Abstract
The cellular mechanisms that determine the frequency of spontaneous activity were investigated in gastric smooth muscles isolated from the guinea-pig. Intact antral muscle generated slow waves periodically; the interval between slow waves was decreased exponentially by depolarization of the membrane to reach a steady interval value of about 7 s. Isolated circular muscle bundles produced slow potentials spontaneously or were evoked by depolarizing current stimuli. Evoked slow potentials appeared in an all-or-none fashion, with a refractory period of ∼2–3 s. Low concentrations of chemicals that modify intracellular signalling revealed that the refractory period was causally related to the activity of protein kinase C (PKC). Activation of PKC increased and inhibition of PKC activity decreased the frequency of slow potentials. Chemicals that inhibit mitochondrial functions reduced the frequency of slow waves. Inhibition of internal Ca2+-store activity decreased the amplitude, but not the frequency of slow potentials, suggesting that the amplitude is causally related to Ca2+ release from the internal store. The results suggest that changes in [Ca2+]i caused by the activity of mitochondria may play a key role in determining the frequency of spontaneous activity in gastric pacemaker cells.
Many smooth muscle tissues isolated from many laboratory animals generate spontaneous activity in the form of slow waves or spike potentials; many of these activities are thought to originate from interstitial cells of Cajal (ICC) distributed in the tissues (Sanders, 1996). Electrical activity of smooth muscle membranes induces an increase in intracellular concentration of Ca2+ ([Ca2+]i), which elicits muscle contractions for peristaltic activity. It is therefore important to understand the cellular mechanism of spontaneous activity in gastrointestinal smooth muscle.
We are currently working on the properties of rhythmic activity in stomach, using small laboratory animals such as guinea-pig and mouse. Smooth muscle tissues isolated from these animals show the rhythmical generation of slow waves; in the pyloric region, spike potentials are superimposed on each slow wave (Tomita, 1981; Ishikawa et al. 1985). Structural studies of the stomach show that at least two types of ICC are present: one type forms a network distributed in the myenteric layer between circular and longitudinal muscle layers (ICC-MY); the another type is distributed within muscle bundles (ICC-IM) (Burns et al. 1995). It is thought that these ICC may play an important role in the generation of spontaneous activity by the stomach.
Properties of spontaneous activity in gastric muscle
Electrical recordings from isolated gastric muscle of the guinea-pig revealed that there are three types of wave forms: slow waves, large square-shaped potentials (driving potentials) and smaller square-shaped potentials (follower potentials) (Fig. 1, A–C, respectively). Identification of the cell recorded from, using dye injection, indicated that slow waves were recorded from circular muscle, driving potentials from ICC-MY and follower potentials from longitudinal muscle, respectively (Dickens et al. 1999). These responses appear at a frequency of ∼3–5 cycles min−1, and each of these potentials is synchronized, suggesting that the cell layers are electrically coupled to each other (Dickens et al. 1999; Cousins et al. 2003; Hirst et al. 2006). As driving potentials appear just prior to the start of slow waves or follower potentials, it is considered that potentials produced in ICC-MY generate pacemaker activity in gastric smooth muscle (Dickens et al. 1999).
Figure 1. Spontaneous electrical responses recorded from gastric smooth muscle tissue.
Spontaneous electrical responses were recorded from intact smooth muscle tissue (A–C) or isolated circular muscle bundle from antrum (D) or corpus (E). Slow waves were recorded from circular muscle (A), driving potentials were recorded from ICC-MY (B), and follower potentials were recorded from longitudinal muscle (C). Slow potentials were recorded from circular muscle of antrum (D) and corpus (E). All responses were recorded from different tissues, in the presence of 1 μm nifedipine.
Isolated circular muscle bundles, which are free from ICC-MY or longitudinal muscle layer, are also spontaneously active; these generate unitary potentials and regenerative-type potentials (slow potentials, Fig. 1D) (Suzuki & Hirst, 1999). Slow potentials are generated periodically, with much lower frequencies and more irregularly than are slow waves (∼0.2–2 cycles min−1). Unitary potentials are generated in a random fashion, and power spectral frequency analysis suggests that unitary potentials sum to give rise to slow potentials (Edwards et al. 1999). The different properties of slow waves recorded from W/WV mutant and wild-type mice suggested that unitary potentials and slow potentials originate in ICC-IM, and give rise to the 2nd component of slow waves (Dickens et al. 2001). These results indicate that slow potentials and unitary potentials recorded from circular smooth muscle are good indicators of the activity of ICC-IM.
Recently, Hashitani et al. (2005) examined the distribution of spontaneous activity in the guinea-pig stomach and found that the most active region (i.e. the region where the frequency of slow waves is the highest) was located along the greater curvature of the corpus. Immunohistochemical examination revealed that this region contains only ICC-IM. Slow waves recorded from the corpus region occurred at a higher frequency (∼5–6 cycles min−1) than in the isolated antral region (equal to ∼3–4 cycles min−1) (Fig. 1E); consequently no stable diastolic phase occurred between corporal slow waves, i.e. the sequence of membrane potential changes resembled that of cardiac pacemaker cells distributed in the sino-atrial node, albeit at a lower frequency. Thus, there are at least three different types of spontaneously active cells in the stomach: ICC-MY producing driving potentials, circular muscle of the antrum producing slow potentials and corpus muscle producing slow potentials with high frequency, with the latter two appearing to originate from ICC-IM distributed in circular muscles. The highest activity would dominate the movement of whole stomach, and it is thus reasonable to consider the corpus as the ‘pacemaker region’ of the gastric movements. These observations reported by Hashitani et al. (2005) agree with the propagation of electrical activity in canine stomach, in which slow waves appear first in the body region and then propagate down to the antrum region with increasing speeds (Kelly et al. 1969).
Cellular mechanism of spontaneous activity
Chemicals that block spontaneous activity in gastric muscle include 2-aminoethoxydiphenyl borate (2-APB), BAPTA, cyclopiazonic acid (CPA), carbonyl cyanide m-chlorophenylhydrazone (CCCP) and thapsigargin. 2-APB, a known inhibitor of IP3 receptors, blocks the generation of slow waves in the guinea-pig stomach (Hirst & Edwards, 2001). The related observation made in gastric muscle of mice lacking the expression of IP3 receptor (Suzuki et al. 2000), suggests that IP3 may be one of the key factors involved in the generation of spontaneous activity in stomach. IP3 is known to act as an intracellular 2nd messenger causing the release of Ca2+ from internal store in response to IP3 receptor activation (Berridge, 1993). As the production and functioning of IP3 can be modulated by membrane potential changes (Itoh et al. 1992; GanitkechVY & Isenberg, 1993), it is reasonable to speculate that depolarization elevates the concentration of IP3 and thus increases [Ca2+]i in pacemaker cells. Intracellular concentration of Ca2+ is also one of the important factors for maintaining the generation of slow waves (Tomita, 1981), and reduction of [Ca2+]i by chemicals such as BAPTA or MAPTA inhibits rhythmic activity in gastric muscle (Fukuta et al. 2001; Hirst et al. 2002; Kito et al. 2002). The likely involvement of Ca2+ release from internal stores in the generation of gastric spontaneous activity is suggested from the inhibitory actions of CPA or thapsigargin (inhibitors of Ca2+-ATPase the internal Ca2+ stores) on slow waves (Kito et al. 2000). Ryanodine, however, does not modify slow waves or slow potentials in the guinea-pig stomach (Fukuta et al. 2002; Kito & Suzuki, 2003b), suggesting that ryanodine receptors do not play an important role in the generation of rhythmic activity.
CCCP (an mitochondrial protonophore) also blocks the generation of slow waves in gastric smooth muscle of the guinea-pig (Kito et al. 2000) and mouse (Ward et al. 2000). Handling of Ca2+ between intracellular organelles has a pivotal role in cell functioning (Duchen, 1999), and the inhibitory effects of CCCP on slow waves suggests that translocation of Ca2+ between internal Ca2+ stores and mitochondria is involved in the generation of rhythmic activity. Thus, periodic alteration of intracellular Ca2+ level through functioning of the IP3 receptor, release of Ca2+ from the internal stores and mitochondrial function appear to be causally related in the generation of rhythmic activity in gastric muscle.
Factors modifying the frequency of spontaneous activity
Attempts were made to investigate the factors which determine the frequency of spontaneous activity in gastric muscle, since one or more of these factors may play a key role in the initiation of spontaneous activity. In gastric muscle of the guinea-pig, slow waves are generated periodically at a frequency of ∼3–5 cycles min−1 (Tomita, 1981). Pharmacological inhibition of slow waves is often accompanied by membrane depolarization, which is also one of the important factors which determines their amplitudes and frequency of occurrence. Thus, membrane depolarization by either current flow or elevation of the concentration of potassium ions (Fig. 2A), increases the frequency and decreases the amplitude of slow waves (Nose et al. 2000). The amplitude decreases linearly with depolarization of the membrane and slow waves are not detected at membrane potentials around −30 ∼−20 mV (Fig. 2B), a potential close to the expected equilibrium potential for chloride ions. Although the frequency of slow waves increases linearly with membrane depolarization, the interval between slow waves decreases exponentially and approaches to a stable value of around 7 s, at about −40 mV (Fig. 2C). The depolarization-induced change in slow waves is associated with an increase in [Ca2+]i, even in the presence of Ca2+ antagonists (Fukuta et al. 2002; Hirst et al. 2002), suggesting that the increase in [Ca2+]i does not result from the activation of voltage-sensitive L-type Ca2+ channels.
Figure 2. Changes in amplitude and frequency of slow waves during depolarization with high-K+ solution.
Slow waves were recorded from smooth muscle isolated from the guinea-pig stomach antrum, during stimulation with 15.3 mm K+ solution (A). Amplitude (B) and interval of slow waves (C) generated at different membrane potentials were plotted. All data from the same tissue.
As these voltage-dependent changes in frequency were also observed in slow potentials recorded from the isolated circular muscle (Nose et al. 2000), subsequent analyses were carried out on individual circular muscle bundles. Measurements of the interval required before an evoked slow potential could be produced after termination of a preceding slow potential (Fig. 3) indicated that slow potentials were generated in an all-or-none fashion and they had a refractory period of ∼2–3 s (Nose et al. 2000; Suzuki et al. 2002a; Kito et al. 2002). The refractory period was changed by inhibiting the activity of protein kinase C (PKC; Kito et al. 2002) or Ca2+ pump at the internal stores with CPA (Suzuki et al. 2002a) or thapsigargin (Kito et al. 2002; Kito & Suzuki, 2003a), but was not changed by inhibiting IP3 receptors with 2-APB (Kito et al. 2002). These results suggest that PKC is one of the important factors for the regulation of the frequency of slow potentials. Moreover, activation of PKC indirectly by elevating the concentration of diacyl glycerol with an inhibitor of diacyl glycerol lipase (Suzuki et al. 2002b) or directly with phorbol esters (Nakamura & Suzuki, 2005) increases the frequency of slow potentials. Many subtypes of PKC have been described, and PKCα is the most likely candidate, since it is a Ca2+-sensitive isoform and the generation of slow potentials is associated with an increase in [Ca2+]i (Fukuta et al. 2002; Hirst et al. 2002).
Figure 3. Modulation of refractory period for slow potentials by inhibition of PKC.
In a circular muscle bundle, isolated from the antrum of guinea-pig stomach, depolarizing current stimulation (3 nA intensity, 1.5 s duration) was applied in the absence (A and B) and presence of 0.3 μm chelerythrine (C and D). In control conditions, stimulation at about 4 s (A) did not, but at 5.5 s (B) did produce slow potential, while in the presence of chelerythrine, stimulation at about 20 s (D) did but at 12 s (C) did not evoke slow potential. The relationship between the time of stimulation after cessation of current stimulation and amplitude of the evoked response (•, control; ○, in the presence of chelerythrine) indicates that the threshold time for the generation of slow potential (T1, equal to refractory period) was increased by about 3 s (T2) by chelerythrine (E).
The effects of 2-APB, CPA and CCCP on slow potentials were investigated, using low concentrations which did not cause membrane depolarization. Both 2-APB and CPA reduced the amplitude of slow potentials, without significantly changing their frequency (Nakamura & Suzuki, 2004); this suggests that the amount of Ca2+ released from internal stores influences the amplitude but not the frequency of slow potentials. The effects of CCCP on slow potentials were unclear: concentrations less than those that caused membrane depolarization failed to alter slow potential frequency or amplitude the membrane. The reason why CCCP failed to affect slow potentials remains undetermined, it may be that elevation of [Ca2+]i due to inhibition of Ca2+ uptake into mitochondria (Duchen, 1999) is important.
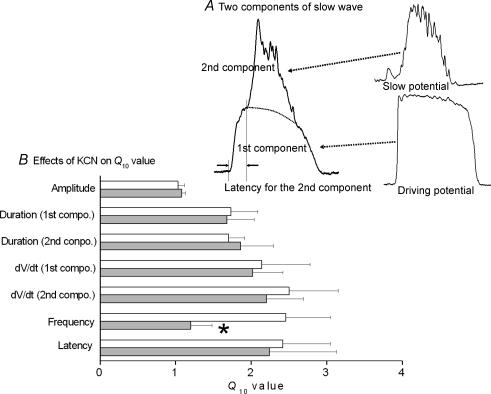
The frequency of slow waves is very sensitive to changes in temperature, a high temperature coefficient (Q10) for slow waves has been reported in the guinea-pig stomach (Ohba et al. 1977; Tomita, 1981; Nakamura et al. 2006). The duration, rate of rise of the upstroke phase and frequency of slow waves all show marked temperature sensitivity (Q10 > 2; Fig. 4). Slow waves consist of two components (Tomita, 1981): the 1st component results from the electrotonic propagation of a driving potential generated in ICC-MY and the 2nd component is a slow potential formed by summation of unitary potentials generated in ICC-IM (Hirst & Ward, 2003). Both components were equally sensitive to temperature, and the latency for the generation of the 2nd component was also sensitive to temperature (Fig. 4). In the presence of KCN, an inhibitor of mitochondrial metabolism, only changed temperature sensitivity of slow wave frequency (Fig. 4B); these results again suggest that mitochondrial activity is coupled to the production of rhythm in gastric muscle. Ward et al. (2000) showed that in cultured ICC isolated from mouse intestine, mitochondrial Ca2+ transients are co-related with depolarization of the plasma membrane, although no successful data indicating direct connection between mitochondrial activity and changes in ionic conductance of plasma membrane is available in gastric muscle.
Figure 4. Effects of KCN on the temperature sensitivity of slow wave components.
Slow wave is formed by 1st and 2nd components, and the 1st component may be formed by electrotonic propagation of driving potential produced in ICC-MY, while the 2nd component is formed by slow potential produced as a result of summation of unitary potentials generated in ICC-IM (A). The temperature coefficient (Q10 value) was measured for parameters of slow waves (amplitude, durations of 1st and 2nd components, rate of rise (dV/dt) of the rising phase of 1st and 2nd components, frequency and the latency between 1st and 2nd components) in the absence (open column) and presence of 30 μm KCN (grey column) (mean ± s.d.). Only the Q10 value for the frequency of slow waves (*) was significantly decreased by KCN.
Conclusion
Our current hypothesis to indicate the cellular mechanism of the generation of rhythmic activity in pacemaker cell of the stomach is shown in Fig. 5. Metabolic activity in mitochondria produces a local change in [Ca2+]i, which in turn activates PKC to facilitate its translocation from a cytosolic site to a membrane site. Although no supporting evidence is available, the activated PKC may elevate production of IP3 to increase the release of Ca2+ from the internal stores through activation of IP3 receptors. Spontaneous electrical activity generated in gastric muscles such as driving potentials (plateau phase), unitary potentials or slow potentials result from the activation of Ca2+-sensitive Cl− channels (Tokutomi et al. 1995; Kito et al. 2000; Hirst et al. 2002; Hotta et al. 2005), and it is therefore reasonable to consider that the elevated [Ca2+]i will activate Ca2+-sensitive Cl− channels to produce voltage change in pacemaker cells. The results indicate that membrane potential changes, temperature changes and pharmacological agents which modulate intracellular signalling (CCCP, BAPTA) can change the frequency of slow waves or slow potentials in stomach smooth muscles. In the latter, CCCP inhibits Ca2+ handling in mitochondria by inhibiting the proton pump (Duchen, 1999). As BAPTA reduces [Ca2+]i, effects of this agent may be related to the change in [Ca2+]i in ICC-IM. PKC, a factor involved in the determination of the refractory period for slow potentials, is also sensitive to Ca2+. Change in membrane potential modulate [Ca2+]i, even in the presence of nifedipine (Fukuta et al. 2002). Thus, intracellular Ca2+ handling in relation to mitochondrial activity may be the key factor to regulate the frequency of spontaneous activity in gastric muscle tissues.
Figure 5. Signalling scheme for producing rhythmic activity in stomach.
In gastric pacemaker cells, mitochondrial metabolic activity produces protons (H+) with associated synthesis of ATP, and the produced H+ is pumped out to cytosolic space by the proton pump (P). The proton pumping produces a large inside-negative voltage gradient across the inner membrane of mitochondria, and this potential gradient allows influx of Ca2+ from the cytosolic space. Elevation of mitochondrial Ca2+ will facilitate activities in mitochondria by consuming ATP, which will activate ATP-sensitive K+ channels (KATP) at the mitochondrial membrane to allow K+ to flow into mitochondria. The influx of K+ will cancel the potential formed across the inner membrane, which will cause extrusion of Ca2+ from mitochondria. As a consequence, metabolic activity results in a generation of periodic change in the concentration of Ca2+ around mitochondria. PKC distributed in the cytosol will be activated when [Ca2+]i is elevated, and activated PKC will translocate from cytosol to the membrane side, and may activate some enzymatic processes which will facilitate activation of PLC to elevate the production of IP3 from phosphatydil inositol (PI). Elevated IP3 concentration will activate IP3 receptors (IP3R) at the internal store (ER) and accelerate the release of Ca2+; the elevated [Ca2+]i will activate ion channels such as Ca2+-sensitive Cl− channels distributed in the plasma membrane to produce electrical activity, and also will again induce mitochondrial activation by the increased uptake of Ca2+ into mitochondria. Thus, Ca2+ handling between mitochondria and internal Ca2+ store would be the source of the production of rhythm in pacemaker cell.
References
- Berridge MJ. Inositol trisphosphate and calcium signaling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Burns AJ, Herbert TM, Ward SM, Sanders KM. Interstitial cells of Cajal in the guinea-pig gastrointestinal tract as revealed by c-Kit immunohistochemistry. Cell Tissue Res. 1995;290:11–20. doi: 10.1007/s004410050902. [DOI] [PubMed] [Google Scholar]
- Cousins HM, Edwards FR, Hickey H, Hill CE, Hirst GDS. Electrical coupling between the myenteric interstitial cells of Cajal and adjacent muscle layers in the guinea-pig gastric antrum. J Physiol. 2003;550:829–844. doi: 10.1113/jphysiol.2003.042176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens EJ, Edwards FR, Hirst GDS. Selective knockout of intramuscular interstitial cells reveals their role in the generation of slow waves in mouse stomach. J Physiol. 2001;531:827–833. doi: 10.1111/j.1469-7793.2001.0827h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens EJ, Hirst GDS, Tomita T. Identification of rhythmically active cells in guinea-pig stomach. J Physiol. 1999;514:515–531. doi: 10.1111/j.1469-7793.1999.515ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen EJ. Contribution of mitochondria to animal physiology: from homeostatic sensor to calcium signaling and cell death. J Physiol. 1999;516:1–17. doi: 10.1111/j.1469-7793.1999.001aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards FR, Hirst GDS, Suzuki H. Unitary nature of regenerative potentials recorded from circular smooth muscle of guinea-pig antrum. J Physiol. 1999;519:235–250. doi: 10.1111/j.1469-7793.1999.0235o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuta H, Kito Y, Suzuki H. Spontaneous electrical activity and associated changes in calcium concentration in the guinea-pig gastric smooth muscle. J Physiol. 2001;540:249–260. doi: 10.1113/jphysiol.2001.013306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkech VY, Isenberg G. Membrane potential modulates inositol 1,4,5-trisphosphate-mediated Ca2+ transients in guinea-pig coronary artery. J Physiol. 1993;470:35–44. doi: 10.1113/jphysiol.1993.sp019845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Garcia-Londono AP, Hirst GDS, Edwards FR. Atypical slow waves generated in gastric corpus provide dominant pacemaker activity in guinea pig stomach. J Physiol. 2005;569:459–469. doi: 10.1113/jphysiol.2005.097907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GDS, Bramich NJ, Teramoto N, Suzuki H, Edwards FR. Regenerative component of slow waves in the guinea-pig antrum involves a delayed increase in [Ca2+]i and Cl− channels. J Physiol. 2002;540:907–919. doi: 10.1113/jphysiol.2001.014803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GDS, Edwards RF. Generation of slow waves in the antral region of guinea-pig stomach – a stochastic process. J Physiol. 2001;535:165–180. doi: 10.1111/j.1469-7793.2001.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GDS, Garcia-Londono AP, Edwards FR. Propagation of slow waves in the guinea-pig gastric antrum. J Physiol. 2006;571:165–177. doi: 10.1113/jphysiol.2005.100735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GDS, Ward SM. Interstitial cells: involvement in rhythmicity and neural control of gut smooth muscle. J Physiol. 2003;550:337–346. doi: 10.1113/jphysiol.2003.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta A, Kito Y, Suzuki H. The effects of flufenamic acid on spontaneous activity of smooth muscle tissue isolated from the guinea-pig stomach antrum. J Smooth Muscle Res. 2005;41:207–220. doi: 10.1540/jsmr.41.207. [DOI] [PubMed] [Google Scholar]
- Ishikawa S, Komori K, Nagao T, Suzuki H. Effects of diltiazem on electrical responses evoked spontaneously or by electrical stimulation in the antrum smooth muscle cells of the guinea-pig stomach. Br J Pharmacol. 1985;86:789–797. doi: 10.1111/j.1476-5381.1985.tb11100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T, Seki N, Suzuki S, Ito S, Kajikuri J, Kuriyama H. Membrane hyperpolarization inhibits agonist-induced synthesis of inositol 1,4,5-trisphosphate in rabbit mesenteric artery. J Physiol. 1992;451:307–328. doi: 10.1113/jphysiol.1992.sp019166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KA, Code CF, Elveback LR. Patterns of canine gastric electrical activity. Am J Physiol. 1969;217:461–471. doi: 10.1152/ajplegacy.1969.217.2.461. [DOI] [PubMed] [Google Scholar]
- Kito Y, Fukuta H, Suzuki H. Components of pacemaker potentials recorded from the guinea-pig stomach antrum. Pflugers Arch. 2000;445:202–217. doi: 10.1007/s00424-002-0884-z. [DOI] [PubMed] [Google Scholar]
- Kito Y, Fukuta H, Yamamoto Y, Suzuki H. Excitation of smooth muscles isolated from the guinea-pig stomach antrum in response to depolarization. J Physiol. 2002;543:155–167. doi: 10.1113/jphysiol.2002.020875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kito Y, Suzuki H. Modulation of slow waves by hyperpolarization with potassium channel openers in antral smooth muscle of the guinea-pig stomach. J Physiol. 2003a;548:175–189. doi: 10.1113/jphysiol.2002.035550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kito Y, Suzuki H. Electrophysiological properties of gastric pacemaker potentials. J Smooth Muscle Res. 2003b;39:163–173. doi: 10.1540/jsmr.39.163. [DOI] [PubMed] [Google Scholar]
- Nakamura E, Kito Y, Hashitani H, Suzuki H. Metabolic component of the temperature-sensitivity of slow waves recorded from gastric muscle of the guinea-pig. J Smooth Muscle Res. 2006;42:33–48. doi: 10.1540/jsmr.42.33. [DOI] [PubMed] [Google Scholar]
- Nakamura E, Suzuki H. Spontaneous activity and its cholinergic modulation in circular smooth muscle isolated from guinea-pig stomach antrum. Pflugers Arch. 2004;449:205–212. doi: 10.1007/s00424-004-1325-y. [DOI] [PubMed] [Google Scholar]
- Nakamura E, Suzuki H. Concentration-dependent dual effects of phorbol 12,13-dibutylrate on spontaneous and acetylcholine-induced electrical responses recorded from isolated circular smooth muscle of the guinea-pig stomach antrum. J Smooth Muscle Res. 2005;40:259–270. doi: 10.1540/jsmr.40.259. [DOI] [PubMed] [Google Scholar]
- Nose K, Suzuki H, Kannan H. Voltage-dependency of the frequency of slow waves in antrum smooth muscle of the guinea-pig stomach. Jpn J Physiol. 2000;50:625–633. doi: 10.2170/jjphysiol.50.625. [DOI] [PubMed] [Google Scholar]
- Ohba M, Sakamoto Y, Tomita T. Slow wave in the circular muscle of the guinea-pig stomach. J Physiol. 1977;253:505–516. doi: 10.1113/jphysiol.1975.sp011203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterol. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Hirst GDS. Regenerative potentials evoked in circular smooth muscle of the antral region of guinea-pig stomach. J Physiol. 1999;517:563–573. doi: 10.1111/j.1469-7793.1999.0563t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Kito Y, Fukuta H, Yamamoto Y. Dual effects of cyclopiazonic acid on excitation of circular smooth muscle isolated from the guinea-pig gastric antrum. J Smooth Muscle Res. 2002a;38:23–37. doi: 10.1540/jsmr.38.23. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Koto Y, Fukuta H, Yamamoto Y. Effects of RHC-80267, an inhibitor of diacylglycerol lipase, on excitation of circular smooth muscle of the guinea-pig gastric antrum. J Smooth Muscle Res. 2002b;38:153–164. doi: 10.1540/jsmr.38.153. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Takano H, Yamamoto Y, Komuro T, Saito M, Kato K, Mikoshiba K. Properties of gastric smooth muscles obtained from mice which lack inositol trisphosphate receptors. J Physiol. 2000;525:105–111. doi: 10.1111/j.1469-7793.2000.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokutomi N, Maeda H, Tokutomi N, Sato D, Sugita M, Nishikawa S, Nakano J, Imamura T, Nishi K. Rhythmic Cl− current and physiological roles of the intestinal c-kit-positive cells. Pflugers Arch. 1995;431:169–177. doi: 10.1007/BF00410188. [DOI] [PubMed] [Google Scholar]
- Tomita T. Electrical activity (spikes and slow waves) in gastrointestinal smooth muscles. In: Bülbring E, Brading AF, Jones AW, Tomita T, editors. Smooth Muscle. London, UK: Edward Arnold; 1981. pp. 127–156. [Google Scholar]
- Ward SM, Ördög T, Koh SD, Baker SA, Jun JY, Amberg G, Monaghan K, Sanders KM. Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J Physiol. 2000;525:355–361. doi: 10.1111/j.1469-7793.2000.t01-1-00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]