Abstract
5-Hydroxytryptamine (5-HT) is one of the major chemical mediators released in injured and inflamed tissue and is capable of inducing hyperalgesia in vivo. However, the cellular mechanisms of 5-HT-induced hyperalgesia remain unclear. Transient receptor potential V1 (TRPV1) plays a pivotal role in nociceptive receptors. In the present study, we determined whether 5-HT changes TRPV1 functions in cultured dorsal root ganglion (DRG) neurons isolated from neonatal rats, using Ca2+ imaging and whole-cell patch-clamp techniques. In more than 70% of DRG neurons, 5-HT potentiated the increases of [Ca2+]i induced by capsaicin, protons and noxious heat. Capsaicin-induced current and depolarizing responses, and proton-induced currents were also augmented by 5-HT. RT-PCR analysis revealed the expression of 5-HT2A and 5-HT7 receptors in rat DRG neurons. Agonists for 5-HT2A and 5-HT7 receptors mimicked the potentiating effect of 5-HT, and their antagonists decreased it. In DRG ipsilateral to the complete Freund's adjuvant-injected inflammation side, expression levels of 5-HT2A and 5-HT7 mRNAs increased, and the potentiating effect of 5-HT was more prominent than in the contralateral control side. These results suggest that the PKC- and PKA-mediated signalling pathways are involved in the potentiating effect of 5-HT on TRPV1 functions through the activation of 5-HT2A and 5-HT7 receptors, respectively. Under inflammatory conditions, the increases of the biosynthesis of these 5-HT receptors may lead to further potentiation of TRPV1 functions, resulting in the generation of inflammatory hyperalgesia in vivo.
Tissue damage associated with inflammation produces an array of chemical mediators that sensitize nociceptor terminals to elicit exacerbate pain. A number of substances released during inflammation such as bradykinin (BK), prostaglandin E2 (PGE2) and 5-hydroxytryptamine (5-HT) generate pain and hyperalgesia (Kress & Reeh, 1996).
Transient receptor potential V1 receptor (TRPV1) is a non-selective cation channel gated by capsaicin, noxious heat and protons (Caterina et al. 1997; Tominaga et al. 1998). TRPV1 function is regulated by a variety of inflammatory mediators. BK reduces the heat threshold of TRPV1 through protein kinase C (PKC) activation (Cesare & McNaughton, 1996; Sugiura et al. 2002). Nerve growth factor and ATP sensitize TRPV1 in a PKC-dependent manner (Bonnington & McNaughton, 2003; Moriyama et al. 2003). PGE2 potentiates TRPV1-mediated responses via a cyclic AMP/protein kinase A (cAMP/PKA) pathway in rat sensory neurons (Lopshire & Nicol, 1998; Moriyama et al. 2005). In mice lacking TRPV1, heat hyperalgesia fails to occur during inflammation (Caterina et al. 2000; Davis et al. 2000), suggesting that TRPV1 is critical for inflammatory heat hypersensitivity.
5-Hydroxytryptamine is released primarily from descending bulbospinal serotonergic neurons and causes analgesia (Sorkin et al. 1993; Liu et al. 2002). In the periphery, 5-HT is released from platelets, mast cells and endothelial cells into a wound site in response to inflammation and injury (Lehtosalo et al. 1984). This chemical is a potent pro-inflammatory and pro-nociceptive agent since it excites nociceptive afferents (Beck & Handwerker, 1974) and induces hyperalgesia in humans (Ernberg et al. 2000; Schmelz et al. 2003) and rats (Sufka et al. 1992; Taiwo & Levine, 1992). 5-HT receptors are classified into 7 families and 13 subtypes (Hoyer et al. 2002). It is recognized that ionotropic 5-HT3 receptors are located on sensory nerve terminals and are responsible for 5-HT-induced pain and hyperalgesia (Eschalier et al. 1989; Giordano & Rogers, 1989). However, it is also reported that other subtypes of 5-HT receptors participate in inflammatory pain (Ebersberger et al. 1995; Abbott et al. 1996; Espejo & Gill, 1998; Tokunaga et al. 1998; Okamoto et al. 2002). Therefore, despite the potential importance of 5-HT in hyperalgesia, the involvement of 5-HT receptor subtypes in hyperalgesia and cellular mechanisms remained to be clarified.
Recently, some reports have suggested the possibility that the hyperalgesic action of 5-HT is related to TRPV1 functions. Stimulation of spinal 5-HT receptors potentiates the capsaicin-induced release of substance P in the rat dorsal horn in vivo (Bertelsen et al. 2003), and 5-HT enhances TRPV1 function in mouse colon sensory neurons (Sugiura et al. 2004).
The aim of the present experiment was to identify the cellular mechanisms and related receptor subtypes in 5-HT-induced hyperalgesia responsible for TRPV1 functions in dorsal root ganglion (DRG) neurons in vitro. As TRPV1 possess high Ca2+ permeability (Caterina & Julius, 2001), it is possible to monitor their functions by measuring the [Ca2+]i response in individual DRG neurons systematically. In the major part of the present study, therefore, we used a Ca2+-imaging technique to evaluate effects of 5-HT on TRPV1-mediated responses. 5-HT-induced changes of membrane potential and current responses to capsaicin were also investigated. 5-HT receptor subtypes and related intracellular signalling pathways were determined by pharmacological and molecular analyses. Finally, we tested whether 5-HT receptor expression and the actions of 5-HT were changed under inflammatory conditions.
Methods
Isolation and culture of DRG neurons
All protocols for the use of animals were approved by the Committee on Animal Experimentation, Graduate School of Veterinary Medicine, Hokkaido University. Neonatal Wistar rats (1–4 days old) of either sex were decapitated under ethyl ether anaesthesia. The spinal column was opened and DRGs were detached under a dissecting microscope. The DRGs were trimmed of attached fibres in Ca2+,Mg2+-free phosphate-buffered saline (PBS (mm): 136.9 NaCl, 2.7 KCl, 1.47 KH2PO4, 8.1 Na2HPO4), enzymatically digested for 30 min in PBS-containing collagenase (1 mg ml−1, Type I, Worthington, Lakewood, NJ, USA) and DNase I (0.5 mg ml−1, Roche, Indianapolis, in, usa), and then for another 30 min in PBS-containing trypsin (0.25% w/v, Type XI, Sigma, St Louis, MO, USA) and DNase I at 37°C. Subsequently, they were rinsed with culture medium, M199 (Sigma) supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY, USA), penicillin (100 μg ml−1, Bunyu, Japan) and streptomycin (100 u ml−1, Meiji-Seika, Japan). Following enzyme digestion, individual cells were mechanically dispersed by repeated triturations using a fire-polished Pasteur pipette and stood on ice. Then the cell suspension was centrifuged (100 g, 5 min, 4°C) and the pellet containing cells was resuspended with the culture medium. Aliquots were placed onto glass coverslips coated with poly d-lysine (Sigma). Cells were kept in the culture medium without nerve growth factor (NGF) in a humidified atmosphere of 5% CO2 and 95% air at 37°C. DRG neurons were used between 12 and 36 h after the plating. In the experiments using DRG isolated from inflammation-induced rats, fresh uncultured DRG neurons were used.
Measurement of intracellular Ca2+ concentration
The intracellular Ca2+ concentration ([Ca2+]i) in single cells was measured with a fluorescent Ca2+ indicator, fura-2, by dual excitation using a fluorescence imaging system controlling illumination and acquisition with software (Aqua Cosmos, Hamamatsu Photonics, Japan) as previously described (Ohta et al. 2005b). To load fura-2, cells were incubated for 1 h at room temperature with a 10 μm concentration of the acetoxymethyl ester form of fura-2 (fura-2 AM; Molecular Probes, Eugene, OR, USA) in normal external solution (mm): 134 NaCl, 6 KCl, 1.2 MgCl2, 2.5 CaCl2, 10 glucose, 10 Hepes (pH 7.4 with NaOH). A coverslip with fura-2-loaded cells was placed in an experimental chamber mounted on the stage of an inverted microscope (Diaphot 300, Nikon, Japan). In some experiments, before [Ca2+]i measurement, cells were incubated with the plant lectin, isolectin GS-IB4 from Griffonia simplicifolia Alexa Fluor 488 conjugate (IB4; 10 μg ml−1, Molecular Probes) for 10 min and then rinsed for 2 min. IB4 staining was visualized with appropriate filters (excitation 490 nm; emission barrier filter 515–555 nm). A neuron was considered IB4-positive if it had a continuous green ring around the perimeter. Cells were illuminated every 2 s with lights at 340 and 380 nm and the respective fluorescence signals (F340 and F380) were detected. The fluorescence emitted was projected onto a CCD camera, and F340, F380 and its ratio (F340/F380) were stored on the hard disk of a computer (Pro-600 l, Epson, Japan). Since fluorescence signals of fura-2 are influenced by changes in pH and temperature, we adopted data from cells of which the F340 and F380 showed a mirror image. Calibration of fura-2 was performed with a Ca2+ calibration buffer solution (Molecular Probes) containing 5 μm fura-2 (Ohta et al. 2005b). Cells were continuously superfused with the external solution through multibarrelled tubes by gravity at a flow rate of ∼1 ml min−1, and the bath level was adjusted so that the total bath volume was about 0.5 ml. Drugs were applied through different tubes of puffer pipettes. All experiments were carried out at room temperature (20–24°C) except the experiment on heat effects. Noxious heat stimulation was carried out by the application to the cells of an excess volume of external solution heated to the desired temperature, as reported previously (Ohta et al. 2005a).
Whole-cell membrane voltage and current recordings
Membrane currents and potentials were recorded using the conventional whole-cell configuration of the patch-clamp technique (Ohta et al. 2001). Whole-cell recordings were made from small diameter neurons (< 20 μm) using glass electrodes with 4–5 MΩ resistance mounted on the head stage of a patch-clamp amplifier (Axopatch 200B, Axon Instruments, Union City, CA, USA). Current data were filtered at 1 kHz and sampled at 2–5 kHz by an A/D converter (PowerLab System, ADInstruments, Australia) in conjunction with a personal computer (Macintosh G3, Apple, Japan), and stored on the hard disk of the PC. The cell capacitance of DRG neurons was 15.8 ± 0.4 pF (n = 92). After cells were voltage clamped at a holding potential of −60 mV, a step depolarization to 0 mV lasting 50 ms was applied for the activation of voltage-dependent Na+ channels to confirm that they were DRG neurons. The standard pipette solution contained (mm): 130 CsCl, 6 NaCl, 4 Mg-ATP, 0.3 GTPNa3, 10 Hepes and 10 EGTA (pH 7.3 with CsOH). For current-clamp study, K+-based pipette solution was used (mm: 130 potassium gluconate, 6 NaCl, 4 Mg-ATP, 0.3 GTPNa3, 10 Hepes and 0.2 EGTA, pH 7.3 with KOH). A multibarrelled puffer system similar to that used for [Ca2+]i measurement was used for drug application and external perfusion.
Reverse transcription-polymerase chain reaction
The design of the oligonucleotides used for the specific amplification of rat 5-HT receptor cDNAs was based on sequences registered in GenBank for each of the receptors in the rat. The nucleotide sequence and the length of the expected PCR product for each primer pair are shown in Table 1. Total RNA from rat DRG was extracted with Trizol reagent (Isogen, Nippon Gene, Japan), and then treated with DNase I (Promega, Madison, WI, USA). First-strand cDNA was synthesized from oligo(dT)-primed total RNA with Superscript II reverse transcriptase (Gibco). The reaction mixture was then subjected to PCR amplification with the use of Taq DNA polymerase (Promega). Samples were heated to 94°C for 5 min, followed by 30 cycles of 94°C for 1 min, 55°C for 1 min and 72°C for 1 min. To exclude the possibility of genomic DNA giving a false-positive result in the PCR, a reverse transcriptase-free (negative) control was run with each primer pair. The PCR products were resolved on 2% agarose gels and visualized by ethidium bromide staining followed by UV transillumination. We identified the respective subtypes of receptors by extracting them from gel, subcloning with pGEM-T-easy vector (Promega) and sequencing (CEQ8000, Beckman, CA, USA) (Ohta et al. 2005a).
Table 1.
5-HT receptor and β-actin primers for RT-PCR
| Name | Sense (upper row)/ antisense (lower row) | Product size (bp) | Accession no. |
|---|---|---|---|
| 5-HT1A | 5′-TCACCTGCGACCTGTTTATC-3′ | 394 | J05276 |
| 5′-GCTCCCTTCTTTTCCACCTT-3′ | |||
| 5-HT1B | 5′-TCGTGCTGGTGTGGGTCTTCT-3′ | 262 | X62944 |
| 5′-ATCAACTGGGCTCGGGTCAAG-3′ | |||
| 5-HT1D | 5′-CTGAATGCTACAGGGGCTTGG-3′ | 452 | M89953 |
| 5′-CGGTGGGATGGAGATACAGA-3′ | |||
| 5-HT1F | 5′-ACCTTTGGCTGAGTGTTGAC-3′ | 591 | L05596 |
| 5′-TAGTGGCTGCTTTGCGTTCT-3′ | |||
| 5-HT2A | 5′-GCATCGAACTGGACAATTGATGCTGAAAA-3′ | 611 | M30705 |
| 5′-ATGAAAAATGCCACAAAAGAGCCTATGAG-3′ | |||
| 5-HT2B | 5′-GTGATGCCGATTGCTCTCTT-3′ | 297 | X66842 |
| 5′-GATGTTGTGTGCGTTGACCA-3′ | |||
| 5-HT2C | 5′-TTCGTTCTCATCGGGTCCTT-3′ | 441 | U35315 |
| 5′-CACATAGCCAATCCACACAA-3′ | |||
| 5-HT3 | 5′-GAACTACAAGCCCCTACAGC-3′ | 439 | D49395 |
| 5′-TGACACGATGATGAGAAAGA-3′ | |||
| 5-HT4 | 5′-GGGAGATGTTTTGCCTGGTC-3′ | 484 | U20907 |
| 5′-CGATGTGTGCTGTGCTGGTC-3′ | |||
| 5-HT5A | 5′-TTCCACCGAGTACCACACAA-3′ | 757 | L10072 |
| 5′-TGACGGACAGTGAACACCAT-3′ | |||
| 5-HT5B | 5′-ACGTGTGGATCTCCTTCGAC-3′ | 426 | L10073 |
| 5′-CAGACTCCTGAGGTGCTTCC-3′ | |||
| 5-HT6 | 5′-TACTGTAATAGCACCATGAACCCTATCAT-3′ | 374 | L41146 |
| 5′-CTGAGTGGATGCGGCCGTATCTCAGGCTC-3′ | |||
| 5-HT7 | 5′-AGCCCTCCAACTACCTGATT-3′ | 566 | L22558 |
| 5′-ACACTCTTCCACCTCCTTCT-3′ | |||
| β-Actin | 5′-AGCCATGTACGTAGCCATCC-3′ | 294 | V01217 |
| 5′-GCCATCTCTTGCTCGAAGTC-3′ |
Complete Freund's adjuvant-induced inflammation
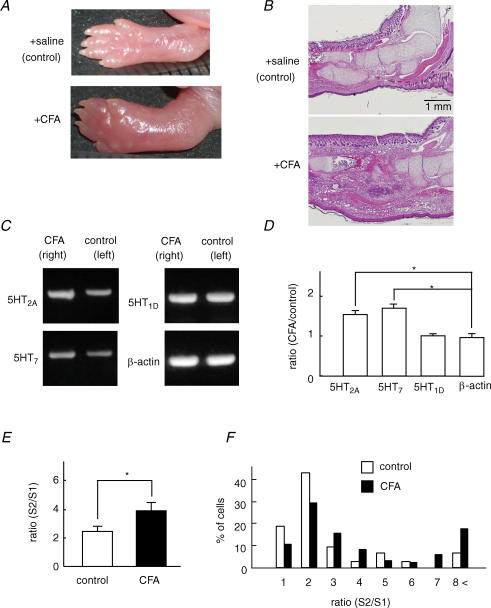
Neonatal rats were injected with complete Freund's adjuvant (CFA, 50 μl; Sigma) into the plantar surface of the right hind paw (Ruda et al. 2000). For the control, the same amount of saline was injected into the left hind paw. Injection of this dose of CFA caused inflammation of the entire hind leg of the rat compared with the control leg (see Fig. 6A). As reported by Ruda et al. (2000), the rats exhibited distinct behavioural responses identical to those observed in an adult model of pain (Abbott et al. 1995). Four days after CFA injection, both inflamed and control sides of DRG at L4–L6, which innervate the hind legs, were separately removed, and enzymatically isolated for the following [Ca2+]i measurement, and mRNA was extracted for the following RT-PCR analysis. For histological observation, dissected legs were fixed with formalin and cross-sections were cut at the base of the leg after paraffin embedding and stained with haematoxylin–eosin by a standard protocol. A total of 12 rats were used for evoking CFA-induced leg inflammation.
Figure 6. Inflammation increased 5-HT receptor expression and the potentiating effect of 5-HT on [Ca2+]i responses to capsaicin.
A and B, complete Freund's adjuvant (CFA) and saline were injected into the right and left hemilateral hind paw of the neonatal rat, respectively. Macroscopic (A) and histological (B) images 4 days after injection of CFA at the CFA-injected site (+CFA) and saline-injected site (control). C, the expression levels of 5-HT2A and 5-HT7 receptors were more pronounced in DRG isolated from the CFA side than the control side. No significant changes occurred in 5-HT1D and β-actin expression in DRG from both sides. D, the ratio of the optical density of PCR products of 5-HT2A, 5-HT7, 5-HT1D and β-actin from the CFA side to that from the control side (N = 5). * P < 0.05. E, the summarized potentiation rate (S2/S1) estimated by the ratio of the capsaicin (30 nm)-induced [Ca2+]i increase during the application of 5-HT (S2) to that before 5-HT application (S1). CFA, n = 114; control, n = 69; N = 5. * P < 0.05. F, histogram of the percentage of cells–S2/S1 ratio relation in response to capsaicin in DRG neurons isolated from the CFA side and control side.
Materials
The following drugs were used: bisindoylmaleimide I (BIM), capsaicin, (±)-2,5-dimethoxy-4-iodoamphetamine (DOI), n-2-hydroxyethyl-piperazine-2-ethanone-sulphonic acid (Hepes) (±)-8-hydroxy-2(di-n-propylamino)tetralin hydrobromide (8-OH-DPAT), 5-hydroxytryptamine (5-HT), ketanserin and SB469970 ((R)-3-(2-(2-(4-methypiperidin-1-yl)ethyl-)-pyrrolidine-1-sulphonyl-phenol) from Sigma. N-[2-(p-bromo-cinnamyl-amino)ethyl]-5-isoquinoline-sulphonamide (H89) was from Seikagaku-Kogyo (Japan). All other drugs used were of analytical grade.
Data analysis
The data are presented as the mean ± s.e.m. (n = number of cells, N = number of animals). The present data were obtained from at least two different coverslips for each different culture (animal) per experiment. Comparisons were made by Student's paired t test when comparing responses before and after the application of test drugs or by one-way ANOVA followed by the Tukey-Kramer test when more than two groups were compared (StatView 5.0, SAS Institute Inc., USA). Differences with a P value of less than 0.05 were considered significant. The EC50 value was determined using Origin software (OriginLab, USA).
Results
5-HT potentiates TRPV1-mediated responses
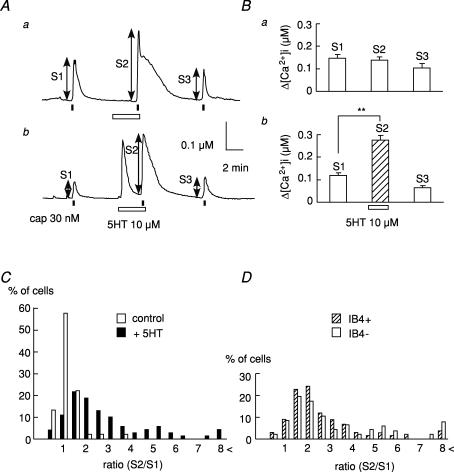
To assess the possibility that 5-HT modulates TRPV1 functions, we examined the effects of 5-HT on the capsaicin-induced [Ca2+]i increase. Approximately 75% of neonatal rat DRG neurons could respond to 1 μm capsaicin (data not shown). DRG neurons were repetitively stimulated with capsaicin (30 nm) for 15 s with an interval of 8 min. 5-HT was applied 3 min prior to and during the second application of capsaicin. Figure 1A shows two representative [Ca2+]i responses to capsaicin and the effect of 5-HT on them. The [Ca2+]i increase induced by capsaicin in the presence of 5-HT (S2) significantly increased in comparison with that in its absence (S1) (Fig. 1B). In a few neurons (6.5%, 15 of 231 cells), a transient [Ca2+]i increase was elicited by 5-HT (Fig. 1Ab). Regardless of whether a 5-HT-induced [Ca2+]i increase occurred, [Ca2+]i responses to capsaicin were augmented by 5-HT. The ratio (S2/S1) of the [Ca2+]i increases before to that after exposure to 5-HT was 2.4 ± 0.3 times. Because the extent of the potentiating effect varied from cell to cell, we determined the ratio (S2/S1) from individual cells, and the percentage of neurons was plotted against each ratio value with and without exposure to 5-HT (Fig. 1C). In control cells (without 5-HT), a large number of cells were distributed below the ratio of 1.5. In the 5-HT treatment group, the proportion of cells showing a ratio of over 2 increased to 75.3%, whereas it was only 6.6% in the control. The histogram showing the percentage of cells–ratio (S2/S1) relation shifted to the right.
Figure 1. Potentiating effects of 5-HT on the [Ca2+]i responses to capsaicin.
A, representative [Ca2+]i increases induced by capsaicin (cap, 30 nm) before, during the application of 5-HT (10 μm), and after its removal. 5-HT failed to produce a [Ca2+]i increase in the majority of cells (a), but in some cells, 5-HT elicited a [Ca2+]i increase (b). B, summarized data on changes in the [Ca2+]i increase induced by capsaicin before (S1), without (a) or with (b) 5-HT (S2) and after its washout of 5-HT (S3). The increment of [Ca2+]i (Δ[Ca2+]i) shown by the double-ended arrows was plotted (control, n = 89, N = 4; +5-HT, n = 231, N = 5). ** P < 0.01. C, histogram showing the percentage of cells versus the ratio (S2/S1) of the capsaicin-induced [Ca2+]i increase. In the control, data were obtained from the 3 repeated applications of capsaicin without 5-HT. D, histogram showing the percentage of cells–S2/S1 ratio relation for IB4-positive (IB4+; n = 129, N = 5) and -negative neurons (IB4−; n = 102, N = 5) in the presence of 5-HT. These data were transcribed from C (+5-HT) after sorting cells by the differences in IB4 staining.
It has been reported that nociceptors can be divided into two main groups; one class expresses trkA receptors and contains neuropeptides such as calcitonin gene-related peptide and substance P, and the other depends on glial cell line-derived neurotrophic factor (GDNF) and contains few neuropeptides but binds isolectin IB4 (Molliver et al. 1997; Bennett et al. 1998). Differential response properties of IB4-positive and -negative sensory neurons have been reported (Dirajlal et al. 2003; Liu et al. 2004; Breese et al. 2005). In the present results, however, there was no clear difference in the distribution of the histograms between IB4-positive and -negative neurons (Fig. 1D).
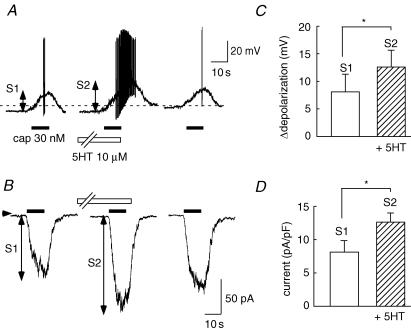
Using the whole-cell patch-clamp technique, the effects of 5-HT on the membrane potential change and inward current in response to capsaicin were investigated. Under current-clamp conditions, the resting membrane potential was −53.9 ± 0.7 and capsaicin (30 nm) depolarized membrane potentials (Fig. 2A). The resting membrane potential did not change in the presence of 10 μm 5-HT (−51.5 ± 0.8 mV, n = 24). After the application of 5-HT, the depolarization induced by capsaicin was clearly increased in 9 of 24 cells, and in 3 cells prominent spike discharges were superimposed on the sustained depolarization (Fig. 2A). Summarized changes in capsaicin-induced depolarization in the absence and the presence of 5-HT are shown in Fig. 2C. Under voltage-clamp conditions, capsaicin evoked an inward current (8.1 ± 1.9 pA pF−1, n = 39) at a holding potential of −60 mV. An obvious augmentation of capsaicin-induced current by 5-HT was observed in 12 of 39 cells. The peak amplitude of capsaicin-induced inward current was significantly increased in the presence of 5-HT (Fig. 2D).
Figure 2. 5-HT potentiates depolarization and the inward current induced by capsaicin.
A, under current-clamp conditions, capsaicin (30 nm) was repetitively applied before and during the application of 5-HT (10 μm), and after its washout. The dashed line indicates the −40 mV level. In this cell, 5-HT increased capsaicin-induced depolarization concomitant with a marked spike discharge. B, at a holding potential of −60 mV, capsaicin evoked inward currents that were augmented by 5-HT. Change in amplitude of membrane potentials (C, n = 24, N = 4) and inward currents (D, n = 39, N = 5) induced by capsaicin before (S1) and after the application of 5-HT (S2). * P < 0.05.
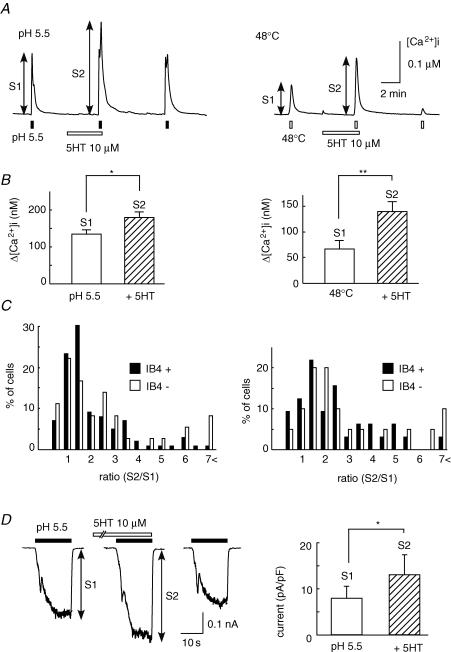
TRPV1 is known as a polymodal receptor, that is activated not only by the vanilloid agonist capsaicin, but also by protons and noxious heat (Caterina et al. 1997; Tominaga et al. 1998). We next examined whether 5-HT produced potentiating effects on the proton- or heat-induced [Ca2+]i responses. As shown in Fig. 3A, a brief application of protons (pH 5.5) or heat (48°C) produced a transient [Ca2+]i increase. After the application of 5-HT (10 μm), each [Ca2+]i response was significantly enhanced (Fig. 3B). Similar to the responses to capsaicin, these potentiating responses occurred in both IB4-positive and -negative neurons (Fig. 3C).
Figure 3. Enhancement of [Ca2+]i responses to protons and noxious heat by 5-HT.
A, representative [Ca2+]i responses to protons (pH 5.5, left) and noxious heat (48°C, right) before and during the application of 5-HT (10 μm), and after its removal. B, summarized changes in [Ca2+]i induced by protons (left, n = 119, N = 4) and noxious heat (right, n = 77, N = 4), before (S1) and after the application of 5-HT (S2). * P < 0.05, ** P < 0.01. C, histograms of the percentage of cells–S2/S1 ratio relation obtained by stimulation with protons (left) and noxious heat (right), between IB4-positive (IB4+) and -negative neurons (IB4−) in the presence of 5-HT. D, representative currents induced by protons (pH 5.5, 15 s) at −60 mV before (S1), during the application of 5-HT (10 μm, S2) and after its removal, and summarized results (n = 25, N = 4). * P < 0.05.
The effects of 5-HT on proton-induced currents at a holding potential of −60 mV were also examined (Fig. 3D). An application of protons (pH 5.5) elicited three distinct inward currents in neonatal rat DRG neurons: a transient current with a rapid inactivation (3 of 25 cells), a transient current followed by a sustained current (12 of 25 cells, shown in Fig. 3D) and only a sustained current (10 of 25 cells). Similar current patterns have been reported in adult rat DRG neurons (Liu et al. 2004). In the presence of 5-HT (10 μm), a sustained component of inward current evoked by protons was significantly augmented. These results indicate that 5-HT potentiates TRPV1 functions in rat DRG neurons.
Involvement of PKC and PKA in the potentiating effect of 5-HT on TRPV1
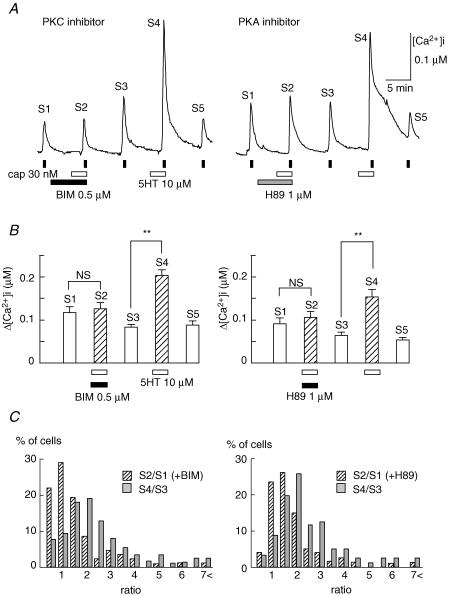
It is known that either BK or PGE2 sensitizes TRPV1 functions in adult rat DRG neurons through the activation of PKC (Cesare & McNaughton, 1996; Cesare et al. 1999; Premkumar & Ahern, 2000) and cAMP/PKA pathways (Lopshire & Nicol, 1998; Gu et al. 2003; Moriyama et al. 2003), respectively. To investigate the mechanism underlying 5-HT-induced augmentations of TRPV functions, we examined the effects of bisindoylmaleimide I (BIM, 0.5 μm), a PKC inhibitor, and H89 (1 μm), a PKA inhibitor, on the potentiating action of 5-HT. As shown in Fig. 4A, each inhibitor was applied for 4 min before 5-HT treatment to the end of the second application of capsaicin. Since the extent of the 5-HT-induced potentiating effect was different among neurons (Fig. 1C), the effects of these kinase inhibitors were estimated by using the data from cells in which clear potentiation by 5-HT occurred after the removal of these inhibitors. Figure 4B depicts summarized data showing that these inhibitors suppressed 5-HT-induced potentiation of [Ca2+]i responses to capsaicin (S1 versus S2). Figure 4C shows histograms of the percentage of cells with the indicated ratio of capsaicin-induced responses in the presence (S2/S1) and after the removal (S4/S3) of BIM or H89. The population of neurons shows that the ratio (>1.5) was reduced from 73.3% to 30.9% by BIM and from 77.6% to 36.2% by H89, and the percentage of cells–ratio relation was shifted leftward. However, even in the presence of these inhibitors, there were neurons showing significant potentiation to an extent almost equal to that after the removal of inhibitors (21 of 84 neurons for BIM, 15 of 58 cells for H89). Moreover, BIM seemed to reduce responses to capsaicin, because 22.8% of cells demonstrated a ratio <0.5 in the presence of BIM but not H89. Taken together, these results suggest that both PKC and PKA pathways are involved in the potentiating effect of 5-HT on TRPV1 functions in rat neonatal DRG neurons.
Figure 4. Effects of inhibitors of PKC and PKA on capsaicin-induced [Ca2+]i increases.
A, representative [Ca2+]i increases induced by capsaicin (cap, 30 nm) in the presence and absence of a kinase inhibitor and/or 5-HT (10 μm). Bisindoylmaleimide I (BIM, 0.5 μm), a PKC inhibitor, and H89 (1 μm), a PKA inhibitor, were used. Cells were stimulated by capsaicin 5 times. The second stimulation was carried out in the presence of 5-HT with a kinase inhibitor and the fifth stimulation in the presence of 5-HT without it. The changes in [Ca2+]i in response to each application of capsaicin, shown as S1–S5, are summarized in B. BIM, n = 84, N = 5; H89, n = 58, N = 4; NS, not significant, ** P < 0.01. C, histograms of the percentage of cells–ratio relations; data obtained in the presence and absence of a kinase inhibitor (S2/S1) and after its washout (S4/S3).
5-HT receptor subtypes in rat DRG neurons
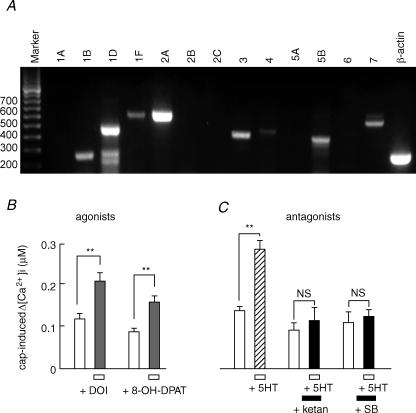
To identify the subtypes of 5-HT receptors expressed in the rat DRG, RT-PCR using 13 pairs of primers for 5-HT receptors was performed. The major PCR products detected in rat DRG were 5-HT1B, 5-HT1D, 5-HT1F, 5-HT2A, 5-HT3, 5-HT4, 5-HT5B and 5-HT7 (Fig. 5A). Based on the examination of intracellular signalling, PKC and PKA systems were suggested to play a role in the potentiating effects of 5-HT on TRPV1. Among the 5-HT receptors detected by PCR, 5-HT2A and 5-HT7 are coupled to Gq/11-PLC/PKC and Gs-adenylate cyclase (AC)/PKA pathways, respectively (Hoyer et al. 2002; Alexander et al. 2006). Therefore, to examine the possibility that 5-HT2A and 5-HT7 receptors were involved in the potentiating effects of 5-HT, we first carried out a pharmacological study using selective agonists and antagonists. A protocol similar to that shown in Fig. 1A was used to investigate whether agonists to 5-HT2A and 5-HT7 receptors produced potentiating effects on TRPV1 function. Changes in the [Ca2+]i increase in response to capsaicin before and after treatment with (±)-2,5-dimethoxy-4-iodoamphetamine (DOI, 20 μm), a 5-HT2A agonist, or (±)-8-hydroxy-2(di-n-propylamino)tetralin hydrobromide (8-OH-DPAT, 20 μm), a 5-HT7 agonist, are summarized in Fig. 5B. Both agonists elicited significant potentiation of the capsaicin-induced [Ca2+]i increase.
Figure 5. Involvement of 5-HT2A and 5-HT7 receptors in the potentiating effects of 5-HT.
A, RT-PCR analysis of 5-HT receptor mRNA. Specific mRNA primers for each 5-HT receptor and β-actin were used for RT-PCR analysis of cDNA synthesis from total RNA of neonatal rat DRG. The expected size of mRNA for each subtype was detected by each primer. Size markers (bp) are shown on the left. B, effects of 5-HT2A and 5-HT7 agonists on the capsaicin-induced [Ca2+]i increases. DOI (20 μm, n = 20, N = 3), a 5-HT2A agonist and 8-OH-DPAT (20 μm, n = 52, N = 4), a 5-HT7 agonist, were used. The panel shows summarized [Ca2+]i increases induced by capsaicin (30 nm) before and during the application of each 5-HT agonist. C, inhibitory effects of 5-HT antagonists on 5-HT-induced potentiation of [Ca2+]i responses to capsaicin. Ketanserin (+ketan, 20 μm, n = 38, N = 4), a 5-HT2A antagonist, and SB269970 (+SB, 20 μm, n = 46, N = 4), a 5-HT7 antagonist, were used. 5-HT significantly potentiated the capsaicin-induced [Ca2+]i increases but it was suppressed by the simultaneous application of a 5-HT2A or 5-HT7 antagonist. ** P < 0.01; NS, not significant. For the experimental protocol, see Results.
Next, we examined the effects of 5-HT2A and 5-HT7 antagonists on 5-HT-induced potentiation of responses to capsaicin. For these experiments, we used a protocol similar to that shown in Fig. 4A. Figure 5C depicts summarized data in the presence and absence of ketanserin (20 μm), a 5-HT2A antagonist, and SB269970 (20 μm), a 5-HT7 antagonist. After exposure to these antagonists, the sensitizing effects of 5-HT were significantly suppressed. The population of neurons showing a ratio > 1.5 was reduced from 78.9% to 48.3% for ketanserin and 74.4% to 36.4% for SB269970. However, even in the presence of each inhibitor there were neurons showing potentiation to the same extent as that after the removal of the inhibitor (8 of 38 cells for ketanserin, 6 of 46 cells for SB269970). These results suggest that 5-HT2A and 5-HT7 receptors are partly involved in the TRPV1 potentiation by 5-HT in rat neonatal DRG neurons.
Change of 5-HT action under inflammatory conditions
To examine the role of 5-HT in the hyperalgesia following peripheral inflammation, we investigated whether the sensitizing effects of 5-HT were changed by inflammation. Inflammatory model animals were made by the injection of complete Freund's adjuvant (CFA) into the right hemilateral hind paw of the neonatal rat. The same volume of saline was injected into the left hemilateral hind paw as a control. Four days after CFA administration, the limb in the CFA-administrated side was prominently swollen in macroscopic observation (Fig. 6A). Histological analysis demonstrated typical inflammation images with the accumulation and invasion of leucocytes in the inflammatory side compared with the control side (Fig. 6B). We isolated DRG of the L4–L6 segment, which innervates the hind limb area, synthesized cDNA, and then performed RT-PCR analysis. As shown in Fig. 6C, mRNA expression of 5-HT2A and 5-HT7, but not 5-HT1D, increased in DRG of the inflamed side. Similar changes in 5-HT receptor expression were observed in four other animals. Quantitative analysis for these PCR products showed significant increases of 5-HT2A and 5-HT7 receptor mRNAs in DRG of the inflammation site (Fig. 6D).
To determine whether functional changes were accompanied by increases in mRNA expression, DRG from both sides were isolated from rats injected with CFA and the potentiating effects of 5-HT were compared. In these experiments, acute isolated DRG neurons were used. A protocol similar to that shown in Fig. 1A was used to estimate possible changes in the TRPV1 potentiation effects of 5-HT. As shown in Fig. 6E, the ratio of potentiation (S2/S1) in DRG neurons isolated from the inflammation side was significantly larger than that from the control side. The histograms of the percentage of cells–ratio relation in DRG neurons of inflammation and control sides are shown in Fig. 6F. There was no significant difference in the magnitude of the capsaicin-induced [Ca2+]i increase in DRG neurons between the inflammation and control sides. These results suggest that the increase of the biosynthesis of at least 5-HT2A and 5-HT7 receptor subtypes may lead to the potentiation of TRPV1 function induced by 5-HT.
Discussion
In the present experiment, 5-HT had a sensitizing action on TRPV1 functions in sensory neurons, since it was capable of potentiating [Ca2+]i increases in response to capsaicin, protons and noxious heat. Membrane depolarization and the inward current induced by capsaicin were also increased by 5-HT. Pharmacological and molecular analyses suggested that at least 5-HT2A and 5-HT7 receptors were involved in 5-HT-induced TRPV1 sensitization through PKC and PKA pathways. Under inflammatory conditions, the expression levels of these 5-HT receptor subtypes increased, and the potentiating effect of 5-HT was augmented, suggesting that sensitization of TRPV1 by 5-HT occurs more markedly under inflammatory conditions. The sensitizing effects of 5-HT on TRPV1 functions may be partly involved in the 5-HT-induced hyperalgesic responses in vivo.
In this study, it was clarified that the [Ca2+]i increase induced by capsaicin was increased by 5-HT in more than 70% of capsaicin-sensitive cells regardless of IB4 staining, though nociceptors having different responsiveness can be cytochemically subdivided by IB4 staining (Dirajlal et al. 2003; Liu et al. 2004; Breese et al. 2005). Similarly, there was no relation between IB4 staining and the potentiating effects of 5-HT on proton- and heat-induced [Ca2+]i responses. Therefore, functional differences reflected by IB4 staining were not associated with the TRPV1 potentiating effects of 5-HT in neonatal rat DRG neurons.
In addition to the augmentation of [Ca2+]i responses to capsaicin, membrane depolarization and current responses to capsaicin were also increased by 5-HT. Under current-clamp conditions, capsaicin-induced depolarization was enhanced by 5-HT. In some cells, capsaicin produced a prominent spike discharge superimposed on the increased sustained depolarization in the presence of 5-HT. Moreover, 5-HT augmented proton-induced inward currents. In many DRG neurons, protons evoked inward currents with different kinetics composed of transient and sustained currents. It is reported that a component of the transient inward current evoked by protons is mediated by the activation of acid-sensing ion channels (ASIC), because of being blocked by amiloride (Dirajlal et al. 2003; Liu et al. 2004). In contrast, the sustained component is mediated predominantly by TRPV1 (Liu et al. 2004). In the present results, a sustained component of proton-induced inward current was augmented by 5-HT. This may provide further evidence that 5-HT potentiates TRPV1-mediated responses. Different amino acid residues are responsible for the recognition of capsaicin and protons (Jordt et al. 2000). Therefore, it is likely that 5-HT increases TRPV1 function regardless of activating factors. These results suggest that 5-HT may participate in the inflammatory hyperalgesia elicited by extracellular acid and heat during inflammation. Under patch-clamp conditions, the percentage of cells showing potentiating effects by 5-HT was smaller (30–40%) than those in [Ca2+]i imaging experiments (∼70%). It may be due to washout of some cellular components related to 5-HT-induced potentiation of TRPV1 function under patch-clamp conditions.
TRPV1 functions are reported to be potentiated through the PLC/PKC (Cesare et al. 1999; Premkumar & Ahern, 2000) and cAMP/PKA pathways (Lopshire & Nicol, 1998; Gu et al. 2003; Moriyama et al. 2003), respectively. Phosphorylation of TRPV1 by these kinases is related to the TRPV1 sensitization (Numazaki et al. 2002; Wang et al. 2004; Ferreira et al. 2005), and putative phosphorylation sites have been demonstrated (Numazaki et al. 2002; Bhave et al. 2002; Mohapatra & Nau, 2003). A PKC inhibitor (BIM) or PKA inhibitor (H89) suppressed the TRPV1 sensitization induced by 5-HT, indicating that both kinase pathways are involved in the 5-HT-induced TRPV1 potentiation in rat DRG neurons. However, there was some diversity in the extent of the inhibitory actions of BIM and H89. This may be due to the differences of dominant signalling pathways in neurons or differences in the expression levels of 5-HT receptors coupled to these intracellular signallings. Alternatively, PKC-independent mechanisms may be involved in the 5-HT-induced TRPV1 potentiation, because BK and NGF stimulate PLC and promote hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2), relieving TRPV1 from PIP2-mediated inhibition (Chuang et al. 2001). It has been also reported that phosphatidylinositol-3-kinase and calcium–calmodulin-dependent protein kinase II are important in mediating the sensitization of TRPV1 (Bonnington & McNaughton, 2003). The BIM treatment produced not only suppression of the potentiation effect of 5-HT but also reduced the capsaicin-induced [Ca2+]i increase. PKCγ, an important isoform in the increased pain sensitivity sensitive to Ca2+ (Martin et al. 2001), can be activated by Ca2+ influx through highly Ca2+-permeable TRPV1 channels. It has also been suggested that background PKC activity is sufficient to phosphorylate TRPV1 (Vellani et al. 2001) and BIM attenuates the capsaicin-evoked current in rat DRG neurons due to inhibition of basal phosphorylation of TRPV1 (Zhou et al. 2001).
RT-PCR analysis demonstrated that 5-HT1B, 5-HT1D, 5-HT1F, 5-HT2A, 5-HT3, 5-HT4, 5-HT5B and 5-HT7 receptor subtypes were expressed in neonatal rat DRG neurons. These results are essentially consistent with the data reported for adult DRG neurons (Wu et al. 2001). The absence of 5-HT1A mRNA is demonstrated in rat lumbar DRG (Chen et al. 1998). Pierce et al. 1996) reported that 5-HT2C, but not 5-HT1F, 5-HT4 or 5-HT5B, are present in adult rat DRG. The reasons for these differences are not clear, but they might be due to the age of the animals or sites of DRG tested. The present results were consistent with previous in situ hybridization and immunohistochemical data showing that 5-HT1B (Doucet et al. 1996), 5-HT2A (Tokunaga et al. 1998), 5-HT3 (Tecott et al. 1993; Kia et al. 1995) and 5-HT7 (Meuser et al. 2002; Doly et al. 2005) are present in rat DRG.
It has been reported that various 5-HT receptor subtypes are related to 5-HT-induced hyperalgesia, as mentioned in the Introduction. Ionotropic 5-HT3 receptors, which when activated promote [Ca2+]i increase, are suggested to be responsible for 5-HT-induced pain and hyperalgesia (Eschalier et al. 1989; Giordano & Rogers, 1989). In the current study, although a few neurons showed a [Ca2+]i increase in response to 5-HT, there was no relation between the 5-HT-induced [Ca2+]i increase and the potentiating action on TRPV1. Therefore, it is unlikely that the potentiating effects of 5-HT on TRPV1 are mediated by ionotropic 5-HT3 receptors. The 5-HT2A receptor is coupled to the PLC/PKC cascade through Gq/11 protein and the 5-HT7 receptor to the AC/PKA cascade through Gs protein (Hoyer et al. 2002; Alexander et al. 2006). In the present results, the sensitization action of 5-HT was mimicked by the 5-HT2A agonist DOI and the 5-HT1A/7 agonist 8OH-DPAT. Furthermore, 5-HT-induced potentiation of the [Ca2+]i response to capsaicin was significantly suppressed by antagonists for 5-HT2A and 5-HT7. 8-OH-DPAT has a high affinity for both 5-HT1A and 5-HT7 receptors, and 5-HT1A and 5-HT7 receptors have been shown to have similar pharmacological profiles (Shen et al. 1993). In the present study, since RT-PCR analysis revealed the absence of 5-HT1A receptor mRNA, it is likely that the potentiating effect of 8OH-DPAT on capsaicin-induced [Ca2+]i increases was mediated by 5-HT7 receptors. In the rat, production of c-fos in the spinal dorsal horn is increased when a 5-HT7 receptor agonist is administered (Meuser et al. 2002). The present data suggest that 5-HT7 receptors in part play a role in hyperalgesia induced by 5-HT. It has been reported that TRPV1 function in mouse colon sensory neurons is enhanced by 5-HT through 5-HT2 and 5-HT4 receptors (Sugiura et al. 2004). In the rat, pain induced by noxious fever, intraperitoneal injection of acetic acid and inflammation of the foot is mediated by 5-HT4 (Espejo & Gill, 1998). Moreover, the present RT-PCR analysis demonstrated the presence of several 5-HT receptors other than 5-HT2A and 5-HT7. Therefore, it leaves open the possibility of the involvement of other 5-HT receptor subtypes in TRPV1 sensitization in rat DRG.
By the administration of CFA to the hind paw, remarkable inflammatory responses occurred. Significant increases of the expression levels of 5-HT2A and 5-HT7 receptor mRNAs, but not that of 5-HT1D, were observed in DRG of the ipsilateral CFA-administered side compared to the contralateral control side. The present results are consistent with a previous report that 5-HT1A, 5-HT1B, 5-HT1F, 5-HT2A, 5-HT3, 5-HT4 and 5-HT7 receptor mRNA expression levels are increased by CFA inflammation in adult rat DRG (Wu et al. 2001). Neurotrophic factors such as NGF (Woolf, 1996) and BDNF (brain-derived neurotrophic factor; Mannion et al. 1999) may contribute to the changes in expression of various proteins under inflammation. In addition, we found that TRPV1 augmentation by 5-HT was more marked in DRG neurons of the inflammation side. These results suggest that the hypersensitivity of nociceptors induced by 5-HT may be mediated by increased expression levels of 5-HT receptor subtypes, at least 5-HT2A and 5-HT7, under inflammatory conditions. The present findings provide information about potential peripheral mechanisms that contribute to inflammatory hyperalgesia mediated by 5-HT. Additional studies are needed to determine whether inhibition of TRPV1 functions or their modulation through 5-HT and/or other signalling pathways may be useful therapeutic targets in the treatment of inflammatory pain syndromes.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan. We would like to thank Ms Y. Izumi for technical support in data analysis.
References
- Abbott FV, Franklin KB, Westbrook RF. The formalin test: scoring properties of the first and second phases of the pain response in rats. Pain. 1995;60:91–102. doi: 10.1016/0304-3959(94)00095-V. [DOI] [PubMed] [Google Scholar]
- Abbott FV, Hong Y, Blier P. Activation of 5-HT2A receptors potentiates pain produced by inflammatory mediators. Neuropharmacology. 1996;35:99–110. doi: 10.1016/0028-3908(95)00136-0. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and chemicals, 7 TM receptors. Br J Pharmacol. 2006;147:S5–S81. doi: 10.1038/sj.bjp.0706651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck PW, Handwerker HO. Bradykinin and serotonin effects on various types of cutaneous nerve fibers. Pflugers Arch. 1974;347:209–222. doi: 10.1007/BF00592598. [DOI] [PubMed] [Google Scholar]
- Bennett DL, Michael GJ, Ramachandran N, Munson JB, Averill S, Yan Q, McMahon SB, Priestley JV. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J Neurosci. 1998;18:3059–3072. doi: 10.1523/JNEUROSCI.18-08-03059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertelsen AK, Afrah AW, Gustafsson H, Tjolsen A, Hole K, Stiller C-O. Stimulation of spinal 5-HT2A/2C receptors potentiates the capsaicin-induced in vivo release of substance P-like immunoreactivity in the rat dorsal horn. Brain Res. 2003;987:10–16. doi: 10.1016/s0006-8993(03)03216-5. [DOI] [PubMed] [Google Scholar]
- Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW., 4th cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. doi: 10.1016/s0896-6273(02)00802-4. [DOI] [PubMed] [Google Scholar]
- Bonnington JK, McNaughton PA. Signalling pathways involved in the sensitisation of mouse nociceptive neurones by nerve growth factor. J Physiol. 2003;551:433–446. doi: 10.1113/jphysiol.2003.039990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese NM, George AC, Pauers LE, Stucky CL. Peripheral inflammation selectively increases TRPV1 function in IB4-positive sensory neurons from adult mouse. Pain. 2005;115:37–49. doi: 10.1016/j.pain.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Peterson-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Cesare P, Dekker LV, Sardini A, Parker PJ, McNaughton PA. Specific involvement of PKCɛ in sensitization of the neuronal response to painful heat. Neuron. 1999;23:617–624. doi: 10.1016/s0896-6273(00)80813-2. [DOI] [PubMed] [Google Scholar]
- Cesare P, McNaughton P. A novel heat-activated current in nociceptive neurons and its sensitization by bradykinin. Proc Natl Acad Sci U S A. 1996;93:15435–15439. doi: 10.1073/pnas.93.26.15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Vasko MR, Wu X, Staeva TP, Baez M, Zgombick JM, Nelson DL. Multiple subtypes of serotonin receptors are expressed in rat sensory neurons in culture. J Pharmacol Exp Ther. 1998;287:1119–1127. [PubMed] [Google Scholar]
- Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5),P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- Davis JB, Gray J, Cunthorpe MJ, Hather JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- Dirajlal S, Pauers LE, Stucky CL. Differential response properties of IB4-positive and -negative unmyelinated sensory neurons to protons and capsaicin. J Neurophysiol. 2003;89:513–524. doi: 10.1152/jn.00371.2002. [DOI] [PubMed] [Google Scholar]
- Doly S, Fischer J, Brisorgueil MJ, Verge D, Conrath M. Pre- and postsynaptic localization of the 5-HT7 receptor in rat dorsal spinal cord: immunocytochemical evidence. J Comp Neurol. 2005;490:256–269. doi: 10.1002/cne.20667. [DOI] [PubMed] [Google Scholar]
- Doucet E, Pohl M, Fattaccini CM, Adrien J, Mestikawy SE, Hamon M. In situ hybridization evidence for the synthesis of 5-HT1B receptor in serotonergic neurons of anterior raphe nuclei in the rat brain. Synapse. 1996;19:18–28. doi: 10.1002/syn.890190104. [DOI] [PubMed] [Google Scholar]
- Ebersberger A, Anton F, Tölle TR, Zieglgänsberger W. Morphine, 5-HT2 and 5-HT3 receptor antagonists reduce c-fos expression in the trigeminal nuclear complex following noxious chemical stimulation of the rat nasal mucosa. Brain Res. 1995;676:336–342. doi: 10.1016/0006-8993(95)00118-a. [DOI] [PubMed] [Google Scholar]
- Ernberg M, Lundeberg T, Kopp S. Effect of propranolol and granisetron on experimentally induced pain and allodynia/hyperalgesia by intramuscular injection of serotonin into the human masseter muscle. Pain. 2000;84:339–346. doi: 10.1016/s0304-3959(99)00221-3. [DOI] [PubMed] [Google Scholar]
- Eschalier A, Kayser V, Guilbaud G. Influence of a specific 5-HT3 antagonist on carrageenan-induced hyperalgesia in rat. Pain. 1989;36:249–255. doi: 10.1016/0304-3959(89)90030-4. [DOI] [PubMed] [Google Scholar]
- Espejo EF, Gill E. Antagonism of peripheral 5-HT4 receptors reduces visceral and cutaneous pain in mice, and induces visceral analgesia after simultaneous inactivation of 5-HT3 receptor. Brain Res. 1998;788:20–24. doi: 10.1016/s0006-8993(97)01510-2. [DOI] [PubMed] [Google Scholar]
- Ferreira J, Triches KM, Medeiros R, Calixto JB. Mechanisms involved in the nociception produced by peripheral protein kinase c activation in mice. Pain. 2005;117:171–181. doi: 10.1016/j.pain.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Giordano J, Rogers LV. Peripherally administered serotonin 5-HT3 receptor antagonists reduce inflammatory pain in rats. Eur J Pharmacol. 1989;28:423–427. doi: 10.1016/0014-2999(89)90137-4. [DOI] [PubMed] [Google Scholar]
- Gu Q, Kwong K, Lee L-Y. Ca2+ transient evoked by chemical stimulation is enhanced by PGE2 in vagal sensory neurons: role of cAMP/PKA signaling pathway. J Neurophysiol. 2003;89:1985–1993. doi: 10.1152/jn.00748.2002. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Jordt S-E, Tominaga M, Julius D. Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc Natl Acad Sci U S A. 2000;97:8134–8139. doi: 10.1073/pnas.100129497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kia HK, Miquel MC, McKernan RM, Laporte AM, Lombard MC, Bourgoin S, Hamon M, Verge D. Localization of the 5-HT3 receptors in the rat spinal cord: immunohistochemistry and in situ hybridization. Neuroreport. 1995;5:257–261. doi: 10.1097/00001756-199501000-00008. [DOI] [PubMed] [Google Scholar]
- Kress M, Reeh PW. Chemical excitation and sensitization in nociceptors. In: Cervero F, Belmonte C, editors. Neurobiology of Nociceptors. New York: Oxford University Press; 1996. pp. 258–297. [Google Scholar]
- Lehtosalo JI, Uusitalo H, Laakso J, Palkama A, Harkonen M. Biochemical and immunohistochemical determination of 5-hydroxytryptamine located in mast cells in the trigeminal ganglion of the rat and guinea pig. Histochemistry. 1984;80:219–223. doi: 10.1007/BF00495769. [DOI] [PubMed] [Google Scholar]
- Liu M, Willmott NJ, Michael GJ, Priestley JV. Differential pH and capsaicin responses of Griffonia simplicifolia IB4 (IB4)-positive and IB4-negative small sensory neurons. Neuroscience. 2004;127:659–672. doi: 10.1016/j.neuroscience.2004.05.041. [DOI] [PubMed] [Google Scholar]
- Liu ZY, Zhuang DB, Lunderberg T, Yu LC. Involvement of 5-hydroxytryptamine1A receptors in the descending anti-nociceptive pathway from periaqueductal gray to the spinal dorsal horn in intact rats, rats with nerve injury and rats with inflammation. Neuroscience. 2002;112:399–407. doi: 10.1016/s0306-4522(02)00038-6. [DOI] [PubMed] [Google Scholar]
- Lopshire JC, Nicol GD. The cAMP transduction cascade mediates the prostaglandin E2 enhancement of the capsaicin-elicited current in rat sensory neurons: whole-cell and single-channel studies. J Neurosci. 1998;18:6081–6092. doi: 10.1523/JNEUROSCI.18-16-06081.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannion RJ, Costigan M, Decosterd I, Amaya F, Ma QP, Holstege JC, Ji RR, Acheson A, Lindsay RM, Wilkinson GA, Woolf CJ. Neurotrophins: peripherally and centrally acting modulators of tactile stimulus-induced inflammatory pain hypersensitivity. Proc Natl Acad Sci U S A. 1999;96:9385–9390. doi: 10.1073/pnas.96.16.9385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WJ, Malmberg AB, Basbaum AI. PKCγ contributes to a subset of the NMDA-dependent spinal circuits that underlie injury-induced persistent pain. J Neurosci. 2001;21:5321–5327. doi: 10.1523/JNEUROSCI.21-14-05321.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuser T, Pietruck C, Gabriel A, Xie G-X, Lim K-J, Palmer PP. 5-HT7 receptors are involved in mediating 5-HT-induced activation of rat primary afferent neurons. Life Sci. 2002;71:2279–2289. doi: 10.1016/s0024-3205(02)02011-8. [DOI] [PubMed] [Google Scholar]
- Mohapatra DP, Nau C. Desensitization of capsaicin-activated currents in the vanilloid receptor TRPV1 is decreased by the cyclic AMP-dependent protein kinase pathway. J Biol Chem. 2003;278:50080–50090. doi: 10.1074/jbc.M306619200. [DOI] [PubMed] [Google Scholar]
- Molliver DC, Wright DE, Leitner ML, Parsadanian AS, Doster K, Wen D, Yan Q, Snider WD. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron. 1997;19:849–861. doi: 10.1016/s0896-6273(00)80966-6. [DOI] [PubMed] [Google Scholar]
- Moriyama T, Higashi T, Togashi K, Iida T, Segi E, Sugimoto Y, Tominaga T, Narumiya S, Tominaga M. Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Mol Pain. 2005;1:3. doi: 10.1186/1744-8069-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama T, Iida T, Kobayashi K, Higashi T, Fukuoka T, Tsumura H, Leon C, Suzuki N, Inoue K, Gachet C, Noguchi K, Tominaga M. Possible involvement of P2Y2 metabotropic receptors in ATP-induced transient receptor potential vanilloid receptor 1-mediated thermal hypersensitivity. J Neurosci. 2003;23:6058–6062. doi: 10.1523/JNEUROSCI.23-14-06058.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numazaki M, Tominaga T, Tominaga M. Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cɛ and identification of two target serine residues. J Biol Chem. 2002;277:13375–13378. doi: 10.1074/jbc.C200104200. [DOI] [PubMed] [Google Scholar]
- Ohta T, Komatsu R, Imagawa T, Otsuguro K, Ito S. Molecular cloning, functional characterization of the porcine transient receptor potential V1 (pTRPV1) and pharmacological comparison with endogenous pTRPV1. Biochem Pharmacol. 2005a;71:173–187. doi: 10.1016/j.bcp.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Ohta T, Kubota A, Murakami M, Otsuguro K, Ito S. P2X2 receptors are essential for [Ca2+]i increases in response to ATP in cultured rat myenteric neurons. Am J Physiol Gastrointest Liver Physiol. 2005b;289:G935–G948. doi: 10.1152/ajpgi.00017.2005. [DOI] [PubMed] [Google Scholar]
- Ohta T, Wakade AR, Nakazato Y, Ito S. Ca2+-dependent K+ current and exocytosis in responses to caffeine and muscarine in voltage-clamped guinea-pig adrenal chromaffin cells. J Neurochem. 2001;78:1243–1255. doi: 10.1046/j.1471-4159.2001.00502.x. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Imbe H, Morikawa Y, Itoh M, Sekimoto M, Nemoto K, Senba E. 5-HT2A receptor subtype in the peripheral branch of sensory fibers is involved in the potentiation of inflammatory pain. Pain. 2002;99:133–143. doi: 10.1016/s0304-3959(02)00070-2. [DOI] [PubMed] [Google Scholar]
- Pierce PA, Xie GX, Levine JD, Peroutka SJ. 5-Hydroxytryptamine receptor subtype messenger RNAs in rat peripheral sensory and sympathetic ganglia: a polymerase chain reaction study. Neuroscience. 1996;70:553–559. doi: 10.1016/0306-4522(95)00329-0. [DOI] [PubMed] [Google Scholar]
- Premkumar LS, Ahern GP. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985–990. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- Ruda MA, Ling QD, Hohmann AG, Peng YB, Tachibana T. Altered nociceptive neuronal circuits after neonatal peripheral inflammation. Science. 2000;289:628–631. doi: 10.1126/science.289.5479.628. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Schmidt R, Weidner C, Hilliges M, Torebjörk HE, Handwerker HO. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. J Neurophysiol. 2003;89:2441–2448. doi: 10.1152/jn.01139.2002. [DOI] [PubMed] [Google Scholar]
- Shen Y, Monsma FJ, Jr, Metcalf MA, Jose PA, Hamblin MW, Sibley DR. Molecular cloning and expression of a 5-hydroxytryptamine 7 serotonin receptor subtype. J Biol Chem. 1993;268:18200–18204. [PubMed] [Google Scholar]
- Sorkin LS, McAdoo DJ, Willis WD. Raphe magnus stimulation-induced antinociception in the cat is associated with release of amino acids as well as serotonin in the lumbar dorsal horn. Brain Res. 1993;618:95–108. doi: 10.1016/0006-8993(93)90433-n. [DOI] [PubMed] [Google Scholar]
- Sufka KJ, Schomburg FM, Giordano J. Receptor mediation of 5-HT-induced inflammation and nociception in rats. Pharmacol Biochem Behav. 1992;41:53–56. doi: 10.1016/0091-3057(92)90058-n. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Bielefeldt K, Gebhart GF. TRPV1 function in mouse colon sensory neurons is enhanced by metabotropic 5-hydroxytryptamine receptor activation. J Neurosci. 2004;24:9521–9530. doi: 10.1523/JNEUROSCI.2639-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Tominaga M, Katsuya H, Mizumura K. Bradykinin lowers the threshold temperature for heat activation of vanilloid receptor 1. J Neurophysiol. 2002;88:544–548. doi: 10.1152/jn.2002.88.1.544. [DOI] [PubMed] [Google Scholar]
- Taiwo YO, Levine JD. Serotonin is a directly-acting hyperalgesic agent in the rat. Neuroscience. 1992;48:485–490. doi: 10.1016/0306-4522(92)90508-y. [DOI] [PubMed] [Google Scholar]
- Tecott LH, Maricq AV, Julius D. Nervous system distribution of the serotonin 5-HT3 receptor mRNA. Proc Natl Acad Sci U S A. 1993;90:1430–1434. doi: 10.1073/pnas.90.4.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga A, Saika M, Senba E. 5-HT2A receptor subtype is involved in the thermal hyperalgesic mechanism of serotonin in the periphery. Pain. 1998;76:349–355. doi: 10.1016/S0304-3959(98)00066-9. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Vellani V, Mapplebeck S, Moriondo A, Davis JB, McNaughton PA. Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. J Physiol. 2001;534:813–825. doi: 10.1111/j.1469-7793.2001.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Kedei N, Wang M, Wang QJ, Huppler AR, Toth A, Tran R, Blumberg PM. Interaction between protein kinase Cμ and the vanilloid receptor type 1. J Biol Chem. 2004;279:53674–53682. doi: 10.1074/jbc.M410331200. [DOI] [PubMed] [Google Scholar]
- Woolf CJ. Phenotypic modification of primary sensory neurons: the role of nerve growth factor in the production of persistent pain. Philos Trans R Soc Lond B Biol Sci. 1996;351:441–448. doi: 10.1098/rstb.1996.0040. [DOI] [PubMed] [Google Scholar]
- Wu W-X, Zhu M, Wang W, Wang Y-Y, Li Y-Q, Yew DT. Changes of the expression of 5-HT receptor subtype mRNAs in rat dorsal root ganglion by complete Freund's adjuvant-induced inflammation. Neurosci Lett. 2001;307:183–186. doi: 10.1016/s0304-3940(01)01946-2. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Zhou ZS, Zhao ZQ. PKC regulates capsaicin-induced currents of dorsal root ganglion neurons in rats. Neuropharmacology. 2001;41:601–608. doi: 10.1016/s0028-3908(01)00106-x. [DOI] [PubMed] [Google Scholar]