Abstract
ICC are found in both the upper and lower urinary tract. They are not found in the ureter itself but are confined to the lamina propria of the renal pelvis and pelvi-calyceal junction. They do not appear to have a primary pacemaker role (this is ascribed to atypical smooth muscle cells in the same location) but rather conduct and amplify the pacemaker signals generated by the atypical smooth muscle cells. In the bladder, ICC are widely distributed in the sub-urothelial region, in the lamina propria and at the margins of the detrusor smooth muscle bundles. Again they appear not to have a pacemaking role and such evidence as there is would suggest that they have a role in the modulation of signal transduction. The strongest evidence that ICC in the urinary tract act as pacemakers comes from studies of those in the urethra. Isolated ICC show regular spontaneous depolarizations in current clamp which resemble very closely the slow waves recorded from intact tissue. In voltage clamp they show abundant calcium-activated chloride current and spontaneous transient inward currents which can be blocked by chloride channel blockers. However, their role in the modulation of urethral tone has yet to be fully elucidated.
The urinary tract has some affinity with the lower gastrointestinal tract in that it could be viewed as the fluid waste disposal system by analogy with the solid waste disposal system that is the lower gastrointestinal tract. Thus the kidney decides how much fluid and solute to retain and what to reject. The rejected fluid must then be propelled by the ureter to the storage depot (the bladder) where it is retained until it can be emptied when a suitable occasion presents itself. Thus the ureter must be capable of efficient peristalsis which is responsive to the rate of urine production. The bladder must be sufficiently compliant to accommodate an adequate volume increase without intravesicular pressure rising to a level where it would inhibit urine flow from the kidney. Lastly the urethra is the guardian of continence and as such makes the final decision as to whether urine is voided or retained in the bladder. What these organs have in common is that, underlying their apparently very different functions, they all have a basic spontaneous electrical rhythm. In the case of the ureter the role of a pacemaker is rather obvious in that it initiates the propulsion of fluid from the renal calyx to the bladder by means of well-coordinated peristaltic waves that have their site of origin in the renal pelvis. It is less obvious why the bladder, which acts as a storage organ most of the time, and the urethra, which remains tonically contracted most of the time, should have any need for spontaneous electrical rhythm. Nevertheless it has been known for a long time that activity recorded using external (Orbeli & Brucke, 1910; Prosser et al. 1955) or intracellular electrodes (Ursillo, 1961; Kuriyama et al. 1967; Creed, 1971; Creed et al. 1983; Hashitani et al. 1996; Bradley et al. 2004) shows rhythmic firing of action potential complexes in all three organs. The purpose of the present review is to explore the origins of this electrical activity and in particular the possible role of ICC or ICC-like cells in its generation or modulation.
Ureter
Electrical activity appears to arise in the most proximal calyceal regions of the renal pelvis. This is not accompanied by a significant contraction since this region is only weakly contractile due to the paucity of smooth muscle cells. It is only when the impulse is conducted to the ureter proper that it initiates a vigorous contraction that is propagated to the uretero-vesical junction as a peristaltic wave. The source of the electrical impulse has been extensively studied by Lang and colleagues (Lang et al. 1998, 2001; Klemm et al. 1999) and is the subject of several reviews by these authors (Lang et al. 2002; Lang & Klemm, 2005). Using intracellular recording and by injecting neurobiotin they identified spindle-shaped ‘pacemaker’ cells in pelvi-calyceal junction and the proximal renal pelvis. These were approximately 160 μm in length and fired oscillations at a frequency of 8 min−1. Their morphology was closer to that of smooth muscle cells than to typical ICC and they were described as atypical smooth muscle cells. These cells have not yet been studied in isolation in voltage clamp so their detailed electrophysiology and the nature of the pacemaking current have not been described. In the lamina propria of the renal pelvis and pelvi-calyceal junction a cell type similar to typical ICC was found. They fired ‘intermediate’ action potentials at a frequency of 3–4 min−1. These cells were not regarded as the primary pacemaker but rather were thought responsible for conducting and amplifying pacemaker signals to initiate action potentials in smooth muscle cells. The observation that c-kit expression is up-regulated in the developing ureter prior to its ability to undergo unidirectional contractions (David et al. 2005) and that anti-c-kit antibodies inhibit peristaltic contractions would suggest that these cells, even if they are not the primary pacemakers, do play an important role in normal ureteral rhythmic activity.
Bladder
The bladder has two essential functions: it must store the urine that is continually produced by the kidney without its pressure rising above the kidney filtration pressure and it must be able to empty quickly when required. However, the bladder is not simply a compliant bag but is rather a spontaneously active muscular organ. After voiding, spontaneous contractions are minimal and filling occurs with little increase in pressure but against a background of small phasic increases in pressure. These rhythmic pressure oscillations are myogenic resulting from poorly coordinated contractions which arise at many different sites throughout the bladder. As filling proceeds the pressure waves increase in magnitude and eventually cause the urge to micturate. The bladder must at this point change from an ill-coordinated set of oscillators which provide background tone to a well-coordinated propulsive system capable of efficient emptying. How both of these functions are controlled is not yet fully understood but it is clear that there must be many pacemaking loci which generate the fundamental rhythmic activity. ICC would be obvious candidates for such a role and there is ample evidence of their presence in the sub-urothelial region, in the lamina propria and on the peripheries of the muscle bundles of the detrusor (Sui et al. 2002; Wiseman et al. 2003; Davidson & McCloskey, 2005). These authors all speculated that the different types of ICC (or myofibroblasts), which had been identified using morphological techniques, were potential pacemaker cells but they presented no convincing evidence in support of these speculations. In a careful study using microelectrodes and intracellular calcium imaging, Hashitani et al. (2004) could find no evidence of a pacemaking role for ICC in the guinea-pig bladder. The latter authors suggested that spontaneous excitation in the bladder might be initiated by detrusor smooth muscle cells themselves with the main role of ICC being to modulate the signal transmission. Clearly we are not yet in possession of enough detailed information to define the role of ICC in the bladder.
Urethra
The mechanism by which urinary continence is achieved is complex and has at least three components: (1) external pressure on the urethra due to contraction of the abdominal and pelvic muscles; (2) neurogenic tone due to sustained contraction of both smooth and striated muscle in response to excitation of cholinergic and noradrenergic nerves; and (3) myogenic tone due to sustained contraction of urethral smooth muscle. While all of these undoubtedly play a part, it is likely that contraction of striated muscle is more important in the increase in urethral tone that accompanies increases in bladder pressure secondary to coughing or other sudden increases in intra-abdominal pressure than in the long-term maintenance of tone. On the other hand, smooth muscle in the urethral wall is better adapted to this latter task since it can achieve it at relatively low energy cost.
There is no doubt that the smooth muscle of the urethra is capable of generating significant tone in the absence of neural input. We have recently demonstrated that an isolated cannulated rat urethra can maintain sufficient tone to limit flow from a reservoir (held at a constant pressure of 20 cmH2O) to less than 25% of the flow encountered when the urethra is maximally dilated (Fig. 1). Under these conditions nerve and striated muscle contributions are likely to be minimal so tone is largely due to sustained contraction of the intramural smooth muscle. That this is so can be demonstrated by applying wortmanin (a myosin light chain kinase inhibitor) which has the effect of fully dilating the urethra within 30 min. Prior to wortmanin application, electrical field stimulation of the inhibitory nerves (0.3 ms pulse width at 0.5 Hz in the presence of atropine and guanethidine) caused a similar increase in flow.
Figure 1. Flow through a cannulated rat urethra that is connected to a constant pressure reservoir of Krebs solution.
Under resting conditions flow was about 20% of maximum. Stimulation of the inhibitory nerves caused the urethra to relax and flow to exceed 80% of maximum. When 30 μm wortmanin was added, flow increased almost to the level seen when the inhibitory nerves were stimulated.
The mechanism of myogenic tone in the urethra is still not fully understood. It has been assumed in the past that tone was due to steady influx of Ca2+ through L-type channels during a window current, a mechanism similar to that proposed for arterial smooth muscle (Smirnov & Aaronson, 1992; Fleishmann et al. 1994). However, a number of recent studies have suggested that urethral tone is associated with the generation of spontaneous transient depolarizations and large regularly occurring slow waves (Hashitani et al. 1996; Hashitani & Edwards, 1999). These events appear to be due to spontaneous release of calcium from intracellular stores and this in turn activates a calcium-activated chloride current which provides the depolarizing current responsible for slow-wave activation. The above studies were carried out by impaling the urethras of rabbits or guinea-pigs with sharp electrodes so it was not possible to determine the exact source of the electrical activity that was being measured. Hashitani et al. (1996) drew attention to the similarities of the slow-wave activity in urethra to that of the gastrointestinal tract, where spontaneous activity originates in specialized pacemaker cells or interstitial cells of Cajal (ICC). They noted that no such cells had been found in urethra. However, in the same year, Smet et al. (1996) demonstrated that the human bladder and urethra had interstitial cells which bore a striking resemblance to the ICC in the digestive tract (Thuneberg, 1982; Sanders, 1996).
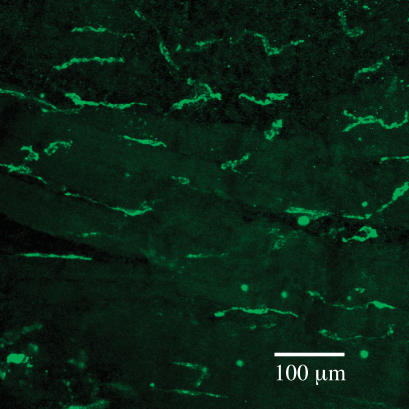
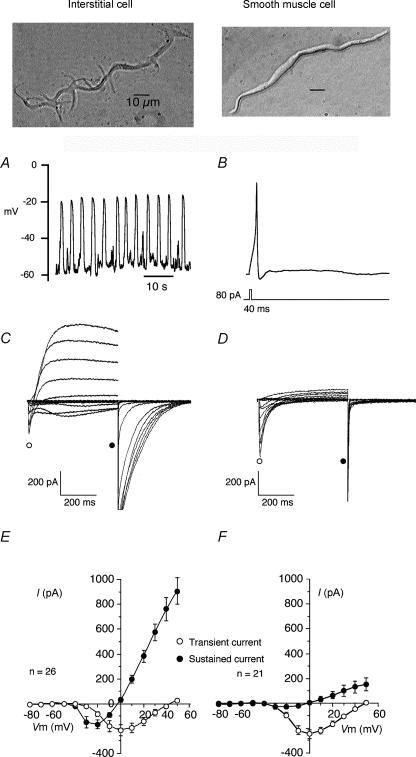
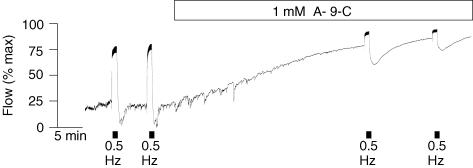
Sergeant et al. (2000) reported that collagenase dispersal of strips of rabbit urethra yielded, in addition to normal spindle-shaped smooth muscle cells, a small proportion of branched cells which resembled the interstitial cells of Cajal dispersed from canine colon (Langton et al. 1989). These were clearly distinguishable from smooth muscle in their appearance under the phase contrast microscope, their immunohistochemistry and ultrastructure. They had abundant vimentin filaments but no myosin, a discontinuous basal lamina, sparse rough endoplasmic reticulum, many mitochondria and well-developed smooth endoplasmic reticulum. At the time it was reported that the cells were not c-kit positive but with improved procedures it has recently been demonstrated that they are. Figure 2 shows a whole mount preparation of rabbit proximal urethra stained with Kit antibody. ICC are evident as irregular elongated cells of about 80–100 μm in length between and surrounding the smooth muscle bundles (the latter are evident because of their weak autofluoresence). ICC generated spontaneous depolarizations, whereas isolated SMCs from the same preparation were electrically quiescent, but could respond to injection of depolarizing current by producing action potentials (Fig. 3, taken from Sergeant et al. 2000). Clues to the mechanisms underlying these different responses were provided by the results of voltage clamp studies, which showed that ICC possessed an abundance of Ca2+-activated chloride current (IClCa) whereas SMCs in comparison did not. Moreover, when ICC were held at −60 mV they generated spontaneous transient inward currents (STICs; Sergeant et al. 2000) which were notably similar to pacemaker currents recorded from ICC in the gastrointestinal tract (Thomsen et al. 1998; Koh et al. 1998). This property, coupled with other fundamental structural and morphological similarities, opens up the intriguing possibility that urethral interstitial cells serve a similar purpose and are therefore key determinants of urethral tone. Our hypothesis therefore is that tone in the urethra is initiated by the oscillatory release of calcium from intracellular stores in the interstitial cells. This, in turn, causes activation of calcium-activated chloride channels resulting in the spontaneous depolarizations referred to above. These, in turn, activate in an asynchronous fashion the smooth muscle bundles to which they are electrically coupled to produce a sustained tone. Recent experiments with the isolated cannulated urethra preparation lend some support to this idea (Fig. 4). Anthracene-9-carboxylic acid (which is known to block Ca2+-activated chloride channels and spontaneous depolarizations in the isolated interstitial cells) was almost as effective as wortmanin in relaxing urethral tone.
Figure 2. Whole mount of rabbit urethra labelled with anti-Kit antibody.
ICC are evident as irregular elongated cells of about 80–100 μm in length between and surrounding the smooth muscle bundles (the latter are evident because of their weak autofluoresence).
Figure 3. Contrasting electrical properties of interstitial and smooth muscle cells.
Interstitial cells showed regular ‘slow-wave’ depolarizations in current clamp (A) while smooth muscle cells were quiescent, although they could produce an action potential in response to depolarizing current (B). Under voltage clamp, interstitial cells exhibited both L-type calcium currents and calcium-activated chloride currents (C) while the smooth muscle cells showed only L-type calcium currents (D). E and F show summaries of the currents measured in 26 interstitial and 21 smooth muscle cells (redrawn from Sergeant et al. 2000).
Figure 4. The effect of anthracene-9-carboxcylic acid on flow through the isolated urethra.
A-9-C (1 μm), like wortmanin, caused an increase in flow almost equal to the effect of stimulation of the inhibitory nerves.
Conclusions
The urinary tract shows evidence of rhythmical electrical and mechanical activity at all levels. The purpose of this is evident in the ureter where it is important for pumping urine but rather less so in the bladder and in the urethra. It would seem that the bladder's ability to expand in a controlled way is dependent on the tone generated by asynchronous firing of many pacemakers. Clearly if these pacemakers could then be coordinated (under neural influence) one has the basis of an efficient emptying system. The urethra, on the other hand, exists only to produce tone and this may also occur by asynchronous firing of many pacemakers.
Acknowledgments
The authors wish to acknowledge support from grant number 064212 from the Wellcome Trust and NIH RO1 DK68565. G.P.S. is in receipt of a Research Fellowship awarded by the Health Research Board, Ireland.
References
- Biers SM, Reynard JM, Doore T, Brading AF. The functional effects of a c-kit tyrosine inhibitor on guinea-pig and human detrusor. BJU Int. 2006;97(3):612–616. doi: 10.1111/j.1464-410X.2005.05988.x. [DOI] [PubMed] [Google Scholar]
- Bradley JE, Anderson UA, Woolsey SM, Thornbury KD, McHale NG, Hollywood MA. Characterization of T-type calcium current and its contribution to electrical activity in rabbit urethra. Am J Physiol Cell Physiol. 2004;286:C1078–C1088. doi: 10.1152/ajpcell.00463.2003. [DOI] [PubMed] [Google Scholar]
- Creed KE. Membrane properties of smooth muscle membrane of the guinea-pig urinary bladder. Pflügers Arch. 1971;326:115–126. doi: 10.1007/BF00586904. [DOI] [PubMed] [Google Scholar]
- Creed KE, Isikawa S, Ito Y. Electrical and mechanical activity recorded from rabbit urinary bladder in response to nerve stimulation. J Physiol. 1983;338:149–164. doi: 10.1113/jphysiol.1983.sp014666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SG, Cebrian C, Vaughan ED, Herzlinger D. C-kit and ureteral peristalsis. J Urol. 2005;173:292–295. doi: 10.1097/01.ju.0000141594.99139.3d. [DOI] [PubMed] [Google Scholar]
- Davidson RA, McClosky KD. Morphology and localization of interstitial cells in the guinea pig-bladder: structural relationships with smooth muscle and neurons. J Urol. 2005;173:1385–1390. doi: 10.1097/01.ju.0000146272.80848.37. [DOI] [PubMed] [Google Scholar]
- Fleishmann BK, Murray RK, Kotlikoff MI. Voltage window for sustained elevation of cytosolic calcium in smooth muscle cells. Proc Natl Acad Sci U S A. 1994;91:11914–11918. doi: 10.1073/pnas.91.25.11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Edwards FR. Spontaneous and neurally activated depolarizations in smooth muscle cells of the guinea-pig urethra. J Physiol. 1999;514:459–470. doi: 10.1111/j.1469-7793.1999.459ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Van Helden DF, Suzuki H. Properties of spontaneous depolarizations in circular smooth muscle cells of rabbit urethra. Br J Pharmacol. 1996;118:1627–1632. doi: 10.1111/j.1476-5381.1996.tb15584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Yanai Y, Suzuki H. Role of interstitial cells and gap junctions in the transmission of spontaneous Ca2+ signals in detrusor smooth muscles of the guinea-pig urinary bladder. J Physiol. 2004;559:567–581. doi: 10.1113/jphysiol.2004.065136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm MF, Exintaris B, Lang RJ. Identification of the cells underlying pacemaking activity in the guinea-pig upper urinary tract. J Physiol. 1999;519:867–884. doi: 10.1111/j.1469-7793.1999.0867n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Biers SM, Kohri K, Brading AF. Effects of imatinib mesylate (Glivec) as a c-kit tyrosine kinase inhibitor in the guinea-pig urinary bladder. Neurourol Urodyn. 2006;25:205–210. doi: 10.1002/nau.20085. [DOI] [PubMed] [Google Scholar]
- Kuriyama H, Osa T, Toida N. Membrane properties of the smooth muscle of the guinea-pig ureter. J Physiol. 1967;191:225–238. doi: 10.1113/jphysiol.1967.sp008247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang RJ, Davidson ME, Exintaris B. Pyeloureteral motility and ureteral peristalsis: essential role of sensory nerves and endogenous prostaglandins. Exp Physiol 87. 2002;2:129–146. doi: 10.1113/eph8702290. [DOI] [PubMed] [Google Scholar]
- Lang RJ, Exintaris B, Teele ME, Harvey J, Klemm MF. Electrical basis of peristalsis in the mammalian upper urinary tract. Clin Exp Pharmacol Physiol. 1998;25:310–321. doi: 10.1111/j.1440-1681.1998.tb02357.x. [DOI] [PubMed] [Google Scholar]
- Lang RJ, Klemm MF. Interstitial cell of Cajal-like cells in the upper urinary tract. J Cell Mol Med. 2005;9:543–556. doi: 10.1111/j.1582-4934.2005.tb00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang RJ, Takano H, Davidson ME, Suzuki H, Klemm MF. Characterization of the spontaneous electrical and contractile activity of smooth muscle cells in the rat upper urinary tract. J Urol. 2001;166:329–334. [PubMed] [Google Scholar]
- Langton P, Ward SM, Carl A, Norell MA, Sanders KM. Spontaneous electrical activity of interstitial cells of Cajal isolated from canine proximal colon. Proc Natl Acad Sci U S A. 1989;86:7280–7284. doi: 10.1073/pnas.86.18.7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orbeli L, Brucke TE. Beitragezur Physiologie der autonomen innervierten Muskulatur. II. Die Actionsstrome der Uretermuskulatur wahrend des Ablaufs spontaner Wellen. Arch Ges Physiol. 1910;133:341–364. [Google Scholar]
- Prosser CL, Smith CE, Melton CE. Conduction of action potentials in the ureter of the rat. Am J Physiol. 1955;181:651–660. doi: 10.1152/ajplegacy.1955.181.3.651. [DOI] [PubMed] [Google Scholar]
- Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterol. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- Sergeant GP, Hollywood MA, McCloskey KD, Thornbury KD, McHale NG. Specialised pacemaking cells in the rabbit urethra. J Physiol. 2000;526:359–366. doi: 10.1111/j.1469-7793.2000.t01-2-00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smet PJ, Jonavicious J, Marshall VR, DeVente J. Distribution of nitric oxide synthase-immunoreactive nerves and identification of the cellular targets of nitric oxide in guinea-pig and human urinary bladder by cGMP immunohistochemistry. J Neurosci. 1996;71:337–348. doi: 10.1016/0306-4522(95)00453-x. [DOI] [PubMed] [Google Scholar]
- Smirnov SV, Aaronson PI. Ca2+ currents in single myocytes from human mesenteric arteries: evidence for a physiological role of L-type channels. J Physiol. 1992;457:455–475. doi: 10.1113/jphysiol.1992.sp019387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui GP, Rothery S, Dupont E, Fry CH, Severs NJ. Gap junctions and connexin expression in human suburothelial interstitial cells. BJU Int. 2002;90:118–129. doi: 10.1046/j.1464-410x.2002.02834.x. [DOI] [PubMed] [Google Scholar]
- Thuneberg L. Interstitial cells of Cajal: intestinal pacemaker cells? Adv Anat Embryol Cell Biol. 1982;71:1–130. [PubMed] [Google Scholar]
- Ursillo RC. Electrical activity of the isolated nerve-urinary bladder strip preparation of the rabbit. Am J Physiol. 1961;201:408–412. doi: 10.1152/ajplegacy.1961.201.3.408. [DOI] [PubMed] [Google Scholar]
- Wiseman OJ, Fowler CJ, Landon DN. The role of the human bladder lamina propria myofibroblast. BJU Int. 2003;91:89–93. doi: 10.1046/j.1464-410x.2003.03802.x. [DOI] [PubMed] [Google Scholar]