Abstract
Skeletal muscle size is tightly regulated by the synergy between anabolic and catabolic signalling pathways which, in humans, have not been well characterized. Akt has been suggested to play a pivotal role in the regulation of skeletal muscle hypertrophy and atrophy in rodents and cells. Here we measured the amount of phospho-Akt and several of its downstream anabolic targets (glycogen synthase kinase-3β (GSK-3β), mTOR, p70s6k and 4E-BP1) and catabolic targets (Foxo1, Foxo3, atrogin-1 and MuRF1). All measurements were performed in human quadriceps muscle biopsies taken after 8 weeks of both hypertrophy-stimulating resistance training and atrophy-stimulating de-training. Following resistance training a muscle hypertrophy (∼10%) and an increase in phospho-Akt, phospho-GSK-3β and phospho-mTOR protein content were observed. This was paralleled by a decrease in Foxo1 nuclear protein content. Following the de-training period a muscle atrophy (5%), relative to the post-training muscle size, a decrease in phospho-Akt and GSK-3β and an increase in Foxo1 were observed. Atrogin-1 and MuRF1 increased after the hypertrophy and decreased after the atrophy phases. We demonstrate, for the first time in human skeletal muscle, that the regulation of Akt and its downstream signalling pathways GSK-3β, mTOR and Foxo1 are associated with both the skeletal muscle hypertrophy and atrophy processes.
The control of skeletal muscle size is tightly regulated by the synergy between hypertrophy (anabolic) and atrophy (catabolic) processes. Human skeletal muscle hypertrophy occurs with an increase in functional demand as seen with resistance training (for review see Fry, 2004), and with functional electrical stimulation after spinal cord injury (Hjeltnes et al. 1997; Baldi et al. 1998) and in the elderly following surgery (Suetta et al. 2004). In contrast, skeletal muscle atrophy is a devastating condition seen after musculoskeletal trauma, in many catabolic diseases such as cancer, diabetes, sepsis and AIDS (Mitch & Goldberg, 1996; Lecker et al. 1999; Tisdale, 2005), denervation and sarcopenia (Zinna & Yarasheski, 2003), in neuromuscular disorders such as Duchenne muscular dystrophy and amyotrophic lateral sclerosis (ALS) (Lynch, 2001) and in critical illness myopathy (Di Giovanni et al. 2004).
Recently, significant advances have been made in understanding the factors controlling skeletal muscle hypertrophy and atrophy using pharmacological and genetic manipulation in cellular and rodent models (Bodine et al. 2001a,b; Gomes et al. 2001; Rommel et al. 2001; Pallafacchina et al. 2002; Sandri et al. 2004; Stitt et al. 2004; Latres et al. 2005). Combined, these studies have underlined Akt (also called PKB; protein kinase B), a serine/threonine kinase, as a pivotal point in the hypertrophy, and more recently, in the atrophy signalling pathways (Stitt et al. 2004; Latres et al. 2005). However, the molecular targets and signalling pathways influencing human muscle hypertrophy and atrophy are not well understood.
Akt is activated following a series of intracellular signalling cascades involving insulin-like growth factor 1 (IGF-1) and phosphatidylinositol 3-kinase (PI3K) (Datta et al. 1999; Rommel et al. 2001; Vivanco & Sawyers, 2002). A downstream target of Akt is glycogen synthase kinase-3β (GSK-3β). The phosphorylation of GSK-3β by Akt (Welsh et al. 1997; Jefferson et al. 1999) releases its inhibition of the translation initiation factor eIF2B (Rhoads, 1999). Akt also phosphorylates and activates the mammalian target of rapamycin (mTOR) (Pallafacchina et al. 2002), with the latter phosphorylating and activating p70S6K as well as phosphorylating and releasing the inhibitory effect of PHAS-1/4E-BP1 (Terada et al. 1994; Lin & Lawrence, 1996; Rhoads, 1999). Phosphorylation of both p70S6K and PHAS-1/4E-BP1 leads to the activation of pathways promoting protein synthesis and translation initiation, respectively. Hence the Akt–GSK-3β and Akt–mTOR pathways are important for muscle hypertrophy.
In situations of skeletal muscle atrophy, either in rodents or myotubes, the down-regulation of Akt signalling permits the transcription of atrogin-1 (also known as MAFbx) and MuRF1, two muscle specific E3 ubiquitin ligases that play a role in muscle atrophy (Sandri et al. 2004; Latres et al. 2005). The dephosphorylation (inactivation) of Akt removes its inhibitory effect on the forkhead (FKHR; Foxo) family of transcription factors, with the latter permitted to translocate from the cytosol to the nucleus (Brunet et al. 1999; Ramaswamy et al. 2002). Studies in cellular and rodent models of muscle atrophy and hypertrophy, using pharmacological and/or gene inhibition techniques, have shown that the transcriptional regulation of atrogin-1 and MuRF1 is controlled by an Akt/Foxo-dependent signalling pathway (Lee et al. 2004; Sandri et al. 2004; Stitt et al. 2004; Latres et al. 2005). Atrogin-1 has also been shown to be regulated, in C2C12 myotubes and adult mice, via a TNFα/p38 MAPK pathway (Li et al. 2005). Recently we observed in ALS patients and ALS G93A mice, severe models of muscle atrophy, that Akt was reduced while atrogin-1 mRNA and protein levels were increased (Léger et al. 2006). This occurred without a change in nuclear Foxo levels, suggesting that atrogin-1 transcription, at least in ALS human and mouse muscle, may be regulated by another Akt stimulated pathway.
The observation that Akt signalling is capable of activating anabolic and suppressing catabolic pathways presents this protein as a potential therapeutic target. However the regulation of Akt signalling in human skeletal muscle, when placed under hypertrophy and atrophy stimuli, is poorly understood. Therefore, the aims of the present study were to measure the active phosphorylated protein content of Akt and its downstream hypertrophy signalling targets, such as GSK-3β, mTOR, p70S6K and 4E-BP1, as well as its atrophy regulatory targets, including Foxo1, Foxo3, atrogin-1 and MuRF1 in human skeletal muscle. TNFα mRNA and phosphorylated p38 MAPK were also measured. These measurements were made before and after a period of resistance training (hypertrophy) and again after a period of de-training (atrophy) in human skeletal muscle (Campos et al. 2002).
Methods
Twenty-five healthy males participated in the study: age (mean ± s.d.) 36 ± 4.9 years, maximal oxygen consumption 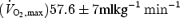 , mass 75.3 ± 9 kg, body fat 16.4 ± 4.5%. The study was approved by the local medical society ethical committee and conformed with the Declaration of Helsinki. All participants gave their informed consent and agreed to muscle biopsies and physiological testing. The subjects were physically active but had not participated in a resistance training programme for more than 12 months. Subjects were divided into two training groups (low repetitions or high repetitions) that were matched for age, height, weight,
, mass 75.3 ± 9 kg, body fat 16.4 ± 4.5%. The study was approved by the local medical society ethical committee and conformed with the Declaration of Helsinki. All participants gave their informed consent and agreed to muscle biopsies and physiological testing. The subjects were physically active but had not participated in a resistance training programme for more than 12 months. Subjects were divided into two training groups (low repetitions or high repetitions) that were matched for age, height, weight, 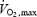 and muscular strength and endurance (Table 1). Prior to all biopsies and during the 48–72 h before the post-training biopsy the subjects were asked to eat a diet consisting of pasta, meat and vegetables with no alcohol. The portions were left to the discretion of the subjects; however, they were asked to have same meal prior to each biopsy.
and muscular strength and endurance (Table 1). Prior to all biopsies and during the 48–72 h before the post-training biopsy the subjects were asked to eat a diet consisting of pasta, meat and vegetables with no alcohol. The portions were left to the discretion of the subjects; however, they were asked to have same meal prior to each biopsy.
Table 1.
Characteristics of the subjects in the strength and endurance training groups before training
| Parameter | Strength group | Endurance group |
|---|---|---|
| Age (years) | 36.8 ± 5.5 | 32.8 ± 2.5 |
| Height (cm) | 177 ± 7 | 180 ± 7 |
| Weight (kg) | 80 ± 13 | 77 ± 13 |
| 45.1 ± 6.3 | 47.7 ± 8.0 | |
| Leg Extension | ||
| Maximal strength (kg) | 114 ± 19 | 125 ± 30 |
| Endurance (repetitions) | 22 ± 5 | 22 ± 8 |
| Leg Press | ||
| Maximal strength (kg) | 251 ± 49 | 245 ± 55 |
| Endurance (repetitions) | 30 ± 8 | 37 ± 6 |
| Squat | ||
| Maximal strength (kg) | 183 ± 15 | 190 ± 40 |
| Endurance (repetitions) | 33 + 5 | 38 ± 7 |
Muscle biopsies
Skeletal muscle samples were obtained under local anaesthesia (Rapidocaine, 1% plain) from the belly of the vastus lateralis muscle using a percutaneous needle biopsy technique (Pro-Mag, Medical Device Technologies Inc., Gainseville, FL, USA) (Cartoni et al. 2005). A single incision was made in the skin and three individual muscle samples were taken. The pretraining, post-training and post-de-training muscle biopsies were taken, respectively, 1 week before training, 48–72 h following the last training session of the 8 week programme and 8 weeks following the last training session. Protein synthesis rates have been shown to remain elevated in skeletal muscle 72 h post-exercise (Miller et al. 2005). The muscle samples were washed in saline then immediately frozen in liquid nitrogen and used for RNA and protein extraction.
Measurement of oxygen consumption
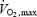 was measured using a Quark B2 metabolic cart (Cosmed, Rome, Italy) while subjects were cycling on an ergometer (Ergoline 900, Sensor Medic, Bitz, Germany). The subjects began cycling at a power of 90 W. The power was increased by 30 W every 3 min until the subject could not maintain a minimal revolution of 75 r.p.m. At the end of each step, lactate concentration was obtained (Lactate Pro, Axon Lab, Baden-Dättwil, Switzerland). The duration of the test was between 20 and 30 min. Heart rate (Polar) and oxygen consumption were measured continually throughout the test.
was measured using a Quark B2 metabolic cart (Cosmed, Rome, Italy) while subjects were cycling on an ergometer (Ergoline 900, Sensor Medic, Bitz, Germany). The subjects began cycling at a power of 90 W. The power was increased by 30 W every 3 min until the subject could not maintain a minimal revolution of 75 r.p.m. At the end of each step, lactate concentration was obtained (Lactate Pro, Axon Lab, Baden-Dättwil, Switzerland). The duration of the test was between 20 and 30 min. Heart rate (Polar) and oxygen consumption were measured continually throughout the test. 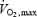 was calculated as the highest value averaged over a 30 s period (Russell et al. 2003a; Cartoni et al. 2005).
was calculated as the highest value averaged over a 30 s period (Russell et al. 2003a; Cartoni et al. 2005).
Maximal power and endurance tests
The subjects were familiarized with the equipment (Technogym®, Gambettola, Italy) and correct lifting technique for the exercises, which included leg press, squat and leg extension. Maximal strength load was estimated as the load (kg) which corresponded to the estimated hypothetical isometric maximal force determined from the force–velocity curve (Rahmani et al. 2001) using a Myotest® accelerometer (Acceltec SA, Sion, Switzerland). For the leg press the subjects started with a load of 60 kg, which was increased by 20 kg per lift until the power–resistance graph demonstrated a decrease in power. For the squat and leg extensions the starting resistance was 25 and 40 kg, respectively, which was increased by 20 and 10 kg, respectively, per lift. Approximately 3 min rest was provided between each lift. Once the estimated maximal strength load was determined, 50% of this value was calculated for the local muscular endurance test. Following a 10 min rest the subjects completed the maximal number of repetitions possible when lifting 50% of the estimated maximal strength load.
Muscle cross-sectional area
Computed tomography (General Electric Medical Systems) was used to determine the cross-sectional area of the quadriceps. While in the supine position, an image was taken at a distance of 20 cm from the roof of the acetabulum. The cross-sectional area was determined by measuring the surface area of the tissue by two observers.
Training protocols
All subjects completed an 8 week resistance training programme as previously described (Campos et al. 2002). The training was performed 2 days per week for the first 4 weeks and 3 days per week for the final 4 weeks. Each session was supervised by a qualified instructor. The subjects were divided into two groups which performed either a LOW number (3–5) or a HIGH number (20–28) of repetitions for each exercise, until fatigue. Therefore the resistance for the LOW group was considerably heavier than the resistance used by the HIGH group. Different training intensities have been shown to have different responses on the activity of intracellular signalling pathways in rat skeletal muscle (Atherton et al. 2005). The training programme was adapted from previous studies (Anderson & Kearney, 1982; Jackson et al. 1990; Campos et al. 2002) and designed to be approximately equal in volume (resistance × repetitions × sets) with the rest periods between sets adjusted according to the strength–endurance continuum (Fleck & Kraemer, 1997). For example, the LOW group performed three to five repetitions for four sets with 3 min rest between each set, while the HIGH group performed 20–28 repetitions for two sets with 1 min rest between each set (Campos et al. 2002). The exercises were performed in the fixed order of leg press, squat and leg extension. The resistance was continually increased during the training programme so that the ranges of repetitions could be respected.
RNA extraction and real time quantitative PCR
RNA from skeletal muscle (approximately 25 mg of muscle) was extracted using a commercially available preparation, peqGOLD Tri-Fast (Peqlab, Erlangen, Germany). PCR primers and conditions were used as published previously (Russell et al. 2003b; Léger et al. 2006). We also measured the mRNA levels of the proteasome 20S α-1 subunit (Psma1) and ubiquitin protein (UBB), two other genes encoding proteins in the ubiquitin signalling pathway. The PCR conditions for UBB and Psma1 were the same as in (Léger et al. 2006); however, the primer sequences were as follows: UBB forward, CGG CAA ACA GCT GGA GGA T; reverse, GGT GCA GGG TGG ACT CTT TC; probe, 5′-FAM-CCG CAC TCT CTC AGA CTA CAA CAT CC- BHQ-3′; Psma1 forward, CAG CCA CAG TTG GTC TGA-AA, reverse, CCA AAC ACT CCT GAC GCA TA. To control for any variations due to efficiencies of the reverse transcription and PCR, acidic ribosomal phosphoprotein PO (RPLPO; 36B4) was used as an internal control.
Protein extraction and Western blotting
Cytosolic and nuclear proteins were extracted from approximately 20 mg of skeletal muscle using the NE-PER® kit (Pierce Biotechnology, Rockford, USA). Two microlitres of phosphatase inhibitor cocktails I, II and III and protease inhibitor cocktail (Sigma) were added before the extraction of both cytosolic and nuclear proteins. In brief, the samples were weighed, cut into small pieces and homogenized on ice directly in the CER I solution provided in the NE-PER kit. The homogenization was performed using a Polytron PT 2100 (Kinematica AG, Luzern, Switzerland). The next steps of the method were performed in accordance with the manufacturer's protocol. The BCA Protein Assay was used to determine protein concentration (Pierce Biotechnology). Aliquots were stored at −20°C until required. Electrophoresis was performed using a 10–15% SDS-PAGE gel in cold (4°C) buffer containing 25 mm Tris pH 8.8, 192 mm glycine and 20% methanol as published previously (Léger et al. 2006). Antibodies and the conditions of incubation have been published previously (Léger et al. 2006). The phospho site-specific antibodies raised against p70S6K Thr389 (1 : 1000) was purchased from Santa Cruz, while mTOR (Ser2448), 4E-BP1 Thr37/46 (1 : 2000) and p38 MAPK (Thr180/Tyr182) (dilution 1 : 2000) were purchased from Cell Signalling Technologies. Protein loading was controlled for using a Ponceau stain. Additionally, the blots were normalized against the tubulin (cytosolic proteins) and lamin A proteins (nuclear proteins) (Fig. 5).
Figure 5. Representative images of Ponceau staining and Tubulin protein content for cytosolic proteins (A) and Ponceau staining and lamin A protein content for nuclear proteins (B).
Statistics
A two-way ANOVA was used to test for the interaction between time and training intensity. Following this a one-way ANOVA with repeated measures was used to test for differences between groups. The level of significance was set at P < 0.05.
Results
There was no significant effect of training intensity on any of the dependent variables. Therefore the two groups of subjects were pooled for all analyses.
Functional measurements indicated that resistance training resulted in a 20% and 15% increase in maximal strength for the leg extension and squat exercises, respectively (P < 0.01). There was no change in maximal strength when performing the leg press exercise (P > 0.05). In addition resistance training resulted in a 30%, 100% and 60% increase in muscular endurance for the leg extension, leg press and squat exercises, respectively (P < 0.01; Table 2).
Table 2.
Effect of training and de-training on quadriceps maximal strength and endurance for leg extension, leg press and squat exercises
| Exercise | Pre-Tr | Post-Tr |
|---|---|---|
| Maximal strength (kg) | ||
| Leg extension | 120 ± 25 | 179 ± 15** |
| Leg press | 248 ± 51 | 252 ± 55 |
| Squat | 187 ± 30 | 214 ± 32** |
| Endurance (repetitions) | ||
| Leg extension | 22 ± 8 | 29 ± 9* |
| Leg press | 34 ± 8 | 71 ± 7* |
| Squat | 35 + 5 | 56 ± 6* |
Pre-Tr, pre-training; Post-Tr, post-training
significantly different from Pre-Tr, P < 0.05
significantly different from Pre-Tr, P < 0.01.
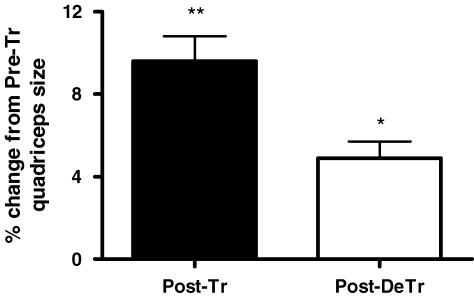
We observed approximately a 10% increase in quadriceps size after training when compared to the pretraining levels (P < 0.01). After 8 weeks of de-training a muscle atrophy of 5% was observed when compared with the post-training hypertrophy (P < 0.05) (Fig. 1).Muscle size following de-training was not significantly different to the pretraining size (P = 0.1)
Figure 1. Percentage change in quadriceps size following 8 weeks of resistance training (Post-Tr) and 8 weeks of de-training (Post-DeTr).
Muscle size was determine using a CT scan as mentioned in the methods section. *significantly different from post-training levels, P < 0.05; **Significantly different from pre-training levels, P < 0.01.
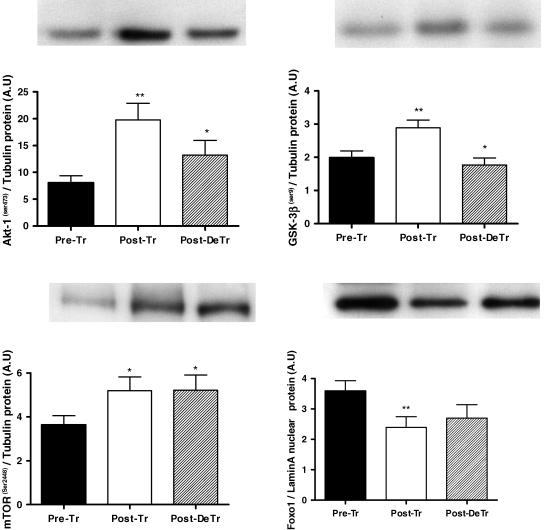
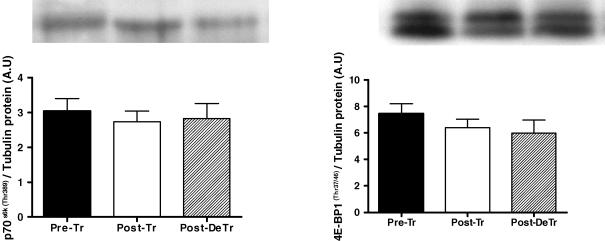
Following 8 weeks of resistance training there was a 1.4-fold increase in phosphorylated Akt (P < 0.01). After the 8 week de-training period, phosphorylated Akt was reduced by 33% when compared to post-training (P < 0.05) (Fig. 2). GSK-3β, a downstream target of Akt followed a similar pattern in that phosphorylated GSK-3β was increased by 42% and then decreased by 33%, respectively, following the training and de-training periods (P < 0.05) (Fig. 2). Phosphorylated mTOR is another downstream target of Akt and was increased by 44% following training and remained elevated after the de-training period. p70s6k and 4E-BP1 are downstream of mTOR and no change in their phosphorylation status (p70s6k Thr389; 4E-BP1 Thr37/46) was observed (Fig. 3).
Figure 2. Effect of 8 weeks of resistance training (Post-Tr) and 8 weeks of de-training (Post-DeTr) on the protein content of phospho-Akt, phospho-GSK-3β, phospho-mTOR and Foxo1.
*Significantly different from post-training levels, P < 0.05; **significantly different from Pre-training levels, P < 0.01.
Figure 3. Effect of 8 weeks of resistance training (Post-Tr) and 8 weeks of de-training (Post-DeTr) on the protein content of phospo-p70s6k and phospho-4E-BP1.
The nuclear protein contents of Foxo1 (FKHR) and Foxo3 (FKHRL1), other downstream targets of Akt, were also measured. Foxo1 was reduced by 33% post-training (P < 0.05). There was a tendency for Foxo1 to increase after the de-training period, but this did not reach statistical significance (P = 0.07) (Fig. 2). There was no significant effect on the nuclear protein content of Foxo3 following resistance training. However, a 46% decrease in Foxo3 was observed following the de-training period, although this did not reach statistical significance (P = 0.08) (not shown).
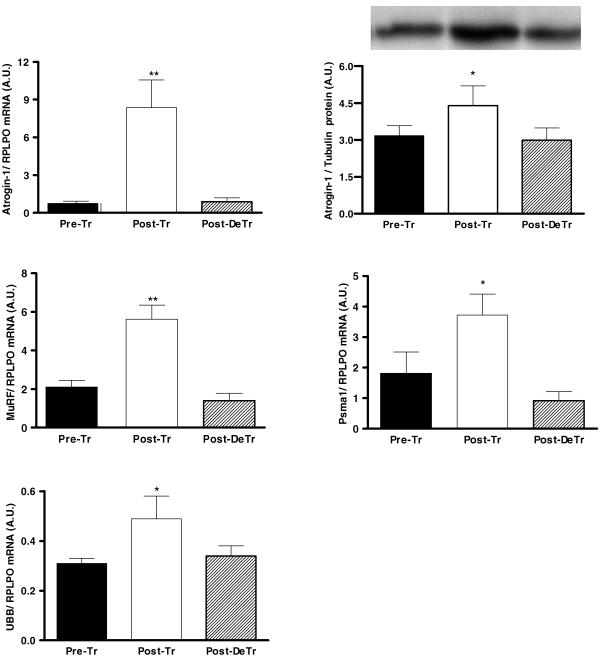
Following 8 weeks of resistance training there was a 10-and 2.5-fold increase in atrogin-1 and MuRF1 mRNA, respectively (P < 0.01). Atrogin-1 and MuRF1 mRNA had returned to baseline levels following the 8 weeks of de-training (Fig. 4). In line with the increase in atrogin-1 mRNA, we also observed a 39% increase in the atrogin-1 protein (P < 0.05) content. Additionally, following the de-training period the atrogin-1 protein content returned to basal levels (Fig. 4). To establish if these increases were specific to atrogin-1 and MuRF1, E3 ligases of the ubiquitin proteasome pathway (UPP), we decided to measure the mRNA expression of two other genes which encode proteins implicated in the UPP. These targets included the proteasome 20S α-1 subunit (Psma1) and ubiquitin protein (UBB). There was a 6- and 3-fold increase in both Psma1 and UBB mRNA following training. Both Psma1 and UBB mRNA returned to basal levels following the de-training period (Fig. 4). All mRNA results were normalized against acid ribosomal phosphoprotein PO (RPLPO), which was shown to be stable across the training and de-training periods. The RPLPO values for the Pre-Tr, Post-Tr and Post-De Tr periods were 3.07 ± 0.63, 3.40 ± 0.74 and 3.40 ± 0.79 (A.U.), respectively.
Figure 4. Regulation of atrogin-1 mRNA and protein as well as MuRF1, Psma1 and UBB mRNA following 8 weeks of resistance training (Post-Tr) and 8 weeks of de-training (Post-DeTr).
*Significantly different from Pre-training and Post-de-training levels (P < 0.05); **significantly different from Pre-training and Post-de-training levels (P < 0.01).
It has been suggested that atrogin-1 transcription may also be regulated via TNFα/p38 MAPK signalling (Li et al. 2005). No significant difference in TNFα mRNA or phosphorylated p38 MAPK protein content was observed following the training or the de-training periods (data not shown).
Figure 5 shows a representative Ponceau stain which was used as a control for the amount of cytoplasmic and nuclear proteins loaded for protein analysis. Tubulin (Fig. 5A) and lamin A (Fig. 5B) proteins were also detected on these blots and used to normalize the protein measurements in the cytoplasic and nuclear fractions, respectively. The blots show that protein loading was similar for the samples and that Tubulin and lamin A protein content did not change with training and de-training. Furthermore the blots were not saturated. Therefore the increase and decrease in the target proteins measured in the present study were due to the effects of exercise and not an artifact of protein extraction, loading or saturation of the Western blot signal.
Discussion
Understanding the signalling mechanisms which control skeletal muscle development is important for combating catabolic diseases and neuromuscular disorders, as well as for reducing the duration of rehabilitation following musculo-skeletal trauma. At present, the molecular targets and signalling pathways influencing human muscle hypertrophy and atrophy are not well understood. In the present study we measured the amount of phosphorylated Akt and several of its downstream targets in human skeletal muscle following resistance training and de-training – stimuli which results in muscle hypertrophy and atrophy, respectively. Several novel and important findings were observed. Firstly, following 8 weeks of resistance training, human skeletal muscle hypertrophy was associated with an increase in the quantity of phosphorylated Akt, GSK-3β and mTOR proteins. Secondly, the increase in Akt was also associated with a decrease in FOXO1 – a transcription factor shown to increase atrophy-stimulating genes in vitro and in vivo in rodents. Thirdly, these observations were all reversed, except for mTOR, which remained elevated, during the atrophy phase of de-training. Lastly, the two atrophy genes, atrogin-1 and MuRF1, as well as Psma1 and UBB, were increased during the hypertrophy phase and decreased during the atrophy phase.
Resistance training and de-training programmes, similar to those used in the present study, are known to cause muscle hypertrophy and atrophy, respectively, as well as an increase in muscular strength and endurance (Hakkinen & Komi, 1983; Campos et al. 2002). We implemented two different resistance training programmes which have been shown to result in functional increases in muscle strength and endurance (Campos et al. 2002). Additionally, this resistance programme consisting of LOW repetitions with heavy resistance has been shown to results in a significant muscle hypertrophy. On the other hand the resistance programme consisting of HIGH repetitions with low resistance has been shown not to result in a significant muscle hypertrophy (Campos et al. 2002). In accordance with these previously published studies we observed an increase in muscle strength and endurance following the completion of both the resistance training programmes. We also observed a significant muscle hypertrophy in the LOW repetition training group. What was unexpected was the development of muscle hypertrophy in the HIGH repetition group (Campos et al. 2002). A possible explanation is that the subjects used in the present study were older than those in the study by Campos et al. (2002) and may have been significantly less trained. Therefore any type of resistance training may have provided significant functional overload to induce an increase in muscle size.
Recently, Akt has been suggested to be a key link in the crossroads between skeletal muscle hypertrophy and atrophy. The overexpression of constitutively active Akt leads to hypertrophy of myotubes (Rommel et al. 2001) as well as in rats in vivo (Bodine et al. 2001b; Pallafacchina et al. 2002) due to downstream signalling which activates GSK-3β, p70s6k and 4E-BP1 (Glass, 2003). The skeletal muscle hypertrophy observed in the present study was associated with an increase in active phosphorylated Akt protein content. The increase in phosphorylated Akt protein content in the present study was also associated with an increase in phosphorylated GSK-3β. By analogy with previous observations made in vivo the increase in phosphorylated GSK-3β would relieve its inhibitory effect on eIF2B and promote protein translation (Welsh et al. 1997; Jefferson et al. 1999). mTOR, another Akt-activated protein, was also increased following resistance training. The Akt activation of mTOR has been shown to phosphorylate both p70s6kinase and 4E-BP1 in mice (Bodine et al. 2001b). However, it is important to note that mechanically induced signalling through mTOR and protein synthesis may also occur in an Akt-independent manner (Hornberger et al. 2004). These observations are similar to the effects of high frequency electrical stimulation, known to cause hypertrophy, which increases the phosphorylation of Akt and several of its downstream targets such as mTOR, GSK-3β, p70s6k, and 4E-BP1 and eIF-2B in rat skeletal muscle (Atherton et al. 2005). However, unlike in the present study, a low frequency electrical stimulation does not effect these proteins in rat skeletal muscle. The phosphorylation of p70s6kinase leads to an activation of protein synthetic pathways, while the phosphorylation of 4E-BP1 releases its inhibitory effect on the translation initiation factor eIF4E promoting protein synthesis (Terada et al. 1994; Lin & Lawrence, 1996; Rhoads, 1999). We did not observe an increase in the phosphorylation status of p70s6kinase or 4E-BP1 when measured 48–72 h after the last bout of an 8 week resistance training programme. This observation does not mean that p70s6kinase and 4E-BP1 are not activated by Akt/mTOR signalling during human skeletal muscle hypertrophy as p70s6kinase or 4E-BP1 are known to be auto-regulated by eIF4E. It has been demonstrated that p70s6kinase and 4E-BP1 are gradually de-phosphorylated when eIF4E levels increase (Khaleghpour et al. 1999). It was suggested that this negative feedback loop may act to maintain cellular translational homeostasis and prevent uncontrolled growth. Furthermore, it was demonstrated that this dephosphorylation of p70s6kinase and 4E-BP1, via eIF4E, was independent of changes in Akt and mTOR (Khaleghpour et al. 1999).
The activation of Akt has been shown to phosphorylate and inactivate members of the Foxo family of transcription factors. When phosphorylated the Foxo proteins are sequestered to the cytosol where they are unable to transcribe genes involved in the atrophy process (Sandri et al. 2004; Stitt et al. 2004; Latres et al. 2005). We therefore measured the nuclear protein content of two Foxo family members, Foxo1 (FKHR) and Foxo3 (FKHRL1) following the resistance training. We show for the first time that Foxo1 is reduced in hypertrophied human skeletal muscle, when compared with pretraining levels, which supports observations made in rodents and cells undergoing hypertrophy (Stitt et al. 2004; Latres et al. 2005). Our results strongly suggest that muscle hypertrophy in healthy skeletal muscle, induced by resistance training, is regulated, in part, by the Akt inhibition of Foxo1.
In situations of muscle atrophy in rodents and cells a reduction in Akt activity has been observed, suggesting that Akt may also be involved in the muscle atrophy process (Sandri et al. 2004). Following the 8 weeks of de-training a small, but significant, atrophy in the skeletal muscle, relative to the post-training size was observed. Contrary to what was observed after the stimulation of muscle hypertrophy, we observed that muscle atrophy was associated with a decrease in phosphorylated Akt. This supports our recent observation in ALS patients (Léger et al. 2006). In addition to Akt, phosphorylated GSK-3β, but not mTOR, was reduced after de-training when compared with the pretraining hypertrophy levels. The muscle atrophy observed following the de-training period did not result in the muscle returning to the basal pretraining size.
A reduction in the activity of Akt has been suggested to assist in the development of skeletal muscle atrophy as it removes the inhibitory phosphorylation of Foxo transcription factors (Brunet et al. 1999; Burgering & Medema, 2003). In the present study the muscle atrophy observed following the de-training period was associated with a tendency for Foxo1 to increase, when compared with the post-training levels. However Foxo1 did not return to the basal pretraining levels. Interestingly, this small increase in Foxo1 following de-training was in parallel to the decrease in active Akt, which similarly had not returned to basal pretraining levels. Combined, the results observed in both the hypertrophy and atrophy phases of this study strongly support, and for the first time extend in human skeletal muscle, the recent findings in rodents and cells (Bodine et al. 2001b; Latres et al. 2005), demonstrating that Akt plays a role in both the skeletal muscle hypertrophy and atrophy processes.
We also measured the content of atrogin-1 and MuRF1 mRNA and atrogin-1 protein. Based on the regulation of Akt and Foxo1 in the present study, and results from previous rodent and cells studies (Sandri et al. 2004; Stitt et al. 2004; Latres et al. 2005), we expected that atrogin-1 and MuRF1 mRNA may have been decreased after the post-training muscle hypertrophy phase and increased, relative to pretraining, following the atrophy de-training phase. Contrary to our expectations atrogin-1 mRNA and protein and MuRF1 mRNA increased following the muscle hypertrophy phase. Additionally, atrogin-1 mRNA and protein and MuRF1 mRNA decreased, when compared to the post-training levels, following the atrophy inducing de-training period. It appears that both atrogin-1 and MuRF1 can be regulated by pathways other than Akt/Foxo. Our observations, surrounding the regulation of atrogin-1 and MuRF1 mRNA in hypertrophy and atrophy conditions, seem to be in contrast with what has been previously been reported in genetically or pharmacologically stimulated rodent and cells models of both muscle hypertrophy and disease and disuse atrophy (for review see (Glass, 2003). These conflicting results may be explained by the fact that our model of hypertrophy and atrophy was induced in healthy skeletal muscle. Atrogin-1 and MuRF1 are E3 ligases in the ubiquitin-proteasome pathway (Bodine et al. 2001a; Gomes et al. 2001). This pathway is constitutively active in muscle fibres in order to mediate intracellular signalling and normal protein turnover (Reid, 2005). Both atrogin-1 and MuRF1 are expressed in wild-type myotubes and rodents and in healthy human skeletal muscle (Bodine et al. 2001a; Gomes et al. 2001; Jones et al. 2004; Stitt et al. 2004; Latres et al. 2005; Léger et al. 2006), suggesting that the basal activity of atrogin-1 and MuRF1 is biologically important for maintaining a normal level of protein degradation in healthy muscle. Our observed increase in atrogin-1 and MuRF1, in hypertrophied human skeletal muscle following resistance training, may represent a new healthy basal level of these E3-ligases, relative to the increased synthesis of structural and cytoskeletal proteins. The increased levels of atrogin-1 and MuRF1, following hypertrophy in healthy muscle, may be required to maintain a normal level of protein degradation in the environment, which now possesses a greater content of structural proteins. In support of this possibility we also observed an increase in Psam1 and UBB mRNA, which encode proteins implicated in the ubiquitin proteosome pathway (UPP). Similar to our observation, previous studies in working field dogs have demonstrated an up-regulation of specific components of the UPP at the end of the hunting season (Wakshlag et al. 2002). Combined, these results indicate an important role in the proteolytic process associated with skeletal muscle turnover during long-term training. Recent data by Rourke et al. (2004) showed an increase in atrogin-1 mRNA in ground squirrels after 3–4 months of hibernation without muscle atrophy. This suggests that the up-regulation of E3 ligases does not necessarily result in muscle atrophy. During the atrophy phase of the present study, relative to the hypertrophy phase, the rate of protein synthesis would have been decreased as the skeletal muscle size gradually returned towards the healthy pretraining baseline level. As the atrophy occurring was not due to disease or severe trauma, the reduction in both atrogin-1 and MuRF1 levels as well as Psam1 and UBB may have been in co-ordination with the maintenance of a healthy basal level of protein turnover. Future studies are required to establish the regulation of atrogin-1 and MuRF1 with respect to the rate of protein degradation in healthy muscle following hypertrophy in healthy and diseased models of muscle atrophy.
Recent work by Li et al. (2005) has demonstrated, in rodents and cells, that atrogin-1 mRNA can also be up-regulated by TNFα, acting through p38 MAPK. In the present study we did not observe any changes in TNFα mRNA or phosphorylated p38 MAPK protein content. TNFα levels have been shown to be decreased in muscle from patients with heart disease and the elderly following training (Greiwe et al. 2001; Conraads et al. 2002) while phosphorylated p38 MAPK has been shown to increase transiently following acute exercise in some studies (Karlsson et al. 2004) but not in others (Williamson et al. 2003). Our results suggest that in our healthy human model of muscle hypertrophy and atrophy atrogin-1 is not regulated via TNFα/p38 MAPK signalling.
In conclusion, we have established a role for Akt signalling in both human skeletal muscle hypertrophy and atrophy. During muscle hypertrophy, induced by resistance training, the activities of Akt and its downstream targets GSK-3β and mTOR were increased, while the activity of the atrophy signalling factor, Foxo1, was decreased. The response of these hypertrophy and atrophy targets were the inverse after a period of atrophy-inducing de-training, when compared to the post-training levels. The unexpected increase of both atrogin-1 and MuRF1 following the hypertrophy phase and their decrease following the atrophy phase may be linked to the maintenance of normal protein turnover in healthy muscle.
Acknowledgments
We wish to all of the subjects who participated in this study for their continual motivation and commitment. We also thank Martine Zermatten for her technical assistance. This work was supported by the Fonds National Suisse de la Recherche Scientifique (no. 3200B0-105936/1), the Loterie Suisse Romande and Technogym®, Gambettola (Italy).
References
- Anderson T, Kearney JT. Effects of three resistance training programs on muscular strength and absolute and relative endurance. Res Q Exerc Sport. 1982;53:1–7. doi: 10.1080/02701367.1982.10605218. [DOI] [PubMed] [Google Scholar]
- Atherton PJ, Babraj J, Smith K, Singh J, Rennie MJ, Wackerhage H. Selective activation of AMPK-PGC-1α or PKB-TSC2-mTOR signaling can explain specific adaptive responses to endurance or resistance training-like electrical muscle stimulation. FASEB J. 2005;19:786–788. doi: 10.1096/fj.04-2179fje. [DOI] [PubMed] [Google Scholar]
- Baldi JC, Jackson RD, Moraille R, Mysiw WJ. Muscle atrophy is prevented in patients with acute spinal cord injury using functional electrical stimulation. Spinal Cord. 1998;36:463–469. doi: 10.1038/sj.sc.3100679. [DOI] [PubMed] [Google Scholar]
- Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001a;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001b;3:1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Burgering BM, Medema RH. Decisions on life and death: FOXO Forkhead transcription factors are in command when PKB/Akt is off duty. J Leukoc Biol. 2003;73:689–701. doi: 10.1189/jlb.1202629. [DOI] [PubMed] [Google Scholar]
- Campos GE, Luecke TJ, Wendeln HK, Toma K, Hagerman FC, Murray TF, Ragg KE, Ratamess NA, Kraemer WJ, Staron RS. Muscular adaptations in response to three different resistance-training regimens: specificity of repetition maximum training zones. Eur J Appl Physiol. 2002;88:50–60. doi: 10.1007/s00421-002-0681-6. [DOI] [PubMed] [Google Scholar]
- Cartoni R, Leger B, Hock MB, Praz M, Crettenand A, Pich S, Ziltener JL, Luthi F, Deriaz O, Zorzano A, Gobelet C, Kralli A, Russell AP. Mitofusins 1/2 and ERRα expression are increased in human skeletal muscle after physical exercise. J Physiol. 2005;567:349–358. doi: 10.1113/jphysiol.2005.092031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conraads VM, Beckers P, Bosmans J, De Clerck LS, Stevens WJ, Vrints CJ, Brutsaert DL. Combined endurance/resistance training reduces plasma TNF-α receptor levels in patients with chronic heart failure and coronary artery disease. Eur Heart J. 2002;23:1854–1860. doi: 10.1053/euhj.2002.3239. [DOI] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- Di Giovanni S, Molon A, Broccolini A, Melcon G, Mirabella M, Hoffman EP, Servidei S. Constitutive activation of MAPK cascade in acute quadriplegic myopathy. Ann Neurol. 2004;55:195–206. doi: 10.1002/ana.10811. [DOI] [PubMed] [Google Scholar]
- Fleck S, Kraemer W. Designing Resistance Training Programs. Champaign, IL, USA: Human Kinetics; 1997. [Google Scholar]
- Fry AC. The role of resistance exercise intensity on muscle fibre adaptations. Sports Med. 2004;34:663–679. doi: 10.2165/00007256-200434100-00004. [DOI] [PubMed] [Google Scholar]
- Glass DJ. Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nat Cell Biol. 2003;5:87–90. doi: 10.1038/ncb0203-87. [DOI] [PubMed] [Google Scholar]
- Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci U S A. 2001;98:14440–14445. doi: 10.1073/pnas.251541198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiwe JS, Cheng B, Rubin DC, Yarasheski KE, Semenkovich CF. Resistance exercise decreases skeletal muscle tumor necrosis factor α in frail elderly humans. FASEB J. 2001;15:475–482. doi: 10.1096/fj.00-0274com. [DOI] [PubMed] [Google Scholar]
- Hakkinen K, Komi PV. Electromyographic changes during strength training and detraining. Med Sci Sports Exerc. 1983;15:455–460. [PubMed] [Google Scholar]
- Hjeltnes N, Aksnes AK, Birkeland KI, Johansen J, Lannem A, Wallberg-Henriksson H. Improved body composition after 8 wk of electrically stimulated leg cycling in tetraplegic patients. Am J Physiol. 1997;273:R1072–R1079. doi: 10.1152/ajpregu.1997.273.3.R1072. [DOI] [PubMed] [Google Scholar]
- Hornberger TA, Stuppard R, Conley KE, Fedele MJ, Fiorotto ML, Chin ER, Esser KA. Mechanical stimuli regulate rapamycin-sensitive signalling by a phosphoinositide 3-kinase-, protein kinase B- and growth factor-independent mechanism. Biochem J. 2004;380:795–804. doi: 10.1042/BJ20040274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CG, Dickinson AL, Ringel SP. Skeletal muscle fiber area alterations in two opposing modes of resistance exercise training in the same individual. Eur J Appl Physiol Occup Physiol. 1990;61:37–41. doi: 10.1007/BF00236691. [DOI] [PubMed] [Google Scholar]
- Jefferson LS, Fabian JR, Kimball SR. Glycogen synthase kinase-3 is the predominant insulin-regulated eukaryotic initiation factor 2B kinase in skeletal muscle. Int J Biochem Cell Biol. 1999;31:191–200. doi: 10.1016/s1357-2725(98)00141-1. [DOI] [PubMed] [Google Scholar]
- Jones SW, Hill RJ, Krasney PA, O'Conner B, Peirce N, Greenhaff PL. Disuse atrophy and exercise rehabilitation in humans profoundly affects the expression of genes associated with the regulation of skeletal muscle mass. FASEB J. 2004;18:1025–1027. doi: 10.1096/fj.03-1228fje. [DOI] [PubMed] [Google Scholar]
- Karlsson HK, Nilsson PA, Nilsson J, Chibalin AV, Zierath JR, Blomstrand E. Branched-chain amino acids increase p70S6k phosphorylation in human skeletal muscle after resistance exercise. Am J Physiol Endocrinol Metab. 2004;287:E1–E7. doi: 10.1152/ajpendo.00430.2003. [DOI] [PubMed] [Google Scholar]
- Khaleghpour K, Pyronnet S, Gingras AC, Sonenberg N. Translational homeostasis: eukaryotic translation initiation factor 4E control of 4E-binding protein 1 and p70, S6 kinase activities. Mol Cell Biol. 1999;19:4302–4310. doi: 10.1128/mcb.19.6.4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latres E, Amini AR, Amini AA, Griffiths J, Martin FJ, Wei Y, Lin HC, Yancopoulos GD, Glass DJ. Insulin-like growth factor-1 (IGF-1) inversely regulates atrophy-induced genes via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway. J Biol Chem. 2005;280:2737–2744. doi: 10.1074/jbc.M407517200. [DOI] [PubMed] [Google Scholar]
- Lecker SH, Solomon V, Mitch WE, Goldberg AL. Muscle protein breakdown and the critical role of the ubiquitin-proteasome pathway in normal and disease states. J Nutr. 1999;129:227S–237S. doi: 10.1093/jn/129.1.227S. [DOI] [PubMed] [Google Scholar]
- Lee SW, Dai G, Hu Z, Wang X, Du J, Mitch WE. Regulation of muscle protein degradation: coordinated control of apoptotic and ubiquitin-proteasome systems by phosphatidylinositol 3 kinase. J Am Soc Nephrol. 2004;15:1537–1545. doi: 10.1097/01.asn.0000127211.86206.e1. [DOI] [PubMed] [Google Scholar]
- Léger B, Vergani L, Soraru G, Hespel P, Derave W, Gobelet C, D'Ascenzio C, Angelini C, Russell AP. Human skeletal muscle atrophy in amyotrophic lateral sclerosis reveals a reduction in Akt and an increase in atrogin-1. FASEB J. 2006;20:583–585. doi: 10.1096/fj.05-5249fje. [DOI] [PubMed] [Google Scholar]
- Li YP, Chen Y, John J, Moylan J, Jin B, Mann DL, Reid MB. TNF-α acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J. 2005;19:362–370. doi: 10.1096/fj.04-2364com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TA, Lawrence JC., Jr Control of the translational regulators PHAS-I and PHAS-II by insulin and cAMP in 3T3-L1 adipocytes. J Biol Chem. 1996;271:30199–30204. doi: 10.1074/jbc.271.47.30199. [DOI] [PubMed] [Google Scholar]
- Lynch GS. Therapies for improving muscle function in neuromuscular disorders. Exerc Sport Sci Rev. 2001;29:141–148. doi: 10.1097/00003677-200110000-00002. [DOI] [PubMed] [Google Scholar]
- Miller BF, Olesen JL, Hansen M, Dossing S, Crameri RM, Welling RJ, Langberg H, Flyvbjerg A, Kjaer M, Babraj JA, Smith K, Rennie MJ. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol. 2005;567:1021–1033. doi: 10.1113/jphysiol.2005.093690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitch WE, Goldberg AL. Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N Engl J Med. 1996;335:1897–1905. doi: 10.1056/NEJM199612193352507. [DOI] [PubMed] [Google Scholar]
- Pallafacchina G, Calabria E, Serrano AL, Kalhovde JM, Schiaffino S. A protein kinase B-dependent and rapamycin-sensitive pathway controls skeletal muscle growth but not fiber type specification. Proc Natl Acad Sci U S A. 2002;99:9213–9218. doi: 10.1073/pnas.142166599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmani A, Viale F, Dalleau G, Lacour JR. Force/velocity and power/velocity relationships in squat exercise. Eur J Appl Physiol. 2001;84:227–232. doi: 10.1007/PL00007956. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Nakamura N, Sansal I, Bergeron L, Sellers WR. A novel mechanism of gene regulation and tumor suppression by the transcription factor FKHR. Cancer Cell. 2002;2:81–91. doi: 10.1016/s1535-6108(02)00086-7. [DOI] [PubMed] [Google Scholar]
- Reid MB. Response of the ubiquitin-proteasome pathway to changes in muscle activity. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1423–R1431. doi: 10.1152/ajpregu.00545.2004. [DOI] [PubMed] [Google Scholar]
- Rhoads RE. Signal transduction pathways that regulate eukaryotic protein synthesis. J Biol Chem. 1999;274:30337–30340. doi: 10.1074/jbc.274.43.30337. [DOI] [PubMed] [Google Scholar]
- Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3:1009–1013. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- Rourke BC, Yokoyama Y, Milsom WK, Caiozzo VJ. Myosin isoform expression and MAFbx mRNA levels in hibernating golden-mantled ground squirrels (Spermophilus lateralis) Physiol Biochem Zool. 2004;77:582–593. doi: 10.1086/421753. [DOI] [PubMed] [Google Scholar]
- Russell AP, Feilchenfeldt J, Schreiber S, Praz M, Crettenand A, Gobelet C, Meier CA, Bell DR, Kralli A, Giacobino JP, Deriaz O. Endurance training in humans leads to fiber type-specific increases in levels of peroxisome proliferator-activated receptor-γ coactivator-1 and peroxisome proliferator-activated receptor-α in skeletal muscle. Diabetes. 2003a;52:2874–2881. doi: 10.2337/diabetes.52.12.2874. [DOI] [PubMed] [Google Scholar]
- Russell AP, Gastaldi G, Bobbioni-Harsch E, Arboit P, Gobelet C, Deriaz O, Golay A, Witztum JL, Giacobino JP. Lipid peroxidation in skeletal muscle of obese as compared to endurance-trained humans: a case of good vs. bad lipids? FEBS Lett. 2003b;551:104–106. doi: 10.1016/s0014-5793(03)00875-5. [DOI] [PubMed] [Google Scholar]
- Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell. 2004;14:395–403. doi: 10.1016/s1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- Suetta C, Magnusson SP, Rosted A, Aagaard P, Jakobsen AK, Larsen LH, Duus B, Kjaer M. Resistance training in the early postoperative phase reduces hospitalization and leads to muscle hypertrophy in elderly hip surgery patients – a controlled, randomized study. J Am Geriatr Soc. 2004;52:2016–2022. doi: 10.1111/j.1532-5415.2004.52557.x. [DOI] [PubMed] [Google Scholar]
- Terada N, Patel HR, Takase K, Kohno K, Nairn AC, Gelfand EW. Rapamycin selectively inhibits translation of mRNAs encoding elongation factors and ribosomal proteins. Proc Natl Acad Sci U S A. 1994;91:11477–11481. doi: 10.1073/pnas.91.24.11477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdale MJ. The ubiquitin-proteasome pathway as a therapeutic target for muscle wasting. J Support Oncol. 2005;3:209–217. [PubMed] [Google Scholar]
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- Wakshlag JJ, Kallfelz FA, Barr SC, Ordway G, Haley NJ, Flaherty CE, Kelley RL, Altom EK, Lepine AJ, Davenport GM. Effects of exercise on canine skeletal muscle proteolysis: an investigation of the ubiquitin-proteasome pathway and other metabolic markers. Vet Ther. 2002;3:215–225. [PubMed] [Google Scholar]
- Welsh GI, Stokes CM, Wang X, Sakaue H, Ogawa W, Kasuga M, Proud CG. Activation of translation initiation factor eIF2B by insulin requires phosphatidyl inositol 3-kinase. FEBS Lett. 1997;410:418–422. doi: 10.1016/s0014-5793(97)00579-6. [DOI] [PubMed] [Google Scholar]
- Williamson D, Gallagher P, Harber M, Hollon C, Trappe S. Mitogen-activated protein kinase (MAPK) pathway activation: effects of age and acute exercise on human skeletal muscle. J Physiol. 2003;547:977–987. doi: 10.1113/jphysiol.2002.036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinna EM, Yarasheski KE. Exercise treatment to counteract protein wasting of chronic diseases. Curr Opin Clin Nutr Metab Care. 2003;6:87–93. doi: 10.1097/00075197-200301000-00013. [DOI] [PubMed] [Google Scholar]