Abstract
The stomach generates a characteristic pattern of coordinated activity whereby rings of contraction regularly start in the corpus and migrate slowly down the stomach to the duodenum. This behaviour persists after isolating the stomach and after blocking nervous activity; hence the response is myogenic, resulting from organized contractions of smooth muscle cells lying in the stomach wall. Each ring of contraction is triggered by a long lasting wave of depolarization, termed a slow wave. Slow waves are now known to be generated by sets of interstitial cells of Cajal (ICC), which intermingle with gastric smooth muscle cells. This article describes some studies which identify the roles played by ICC in the on-going generation of coordinated gastric movements. Intramuscular ICC in the corpus generate slow waves and these provide the dominant pacemaker frequency in the stomach. Corporal slow waves, in turn, activate a network of myenteric ICC, which starts in the antrum and slowly conducts waves of depolarization down the stomach. As these waves pass over bundles of circularly orientated muscle cells, they activate a set of intramuscular ICC which lie in the circular muscle layer: these generate slow waves that rapidly spread radially, so triggering each ring of contraction.
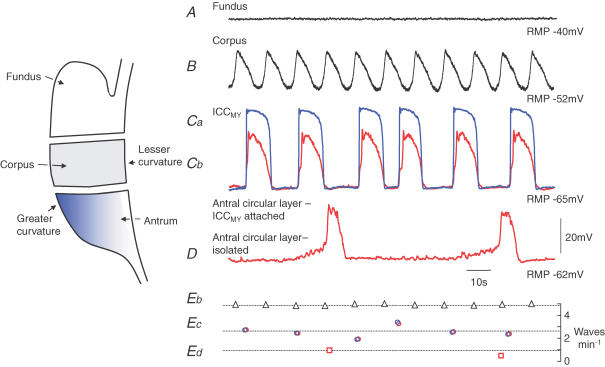
Electrical recordings show that successive descending rings of gastric contraction are triggered by waves of electrical activity which originate in the corpus. The waves occur regularly at 5 waves min−1 and in the antrum conduct slowly in an anal direction but more rapidly in a circumferential direction (Szurszewski, 1981; Sanders & Publicover, 1989). Intracellular recordings from different regions of the stomach indicate that all regions, except the fundus (Komori & Suzuki, 1986; Beckett et al. 2004; Fig. 1A, generate long lasting waves of depolarization, slow waves (Tomita, 1981). The corpus generates slow waves regularly at 5 waves min−1 (Hashitani et al. 2005; Fig. 1B and Eb). When the antrum is separated from the corpus, it generates slow waves irregularly with a mean frequency of ∼3 waves min−1 (Tsugeno et al. 1995; Hirst & Edwards, 2001; Fig. 1Cb and Ec). Isolated bundles of the antral circular layer generate slow waves erratically at ∼1 wave min−1 (Kito et al. 2002b; Fig. 1D and Ed). Thus when dissected apart, different regions of the stomach generate slow waves at different frequencies, with corporal slow waves occurring regularly and most frequently. However, in the intact stomach, all regions discharge slow waves regularly at the same frequency as the corpus, indicating that corporal slow waves normally entrain activity throughout the lower stomach.
Figure 1. Electrical activity recorded from isolated regions of the guinea pig stomach.
The upper trace (A) shows a recording from the circular muscle layer of the isolated fundus: although an ongoing discharge of membrane noise occurred, rhythmical activity was not detected. Trace B shows slow waves recorded from the circular muscle layer of the isolated corpus: slow waves occurred regularly at 5 waves min−1 (Eb). The next set of superimposed traces show simultaneous recordings from an ICCMY (Ca; blue trace) and a nearby smooth muscle cell in the circular layer (Cb; red trace: modified from Hirst & Edwards, 2001): pacemaker potentials and slow waves occurred synchronously and their rate of occurrence fluctuated around 3 waves min−1 (Ec). Trace D shows spontaneous electrical activity recorded from an isolated bundle of antral circular muscle: regenerative potentials occur at about 1 wave min−1 (Ed). The time and voltage calibration bars apply to all traces.
Involvement of ICC in stomach rhythmicity
The wall of the gastrointestinal tract contains at least two muscle layers: a thin outer longitudinal layer, made up of electrically interconnected smooth muscle cells which are orientated in a longitudinal direction; and an inner thicker circular muscle layer, consisting of electrically interconnected smooth muscle cells which are organized into muscle bundles that run in a circular direction. As slow waves have been recorded from these muscle layers for many years, it was thought that they were generated by smooth muscle cells. Recently it has become clear that slow waves are not generated by smooth muscle cells but are generated by ICC. However, as all ICC are electrically coupled to nearby smooth muscle cells, each depolarization that a set of ICC generates will passively depolarize nearby smooth muscle cells: if the depolarization is sufficiently large to activate smooth muscle L-type Ca2+ channels (Farrugia, 1999), a contraction will occur. Conversely, if a set of ICC generates a hyperpolarization, nearby smooth muscle cells will be hyperpolarized, making them less likely to contract.
Gastrointestinal ICC selectively bind antibodies to CD117 and the distribution of ICC can be determined immunohistochemically (Sanders, 1996; Komuro et al. 1999). In part of the stomach, a myenteric network of ICC lies between the muscle layers (ICCMY; Burns et al. 1997). ICCMY appear at the anal end of the corpus at the greater curvature and spread over the antrum and pylorus: their density is highest near the greater curvature and falls towards the lesser curvature (Hirst et al. 2002a; Mazet & Raynier, 2004). The density of ICCMY also falls as the gastro-duodenal junction is approached (Wang et al. 2005). The role of ICC in the generation of slow waves was unequivocally demonstrated in studies on W/WV mutant mice. The gastrointestinal tract of W/WV mice shows regional deficits of ICC: their small intestines selectively lack ICCMY and fail to generate slow waves, unlike those of wild-type mice (Ward et al. 1994; Huizinga et al. 1995). Subsequently, intestinal ICCMY were shown to generate pacemaker potentials (Kito & Suzuki, 2003).
A second set of ICC have an intramuscular location, ICCIM. In all regions of the stomach, ICCIM are scattered amongst the smooth muscle cells of the circular layer. Similarly ICCIM intermingle with longitudinal smooth muscle cells in the fundus and corpus (Burns et al. 1997) but are infrequent in the longitudinal layer of the antrum (Cousins et al. 2003). ICCIM play a key role in both the generation of gastric slow waves and in the pathway between enteric nerve terminals and smooth muscle cells. The stomachs of W/WV mice, unlike their small intestines, contain ICCMY but lack ICCIM (Sanders, 1996). Antral preparations from W/WV mice generate incomplete slow waves (Dickens et al. 2001): gastric preparations from W/WV mice also fail to respond to excitatory cholinergic or inhibitory nitrergic nerve stimulation unlike those from wild types (Hirst & Ward, 2003).
Generation of slow waves in the corpus
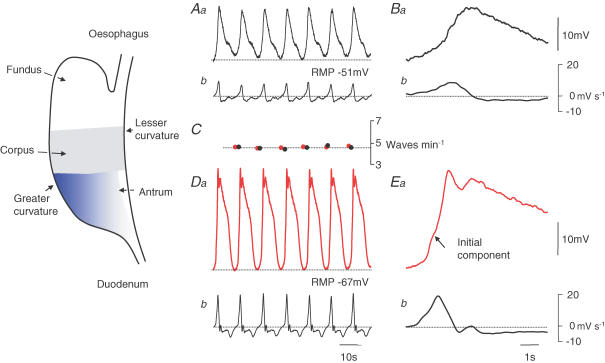
Although electrical activity originates in the corpus, few electrophysiological studies have been carried out on this region of the stomach. The guinea pig corpus lacks ICCMY but contains ICCIM, distributed in the circular and longitudinal layers. The intact corpus and isolated circular bundles of the corpus generate slow waves regularly at 5 waves min−1 (Fig. 1B and Eb). Corporal slow waves have peak amplitudes of ∼15 mV, superimposed on a peak negative potential around −50 mV. They have low rates of rise (< 10 mV s−1) (Fig. 2Ba and Bb) and are abolished by buffering the internal concentration of calcium ions to low levels (Hashitani et al. 2005). Given their similarities with other slow waves, it seems most likely that corporal slow waves are generated by corporal ICCIM. Their high rate of occurrence indicates that corporal slow waves are the source of dominant pacemaker activity in the intact stomach.
Figure 2. Comparison between corporal and antral slow waves recorded from an attached corpus–antrum preparation.
The upper left hand pair of traces shows corporal slow waves recorded near the greater curvature (Aa) and their associated dV/dt (Ab). Corporal slow waves had low rates of rise (Ab) and occurred regularly at 4.8 waves min−1 (C). Expanded regions of these traces (Ba and b) showed that upstroke of the corporal slow wave (Ba) lacked an obvious inflection. The lower left hand pair of traces shows antral slow waves, recorded from the same preparation as Aa, near the greater curvature (Da) and associated dV/dt (Db). Antral slow waves had faster rates of rise (Db) and occurred regularly with the same frequency as corporal slow waves (C). Expanded regions show that the upstroke of the antral slow wave displayed an initial component (Ea). The left hand time calibration bar applies to traces A and D: the right hand time calibration bar applies to traces B and E. Figure modified from Hashitani et al. (2005).
Generation of slow waves in the antrum
Recordings from identified antral ICCMY indicate that they generate large-amplitude pacemaker potentials superimposed on a peak negative potential of some −65 mV (Dickens et al. 1999; Fig. 1Ca). Each pacemaker potential consists of two components, a rapidly rising initial component, lasting about 2 s; this is followed by a plateau component that lasts a further 5–10 s (Hirst & Edwards, 2001; Kito et al. 2002a). In the isolated antrum the discharge of pacemaker potentials occurs irregularly with a mean frequency of 3 waves min−1 (Fig. 1Ec). Simultaneous recordings from ICCMY and either the circular (Fig. 1Ca and Cb) or longitudinal layers show that ICCMY trigger attenuated waves of depolarization in each layer as pacemaker current flows through the electrical connections between ICCMY and adjacent layers (Cousins et al. 2003; Edwards & Hirst, 2005).
In the antral circular layer, each passive wave of pacemaker depolarization gives rise to the primary component of the slow wave which triggers a secondary regenerative component (Ohba et al. 1975; Suzuki & Hirst, 1999; Figs 1Cb and 2Ea). The secondary component is generated by ICCIM, being absent in antrums of W/WV mice which lack ICCIM (Dickens et al. 2001). Characteristically the secondary regenerative component is triggered by membrane depolarization and starts about 1 s after the onset of depolarization; it appears to result from the release of calcium ions from inositol 1,4,5-trisphosphate-dependent internal calcium stores and the subsequent activation of chloride-selective channels (Suzuki & Hirst, 1999; Edwards et al. 1999; Suzuki et al. 2000; Hirst et al. 2002b). For convenience the secondary component of the slow wave will be referred to as a regenerative potential.
In summary, antral slow waves are generated by two separate sets of ICC, ICCMY and ICCIM.
Anal spread of slow waves in the antrum
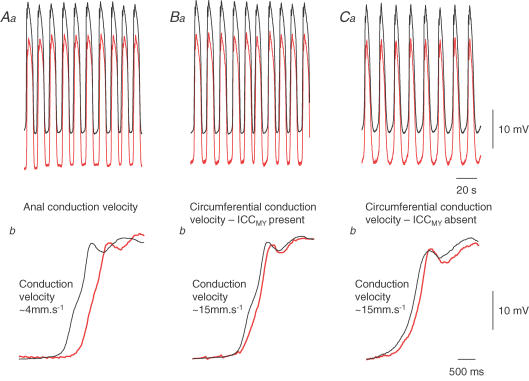
In the isolated antrum, pacemaker potentials start at random points within the ICCMY network and conduct away from the point of initiation, orally, anally or circumferentially, at the same speed, ∼3 mm s−1 (Hennig et al. 2004). Thus in isolated preparations, antral slow waves start at varying locations and conduct away from the point of origin. When the ICCMY network is electrically stimulated, pacemaker potentials conduct from the stimulation point with the same conduction velocity anally or circumferentially (∼3 mm s−1, Hirst et al. 2006). When the antrum and corpus are left in continuity, antral slow waves invariably start at the corporal end of the antrum at the same frequency as in the corpus (Fig. 2; Hirst et al. 2006). Their anal conduction velocity, ∼3 mm s−1 (Fig. 3Aa and Ab), is the same as that of pacemaker potentials in the ICCMY network. Why pacemaker potentials should conduct so slowly in the ICCMY network is not understood: it is unlikely to result from poor coupling between neighbouring ICCMY but may reflect the activation properties of the initial component of the pacemaker potential (Goto et al. 2004; Edwards & Hirst, 2006).
Figure 3. Conduction velocities of slow waves in the anal direction and in the circumferential direction, with ICCMY present and removed.
Upper left hand pair of traces (Aa) shows slow waves recorded simultaneously from the greater curvature of an antral preparation attached to the corpus. The electrodes were separated by 2 mm with the lower recording (red trace) having a more anal location. Expansions of these traces (Ab) show that slow waves were first detected by the electrode nearer the corpus and rising phases were separated by ∼500 ms, giving a conduction velocity of ∼4 mm s−1. Upper central pair of traces (Ba) shows slow waves recorded from antral preparation, attached to the corpus, with intact ICCMY network; electrode separation 2 mm with lower recording (red trace) being circumferentially located. Expansions (Bb) show that slow waves were first detected near the greater curvature and rising phases were separated by ∼140 ms, giving a conduction velocity of ∼15 mm s−1. The upper left hand pair of traces (Ca) show simultaneous recordings of slow waves from an antral preparation attached to the corpus in a region from which ICCMY network had been dissected away; electrode separation 2 mm with the lower recording (red trace) circumferentially located. Expansions (Cb) show that slow waves were again first detected near the greater curvature and the conduction velocity was again ∼15 mm s−1. The upper time calibration bar applies to traces Aa, Ba and Ca: The lower time calibration bar applies to traces Ab, Bb and Cb. Traces modified from Hirst et al. (2006).
The way in which corporal slow waves set the frequency of antral pacemaker potentials requires discussion. ICCMY are electrically excitable; electrical stimulation shortly after the end of a pacemaker potential evokes a premature pacemaker potential (Hirst et al. 2002c), leading to an increased frequency of antral slow waves (Publicover & Sanders, 1986). As ICCMY are electrically coupled to adjacent muscle layers, a depolarization in the circular layer depolarizes the ICCMY network (Cousins et al. 2002) and can trigger a premature pacemaker potential. This occurs when antral ICCIM are activated by neurally released acetylcholine (Hirst et al. 2002c) and will also occur at the corporal–antral interface, where the ICCMY network starts. Corporal slow waves occur more frequently than do pacemaker potentials in the isolated antrum (Fig. 1), hence when corporal slow waves rhythmically depolarize the oral end of the ICCMY network, they will trigger a regular discharge of pacemaker potentials and associated antral slow waves, at the same rate as in the corpus.
Circumferential spread of slow waves in the antrum
When paired recordings of slow waves were made from separated points in the antral circular layer, their circumferential conduction velocity was found to be four to five times faster (Fig. 3Ba and Bb) than their anal conduction velocity (Fig. 3Aa and Ab). As pointed out, this does not occur because pacemaker potentials have different anal and circumferential conduction velocities in the ICCMY network. Although antral slow waves are triggered by ICCMY, once initiated, slow waves of normal amplitude are recorded from regions of the antrum which lack ICCMY. In mouse antrum, the density of ICCMY is highest at the greater curvature and falls to undetectable levels at the lesser curvature but slow waves of normal amplitude are recorded throughout, even when ICCMY are absent. However, in the W/WV mouse, at the lesser curvature, where both ICCMY and ICCIM are absent, slow waves are not detected (Hirst et al. 2002a). These observations suggest that ICCIM alone sustain the circumferential spread of slow waves. To test this idea further, slow waves were recorded from preparations where all but a narrow band of ICCMY at the greater curvature had been dissected away. Slow waves had similar amplitudes whether ICCMY were present or had been removed (Fig. 3Ba and Ca); furthermore removing the ICCMY network did not alter the circumferential conduction velocity (Fig. 3Bb and Cb; Hirst et al. 2006). Similarly, isolated bundles of the pyloric circular layer readily conduct regenerative potentials over long lengths with the same conduction velocity as in the antrum (van Helden & Imtiaz, 2003). The simplest explanation is that in the intact stomach pacemaker potentials maximally depolarize the circular layer at the greater curvature, where the density of ICCMY is highest (Hirst et al. 2002a). Once a band of ICCIM has been excited, they support a circumferentially directed regenerative potential, with the long refractory period of regenerative potentials preventing backfiring (Suzuki & Hirst, 1999). In the antral circular layer, circumferential conduction is aided by the long electrical length constant of the circular bundles but extensive oro-anal conduction does not occur as the bundles are organized into functional units which are electrically isolated from each other (Hirst et al. 2006).
Summary
The slow descending rings of contraction that occur regularly in the intact stomach are triggered by electrical activity generated in successive sets of ICC. The corpus acts as the dominant pacemaker with corporal ICCIM generating a regular high frequency discharge of slow waves (Fig. 4). At the junction between the corpus and the antrum, each corporal slow wave triggers a pacemaker potential in the ICCMY network which conducts slowly down the ICCMY network (Fig. 4). Given that the dominant pacemaker region in the stomach resides in the corpus, it may be misleading to refer to the signals generated by ICCMY as pacemaker potentials. It may be more appropriate to refer to the signals generated by antral ICCMY as ‘driving potentials’ since their function in the intact stomach is simply to convey excitation in a fixed direction down the wall of the stomach and not to initiate spontaneous activity. As each driving/pacemaker potential passes over a circular muscle bundle, ICCIM are depolarized and they initiate the secondary regenerative component of the slow wave. Once initiated, the regenerative potential spreads rapidly in a circumferential direction (Fig. 4), concurrently depolarizing circular smooth muscle cells and causing a ring of contraction to develop. As the driving/pacemaker potential passes over successive anally located bundles, the process repeats, so causing successive bundles of circular muscle to contract. Mechanically this manifests itself as a slowly descending ring of contraction, moving the stomach contents towards the gastro-duodenal junction.
Figure 4. Propagation of slow waves down the guinea pig stomach.
ICCIM in the corpus generate a regular discharge of corporal slow waves. At the junction of the corpus and antrum, where ICCMY first appear, each corporal slow wave depolarizes the ICCMY network and triggers a driving/pacemaker potential. Each driving/pacemaker potential propagates anally from the point of initiation through the ICCMY network with a characteristically slow conduction velocity. As driving/pacemaker potentials pass over bundles of circular muscle, the bundles are depolarized, antral ICCIM are activated and the regenerative component of the slow wave is generated. The regenerative potential rapidly conducts circumferentially, depolarizing nearby smooth muscle cells, so triggering a ring of contraction. Electrical connections between adjacent circular muscle bundles are illustrated as closed rectangles: the occasional lack of such connections prevents extensive anal conduction of slow waves within the circular layer. Figure modified from Hirst et al. (2006).
More detailed descriptions of the organization of gastric ICC and their role in neuroeffector transmission appear in the symposium articles by Komuro (2006) and Ward & Sanders (2006), respectively, in this special issue of The Journal of Physiology. A detailed description of the generation of gastrointestinal pacemaker potentials appears in the symposium article by Suzuki et al. (2006).
Acknowledgments
This project was supported by a grant from the Australian NH and MRC.
References
- Beckett EAH, Bayguinov YR, Sanders KM, Ward SM, Hirst GDS. Properties of unitary potentials generated by intramuscular interstitial cells of Cajal in the murine and guinea-pig gastric fundus. J Physiol. 2004;559:259–269. doi: 10.1113/jphysiol.2004.063016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns AJ, Herbert TM, Ward SM, Sanders KM. Interstitial cells of Cajal in the guinea-pig gastrointestinal tract as revealed by c-Kit. Cell Tissue Res. 1997;290:11–20. doi: 10.1007/s004410050902. [DOI] [PubMed] [Google Scholar]
- Cousins HM, Edwards FR, Hickey H, Hill CE, Hirst GDS. Electrical coupling between the myenteric interstitial cells of Cajal and adjacent muscle layers in the guinea-pig gastric antrum. J Physiol. 2003;550:829–844. doi: 10.1113/jphysiol.2003.042176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens EJ, Edwards FR, Hirst GDS. Selective knockout of intramuscular interstitial cells reveals their role in the generation of slow waves in mouse stomach. J Physiol. 2001;531:827–833. doi: 10.1111/j.1469-7793.2001.0827h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens EJ, Hirst GDS, Tomita T. Identification of rhythmically active cells in guinea-pig stomach. J Physiol. 1999;514:515–531. doi: 10.1111/j.1469-7793.1999.515ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards FR, Hirst GDS. An electrical description of the generation of slow waves in the antrum of the guinea pig. J Physiol. 2005;564:213–232. doi: 10.1113/jphysiol.2004.077123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards FR, Hirst GDS. An electrical analysis of slow wave propagation in the guinea pig gastric antrum. J Physiol. 2006;571:179–189. doi: 10.1113/jphysiol.2005.100743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards FR, Hirst GDS, Suzuki H. Unitary nature of regenerative potentials recorded from circular smooth muscle of guinea-pig antrum. J Physiol. 1999;519:235–250. doi: 10.1111/j.1469-7793.1999.0235o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrugia G. Ionic conductances in gastrointestinal smooth muscles and interstitial cells of Cajal. Ann Rev Physiol. 1999;61:45–84. doi: 10.1146/annurev.physiol.61.1.45. [DOI] [PubMed] [Google Scholar]
- Goto K, Matsuoka S, Noma A. Two types of spontaneous depolarizations in the interstitial cells freshly prepared from the murine small intestine. J Physiol. 2004;559:411–422. doi: 10.1113/jphysiol.2004.063875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Garcia-Londoño AP, Hirst GDS, Edwards FR. Atypical slow waves generated in gastric corpus provide dominant pacemaker activity in guinea pig stomach. J Physiol. 2005;569:459–465. doi: 10.1113/jphysiol.2005.097907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig GW, Hirst GDS, Park KJ, Smith CB, Sanders KM, Ward SM, Smith TK. Propagation of pacemaker activity in the guinea-pig antrum. J Physiol. 2004;556:585–599. doi: 10.1113/jphysiol.2003.059055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GDS, Beckett EAH, Sanders KM, Ward SM. Regional variation in contribution of myenteric and intramuscular interstitial cells of Cajal to generation of slow waves in mouse gastric antrum. J Physiol. 2002a;540:1003–1012. doi: 10.1113/jphysiol.2001.013672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GDS, Bramich NJ, Teramoto N, Suzuki H, Edwards FR. Regenerative component of slow waves in the guinea-pig gastric antrum involves a delayed increase in [Ca2+]i and Cl− channels. J Physiol. 2002b;540:907–919. doi: 10.1113/jphysiol.2001.014803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GDS, Dickens EJ, Edwards FR. Pacemaker shift in the gastric antrum of guinea-pigs produced by excitatory vagal stimulation involves intramuscular interstitial cells. J Physiol. 2002c;541:917–928. doi: 10.1113/jphysiol.2002.018614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GDS, Edwards FR. Generation of slow waves in the antral region of guinea-pig stomach – a stochastic process. J Physiol. 2001;535:165–180. doi: 10.1111/j.1469-7793.2001.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GDS, Garcia-Londoño AP, Edwards FR. Propagation of slow waves in the guinea-pig gastric antrum. J Physiol. 2006;571:165–177. doi: 10.1113/jphysiol.2005.100735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GDS, Ward SM. Interstitial cells: involvement in rhythmicity and neural control of gut smooth muscle. J Physiol. 2003;550:337–346. doi: 10.1113/jphysiol.2003.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- Kito Y, Fukuta H, Suzuki H. Components of pacemaker potentials recorded from the guinea pig stomach antrum. Pflugers Arch. 2002a;445:202–217. doi: 10.1007/s00424-002-0884-z. [DOI] [PubMed] [Google Scholar]
- Kito Y, Fukuta H, Yamamoto Y, Suzuki H. Excitation of smooth muscles isolated from the guinea-pig gastric antrum in response to depolarization. J Physiol. 2002b;543:155–167. doi: 10.1113/jphysiol.2002.020875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kito Y, Suzuki H. Properties of pacemaker potentials recorded from myenteric interstitial cells of Cajal distributed in the mouse small intestine. J Physiol. 2003;553:803–818. doi: 10.1113/jphysiol.2003.051334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori K, Suzuki H. Distribution and properties of excitatory and inhibitory junction potentials in circular muscle of the guinea-pig stomach. J Physiol. 1986;370:339–355. doi: 10.1113/jphysiol.1986.sp015938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro T. Structure and organization of interstitial cells of Cajal in the gastrointestinal tract. J Physiol. 2006;576:653–658. doi: 10.1113/jphysiol.2006.116624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro T, Seki K, Horiguchi K. Ultrastructural characterization of the interstitial cells of Cajal. Arch Histol Cytol. 1999;62:295–316. doi: 10.1679/aohc.62.295. [DOI] [PubMed] [Google Scholar]
- Mazet B, Raynier C. Interstitial cells of Cajal in the guinea pig gastric antrum: distribution and regional density. Cell Tissue Res. 2004;316:23–34. doi: 10.1007/s00441-003-0835-9. [DOI] [PubMed] [Google Scholar]
- Ohba M, Sakamoto Y, Tomita T. The slow wave in the circular muscle of the guinea-pig stomach. J Physiol. 1975;253:505–516. doi: 10.1113/jphysiol.1975.sp011203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Publicover NG, Sanders KM. Effects of frequency on the wave form of propagated slow waves in canine gastric antral muscle. J Physiol. 1986;371:179–189. doi: 10.1113/jphysiol.1986.sp015967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- Sanders KN, Publicover NG. Handbook of Physiology, Section 6, Gastrointestinal System. Part 1. Vol. 1. Bethesda, MA: American Physiological Society; 1989. Electrophysiology of the gastric musculature; pp. 187–216. [Google Scholar]
- Suzuki H, Hirst GDS. Regenerative potentials evoked in circular smooth muscle of the antral region of guinea-pig stomach. J Physiol. 1999;517:563–573. doi: 10.1111/j.1469-7793.1999.0563t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Takano H, Yamamoto Y, Komuro T, Saito M, Kato K, Mikoshiba K. Properties of gastric smooth muscles obtained from mice which lack inositol trisphosphate receptor. J Physiol. 2000;525:105–111. doi: 10.1111/j.1469-7793.2000.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Kito Y, Hashitani H, Nakamura E. Factors modifying the frequency of spontaneous activity in gastric muscle. J Physiol. 2006;576:667–674. doi: 10.1113/jphysiol.2006.117093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szurszewski JH. Electrical basis for gastrointestinal motility. In: Johnson R, editor. Physiology of the Gastrointestinal Tract. New York: Raven Press; 1981. pp. 1435–1465. [Google Scholar]
- Tomita T. Electrical activity (spikes and slow waves) in gastrointestinal smooth muscles. In: Bulbring E, Brading AF, Jones AW, Tomita T, editors. Smooth Muscle: an Assessment of Current Knowledge. London: Edward Arnold; 1981. pp. 127–156. [Google Scholar]
- Tsugeno M, Huang S-M, Pang Y-W, Chowdhury JU, Tomita T. Effects of phosphodiesterase inhibitors on spontaneous electrical activity (slow waves) in guinea pig gastric muscle. J Physiol. 1995;485:493–502. doi: 10.1113/jphysiol.1995.sp020745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Helden DF, Imtiaz MS. Ca2+ phase waves: a basis for cellular pacemaking and long-range synchronicity in the guinea-pig gastric pylorus. J Physiol. 2003;548:271–296. doi: 10.1113/jphysiol.2002.033720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X-Y, Lammers WJEP, Bercik P, Huizinga JD. Lack of pyloric interstitial cells of Cajal explains distinct peristaltic motor patterns in stomach and small intestine. Am J Physiol. 2005;289:G539–G549. doi: 10.1152/ajpgi.00046.2005. [DOI] [PubMed] [Google Scholar]
- Ward SM, Sanders KM. Involvement of intramuscular ICC in neuroeffector transmission in the gastrointestinal tract. J Physiol. 2006 doi: 10.1113/jphysiol.2006.117390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994;480:91–97. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]