energy expenditure and conserve body fat, causing weight gain and adipogenesis (4–7). However, if exogenous ghrelin actions are well established, the role of endogenous ghrelin is still unclear. Ghrelin antagonists have significant effects on these two functions (8–10) and active vaccination against ghrelin immunoconjugates decreases feed efficiency, relative adiposity and body weight gain in mature rats (11). Mice invalidated for the preproghrelin or ghrelin receptor gene do not display a major phenotype in term of body growth (12, 13) but are protected against early-onset obesity (14, 15). The recent identification of obestatin, a new peptide derived from preproghrelin which has been reported to bind to and activates the orphan receptor GPR39 (16, 17) may explain such discrepancies. Indeed, obestatin exhibits opposite effects of ghrelin on energy homeostasis and gastrointestinal function but appears ineffective on GH secretion (17).
In the present work we further investigated obestatin’s ability to modulate spontaneous or ghrelin induced food intake and GH secretion. Plasma obestatin, ghrelin and GH levels were monitored by selective assays. Food intake was measured at the onset of the dark phase in fed and in 24h-fasted/refed mice. GH secretion was evaluated ex vivo from superfused rat pituitaries and in vivo in freely moving male rats. Finally, the relationship between obestatin, (18–20) ghrelin and GH secretion was assessed by sampling blood every 20 min for 6 consecutive hours, the first three corresponding to the end of the light-on period and the last three to the beginning of the light-off period.
Materials and Methods
Animals
Four weeks before experiments, adult male C57Bl/6 mice and Sprague-Dawley rats (Charles River Laboratories, Inc., L’Arbresle, France) weighing respectively between 20 and 25g and 100 and 125 g at the onset of the experiment were housed individually in transparent plastic containers placed in a sound proof room with controlled temperature (22–24 C) and illumination (12 h light, 12 h dark schedule with lights off at 1700 h). They had free access to food and water and they were regularly weighed and handled in order to minimize manipulation stress. Animal experiments were performed according to the guidelines of the French Act of Animal Care and Experimentation (1990; registration number 75–343). All efforts were made to minimize pain and suffering and to reduce the number of animals used.
In vivo experiments
Mouse experiments
For the food intake experiments, C57Bl/6 mice had free access to food and water until the fasting period. Every other day during the two weeks before the experiments, mice received intra-peritoneal saline injections in order to minimize stress. They were assigned randomly to the fast and fed groups. One day before the experiment a group was fasted for 24h. On the day of the experiment, animals received an intra-peritoneal (ip) injection of saline, ghrelin, obestatin or obestatin + ghrelin (1μmol/kg each; NeoMPS Strasbourg, France) one hour before the onset of the dark period. Fasted animals were given access to food just after the injection. In a first set of experiments, blood samples were withdrawn from the jugular vein at 1600h after anaesthesia with ketamine-xylazine in order to determine endogenous levels of ghrelin, obestatin, and glucose at injection time. In a second one, food intake was monitored 1, 3, 5 and 18h after the injection. Finally, in a third one, animals were decapitated one hour after peptide injection and blood was sampled in order to determine glucose levels (measured by glucose oxydase; Beckman analyzer II; Beckman Coulter, Fullerton, CA).
Rat experiments
Two days before blood sampling, an indwelling cannula was inserted into the right atrium as previously described (21). Two hours before the sampling period, the distal extremity of the cannula was connected to a polyethylene catheter filled with 25 IU/ml heparinized saline. Blood samples were collected on EDTA (1mg/ml) and p-hydroxy-mercuribenzoic acid (PHMB, Sigma, Saint Quentin Fallavier, France) (0.36mg/ml) to avoid ghrelin degradation, immediately centrifuged and plasma was stored at −20°C until hormone assays. Blood from donor rats was regularly reinjected to attenuate hemodynamic modifications.
In experiment 2, saline, obestatin (10μg/rat), ghrelin (10μg/rat) or obestatin plus ghrelin were administered intravenously at 1000h and blood samples withdrawn just before and 5, 10, 20, 30, 45 and 60 min after injection for GH determination.
In experiment 3, the half life of obestatin in plasma was determined. Blood samples were collected 0, 5, 10, 20, 30, 45 and 60 minutes after IV injection of 10 μg of obestatin and plasma obestatin levels were measured. Half life was calculated as t ½ =ln(2)/Kel where Kel=−slope.
In experiment 4, blood was sampled every 20 min from 1400h to 2000h, in order to compare endogenous obestatine, octanoylated ghrelin and GH secretory parameters.
Ex vivo experiments
Rats were sacrificed by decapitation. Pituitaries were rapidly dissected, washed for 30 min in oxygenated Dulbecco’s modified Eagle’s medium (with L-glutamine, 4.5 g glucose, L-1 and 25 mM HEPES) containing 0.1% bovine serum albumin, placed in perifusion chambers (vol 0.3 ml) and superfused at a rate of 0.1 ml/min with the same medium. After a 120-min equilibration period, effluents were collected every 5 min. Peptides were added to the medium during 15-min periods. Samples were frozen until GH determinations.
Hormone assays
Octanoylated ghrelin was measured by an in-house immuno-enzymatic assay using polyclonal rabbit antibodies made against N-terminal rat ghrelin (kindly provided by Dr. Hosoda, Osaka, Japan), and human ghrelin coupled to acetylcholinesterase (Spibio, Saclay, France) as tracer. The sensitivity was 6 pmole/L and the intraassay coefficient of variation was 7 %.
Obestatin levels were determined with a commercial RIA kit (Phoenix, Belmont, CA). We have verified that rat pre-proghrelin 52–85 and 86–117 (Phoenix, Belmont, CA) do not cross-react up to the concentration of 3973 pmole/L. The sensitivity of this assay was 4 pmole/Land the intraassay coefficient of variation was 8 %.
Plasma GH concentrations were evaluated by EIA as previously described (22). Values are reported in terms of rGH-RP2, with sensitivity of 0.6 ng/ml and intra- and interassay coefficients of variation were 4 and 14 % respectively
Statistical analysis
Acylated ghrelin, obestatin and GH pulse analysis was performed using the Cluster 8 software (23) with the t value set to 2 to maintain false positive rates under 1%. Number of point for a peak and number of point for a nadir were set to 1 and 2 respectively.
Approximative entropy was calculated using the MC-ApEn software using R-value set to 0.2 and number of MC cycle set to 1000.
These programs are avaible from: http://mljohnson.pharm.virginia.edu/home.html.
Values are given as means ± sem, and statistical analysis was performed by ANOVA and paired t test using the JMP IN 5.1 software (SAS Institute Inc., Cary, NC).
Results
24 hours of fasting significantly reduced glycaemia (131±18 vs 313±44 mg/dl in ad libitum fed mice; P<0.01) and obestatin levels (256±6 vs 320±12 pmole/L; P<0.01) while it increased octanoylated ghrelin (787±225 vs 279±105 pmole/L; P= 0.087) (n=4).
Obestatin effects on spontaneous and ghrelin induced food intake at the onset of the dark period, in fed and 24h fasted mice
As shown on figure 1, during the light-off period, spontaneous cumulative food consumption (after 1,3, 5 and 18h) was lower in ad libitum fed than in fasted mice given access to food. Administration of obestatin (1 μmol/kg body weight ip) one hour before light-off was ineffective to modify food intake in fed (fig 1B, 1C) or fasted/refed (fig 1A, 1C) mice during the following 18 hours. In the same conditions ghrelin (1 μmol/kg body weight ip) significantly stimulated food intake in fed mice only and co-administration of obestatin inhibited this effect. In fasted/refed mice, cumulative food intake was similar to that of ghrelin- treated fed mice.
Figure 1. Obestatin effects on spontaneous and ghrelin-induced food intake, in fed and 24h fasted mice, during the dark period.
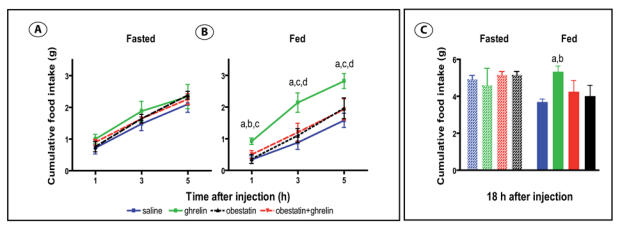
Cumulative food intake 1, 3, 5 hours after injection of saline (blue square), 1μmol/kg ghrelin (green square), 1μmol/kg obestatin (dark triangle) or 1μmol/kg obestatin + ghrelin (red triangle) : Panel A : in 24 h refeeding fasted mice (n= 20, 5 in each group) ; Panel B : in ad libitum fed mice (n= 20, 5 in each group)
a: ghrelin vs saline, P<0.01 ; b : ghrelin vs obestatin, P<0.01 ; c : ghrelin vs obestatin + ghrelin, P<0.05 ; d : ghrelin vs obestatin, P<0.05
Panel C : Cumulative food intake 18 h post injection in 24 h fasted (left) and ad libitum fed (right) mice receiving saline (blue), 1μmol/kg ghrelin (green), 1μmol/kg obestatin (dark) or 1μmol/kg obestatin + ghrelin (red)
a : ghrelin vs saline, P<0.05 ; b : ghrelin vs obestatin, P=0.06
In fed and fasted animals, ghrelin increased plasma glucose levels, one hour after the injection (Fed: saline 177.0±6.6 mg/dl, ghrelin: 221.4±18.0 mg/dl; P<0.05 vs saline; Fasted: saline: 249.6±26.7 mg/dl, ghrelin: 342.4±24.8 mg/dl; P<0.01 vs saline). Obestatin did not affect basal (Fed: 204.6±8.9 mg/dl; Fasted: 240.0±26.0 mg/dl) or ghrelin-induced glucose levels (Fed: 222.2±10.3 mg/dl; Fasted: 321.6±27.5 mg/dl).
Ex vivo and in vivo effects of obestatin on spontaneous and ghrelin induced GH release
As shown on figure 2A, ghrelin (10−7M) rapidly stimulated GH release from superfused pituitaries ex vivo. Obestatin (10−7 and 10−6 M) did not affect spontaneous or ghrelin-induced GH release.
Figure 2. Obestatin effects on spontaneous or ghrelin induced GH secretion in the rat.
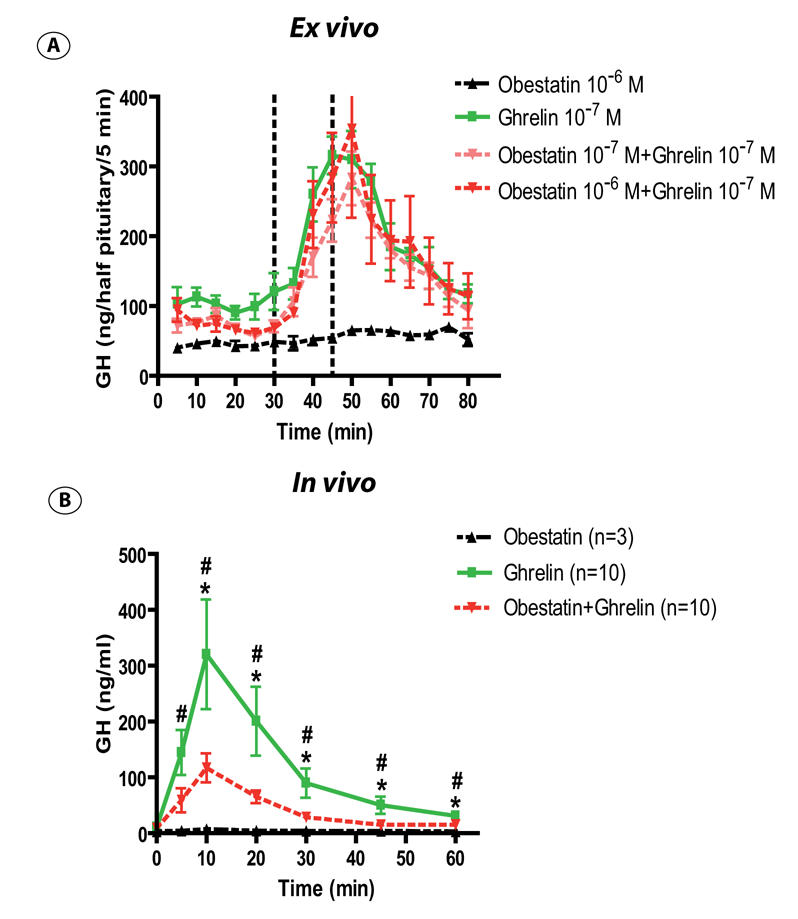
Panel A : Mean profile of GH release from superfused rat pituitaries in basal condition and after infusion of obestatin 10−6M (dark triangle), ghrelin 10−7M (green square), obestatin 10−7M + ghrelin 10−7M (pink triangle), obestatin 10−6M + ghrelin 10−7M (red triangle). The different peptides were infused during 15 minutes (materialised by dotted line). Each point and vertical bar indicates mean +/− sem of 4 chambers.
Panel B : Effect of 10 μg obestatin (dark triangle), 10 μg ghrelin (green square) or 10 μg obestatin + ghrelin (red triangle) on GH secretion in freely moving rats. The peptides were injected at t=0, and blood were collected pre-injection and 5, 10, 20, 30, 45, 60 minutes post injection. Data are means +/− sem. Number of animals are indicated in parentheses.
*: Ghrelin vs Obestatin + Ghrelin, P<0.05; #: Ghrelin vs Obestatin, P<0.05
In vivo, intravenous administration of rat/mouse ghrelin (10μg/rat) increased plasma GH levels as early as 5 min after the injection. The effect was maximal at 10 min and returned to basal levels after 45 to 60 minutes. Under the same conditions, obestatin (10μg/rat) did not change basal GH levels but markedly inhibited ghrelin-induced GH secretion (Figure 2B).
Obestatin, ghrelin and GH levels in freely moving rats
Determination of plasma obestatin concentrations from 5 to 60 min after synthetic obestatin administration (10 μg, iv) in freely moving rats showed that the peptide half life in plasma was 22±2 minutes (n=6) (Figure 3). This permitted to use a 20 min sampling periodicity during 6 hours to compare obestatin and ghrelin secretory profiles.
Figure 3. Determination of exogenous obestatin half-life in rat plasma.
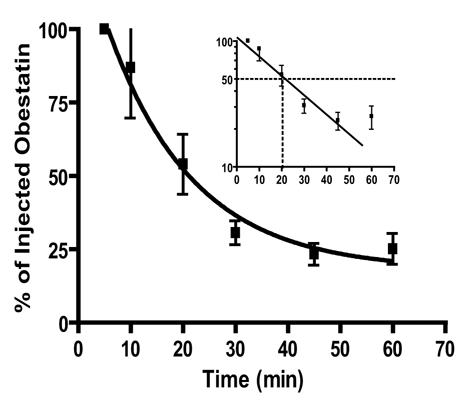
Obestatin immunoreactivity was detected by RIA in rat plasma after iv injection of 10 μg synthetic peptide. Data are expressed as % obestatin concentrations measured immediately after the injection. Values are the means±sem of six determinations. A semilogarithmic plot of the data is given in the inset.
Individual profiles of obestatin, ghrelin and GH secretion are displayed on figure 4. Plasma obestatin levels exhibited pulsatile variations of moderate amplitude, comparable with those of ghrelin.
Figure 4. Ghrelin, obestatin and GH profiles in freely moving rats.
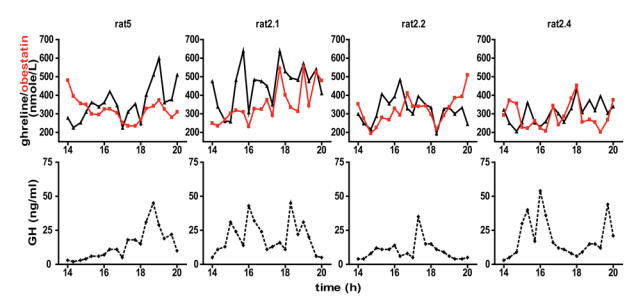
Representative octanoylated ghrelin (triangle), obestatin (red square) and GH (diamond-shaped) secretory patterns during a 6-h sampling period (3-h during the light period and 3-h during the dark period) in four freely moving male rats.
Pulsatility parameters for obestatin, ghrelin and GH secretory profiles are indicated in table 1. Peak amplitudes are similar for ghrelin and obestatin (increase of 54% for obestatin and 81% for ghrelin) and of lesser amplitude than those of GH (increase of 370%). Obestatin pulse frequency is slightly lower than that of octanoylated ghrelin or GH. Regularity of secretion determined by approximative entropy (ApEn) is very similar for obestatin, ghrelin, and GH.
Table 1.
Analysis of octanoylated ghrelin, obestatin and GH pulsatility parameters. Data are mean±sem of 8 rats.
| Mean levels (pmole/l) | Nadir (pmole/l) | Peak amplitude (pmole/l) | Peak frequency/360 min | Peak interval (min) | ApEn (1,20) | |
|---|---|---|---|---|---|---|
| Ghrelin | 358±25 | 259±21 | 452±21 | 2.25±0 | 25 85±14 | 0.739±0.051 |
| Obestatin | 317± 8 | 253±12 | 385±17 | 1.50±0.27a,b | 117±52 | 0.758±0.029 |
| (ng/ml) | (ng/ml) | (ng/ml) | (min) | |||
| GH | 19±3 | 12±4 | 40±7 | 2.28±0.36 | 89±16 | 0.810±0.060 |
Data are mean±sem of eight rats
P=0.073 vs ghrelin;
P=0.077 vs obestatin
When all samples were pooled, obestatin and ghrelin levels were significantly but weakly correlated (r2=0.0297, P=0.0344). When data from each rat were treated separately, significant correlations between ghrelin and obestatin were only observed in 3 out of 8 rats (individual r2=0.3147; 0.3990; 0.3566, with P=0.0125, 0.0049, 0.0069 respectively; all others r2<0.05; overall correlation for these 3 rats, r2=0.1272, P=0.007).
Discussion
In the present study we observe that, in some conditions, obestatin can inhibit ghrelin effects on food intake, depending on the feeding status, and on GH secretion. However, it is ineffective per se on these parameters.
Our results on food intake are slightly different from those originally reported by Zhang et al. who observed an inhibitory effect of obestatin on spontaneous food intake in refed mice (17). In their study, obestatin effects were only observed in refed animals, sacrificed 2 hours after light onset whether our data were obtained just after the onset of the light-off period when animals begin to eat spontaneously (24). More recently, the vast majority of studies (18, 20, 25–29) with one exception (30), reported no effect of obestatin per se or on ghrelin-induced food intake in rats and mice (fed or fasted/refed), whatever the route of administration and mode of obestatin dilution. Effects of orexigenic/anorexigenic peptides such as ghrelin and obestatin likely depends upon the relative concentrations of other orexigenic/anorexigenic factors at the time of injection. Indeed, we observed that, injected one hour before light off, ghrelin stimulates food intake in fed animals but is ineffective at the same dose in fasted ones given access to food. Similar results were reported when experiments were performed during the light-on period with a very low increase of food intake in refed animals compared with that of ad libitum ones (30% vs 320%) (17, 31). Endogenous ghrelin concentrations are markedly increased during fasting (125% after 24h fasting) in mice (32) and to a lesser extent just before light off (20 to 30%) in rats (33) (and Zizzari et al, unpublished data). The fact that ghrelin is effective only in fed mice and that in refed mice cumulative food intake is of the same magnitude than in ghrelin treated fed ones suggests that, beyond a certain threshold, additional exogenous ghrelin cannot further increase an already elevated food consumption. Reciprocally, the inefficiency of obestatin to alter spontaneous food intake at the beginning of the light-off period is probably not due to plasma ghrelin level increases since obestatin is able to counteract the effect of very high doses of exogenous ghrelin (1 μmol/kg). Other orexigenic/anorexigenic factors exhibit circadian variations which could modify obestatin or ghrelin responses. Numerous studies show modifications of NPY, GAL, β-endorphin and POMC gene expression at the onset of the dark phase (34–36) and it will be of interest to test obestatin and ghrelin responses in presence of variable concentrations of these factors.
It is generally believed that ghrelin exerts its orexigenic effect mainly by activating GHSR1a on hypothalamic arcuate nucleus NPY/AgRP neurons (37–40) but the mechanisms by which obestatin could modulate food intake are not yet known. Obestatin exerts its anorectic effect after intracerebroventricular administration at a low dose (8nmol/kg) suggesting a central action (17). Obestatin was originally reported to bind to GPR39, an orphan receptor, which shares similarities with GHSR1a (16, 17). GPR39 mRNA has been detected by reverse transcriptase polymerase chain reaction in the hypothalamus and 125I obestatin binding sites were reported in the same region (16, 17). Nevertheless, recent studies failed to confirm the presence of specific radio-labelled obestatin binding on GPR39 or activation of this receptor by obestatin (18–20). In preliminary experiments, we also observed that obestatin did not modify several signal transduction pathways in GPR-39 transfected cells (Pantel, et al unpublished data). It remains to determine which is the receptor for obestatin and how obestatin interferes with GHSR 1a to inhibit ghrelin stimulated food consumption. Since obestatin inhibits jejunal contractile activity and suppress gastric empting activity (17), it cannot be excluded that its anorectic effect relies mostly on peripheral sites of action. The inhibition of jejunal contraction could generate an afferent vagus signal to induce satiety in the CNS.
As already reported (17, 27), we did not observe any effect of obestatin on spontaneous or ghrelin-induced GH release ex vivo. In contrast, when administered iv to rats, obestatin significantly inhibited ghrelin stimulation of GH secretion. Ghrelin increases plasma GH levels by acting directly on pituitary but also indirectly at the hypothalamic level where it stimulates GHRH secretion (41) and decreases somatostatin release (3). Alternately, gastric vagal afferents are also an important pathway conveying ghrelin signals for GH secretion to the brain (42).
The present study shows, for the first time, that obestatin secretion is pulsatile and displays an ultradian rhythmicity, very similar to ghrelin and GH secretion. Interestingly however, plasma ghrelin and obestatin levels are not strictly correlated and the number of obestatin pulsatile episodes may seem slightly lower than the one observed for ghrelin and GH secretion. As obestatin and ghrelin are derived from the same gene, this lack of strict correlation supports the notion that obestatin is a physiologically relevant peptide and not only a non-functional connective peptide. Such an hypothesis is further substantiated by the differential effect of fasting on ghrelin and obestatin levels, ghrelin being markedly increased and obestatin slightly decreased after 24 hours of fasting. This strongly suggests that the secretion of the two peptides is regulated by the nutritional status in an opposite manner. It was previously shown that proopiomelanocortin, another multipeptide precursor, is cleaved into several bioactive fragments that include the anorectic α and β melanocyte-stimulating hormones and the orexigenic β-endorphin and that tissue-specific enzymes determine which of those peptides are generated (43). It remains to be determined whether a differential tissue specific post-translational process exists in the case of proghrelin and obestatin processing.
Acknowledgments
We thank A. Cougnon for technical assistance. We are grateful to the NIDDK for providing with GH assay reagents.
Footnotes
DISCLOSURE STATEMENT: The authors have nothing to disclose
References
- 1.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 2.Arvat E, Maccario M, Di Vito L, Broglio F, Benso A, Gottero C, Papotti M, Muccioli G, Dieguez C, Casanueva FF, Deghenghi R, Camanni F, Ghigo E. Endocrine activities of ghrelin, a natural growth hormone secretagogue (GHS), in humans: comparison and interactions with hexarelin, a nonnatural peptidyl GHS, and GH-releasing hormone. J Clin Endocrinol Metab. 2001;86:1169–1174. doi: 10.1210/jcem.86.3.7314. [DOI] [PubMed] [Google Scholar]
- 3.Tolle V, Zizzari P, Tomasetto C, Rio MC, Epelbaum J, Bluet-Pajot MT. In vivo and in vitro effects of ghrelin/motilin-related peptide on growth hormone secretion in the rat. Neuroendocrinology. 2001;73:54–61. doi: 10.1159/000054620. [DOI] [PubMed] [Google Scholar]
- 4.Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 5.Tolle V, Bassant MH, Zizzari P, Poindessous-Jazat F, Tomasetto C, Epelbaum J, Bluet-Pajot MT. Ultradian rhythmicity of ghrelin secretion in relation with GH, feeding behavior, and sleep-wake patterns in rats. Endocrinology. 2002;143:1353–1361. doi: 10.1210/endo.143.4.8712. [DOI] [PubMed] [Google Scholar]
- 6.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 7.Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, Kennedy AR, Roberts GH, Morgan DG, Ghatei MA, Bloom SR. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141:4325–4328. doi: 10.1210/endo.141.11.7873. [DOI] [PubMed] [Google Scholar]
- 8.Beck B, Richy S, Stricker-Krongrad A. Feeding response to ghrelin agonist and antagonist in lean and obese Zucker rats. Life Sci. 2004;76:473–478. doi: 10.1016/j.lfs.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Kobelt P, Helmling S, Stengel A, Wlotzka B, Andresen V, Klapp BF, Wiedenmann B, Klussmann S, Monnikes H. Anti-ghrelin Spiegelmer NOX-B11 inhibits neurostimulatory and orexigenic effects of peripheral ghrelin in rats. Gut. 2006;55:788–792. doi: 10.1136/gut.2004.061010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zizzari P, Halem H, Taylor J, Dong JZ, Datta R, Culler MD, Epelbaum J, Bluet-Pajot MT. Endogenous ghrelin regulates episodic growth hormone (GH) secretion by amplifying GH Pulse amplitude: evidence from antagonism of the GH secretagogue-R1a receptor. Endocrinology. 2005;146:3836–3842. doi: 10.1210/en.2005-0212. [DOI] [PubMed] [Google Scholar]
- 11.Zorrilla EP, Iwasaki S, Moss JA, Chang J, Otsuji J, Inoue K, Meijler MM, Janda KD. From the Cover: Vaccination against weight gain. Proc Natl Acad Sci U S A. 2006;103:13226–13231. doi: 10.1073/pnas.0605376103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun Y, Ahmed S, Smith RG. Deletion of ghrelin impairs neither growth nor appetite. Mol Cell Biol. 2003;23:7973–7981. doi: 10.1128/MCB.23.22.7973-7981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wortley KE, Anderson KD, Garcia K, Murray JD, Malinova L, Liu R, Moncrieffe M, Thabet K, Cox HJ, Yancopoulos GD, Wiegand SJ, Sleeman MW. Genetic deletion of ghrelin does not decrease food intake but influences metabolic fuel preference. Proc Natl Acad Sci U S A. 2004;101:8227–8232. doi: 10.1073/pnas.0402763101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wortley KE, del Rincon JP, Murray JD, Garcia K, Iida K, Thorner MO, Sleeman MW. Absence of ghrelin protects against early-onset obesity. J Clin Invest. 2005;115:3573–3578. doi: 10.1172/JCI26003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, Jones JE, Deysher AE, Waxman AR, White RD, Williams TD, Lachey JL, Seeley RJ, Lowell BB, Elmquist JK. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest. 2005;115:3564–3572. doi: 10.1172/JCI26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKee KK, Tan CP, Palyha OC, Liu J, Feighner SD, Hreniuk DL, Smith RG, Howard AD, Van der Ploeg LH. Cloning and characterization of two human G protein-coupled receptor genes (GPR38 and GPR39) related to the growth hormone secretagogue and neurotensin receptors. Genomics. 1997;46:426–434. doi: 10.1006/geno.1997.5069. [DOI] [PubMed] [Google Scholar]
- 17.Zhang JV, Ren PG, Avsian-Kretchmer O, Luo CW, Rauch R, Klein C, Hsueh AJ. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake. Science. 2005;310:996–999. doi: 10.1126/science.1117255. [DOI] [PubMed] [Google Scholar]
- 18.Holst B, Egerod KL, Schild E, Vickers SP, Cheetham S, Gerlach LO, Storjohann L, Stidsen CE, Jones R, Beck-Sickinger AG, Schwartz TW. GPR39 signaling is stimulated by zinc ions but not by obestatin. Endocrinology. 2006 doi: 10.1210/en.2006-0933. [DOI] [PubMed] [Google Scholar]
- 19.Lauwers E, Landuyt B, Arckens L, Schoofs L, Luyten W. Obestatin does not activate orphan G protein-coupled receptor GPR39. Biochem Biophys Res Commun. 2006;351:21–25. doi: 10.1016/j.bbrc.2006.09.141. [DOI] [PubMed] [Google Scholar]
- 20.Tremblay F, Perreault M, Klaman LD, Tobin JF, Smith E, Gimeno RE. Normal food intake and body weight in mice lacking the G protein-coupled receptor GPR39. Endocrinology. 2006 doi: 10.1210/en.2006-1275. [DOI] [PubMed] [Google Scholar]
- 21.Bluet-Pajot MT, Durand D, Drouva SV, Mounier F, Pressac M, Kordon C. Further evidence that thyrotropin-releasing hormone participate in the regulation of growth hormone secretion in the rat. Neuroendocrinology. 1986;44:70–75. doi: 10.1159/000124624. [DOI] [PubMed] [Google Scholar]
- 22.Ezan E, Laplante E, Bluet-Pajot MT, Mounier F, Mamas S, Grouselle D, Grognet JM, Kordon C. An enzyme immunoassay for rat growth hormone: validation and application to the determination of plasma levels and in vitro release. J Immunoassay. 1997;18:335–356. doi: 10.1080/01971529708005826. [DOI] [PubMed] [Google Scholar]
- 23.Veldhuis JD, Johnson ML. Cluster analysis: a simple, versatile, and robust algorithm for endocrine pulse detection. Am J Physiol. 1986;250:E486–493. doi: 10.1152/ajpendo.1986.250.4.E486. [DOI] [PubMed] [Google Scholar]
- 24.Armstrong S. A chronometric approach to the study of feeding behavior. Neurosci Biobehav Rev. 1980;4:27–53. doi: 10.1016/0149-7634(80)90024-x. [DOI] [PubMed] [Google Scholar]
- 25.Gourcerol G, Million M, Adelson DW, Wang Y, Wang L, Rivier J, St-Pierre DH, Tache Y. Lack of interaction between peripheral injection of CCK and obestatin in the regulation of gastric satiety signaling in rodents. Peptides. 2006;27:2811–2819. doi: 10.1016/j.peptides.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Nogueiras R, Pfluger P, Tovar S, Myrtha A, Mitchell S, Morris A, Perez-Tilve D, Vazquez MJ, Wiedmer P, Castaneda TR, Dimarchi R, Tschop M, Schurmann A, Joost HG, Williams LM, Langhans W, Dieguez C. Effects of obestatin on energy balance and growth hormone secretion in rodents. Endocrinology. 2006 doi: 10.1210/en.2006-0915. [DOI] [PubMed] [Google Scholar]
- 27.Samson WK, White MM, Price C, Ferguson AV. Obestatin acts in brain to inhibit thirst. Am J Physiol Regul Integr Comp Physiol. 2006 doi: 10.1152/ajpregu.00395.2006. [DOI] [PubMed] [Google Scholar]
- 28.Seoane LM, Al-Massadi O, Pazos Y, Pagotto U, Casanueva FF. Central obestatin administration does not modify either spontaneous or ghrelin-induced food intake in rats. J Endocrinol Invest. 2006;29:RC13–15. doi: 10.1007/BF03344174. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto D, Ikeshita N, Daito R, Herningtyas EH, Toda K, Takahashi K, Iida K, Takahashi Y, Kaji H, Chihara K, Okimura Y. Neither intravenous nor intracerebroventricular administration of obestatin affects the secretion of GH, PRL, TSH and ACTH in rats. Regul Pept. 2006 doi: 10.1016/j.regpep.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Bresciani E, Rapetti D, Dona F, Bulgarelli I, Tamiazzo L, Locatelli V, Torsello A. Obestatin inhibits feeding but does not modulate GH and corticosterone secretion in the rat. J Endocrinol Invest. 2006;29:RC16–18. doi: 10.1007/BF03344175. [DOI] [PubMed] [Google Scholar]
- 31.Neary NM, Druce MR, Small CJ, Bloom SR. Acylated ghrelin stimulates food intake in the fed and fasted states but desacylated ghrelin has no effect. Gut. 2006;55:135. [PMC free article] [PubMed] [Google Scholar]
- 32.Nonogaki K, Ohashi-Nozue K, Oka Y. A negative feedback system between brain serotonin systems and plasma active ghrelin levels in mice. Biochem Biophys Res Commun. 2006;341:703–707. doi: 10.1016/j.bbrc.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 33.Drazen DL, Vahl TP, D’Alessio DA, Seeley RJ, Woods SC. Effects of a fixed meal pattern on ghrelin secretion: evidence for a learned response independent of nutrient status. Endocrinology. 2006;147:23–30. doi: 10.1210/en.2005-0973. [DOI] [PubMed] [Google Scholar]
- 34.Akabayashi A, Koenig JI, Watanabe Y, Alexander JT, Leibowitz SF. Galanin-containing neurons in the paraventricular nucleus: a neurochemical marker for fat ingestion and body weight gain. Proc Natl Acad Sci U S A. 1994;91:10375–10379. doi: 10.1073/pnas.91.22.10375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steiner RA, Kabigting E, Lent K, Clifton DK. Diurnal rhythm in proopiomelanocortin mRNA in the arcuate nucleus of the male rat. J Neuroendocrinol. 1994;6:603–608. doi: 10.1111/j.1365-2826.1994.tb00625.x. [DOI] [PubMed] [Google Scholar]
- 36.Xu B, Kalra PS, Farmerie WG, Kalra SP. Daily changes in hypothalamic gene expression of neuropeptide Y, galanin, proopiomelanocortin, and adipocyte leptin gene expression and secretion: effects of food restriction. Endocrinology. 1999;140:2868–2875. doi: 10.1210/endo.140.6.6789. [DOI] [PubMed] [Google Scholar]
- 37.Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 38.Seoane LM, Lopez M, Tovar S, Casanueva FF, Senaris R, Dieguez C. Agouti-related peptide, neuropeptide Y, and somatostatin-producing neurons are targets for ghrelin actions in the rat hypothalamus. Endocrinology. 2003;144:544–551. doi: 10.1210/en.2002-220795. [DOI] [PubMed] [Google Scholar]
- 39.Shintani M, Ogawa Y, Ebihara K, Aizawa-Abe M, Miyanaga F, Takaya K, Hayashi T, Inoue G, Hosoda K, Kojima M, Kangawa K, Nakao K. Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway. Diabetes. 2001;50:227–232. doi: 10.2337/diabetes.50.2.227. [DOI] [PubMed] [Google Scholar]
- 40.Willesen MG, Kristensen P, Romer J. Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology. 1999;70:306–316. doi: 10.1159/000054491. [DOI] [PubMed] [Google Scholar]
- 41.Tannenbaum GS, Epelbaum J, Bowers CY. Interrelationship between the novel peptide ghrelin and somatostatin/growth hormone-releasing hormone in regulation of pulsatile growth hormone secretion. Endocrinology. 2003;144:967–974. doi: 10.1210/en.2002-220852. [DOI] [PubMed] [Google Scholar]
- 42.Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, Kangawa K, Nakazato M. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–1128. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- 43.Castro MG, Morrison E. Post-translational processing of proopiomelanocortin in the pituitary and in the brain. Crit Rev Neurobiol. 1997;11:35–57. doi: 10.1615/critrevneurobiol.v11.i1.30. [DOI] [PubMed] [Google Scholar]


