Abstract
In hair cells of all vertebrates, a mechanosensory bundle is formed by stereocilia with precisely graded heights. Unconventional myosin-XVa is critical for formation of this bundle because it transports whirlin and perhaps other molecular components responsible for programmed elongation of stereocilia to the stereocilia tips. A tip of a stereocilium is the site of stereocilia growth and one of the proposed sites of mechano-electrical transduction. In adult shaker 2 mice, a mutation that disables the motor function of myosin-XVa results in profound deafness and abnormally short stereocilia that lack stereocilia links, an indispensable component of mechanotransduction machinery. Therefore, it was assumed that myosin-XVa is required for proper formation of the mechanotransduction apparatus. Here we show that in young postnatal shaker 2 mice, abnormally short stereocilia bundles of auditory hair cells have numerous stereocilia links and ‘wild type’ mechano-electrical transduction. We compared the mechanotransduction current in auditory hair cells of young normal-hearing littermates, myosin-XVa-deficient shaker 2 mice, and whirler mice that have similarly short stereocilia but intact myosin-XVa at the stereocilia tips. This comparison revealed that the absence of functional myosin-XVa does not disrupt adaptation of the mechanotransduction current during sustained bundle deflection. Thus, the hair cell mechanotransduction complex forms and functions independently from myosin-XVa-based hair bundle morphogenesis.
Hair cell stereocilia are microvilli-like projections filled with unidirectionally aligned actin filaments, which elongate through the addition of actin monomers at their tips (Schneider et al. 2002). In the mechanosensory bundle, stereocilia are interconnected with numerous extracellular filaments, including a tip-link that runs obliquely from the tip of a shorter stereocilium to a neighbouring taller stereocilium (Pickles et al. 1984). Current models postulate that deflection of the hair bundle toward the tallest row of stereocilia increases the tension of the tip-links and mechanically gates transduction channels located at either or both ends of a tip-link (Denk et al. 1995). It has also been proposed that these mechanotransduction channels are coupled extracellularly to the tip-link and intracellularly to the actin core of the stereocilia (Gillespie & Walker, 2001).
Unconventional myosins-Ic, -VIIa and -XVa are attractive candidates for establishing mechanical links between the actin core of a stereocilium and various plasma membrane components. In mammalian hair cells, myosins-Ic and -VIIa are located along the length of stereocilia (Hasson et al. 1997; Dumont et al. 2002) and both influence mechanotransduction by changing the adaptation of mechanotransduction current during sustained bundle deflections (Holt et al. 2002; Kros et al. 2002; Stauffer et al. 2005). In the organ of Corti, myosin-XVa is localized exclusively at the tips of stereocilia (Belyantseva et al. 2003). Mutations of Myo15a encoding myosin-XVa cause profound deafness in mice (Liang et al. 1998; Probst et al. 1998) and humans (Wang et al. 1998). Myosin-XVa-deficient mice were reported to lack any links between stereocilia (Beyer et al. 2000), suggesting a complete disruption of the mechanotransduction machinery. Therefore, it was assumed that myosin-XVa is required for proper formation and function of the mechanotransduction apparatus. Myosin-XVa transports to the stereocilia tips a scaffold protein, whirlin, contributing to a proposed macromolecular complex that regulates the growth of stereocilia actin filaments (Belyantseva et al. 2005). In mouse vestibular (utricular) hair cells, myosin-XVa appears at the stereocilia tips at embryonic day 14.5 (Belyantseva et al. 2003), 2 days before the acquisition of mechano-electrical transduction (Geleoc & Holt, 2003). Therefore, myosin-XVa may represent an essential component of the mechanotransduction complex or deliver such components to the tip of the stereocilium.
Here we investigated mechanosensitivity in cochlear outer hair cells (OHCs) of young postnatal homozygous shaker 2 (Myo15sh2/sh2) and whirler (Whrnwi/wi) mice. In Myo15sh2/sh2 mice, a recessive mutation disables the motor domain of myosin-XVa, preventing its translocation to stereocilia tips (Belyantseva et al. 2005), resulting in abnormally short stereocilia (Probst et al. 1998). In Whrnwi/wi mice, a truncating mutation of whirlin results in similarly short stereocilia (Mburu et al. 2003), although they have intact myosin-XVa at their tips (Belyantseva et al. 2005). We observed essentially normal hair cell mechanotransduction in both of these young postnatal mutants, suggesting that formation and function of the mechanotransduction complex are independent of the presence of myosin-XVa at the stereocilia tips.
Methods
Organ of Corti explants
Mice were derived from the colony reported previously (Belyantseva et al. 2005). Mouse pups at postnatal days 2–4 (P2–4) were cooled on an ice bed, killed by CO2 and decapitated. Organs of Corti explants were dissected and placed in glass-bottomed Petri dishes (WillCo Wells, the Netherlands) covered with a thin layer of type I collagen (Upstate, Lake Placid, NY, USA). The organs of Corti were cultured in DMEM medium supplemented with 25 mm Hepes and 7% fetal bovine serum (Invitrogen, Carlsbad, CA, USA) at 37°C and 5% CO2. Cultured explants were used in experiments within 1–5 days. In some experiments, 10 μg ml−1 of ampicillin (Calbiochem, La Jolla, CA, USA) was added to the medium with no observed effects on mechanotransduction currents. All animal procedures were approved by the University of Kentucky Animal Care and Use Committee.
Dye loading
Solutions of FM1-43 (10 mm) and FM3-25 (5 mm) (Biotium, Hayward, CA, USA) in DMSO were diluted to 5 μm in Ca2+-containing standard Hank's balanced salt solution (HBSS: Invitrogen). Cover slips with the cultured organs of Corti were transferred to HBSS, then to dye-containing HBSS for the indicated time, and washed 2–3 times in HBSS. For time course investigations, dye was pressure applied from a puff pipette (∼1 μm tip diameter) to the organ of Corti cultured in the glass-bottomed Petri dish. Samples were observed with epifluorescent illumination using a 100 ×, 1.3 NA, 0.2 mm working distance (WD) oil-immersion objective. Images were acquired with an ORCA-II-ER camera (Hamamatsu, Japan) using MetaMorph software (Molecular Devices, Sannyvale, CA, USA) and spinning-wheel confocal attachment (CARV, BD Biosciences, San Jose, CA, USA).
Scanning electron microscopy (SEM)
Cultured organs of Corti or freshly dissected temporal bones were immersed in 2.5% glutaraldehyde in 0.1 m cacodylate buffer supplemented with 2 mm CaCl2 for 1–2 h at room temperature. Organs of Corti were then dissected in HBSS, dehydrated in a graded series of acetone, and critical-point dried from liquid CO2. Specimens were sputter-coated with platinum (5.5 nm) and observed with a field-emission SEM (S-4500, Hitachi, Japan).
Whole-cell patch-clamp recording
Experiments were performed at room temperature in L-15 cell culture medium (Invitrogen) containing the following inorganic salts (mm): NaCl 137, KCl 5.4, CaCl2 1.3, MgCl2 1.0, Na2HPO4 1.0, KH2PO4 0.44 and MgSO4 0.81. OHCs were observed with an inverted microscope using a 100 × 1.3 NA 0.2 WD oil-immersion objective and differential interference contrast. To access the OHC basolateral plasma membrane (Fig. 2A), outermost non-sensory cells were removed by gentle suction with a ∼5 μm micropipette. Smaller pipettes for whole-cell patch-clamp recordings were filled with intracellular solution containing (mm): CsCl 140, MgCl2 2.5, Na2ATP 2.5, EGTA 1.0 and Hepes 5. Osmolarity and pH of the intrapipette solution were adjusted with d-glucose and CsOH to match corresponding values of the bath (325 mosmol l−1, pH = 7.35). The pipette resistance was typically 8–10 MΩ when measured in the bath. During whole-cell recordings, the access and membrane resistances were correspondingly ∼50 MΩ and ∼1 GΩ, as determined by ‘membrane test’ feature of pCLAMP 9.0 software (Molecular Devices, Sunnyvale, CA, USA).
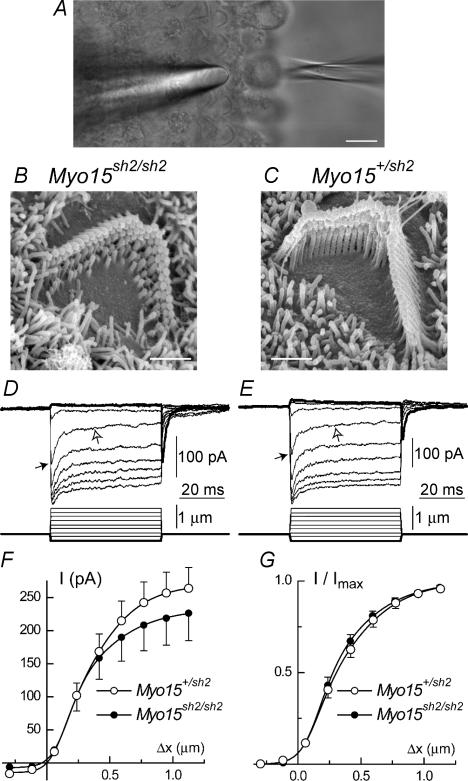
Figure 2. Intact mechano-electrical transduction in young Myo15sh2/sh2 OHCs.
A, organ of Corti explant with a piezo-driven probe (left) and a patch pipette (right). B and C, typical SEM images of OHC stereocilia bundles at the end of the first (apical) cochlear turn in Myo15sh2/sh2 (B) and Myo15+/sh2 (C) organ of Corti explants. D and E, representative whole-cell currents (top traces) evoked by the graded deflections of stereocilia at a holding potential of −90 mV in Myo15sh2/sh2 (D) and Myo15+/sh2 (E) mice. Mechanotransduction current rapidly reaches a peak (black arrows) and then decays on a fast and then slow (open arrows) time scale. Responses to the inhibitory bundle deflections are shown in bold. Relatively large access resistances (D −74 MΩ, E −73.5 MΩ) may somewhat decrease the driving force and maximum amplitude of transduction current. F, average relationships between peak transduction current and probe displacement in Myo15+/sh2 (○, n = 7) and Myo15sh2/sh2 (•, n = 7) OHCs. Data were fitted with a second order Boltzmann function: I = Imax/[1 + exp(α2*(p2 −Δx))*(1 + exp(α1*(p1 −Δx)))] − Imin. Parameters of the fit (Myo15+/sh2/Myo15sh2/sh2): Imax = 285/240 pA; Imin = 14.4/6.9 pA; α1 = 12.6/11.7 μm−1; p1 = 0.11/0.18 μm; α2 = 4.5/3.7 μm−1; p2 = 0.28/0.18 μm. Access resistances (Myo15+/sh2/Myo15sh2/sh2): 53 ± 6/51 ± 4 MΩ. G, normalized current-displacement relationships in OHCs of Myo15+/sh2 and Myo15sh2/sh2 mice at −90 mV holding potential. The averages of individual normalized current-displacement curves are shown. Parameters of the fit (Myo15+/sh2/Myo15sh2/sh2): α1 = 10.7/10.5 μm−1; p1 = 0.14/0.18 μm; α2 = 3.8/3.6 μm−1; p2 = 0.26/0.19 μm. The same OHCs contributed to F and G. Vertical bars indicate Standard Errors. Scale bars: 10 μm (A); 1 μm (B and C). Hair cell ages: B–E: P2 plus 3–4 days in vitro, F and G: P2–3 plus 2–4 days in vitro.
Hair bundle stimulation
We reproduced a previously described technique (Kennedy et al. 2003) of hair bundle deflection by a stiff glass probe that had been fire-polished to a diameter of 3–5 μm matching the V-shape of the bundle. The probe was driven by a fast piezo actuator (PA 8/12, Piezosystem Jena, Germany). Probe movement was monitored with a differential photodiode. To eliminate resonant oscillations, the command voltage was low-pass filtered (1–5 kHz), which resulted in a rise time of 0.15–0.75 ms required to span 10–90% of the step-like deflection. Due to a shallow approach angle to the short Myo15sh2/sh2 stereocilia, we had to use a relatively long stimulating probe, which resulted in additional lateral resonances, limiting the speed of bundle deflection in our experiments. Identical probes and filtering frequencies were used for recording mechanotransduction responses in OHCs of both Myo15sh2/sh2 and Myo15+/sh2 mice of the same litter. Movement of the probe was calibrated by video recording and command voltage was converted to displacement units. The actual deflection of the stereocilia was not possible to record, since the probe image obscured the stereocilia. In most experiments, a freshly prepared probe adhered to the stereocilia bundle allowing us not only to push it, but also to pull it in the inhibitory direction. Resting (zero) position of the bundle was determined by manually advancing the piezo probe toward the bundle in the excitatory direction until the mechanotransduction current appeared and then stepping back until the cell current returned to normal. Maximal sensitivity to small displacements at resting position and current saturation at displacements larger than 0.5 μm indicated the best fit of the probe to the V-shaped bundle.
Auditory brainstem responses (ABR)
Mice were anaesthetized with an intraperitoneal injection of 2.5% Avertin (0.015 ml g−1) and placed on a heating pad in a sound-attenuated chamber. The ‘Smart EP’ auditory evoked potential diagnostic system (Intelligent Hearing Systems, Miami, FL, USA) was used to record brainstem activity with subdermal needle electrodes placed at the forehead and mastoid locations. Stimuli were either of 31 μs duration clicks or 8, 16 and 32 kHz tone bursts (1.5 ms duration, Blackman envelope) presented at 20 Hz with alternating polarity. The recorded signal was bandpass filtered between 30 and 5000 Hz and averaged from 512 to 2048 times. After the experiment, the animal was placed in a heated cage and allowed to recover from anaesthesia.
Results
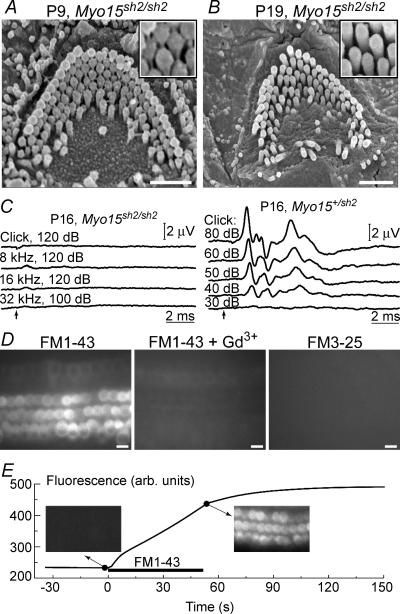
In young postnatal Myo15sh2/sh2 mice, we found numerous stereocilia links in auditory outer (Fig. 1A) and inner (data not shown) hair cells. By approximately the third week of age, when hearing assessment by ABR analysis becomes possible, stereocilia links are no longer observed (Fig. 1B) and Myo15sh2/sh2 mice exhibit no detectable ABR waveforms (Fig. 1C). At this age, OHC stereocilia also begin to retract at the base of the cochlea, indicating progressive base-to-apex hair bundle degeneration (data not shown). In contrast to older OHCs that start loosing their stereocilia, young postnatal Myo15sh2/sh2 OHCs seem to be functional. At any cochlear location these cells rapidly accumulated FM1-43 (Fig. 1D and E), a styryl dye that permeates mechanotransduction channels (Gale et al. 2001; Meyers et al. 2003). This fast accumulation was inhibited by 1 mm Gd3+, a blocker of mechanotransduction channels (Kimitsuki et al. 1996). FM3-25, a larger styryl dye that does not permeate through mechanotransduction channels (Gale et al. 2001; Meyers et al. 2003), did not accumulate in Myo15sh2/sh2 hair cells even after longer incubations (Fig. 1D). These results indicate that Myo15sh2/sh2 hair cells may have functional mechanotransduction channels at birth and throughout the first two postnatal weeks.
Figure 1. Auditory mechanotransduction phenotype of young postnatal Myo15sh2/sh2 mice.
A and B, SEM images of Myo15sh2/sh2 OHCs at postnatal day 9 (P9) (A) and 19 (B). Insets show magnified images of stereocilia. Note stereocilia links in A and their absence in B. C, auditory brainstem responses in P16 homozygous (left) and heterozygous (right) Myo15sh2 mice. Stimulus type and intensity (dB SPL, sound pressure level) are indicated. Arrows mark the stimulus onset. D, epifluorescent images of Myo15sh2/sh2 organ of Corti explants (harvested at P3 and kept 3 days in vitro) after incubations with 5 μm FM1-43 for 60 s (left), FM1-43 and 1 mm GdCl3 for 60 s (middle), or FM3-25 for 6 min (right). E, time course of FM1-43 uptake in Myo15sh2/sh2 hair cells measured at the confocal optical plane below the cuticular plate. Insets show the actual fluorescent images before and immediately after application of 5 μm of the dye to the apical surface of OHCs. Scale bars: 1 μm (A, B); 10 μm (D). All cells (A and B, D and E) were located approximately at the end of the first (apical) cochlear turn.
Next, we used the whole-cell patch-clamp technique to record mechanotransduction currents in OHCs (Fig. 2A). In P3–P7 Myo15sh2/sh2 mice, only OHCs in the apical cochlear turn possess a substantial staircase arrangement of stereocilia, although with a decreased overall height of the bundle that was evident in all organ of Corti explants used in our study (Fig. 2B and C). Therefore, these cells were chosen to study the role of myosin-XVa in mechanotransduction with minimal confounding effects of abnormal hair bundle morphology and progressive base-to-apex degeneration. In Myo15sh2/sh2 apical OHCs, we observed a fast mechanically gated current (Fig. 2D), indistinguishable from the mechanotransduction current in hair cells of normal-hearing heterozygous littermates (Fig. 2E). Mechanotransduction current in Myo15sh2/sh2 and control Myo15+/sh2 OHCs approaches saturation when the probe displacement amplitude exceeded 0.5 μm (Fig. 2D and E). On average, Myo15sh2/sh2 OHCs had a smaller amplitude of saturating transduction current (Fig. 2F) but these differences were not statistically significant and normalized current–displacement relationships were almost identical (Fig. 2G).
The Myo15sh2/sh2 mechanotransduction current showed a prominent adaptation, a characteristic decay during sustained deflection of the bundle (Fig. 2D). In all recorded cells, the adaptation was best fitted with a double exponential function, consistent with the presence in OHCs of both ‘fast’ and ‘slow’ adaptation components (Kros et al. 1992, 2002; Kennedy et al. 2003). The time constants of this function and the maximal amplitude of the transduction current were not significantly different among apical OHCs of Myo15sh2/sh2 mice, their normal-hearing heterozygous Myo15+/sh2 littermates, or wild type (Myo15+/+) mice (Table 1). We also recorded mechanotransduction responses in apical OHCs of young postnatal Whrnwi/wi mice, which have wild type myosin-XVa at the tips of similarly short stereocilia, but lack whirlin, another component of a stereocilia elongation complex (Belyantseva et al. 2005). Mechanotransduction currents in Whrnwi/wi hair cells had similar amplitudes and adaptation constants (Table 1).
Table 1.
Quantitative characteristics of mechano-electrical transduction
| Adaptation | ||||
|---|---|---|---|---|
| Genotype | Maximal current (pA)* | τfast (ms)** | τslow (ms)** | No of cells/animal |
| Myo15sh2/sh2 | 226 ± 41 (124–439) | 2.07 ± 0.25 (1.23–3.42) | 20.5 ± 3.0 (11.9–33.1) | 7/4 |
| Myo15+/sh2 | 264 ± 32 (161–384) | 2.10 ± 0.33 (1.15–3.65) | 24.3 ± 3.4 (11.6–34.6) | 7/3 |
| Myo15+/+ | 228 ± 36 (125–288) | 2.06 ± 0.21 (1.62–2.55) | 22.1 ± 6.0 (11.3–36.0) | 4/3 |
| Whrnwi/wi | 256 ± 79 (105–469) | 2.00 ± 0.29 (1.30–2.54) | 24.2 ± 2.7 (19.4–31.6) | 5/2 |
The data are shown as mean ±s.e.m. (Min–Max). There were no statistically significant differences (P > 0.05, two-sided Student's t test) in these parameters among any pairs of the genotype groups.
Maximal mechanotransduction current was defined as a peak current evoked by the largest deflection (∼1 μm) of the stereocilia bundle at the holding potential of −90 mV.
Time constants were determined from the double-exponential fit of the decay of the transduction current at a displacement corresponding to half-maximal activation of this current.
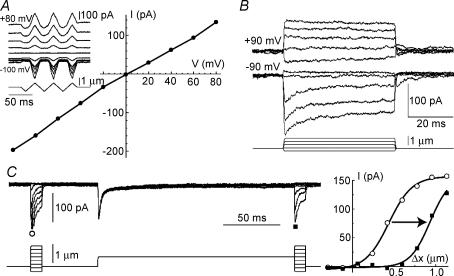
The reversal potential of mechanotransduction current (Fig. 3A) in Myo15sh2/sh2 OHCs was close to zero (Myo15sh2/sh2: 3.1 ± 1.6 mV, n = 10; Myo15+/sh2: 5.4 ± 1.7 mV, n = 7), consistent with the non-selective cation conductance of mechanotransduction channels (Gillespie & Walker, 2001). Adaptation in Myo15sh2/sh2 hair cells existed at negative but not positive holding potentials (Fig. 3B), represented a shift in displacement–current relationship (Fig. 3C), and reduced the resting mechanotransduction current (Fig. 3B; compare the responses to inhibitory deflections at positive and negative voltages). The same adaptation properties were previously reported for wild type non-mammalian hair cells (Assad et al. 1989; Crawford et al. 1989) and mammalian OHCs (Kros et al. 1992, 2002; Kennedy et al. 2003, 2006).
Figure 3. Properties of mechano-electrical transduction in Myo15sh2/sh2 OHCs.
A, voltage–current relationship of the maximal mechanotransduction current evoked by ramp deflections of the bundle at different holding potentials (inset). B, mechanotransduction currents recorded at −90 mV and +90 mV holding potentials in the same cell. C, mechanotransduction current (left) evoked by a brief bundle deflection before (○) and after (▪) a longer step-like deflection of stereocilia by 0.6 μm at −70 mV holding potential. Displacement–current relationships (right) were fitted to a first order Boltzmann function to determine a shift due to adaptation (0.5 μm, indicated by arrow). Access resistances (MΩ): A −40.6; B −37.8; C −74.
Discussion
Our observations of normal mechano-electrical transduction in OHCs of Myo15sh2/sh2 mice were unexpected for several reasons. First, these mice do not have detectable ABRs even to strong acoustical stimuli (Liang et al. 1998; Beyer et al. 2000). Second, the precise architecture of a mature stereocilia bundle is considered a prerequisite of normal hair cell mechanosensitivity and, so far, congenital abnormalities of this structure were associated with the loss or substantial alteration of mechano-electrical transduction (Frolenkov et al. 2004). Third, the previously reported absence of tip-links in the stereocilia of adult Myo15sh2/sh2 mice (Beyer et al. 2000) indicated that myosin-XVa is likely to participate in formation or maintenance of these structures, which are vital for mechanotransduction (Assad et al. 1991). This hypothesis was further supported by the appearance of myosin-XVa at the tips of vestibular hair cell stereocilia 2 days before the onset of mechanotransduction in these cells (Belyantseva et al. 2003; Geleoc & Holt, 2003). Finally, other stereocilia myosins (VIIa and Ic) are known to influence mechanotransduction (Holt et al. 2002; Kros et al. 2002; Stauffer et al. 2005).
Intact mechanotransduction before onset of hearing and profound deafness of Myo15sh2/sh2 mice
Abnormally short stereocilia of Myo15sh2/sh2 OHCs are unlikely to be tightly coupled to the overlying tectorial membrane, which is essential for normal hearing (Legan et al. 2000). In addition, the viscous drag force on short Myo15sh2/sh2 stereocilia is likely to be significantly reduced. Therefore, even if the Myo15sh2/sh2 hair cells could transduce normally throughout the life of the animal, significant hearing loss would still be expected. However, all our recordings of mechanotransduction current in Myo15sh2/sh2 OHCs were obtained in cultured organ of Corti explants with an equivalent age of not more than P9. After this age, there is a gradual degeneration of the OHC bundle that starts with the disappearance of stereocilia links (Fig. 1B) and continues with subsequent stereocilia retraction (data not shown). These degenerative processes should disrupt mechanotransduction in Myo15sh2/sh2 OHCs by the time normal hearing thresholds are established in wild type mice (P14–P18). All of these factors are likely to contribute to the profound deafness of Myo15sh2/sh2 mice.
Abnormally short Myo15sh2/sh2 hair bundles and operating range of mechanotransduction
We were surprised to observe similar current–displacement relationships in Myo15sh2/sh2 and Myo15+/sh2 OHCs (Fig. 2G). According to our SEM examination, the heights of the hair bundles in the recorded OHCs were about 2.5 μm in Myo15+/sh2 mice and about 1 μm in Myo15sh2/sh2 mice. Assuming that equal angular stereocilia deflections produce the same transduction current in the bundles of different heights (Geleoc et al. 1997), a current–displacement relationship should be about 2.5 times steeper in Myo15sh2/sh2 than in Myo15+/sh2 OHCs. However, this assumption is based on the experiments with wild type hair cells. In Myo15sh2/sh2 OHCs, a reduced staircase arrangement of stereocilia may be associated with a significant percentage of the links that run more perpendicular to the actin core of a stereocilium than the tip-links in wild type OHCs. This would result in less efficient tensioning of the links, extending the operating range of mechanotransduction. In addition, short Myo15sh2/sh2 stereocilia may slide beneath the stimulating probe, which would result in stereocilia deflections smaller than probe movement, increasing the apparent operating range. However, the sustained mechanotransduction responses at positive holding potentials (Fig. 3B) strongly suggest that our stimulation produced true step-like deflections of stereocilia even in Myo15sh2/sh2 OHCs. Furthermore, robust responses to negative bundle displacements (Fig. 2D) indicated adhesive coupling between Myo15sh2/sh2 stereocilia and the probe. Therefore, we tentatively assume that Myo15sh2/sh2 hair bundles have less steep angular deflection sensitivity due to a decreased staircase.
Myosin-XVa and adaptation of the mechano-electrical transduction
Since the shallow approach angle to the short Myo15sh2/sh2 stereocilia requires a relatively long stimulating probe, we were not able to deflect stereocilia sufficiently fast to fully reveal the largest, fast component of transduction current (Kennedy et al. 2003). However, transduction current amplitudes and the fastest time constants, determined in our study of mouse OHCs, were within or at the edge of the ranges reported by Kennedy et al. (2003) for rat OHCs. More importantly, we observed similar two-component kinetics of adaptation in all normal and mutant OHCs. A quantitative comparison revealed virtually identical time constants of adaptation in Myo15+/+, Myo15sh2/sh2, Myo15+/sh2 and Whrnwi/wi OHCs (Table 1). Therefore, we conclude that the absence of functional myosin-XVa is unlikely to eliminate either adaptation component.
Hair bundle morphogenesis and mechanotransduction
Normal mechano-electrical transduction in Myo15sh2/sh2 hair cells demonstrates that myosin-XVa is not needed for formation and function of the mechanotransduction apparatus. Myosin-XVa delivers whirlin, and probably other molecules regulating the growth of stereocilia actin filaments (Belyantseva et al. 2005), to the tips of stereocilia. Our observations, including normal mechanotransduction in Whrnwi/wi hair cells, indicate that neither whirlin nor these other molecules are directly essential for hair cell mechanotransduction. Thus, formation of the mechanotransduction complex in hair cells is likely to be independent from the molecular mechanisms underlying myosin-XVa-based stereocilia elongation. It is still unknown, however, whether the myosin-XVa-based growth of stereocilia is, in turn, independent from the operation of mechanotransduction channels. Gating of mechanotransduction channels in Myo15sh2/sh2 hair cells is apparently mediated by some of the links interconnecting stereocilia of these cells at the early postnatal age (Fig. 1A). These links disappear later in development (Fig. 1B) and therefore they may represent ‘transient’ links observed in early postnatal wild type outer hair cells, but not tip-links that persist throughout the life of the animal (Goodyear et al. 2005). Alternatively, ‘true’ tip-links may form normally in hair cells of young postnatal Myo15sh2/sh2 mice together with ‘transient’ links but eventually disappear due to overall degeneration of the abnormally short hair bundles that occurs in late postnatal development. In any case, our data suggest that the stereocilia links in young Myo15sh2/sh2 hair cells are sufficient to mediate normal mechano-electrical transduction. Our data also demonstrate that the precisely controlled overall height of the hair bundle does not influence essential features of mechanotransduction but may serve another purpose such as determining the individual bending rigidity of the bundle (Flock & Strelioff, 1984). Future studies will determine whether other bundles without staircase stereocilia morphology, such as Myo15sh2/sh2 cochlear inner or vestibular hair cells, are also mechanosensitive.
In conclusion, our study demonstrates intact mechanotransduction in the absence of functional myosin-XVa. Therefore, the molecular mechanisms of formation and operation of the hair cell mechanotransduction complex are independent of the myosin-XVa-based stereocilia elongation that creates the precise shape of the mature hair bundle.
Acknowledgments
G.I.F. is supported by the University of Kentucky funds and by the Deafness Research Foundation. A.J.G., I.A.B and T.B.F. are supported by NIDCD/NIH intramural funds. We thank Hitachi and Richard Leapman for providing access to SEMs.
References
- Assad JA, Hacohen N, Corey DP. Voltage dependence of adaptation and active bundle movement in bullfrog saccular hair cells. Proc Natl Acad Sci U S A. 1989;86:2918–2922. doi: 10.1073/pnas.86.8.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assad JA, Shepherd GM, Corey DP. Tip-link integrity and mechanical transduction in vertebrate hair cells. Neuron. 1991;7:985–994. doi: 10.1016/0896-6273(91)90343-x. [DOI] [PubMed] [Google Scholar]
- Belyantseva IA, Boger ET, Friedman TB. Myosin XVa localizes to the tips of inner ear sensory cell stereocilia and is essential for staircase formation of the hair bundle. Proc Natl Acad Sci U S A. 2003;100:13958–13963. doi: 10.1073/pnas.2334417100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyantseva IA, Boger ET, Naz S, Frolenkov GI, Sellers JR, Ahmed ZM, Griffith AJ, Friedman TB. Myosin-XVa is required for tip localization of whirlin and differential elongation of hair-cell stereocilia. Nat Cell Biol. 2005;7:148–156. doi: 10.1038/ncb1219. [DOI] [PubMed] [Google Scholar]
- Beyer LA, Odeh H, Probst FJ, Lambert EH, Dolan DF, Camper SA, Kohrman DC, Raphael Y. Hair cells in the inner ear of the pirouette and shaker 2 mutant mice. J Neurocytol. 2000;29:227–240. doi: 10.1023/a:1026515619443. [DOI] [PubMed] [Google Scholar]
- Crawford AC, Evans MG, Fettiplace R. Activation and adaptation of transducer currents in turtle hair cells. J Physiol. 1989;419:405–434. doi: 10.1113/jphysiol.1989.sp017878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk W, Holt JR, Shepherd GM, Corey DP. Calcium imaging of single stereocilia in hair cells: localization of transduction channels at both ends of tip links. Neuron. 1995;15:1311–1321. doi: 10.1016/0896-6273(95)90010-1. [DOI] [PubMed] [Google Scholar]
- Dumont RA, Zhao YD, Holt JR, Bahler M, Gillespie PG. Myosin-I isozymes in neonatal rodent auditory and vestibular epithelia. J Assoc Res Otolaryngol. 2002;3:375–389. doi: 10.1007/s101620020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flock A, Strelioff D. Graded and nonlinear mechanical properties of sensory hairs in the mammalian hearing organ. Nature. 1984;310:597–599. doi: 10.1038/310597a0. [DOI] [PubMed] [Google Scholar]
- Frolenkov GI, Belyantseva IA, Friedman TB, Griffith AJ. Genetic insights into the morphogenesis of inner ear hair cells. Nat Rev Genet. 2004;5:489–498. doi: 10.1038/nrg1377. [DOI] [PubMed] [Google Scholar]
- Gale JE, Marcotti W, Kennedy HJ, Kros CJ, Richardson GP. FM1-43 dye behaves as a permeant blocker of the hair-cell mechanotransducer channel. J Neurosci. 2001;21:7013–7025. doi: 10.1523/JNEUROSCI.21-18-07013.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geleoc GS, Holt JR. Developmental acquisition of sensory transduction in hair cells of the mouse inner ear. Nat Neurosci. 2003;6:1019–1020. doi: 10.1038/nn1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geleoc GS, Lennan GW, Richardson GP, Kros CJ. A quantitative comparison of mechanoelectrical transduction in vestibular and auditory hair cells of neonatal mice. Proc Biol Sci. 1997;264:611–621. doi: 10.1098/rspb.1997.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie PG, Walker RG. Molecular basis of mechanosensory transduction. Nature. 2001;413:194–202. doi: 10.1038/35093011. [DOI] [PubMed] [Google Scholar]
- Goodyear RJ, Marcotti W, Kros CJ, Richardson GP. Development and properties of stereociliary link types in hair cells of the mouse cochlea. J Comp Neurol. 2005;485:75–85. doi: 10.1002/cne.20513. [DOI] [PubMed] [Google Scholar]
- Hasson T, Gillespie PG, Garcia JA, MacDonald RB, Zhao Y, Yee AG, Mooseker MS, Corey DP. Unconventional myosins in inner-ear sensory epithelia. J Cell Biol. 1997;137:1287–1307. doi: 10.1083/jcb.137.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt JR, Gillespie SK, Provance DW, Shah K, Shokat KM, Corey DP, Mercer JA, Gillespie PG. A chemical-genetic strategy implicates myosin-1c in adaptation by hair cells. Cell. 2002;108:371–381. doi: 10.1016/s0092-8674(02)00629-3. [DOI] [PubMed] [Google Scholar]
- Kennedy HJ, Evans MG, Crawford AC, Fettiplace R. Fast adaptation of mechanoelectrical transducer channels in mammalian cochlear hair cells. Nat Neurosci. 2003;6:832–836. doi: 10.1038/nn1089. [DOI] [PubMed] [Google Scholar]
- Kennedy HJ, Evans MG, Crawford AC, Fettiplace R. Depolarization of cochlear outer hair cells evokes active hair bundle motion by two mechanisms. J Neurosci. 2006;26:2757–2766. doi: 10.1523/JNEUROSCI.3808-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimitsuki T, Nakagawa T, Hisashi K, Komune S, Komiyama S. Gadolinium blocks mechano-electric transducer current in chick cochlear hair cells. Hear Res. 1996;101:75–80. doi: 10.1016/s0378-5955(96)00134-7. [DOI] [PubMed] [Google Scholar]
- Kros CJ, Marcotti W, van Netten SM, Self TJ, Libby RT, Brown SD, Richardson GP, Steel KP. Reduced climbing and increased slipping adaptation in cochlear hair cells of mice with Myo7a mutations. Nat Neurosci. 2002;5:41–47. doi: 10.1038/nn784. [DOI] [PubMed] [Google Scholar]
- Kros CJ, Rusch A, Richardson GP. Mechano-electrical transducer currents in hair cells of the cultured neonatal mouse cochlea. Proc Biol Sci. 1992;249:185–193. doi: 10.1098/rspb.1992.0102. [DOI] [PubMed] [Google Scholar]
- Legan PK, Lukashkina VA, Goodyear RJ, Kossi M, Russell IJ, Richardson GP. A targeted deletion in alpha-tectorin reveals that the tectorial membrane is required for the gain and timing of cochlear feedback. Neuron. 2000;28:273–285. doi: 10.1016/s0896-6273(00)00102-1. [DOI] [PubMed] [Google Scholar]
- Liang Y, Wang A, Probst FJ, Arhya IN, Barber TD, Chen KS, et al. Genetic mapping refines DFNB3-17p11.2, suggests multiple alleles of DFNB3, and supports homology to the mouse model shaker-2. Am J Hum Genet. 1998;62:904–915. doi: 10.1086/301786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mburu P, Mustapha M, Varela A, Weil D, El-Amraoui A, Holme RH, et al. Defects in whirlin, a PDZ domain molecule involved in stereocilia elongation, cause deafness in the whirler mouse and families with DFNB31. Nat Genet. 2003;34:421–428. doi: 10.1038/ng1208. [DOI] [PubMed] [Google Scholar]
- Meyers JR, MacDonald RB, Duggan A, Lenzi D, Standaert DG, Corwin JT, Corey DP. Lighting up the senses: FM1-43 loading of sensory cells through nonselective ion channels. J Neurosci. 2003;23:4054–4065. doi: 10.1523/JNEUROSCI.23-10-04054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickles JO, Comis SD, Osborne MP. Cross-links between stereocilia in the guinea pig organ of Corti, and their possible relation to sensory transduction. Hear Res. 1984;15:103–112. doi: 10.1016/0378-5955(84)90041-8. [DOI] [PubMed] [Google Scholar]
- Probst FJ, Fridell RA, Raphael Y, Saunders TL, Wang A, Liang Y, et al. Correction of deafness in shaker-2 mice by an unconventional myosin in a BAC transgene. Science. 1998;280:1444–1447. doi: 10.1126/science.280.5368.1444. [DOI] [PubMed] [Google Scholar]
- Schneider ME, Belyantseva IA, Azevedo RB, Kachar B. Rapid renewal of auditory hair bundles. Nature. 2002;418:837–838. doi: 10.1038/418837a. [DOI] [PubMed] [Google Scholar]
- Stauffer EA, Scarborough JD, Hirono M, Miller ED, Shah K, Mercer JA, Holt JR, Gillespie PG. Fast adaptation in vestibular hair cells requires Myosin-1c activity. Neuron. 2005;47:541–553. doi: 10.1016/j.neuron.2005.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Liang Y, Fridell RA, Probst FJ, Wilcox ER, Touchman JW, et al. Association of unconventional myosin MYO15 mutations with human nonsyndromic deafness DFNB3. Science. 1998;280:1447–1451. doi: 10.1126/science.280.5368.1447. [DOI] [PubMed] [Google Scholar]