Abstract
The morphological features of interstitial cells of Cajal (ICC) in the gastrointestinal (GI) tract are described based on observations of laboratory animals including mice, rats and guinea-pigs, using immunohistochemical staining for Kit and electron microscopy. ICC show a specific distribution, arrangement and cell shape depending on their location within various regions and tissue layers of the GI tract. Hence they are classified into several subtypes. The stomach shows distinct regional variations in the distribution of subtypes of ICC from the cardia to pylorus, whereas the small intestine and colon both seem to retain nearly the same distribution pattern of subtypes of ICC throughout each organ. All subtypes of ICC share common ultrastructural features, such as the presence of numerous mitochondria, abundant intermediate filaments, and formation of gap junctions with the same type of cells and with smooth muscle cells. In addition, depending on their species and anatomical location, some subtypes of ICC show some features typical of smooth muscle cells including a basal lamina, caveolae, subsurface cisterns and dense bodies. ICC are somewhat heterogeneous morphologically. A question is raised on a special relationship between their ultrastructural features and dependency on Kit/stem cell factor system. As the neuromediator function of ICC, reciprocal distribution of ICC and gap junctions in the muscle coat is demonstrated by the comparison of Kit immunoreactive cells and gap junction protein connexin 43 in both small intestine and colon.
In 1982, Thuneberg surprised the researchers of the GI tract by proposing the hypothesis that interstitial cells of Cajal (ICC) might act as pacemaker cells and as an impulse conduction system in the gut musculature in a way analogous to the pacemaker cells in the heart (Thuneberg, 1982). Since then, an accumulation of evidence from morphological and physiological studies has made it clear that this hypothesis is compatible with experimental observations and that ICC are a distinct mesenchymal cell type (Lecoin et al. 1996; Young et al. 1996) with functions of either pacemaker or neuromediator cells in the tunica muscularis of the GI tract (Thuneberg, 1989; Sanders, 1996; Huizinga et al. 1997; Komuro et al. 1999; Ward & Sanders, 2001). As further evidence for their neuromediator role, synaptic specializations beween ICC and nerves were recently demonstrated by immunohistochemical staining for synapse-associated proteins (Beckett et al. 2005).
ICC have been found throughout the digestive tract from the oesophagus (Faussone-Pellegrini & Cortesini, 1985; Torihashi et al. 1999) to the inner sphincter region of the anus in the human (Hagger et al. 1998), but they show different distribution patterns and morphological features depending on their anatomical locations, and according to which they are classified into several subtypes (Hanani et al. 2005). The structural arrangement of ICC appears to reflect their physiological tasks and thus provides a clue for critical understanding of intestinal motility. This article deals with the morphological features of each subtypes of ICC revealed by the immunohistochemical and ultrastructural observations of the stomach, small intestine and colon of laboratory animals such as mouse, rat and guinea-pig.
Organization of ICC networks
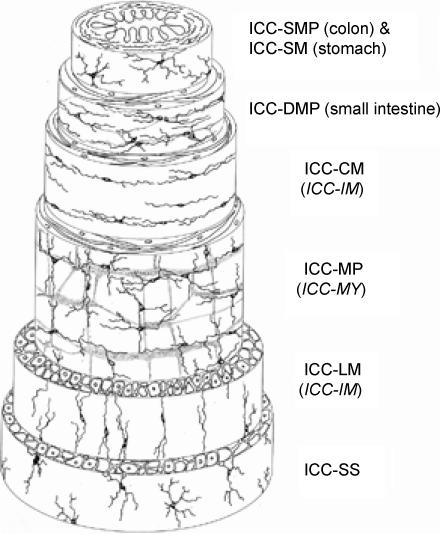
The location of the different subtypes of ICC is shown schematically in Fig. 1. The cell shape and arrangement of each subtype of ICC is mainly determined by their relationships to local nerve plexuses, the orientation of the smooth muscle layer in which they are contained, and the frequency of connections between ICC themselves.
Figure 1. Schematic representation of the types of ICC located in different tissue layers of the GI tract.
ICC-SMP and ICC-SM are seen at the interface between the submucosa and circular muscle layer in the colon and gastric pylorus, respectively. ICC-DMP are associated with the deep muscular plexus located between the inner and outer circular muscle sublayers in the small intestine. ICC-CM and ICC-LM are found within the circular and longitudinal muscle layers, respectively. ICC-MP are associated with the myenteric plexus between the circular and longitudinal muscle layers. ICC-SS are found in the subserosal connective tissue space. (Reproduced with permission from Hanani et al. 2005.). As to terminology in this article, ICC located in the myenteric (Auerbach's) plexus are designated as ICC-MP instead of ICC-MY or ICC-AP, to keep consistency with the abbreviation for ICC-DMP (deep muscular plexus) and ICC-SMP (submuscular plexus) and because of uncommon usage of the term Auerbach's plexus in the recent literature. ICC-CM and ICC-LM are separately designated, because some morphological and functional differences between them have not been ruled out.
ICC of the myenteric plexus (ICC-MP)
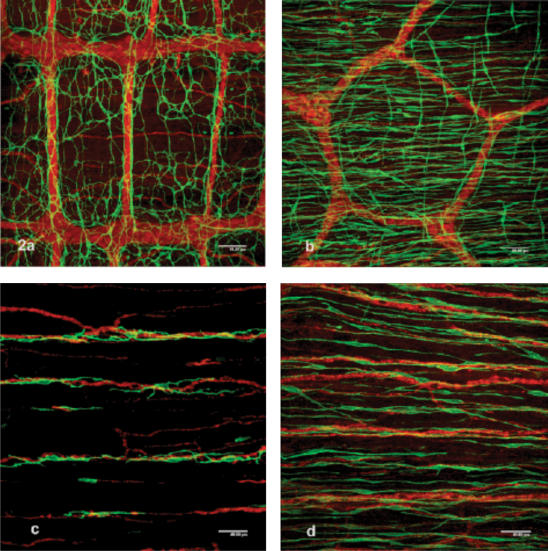
ICC-MP are multipolar cells with three to five primary cytoplasmic processes that project secondary, tertiary and further branching processes that interconnect with their counterparts. They form a cellular network around the myenteric plexus in the space between the circular and longitudinal muscle layers (Fig. 2a and b). The size of ganglia and pattern of the myenteric plexus vary greatly between different parts of the GI tract (Gabella, 1979). In addition to features reflecting the local myenteric plexus, ICC-MP are fewer in number and their cellular networks are relatively looser in the gastric corpus and colon than in the small intestine (Fig. 2b).
Figure 2. Immunohistochemical staining for Kit and PGP 9.5 to demonstrate cellular networks of ICC (green fluorescence of Alexa 488) and nerves (red fluorescence of TRITC), respectively.
a, the network of ICC-MP over the myenteric plexus of the guinea-pig small intestine in which ganglia are orientated parallel to the circular muscle fibres and are connected with thick nerve bundles orientated almost perpendicularly to the ganglia. Scale bar, 80 μm. b, around the myenteric plexus of the guinea-pig colon, ICC-MP are not clearly observed because of their loose arrangement and dense distribution of ICC-CM orientated in a horizontal direction. A few ICC-LM are also seen perpendicularly. Scale bar, 80 μm. c, sparsely distributed ICC–CM in association with rather thick nerve bundles within the circular muscle layer of the guinea-pig small intestine. Scale bar, 40 μm. d, ICC-CM within the circular muscle layer of the guinea-pig gastric corpus. Scale bar, 40 μm.
ICC of the circular muscle (ICC-CM)
ICC-CM of the circular muscle layers are mainly bipolar cells orientated along the long axis of surrounding smooth muscle cells. However, the distribution and cell density of ICC-CM differs considerably from organ to organ. ICC-CM of the small intestine often show secondary cytoplasmic branches and are sparsely distributed in association with rather thicker nerve bundles (Fig. 2c). They appear not to form their own developed cellular network. In contrast, ICC-CM of the stomach and colon show a simple elongated spindle shape, but are densely distributed along nerve bundles (Fig. 2d). They are also found in the connective tissue septa, where they have been specially designated as ICC-SEP in the literature (Horiguchi et al. 2001; Mazet & Raynier, 2004).
ICC of the longitudinal muscle (ICC-LM)
ICC-LM are similar to ICC-CM in cell shape but are usually less numerous than the latter in nearly the whole GI tract, i.e. in the stomach, small intestine and colon (Komuro, 2004). ICC-CM and ICC-LM are often collectively termed ICC-IM.
ICC of the deep muscular plexus (ICC-DMP)
ICC-DMP are closely associated with the nerve bundles of the deep muscular plexus of the small intestine that extends two-dimensionally in a plane between the inner thin and outer thick sublayers of the circular muscle (Rumessen et al. 1982; Zhou & Komuro, 1992). ICC-DMP are also multipolar cells, but the majority of their processes show a distinct unidirectional orientation along the circumference, due to their close association with both nerve bundles and circular muscle fibres (Komuro, 2004).
ICC of submucosa and sumucosal plexus (ICC-SM and ICC-SMP)
ICC-SM and ICC-SMP are found at the interface between the submucosal connective tissue and the innermost circular muscle layer of the pyloric region of the stomach (Horiguchi et al. 2001; Seki & Komuro, 2002; Mitsui & Komuro, 2003) and of the colon, respectively (Berezin et al. 1988; Ishikawa & Komuro, 1996). Their cell axes are parallel with those of adjacent circular muscle cells, but they contain multipolar cells with a few secondary processes and seem to form a loose network with each other (Kunisawa & Komuro, 2004), unlike the adjacent ICC-CM.
ICC of the subserosa (ICC-SS)
Stellate interstitial cells in the subserosal layer were observed in the mouse small intestine with supravital methylene blue staining (Thuneberg, 1982) and in the mouse colon by Kit immunohistochemistry (Vanderwinden et al. 2000).
Distribution of ICC
Each subtype of ICC is almost uniformly found in its own tissue layer throughout the entire length of the small intestine and colon, though some differences have been reported in the mouse colon (Ward et al. 2002) and human colon (Horisawa et al. 1998). In this respect, the stomach is unique in that ICC have a different distribution in proximal and distal regions of the same organ (Burns et al. 1997; Seki & Komuro, 2002). In the mouse stomach, for example, ICC-CM and ICC-LM are densely distributed throughout the thick circular and longitudinal muscle layers of the cardia, fundus and most of the squamous epithelial portion of the corpus. However, ICC-MP are completely lacking from the myenteric region in these areas. ICC-MP emerge in the area adjacent to the glandular corpus and become well-developed in the pyloric antrum, while both ICC-CM and ICC-LM decrease in number in this area. Along the circumferential axis of the antrum, ICC-MP are distributed more densely in the greater curvature than in the lesser curvature in the mouse (Hirst et al. 2002) and the guinea-pig (Kunisawa & Komuro, 2004; Mazet & Raynier, 2004).
Another characteristic feature of the pylorus is the presence of ICC-SM at the submucosal border of the circular muscle layer in a confined area directly adjacent to the sphincter (Horiguchi et al. 2001; Mitsui & Komuro, 2003).
Coupling of ICC and smooth muscle cells
Ultrastructural observations revealed that certain types of ICC are intercalated between nerves and smooth muscle cells (Thuneberg, 1982; Zhou & Komuro, 1992; Ishikawa et al. 1997; Seki & Komuro, 1998) and indicated that they may act as a route for neurotransmission. Such ICC make close contacts with nerve varicosities containing many synaptic vesicles on the one-hand and form gap junctions with neighbouring muscle cells on the other (Fig. 3). More concrete morphological evidence for the neuromediatory function of such ICC was recently provided by immunohistochemical staining for synaptic molecules (Beckett et al. 2005).
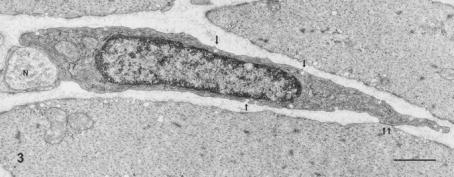
Figure 3. ICC-CM.
ICC-CM in the rat stomach characterized by electron-dense cytoplasm, caveolae (arrows), a gap junction with a smooth muscle cell (double arrow) and a close contact with nerve terminal (N). × 16 000. (Reproduced with permission from Ishikawa et al. 1997.) Scale bar, 1 μm.
A clear difference between the distribution patterns of immunoreactivity for Kit-positive cells (ICC) and of the gap junction protein connexin 43 (Cx43) was demonstrated in the muscle coat of the guinea-pig digestive tract (Seki et al. 1998). The dense staining for Cx43 in the circular muscle layer of the small intestine indicates that muscle cells were coupled well with each other by gap junctions, but were not intercalated by ICC, because Kit immunoreactive cells were rarely observed in the outer circular muscle layer. On the other hand, the colon contained a regular distribution of Kit immunoreactive cells, and had much less Cx43 immunoreactivity in the circular muscle layer. These data suggest that circular muscle cells of the colon are not well coupled with each other by gap junctions, but are interconnected by intercalated ICC. The reciprocal distribution of Kit and Cx43 immunoreactivity was also shown within the mouse stomach (Seki & Komuro, 2002). The thick circular muscle layer of the fundus had a dense population of ICC-CM, but only weak Cx43 immunoreactivity, whereas the thin muscle portion of the corpus was characterized by a sparse population of ICC-CM, but a dense Cx43 immunoreactivity. These observations suggest that muscle cells that are not well coupled to each other by gap junctions receive nerve signals via a rich network of ICC-CM, whereas muscle cells that are well coupled with each other to form large units of an electrical syncytium receive nerve signals at fewer contacts with ICC-CM. Regarding coupling between ICC-MP and smooth muscle cells, ultrastructural observations failed to provide good enough evidence for their coupling by gap junctions hitherto, except one report in the rat small intestine (Komuro, 1989).
Ultrastructure and dependency on the Kit/stem cell factor system
ICC are generally characterized by ultrastructural features such as the presence of numerous mitochondria, abundant intermediate filaments, moderately developed Golgi apparatus, rough and smooth endoplasmic reticulum, close contacts with nerve varicosities and formation of gap junctions with each other and with smooth muscle cells (Komuro, 1999). However, ICC show a certain range of morphological heterogeneity ranging from features similar to the fibroblasts to those specific to smooth muscles cells such as caveolae, a basal lamina and subsurface cistern, depending on anatomical location and species (Table 1; Komuro et al. 1999; Mitsui & Komuro, 2003).
Table 1.
Three types of ICC classified by their ultrastructural features
| ICC type | Basal lamina | Caveolae | Gap junctions | Intermediate filaments | Mitochondria | Nerve contacts |
|---|---|---|---|---|---|---|
| Type 1 ICC | − | +/− | ++ | ++ | ++ | ++ |
| (least like smooth muscle cells) | ||||||
| Type 2 ICC | +/− | ++ | ++ | ++ | ++ | ++ |
| (intermediate type) | ||||||
| Type 3 ICC | ++ | ++ | ++ | ++ | ++ | ++ |
| (most like smooth muscle cells) | ||||||
Abundance, (++) present or numerous; (+/−), fuzzy or few; (−) absent. All types of ICC are positive for Kit immunoreactivity.
In spite of the fact that in normal development ICC express Kit and their cell maturation depends on signalling between the Kit receptor and its ligand, stem cell factor (SCF), many reports indicate that a certain population of ICC can survive in the absence of proper Kit/SCF signalling. Such ICC were observed in both c-kit and stem cell factor mutant animals. Those are ICC-MP in the pylorus of W/Wv mouse (Ward et al. 1998; Seki & Komuro, 2002), and ICC-MP and ICC-SM in the pylorus of Ws/Ws rat (Mitsui & Komuro, 2003). Other examples include the ICC-DMP of the Ws/Ws rat small intestine (Horiguchi et al. 1995; Horiguchi & Komuro, 1998), the ICC-DMP of the small intestine of the Sl/Sld mouse (Ward et al. 1995) and W/Wv mouse (Malysz et al. 1996) and ICC-SMP of the Ws/Ws rat colon (Ishikawa et al. 1997).
These ICC share common ultrastructural features and are classified into Type 3 ICC (Table 1), the most similar to smooth muscle cells, in respect of the presence of many caveolae and a distinct basal lamina (Komuro et al. 1999), regardless of the organ or tissue layer concerned. The evidence suggests that the most muscle-like Type 3 ICC can develop and mature cytologically independent of the Kit/SCF system, or that some other system can compensate in their cell maturation. These observations appear to raise important questions for future studies on why ICC show a fairly wide range of phenotypes and which factors determine the ultrastructural features of particular type of ICC, together with studies to demonstrate if ICC and smooth muscle cells are derived from the same mesechymal progenitor cells (Kluppel et al. 1998) and that blockade of Kit signalling induces a smooth muscle cell phenotype (Torihashi et al. 1999).
References
- Beckett EAH, Takeda Y, Yanase H, Sanders KM, Ward SM. Synaptic specializations exist between enteric motor nerves and interstitial cells of Cajal in the murine stomach. J Comp Neurol. 2005;493:193–206. doi: 10.1002/cne.20746. [DOI] [PubMed] [Google Scholar]
- Berezin I, Huizinga JD, Daniel EE. Interstitial cells of Cajal in the canine colon: a special communication network at the inner border of the circular muscle. J Comp Neurol. 1988;373:42–51. doi: 10.1002/cne.902730105. [DOI] [PubMed] [Google Scholar]
- Burns AJ, Herbert TM, Ward SM, Sanders KM. Interstitial cells of Cajal in the guinea-pig gastrointestinal tract as revealed by c-Kit immunohistochemistry. Cell Tissue Res. 1997;290:11–20. doi: 10.1007/s004410050902. [DOI] [PubMed] [Google Scholar]
- Faussone-Pellegrini MS, Cortesini C. Ultrastructural features and localization of the interstitial cells of Cajal in the smooth muscle coat of human esophagus. J Submicrosc Cytol Patol. 1985;17:187–197. [PubMed] [Google Scholar]
- Gabella G. Innervation of the gastrointestinal tract. Int Rev Cytol. 1979;59:129–193. doi: 10.1016/s0074-7696(08)61662-9. [DOI] [PubMed] [Google Scholar]
- Hagger R, Gharaie S, Finlayson C, Kumar D. Distribution of the interstitial cells of Cajal in the human anorectum. J Auton Nerv Syst. 1998;73:75–79. doi: 10.1016/s0165-1838(98)00038-1. [DOI] [PubMed] [Google Scholar]
- Hanani M, Farrugia G, Komuro T. Intercellular coupling of interstitial cells of Cajal in the digestive tract. Int Rev Cytol. 2005;242:249–282. doi: 10.1016/S0074-7696(04)42006-3. [DOI] [PubMed] [Google Scholar]
- Hirst GDS, Beckett EAH, Sanders KM, Ward SM. Regional variation in contribution of myenteric and intramuscular interstitial cells of Cajal to generation of slow waves in mouse gastric antrum. J Physiol. 2002;540:1003–1012. doi: 10.1113/jphysiol.2001.013672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi K, Komuro T. Ultrastructural characterization of interstitial cells of Cajal in the rat small intestine using control and Ws/Ws mutant rats. Cell Tissue Res. 1998;293:277–284. doi: 10.1007/s004410051119. [DOI] [PubMed] [Google Scholar]
- Horiguchi K, Semple GS, Sanders KM, Ward SM. Distribution of pacemaker function through the tunica muscularis of the canine gastric antrum. J Physiol. 2001;537:237–250. doi: 10.1111/j.1469-7793.2001.0237k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi K, Zhou DS, Seki K, Komuro T, Hirota S, Kitamura Y. Morphological analysis of c-kit expressing cells, with reference to interstitial cells of Cajal. J Smooth Muscle Res. 1995;31:303–305. [Google Scholar]
- Horisawa M, Watanabe Y, Torihashi S. Distribution of c-kit immunopositive cells in normal human colon and in Hirschsprung's disease. J Pediatr Surg. 1998;33:1209–1214. doi: 10.1016/s0022-3468(98)90152-x. [DOI] [PubMed] [Google Scholar]
- Huizinga JD, Thuneberg L, Vanderwinden JM, Rumessen JJ. Interstitial cells of Cajal as targets for pharmacological intervention in gastrointestinal motor disorders. TIPS. 1997;18:393–403. doi: 10.1016/s0165-6147(97)01108-5. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Komuro T. Characterization of the interstitial cells associated with the submuscular plexus of the guinea-pig colon. Anat Embryol. 1996;194:49–55. doi: 10.1007/BF00196314. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Komuro T, Hirota S, Kitamura Y. Ultrastructural identification of the c-kit-expressing interstitial cells in the rat stomach: a comparison of control and Ws/Ws mutant rats. Cell Tissue Res. 1997;289:137–143. doi: 10.1007/s004410050859. [DOI] [PubMed] [Google Scholar]
- Kluppel MJ, Huizinga JD, Malysz J, Bernstein A. Developmental origin and kit-dependent development of the interstitial cells of Cajal in the mammalian small intestine. Devel Dynamics. 1998;211:60–71. doi: 10.1002/(SICI)1097-0177(199801)211:1<60::AID-AJA6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Komuro T. Three-dimensional observation of the fibroblast-like cells associated with the rat myenteric plexus, with special reference to the interstitial cells of Cajal. Cell Tissue Res. 1989;255:343–351. doi: 10.1007/BF00224117. [DOI] [PubMed] [Google Scholar]
- Komuro T. Comparative morphology of interstitial cells of Cajal: ultrastructural characterization. Microsc Res Tech. 1999;47:267–285. doi: 10.1002/(SICI)1097-0029(19991115)47:4<267::AID-JEMT5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Komuro T. Morphological features of interstitial cells of Cajal. In: Kitamura Y, Miettinenn M, Hirota S, Kanakura Y, editors. In Gastrointestinal Stromal Tumor (GIST): from pathology to molecular target therapy. Tokyo, Basel: Japan Scientific Societies Press & Karger; 2004. pp. 109–134. Gann Monograph on Cancer Research No. 53. [Google Scholar]
- Komuro T, Seki K, Horiguchi K. Ultrastructural characterization of the interstitial cells of Cajal. Arch Histol Cytol. 1999;62:295–316. doi: 10.1679/aohc.62.295. [DOI] [PubMed] [Google Scholar]
- Kunisawa Y, Komuro T. Interstitial cells of Cajal in the guinea-pig gastric antrum. Distribution and Ultrastructure. Proceedings of the 8APEM. 2004:945–946. [Google Scholar]
- Lecoin L, Gabella G, Douarin NL. Origin of the c-kit-positive interstitial cells in the avian bowel. Development. 1996;122:725–733. doi: 10.1242/dev.122.3.725. [DOI] [PubMed] [Google Scholar]
- Malysz J, Thuneberg L, Mikkelsen HB, Huizinga JD. Action potential generation in the small intestine of W mutant mice that lack interstitial cells of Cajal. Am J Physiol. 1996;271:G387–G399. doi: 10.1152/ajpgi.1996.271.3.G387. [DOI] [PubMed] [Google Scholar]
- Mazet B, Raynier C. Interstitial cells of Cajal in the guinea pig gastric antrum: distribution and regional density. Cell Tissue Res. 2004;316:23–34. doi: 10.1007/s00441-003-0835-9. [DOI] [PubMed] [Google Scholar]
- Mitsui R, Komuro T. Distribution and ultrastructure of interstitial cells of Cajal in the gastric antrum of wild-type and Ws/Ws rats. Anat Embryol. 2003;206:453–460. doi: 10.1007/s00429-003-0323-8. [DOI] [PubMed] [Google Scholar]
- Rumessen JJ, Thuneberg L, Mikkelsen HB. Plexus muscularis profundus and associated interstitial cells. II. Ultrastructural studies of mouse small intestine. Anat Record. 1982;203:129–146. doi: 10.1002/ar.1092030112. [DOI] [PubMed] [Google Scholar]
- Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- Seki K, Komuro T. Further observation of the gap junction-rich cells in the deep muscular plexus of the rat small intestine. Anat Embryol. 1998;197:135–141. doi: 10.1007/s004290050125. [DOI] [PubMed] [Google Scholar]
- Seki K, Komuro T. Distribution of interstitial cells of Cajal and gap junction protein, Cx 43 in the stomach of wild-type and W/Wv mutant mice. Anat Embryol. 2002;206:57–65. doi: 10.1007/s00429-002-0279-0. [DOI] [PubMed] [Google Scholar]
- Seki K, Zhou DS, Komuro T. Immunohistochemical study of the c-kit expressing cells and connexin 43 in the guinea-pig digestive tract. J Auton Nerv Syst. 1998;68:182–187. doi: 10.1016/s0165-1838(97)00134-3. [DOI] [PubMed] [Google Scholar]
- Thuneberg L. Interstitial cells of Cajal: Intestinal pacemaker cells? Adv Anat Embryol Cell Biol. 1982;71:1–130. [PubMed] [Google Scholar]
- Thuneberg L. Interstitial cells of Cajal. In: Wood JD, editor. Handbook of Physiology. The Gastrointestinal System. Vol. 1. Bethesda, Maryland: American Physiological Society; 1989. pp. 349–386. [Google Scholar]
- Torihashi S, Horisawa M, Watanabe Y. c-Kit immunoreactive interstitial cells in the human gastrointestinal tract. J Auton Nerv Syst. 1999;75:38–50. doi: 10.1016/s0165-1838(98)00174-x. [DOI] [PubMed] [Google Scholar]
- Vanderwinden JM, Rumessen JJ, Bernex F, Schiffmann SN, Panthier JJ. Distribution and ultrastructure of interstitial cells of Cajal in the mouse colon, using antibodies to Kit and Kit (W-lacZ) mice. Cell Tissue Res. 2000;302:155–170. doi: 10.1007/s004419900170. [DOI] [PubMed] [Google Scholar]
- Ward SM, Burns AJ, Torihashi S, Harney SC, Sanders KM. Impaired development of interstitial cells and intestinal electrical rhythmicity in steel mutants. Am J Physiol. 1995;269:C1577–C1585. doi: 10.1152/ajpcell.1995.269.6.C1577. [DOI] [PubMed] [Google Scholar]
- Ward SM, Gershon MD, Keef K, Bayguinov YR, Nelson C, Sanders KM. Interstitial cells of Cajal and electrical activity in ganglionic and aganglionic colons of mice. Am J Physiol Gastrointest Liver Physiol. 2002;283:G445–G456. doi: 10.1152/ajpgi.00475.2001. [DOI] [PubMed] [Google Scholar]
- Ward SM, Morris G, Reese L, Wang XY, Sanders KM. Interstitial cells of Cajal mediate enteric inhibitory neurotransmission in the lower esophageal and pyloric sphincters. Gastroenterology. 1998;115:314–329. doi: 10.1016/s0016-5085(98)70198-2. [DOI] [PubMed] [Google Scholar]
- Ward SM, Sanders KM. Interstitial cells of Cajal: primary target of enteric motor innervation. Anat Rec. 2001;262:125–135. doi: 10.1002/1097-0185(20010101)262:1<125::AID-AR1017>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Young HM, Ciampoli D, Southwell BR, Newgreen DF. Origin of interstitial cells of Cajal in the mouse intestine. Dev Biol. 1996;180:97–107. doi: 10.1006/dbio.1996.0287. [DOI] [PubMed] [Google Scholar]
- Zhou DS, Komuro T. Interstitial cells associated with the deep muscular plexus of the guinea-pig small intestine, with special reference to the interstitial cells of Cajal. Cell Tissue Res. 1992;268:205–216. doi: 10.1007/BF00318788. [DOI] [PubMed] [Google Scholar]