Abstract
Smooth muscles in the lower urinary tract and corporal tissue exhibit spontaneous contractile activity which depends on L-type Ca2+ channels. The mechanism underlying this activity is spontaneous electrical activity which shows varied form and property between these tissues. Recent studies revealed that interstitial cells (ICs) are widely distributed in the genitourinary system, and suggested their involvement in spontaneous muscle activity. ICs in the system are not a simple analogy of interstitial cells of Cajal (ICC) in the gut, which act as electrical pacemaker, but represent variability amongst tissues which may account for individual characteristics of each organ. In the bladder and corporal tissue, where smooth muscle cells are capable of generating spontaneous electrical activity, ICs may modulate smooth muscle activity. ICs in corporal tissue release prostaglandins via cyclooxygenase-2 (COX-2) activity and reinforce not only spontaneous but also nerve-mediated α-adrenergic contractions. In the bladder, their fundamental role in the integration of signals between populations of cells has been proposed, and thus changes in ICs may contribute to an overactive bladder, a pathological condition which results from increased excitability in detrusor smooth muscles. In the urethra, ICs may act as electrical pacemakers as do ICC. However, overall contractility of urethral smooth muscles does not necessarily rely on pacemaking of ICs, and thus some population of smooth muscles may also have their own excitability.
Interstitial cells (ICs) have been identified in the lower urinary tract and corporal tissue by their immunoreactivity against Kit receptor tyrosine kinase, a cell surface marker specific for ICC/ICs, or vimentin filament, a marker for cells of mesenchymal origin (Brading & McCloskey, 2005). Since smooth muscles in these tissues exhibit spontaneous excitation, ICs may drive smooth muscles as do ICC in the gut (Sanders, 1996). In the urethra, isolated ICs generate spontaneous electrical activity which is very similar to that recorded from intact urethral smooth muscles, suggesting that ICs may act as electrical pacemakers (Hashitani et al. 1996; Sergeant et al. 2000). In the bladder and corpus cavernosum, however, dispersed smooth muscle cells are capable of generating spontaneous electrical activity (Montgomery & Fry, 1992; Karkanis et al. 2003), and thus the functional role of ICs in these tissues may be different from that in the gut or the urethra. Therefore, besides intrinsic properties of smooth muscles and autonomic innervation, varied functions of ICs amongst these tissues may also contribute to individual characteristics of each tissue.
Whilst recognizing the variation of ICs in the lower urinary tract and corporal tissue, we may also need to consider the commonality of ICs in these tissues, particularly as a basis for pathological excitability of smooth muscles. Lower urinary tract symptoms (LUTS) and erectile dysfunction (ED) are extremely prevalent in ageing men. However, accumulated epidemiological surveys demonstrated the independent relationships of age to LUTS and to ED (McVary, 2006), and thus it is important to establish a reasonable pathophysiological basis to explain the association between LUTS and ED. Although several common factors have been already hypothesized for it, general distribution of ICs in the genitourinary system suggests that pathological changes in their property may also be crucial.
Corporal tissue
ICs have been identified in the corporal tissue by their immunoreactivity for Kit receptor tyrosinekinase (Hashitani & Suzuki, 2004). Spontaneous Ca2+ transients recorded from corpus spongiosum smooth muscle of the guinea-pig are readily blocked by pharmacological disruption of Ca2+ release from intracellular Ca2+ stores (Hashitani & Suzuki, 2004). Since spontaneous activity originating from ICs generally depends on Ca2+ store function, ICs in the corporal tissue could be primary pacemakers as with ICC in the gut. However, dispersed corporal smooth muscle cells are capable of generating spontaneous electrical activity by the opening of Ca2+-activated Cl− channels which are stimulated by Ca2+ release from intracellular stores (Karkanis et al. 2003; Craven et al. 2004). Therefore, ICs in the corporal tissue may have a functional role other than that of electrical pacemaker.
Spontaneous contractions of corporal smooth muscle are known to be inhibited by indomethacin, and thus background formation of prostaglandins may be involved in their generation (Christ et al. 1990). In the rabbit corpus cavernosum smooth muscle (CCSM), spontaneous contractions were largely attenuated by NS 398, an inhibitor for COX-2, suggesting that spontaneous production of prostaglandins via COX-2 may contribute to the generation of spontaneous activity (Fig. 1A; Hashitani et al. 2005). In addition, inhibition of COX-2 suppressed nerve-evoked α-adrenergic contractions. It should be noted that the COX-2 inhibitor reduced the amplitude of α-adrenergic contractions with a relatively small reduction in corresponding Ca2+ transients (Fig. 1B–C). Since PGF2α-induced contractions of CCSM are greatly suppressed by Y-27632 Rho kinase inhibitor, PGF2α may reinforce the α-adrenergic contractions through Ca2+-independent mechanisms, presumably by the activation of Rho kinase.
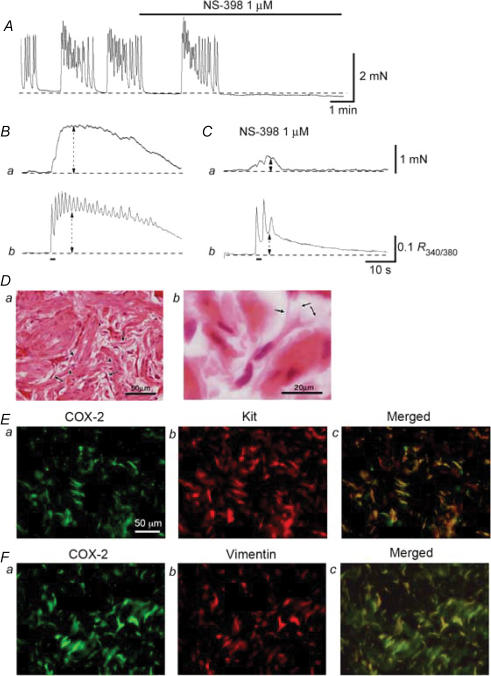
Figure 1. Function and distribution of COX-2-immunoreactive interstitial cells in corpus cavernosum.
In a CCSM preparation that generated spontaneous contractions, NS-398 (1 μm) abolished the contractions (A). When changes in tension were measured simultaneously with changes in [Ca2+]i in another preparation, transmural stimulation evoked phasic contractions (Ba) and corresponding increases in [Ca2+]i (Bb). NS-398 (1 μm) strongly suppressed nerve-evoked contractions (Ca), and also reduced the amplitude of the initial phase of Ca2+ transients by about 50% (Cb). HE staining of the smooth muscle cells and ICs in corpus cavernosum (Da). Smooth muscle cells had a large, clear nucleus (arrowheads), while ICs were characterized by their smaller, darker nucleus (arrows). ICs typically had spindle- or star-shaped cell bodies and had some branches (arrows) which connected with neighbouring cells (Db). COX-2-immunoreactive cells, which had spindle- or star-shaped cells with some branches, were widely distributed throughout the corpus cavernosum (Ea). COX-2-immunoreactive cells were also stained for Kit antibody (Eb and Ec). COX-2-immunoreactive ICs in the corpus cavernosum (Fa) were also stained for vimentin (Fb and Fc).
Immunohistochemical studies showed that COX-2 was highly expressed in ICs in the corpus cavernosum and the corpus spongiosum (Hashitani et al. 2005). Haematoxylin & Eosin (HE) staining revealed that cells with COX-2 immunoreactivity were spindle-or star-shaped with some branches and were interconnected (Fig. 1D). COX-2-immunoreactive cells are scattered over corporal smooth muscle and background smooth muscle expresses no or very weak COX-2 immunoreactivity (Fig. 1Ea and Fa). COX-2-positive cells are also shown to express strong immunoreactivity against Kit (Fig. 1Eb and c) or vimentin (Fig. 1Fb and c) antibody. Therefore, ICs in CCSM may be a modulator of spontaneous activity, which originate from smooth muscle cells, by releasing prostaglandins via COX-2 activity.
Several studies indicated that the disturbed balance between spontaneously active NO–cGMP pathway and prostaglandins may be crucial for pathophysiology of erectile dysfunction (Fig. 2). The blockade of guanylate cyclase in CCSM has been reported to increase both resting tension and noradrenaline-induced contraction, suggesting that the tonic production of cGMP inhibits CCSM excitability (Minhas et al. 2000). Since the effects of guanylate cyclase inhibition were reversed by indomethacin, interaction and functional antagonism between cGMP and prostaglandins was suggested. Furthermore, increased cyclooxygenase activity is reported to account for ischaemia-induced increased contractions of CCSM in the rabbit (Azadzoi et al. 1992). It has also been reported that diminished NO production in diabetic CCSM is associated with increased Rho kinase activity, and that increased Rho kinase activity may suppress the production of NO (Bivalacqua et al. 2004). Since prostaglandins contract CCSM partly by increasing Rho kinase activity, increased Rho kinase activity in association with suppressed NO production may attribute to overexpression of COX-2 in ICs.
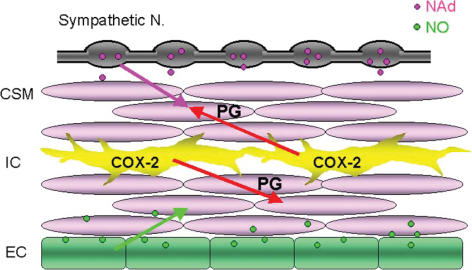
Figure 2. Role of interstitial cells in regulating cavernosal smooth muscle (CSM) tone.
In corpus cavernosum, both spontaneous activity of CSM and neurally released noradrenaline (NAd; pink arrow) contract CSM. Spontaneously produced prostaglandins (PGs) via COX-2 activity in interstitial cells (ICs) not only reinforce spontaneous excitation of CSM (lower red arrow) but also facilitate nerve-mediated α-adrenergic contractions (upper red arrow). Conversely, spontaneously released nitric oxide (NO) from endothelium (EC) suppresses excitation (green arrow). Thus, the balance between spontaneously released PGs and NO is important in regulating CCSM tone to determine the contractile state of the penis.
Urinary bladder
ICs are distributed throughout the bladder wall (Davidson & McCloskey, 2005). In the suburothelium region, a network of vimentin-positive ICs connecting thorough gap junction protein connexin43 (Cx43) has been identified (Sui et al. 2002; Wiseman et al. 2003). Suburothelial ICs form very close association with afferent nerves, and may play a modulatory role in the process of bladder sensation, i.e. signal transmission from urothelium to afferent nerves.
In detrusor muscle layer, ICs are found preferentially located on the boundary of muscle bundles from where spontaneous Ca2+ transients originate, suggesting that they may be crucial in generating spontaneous excitation (Fig. 3A; see also McCloskey & Gurney, 2002). However, spontaneous Ca2+ transients recorded from ICs in fact occurred independently of those of smooth muscles even when synchronous Ca2+ waves swept across muscle bundles (Fig. 3B; Hashitani et al. 2004). Therefore, although the location of boundary ICs seems to be ideal to drive the bulk of smooth muscles, they may not be electrical pacemaking cells. Indeed, electrophysiological recordings have demonstrated that isolated detrusor smooth muscle cells of the bladder are capable of generating spontaneous action potentials which are almost identical to those recorded from intact preparations (Montgomery & Fry, 1992; Hashitani et al. 2001). Unlike Ca2+ transients recorded from detrusor smooth muscles, those from ICs persisted in the presence of nifedipine (Fig. 3C) as did carbachol-induced Ca2+ oscillations in isolated ICs (McCloskey & Gurney, 2002), suggesting that voltage-dependent L-type Ca2+ channels are not involved in the generation of this activity.
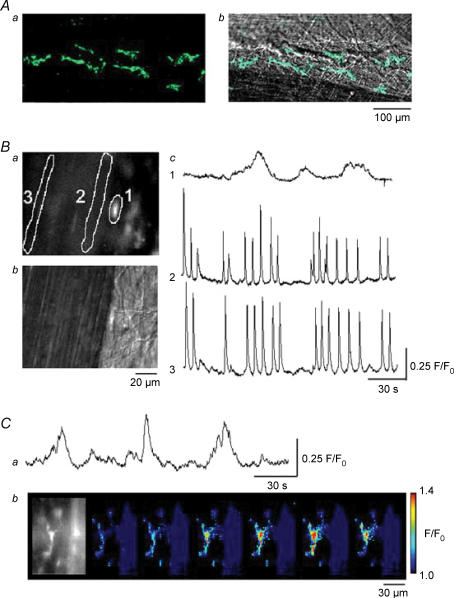
Figure 3. Ca2+ transients recorded from smooth muscles and interstitial cells in the guinea-pig bladder.
Kit-positive ICs having spindle-shaped cell bodies, some 80 μm in length and less than 10 μm in width, are shown located adjacent to a pair of muscle bundles (Aa). The same images were superimposed on the plane images of the smooth muscle bundles (Ab). In a preparation loaded with fluo-4, IC located near the muscle boundary had a higher fluorescence intensity than that of smooth muscle (Ba). A plane image with Nomarski optics visualized the cell body of IC (Bb). When Ca2+ transients were recorded from the IC (area 1) and from two smooth muscle areas (areas 2 and 3), synchronous Ca2+ waves were detected at areas 2 and 3 (Bc). However, IC generated slow Ca2+ transients independently from those of smooth muscles (Bc). In another fluo-4-loaded preparation which had been exposed to nifedipine (10 μm) for 30 min, IC continued to generate slow Ca2+ transients (Ca). A series of frames with intervals of 2 s demonstrates a Ca2+ transient originating from the IC (Cb).
ICs are closely associated with intramural nerves (Davidson & McCloskey, 2005). Following stimulation with sodium nitroprusside (SNP), an NO donor, ICs throughout the bladder demonstrated an intense induction of cGMP immunoreactivity, but detrusor muscle cells remained uniformly negative (Smet et al. 1996; Gillespie et al. 2004). Thus, ICs in the bladder may be involved in the neuromuscular transmission as do ICC in the gut (Hirst & Ward, 2003), and thus changes in ICs may account for increased excitability of detrusor smooth muscles. Our preliminary studies showed that sildenafil, a phosphodiesterase type 5 (PDE5) inhibitor, suppressed spontaneous contractions recorded from multiple detrusor smooth muscle preparations, whilst invariably unaffecting spontaneous activity in single muscle bundles. Interestingly, SNP rather enhanced spontaneous excitations in detrusor smooth muscles, and this was consistent with the results of whole bladder experiments (Gillespie & Drake, 2004). These results suggested that sildenafil may accumulate endogenous cGMP in ICs and diminish the synchronicity between muscle bundles.
Bladder overactivity has been suggested to result from increased coupling between detrusor smooth muscle cells (Brading, 1997). Increased connexin43-mediated intercellular communications in a rat model of bladder overactivity has been reported (Haefliger et al. 2002; Christ et al. 2003). Micromotion of the bladder wall, which may be attributed to spontaneous contractions of a unit of muscle bundles, has also been reported to be enhanced in a rat model of bladder overactivity (Drake et al. 2003). Therefore, either quantitative or qualitative changes in ICs may account for the increased excitability in the overactive bladder. The finding in human bladder that Kit-positive cells are increased in number in samples of bladder taken from patients with overactive bladder may support this idea (Biers et al. 2006). An unpublished observation from our group using a guinea-pig with bladder outlet obstruction demonstrated that populations of Kit/vimentin-positive ICs were dramatically increased in the obstructed bladder.
Urethra
In circular smooth muscles of the rabbit urethra, spontaneous excitation may originate from ICs by mean of the spontaneous release of Ca2+ from intracellular stores, which activates Ca2+-activated Cl− channels (Sergeant et al. 2000, 2001; Johnston et al. 2005). Resultant depolarizations may be transmitted to smooth muscle cells, and thus spontaneous transient depolarizations (STDs) recorded from intact urethral smooth muscle may attribute to spontaneous inward currents generated by ICs (Hashitani et al. 1996). Summed STDs result in larger depolarizations which reach the threshold for the opening of L-type Ca2+ channels (Hashitani & Edwards, 1999). The activation of L-type Ca2+ channels in smooth muscle cells contributes to the plateau phase of slow waves and leads to spontaneous contractions (Hashitani et al. 1996; Hashitani & Edwards, 1999). Therefore, spontaneous contractions are expected to be inhibited as a consequence of the inhibition of the primary step of spontaneous excitation, i.e. Ca2+ release from stores in ICs.
Cyclopiazonic acid (CPA is known to inhibit Ca2+ uptake into intracellular stores via SERCA pumps, and is known to suppress both spontaneous transient inward currents (STICs) in isolated interstitial cells and STDs in intact circular smooth muscle (Hashitani et al. 1996; Sergeant et al. 2001). Therefore, the blockade of SERCA pumps with CPA may suppress spontaneous contractions of the urethral smooth muscles. However, CPA increased the amplitude and duration of phasic contractions in 70–90% of preparations, although it largely reduced their frequency (Hashitani et al. 2006). CPA prevented the generation of spontaneous contractions in the remaining preparations. Since CPA abolished caffeine-induced Ca2+ transients in nominally Ca2+-free solution, it may have effectively depleted intracellular Ca2+ stores. Thus, it is unlikely that CPA-treated preparations generated spontaneous contractions through a Ca2+ store-dependent pacemaker, i.e. through ICs.
Consistent with the results of contractile studies, CPA increased the amplitude and duration of spontaneous depolarizations and corresponding Ca2+ transients in some 40–60% of preparations, whilst suppressing their generation in the remainder (Hashitani et al. 2006). The frequency of spontaneous depolarizations and corresponding Ca2+ transients was again largely decreased in CPA-treated preparations. In preparations in which spontaneous depolarizations and Ca2+ transients were not abolished by CPA, nicardipine greatly diminished Ca2+ transients but not depolarizations. After the blockade of L-type Ca2+ channels, CPA invariably abolished residual depolarizations and Ca2+ transients, suggesting that these events may result from Ca2+ store-dependent mechanisms. After all, this heterogeneous CPA sensitivity of spontaneous excitation of urethral smooth muscles may attribute to the existence of a secondary pacemaker which relies on L-type Ca2+ channels.
In longitudinal smooth muscle of the urethra, spontaneous action potentials are generated, and inhibited by nicardipine but not CPA, suggesting that L-type Ca2+ channels play a principal role in their generation (Hashitani et al. 2006). Thus, after disrupting primary pacemaking by ICs which depend on Ca2+ release, L-type Ca2+ channel-dependent pacemaking depolarizations arising from longitudinal smooth muscles may drive the circular smooth muscles. Alternatively, small populations of circular smooth muscles which are capable of generating spontaneous depolarization might dominate the pacemaking system.
To further explore the origin of CPA-sensitive spontaneous excitations, attempts were made to visualize Ca2+ transients from intact ICs in urethral smooth muscle preparations which had been loaded with fluo-4 AM. Spontaneous Ca2+ transients recorded from ICs occurred at the frequency of 1–10 min−1, and had a longer duration (5–30 s) than those of urethral smooth muscle cells (1–3 s) which were measured in similar preparations (Fig. 4A, Ba and Ca). ICs were either distributed individually or several ICs were clustered, but did not compose an extensive network. Nicardipine (1 μm), which either abolished Ca2+ transients in smooth muscles or greatly reduced their amplitude (Fig. 4Ba and b), did not suppress Ca2+ transients of ICs (Fig. 4Ca and b). Although Ca2+ transients of ICs were virtually insensitive to nicardipine, their generation was prevented by switching from normal physiological saline to nominally Ca2+-free solution. They were also readily abolished by CPA (10 μm), ryanodine (30 μm) or caffeine (10 mm), and their amplitude was greatly reduced 2-APB (50 μm), suggesting that their generation can be attributed to the Ca2+ release from intracellular Ca2+ stores through both ryanodine-sensitive and InsP3-sensitive Ca2+ channels. These results were consistent with properties of isolated urethral ICs (Johnston et al. 2005), and again confirmed that spontaneous activity of ICs relies on Ca2+ handling by intracellular Ca2+ stores.
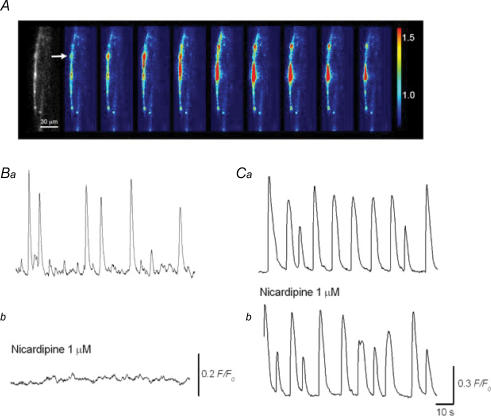
Figure 4. Ca2+ transients recorded from smooth muscles and interstitial cells in the rabbit urethra.
In a preparation loaded with fluo-4, urethral IC located near the muscle boundary generated slow Ca2+ transients (A). A series of frames with intervals of 2 s demonstrates a Ca2+ transient originating from the IC (A). Note that Ca2+ transients were initiated at the point indicated by an arrow, and then propagated upward and downward. In a different preparation, Ca2+ transients recorded from urethral smooth muscles were largely attenuated by nicardipine (1 μm) (Ba and C). In another preparation, Ca2+ transients recorded from urethral IC were virtually insensitive to nicardipine (1 μm) (Ca and b). The scale bar in Bb also refers to Ba. The scale bar in Cb also refers to Ca.
Conclusion
ICs are widely distributed in the lower urinary tract and corporal tissues. Corporal ICs reinforce both spontaneous and nerve-mediated α-adrenergic excitations by releasing prostaglandins via COX-2 activity. In detrusor smooth muscle layer, ICs generate spontaneous excitations independent from those of smooth muscles. They may be targeted by nitrergic nerves and modulate communications between muscle bundles, and thus increasing their population may account for pathological excitability of detrusor smooth muscle. Urethral ICs may be the primary pacemaker to drive smooth muscles by a Ca2+-store-dependent mechanism. However, there may also be L-type Ca2+ channel-dependent pacemaking presumably arising from smooth muscles. In the genitourinary system, overactivity of ICs may result in increased excitability of smooth muscles in individual organs, and thus may account for the association between LUTS and ED.
Acknowledgments
This project was supported by research grants from the Japan Society for the Promotion of Science (No. 15591704 and No.17390443) to H.H.
References
- Azadzoi KM, Kim N, Brown ML, Goldstein I, Cohen RA, Saenz De Tejada I. Endothelium-derived nitric oxide and cyclooxygenase products modulate corpus cavernosum smooth muscle tone. J Urol. 1992;147:220–225. doi: 10.1016/s0022-5347(17)37201-4. [DOI] [PubMed] [Google Scholar]
- Biers SM, Reynard JM, Doore T, Brading AF. The functional effects of a c-kit tyrosine inhibitor on guinea-pig and human detrusor. BJU Int. 2006;97:612–616. doi: 10.1111/j.1464-410X.2005.05988.x. [DOI] [PubMed] [Google Scholar]
- Bivalacqua TJ, Champion HC, Usta MF, Cellek S, Chitaley K, Webb RC, Lewis RL, Mills TM, Hellstrom WJ, Kadowitz PJ. RhoA/Rho-kinase suppresses endothelial nitric oxide synthase in the penis: a mechanism for diabetes-associated erectile dysfunction. Proc Natl Acad Sci U S A. 2004;101:9121–9126. doi: 10.1073/pnas.0400520101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading AF. A myogenic basis for overactive bladder. Urology. 1997;50:57–67. doi: 10.1016/s0090-4295(97)00591-8. [DOI] [PubMed] [Google Scholar]
- Brading AF, McCloskey KD. Mechanisms of disease: specialized interstitial cells of the urinary tract – an assessment of current knowledge. Nat Clin Pract Urol. 2005;2:546–554. doi: 10.1038/ncpuro0340. [DOI] [PubMed] [Google Scholar]
- Christ GJ, Day NS, Day M, Zhao W, Person K, Pandita RK, Andersson KE. Increased connexin43-mediated intercellular communication in a rat model of bladder overactivity in vivo. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1241–R1248. doi: 10.1152/ajpregu.00030.2002. [DOI] [PubMed] [Google Scholar]
- Christ GJ, Maayani S, Valcic M, Melman A. Pharmacological studies of human erectile tissue: characteristics of spontaneous contractions and alterations in alpha-adrenoceptor responsiveness with age and disease in isolated tissues. Br J Pharmacol. 1990;101:375–381. doi: 10.1111/j.1476-5381.1990.tb12717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven M, Sergeant GP, Hollywood MA, McHale NG, Thornbury KD. Modulation of spontaneous Ca2+-activated Cl− currents in the rabbit corpus cavernosum by the nitric oxide–cGMP pathway. J Physiol. 2004;556:495–506. doi: 10.1113/jphysiol.2003.058628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RA, McCloskey KD. Morphology and localization of interstitial cells in the guinea pig bladder: structural relationships with smooth muscle and neurons. J Urol. 2005;173:1385–1390. doi: 10.1097/01.ju.0000146272.80848.37. [DOI] [PubMed] [Google Scholar]
- Drake MJ, Hedlund P, Harvey IJ, Pandita RK, Andersson KE, Gillespie JL. Partial outlet obstruction enhances modular autonomous activity in the isolated rat bladder. J Urol. 2003;170:276–279. doi: 10.1097/01.ju.0000069722.35137.e0. [DOI] [PubMed] [Google Scholar]
- Gillespie JI, Drake MJ. The actions of sodium nitroprusside and the phosphodiesterase inhibitor dipyridamole on phasic activity in the isolated guinea-pig bladder. BJU Int. 2004;93:851–858. doi: 10.1111/j.1464-410X.2003.04727.x. [DOI] [PubMed] [Google Scholar]
- Gillespie JI, Markerink-van Ittersum M, de Vente J. cGMP-generating cells in the bladder wall: identification of distinct networks of interstitial cells. BJU Int. 2004;94:1114–1124. doi: 10.1111/j.1464-410X.2004.05186.x. [DOI] [PubMed] [Google Scholar]
- Haefliger JA, Tissieres P, Tawadros T, Formenton A, Beny JL, Nicod P, Frey P, Meda P. Connexins 43 and 26 are differentially increased after rat bladder outlet obstruction. Exp Cell Res. 2002;274:216–225. doi: 10.1006/excr.2001.5465. [DOI] [PubMed] [Google Scholar]
- Hashitani H, Edwards FR. Spontaneous and neurally activated depolarizations in smooth muscle cells of the guinea-pig urethra. J Physiol. 1999;514:459–470. doi: 10.1111/j.1469-7793.1999.459ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Fukuta H, Takano H, Klemm M, Suzuki H. Origin and propagation of spontaneous excitation in smooth muscle of the guinea-pig urinary bladder. J Physiol. 2001;530:273–286. doi: 10.1111/j.1469-7793.2001.0273l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Suzuki H. Identification of interstitial cells of Cajal in corporal tissues of the guinea-pig penis. Br J Pharmacol. 2004;141:199–204. doi: 10.1038/sj.bjp.0705622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Van Helden DF, Suzuki H. Properties of spontaneous depolarizations in circular smooth muscle cells of rabbit urethra. Br J Pharmacol. 1996;118:1627–1632. doi: 10.1111/j.1476-5381.1996.tb15584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Yanai Y, Kohri K, Suzuki H. Heterogeneous CPA sensitivity of spontaneous excitation in smooth muscle of the rabbit urethra. Br J Pharmacol. 2006;148:340–349. doi: 10.1038/sj.bjp.0706729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Yanai Y, Shirasawa N, Soji T, Tomita A, Kohri K, Suzuki H. Interaction between spontaneous and neurally mediated regulation of smooth muscle tone in the rabbit corpus cavernosum. J Physiol. 2005;569:723–735. doi: 10.1113/jphysiol.2005.099309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Yanai Y, Suzuki H. Role of interstitial cells and gap junctions in the transmission of spontaneous Ca2+ signals in detrusor smooth muscles of the guinea-pig urinary bladder. J Physiol. 2004;559:567–581. doi: 10.1113/jphysiol.2004.065136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GDS, Ward SM. Interstitial cells: involvement in rhythmicity and neural control of gut smooth muscle. J Physiol. 2003;550:337–346. doi: 10.1113/jphysiol.2003.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston L, Sergeant GP, Hollywood MA, Thornbury KD, McHale NG. Calcium oscillations in interstitial cells of the rabbit urethra. J Physiol. 2005;565:449–461. doi: 10.1113/jphysiol.2004.078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkanis T, DeYoung L, Brock GB, Sims SM. Ca2+-activated Cl− channels in corpus cavernosum smooth muscle: a novel mechanism for control of penile erection. J Appl Physiol. 2003;94:301–313. doi: 10.1152/japplphysiol.00660.2002. [DOI] [PubMed] [Google Scholar]
- McCloskey KD, Gurney AM. Kit positive cells in the guinea pig bladder. J Urol. 2002;168:832–836. [PubMed] [Google Scholar]
- McVary K. Lower urinary tract symptoms and sexual dysfunction: epidemiology and pathophysiology. BJU Int. 2006;97(Suppl. 2):23–28. doi: 10.1111/j.1464-410X.2006.06102.x. discussion 44–45. [DOI] [PubMed] [Google Scholar]
- Minhas S, Eardley I, Joyce AD, Morrison JB. The effect of cyclic GMP on rabbit corporal smooth muscle tone and its modulation by cyclo-oxygenase products. Prostaglandins Leukot Essent Fatty Acids. 2000;62:153–160. doi: 10.1054/plef.2000.0135. [DOI] [PubMed] [Google Scholar]
- Montgomery BS, Fry CH. The action potential and net membrane currents in isolated human detrusor smooth muscle cells. J Urol. 1992;147:176–184. doi: 10.1016/s0022-5347(17)37192-6. [DOI] [PubMed] [Google Scholar]
- Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- Sergeant GP, Hollywood MA, McCloskey KD, McHale NG, Thornbury KD. Role of IP3 in modulation of spontaneous activity in pacemaker cells of rabbit urethra. Am J Physiol. 2001;280:C1349–C1356. doi: 10.1152/ajpcell.2001.280.5.C1349. [DOI] [PubMed] [Google Scholar]
- Sergeant GP, Hollywood MA, McCloskey KD, Thornbury KD, McHale NG. Specialised pacemaking cells in the rabbit urethra. J Physiol. 2000;526:359–366. doi: 10.1111/j.1469-7793.2000.t01-2-00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smet PJ, Jonavicius J, Marshall VR, de Vente J. Distribution of nitric oxide synthase-immunoreactive nerves and identification of the cellular targets of nitric oxide in guinea-pig and human urinary bladder by cGMP immunohistochemistry. Neuroscience. 1996;71:337–348. doi: 10.1016/0306-4522(95)00453-x. [DOI] [PubMed] [Google Scholar]
- Sui GP, Rothery S, Dupont E, Fry CH, Severs NJ. Gap junctions and connexin expression in human suburothelial interstitial cells. BJU Int. 2002;90:118–129. doi: 10.1046/j.1464-410x.2002.02834.x. [DOI] [PubMed] [Google Scholar]
- Wiseman OJ, Fowler CJ, Landon DN. The role of the human bladder lamina propria myofibroblast. BJU Int. 2003;91:89–93. doi: 10.1046/j.1464-410x.2003.03802.x. [DOI] [PubMed] [Google Scholar]