Abstract
Sex hormones modulate plasticity in the central nervous system, including respiratory long-term facilitation (LTF), a form of serotonin-dependent respiratory plasticity induced by intermittent hypoxia. Since gonadectomy (GDX) attenuates LTF in male rats, we tested the hypotheses that: (1) testosterone replenishment restores LTF in gonadectomized male rats, and (2) that the conversion of testosterone to oestradiol (under the influence of aromatase) is required for these effects. Intact and sham operated male F344 rats were compared to gonadectomized rats implanted with Silastic tubing containing testosterone (T), T plus an aromatase inhibitor (ADT), or 5α-dihydrotestosterone (DHT), a form of testosterone not converted to oestradiol. Seven days postsurgery, LTF was studied in anaesthetized, neuromuscularly blocked and ventilated rats while monitoring integrated phrenic and hypoglossal (XII) motor output. LTF was elicited by three 5 min hypoxic episodes (Pa,O2 = 35 − 45 mmHg). Although significant phrenic and XII LTF were observed in all rat groups, GDX reduced both phrenic and XII LTF, an effect reversed by T. In contrast, LTF was not restored in T + ADT or DHT-treated gonadectomized rats. We conclude that the conversion of testosterone to oestradiol modulates phrenic and XII LTF in male F344 rats.
Long-term facilitation (LTF) is a form of respiratory plasticity expressed as a persistent augmentation of phrenic and/or hypoglossal (XII) nerve activity following intermittent hypoxia. The fundamental mechanisms of LTF have been studied extensively in recent years (Mitchell et al. 2001; Feldman et al. 2003). Spinal serotonin (5-HT) receptor activation during hypoxic episodes initiates LTF (Fuller et al. 2001; Baker-Herman & Mitchell, 2002), whereas new synthesis of brain derived neurotrophic factor (BDNF) maintains it (Baker-Herman et al. 2004). Thus, factors that affect serotonergic function and BDNF synthesis may have the capacity to modulate LTF.
Sex hormones influence respiratory plasticity. For example, hypoglossal LTF is (1) reduced by gonadectomy in male rats (Zabka et al. 2005), (2) correlated with serum testosterone levels in male rats (Zabka et al. 2005), and (3) correlated with serum oestradiol and progesterone levels in female rats (Zabka et al. 2003). Sex hormones enhance serotonergic function in many brain regions (Aylward, 1973; Poirier et al. 1985; Lopez-Jaramillo & Teran, 1999; Bethea et al. 2000; Klink et al. 2002; Kugaya et al. 2003). Thus, gonadectomy in male rats reduces 5-HT concentration in the caudal raphe nuclei (Long et al. 1983), and decreases 5-HT immunoreactivity in the XII motor nucleus (M. Behan, unpublished observations). In female rats, serotonin concentrations vary with the oestrus cycle in phrenic and hypoglossal motor nuclei, and are associated with oestrus-cycle related differences in phrenic and hypoglossal LTF (Zabka et al. 2001b; Behan et al. 2003). Consequently, sex hormones have the potential to modulate hypoglossal and phrenic LTF via effects on serotonergic function within the respective motor nuclei.
Sex hormones also influence BDNF gene expression (Gibbs, 1999; Scharfman et al. 2003; Zhao et al. 2004; Scharfman & MacLusky, 2005). Since BDNF plays a key role in LTF (Baker-Herman et al. 2004), oestradiol and progesterone may influence LTF by altering BDNF expression in respiratory motor nuclei. Furthermore, since BDNF exerts complex trophic effects on serotonergic neurons (Mamounas et al. 1995, 2000; Mattson et al. 2004), and serotonin receptor activation increases BDNF synthesis within respiratory motor nuclei (Baker-Herman et al. 2004), sex hormone effects on BDNF and serotonin may have complex, interactive effects on respiratory plasticity.
Since oestrogen modulates many forms of neuroplasticity (Woolley & McEwen, 1994; Murphy et al. 1998; Brinton, 2001; Scharfman et al. 2003), and exerts potent effects on both 5-HT and BDNF, an important question is whether the effect of testosterone on respiratory LTF in male rats is direct, or indirect by conversion to oestradiol due to aromatase activity. For example, testosterone influences 5-HT2A receptor expression in the rat forebrain only through the conversion of testosterone to oestrogen (Sumner & Fink, 1998). In this study, we tested the hypotheses that decreased LTF following gonadectomy in male rats can be reversed by restoring normal circulating testosterone levels, and that this effect requires the conversion of testosterone to oestrogen under the influence of aromatase activity. Differential influences of testosterone and oestrogen on respiratory plasticity may contribute to an understanding of sexual dimorphisms in age-related human breathing disorders, such as age and sex-specific manifestations of obstructive sleep apnoea (Redline et al. 1994; Ware et al. 1999; Bixler et al. 2001).
Methods
Experimental groups
Experiments were performed on young adult male Fischer 344 rats (National Institutes of Health, National Institute of Ageing Colony). Six groups of male rats (3–4 months old) were used for this study: intact, unoperated (Intact, n (phr) = 7, n (XII) = 6), sham operated (Sham n (phr) = 6, n (XII) = (8), gonadectomized (GDX n (phr) = 9, n (XII) = (6), gonadectomized with testosterone supplement (T, n (phr) = 8, n (XII) = 8), gonadectomized with testosterone and an aromatase inhibitor (ADT, n (phr) = 8, n (XII) = 11), and gonadectomized with 5α-dihydrotestosterone supplement (DHT, n (phr) = 10, n (XII) = 8), a form of testosterone that cannot be converted to oestradiol. n differed between phrenic and XII analyses since only one neurogram was usable for data analysis in some rats. All experimental procedures were approved by the University of Wisconsin–Madison Animal Care and Use Committee.
Gonadectomy
Anaesthesia was induced with isoflurane in an induction chamber and maintained (2.0–2.5% isoflurane in 30% O2) using a nose cone. All operated rats received 0.05 mg kg−1 buprenorphine i.m. prior to surgery to prevent postsurgical pain. Rats were positioned in dorsal recumbency on a heated pad. The scrotum was clipped and cleaned with an antiseptic detergent. A skin incision of 1 cm was made on the raphe scroti followed by a 0.5 cm incision in the muscle layer over the testicles. Testicles were removed by separating them distally from a ligature around the ductus deferens and accompanying blood vessels with a scalpel. The muscle layer and the skin incision were closed with reabsorbable suture and staples, respectively.
Hormone implants
Silastic® laboratory tubing (Dow Corning, Midland, MI, USA) was used for testosterone (crystalline testosterone (T), Steraloids, Inc. (Newport, RI, USA) and aromatase inhibitor (crystalline 1,4,9-androstatriene-3,17-dione (ADT), Steraloids implants. Prior to implantation, T and ADT implants were submerged in 0.1 mol l−1 phosphate buffered saline at room temperature for 24 h. GDX and Sham rats received one empty Silastic implant (T implant size). DHT rats received one pellet of 5α-dihydrotestosterone (10.0 mg per pellet, 21 day release, Innovative Research of America, Sarasota, FL, USA) subcutaneously.
Two pilot studies were conducted to determine the number of implants that would produce serum hormone levels in the middle of the physiological range 7 days following surgery (GDX and hormone replacement). In pilot studies, physiological serum testosterone levels in intact animals were 11.7 ± 4.7 and 11.6 ± 3.5 ng ml−1, respectively (range 1.7–27.7 and 1.8–26.8 ng ml−1, respectively; n = 3). In gonadectomized rats, two testosterone implants (inner diameter 0.16 cm, outer diameter 0.32 cm, length 3 cm) per rat resulted in testosterone levels of 5.3 ± 0.3 ng ml−1. One ADT (inner diameter 0.15 cm, outer diameter 0.20 cm, length 3 cm) and one testosterone implant resulted in testosterone levels of 5.0 ± 0.2 ng ml−1. Two ADT implants and one testosterone implant resulted in testosterone levels of 7.3 ± 0.4 ng ml−1. Based on these pilot experiments, for the T group of rats we implanted three testosterone implants per rat. For the ADT group of rats, we implanted three ADT and one testosterone implant per rat.
Hormone replacement
Following gonadectomy or sham surgery, rats were placed in ventral recumbency. One cm distal to the shoulder blades, the skin was clipped and cleaned with an antiseptic detergent in an area 2 cm × 2 cm. A skin incision of 1 cm was made parallel to the spine. The skin was undermined bluntly and Silastic implants or DHT pellets were inserted. The incision was closed using reabsorbable suture (Vicryl, 3–0). Seven days postgonadectomy, after initiating anaesthesia for the acute neurophysiological experimental protocol (LTF protocol), blood was collected to assess levels of testosterone, 5α-DHT, oestrogen and progesterone. Hormone levels were compared to levels in intact, unoperated animals that were used for the neurophysiological experiments.
Experimental preparation
Methods have been extensively described in previous publications (Bach & Mitchell, 1996; Baker & Mitchell, 2000; Fuller et al. 2001; Zabka et al. 2001a,b, 2003, 2005). In brief, animals were anaesthetized with urethane, subjected to neuromuscular blockade (pancuronium bromide, 2.5 mg/kg i.v.), bilaterally vagotomized and pump ventilated. Blood samples (0.2 ml in a 0.5 ml heparinized glass syringe) were drawn to determine arterial blood gases (Pa,O2 and Pa,CO2), pH and base excess (ABL 500; Radiometer, Copenhagen, Denmark). Body temperature was maintained between 37 and 38°C using a heated table. End-tidal CO2 was measured with a flow-through capnograph (Capnogard, Novametrix, Wallingford, CT, USA). The left phrenic and XII nerves were isolated via a dorsal approach, cut distally, desheathed, submerged in mineral oil and placed on bipolar silver wire electrodes. Nerve activities were amplified (×10 000), band pass filtered (100 Hz to 10 kHz) (Model 1700, A-M Systems, Inc., Carlsborg, WA, USA) and integrated (time constant = 50 ms, Model MA-821RSP, CWE Inc., Ardmore, PA, USA).
Experimental protocol
Before recording, phrenic and XII nerve activities were allowed to stabilize for approximately 90 min following surgery under hyperoxia and normocapnia (Pa,O2 > 150 mmHg). Subsequently, the CO2 apnoeic/recruitment threshold was determined and baseline nerve activities were established 2 mmHg above this threshold. Baseline blood gas values were assessed before starting the protocol. All subsequent blood samples were compared to this initial baseline value. Strict isocapnia (± 1 mmHg from baseline Pa,CO2) was maintained throughout an experiment. An LTF protocol started with three 5 min hypoxic episodes (FI,O2 = 0.11, target Pa,O2 35–45 mmHg) separated by 5 min intervals, and followed by 60 min of isocapnic hyperoxic baseline conditions. A protocol ended with 5 min of hypercapnia (PET,CO2 = 80 − 90 mmHg) to assess maximal hypercapnia-stimulated nerve activity. Arterial blood samples were drawn during the last minute of the first hypoxic episode to determine the severity of hypoxia, and 15, 30 and 60 min after the final hypoxic episode to confirm isocapnic conditions. Rats were excluded from analysis if Pa,CO2 deviated from baseline by more than 3 mmHg during hypoxia or 1 mmHg during post-hypoxic episodes from baseline. Therefore, changes in Pa,CO2 had minimal impact on the results of this study. Experiments were also excluded if arterial blood pressure dropped by more than 30 mmHg from baseline at the end of a protocol. At the end of each experiment, rats were killed with an overdose of urethane (i.v.).
Sex hormone levels
Arterial blood samples (1 ml) were taken as soon as the arterial catheter was placed. Subsequently, blood samples were centrifuged to collect serum. Serum was immediately frozen at −80°C. After collection of all serum samples, total testosterone, oestradiol and progesterone levels were analysed using radioimmunoassay (RIA) (Testosterone Coat-a-Count, Oestradiol Coat-a-Count, Progesterone Coat-a-Count; Diagnostic Products, Los Angeles, CA, USA). 5α-Dihydrotestosterone was analysed using enzyme-linked immunosorbent assay (ELISA) (Immuno-Biological Laboratories Inc., Minneapolis, MN, USA). Prior to analysing the samples, the assays were validated with pooled serum from 10 rats to create a standard curve.
Data analysis
Phrenic and XII nerve activities were recorded throughout the protocol. Peak integrated amplitude (ΔPhr and ΔXII), burst frequency (bursts min−1), and mean arterial blood pressure (MAP) were measured at the following time points: baseline, last minute of first hypoxic episode (short-term hypoxic response), 15, 30 and 60 min after the final hypoxic episode, and the last minute of the hypercapnic response (max CO2 response; Fig. 1). Nerve activity was averaged over approximately 60 s at each measurement. Changes in amplitude from baseline were normalized as a percentage of baseline nerve activity (% baseline), and as a percentage of the hypercapnic response (% maximum). All conclusions were the same, regardless of the normalization used. Thus, only percentage baseline data are presented in this paper. Changes in burst frequency were expressed as a difference from baseline in bursts per minute.
Figure 1. Representative phrenic LTF protocols from Intact, GDX and GDX-T rats.
After establishing baseline conditions, three episodes of hypoxia (11% O2) were applied. Arterial blood samples for blood gas analysis (arrows) were taken under baseline conditions (BL), during the last minute of the first hypoxia, and 15, 30 and 60 min after the last hypoxic episode. A protocol ended with 5 min of hypercapnia to assess maximal nerve activity. The upper panel shows a compressed tracing from a young adult male rat revealing an LTF magnitude typically seen in young male rats. Tracings from Intact and Sham rats were comparable (not shown). The middle tracing shows reduced LTF in a gonadectomized (GDX) young male rat. Tracings from GDX and T/ADT or DHT rats were comparable (not shown). The bottom tracing shows LTF in a gonadectomized rat supplemented with testosterone (T). PET,CO2: end-tidal CO2
Depending on the variable, either a one-way or a two-way ANOVA with a repeated measures design (SigmaStat v. 2.0, Jandel Corporation, San Rafael, CA, USA) was performed, followed by post hoc inferences for individual comparisons using the least significant difference test (Fisher's LSD method). If the normality test failed, a Kruskal–Wallis one-way ANOVA on ranks was performed followed by an all pair-wise multiple comparisons test (Dunn's Method) if differences were significant. Differences were considered significant if P < 0.05. All data reported are means ± s.e.m. Serum levels of testosterone, 5α-DHT, oestradiol and progesterone in individual rats were related to the magnitude of phrenic and XII LTF via multiple and/or simple linear regressions. A variable was considered to contribute significantly to the model if P < 0.05.
Results
Experimental animals
Mean weights of rats in all groups were statistically indistinguishable, either at the time of surgery, or at the time of acute LTF studies (range: 263–300 g; P > 0.05).
Sex hormone levels
After gonadectomy, serum testosterone levels were reduced, although to a lesser extent than in previous studies from our laboratory (Behan et al. 2003; Zabka et al. 2005). Serum testosterone levels were at the high end of the physiological range in T and ADT rats (2.34–18.2 and 5.03–11.4 ng ml−1, respectively) (Fig. 2A). Serum testosterone levels were significantly higher in T and ADT rats than in GDX or DHT rats (P < 0.05), but not different from Intact or Sham animals (P > 0.05; Fig. 2A). Serum oestradiol and progesterone levels were not significantly different among groups (P > 0.05; Fig. 2A). Serum 5α-DHT levels were similar in T and ADT rats but significantly greater than in Intact, Sham, GDX and DHT rat (Fig. 2B).
Figure 2. Serum levels of testosterone, oestradiol, progesterone and 5α-DHT during baseline conditions.
A, testosterone levels (ng ml−1) were significantly greater in T (*) and ADT (#) rats than in GDX or DHT rats (P < 0.05), but not different from Intact or Sham animals (P > 0.05). Oestradiol levels (pg ml−1) and progesterone levels (ng ml−1) did not differ among groups (P > 0.05). B, 5α-DHT levels (pg ml−1) were significantly greater in T (*) and ADT (#) rats than all other groups (P < 0.05). Values are means ± s.e.m. from all animals used for XII LTF analysis.
Apnoeic/recruitment threshold, baseline conditions and CO2 regulation
Baseline conditions were standardized through individual determinations of individual CO2 apnoeic/recruitment thresholds, and establishing baseline conditions 2 mmHg above this level. Overall, the CO2 apnoeic/recruitment threshold did not differ among rat groups (Intact = 41 ± 2; Sham = 41 ± 2; GDX = 41 ± 1; T = 40 ± 1; ADT = 39 ± 1; DHT = 41 ± 1 mmHg; P > 0.05). Throughout protocols, strict isocapnic conditions were maintained to prevent CO2-based changes in phrenic or XII nerve activity. The ratio of baseline/maximal CO2 response was similar in all rat groups for phrenic and XII nerve activity indicating a similar dynamic range and baseline ventilatory drive in all rats (Phrenic: P > 0.05; XII: P > 0.05).
Short-term hypoxic responses
Pa,O2 during hypoxic episodes did not differ among rat groups (P > 0.05). Phrenic and XII short-term hypoxic response amplitude when expressed as a percentage change from baseline (Table 1), and frequency when expressed as a change from baseline (bursts min−1) were not different among groups (all P > 0.05). Thus, sex hormone changes have little effect on phrenic or XII short-term hypoxic responses in anaesthetized rats.
Table 1.
Short-term hypoxic responses
| Phrenic amplitude (% change from baseline) | XII amplitude (% change from baseline) | |
|---|---|---|
| Intact | 65 ± 11 | 161 ± 22 |
| Sham | 48 ± 2 | 143 ± 17 |
| GDX | 64 ± 14 | 130 ± 21 |
| T | 76 ± 18 | 169 ± 16 |
| ADT | 59 ± 9 | 150 ± 18 |
| DHT | 75 ± 11 | 155 ± 23 |
Phrenic long-term facilitation (LTF)
All six groups of rats revealed phrenic LTF (ΔPhr significantly increased versus baseline; Δ percentage BL) and showed a significant time–treatment interaction (P = 0.003). In individual groups, LTF was observed in Intact, GDX, T and DHT animals at 15, 30 and 60 min, in Sham animals at 30 and 60 min, and in ADT animals at 15 and 30 min following episodic hypoxia (P < 0.001; Fig. 3A). The magnitude of phrenic LTF was significantly different among groups at 30 and 60 min post-episodic hypoxia (P < 0.05) such that T rats had significantly greater LTF compared to Sham, GDX, ADT, and DHT rats at 30 and 60 min (P < 0.05). Furthermore, at 60 min post-hypoxia, Intact rats developed greater LTF than ADT and DHT rats (P < 0.05); LTF in Sham animals was greater than ADT (P = 0.027). Intact and T animals were not different at any time post-episodic hypoxia.
Figure 3. Long-term facilitation (LTF) measured at 15, 30, and 60 min post-episodic hypoxia.
A, at 30 and 60 min, phrenic LTF in T (*) was significantly greater than in Sham, GDX, ADT and DHT rats. At 60 min, phrenic LTF in Intact (#) was significantly greater than in ADT and DHT rats, and LTF in Sham (‡) was significantly greater than in ADT rats (all P < 0.05). B, at 30 min, XII LTF in T (*) was significantly greater than in GDX and DHT rats. LTF in Sham (#) was significantly greater than in GDX rats (all P < 0.05). At 60 min, XII LTF in Intact, Sham and T (‡) was significantly greater than in GDX, ADT and DHT rats (all P < 0.05).
XII long-term facilitation
All six groups of rats revealed XII LTF (ΔXII significantly increased versus baseline; Δ percentage BL) and showed a significant time–treatment interaction (P = 0.008). In individual groups, LTF was observed in Intact, Sham, GDX, T and DHT animals at 15, 30 and 60 min, and in ADT animals at 15 and 30 min following episodic hypoxia (P < 0.001; Fig. 3B). The magnitude of XII LTF was significantly different among groups at 30 and 60 min post-episodic hypoxia (P < 0.05). T rats had significantly greater LTF compared to GDX and DHT rats at 30 min (P < 0.03), and compared to GDX, ADT and DHT rats at 60 min (P = 0.001). Furthermore, at 30 min, Sham showed greater LTF than GDX rats (P = 0.018) and at 60 min, Intact and Sham rats developed greater LTF than GDX, ADT and DHT rats (P < 0.04). Intact, Sham and T animals were not different at any time post-episodic hypoxia.
Burst frequency LTF
There was a small but significant increase in burst frequency versus baseline post-hypoxia (i.e. frequency LTF) including all groups at 30 and 60 min and in T animals only at 15 min (P < 0.05; data not shown). LTF in T animals was significantly greater versus DHT at 15 min, versus ADT at 30 min, and versus GDX at 60 min (P < 0.05).
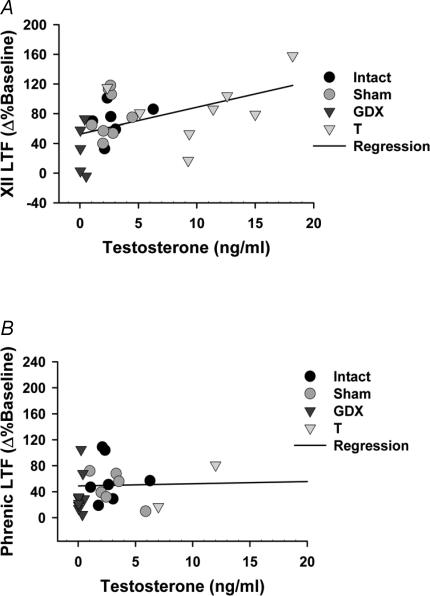
Sex hormone levels correlate with LTF
There was no correlation between the magnitude of phrenic LTF and any sex hormone measured in serum at the beginning of experiments (Fig. 4A). However, there was a weak, but significant positive correlation between XII LTF and serum testosterone in Intact, Sham, GDX and T rats, but not in DHT and ADT rats (Fig. 4B). The linear model expressing this relationship was:
Figure 4. Correlation of Phrenic and XII LTF with serum testosterone levels.
A, the magnitude of XII LTF measured in Intact, Sham, GDX, T and DHT rats was positively correlated with serum testosterone levels (R2 = 0.208; P = 0.015). B, no correlation was observed between phrenic LTF in and serum testosterone levels (R2 = 0.0495; P = 0.237).
Mean arterial blood pressure (MAP)
MAP did not differ between rat groups at any time before, during or following hypoxic episodes (P > 0.05; data not shown). During hypoxic episodes, MAP decreased significantly in all groups, as typically observed during hypoxia in anaesthetized rats.
Discussion
The major finding of this study is that phrenic and XII LTF are restored by testosterone replacement in gonadectomized male rats, and that this effect requires the conversion of testosterone to oestrogen by the actions of aromatase. This latter conclusion is supported by observations that testosterone replacement with an aromatase inhibitor, and application of a testosterone derivate that is not converted to oestrogen, fail to restore LTF in either neurogram. We speculate that testosterone-derived oestrogen exerts indirect effects on the serotonergic system and/or BDNF within the respective respiratory motor nuclei, thereby restoring phrenic and XII LTF.
Sex hormone levels
In this study, gonadectomy failed to decrease serum testosterone levels significantly as observed previously in our laboratory (Behan et al. 2003; Zabka et al. 2005). Such interstudy variations may result from: (1) incomplete gonadectomy, particularly in animals with testosterone levels > 1 pg ml−1; or (2) relatively low serum testosterone levels in the Intact and Sham rats used in this study (range 1.08–6.27 and 1.01–5.89 ng ml−1, respectively) compared with levels measured in two pilot studies (see Methods), although serum testosterone levels in the present study are similar to levels reported in several other studies (Dohler & Wuttke, 1974; Smith et al. 1992; Neill, 2006). Nonetheless, significantly greater serum testosterone levels in T and ADT rats compared with GDX or DHT rats that were not different from Intact or Sham animals suggest that reasonable and appropriate manipulations of sex hormone levels were achieved in this study (Fig. 2A). Since serum oestradiol and progesterone levels were not significantly different among groups, differences in circulating levels of these hormones cannot explain differences caused by testosterone replacement. However, this finding does not rule out localized differences in oestrogen and progesterone levels in other body compartments, such as the central nervous system. On the other hand, serum oestradiol levels are quite low in male rats, and are at the edge of the detectable range using our assay procedures. Thus, potential increases in serum oestradiol may have been obscured by a lack of sensitivity in the bioassay and/or by oestradiol binding to globulin and albumin (Davison et al. 2005). In a study by Robaire et al. (1979), testosterone implants of differing strengths failed to alter serum oestradiol levels in male rats.
Short-term hypoxic responses
Phrenic and XII short-term hypoxic responses were unaffected by sex hormones, as shown previously in this rat strain (Zabka et al. 2005). Other studies have demonstrated that the short-term ventilatory and/or carotid chemoafferent neuron responses to hypoxia are influenced by sex hormones in some species (Schlenker & Goldman, 1985; Hannhart et al. 1990; Tatsumi et al. 1994, 1997). Apparent discrepancies between our study and previous reports might arise from species and/or experimental preparation differences, or may be due to the direct assessment of respiratory motor output in the phrenic and XII nerves in this study versus measurements of carotid sinus nerve activity and breathing.
Long-term facilitation (LTF)
Earlier studies on Sprague-Dawley and F344 rats report that XII LTF is reduced by gonadectomy (Behan et al. 2003; Zabka et al. 2005) whereas phrenic LTF is unaffected (Zabka et al. 2005). Consistent with these earlier studies, gonadectomy reduced XII LTF in the present experiments. On the other hand, in contrast to these earlier reports, we found that phrenic LTF is also reduced following gonadectomy in the present study. This discrepancy in the phrenic LTF response to gonadectomy in the two studies is difficult to explain. In general, there is greater variability in reported phrenic motoneuron responses to age and/or sex hormones in male and female rats, whereas XII responses have been more consistent (Zabka et al. 2001a,b, 2003, 2005; Behan et al. 2002, 2003). Both motoneuron pools have robust expression of androgen and oestrogen receptors (Behan & Thomas, 2005), leading us to speculate that their differing responses to sex hormones may be regulated at the level of neuromodulatory inputs such as 5-HT in the caudal raphe nuclei. Since increased androgen levels enhance aromatase activity (Roselli, 1991; Wagner & Morrell, 1997; Roselli et al. 1998; Bourguiba et al. 2003), pharmacological testosterone administration may enhance its own conversion to oestradiol, and the possibility exists that this response is differentially expressed in phrenic and XII motor nuclei, thereby explaining differential responses in phrenic and XII LTF.
Correlation between sex hormones and LTF
Serum testosterone levels correlated more robustly with XII versus phrenic LTF, suggesting that testosterone has a greater impact on plasticity in motoneurons innervating upper airway versus respiratory pump muscles. These male F344 rats demonstrated positive correlations between XII LTF and testosterone, the ratio testosterone/oestradiol and the ratio testosterone/progesterone. Female rats show a stronger correlation of XII (versus phrenic) LTF with serum oestrogen and progesterone levels (Zabka et al. 2003). In contrast to XII LTF, phrenic LTF was not significantly correlated with any sex hormone or hormone ratio (this study; Zabka et al. 2005). Reasons for this difference in the correlation with sex hormones in XII and phrenic LTF are not known. However, an important caveat is that sex hormone levels measured in serum may not accurately reflect hormone levels in respiratory-related motor nuclei. Measurements of aromatase activity or measurements of testosterone and oestradiol levels in XII and phrenic nuclei could provide important insights concerning the testosterone-derived oestradiol levels achieved. Thus, additional investigations are necessary to define the mechanistic basis of differential hormone influence on respiratory motoneuron pools.
Possible mechanisms
Serotonergic neurons in the caudal raphe nuclei project to the XII and phrenic motor nuclei (Manaker et al. 1992; Manaker & Tischler, 1993), and there is some suggestive evidence that sex hormones modulate respiratory plasticity indirectly via the serotonergic system. For example, gonadectomy decreases 5-HT immunoreactivity in the XII nucleus of young male Sprague-Dawley rats (M. Behan, unpublished observations). Thus, depletion of gonadal hormones may decrease serotonergic input to the XII and/or the phrenic motor nuclei, thereby diminishing LTF. Androgen effects on the serotonergic system are thought to be indirect, reflecting the conversion of testosterone to oestradiol in the CNS (Celotti et al. 1991; Fink et al. 1998; this study). Depletion of oestradiol decreases the synthesis, release, reuptake and degradation of serotonin, and decreases serotonin terminal and receptor density in several brain regions (Aylward, 1973; Poirier et al. 1985; Sohrabji et al. 1995; Gibbs, 1998, 1999; Lopez-Jaramillo & Teran, 1999; Kugaya et al. 2003; Scharfman et al. 2003; Blurton-Jones et al. 2004). Thus, oestradiol may be a key player in modulating serotonergic function, thereby influencing respiratory LTF.
BDNF is another molecule critical to respiratory LTF (Baker-Herman et al. 2004) that is regulated by oestradiol. Oestradiol directly affects BDNF gene expression via an oestrogen response element encoding sequence in the BDNF gene (Sohrabji et al. 1995). Hippocampal BDNF protein fluctuates in parallel with oestradiol levels during the oestrus cycle in female rats (Scharfman et al. 2003). Oestrogen and progesterone supplementation elevates BDNF in multiple brain regions of ovariectomized rats (Gibbs, 1999). In male rats, increases in spinal BDNF protein due to chronic pain are attenuated by gonadectomy (Zhao et al. 2004). Thus, the regulation of BDNF expression by testosterone-derived oestradiol has the potential to modulate LTF. Testosterone-derived oestradiol could alter BDNF expression directly, or indirectly by actions on serotonergic function (Baker-Herman et al. 2004).
Oestradiol could also affect respiratory plasticity by direct effects on motoneurons or interneurons or glia in respiratory motor nuclei. Androgen and oestrogen receptors are present in identified XII and phrenic motoneurons (Behan & Thomas, 2005). By acting on these receptors, sex hormones could enhance synaptic density, as shown in other CNS regions (Woolley & McEwen, 1994; Brinton, 2001; Leranth et al. 2004). Oestrogen also causes changes in GABAergic interneurons, transiently inhibiting the limiting enzyme for GABA synthesis, and up-regulating glutamatergic and GABAergic synapses onto hippocampal neurons (Murphy et al. 1998). A similar mechanism is possible in respiratory motor nuclei, as both GABAergic and glutamatergic inputs play key roles in regulating respiratory motoneuron activity (McCrimmon et al. 1997).
Significance
Respiratory LTF provides an important model, enabling the investigation of sex hormone effects of on neuroplasticity in general, and respiratory plasticity in particular. By studying mechanisms underlying the effects of sex hormones on the control of breathing, we may gain new insights into breathing disorders with distinct age/sex patterns, such as obstructive sleep apnoea (OSA). OSA is most prevalent in middle-aged men and postmenopausal women who are not taking hormone replacement therapy (Hla et al. 1994; Redline et al. 1994; Ware et al. 1999; Bixler et al. 2001; Young et al. 2003). Besides anatomical factors such as narrowed airways and obesity, OSA is caused by relaxation of upper airway muscle tone during sleep, accompanied by prolapse of the tongue and soft palate, which obstructs the upper airway (Partinen et al. 1988). By stiffening the upper airway muscles, respiratory LTF may serve to stabilize breathing under conditions that would otherwise lead to upper airway obstruction and apnoea.
Although a specific role for LTF (or diminished LTF with age) has not been clearly identified as a causal factor in OSA, diminished capacity for LTF due to decreasing sex hormone levels with ageing may contribute to the progression of this syndrome. The occurrence of OSA is increased in men with low serum testosterone levels (Luboshitzky et al. 2002; Meston et al. 2003), in middle aged men, and in women with low oestrogen and progesterone levels (Netzer et al. 2003). Interestingly, administration of testosterone to individuals with low testosterone levels actually worsens the symptoms of OSA (Matsumoto et al. 1985; Cistulli et al. 1994), possibly suggesting a defect in the conversion of testosterone to oestradiol in these individuals. In conclusion, low androgen levels are correlated with greater occurrence of OSA in men, but testosterone application does not protect against OSA. In contrast, oestrogen appears to protect against OSA in women, as postmenopausal women on hormone replacement therapy (HRT) are less likely to have OSA than similarly aged women without HRT (Bixler et al. 2001; Young et al. 2003). Ultimately, testosterone may be essential to prevent OSA in men, but only if it can be converted to oestradiol. Thus, it may be that the critical deficit in middle-aged men with OSA is an inability to produce oestradiol in the CNS due to inadequate aromatase activity. This hypothesis remains to be tested.
Acknowledgments
This study was supported by NIH grants AG18760, HL80209 and HL69064.
References
- Aylward M. Plasma tryptophan levels and mental depression in postmenopausal subjects: effects of oral piperazine-oestrone sulphate. IRCS Med Sci. 1973;1:30–34. [Google Scholar]
- Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol. 1996;104:251–260. doi: 10.1016/0034-5687(96)00017-5. [DOI] [PubMed] [Google Scholar]
- Baker TL, Mitchell GS. Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. J Physiol. 2000;529:215–219. doi: 10.1111/j.1469-7793.2000.00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nature Neurosci. 2004;7:48–55. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci. 2002;22:6239–6246. doi: 10.1523/JNEUROSCI.22-14-06239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan M, Thomas CF. Sex hormone receptors are expressed in identified respiratory motoneurons in male and female rats. Neurosci. 2005;130:725–734. doi: 10.1016/j.neuroscience.2004.09.058. [DOI] [PubMed] [Google Scholar]
- Behan M, Zabka AG, Mitchell GS. Age and gender effects on serotonin-dependent plasticity in respiratory motor control. Respir Physiol Neurobiol. 2002;131:65–77. doi: 10.1016/s1569-9048(02)00038-1. [DOI] [PubMed] [Google Scholar]
- Behan M, Zabka AG, Thomas CF, Mitchell GS. Sex steroid hormones and the neural control of breathing. Respir Physiol Neurobiol. 2003;136:249–263. doi: 10.1016/s1569-9048(03)00086-7. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Gundlah C, Mirkes SJ. Ovarian steroid action in the serotonin neural system of macaques. Novartis Found Symp. 2000;230:112–130. doi: 10.1002/0470870818.ch9. [DOI] [PubMed] [Google Scholar]
- Bixler EO, Vgontzas AN, Lin H-M, Have TT, Rein J, Vela-Bueno A, Kales A. Prevalence of sleep disordered breathing in women. Effects of gender. Am J Respir Crit Care Med. 2001;163:608–613. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- Blurton-Jones M, Kuan PN, Tuszynski MH. Anatomical evidence for transsynaptic influences of estrogen on brain-derived neurotrophic factor expression. J Comp Neurol. 2004;468:347–360. doi: 10.1002/cne.10989. [DOI] [PubMed] [Google Scholar]
- Bourguiba S, Lambard S, Carreau S. Steroids control the aromatase gene exression in purified germ cells from the adult male rat. J Mol Endocinol. 2003;31:83–94. doi: 10.1677/jme.0.0310083. [DOI] [PubMed] [Google Scholar]
- Brinton RD. Cellular and molecular mechanisms of estrogen regulation of memory function and neuroprotection against Alzheimer's disease: Recent insights and remaining challenges. Learning Memory. 2001;8:121–133. doi: 10.1101/lm.39601. [DOI] [PubMed] [Google Scholar]
- Celotti F, Melcangi RC, Negri-Cesi P, Poletti A. Testosterone metabolism in brain cells and membranes. J Steroid Biochem Mol Biol. 1991;40:673–678. doi: 10.1016/0960-0760(91)90289-h. [DOI] [PubMed] [Google Scholar]
- Cistulli PA, Grunstein RR, Sullivan CE. Effect of testosterone administration on upper airway collapsibility during sleep. Am J Respir Crit Care Med. 1994;149:530–532. doi: 10.1164/ajrccm.149.2.8306057. [DOI] [PubMed] [Google Scholar]
- Davison S, Thipphawong J, Blanchard J, Liu K, Morishige R, Gonda I, Okikawa J, Adams J, Evans A, Babatunde O, Davis S. Pharmacokinetics and acute safety of inhaled testosterone in postmenopausal women. J Clin Pharmacol. 2005;45:177–184. doi: 10.1177/0091270004269840. [DOI] [PubMed] [Google Scholar]
- Dohler KD, Wuttke W. Changes with age in levels of serum gonadotropins, prolactin and gonadal steroids in prepubertal male and female rats. Endocrinology. 1974;94:898–907. doi: 10.1210/endo-97-4-898. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Ann Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink G, Sumner BE, McQueen JK, Wilson H, Rosie R. Sex steroid control of mood, mental state and memory. Clin Exp Pharmacol Physiol. 1998;25:764–775. doi: 10.1111/j.1440-1681.1998.tb02151.x. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Baker TL, Behan M, Mitchell GS. Expression of hypoglossal long term facilitation differs between sub-strains of Sprague-Dawley rat. Physiol Genom. 2001;4:175–181. doi: 10.1152/physiolgenomics.2001.4.3.175. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Levels of trkA and BDNF mRNA, but not NGF mRNA, fluctuate across the estrous cycle and increase in response to acute hormone replacement. Brain Res. 1998;787:259–268. doi: 10.1016/s0006-8993(97)01511-4. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Treatment with estrogen and progesterone affects relative levels of brain-derived neurotrophic factor mRNA and protein in different regions of the adult rat brain. Brain Res. 1999;844:20–27. doi: 10.1016/s0006-8993(99)01880-6. [DOI] [PubMed] [Google Scholar]
- Hannhart B, Pickett CL, Moore LG. Effects of estrogen and progesterone on carotid body neural output responsiveness to hypoxia. J Appl Physiol. 1990;68:1909–1916. doi: 10.1152/jappl.1990.68.5.1909. [DOI] [PubMed] [Google Scholar]
- Hla KM, Young TB, Bidwell T, Palta M, Skatrud JB, Dempsey J. Sleep apnea and hypertension: a population-based study. An Intern Med. 1994;120:382–388. doi: 10.7326/0003-4819-120-5-199403010-00005. [DOI] [PubMed] [Google Scholar]
- Klink R, Robichaud M, Debonnel G. Gender and gonadal status modulation of dorsal raphe nucleus serotonergic neurons. Part I: effects of gender and pregnancy. Neuropharmacol. 2002;43:1119–1128. doi: 10.1016/s0028-3908(02)00219-8. [DOI] [PubMed] [Google Scholar]
- Kugaya A, Epperson CN, Zoghbi S, Van Dyck CH, Hou Y, Fujita M, Staley JK, Garg PK, Seibyl JP, Innis RB. Increase in prefrontal cortex serotonin 2A receptors following estrogen treatment in postmenopausal women. Am J Psych. 2003;160:1522–1524. doi: 10.1176/appi.ajp.160.8.1522. [DOI] [PubMed] [Google Scholar]
- Leranth C, Hajszan T, Maclusky NJ. Androgens increase spine synapse density in the CA1 hippocampal subfield of ovariectomized female rats. J Neurosci. 2004;24:495–499. doi: 10.1523/JNEUROSCI.4516-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JB, Youngblood WW, Kitzer JS. Effects of castration and adrenalectomy on in vitro rates of tryptophan hydroxilation and levels of serotonin in microdissected brain nuclei of adult male rats. Brain Res. 1983;277:289–297. doi: 10.1016/0006-8993(83)90936-8. [DOI] [PubMed] [Google Scholar]
- Lopez-Jaramillo P, Teran E. Improvement in functions of the central nervous system by estrogen replacement therapy might be related with an increased nitric oxide production. Endothelium. 1999;6:263–266. doi: 10.3109/10623329909078493. [DOI] [PubMed] [Google Scholar]
- Luboshitzky R, Aviv A, Hefetz A, Herer P, Shen-Orr Z, Lavie L, Lavie P. Decreased pituitary-gonadal secretion in men with obstructive sleep apnea. J Clin Endocrinol Metab. 2002;87:3394–3398. doi: 10.1210/jcem.87.7.8663. [DOI] [PubMed] [Google Scholar]
- Mamounas LA, Altar CA, Blue ME, Kaplan DR, Tessarollo L, Lyons WE. BDNF promotes the regenerative sprouting, but not survival, of injured serotonergic axons in the adult rat brain. J Neurosci. 2000;20:771–782. doi: 10.1523/JNEUROSCI.20-02-00771.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamounas LA, Blue ME, Siuciak JA, Altar CA. Brain derived neurotrophic factor promotes the survival and sprouting of serotonergic axons in rat brain. J Neurosci. 1995;15:7929–7939. doi: 10.1523/JNEUROSCI.15-12-07929.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manaker S, Tischler LJ. Origin of serotonergic afferents to the hypoglossal nucleus in the rat. J Comp Neurol. 1993;334:466–476. doi: 10.1002/cne.903340310. [DOI] [PubMed] [Google Scholar]
- Manaker S, Tischler LJ, Morrison AR. Raphespinal and reticulospinal axon collaterals to the hypoglossal nucleus in the rat. J Comp Neurol. 1992;322:68–78. doi: 10.1002/cne.903220106. [DOI] [PubMed] [Google Scholar]
- Matsumoto AM, Sandblom RE, Schoene RB, Lee KA, Giblin EC, Pierson DJ, Bremner WJ. Testosterone replacement in hypogonadal men: effects on obstructive sleep apnoea, respiratory drives, and sleep. Clin Endocrinol (Oxf) 1985;22:713–721. doi: 10.1111/j.1365-2265.1985.tb00161.x. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004;27:589–594. doi: 10.1016/j.tins.2004.08.001. [DOI] [PubMed] [Google Scholar]
- McCrimmon DR, Zuperku EJ, Hayashi F, Dogas Z, Hinrichsen CF, Stuth EA, Toncovic-Capin M, Krolo M, Hopp FA. Modulation of the synaptic drive to respiratory premotor and motor neurons. Respir Physiol. 1997;110:161–176. doi: 10.1016/s0034-5687(97)00081-9. [DOI] [PubMed] [Google Scholar]
- Meston N, Davies RJ, Mullins R, Jenkinson C, Wass JA, Stradling JR. Endocrine effects of nasal continuous positive airway pressure in male patients with obstructive sleep apnoea. Intern Med. 2003;254:447–454. doi: 10.1046/j.1365-2796.2003.01212.x. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB., Jr Intermittent hypoxia and respiratory plasticity. J Appl Physiol. 2001;90:2466–2475. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- Murphy DD, Cole NB, Greenberger V, Segal M. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J Neurosci. 1998;18:2550–2559. doi: 10.1523/JNEUROSCI.18-07-02550.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill JD, editor. Knobil and Neill's Physiology of Reproduction. 3. Elsevier, Amsterdam: 2006. p. 1027. [Google Scholar]
- Netzer NC, Eliasson AH, Strohl KP. Women with sleep apnea have lower levels of sex hormones. Sleep Breathing. 2003;7:25–29. doi: 10.1007/s11325-003-0025-8. [DOI] [PubMed] [Google Scholar]
- Partinen M, Guilleminault C, Quera-Salva MA, Jamieson A. Obstructive sleep apnea and cephalometric toentgenograms: the role of anatomic upper airway abnormalities in the definition of abnormal breathing during sleep. Chest. 1988;93:1199–1205. doi: 10.1378/chest.93.6.1199. [DOI] [PubMed] [Google Scholar]
- Poirier MF, Loo H, Dennis T, Le FG, Scatton B. Platelet monoaminooxidase activity and plasma 3,4-dihydroxyphenylethylene glycol levels during the menstrual cycle. Neuropsychobiol. 1985;14:165–169. doi: 10.1159/000118222. [DOI] [PubMed] [Google Scholar]
- Redline S, Kump K, Tishler PV, Browner I, Ferrett V. Gender differences in sleep disordered breathing in a community-based sample. Am J Respir Crit Care Med. 1994;149:722–726. doi: 10.1164/ajrccm.149.3.8118642. [DOI] [PubMed] [Google Scholar]
- Robaire B, Ewing LL, Irby C, Desjardins C. Interactions of testosterone and estradiol-17β on the reproductive tract of the male rat. Biol Reprod. 1979;21:455–463. doi: 10.1095/biolreprod21.2.455. [DOI] [PubMed] [Google Scholar]
- Roselli CE. Synergistic induction of aromatase activity in the rat brain by estradiol and 5α-dihydrotestosterone. Neuroendocrinol. 1991;53:79–84. doi: 10.1159/000125701. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Abdelgadir SE, Ronnekleiv OK, Klosterman SA. Anatomic distribution and regulation of aromatase gene expression in the rat brain. Biol Reprod. 1998;58:79–87. doi: 10.1095/biolreprod58.1.79. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Maclusky NJ. Similarities between actions of estrogen and BDNF in the hippocampus: coincidence or clue? Trends Neurosci. 2005;28:79–85. doi: 10.1016/j.tins.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Mercurio TC, Goodman JH, Wilson MA, Maclusky NJ. Hippocampal excitability increases during the estrous cycle in the rat: a potential role for brain-derived neurotrophic factor. J Neurosci. 2003;23:11641–11652. doi: 10.1523/JNEUROSCI.23-37-11641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlenker HE, Goldman M. Ventilatory responses of aged male and female rats to hypercapnia and to hypoxia. Gerontology. 1985;31:301–308. doi: 10.1159/000212713. [DOI] [PubMed] [Google Scholar]
- Smith ER, Stefanick ML, Clark JT, Davidson JM. Hormones and sexual behavior in relationship to aging in male rats. Hormones Behav. 1992;26:110–135. doi: 10.1016/0018-506x(92)90035-t. [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Miranda RC, Toran-Allerand CD. Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proc Natl Acad Sci USA. 1995;92:11110–11114. doi: 10.1073/pnas.92.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner BE, Fink G. The density of 5-hydoxytryptamine2A receptors in forebrain is increased at pro-oestrus in intact female rats. Neurosci Lett. 1998;234:7–10. doi: 10.1016/s0304-3940(97)00651-4. [DOI] [PubMed] [Google Scholar]
- Tatsumi K, Pickett CK, Jacoby CR, Weil JV, Moore LG. Effects of testosterone on hypoxic ventilatory and carotid body neural responsiveness. Am J Respir Crit Care Med. 1994;149:1248–1253. doi: 10.1164/ajrccm.149.5.8173766. [DOI] [PubMed] [Google Scholar]
- Tatsumi K, Pickett CK, Jacoby CR, Weil JV, Moore LG. Role of endogenous female hormones in hypoxic chemosensitivity. J Appl Physiol. 1997;83:17060–17070. doi: 10.1152/jappl.1997.83.5.1706. [DOI] [PubMed] [Google Scholar]
- Wagner CK, Morrell JI. Neuroanatomical distribution of aromatase mRNA in the rat brain: indications of regional regulation. J Steroid Biochem Mol Biol. 1997;61:307–314. [PubMed] [Google Scholar]
- Ware JC, McBrayer RH, Scott JA. Influence of sex and age on duration and frequency of sleep apnea events. Sleep. 1999;23:165–170. [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol regulates hippocampal dendritic spine density via an N-methyl-D-aspartate receptor-dependent mechanism. J Neurosci. 1994;14:7680–7687. doi: 10.1523/JNEUROSCI.14-12-07680.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin sleep cohort study. Am J Respir Crit Care Med. 2003;167:1181–1185. doi: 10.1164/rccm.200209-1055OC. [DOI] [PubMed] [Google Scholar]
- Zabka AG, Behan M, Mitchell GS. Time-dependent hypoxic respiratory responses in female rats are influenced by age and by the estrus cycle. J Appl Physiol. 2001a;91:2831–2838. doi: 10.1152/jappl.2001.91.6.2831. [DOI] [PubMed] [Google Scholar]
- Zabka AG, Behan M, Mitchell GS. Long-term facilitation of respiratory motor output decreases with age in male rats. J Physiol. 2001b;531:509–514. doi: 10.1111/j.1469-7793.2001.0509i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabka AG, Behan M, Mitchell GS. Chronic intermittent hypoxia enhances respiratory long-term facilitation in geriatric female rats. J Appl Physiol. 2003;95:2614–2623. doi: 10.1152/japplphysiol.00476.2003. [DOI] [PubMed] [Google Scholar]
- Zabka AG, Behan M, Mitchell GS. Aging and gonadectomy have similar effects on hypoglossal long-term facilitation in male Fischer rats. J Physiol. 2005;563:557–568. doi: 10.1113/jphysiol.2004.077511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Wang X, Zheng SY, Zu JG. Gonadectomy affects brain derived neurotrophic factor in rats after chronic constriction nerve injury. Acta Pharmacol Sin. 2004;25:286–292. [PubMed] [Google Scholar]