Abstract
Vasomotor sympathetic activity plays an important role in arterial pressure maintenance via the baroreflex during acute orthostasis in humans. If orthostasis is prolonged, blood pressure may be supported additionally by humoral factors with a possible reduction in sympathetic baroreflex sensitivity. We tested the hypothesis that baroreflex control of muscle sympathetic nerve activity (MSNA) decreases during prolonged upright posture. MSNA and haemodynamics were measured supine and during 45 min 60 deg upright tilt in 13 healthy individuals. Sympathetic baroreflex sensitivity was quantified using the slope of the linear correlation between MSNA and diastolic pressure during spontaneous breathing. It was further assessed as the relationship between MSNA and stroke volume, with stroke volume derived from cardiac output (C2H2 rebreathing) and heart rate. Total peripheral resistance was calculated from mean arterial pressure and cardiac output. We found that MSNA increased from supine to upright (17 ± 8 (s.d.) versus 38 ± 12 bursts min−1; P < 0.01), and continued to increase to a smaller degree during sustained tilt (39 ± 11, 41 ± 12, 43 ± 13 and 46 ± 15 bursts min−1 after 10, 20, 30 and 45 min of tilt; between treatments P < 0.01). Sympathetic baroreflex sensitivity increased from supine to upright (−292 ± 180 versus −718 ± 362 units beat−1 mmHg−1; P < 0.01), but remained unchanged as tilting continued (−611 ± 342 and −521 ± 221 units beat−1 mmHg−1 after 20 and 45 min of tilt; P = 0.49). For each subject, changes in MSNA were associated with changes in stroke volume (r = 0.88 ± 0.13, P < 0.05), while total peripheral resistance was related to MSNA during 45 min upright tilt (r = 0.82 ± 0.15, P < 0.05). These results suggest that the vasoconstriction initiated by sympathetic adrenergic nerves is maintained by ongoing sympathetic activation during sustained (i.e. 45 min) orthostasis without obvious changes in vasomotor sympathetic neural control.
Vasomotor sympathetic activity plays an important role in arterial pressure maintenance mainly through baroreflex-mediated vasoconstriction during short-term orthostasis in humans (Johnson et al. 1974; Wallin & Sundlof, 1982; Fu et al. 2004). If upright posture is prolonged, blood pressure may be supported additionally by vasoactive humoral factors (Rowell, 1993), with a possible reduction in baroreflex regulation of vasomotor sympathetic activity (Sanderford & Bishop, 2000, 2002).
Conversely, despite the importance of neural control in the rapid stabilization of arterial pressure, it has been proposed that the sympathetic baroreflex is not important in the long term regulation of blood pressure (Cowley, 1992). For example, previous studies in animals have shown that the strength or sensitivity of the baroreceptor feedback control system is insufficient to account for the prolonged constancy of blood pressure (Donald & Edis, 1971; Cowley et al. 1973); moreover, the arterial baroreceptors adapt quickly (Cowley et al. 1973; Fisher et al. 1984). Furthermore, one human study showed that patients with baroreflex failure have little orthostatic hypotension (Robertson et al. 1993). It seems likely that the vasoconstriction in the upright posture may be initiated by sympathetic adrenergic nerves and maintained by circulating vasoactive humoral factors in humans. As direct intraneural sympathetic recordings during sustained orthostasis have never been measured in healthy individuals, this speculation has not yet been proven.
We speculated that an increase in vasomotor sympathetic activity, which can be recorded as muscle sympathetic nerve activity in humans (Wallin et al. 1974; Vallbo et al. 1979), may not be well maintained during prolonged upright posture, and therefore vasomotor sympathetic control may play a smaller role during sustained compared with acute orthostasis. Thus, the present study was performed to test the hypothesis that baroreflex control of muscle sympathetic nerve activity decreases during prolonged upright posture in healthy humans. Specifically, we determined (1) whether the increase in muscle sympathetic nerve activity was attenuated, and (2) whether sympathetic baroreflex sensitivity decreased during sustained upright posture in humans.
Methods
Subjects
Twenty healthy volunteers aged from 19 to 51 years were studied. Thirteen of these completed a 45 min 60 deg upright tilt test (9 men, 4 women), while 7 subjects (2 men, 5 women) developed presyncope during tilting. The data reported in this study are from those 13 subjects who did not have presyncope to avoid the confounding effects of triggered sympathetic withdrawal and hypotension (Kamiya et al. 2005a) on the calculations of baroreflex sensitivity. The mean age (± s.d.) of this group was 32 ± 10 years, mean body weight 73 ± 14 kg and mean height 175 ± 7 cm. No subject smoked, used recreational drugs, or had significant medical problems. None was an endurance-trained athlete (Levine et al. 1991). All females were normally menstruating (∼28 days cycle), and had never taken or had not taken oral contraceptives for ≥ 6 months. They were not pregnant during the study. The subjects were screened with a careful medical history, physical examination, electrocardiogram and a 15 min 60 deg upright tilt test. Individuals with a history of fainting or neurally mediated syncope were excluded. All subjects were informed of the purpose and procedures used in the study and gave their written informed consent to a protocol approved by the Institutional Review Boards of the University of Texas Southwestern Medical Center and Presbyterian Hospital of Dallas. The study followed guidelines set forth in the Declaration of Helsinki.
Measurements
Muscle sympathetic nerve activity
Muscle sympathetic nerve activity signals were obtained with the microneurographic technique (Wallin et al. 1974; Vallbo et al. 1979). Briefly, a recording electrode was placed in the peroneal nerve at the popliteal fossa, and a reference electrode was placed subcutaneously 2–3 cm from the recording electrode. The nerve signals were amplified (gain 70 000–160 000), band-pass filtered (700–2000 Hz), full-wave rectified, and integrated with a resistance-capacitance circuit (time constant 0.1 s). Criteria for adequate muscle sympathetic nerve activity recording included: (1) pulse synchrony; (2) facilitation during the hypotensive phase of the Valsalva manoeuvre, and suppression during the hypertensive overshoot after release; (3) increases in response to breath holding; and (4) insensitivity to emotional stimuli (Wallin et al. 1974; Vallbo et al. 1979).
Heart rate and blood pressure
Heart rate was determined from lead II of the electrocardiogram, and beat-by-beat arterial pressure was measured non-invasively from the middle finger using photoplethysmography (Finapres, Ohmeda, A BOC Health Care Company, Louisville, CO, USA), supported by an arm board at heart level to standardize hydrostatic pressure effects between supine and upright positions. Cuff blood pressure was measured by electrosphygmomanometry (model 4240, SunTech Medical Instruments Inc., Raleigh, NC, USA), with a microphone placed over the brachial artery to detect Korotkoff sounds. Respiratory excursions were detected by a nasal cannula (model 1265, Respironics California Inc., Carlsbad, CA, USA).
Cardiac output
Cardiac output was measured with the acetylene rebreathing technique (Triebwasser et al. 1977). Cardiac output is calculated from the disappearance rate of acetylene in expired air, measured with a mass spectrometer (model MGA1100, Marquette, Milwaukee, WI, USA), after adequate mixing in the lung has been confirmed by a stable helium concentration. This method has been validated against standard invasive techniques, including thermodilution and direct Fick at rest and during orthostasis with a typical error (expressed as coefficient of variation) of 4–5% (Fu et al. 2005a).
Stroke volume was calculated from cardiac output and the heart rate measured during the rebreathing. Total peripheral resistance was calculated as the quotient of mean arterial pressure and cardiac output, multiplied by 80 (expressed as dyn s cm−5). Mean arterial pressure was calculated as [(systolic blood pressure – diastolic blood pressure)/3] + diastolic blood pressure, where blood pressure was measured by arm cuff during the rebreathing.
Protocol
The experiment was performed in the morning or afternoon ≥ 2 h after a light breakfast or lunch, and ≥ 12 h after the last caffeinated or alcoholic beverage in a quiet, environmentally controlled laboratory with an ambient temperature of ∼25°C. Three females were studied during the luteal phase (from 20 to 22 days after the onset of menstruation, when both oestrogen and progesterone were high), and one was studied during the early follicular phase (2 days after the onset of menstruation, when both sex hormones were low) of their menstrual cycles.
After ≥ 30 min of quiet rest in the supine position, baseline data were collected for 6 min. The subject was then tilted passively to 60 deg upright tilt for 45 min. A belt was placed across the subject's waist to make sure he or she would not fall. A bicycle saddle was used to support approximately two-thirds of the body weight, while the subject stood on a plate at the end of the tilt bed on one leg, allowing the other leg to be relaxed for microneurography. After that, the subject was returned to the supine position for recovery. Heart rate, blood pressure, respiratory waveforms and muscle sympathetic nerve activity were recorded continuously. Cardiac output was measured while supine, and at the 5th, 10th, 20th, 30th and 40th minutes of tilting.
Data analysis
Data were sampled at 500 Hz with a commercial data acquisition system (Biopac System, Santa Barbara, CA, USA) and analysed using LabView software (National Instruments, Austin, TX, USA). Beat-by-beat heart rate was calculated from the R–R interval of the electrocardiogram. Beat-by-beat systolic and diastolic blood pressures were obtained from the arterial pressure waveform.
Sympathetic bursts were identified by a computer program using a 3: 1 signal-to-noise ratio threshold within a 0.5 s search window and an expected burst reflex latency from the preceding R-wave of 1.3 s (Cui et al. 2001), and then were confirmed by an experienced microneurographer. The integrated neurogram was normalized by assigning a value of 100 to the largest amplitude of a sympathetic burst during the 6 min resting supine baseline. All bursts for that trial were then normalized against that value (Halliwill, 2000). Burst areas of the integrated neurogram, and systolic and diastolic blood pressure were measured simultaneously on a beat-by-beat basis. Total activity of the burst was defined as the burst area of the rectified and integrated neurogram. The number of bursts per minute (burst frequency) and total activity were used as quantitative indices.
Assessment of sympathetic baroreflex control
Baroreflex control of muscle sympathetic nerve activity was assessed by using the slope of the linear correlation between total activity and diastolic blood pressure during spontaneous breathing (Wallin et al. 1974; Sundlof & Wallin, 1978; Kienbaum et al. 2001), in the supine position and during the early (from the 2nd to the 5th minute), middle (from the 17th to the 20th minute), and late stages (from the 42nd to the 45th minute) of upright tilt. To perform a linear regression, values for total activity were averaged over a 3 mmHg diastolic pressure bin. This pooling procedure reduces the statistical impact of inherent beat-by-beat variability in nerve activity attributable to non-baroreflex influences (e.g. respiration) (Sundlof & Wallin, 1978). However, minor variations of bin width or bin position may affect the baroreflex sensitivity (Kienbaum et al. 2001). To minimize such variations, a statistical weighting procedure was adopted (Kienbaum et al. 2001); each data point was entered once for each heart beat in the bin, and total activity was expressed as arbitrary units per heart beat (i.e. units beat−1). The fluctuation of diastolic blood pressure during spontaneous breathing was quantified as the difference between the maximal and the minimal values of the bin.
Additionally, we used the muscle sympathetic nerve activity and stroke volume relationship to further evaluate sympathetic baroreflex control for each subject during changes in posture and 45 min upright tilt, since muscle sympathetic nerve activity has been demonstrated to be related to the change in stroke volume in the supine position (Charkoudian et al. 2005) and during orthostasis (Levine et al. 2002; Convertino et al. 2004; Fu et al. 2005a,b).
Statistical analysis
Data are expressed as mean ± standard deviation (s.d.). Supine baseline muscle sympathetic nerve activity, blood pressure and heart rate were averaged for 6 min. During upright tilt, data were collected and averaged from the 2nd to 5th minutes (Tilt5), 7th to 10th minutes (Tilt10), 17th to 20th minutes (Tilt20), 26th to 29th minutes (Tilt30), 36th to 39th minutes (Tilt40) and 42nd to 45th minutes (Tilt45). Haemodynamic and muscle sympathetic nerve activity responses to upright tilt were analysed using Friedman repeated-measures analysis of variance on rank. The Student-Newman-Keuls method was used post hoc for multiple comparisons. The relationship between total activity and diastolic pressure during spontaneous breathing in the supine and upright positions was determined for each subject by least-squares linear regression analysis, and the slopes were compared using Friedman repeated-measures analysis of variance on rank. The relationship between muscle sympathetic nerve activity and stroke volume as well as total peripheral resistance in the supine position and during 45 min upright tilt or in the middle and the late stages of tilt (e.g. Tilt20, Tilt30 and Tilt40) was determined by least-squares linear regression for each subject. All statistical analyses were performed with a personal computer-based analysis program (SigmaStat, SPSS). A P value of < 0.05 was considered statistically significant.
Results
Vasomotor sympathetic and haemodynamic responses during 45 min upright tilt
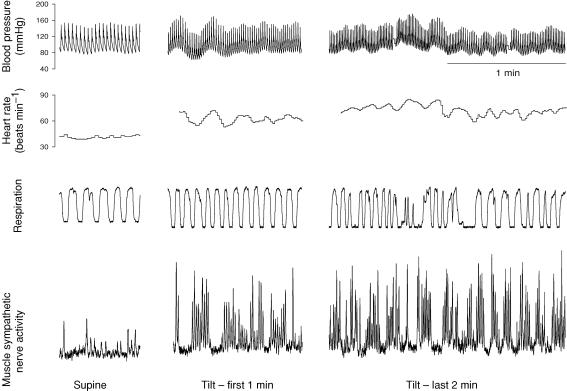
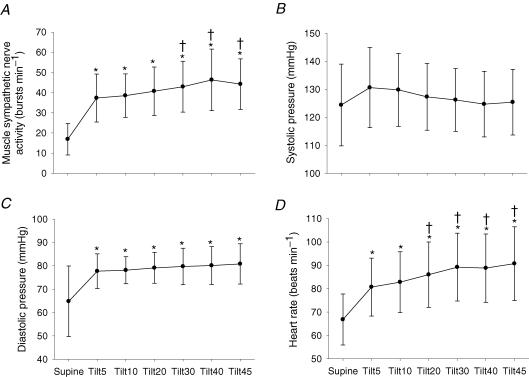
Figure 1 shows original tracings of blood pressure, heart rate, respiratory waveforms and muscle sympathetic nerve activity from one subject. Muscle sympathetic nerve activity increased on moving from supine to upright; it continued to increase, and reached a peak at 42.5 ± 2.4 min during tilt (Fig. 2A, P < 0.01). Systolic blood pressure was well maintained (Fig. 2B), while diastolic blood pressure increased during 45 min upright tilt (Fig. 2C, P < 0.01). Heart rate increased from supine to upright, and continued to increase during 45 min tilt (Fig. 2D, P < 0.01).
Figure 1.
Original tracings of blood pressure, heart rate, respiratory waveforms, and muscle sympathetic nerve activity from one subject in the supine position, and during the initial 1 min and the last 2 min of 60 deg upright tilt.
Figure 2.
Muscle sympathetic nerve activity (A), systolic blood pressure (B), diastolic blood pressure (C), and heart rate (D) responses during 45 min of 60 deg upright tilt. Values are mean ±s.d.*P < 0.05 compared to the supine position. †P < 0.05 compared to the 5th minute of upright tilt.
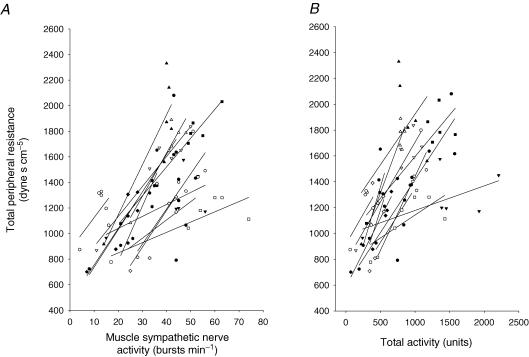
Table 1 shows haemodynamic responses to 45 min upright tilt. Both stroke volume and cardiac output decreased from supine to upright, and further decreased during 45 min tilt. Total peripheral resistance increased during changes in posture, and it continued to increase during 45 min upright tilt. Total peripheral resistance was positively related to muscle sympathetic nerve activity during changes in posture and 45 min upright tilt in each subject, and the correlation coefficient (r) was 0.82 ± 0.15 and 0.75 ± 0.18 for Fig. 3A and B. The positive linear correlation still existed between total peripheral resistance and muscle sympathetic nerve activity in the middle and the late stages of tilt, and the mean of the individual correlation coefficient was 0.70 ± 0.31.
Table 1.
Haemodynamic responses to 45 min 60 deg upright tilt
| 60 deg upright tilt | ||||||
|---|---|---|---|---|---|---|
| Variables | Supine | 5th minute | 10th minute | 20th minute | 30th minute | 40th minute |
| Stroke volume (ml) | 103 ± 23 | 75 ± 23* | 61 ± 15*† | 61 ± 21*† | 57 ± 22*† | 55 ± 16*† |
| Cardiac output (l min−1) | 7.74 ± 1.23 | 6.31 ± 1.71* | 5.24 ± 0.97*† | 5.24 ± 1.08*† | 4.89 ± 1.18*† | 4.91 ± 1.08*† |
| Total peripheral resistance (dyn s cm−5) | 873 ± 126 | 1247 ± 349* | 1445 ± 256*† | 1453 ± 306*† | 1579 ± 362*† | 1541 ± 279*† |
Data are mean ±s.d.
P < 0.05 compared to the supine position
P < 0.05 compared to the 5th minute of upright tilt.
Figure 3.
Correlation between total peripheral resistance and muscle sympathetic nerve activity in the supine position and during 45 min upright tilt in each subject.
Sympathetic baroreflex control during 45 min upright tilt
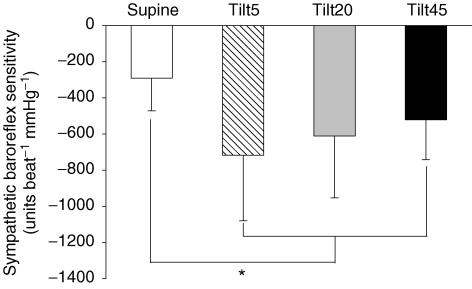
The fluctuation of diastolic blood pressure during spontaneous breathing was 15 ± 6 mmHg in the supine position, and 19 ± 5, 21 ± 4 and 22 ± 5 mmHg during the early, middle and late stages of tilting. Sympathetic baroreflex sensitivity increased on moving from supine to upright (P < 0.01); however, the sensitivity remained unchanged during the middle and the late stages of sustained upright tilt (between treatments, P = 0.49; Fig. 4). The correlation coefficient for the linear relationship between total activity and diastolic pressure was 0.84 ± 0.23 in the supine position, 0.92 ± 0.12, 0.91 ± 0.13 and 0.94 ± 0.04 during the early, middle and late stages of 45 min upright tilt.
Figure 4.
Sympathetic baroreflex sensitivity in the supine position, and during the early, middle and late stages of 60 deg upright tilt. Values are mean ±s.d. Tilt5, between the 2nd and 5th minute; Tilt20, between the 17th and 20th minute; Tilt45, between the 42nd and 45th minute of upright tilt. *P < 0.05 compared to the supine position.
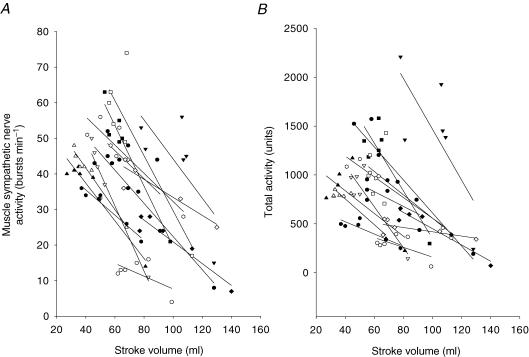
Muscle sympathetic nerve activity was inversely related to stroke volume during changes in posture and 45 min upright tilt (Fig. 5). A strong linear relationship was observed in each subject, and the mean correlation coefficient was 0.88 ± 0.13. A negative linear correlation still existed between stroke volume and muscle sympathetic nerve activity in the middle and the late stages of tilt, and the mean correlation coefficient was 0.79 ± 0.23.
Figure 5.
Correlation between muscle sympathetic nerve activity and stroke volume in the supine position and during 45 min upright tilt in each subject.
Discussion
The major findings from this study are that (1) muscle sympathetic nerve activity increased progressively during sustained upright posture; (2) sympathetic baroreflex sensitivity increased immediately after tilting, but did not change further during 45 min upright tilt; and (3) increases in muscle sympathetic nerve activity were associated with decreases in stroke volume during acute and prolonged upright posture in healthy individuals. These results do not support our hypothesis. Rather, they suggest that the vasoconstriction initiated by sympathetic adrenergic nerves is maintained by ongoing sympathetic activation. Therefore vasomotor sympathetic neural control plays an important role not only in acute but also in sustained (i.e. 45 min) arterial pressure maintenance in humans.
Unaltered vasomotor sympathetic control during sustained upright posture
Despite much research on the neural, hormonal and intrinsic mechanisms involved in the regulation of arterial pressure in humans, our understanding of how blood pressure is controlled over the long-term is limited (Barrett & Malpas, 2005). We found that vasomotor sympathetic neural control still plays an important role in arterial pressure maintenance during prolonged orthostasis. The underlying mechanism(s) are unknown. One potential explanation may be that physiological levels of increases in humoral factors do not affect vasomotor sympathetic control. Indeed, in contrast to animal studies (Sanderford & Bishop, 2000, 2002), some earlier studies showed that physiological elevations of arginine vasopressin did not alter cardiac or sympathetic baroreflex function, while much higher levels of vasopressin enhanced the vasomotor sympathetic response to unloading of baroreceptors in healthy humans (Ebert & Cowley, 1992; Goldsmith, 1994). It was also found that subpressor doses of angiotensin II did not affect plasma noradrenaline (norepinephrine) concentration (Goldsmith & Hasking, 1990); however, much higher doses of angiotensin II increased muscle sympathetic nerve activity (Matsukawa et al. 1991). Although humoral changes were not measured in our subjects, the data suggest that their influences on vasomotor sympathetic neural control were limited.
The continuous increase in muscle sympathetic nerve activity during sustained upright posture was related to the progressive decrease in stroke volume. This result expands previous findings from our laboratory (Levine et al. 2002; Fu et al. 2005a,b) and others (Convertino et al. 2004; Charkoudian et al. 2005), and further suggests that changes in muscle sympathetic nerve activity may be associated with changes in stroke volume. During prolonged orthostasis, transudation of fluid out of the capillaries and into the tissue space in the legs gradually decreases central blood volume and subsequently stroke volume in sustained upright humans (Watenpaugh et al. 1995). Recently, we found in healthy subjects that an index of carotid artery distortion, assessed by high resolution ultrasonography, was closely correlated to stroke volume during a graded upright tilt, while the increase in muscle sympathetic nerve activity was related to the decrease in this index of carotid distortion (Hastings et al. 2006). These preliminary data support the notion that stroke volume may influence the primary stimulus to the baroreceptors (distortion) during orthostasis. It has been demonstrated that stroke volume changes translate into arterial pulse amplitude and pressure changes, which modulate arterial baroreceptor activity (Angell James, 1971; Chapleau & Abboud, 1989). In addition, stroke volume is one of the determinants of flow in baroreceptive arteries (Hajduczok et al. 1988). Moreover, stroke volume is a function of central blood volume and left ventricular end-diastolic volume, and thereby may reflect the stimulus to the myriad receptor populations termed ‘cardiopulmonary’ (Persson et al. 1988).
Since the upright tilt test lasted for only 45 min in the present study, we cannot exclude the possibility that vasomotor sympathetic neural control may decrease during more prolonged (i.e. several hours or days) upright posture in humans. It was difficult for the subjects to stand on one leg, allowing the other leg to be completely relaxed for microneurography for over 45 min in the upright position. Future investigations with improved microneurographic technique, improved tilt beds, and a much longer period of upright tilt are needed to evaluate sympathetic control of vascular resistance over longer time periods.
Baroreflex sensitivity assessment during spontaneous breathing
Sympathetic baroreflex sensitivity was quantified during spontaneous breathing. We recognize that the blood pressure fluctuations during spontaneous breathing were not as large as those obtained using other methods such as the neck-chamber technique or invasive pharmacological manipulation. Therefore the entire baroreflex stimulus–response curve cannot be evaluated in this study. Consequently, we cannot determine whether the operating point on this stimulus–response curve has simply shifted to a steeper part of the curve during upright posture or whether an entirely new relationship is achieved. This problem is compounded by the differential effect of upright posture on hydrostatic gradients at the carotid and aortic baroreceptor populations. Still, a 20 mmHg change in diastolic pressure is within the physiological range and should be a good reflection of the dynamic baroreflex control of muscle sympathetic nerve activity under physiological conditions regardless of whether the curve has shifted or not. Additionally, previous studies have demonstrated that pharmacological and spontaneous baroreflex sensitivity values are closely correlated in most instances (Parlow et al. 1995; Pitzalis et al. 1998). Thus, our data can be used to reveal the physiological modulation of vasomotor sympathetic control around the prevailing, regulated operating point in upright humans.
The baroreflex system is a feedback control system from baroreceptor distortion to systemic arterial pressure. It has been proposed that the baroreflex system has two subsystems; the central arc from baroreceptor distortion to efferent sympathetic nerve activity via the central nervous system and the peripheral arc from efferent sympathetic nerve activity to systemic arterial pressure (Kamiya et al. 2005b). Thus spontaneous arterial pressure and muscle sympathetic nerve activity in a closed-loop condition theoretically result from both central and peripheral arcs. However, due to the limitations of the methodology, it is impossible to isolate one unique subsystem (i.e. baroreflex control of muscle sympathetic nerve activity) from the total system output in human research.
Although diastolic pressure has been found to have the best correlation to the occurrence of sympathetic bursts (Sundlof & Wallin, 1978), it cannot be the primary parameter that determines when a burst occurs. For example, it was observed that the start of the burst began approximately 0.1–0.3 s before the end-diastolic point was reached (B. G. Wallin, personal communication). Therefore, other factors which have an intimate relationship to diastolic pressure may be the primary stimulus to the baroreceptors. We assumed but did not verify in this study that stroke volume may be an index of the primary stimulus (distortion of baroreceptor populations) and assessed sympathetic baroreflex control as the relationship between muscle sympathetic nerve activity and stroke volume. Further studies are needed to verify this assumption.
In conclusion, muscle sympathetic nerve activity increased from supine to upright and continued to increase during sustained upright posture. Sympathetic baroreflex sensitivity increased during changes in posture, but it remained unchanged as upright posture continued. The increase in muscle sympathetic nerve activity was associated with the decrease in stroke volume. The positive relationship between total peripheral resistance and muscle sympathetic nerve activity on an individual basis indicates that vasomotor sympathetic neural control is still important for ‘prolonged’ (i.e. 45 min) arterial pressure control during upright posture in healthy humans.
Acknowledgments
The time and effort put forth by the subjects is greatly appreciated. The authors thank Emily R. Martini, Diane Bedenkop, Peggy Fowler, Murugappan Ramanathan, Cyrus Oufi, and Dak Quarles for their valuable laboratory assistance. This study was supported partially by the American Heart Association Texas Affiliate Post Doctoral Fellowship grant (No. 0225017Y), National Institutes of Health K23 grant (HL075283), and the Wallace, Barbara, and Kelly King Foundation trust. It was also supported by a General Clinical Research Center grant (RR00633).
References
- Angell James JE. The effects of altering mean pressure, pulse pressure and pulse frequency on the impulse activity in baroreceptor fibres from the aortic arch and right subclavian artery in the rabbit. J Physiol. 1971;214:65–88. doi: 10.1113/jphysiol.1971.sp009419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett CJ, Malpas SC. Problems, possibilities, and pitfalls in studying the arterial baroreflexes' influence over long-term control of blood pressure. Am J Physiol Regul Integr Comp Physiol. 2005;288:R837–R845. doi: 10.1152/ajpregu.00456.2004. [DOI] [PubMed] [Google Scholar]
- Chapleau MW, Abboud FM. Determinants of sensitization of carotid baroreceptors by pulsatile pressure in dogs. Circ Res. 1989;65:566–577. doi: 10.1161/01.res.65.3.566. [DOI] [PubMed] [Google Scholar]
- Charkoudian N, Joyner MJ, Johnson CP, Eisenach JH, Dietz NM, Wallin BG. Balance between cardiac output and sympathetic nerve activity in resting humans: role in arterial pressure regulation. J Physiol. 2005;568:315–321. doi: 10.1113/jphysiol.2005.090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Convertino VA, Ludwig DA, Cooke WH. Stroke volume and sympathetic responses to lower-body negative pressure reveal new insight into circulatory shock in humans. Auton Neurosci. 2004;111:127–134. doi: 10.1016/j.autneu.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Cowley AW., Jr Long-term control of arterial blood pressure. Physiol Rev. 1992;72:231–300. doi: 10.1152/physrev.1992.72.1.231. [DOI] [PubMed] [Google Scholar]
- Cowley AW, Jr, Liard JF, Guyton AC. Role of baroreceptor reflex in daily control of arterial blood pressure and other variables in dogs. Circ Res. 1973;32:564–576. doi: 10.1161/01.res.32.5.564. [DOI] [PubMed] [Google Scholar]
- Cui J, Wilson TE, Shibasaki M, Hodges NA, Crandall CG. Baroreflex modulation of muscle sympathetic nerve activity during posthandgrip muscle ischemia in humans. J Appl Physiol. 2001;91:1679–1686. doi: 10.1152/jappl.2001.91.4.1679. [DOI] [PubMed] [Google Scholar]
- Donald DE, Edis AJ. Comparison of aortic and carotid baroreflexes in the dog. J Physiol. 1971;215:521–538. doi: 10.1113/jphysiol.1971.sp009483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert TJ, Cowley AW., Jr Baroreflex modulation of sympathetic outflow during physiological increases of vasopressin in humans. Am J Physiol Heart Circ Physiol. 1992;262:H1372–H1378. doi: 10.1152/ajpheart.1992.262.5.H1372. [DOI] [PubMed] [Google Scholar]
- Fisher SJ, Scher AM, Wyss CR. Long-term responses of atrial rate and peripheral resistance to changes in ventricular pacing rate in awake dogs with atrioventricular block. Circ Res. 1984;54:196–203. doi: 10.1161/01.res.54.2.196. [DOI] [PubMed] [Google Scholar]
- Fu Q, Witkowski S, Levine BD. Vasoconstrictor reserve and sympathetic neural control of orthostasis. Circulation. 2004;110:2931–2937. doi: 10.1161/01.CIR.0000146384.91715.B5. [DOI] [PubMed] [Google Scholar]
- Fu Q, Witkowski S, Okazaki K, Levine BD. Effects of gender and hypovolemia on sympathetic neural responses to orthostatic stress. Am J Physiol Regul Integr Comp Physiol. 2005a;289:R109–R116. doi: 10.1152/ajpregu.00013.2005. [DOI] [PubMed] [Google Scholar]
- Fu Q, Zhang R, Witkowski S, Arbab-Zadeh A, Prasad A, Okazaki K, Levine DB. Persistent sympathetic activation during chronic antihypertensive therapy. A potential mechanism for long term morbidity? Hypertension. 2005b;45:513–521. doi: 10.1161/01.HYP.0000158312.63381.c1. [DOI] [PubMed] [Google Scholar]
- Goldsmith SR. Physiological arginine vasopressin levels do not enhance baroreflex function in normal humans. Am J Physiol Heart Circ Physiol. 1994;266:H2374–H2379. doi: 10.1152/ajpheart.1994.266.6.H2374. [DOI] [PubMed] [Google Scholar]
- Goldsmith SR, Hasking GJ. Subpressor angiotensin II infusions do not stimulate sympathetic activity in humans. Am J Physiol Heart Circ Physiol. 1990;258:H179–H182. doi: 10.1152/ajpheart.1990.258.1.H179. [DOI] [PubMed] [Google Scholar]
- Hajduczok G, Chapleau MW, Abboud FM. Rheoreceptors in the carotid sinus of dog. Proc Natl Acad Sci U S A. 1988;85:7399–7403. doi: 10.1073/pnas.85.19.7399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwill JR. Segregated signal averaging of sympathetic baroreflex responses in humans. J Appl Physiol. 2000;88:767–773. doi: 10.1152/jappl.2000.88.2.767. [DOI] [PubMed] [Google Scholar]
- Hastings J, Shibata S, Shook R, Okazaki K, Conner C, Palmer MD, Fu Q, Levine BD. Changes in stroke volume directly alter carotid artery distortion during upright posture in humans. FASEB J. 2006;20:LB167. doi: 10.1113/jphysiol.2006.118158. Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JM, Rowell LB, Niederberger M, Eisman MM. Human splanchnic and forearm vasoconstrictor responses to reductions of right atrial and aortic pressures. Circ Res. 1974;34:515–524. doi: 10.1161/01.res.34.4.515. [DOI] [PubMed] [Google Scholar]
- Kamiya A, Hayano J, Kawada T, Michikami D, Yamamoto K, Ariumi H, Shimizu S, Uemura K, Miyamoto T, Aiba T, Sunagawa K, Sugimachi M. Low-frequency oscillation of sympathetic nerve activity decreases during development of tilt-induced syncope preceding sympathetic withdrawal and bradycardia. Am J Physiol Heart Circ Physiol. 2005a;289:H1758–H1769. doi: 10.1152/ajpheart.01027.2004. [DOI] [PubMed] [Google Scholar]
- Kamiya A, Kawada T, Yamamoto K, Michikami D, Ariumi H, Uemura K, Zheng C, Shimizu S, Aiba T, Miyamoto T, Sugimachi M, Sunagawa K. Resetting of arterial baroreflex increases orthostatic sympathetic activation and prevents postural hypotension in rabbits. J Physiol. 2005b;566:237–246. doi: 10.1113/jphysiol.2005.086512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienbaum P, Karlssonn T, Sverrisdottir YB, Elam M, Wallin BG. Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol. 2001;531:861–869. doi: 10.1111/j.1469-7793.2001.0861h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine BD, Buckey JC, Fritsch JM, Yancy CW, Jr, Watenpaugh DE, Snell PG, Lane LD, Eckberg DL, Blomqvist CG. Physical fitness and cardiovascular regulation: mechanisms of orthostatic intolerance. J Appl Physiol. 1991;70:112–122. doi: 10.1152/jappl.1991.70.1.112. [DOI] [PubMed] [Google Scholar]
- Levine BD, Pawelczyk JA, Ertl AC, Cox JF, Zuckerman JH, Diedrich A, Biaggioni I, Ray CA, Smith ML, Iwase S, Saito M, Sugiyama Y, Mano T, Zhang R, Iwasaki K, Lane LD, Buckey JC, Jr, Cooke WH, Baisch FJ, Eckberg DL, Blomqvist CG. Human muscle sympathetic neural and haemodynamic responses to tilt following spaceflight. J Physiol. 2002;538:331–340. doi: 10.1113/jphysiol.2001.012575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukawa T, Gotoh E, Minamisawa K, Kihara M, Ueda S, Shionoiri H, Ishii M. Effects of intravenous infusions of angiotensin II on muscle sympathetic nerve activity in humans. Am J Physiol Regul Integr Comp Physiol. 1991;261:R690–R696. doi: 10.1152/ajpregu.1991.261.3.R690. [DOI] [PubMed] [Google Scholar]
- Parlow J, Viale JP, Annat G, Hughson R, Quintin L. Spontaneous cardiac baroreflex in humans. Comparison with drug-induced responses. Hypertension. 1995;25:1058–1068. doi: 10.1161/01.hyp.25.5.1058. [DOI] [PubMed] [Google Scholar]
- Persson P, Ehmke H, Kirchheim H, Seller H. Effect of sino-aortic denervation in comparison to cardiopulmonary deafferentiation on long-term blood pressure in conscious dogs. Pflugers Arch. 1988;411:160–166. doi: 10.1007/BF00582309. [DOI] [PubMed] [Google Scholar]
- Pitzalis MV, Mastropasqua F, Passantino A, Massari F, Ligurgo L, Forleo C, Balducci C, Lombardi F, Rizzon P. Comparison between noninvasive indices of baroreceptor sensitivity and the phenylephrine method in post-myocardial infarction patients. Circulation. 1998;97:1362–1367. doi: 10.1161/01.cir.97.14.1362. [DOI] [PubMed] [Google Scholar]
- Robertson D, Hollister AS, Biaggioni I, Netterville JL, Mosqueda-Garcia R, Robertson RM. The diagnosis and treatment of baroreflex failure. N Engl J Med. 1993;329:1449–1455. doi: 10.1056/NEJM199311113292003. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Human Cardiovascular Control. New York, Oxford: Oxford University Press; 1993. [Google Scholar]
- Sanderford MG, Bishop VS. Angiotensin II acutely attenuates range of arterial baroreflex control of renal sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 2000;279:H1804–H1812. doi: 10.1152/ajpheart.2000.279.4.H1804. [DOI] [PubMed] [Google Scholar]
- Sanderford MG, Bishop VS. Central mechanisms of acute ANG II modulation of arterial baroreflex control of renal sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 2002;282:H1592–H1602. doi: 10.1152/ajpheart.00222.2001. [DOI] [PubMed] [Google Scholar]
- Sundlof G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol. 1978;274:621–637. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triebwasser JH, Johnson RL, Burpo RP, Campbell JC, Reardon WC, Blomqvist CG. Noninvasive determination of cardiac output by a modified acetylene rebreathing procedure utilizing mass spectrometer measurements. Aviat Space Environ Med. 1977;48:203–209. [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev. 1979;59:919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Delius W, Sundlof G. Human muscle nerve sympathetic activity in cardiac arrhythmias. Scand J Clin Lab Invest. 1974;34:293–300. doi: 10.3109/00365517409049883. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Sundlof G. Sympathetic outflow to muscles during vasovagal syncope. J Auton Nerv Syst. 1982;6:287–291. doi: 10.1016/0165-1838(82)90001-7. [DOI] [PubMed] [Google Scholar]
- Watenpaugh DE, Vissing SF, Lane LD, Buckey JC, Firth BG, Erdman W, Hargens AR, Blomqvist CG. Pharmacologic atrial natriuretic peptide reduces human leg capillary filtration. J Cardiovasc Pharmacol. 1995;26:414–419. doi: 10.1097/00005344-199509000-00011. [DOI] [PubMed] [Google Scholar]