Abstract
It is well established that metabolic inhibition of adrenergic vasoconstriction contributes to the maintenance of adequate perfusion to exercising skeletal muscle. However, little is known regarding nonadrenergic vasoconstriction during exercise. We tested the hypothesis that a non-adrenergic vasoconstrictor, angiotensin II (AngII), would be less sensitive to metabolic inhibition than an α1-agonist, phenylephrine (PE), in the exercising human thigh. In 11 healthy men, femoral blood flow (FBF, ultrasound Doppler and thermodilution) and blood pressure were evaluated during wide-ranging doses of intra-arterial (femoral) infusions of PE and AngII at rest and during two workloads of steady-state knee-extensor exercise (7 W and 27 W). At rest, the maximal decrease in femoral artery diameter (FAD) during AngII (9.0 ± 0.2 to 8.4 ± 0.4 mm) was markedly less than during PE (9.0 ± 0.3 to 5.7 ± 0.5 mm), whereas maximal reductions in FBF and femoral vascular conductance (FVC) were similar during AngII (FBF: −65 ± 6 and FVC: −66 ± 6%) and PE (−57 ± 5 and −59 ± 4%). During exercise, FAD was not changed by AngII, but moderately decreased by PE. The maximal reductions in FBF and FVC were blunted during exercise compared to rest for both AngII (7 W: −28 ± 5 and −40 ± 5%; 27 W: −15 ± 4% and −29 ± 5%) and PE (7 W: −30 ± 4 and −37 ± 6%; 27 W: −15 ± 2 and −24 ± 6%), with no significant differences between drugs. The major new findings are (1) an exercise-induced intensity-dependent metabolic attenuation of non-adrenergic vasoconstriction in the human leg; and (2) functional evidence that AngII-vasoconstriction is predominantly distal, whereas α1-vasoconstriction is proximal and distal within the muscle vascular bed of the human thigh.
Exercise is accompanied by powerful intensity-dependent vasodilation and increases in blood flow to the active muscle (Andersen & Saltin, 1985; Radegran, 1997; Wray et al. 2004). This exercise hyperaemia is challenged by activation of sympatho-adrenergic and nonadrenergic vasoconstrictor systems. During increasing intensities of exercise the concomitant increasing activity in sympathetic vasocontrictor nerves is driven by a combination of central nervous coactivation of motor control and sympathetic outflow (i.e. ‘central command’) and muscle-derived afferents (i.e. ‘mechano- and metaboreflexes’) (Victor et al. 1989, 1995). During higher levels of sympathetic activity, plasma renin acivity increases, leading to increased production of the nonadrenergic vasoconstrictor angiotensin II (AngII) (Staessen et al. 1987; Tidgren et al. 1991; Bocqueraz et al. 2004). Several studies indicate that both resting and exercise-induced sympathetic activity and AngII production are elevated in pathological conditions such as hypertension and heart failure (Kinugawa et al. 1996, 1997; Cheng et al. 2001; Mansfield et al. 2003; Schlaich et al. 2004). In addition, the non-adrenergic vasoconstrictor endothelin-1 seems to have an increased functional significance in patients with cardiovascular disease compared to controls (McEniery et al. 2002; Yousufuddin et al. 2000). Thus, the exercise-induced activation and efficacy of vasodilator and vasoconstrictor systems within skeletal muscle is dependent upon the type and intensity of exercise as well as pathophysiological state.
The interplay between the vasoactive systems results in a complex integrative control of local blood flow within exercising skeletal muscle. In some models of exercise, there is experimental evidence that sympathoexcitation may cause vasoconstriction in the active skeletal muscle (Buckwalter & Clifford, 1999; Joyner et al. 1992). However, it has been acknowledged for decades that the response to adrenergic stimuli may be attenuated in exercising muscle compared to resting muscle (Remensynder et al. 1962), a phenomenon termed functional sympatholysis. More recently, a variety of studies in rodent, canine and human models of exercise have widely agreed that the vasoconstrictor responses to sympathetic nerve activity or intra-arterial sympathomimetic drugs are at least partially inhibited by metabolic products of muscle contraction (Thomas & Victor, 1997; Thomas et al. 1997, 1998, 2001; Hansen et al. 1999; Sander et al. 2000; Dinenno & Joyner, 2003; Buckwalter et al. 2004a; Keller et al. 2004; Wray et al. 2004;). The significance of this phenomenon has been illustrated in rats with experimental heart failure (Thomas et al. 2001) and in children with Duchenne's muscular dystrophy (Sander et al. 2000). In both these conditions, metabolic inhibition of sympathetic vasoconstriction is diminished, which leads to hypoperfusion of the exercising muscles during sympathoexcitation. Thus, subnormal metabolic inhibition of sympathetic vasoconstriction could contribute to development of muscle fatigue. Specifically, it has been hypothesized that this mechanism constitutes part of the ‘peripheral factor’ of exercise intolerance in human heart failure (Hansen et al. 2000).
While the exercise-induced attenuation of adrenergic vasoconstriction has been well characterized, little is known about the extent to which non-adrenergic vasoconstrictor systems are susceptible to the metabolic events of exercise. Thus, the present study focuses on AngII-mediated vasoconstrictor effects in human thigh muscle. Specifically, we hypothesized that in exercising human muscle AngII-vasoconstriction would be significantly less inhibited than α1-vasoconstriction. If proven, this hypothesis would strongly suggest that attenuation of α1-mediated vasoconstriction is related to a specific inhibitory action of metabolic events. If refuted, this would provide evidence in humans that non-adrenergic vasoconstriction is also sensitive to metabolic inhibition. To this end, we measured femoral blood flow directly at rest and during two workloads of dynamic steady-state knee-extensor exercise with superimposed intra-arterial administration of the endogenous angiotensin (AT)-receptor agonist AngII and a selective α1-agonist phenylephrine (PE).
Methods
Subjects and general procedures
Eleven healthy young men (age: 25 ± 2 years; body mass index (BMI), 25 ± 1 kg m−2) gave written informed consent to participate. The study was approved by the local ethics committee of Copenhagen and Frederiksberg and conformed to the Helsinki Declaration. All experiments were performed in a thermoneutral environment, with subjects seated in a semirecumbent position (approximately 45 deg hipflexion). Heart rate (HR) was obtained from an ECG recording (BioAmp, ADInstruments). One-legged knee-extensor exercise was performed at 60 r.p.m. on a modified cycle ergometer as previously described (Anderson & Saltin, 1985; Wray et al. 2004). The knee extensor force and rhythm were recorded via a strain gauge (customized signal processor, FBJ Industries).
Catheterization
Under local anaesthesia (lidocaine; Danish county pharmaceutical corporation), the arterial (Arrow, 20 gauge) and venous (Cook, 18 gauge) catheters were inserted into the femoral artery and vein of the right leg. The catheters were used for intra-arterial drug infusions, direct phasic blood pressure measurements (BP), and femoral blood flow measurements via thermodilution (FBFTD) (Wray et al. 2004). BP was measured at the level of the heart by adjustment of the position of the pressure transducer (Baxter), which was interfaced with a blood pressure amplifier (BPamp, ADInstruments). The thermodilution technique has been previously described and validated in detail (Andersen & Saltin, 1985; Wray et al. 2004), and provides accurate and reproducible measurements of leg blood flow during knee-extensor exercise. Thermodilution was not used during rest because it is not suitable for determination of the low blood flows seen during infusions of vasoconstricting drugs.
Ultrasound imaging and Doppler were used to determine femoral artery diameter (FAD) and blood velocity (FBV) (7.5 MHz mechanical sector transducer, CFM 800, GE Medical). Femoral blood flow by ultrasound (FBFD) was calculated as previously described (Wray et al. 2004).
Drugs
PE (Danish county pharmaceutical corporation) was used as a specific α1-adrenergic agonist. AngII (Clinalfa, Switzerland) was used as an AT-receptor agonist. Drugs were diluted with normal saline and intra-arterial infusion rates ranged from 0.2 to 6 ml min−1.
Experimental protocols
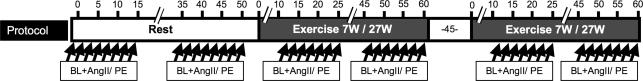
The protocol is outlined in Fig. 1. In all subjects we measured BP, HR, FBF, and FAD during (1) rest; (2) 7 W (low workload); and (3) 27 W one-legged knee-extensor exercise (moderate workload). The resting condition was always first, whereas the order of the two exercise intensities was alternated. Measurements during rest and exercise were obtained before and during continuous intra-arterial infusions of 6–7 incremental doses of PE and AngII. The measurements were made during the last 45 s of each dose (total time was 2–3 min on each dose). The doses used during rest and exercise were: PE, 0.0125–0.8 μg kg−1 min−1 and 0.05–1.6 μg kg−1 min−1; AngII, 0.25–16 and 1.0–32 ng kg−1 min−1. The wide range of drug doses allowed for evaluation of saturation kinetics, and post hoc comparison of doses normalized to femoral blood flow using linear interpolation between individually obtained values. The order of the two vasoconstrictors was balanced, and the drugs were separated by at least 15 min, which was accompanied by restoration of predrug haemodynamic values. To avoid the initial non-steady state, measurements were not made during the first 10 min of each exercise bout. We used recovery periods of at least 45 min between exercise conditions.
Figure 1. Protocol outline for constant intra-arterial (femoral) infusions of 7 AngII doses (0.25–16 ng kg−1 min−1) and 7 PE doses (0.0125–0.8 μg kg−1 min−1) during rest; as well as 6 AngII doses (0.5–32 ng kg−1 min−1) and 6 PE doses (0.05–1.6 μg·kg−1 min−1) during 7 w and 27 W ipsilateral knee-extensor exercise.
The time course is represented on the top axis in minutes. The order of the drugs and of the exercise levels were random and balanced. There was a resting period of at least 45 min between exercise bouts. Measurements of femoral blood flow during baseline and each drug dose are marked by arrows. Each dose was administered as a constant intra-arterial infusion for at least 2 min, with measurements obtained during the last 45 s. Each shift of dose was a doubling of the previous dose. BL, baseline; PE, phenylephrine; AngII, angiotensin II.
Data analysis and statistics
All data were sampled at 400 Hz, recorded on a PC, and analysed offline (PowerLab, ADInstruments). Mean arterial pressure (MAP) was derived from the arterial pressure waveform. Femoral vascular conductance (FVC) was calculated by the formula: FVC = FBF/MAP, and vascular resistance (FVR) by: FVR = MAP/FBF. The variables analysed did not depart from normal distributions by F test, or homogeneity of variances by Bartlett's Chi-square test. Student's t test for paired data was applied to test for differences between baseline values (e.g. steady-state values before drug intervention). Within-group differences were assessed by one- or two-way ANOVA for repeated measures, for drug, dose, and exercise intensity. Post hoc analysis was performed by Dunnett's procedure for one-way ANOVA and by Tukey's procedure for two-way ANOVA. Statistical significance was set at P < 0.05 and adjusted by the Bonferroni method as appropriate.
Results
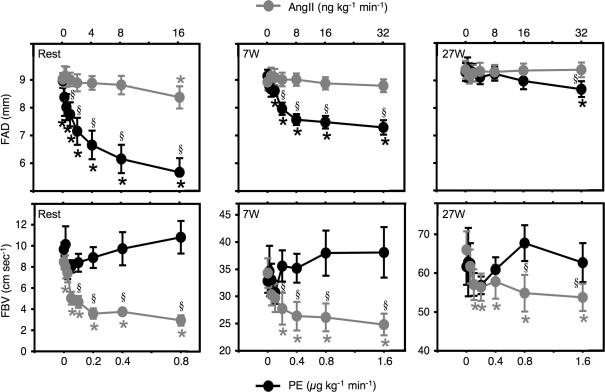
At rest, continuous intra-arterial infusions of PE and AngII had distinct vasoconstrictor effects evidenced by differential effects on femoral artery diameter and blood velocity (Fig. 2), and both drugs showed dose-dependent steady-state decreases in femoral blood flow (Fig. 3). The lower doses of PE caused minor decreases in FBV, and moderate decreases in FAD, whereas the higher doses of PE caused substantial decreases in FAD (from 9.0 ± 0.3 mm at baseline to 5.7 ± 0.5 mm during PE 0.8 μg kg−1 min−1, P < 0.05), and no change in FBV (from 9.7 ± 0.6 at baseline to 10.8 ± 1.5 cm s−1. during PE 0.8 μg kg−1 min−1, P = n.s.). In contrast, AngII had minor effects on FAD (from 9.0 ± 0.2 at baseline to 8.4 ± 0.4 mm during AngII 16 ng kg−1 min−1, P < 0.05), and marked effects on FBV (from 8.5 ± 0.7 at baseline to 2.9 ± 0.5 cm s−1. during AngII 16 ng kg−1 min−1, P < 0.05). The resultant effects on FBF and FVC were similar for the two drugs (Fig. 3 and Table 1). The maximum decreases in flow and conductance were not significantly different for the two drugs (Table 1), and the two dose–response curves both show saturation at 60–65% decreases for both variables (Fig. 3). The maximal decreases were 57 ± 5% and 65 ± 6% for FBF; and 59 ± 4 and 66 ± 6% for FVC during PE and AngII, respectively (P = n.s.). The nominal difference between the two drugs did not reach significance, and was caused mainly by one subject with a low response to PE (around a 20% decrease). There were only minor changes in MAP and no significant changes in HR at rest (Table 1).
Figure 2. Femoral artery diameter and blood velocity.
Dose–response relationships for intra-arterial AngII and PE during rest, 7 W, and 27 W exercise. AngII, angiotensin II; PE, phenylephrine; FAD; femoral artery diameter; FBV, femoral blood velocity. *P < 0.05 compared to baseline; §P < 0.05 between PE and AngII. Error bars are s.e.m.
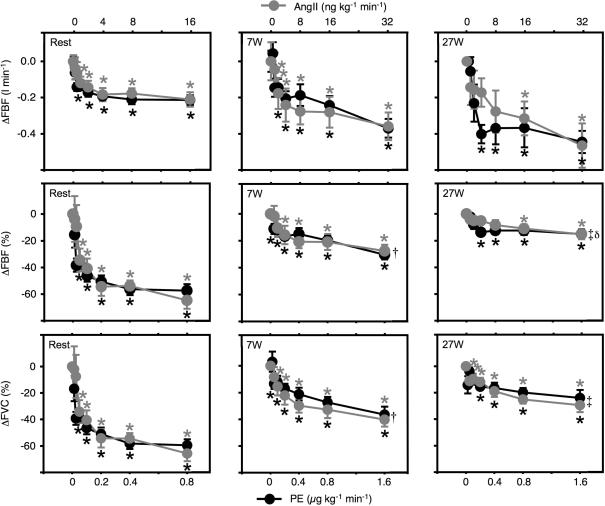
Figure 3. Femoral blood flow and conductance.
Dose–response relationships for intra-arterial AngII and PE during rest, 7 W, and 27 W exercise. AngII, angiotensin II; PE, phenylephrine; ΔFBF, changes in femoral blood flow (absolute in top panels and relative in middle panels); ΔFVC, changes in femoral vascular conductance (relative in bottom panels). *P < 0.05 compared to baseline; †P < 0.05 between rest and 7 W; ‡P < 0.05 between rest and 27 W; δP < 0.05 between rest and 7 W. Error bars are s.e.m.
Table 1.
Values during baseline (BL) and maximal drug (Drug) doses during rest, 7 W, and 27 W
| Resting | 7 W | 27 W | |||||
|---|---|---|---|---|---|---|---|
| BL | Drug | BL | Drug | BL | Drug | ||
| HR (beats min−1) | PE | 55 ± 3 | 52 ± 2 | 67 ± 3 | 54 ± 3* | 77 ± 3 | 65 ± 3* |
| AngII | 55 ± 3 | 55 ± 2 | 65 ± 4 | 59 ± 4* | 76 ± 3 | 73 ± 4* | |
| MAP (mmHg) | PE | 88 ± 3 | 92 ± 3* | 89 ± 4 | 104 ± 6* | 93 ± 4 | 105 ± 7* |
| AngII | 88 ± 3 | 90 ± 4 | 87 ± 3 | 108 ± 6* | 90 ± 3 | 111 ± 6* | |
| FBFD (l min−1) | PE | 0.37 ± 0.03 | 0.16 ± 0.02* | 1.27 ± 0.05 | 0.91 ± 0.07* | 2.50 ± 0.20 | 2.16 ± 0.10* |
| AngII | 0.32 ± 0.03 | 0.09 ± 0.02* | 1.25 ± 0.07 | 0.90 ± 0.07* | 2.68 ± 0.15 | 2.21 ± 0.15* | |
| FBFTD (l min−1) | PE | — | — | 1.06 ± 0.04 | 0.82 ± 0.04* | 2.90 ± 0.14 | 2.46 ± 0.11* |
| AngII | — | — | 1.17 ± 0.11 | 0.81 ± 0.04* | 2.87 ± 0.18 | 2.41 ± 0.15* | |
| FVCD (ml mmHg min−1) | PE | 4.2 ± 0.3 | 1.7 ± 0.2* | 14.4 ± 1.0 | 9.3 ± 1.3* | 28.5 ± 3.5 | 21.1 ± 2.1* |
| AngII | 3.6 ± 0.4 | 1.0 ± 0.2* | 14.5 ± 0.9 | 8.4 ± 0.6* | 30.6 ± 2.7 | 20.8 ± 2.0* | |
| FVCTD (ml mmHg min−1) | PE | — | — | 13.6 ± 1.0 | 8.2 ± 0.5* | 32.8 ± 2.6 | 24.6 ± 2.3* |
| AngII | — | — | 13.6 ± 1.4 | 7.6 ± 0.4* | 32.5 ± 2.7 | 22.5 ± 2.1* | |
AngII, angiotensin II; PE, phenylephrine; HR, heart rate; MAP, mean arterial pressure; FBFD, femoral blood flow by Doppler; FBFTD, femoral blood flow by thermodilution; FVCD, femoral vascular conductance by Doppler; FVCTD, femoral vascular conductance by thermodilution
P < 0.05 for drug compared to baseline.
During both exercise intensities, continuous intra-arterial infusions of PE and AngII caused dose-dependent steady-state decreases in femoral blood flow. These decreases were of a similar magnitude for measurements based on thermodilution and ultrasound (Table 1). The underlying patterns of effects on FAD and FBV seen at rest were also present during both exercise intensities (Fig. 2). Thus, PE caused moderate decreases in FAD (7 W: from 9.1 ± 0.2 to 7.3 ± 0.3 mm, and 27 W: 9.1 ± 0.3–8.7 ± 0.3 mm during PE 1.6 μg kg−1 min−1, P < 0.05 for both), but no change in FBV. In contrast, AngII caused no significant changes in FAD, but decreased FBV (7 W: from 34 ± 3 to 25 ± 2 cm s−1., and 27 W: from 66 ± 5 to 54 ± 3 cm s−1. during AngII 32 ng kg−1 min−1, P < 0.05 for both). Again, the maximal decreases and dose–response curves for changes in FBF and FVC were similar between AngII and PE (Fig. 3). The absolute decreases in femoral blood flow caused by both drugs during exercise were nominally (but not significantly) higher during exercise than at rest (Fig. 3). Thus, the saturation plateaus for the absolute FBF decreases were around 210 ml min−1 at rest; around 350 ml min−1 at 7 W; and around 450 ml min−1 at 27 W, for both drugs. In contrast, the relative decreases in femoral blood flow and vascular conductance were markedly and significantly smaller during exercise compared to the resting condition (Fig. 3). Thus, the saturation plateaus for the relative FBF decreases were around 60% at rest; around 30% at 7 W; and around 15% at 27 W, for both drugs; and the saturation plateaus for the relative FVC decreases were around 60% at rest; around 40% at 7 W; and around 25% at 27 W; for both drugs. The vasoconstrictions obtained were not significantly different between PE and AngII at 7 W or 27 W exercise.
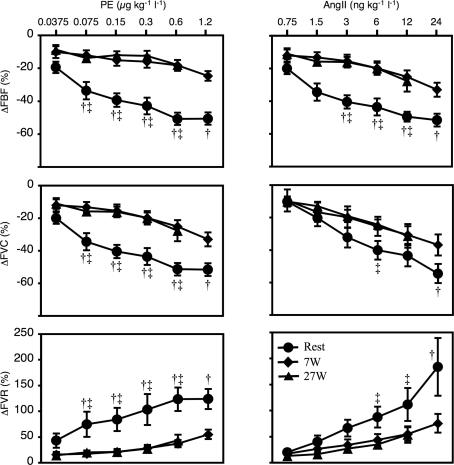
When flow-adjusting PE and AngII doses (post hoc), the relative changes in FBF, FVC, and FVR remained significantly smaller during both exercise intensities compared to rest for both drugs (Fig. 4). There were no statistically significant differences in the responses to either drug between 7 W and 27 W exercise.
Figure 4. Femoral blood flow, conductance and resistance.
Flow-adjusted dose–response relationships for intra-arterial AngII and PE during rest, 7 W, and 27 W exercise. AngII, angiotensin II; PE, phenylephrine; ΔFBF, relative changes in femoral blood flow; ΔFVC, relative changes in femoral vascular conductance; ΔFVR, relative changes in femoral vascular resistance. †P < 0.05 between rest and 7 W; ‡P < 0.05 between rest and 27 W. Error bars are s.e.m.
During exercise, HR increased from 55 ± 3 beats min−1 at rest to 65 ± 3 beats min−1 and 77 ± 3 beats min−1 at 7 W and 27 W, respectively. Both drugs caused larger changes in MAP and HR during both exercise intensities than at rest (Table 1); PE caused increases in MAP of up to 12–14 mmHg with concomitant decreases in HR of 13–14 beats min−1; AngII caused increases in MAP of more than 20 mmHg, but caused only minor decreases in HR of 4–6 beats min−1. The highest drug doses which were not accompanied by significant increases in MAP were PE: 0.2 μg kg−1 min−1 and AngII: 16 ng kg−1 min−1, at rest; PE: 0.2 μg kg−1 min−1 and AngII: 2 ng kg−1 min−1, at 7 W; and PE: 0.4 μg kg−1 min−1 and AngII: 4 ng kg−1 min−1 at 27 W. At these doses of drugs the effects on FBF and FVC were submaximal but remained similar between drugs, and statistically significant for both drugs.
Discussion
The major new finding of the present study is a significant exercise-induced inhibition of AngII-induced vasoconstriction in human thigh muscle. This apparent exercise angiotensinolysis was evident during both mild-and moderate-intensity exercise. This finding was contrary to our hypothesis, and accordingly suggests that metabolic events within exercising human muscle attenuate both adrenergic and non-adrenergic vasoconstriction. The AngII- and PE-induced decreases in blood flow were attenuated to similar degrees during both exercise intensities.
AngII- and PE-induced vasoconstriction
AngII has previously been identified as a potent vasoconstrictor in the resting human forearm (Saris et al. 2000; Nielsen et al. 2004). Pharmacological studies have provided evidence that AngII-vasoconstriction is mediated by AT1 receptors, as the AT1-receptor blocker losartan effectively inhibits AngII-vasoconstriction in the forearm (Saris et al. 2000; Baan et al. 1996). Likewise, PE has been validated extensively as a potent vasoconstrictor operating as an α1-receptor agonist in the human forearm and leg (Wray et al. 2004; Dinenno et al. 2002). Thus, the AngII and PE dose ranges used in the present study probe the maximal responses obtainable with AT1 and α1-receptor stimulation in the resting and exercising human thigh.
In the present study maximal AT1 and α1-stimulation produced a similar degree of vasoconstriction in the human leg both at rest and during exercise, but exhibited differential effects on conduit vessel diameter and blood velocity. Specifically, PE caused an average decrease in femoral artery diameter exceeding 3 mm (−36%), whereas the maximum effect of AngII was around 0.6 mm (−7%) at rest. During both exercise intensities, PE still caused significant decreases in the large artery diameter, whereas AngII became ineffective. Conversely, AngII more potently decreased femoral blood velocity, suggesting a higher maximal response on distal vessel constriction for AngII than for PE-related vasoconstriction. Taken together, these findings suggest anatomically dissimilar locations of the functionally important α1 and AT1 receptors in the human thigh. The responses to PE infusion suggest that α1 receptors are distributed within as well as distal to the conduit artery, whereas the functional effects of AT1 receptor activation suggest a predominantly more distal location within the vascular bed. To our knowledge, these data are the first to identify a heterogeneous distribution of α1 and AT1 receptors by functional responses in human thigh muscle. The findings concur with previous studies in rodents, where functional microvascular studies have indicated that α1-related effects are minor at the level of precapillary arterioles (Faber, 1988), and AngII becomes more potent the smaller the resistance vessel (Vicaut et al. 1989). Using immunohistochemistry, AT1 receptors have been detected throughout the vascular tree of rats (Paxton et al. 1993). Despite these apparent differences, the dose–response curves for AT1 and α1-stimulation versus flow and vascular conductance are remarkably similar during both rest and exercise (Fig. 3), and the maximal effects of the two drugs were also similar under all circumstances. The effects of the drugs during rest were comparable to previously published human studies of PE infusion in the femoral artery (Wray et al. 2004) and AngII infusion in the brachial artery (Saris et al. 2000; Nielsen et al. 2004).
The degrees of metabolic inhibition of AT1- and α1-vasoconstrictor effects produced by both mild (7 W) and moderate (27 W) exercise intensities were also very similar (Fig. 4). The maximal relative decreases in flow were reduced by around 50% during 7 W and around 75% during 27 W exercise for both drugs; and the relative decreases in vascular conductance were 33% during 7 W and around 50% during 27 W exercise for both drugs. When normalized for blood flow (i.e. the dilution-effect of the higher flows), the vasoconstrictor potential of the two drugs during exercise was still reduced by at least 50% compared to rest. Interestingly, there were no apparent differences in the exercise-induced attenuation of effects for either drug between 7 W and 27 W when normalized for blood flow. The present report is the first on the response to intra-arterial AngII in an exercising human limb. The responses to PE in the exercising thigh are similar to our previously published results in this model (Wray et al. 2004), and to responses seen during mild human forearm exercise (Dinenno & Joyner, 2004).
The vasoconstrictive actions of intra-arterial bolus-injections of AngII in a constant-flow dog gracilis muscle preparation have been briefly described (Burcher & Garlick, 1973). In this study, the AngII-induced increase in perfusion pressure was reportedly attenuated by electrically evoked gracilis contractions, but no detailed information was published regarding drug doses or maximal responses. In the same study, the vasoconstrictive actions of intra-arterial vasopressin injections were also attenuated by exercise (Burcher & Garlick, 1973). More recently, the effects of intra-arterial bolus injections of the pharmacologically engineered non-adrenergic vasoconstrictors α,β-methyl-eneadenosine-5-triphosphate lithium salt, a P2X receptor agonist, and [Leu31,Pro34]-neuropeptide Y, a Y1-receptor agonist, were found to be attenuated during exercise in an intensity-dependent manner in a conscious dog model (Buckwalter et al. 2003, 2004b). Together these dog studies have previously suggested that non-adrenergic vasoconstriction may be attenuated during exercise. The present study extends these findings to humans, and extends the conclusion by demonstrating that exercise attenuates the maximal steady state vasoconstrictor effects of a non-adrenergic vasoconstrictor.
Mechanisms of metabolic inhibition of vasoconstriction
While we observed a profound decrease of both AT1- and α1-mediated vasoconstriction during exercise, the underlying mechanisms remain unclear. AT1 and α1 receptors have very similar G-protein coupling, and thus it is possible that these two vasoconstrictor systems could be challenged by the same metabolic event. There is evidence that AT1 and α1 stimulation both cause myosin light chain (MLC) kinase activation as well as MLC phosphatase inhibition (Stull et al. 1998; Hartshorne, 1998; Gohla et al. 2000). Furthermore, functional convergence of AT1- and α1-signal transduction has been illustrated in vivo in animal studies. For example, chronic administration of noradrenaline induces hyposensitivity in the vasculature to both noradrenaline and AngII (Seasholtz et al. 1997), which may be related to downregulated G-protein coupling to both AT1 and α1 receptors.
However, considering the differing sites of functional importance for AT1 receptors (distal) and α1 receptors (proximal and distal) within the vascular bed, their respective exposure to metabolites released from the contracting myocytes may in fact differ. It seems plausible that metabolites released from the contracting myocytes interfere with the distal AngII effects at the level of the resistance arterioles, while the same metabolites are unlikely to directly influence the vasoconstrictor effects at the level of the conduit artery. The inhibition at the level of the femoral artery could conceptually be related to ascending vasodilatation mediated via gap-junction communication between endothelial or smooth muscle cells. However, a recent study using intravital microscopy in isolated hamster vessels lends no support to this possibility, with no evidence of exercise-induced inhibition of feed artery constriction (VanTeeffelen & Segal, 2003). Alternatively, the exercise-induced inhibition of large artery constriction may be unrelated to the metabolic events of the skeletal muscle, but instead due to either physical deformation of the large artery leading to vessel wall relaxation, altered perfusion of the vasa vasorum of the femoral artery, or decreased time for agonist–receptor interaction.
The possibility has been raised that AngII-vasoconstriction could in part be related to enhanced noradrenaline release from sympathetic nerve endings. In vitro studies have provided evidence that stimulation of AT1 receptors may enhance noradrenaline release by 50–60% in certain, but not all, noradrenaline-containing cells or tissues (Cox et al. 1999; Trendelenburg et al. 2003). In one human forearm study, intrabrachial AngII infusion during concomitant nitroprusside infusion to offset vasoconstriction was accompanied by an increased concentration of noradrenaline in the forearm venous blood and an increased forearm noradrenaline spillover (Clemson et al. 1994). However, several other similar studies, including one by the same group, found no evidence for a functionally significant effect of AngII on noradrenaline release in healthy subjects or in patients with heart failure (Chang et al. 1995; Leuenberger et al. 1996; Goldsmith et al. 1998). In addition, more recent studies, using AT1 blockade and investigating several vascular beds in humans, have found no support for this mechanism either (Schlaich et al. 2005; Krum et al. 2006). On balance, AngII may exert an effect on noradrenaline release, but the functional significance of this effect would seem to explain no more than a minor part of AngII-vasoconstriction. Consequently, the exercise-induced inhibition of AngII-vasoconstriction is unlikely to be dependent on inhibition of the effects of noradrenaline.
Methodological considerations
Dynamic knee-extensor exercise as a model for studying human vascular control has important advantages: (i) the fraction of the blood flow through the femoral artery reaching the exercising quadriceps muscle is more than 95% even at mild to moderate intensities; (ii) one-legged knee-extensor exercise (at least up to 30 W which corresponds to about 40% of maximum dynamic effort) can be sustained for hours with steady-state hyperaemia, and without significant increases in blood pressure or sympathetic nervous system activity; (iii) direct reproducible measurements of femoral artery diameter and blood flow by both ultrasound and thermodilution can be obtained in the exercising leg without pausing or otherwise interfering with the exercise.
A basic concern with any comparison between vasoconstrictor responses at rest and during exercise is the sizeable difference in baseline muscle blood flows in the two conditions. Several recent studies have used intra-arterial vasodilators to produce a steady-state pharmacologically induced hyperaemia matching the exercise hyperaemia in a rat hindlimb exercise model (Thomas et al. 1994), a human handgrip (Dinenno & Joyner, 2003; Rosenmeier et al. 2003; Tschakovsky et al. 2002) and in the human knee-extensor model used in the present study (Rosenmeier et al. 2004). These studies have all clearly demonstrated that when the vasoconstrictors PE, clonidine, and tyramine are superimposed on adenosine-, nitroprusside-, or hydralazine-induced hyperaemia the result is invariably large decreases in blood flow, and always significantly larger decreases than the vasoconstrictor responses seen during exercise hyperaemia. Taken together these studies have convincingly shown that a diminished vasoconstrictor response observed during exercise is not merely due to an elevated baseline blood flow, but instead must be related to other effects of exercise.
A specific concern in the present study is that the highest doses of both PE and AngII provoked increases in arterial pressure, thereby engaging the arterial baroreflex. Presumably this was accompanied by withdrawal of sympathetic nerve activity to the vasculature, including the vascular bed of the infused leg. Because the increases in blood pressure were larger during exercise than rest, the possibility existed that a more powerful withdrawal of sympathetic nerve activity during exercise is partially responsible for the attenuation of the maximal drug responses. To address this issue, we also examined the responses to the largest doses of the two drugs that did not elicit a systemic pressor response. While these drug doses were large enough to cause significant vasoconstrictive effects in the infused legs, the responses remained significantly reduced during exercise, indicating that the attenuated vasoconstriction during exercise was present even when blood pressure was unaffected.
Clinical significance of angiotensin during exercise
The functional significance of the observed exercise angiotensinolysis could be of particular interest in a variety of pathological states that are associated with sympathoexcitation such as heart failure, obesity, hypertension, diabetes and ageing. Some of these states are characterized by higher baseline and larger exercise-induced increases in AngII levels compared to healthy controls (Kinugawa et al. 1996, 1997; Cheng et al. 2001; Mansfield et al. 2003; Schlaich et al. 2004). The clinical significance of this may have become apparent in a recent study, where patients with congestive heart failure were exercise tested before and after 6 months add-on therapy with an AT1 receptor blocker or placebo. The submaximal exercise capacity was improved by 26% in the active treatment arm, compared to only 7% in the placebo arm (Blanchet et al. 2005). Based on such findings and the current data, it is tempting to speculate that attenuated exercise angiotensinolysis may be a potential novel mechanism by which skeletal muscle blood flow is limited during demanding exercise in heart failure patients.
Acknowledgments
This study was performed at the Copenhagen Muscle Research Center with the financial support of the Danish National Research Foundation (#504–14). M.S.'s work was supported by grants and fellowships from the Danish Heart Foundation, the Danish Medical Research Council, the Novo Foundation, the Michaelsen Foundation, and the Kaj Hansen Foundation. This study was also supported in part by funding from NIH (#HL045547) to Dr Raven. This work has been completed in partial fulfilment of the requirements for the degree of Doctor of Philosophy for R. Matthew Brothers.
References
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baan J, Jr, Chang PC, Vermeij P, Pfaffendorf M, Van Zwieten PA. Effects of losartan on vasoconstrictor responses to angiotensin II in the forearm vascular bed of healthy volunteers. Cardiovasc Res. 1996;32:973–979. [PubMed] [Google Scholar]
- Blanchet M, Sheppard R, Racine N, Ducharme A, Curnier D, Tardif JC, Sirois P, Lamoureux MC, De Champlain J, White M. Effects of angiotensin-converting enzyme inhibitor plus irbesartan on maximal and submaximal exercise capacity and neurohumoral activation in patients with congestive heart failure. Am J Physiol Heart Circ Physiol. 2005;149:e1–7. doi: 10.1016/j.ahj.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Bocqueraz O, Koulmann N, Guigas B, Jimenez C, Melin B. Fluid-regulatory hormone responses during cycling exercise in acute hypobaric hypoxia. Med Sci Sports Exerc. 2004;36:1730–1736. doi: 10.1249/01.mss.0000142368.56816.e5. [DOI] [PubMed] [Google Scholar]
- Buckwalter JB, Clifford PS. α-Adrenergic vasoconstriction in active skeletal muscles during dynamic exercise. Am J Physiol Heart Circ Physiol. 1999;277:H33–H39. doi: 10.1152/ajpheart.1999.277.1.H33. [DOI] [PubMed] [Google Scholar]
- Buckwalter JB, Hamann JJ, Clifford PS. Vasoconstriction in active skeletal muscles: a potential role for P2X purinergic receptors? J Appl Physiol. 2003;95:953–959. doi: 10.1152/japplphysiol.00173.2003. [DOI] [PubMed] [Google Scholar]
- Buckwalter JB, Hamann JJ, Kluess HA, Clifford PS. Vasoconstriction in exercising skeletal muscles: a potential role for neuropeptide Y? Am J Physiol Heart Circ Physiol. 2004b;287:H144–H149. doi: 10.1152/ajpheart.00071.2004. [DOI] [PubMed] [Google Scholar]
- Buckwalter JB, Taylor JC, Hamann JJ, Clifford PS. Role of nitric oxide in exercise sympatholysis. J Appl Physiol. 2004a;97:417–423. doi: 10.1152/japplphysiol.01181.2003. [DOI] [PubMed] [Google Scholar]
- Burcher E, Garlick D. Antagonism of vasoconstrictor responses by exercise in the gracilis muscle of the dog. J Pharmacol Exp Ther. 1973;187:78–85. [PubMed] [Google Scholar]
- Chang PC, Grossman E, Kopin IJ, Goldstein DS, Folio CJ, Holmes C. On the existence of functional angiotensin II receptors on vascular sympathetic nerve terminals in the human forearm. J Hypertens. 1995;13:1275–1284. doi: 10.1097/00004872-199511000-00009. [DOI] [PubMed] [Google Scholar]
- Cheng C-P, Ukai T, Onishi K, Ohte N, Suzuki M, Zhang Z-S, Cheng H-J, Tachibana H, Igawa A, Little WC. The role of ang II and endothelin-1 in exercise-induced diastolic dysfunction in heart failure. Am J Physiol Heart Circ Physiol. 2001;280:H1853–H1860. doi: 10.1152/ajpheart.2001.280.4.H1853. [DOI] [PubMed] [Google Scholar]
- Clemson B, Gaul Lawrence Gubin SS, Campsey DS, McConville J, Nussberger J, Zelis R. Prejunctional angiotensin II receptors. facilitation of norepinephrine release in the human forearm. J Clin Invest. 1994;93:684–691. doi: 10.1172/JCI117021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox SL, Trendelenburg AU, Starke K. Prejunctional angiotensin receptors involved in the facilitation of noradrenaline release in mouse tissues. Br J Pharmacol. 1999;127:1256–1262. doi: 10.1038/sj.bjp.0702652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinenno FA, Eisenach JH, Dietz NM, Joyner MJ. Post-junctional alpha-adrenoceptors and basal limb vascular tone in healthy men. J Physiol. 2002;540:1103–1110. doi: 10.1113/jphysiol.2001.015297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinenno FA, Joyner MJ. Blunted sympathetic vasoconstriction in contracting skeletal muscle of healthy humans: is nitric oxide obligatory? J Physiol 533. 2003;1:281–292. doi: 10.1113/jphysiol.2003.049940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinenno FA, Joyner MJ. Combined NO and PG inhibition augments alpha-adrenergic vasoconstriction in contracting human skeletal muscle. Am J Physiol Heart Circ Physiol. 2004;287:H2576–H2584. doi: 10.1152/ajpheart.00621.2004. [DOI] [PubMed] [Google Scholar]
- Faber JE. In situ analysis of alpha-adrenoceptors on arteriolar and venular smooth muscle in rat skeletal muscle microcirculation. Circ Res. 1988;62:37–50. doi: 10.1161/01.res.62.1.37. [DOI] [PubMed] [Google Scholar]
- Gohla A, Schultz G, Offermans S. Role for G12/G13 in agonist-induced vascular smooth muscle cell contraction. Circ Res. 2000;87:221–227. doi: 10.1161/01.res.87.3.221. [DOI] [PubMed] [Google Scholar]
- Goldsmith SR, Garr M, McLaurin M. Regulation of regional norepinephrine spillover in heart failure: the effect of angiotensin II and beta-adrenergic agonists in the forearm circulation. J Card Fail. 1998;4:305–310. doi: 10.1016/s1071-9164(98)90236-6. [DOI] [PubMed] [Google Scholar]
- Hansen J, Sander M, Thomas GD. Metabolic modulation of sympathetic vasoconstriction in exercising skeletal muscle. Acta Physiol Scand. 2000;168:489–503. doi: 10.1046/j.1365-201x.2000.00701.x. [DOI] [PubMed] [Google Scholar]
- Hansen J, Sayad D, Thomas GD, Clarke GD, Peshock RM, Victor RG. Exercise-induced attenuation of alpha-adrenoceptor mediated vasoconstriction in humans: evidence from phase-contrast MRI. Cardiovasc Res. 1999;41:220–228. doi: 10.1016/s0008-6363(98)00226-0. [DOI] [PubMed] [Google Scholar]
- Hartshorne DJ. Myosin phosphatase: subunits and interactions. Acta Physiol Scand. 1998;164:483–493. doi: 10.1046/j.1365-201X.1998.00447.x. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Nauss LA, Warner MA, Warner DO. Sympathetic modulation of blood flow and O2 uptake in rhythmically contracting human forearm muscles. Am J Physiol Heart Circ Physiol. 1992;263:H1078–H1083. doi: 10.1152/ajpheart.1992.263.4.H1078. [DOI] [PubMed] [Google Scholar]
- Keller DM, Ogoh D, Green S, Olivencia-Yurvati A, Raven PB. Inhibition of Katp channel activity augments baroreflex mediated vasoconstriction in exercising skeletal muscle. J Physiol. 2004;561:273–282. doi: 10.1113/jphysiol.2004.071993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinugawa T, Endo A, Kato M, Kato T, Ahmmed GU, Omodani H, Osaki S, Ogino K, Hisatome I, Miyakoda H, Fujimoto Y, Yoshida A, Shigemasa C. Responses of plasma catecholamines, renin-angiotensin-aldosterone system, and atrial natriuretic peptide to exercise in patients with essential hypertension. Cardiology. 1997;88:238–245. doi: 10.1159/000177336. [DOI] [PubMed] [Google Scholar]
- Kinugawa T, Ogino K, Kitamura H, Saitoh M, Omodani H, Osaki S, Hisatome I, Miyakoda H. Catecholamines, renin-angiotensin-aldosterone system, and atrial natriuretic peptide at rest and during submaximal exercise in patients with congestive heart failure. Am J Med Sci. 1996;312:110–117. doi: 10.1097/00000441-199609000-00003. [DOI] [PubMed] [Google Scholar]
- Krum H, Lambert E, Windebank E, Campbell DJ, Esler M. Effect of angiotensin II receptor blockade on autonomic nervous system function in patients with essential hypertension. Am J Physiol Heart Circ Physiol. 2006;290:H1706–H1712. doi: 10.1152/ajpheart.00885.2005. [DOI] [PubMed] [Google Scholar]
- Leuenberger U, Gaul L, Noack H, Gubin S, Zelis R. Angiotensin-converting enzyme inhibition and the norepinephrine spillover response to dynamic exercise. J Appl Physiol. 1996;81:1138–1142. doi: 10.1152/jappl.1996.81.3.1138. [DOI] [PubMed] [Google Scholar]
- Mansfield D, Kaye DM, Brunner La Rocca H, Solin P, Esler MD, Naughton MT. Raised sympathetic nerve activity in heart failure and central sleep apnea is due to heart failure severity. Circulation. 2003;107:1396–1400. doi: 10.1161/01.cir.0000056520.17353.4f. [DOI] [PubMed] [Google Scholar]
- McEniery CM, Wilkinson IB, Jenkins DG, Webb DJ. Endogenous endothelin-1 limits exercise-induced vasodilation in hypertensive humans. Hypertension. 2002;40:202–206. doi: 10.1161/01.hyp.0000024218.04872.f3. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Halliwill JR, Joyner MJ, Jensen MD. Vascular response to angiotensin II in upper body obesity. Hypertension. 2004;44:435–441. doi: 10.1161/01.HYP.0000142111.67601.6b. [DOI] [PubMed] [Google Scholar]
- Paxton WG, Runge M, Horaist C, Cohen C, Alexander RW, Bernstein KE. Immunohistochemical localization of rat angiotensin II AT1 receptor. Am J Physiol. 1993;264:F989–F995. doi: 10.1152/ajprenal.1993.264.6.F989. [DOI] [PubMed] [Google Scholar]
- Radegran G. Ultrasound Doppler estimates of femoral artery blood flow during dynamic knee extensor exercise in humans. J Appl Physiol. 1997;83:1383–1388. doi: 10.1152/jappl.1997.83.4.1383. [DOI] [PubMed] [Google Scholar]
- Remensynder JP, Mitchell JH, Sarnoff SJ. Functional sympatholysis during muscular activity. Circ Res. 1962;11:370–380. doi: 10.1161/01.res.11.3.370. [DOI] [PubMed] [Google Scholar]
- Rosenmeier JB, Dinneno FA, Fritzlar SJ, Joyner MJ. α1- and α2-adrenergic vasoconstriction is blunted in contracting human muscle. J Physiol. 2003;547:971–979. doi: 10.1113/jphysiol.2002.037937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmeier JB, Hansen J, Gonzalez-Alonso J. Circulating ATP-induced vasodilation overrides sympathetic vasoconstrictor activity in human skeletal muscle. J Physiol. 2004;558:351–365. doi: 10.1113/jphysiol.2004.063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander M, Chavoshan B, Harris SA, Iannaccone ST, Stull JT, Thomas GD, Victor RG. Functional muscle ischemia in neuronal nitric oxide synthase-deficient skeletal muscle of children with Duchenne's muscular dystrophy. Proc Natl Acad Sci U S A. 2000;97:13818–13823. doi: 10.1073/pnas.250379497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saris JJ, van Dijk MA, Kroon I, Schalekamp MA, Dauser AH. Functional importance of angiotensin-converting-enzyme-dependent in situ angiotensin II generation in the human forearm. Hypertension. 2000;35:764–768. doi: 10.1161/01.hyp.35.3.764. [DOI] [PubMed] [Google Scholar]
- Schlaich MP, Kaye DM, Lambert E, Hastings J, Campbell DJ, Lambert G, Esler MD. Angiotensin II and norepinephrine release: interaction and effects on the heart. J Hypertens. 2005;23:1077–1082. doi: 10.1097/01.hjh.0000166850.80344.cf. [DOI] [PubMed] [Google Scholar]
- Schlaich MP, Lambert E, Kaye DM, Krozowski Z, Campbell DJ, Lambert G, Hastings J, Aggarwal A, Esler MD. Sympathetic augmentation in hypertension: role of nerve firing, norepinephrine reuptake, and angiotensin neuromodulation. Hypertension. 2004;43:169–175. doi: 10.1161/01.HYP.0000103160.35395.9E. [DOI] [PubMed] [Google Scholar]
- Seasholtz TM, Gurdal H, Wang H-Y, Johnson MD, Friedman E. Desensitization of norepinephrine receptor function is associated with G protein uncoupling in the rat aorta. Am J Physiol Heart Circ Physiol. 1997;42:H279–H285. doi: 10.1152/ajpheart.1997.273.1.H279. [DOI] [PubMed] [Google Scholar]
- Staessen J, Fagard R, Hespel P, Lijnen P, Vanhees L, Amery A. Plasma renin system during exercise in normal men. J Appl Physiol. 1987;63:188–194. doi: 10.1152/jappl.1987.63.1.188. [DOI] [PubMed] [Google Scholar]
- Stull JT, Lin PJ, Krueger JK, Trewhella J, Zhi G. Myosin light chain kinase: functional domains and structural motifs. Acta Physiol Scand. 1998;164:471–482. doi: 10.1111/j.1365-201x.1998.tb10699.x. [DOI] [PubMed] [Google Scholar]
- Thomas GD, Hansen J, Victor RG. Inhibition of alpha 2-adrenergic vasoconstriction during contraction of glycolytic, not oxidative, rat hindlimb muscle. Am J Physiol Heart Circ Physiol. 1994;266:H920–H929. doi: 10.1152/ajpheart.1994.266.3.H920. [DOI] [PubMed] [Google Scholar]
- Thomas GD, Hansen J, Victor RG. ATP-sensitive potassium channels mediate contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J Clin Invest. 1997;99:2602–2609. doi: 10.1172/JCI119448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GD, Sander M, Lau KS, Huang PL, Stull JT, Victor RG. Impaired metabolic modulation of α-adrenergic vasoconstriction in dystrophin-deficient skeletal muscle. Proc Natl Acad Sci U S A. 1998;95:15090–15095. doi: 10.1073/pnas.95.25.15090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GD, Victor RG. Nitric oxide mediates contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J Physiol. 1997;506:817–826. doi: 10.1111/j.1469-7793.1998.817bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GD, Zhang W, Victor RG. Impaired modulation of sympathetic vasoconstriction in contracting skeletal muscle of rats with chronic myocardial infarctions: role of oxidative stress. Circ Res. 2001;88:816–823. doi: 10.1161/hh0801.089341. [DOI] [PubMed] [Google Scholar]
- Tidgren B, Hjemdahl P, Theodorsson E, Nussberger J. Renal neurohormonal and vascular responses to dynamic exercise in humans. J Appl Physiol. 1991;70:2279–2286. doi: 10.1152/jappl.1991.70.5.2279. [DOI] [PubMed] [Google Scholar]
- Trendelenburg AU, Meyer A, Klebroff W, Guimaraes S, Starke K. Crosstalk between presynaptic angiotensin receptors, bradykinin receptors and alpha 2-autoreceptors in sympathetic neurons: a study in alpha 2-adrenoceptor-deficient mice. Br J Pharmacol. 2003;138:1389–1402. doi: 10.1038/sj.bjp.0705223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschakovsky ME, Sujirattanawimol K, Ruble SB, Valic Z, Joyner MJ. Is sympathetic neural vasoconstriction blunted in the vascular bed of exercising human muscle? J Physiol. 2002;541:623–635. doi: 10.1113/jphysiol.2001.014431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanTeeffelen JW, Segal SS. Interaction between sympathetic nerve activation and muscle fibre contraction in resistance vessels of hamster retractor muscle. J Physiol. 2003;550:563–574. doi: 10.1113/jphysiol.2003.038984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicaut E, Montalescot G, Hou X, Stucker O, Teisseire B. Arteriolar vasoconstriction and tachyphylaxis with intra-arterial angiotensin-II. Microvasc Res. 1989;37:28–41. doi: 10.1016/0026-2862(89)90070-8. [DOI] [PubMed] [Google Scholar]
- Victor RG, Pryor SL, Secher NH, Mitchell JH. Effects of partial neuromuscular blockade on sympathetic nerve responses to static exercise in humans. Circ Res. 1989;65:468–476. doi: 10.1161/01.res.65.2.468. [DOI] [PubMed] [Google Scholar]
- Victor RG, Secher NH, Lyson T, Mitchell JH. Central command increases muscle sympathetic nerve activity during intense intermittent isometric exercise in humans. Circ Res. 1995;76:127–131. doi: 10.1161/01.res.76.1.127. [DOI] [PubMed] [Google Scholar]
- Wray DW, Fadel PJ, Smith ML, Raven PB, Sander M. Inhibition of α-adrenergic vasoconstriction in exercising human thigh muscles. J Physiol. 2004;555:545–563. doi: 10.1113/jphysiol.2003.054650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousufuddin M, Shamim W, Chambers JS, Henein M, Amin FR, Anker SD, Kemp M, Hooper J, Coats AJS. Superiority of endothelin-1 over norepinephrine in exercise-induced alterations of the conduit artery tone of the non-exercised arm in patients with chronic heart failure. Int J Cardiol. 2000;73:15–25. doi: 10.1016/s0167-5273(99)00200-4. [DOI] [PubMed] [Google Scholar]