Abstract
In patients with high spinal cord injuries autonomic dysfunction can be dangerous, leading to medical complications such as postural hypotension, autonomic dysreflexia and temperature disturbance. While animal models have been developed to study autonomic dysreflexia, associated temperature changes have not been documented. Our aim here was to use radiotelemetry and infrared thermography in rodents to record the development of cardiovascular and skin temperature changes following complete T4 transection. In adult male Wistar rats (n = 5), responses were assessed prior to spinal cord injury (intact) and for 6 weeks following injury. Statistical analysis by a repeated-measure ANOVA revealed that following spinal cord injury (SCI), rats exhibited decreased mean arterial pressure (MAP, average decrease of 26 mmHg; P < 0.035) and elevated heart rate (HR, average increase of 65 bpm, P < 0.035) at rest. The basal core body temperature following SCI was also significantly lower than intact levels (−0.9°C; P < 0.0035). Associated with this decreased basal core temperature following SCI was an increased skin temperature of the mid-tail and hindpaw (+5.6 and +4.0°C, respectively; P < 0.0003) consistent with decreased cutaneous vasoconstrictor tone. Autonomic dysreflexia, in response to a 1 min colorectal distension (25 mmHg), was fully developed by 4 weeks after spinal cord transection, producing increases in MAP greater than 25 mmHg (P < 0.0003). In contrast to the tachycardia seen in intact animals in response to colorectal distension, SCI animals exhibited bradycardia (P < 0.0023). During episodes of autonomic dysreflexia mid-tail surface temperature decreased (approx. −1.7°C, P < 0.012), consistent with cutaneous vasoconstriction. This is the first study to compare cardiovascular dysfunction with temperature changes following spinal cord transection in rats.
Patients with high level spinal cord injuries prioritize the recovery of autonomic functions such as bowel and bladder control, cardiovascular and sexual function above the ability to walk (Anderson, 2004). One factor contributing to the importance of autonomic function for spinally injured patients is the danger posed when autonomic regulation has been disturbed. Common occurrences such as a full bladder or bowel, or an undetected skin lesion can lead to sudden and severe increases in blood pressure which, along with other symptoms, are known as autonomic dysreflexia (Shergill et al. 2004). These other symptoms include throbbing headaches, upper body flushing and sweating due to vasodilatation of upper body blood vessels and cold lower body extremities due to the massive vasoconstriction occurring below the level of the injury (Teasell et al. 2000). In addition, common events such as mild exercise or changes in ambient temperatures can result in dangerous levels of hypothermia or hyperthermia for the patient (McLean et al. 1999; Price & Campbell, 1999).
Cardiovascular complications are common in quadriplegia because injuries in the cervical or upper thoracic regions disrupt the control of sympathetic outflow to major vascular beds and thus impair blood pressure regulation (Gao et al. 2002). Following a high spinal cord injury, patients usually develop long lasting hypotension (Mathias & Frankel, 1999), which is attributed to a decreased level of sympathetic activity (Stjernberg et al. 1986; Mathias & Frankel, 1999). Basal noradrenaline levels during the chronic phase are as low as during spinal shock, suggesting that decreased sympathetic activity continues during this phase (Mathias & Frankel, 1999). However a study in rats has reported that spinalized rats recover normal basal mean arterial pressure (MAP) levels 2 days after a clip compression injury which leaves some decscending pathways intact (Mayorov et al. 2001). It has been reported that the heart rate (HR) of spinal patients does not change much and, if anything, decreases below normal rates (Mathias & Frankel, 1999). However, like the data on basal MAP, variation exists in the HR data with some reports in animals differing from this suggestion (Mayorov et al. 2001; Collins et al. 2006).
Although autonomic dysreflexia is a common occurrence in patients with high SCI, the time course of its development has not been extensively investigated. Symptoms of autonomic dysreflexia can present in patients 2–3 months following the injury (Erickson, 1980; Karlsson, 2006). However, Krassioukov et al. (2003) have reported its occurrence within the first month following injury in 5.7% of at-risk patients. Animal studies have reported that full autonomic dysreflexia develops between 2 and 4 weeks following spinal cord injury (Krenz & Weaver, 1998; Mayorov et al. 2001; Marsh et al. 2002; Marsh & Weaver, 2004). It has been suggested that this delay in development may be related, amongst other changes, to the time course of formation of increased afferent arbours in the lower spinal cord, under the influence of increased intraspinal nerve growth factor levels following the trauma (Krenz & Weaver, 1998). It is proposed that this afferent sprouting leads to amplification of afferent inputs, such as those from the bladder and bowel, to interneurons and thus sympathetic preganglionic neurons (Krenz et al. 1999). Stimulation of these preganglionic neurons leads to increased sympathetic nerve activity, especially that of a major sympathetic bed, the splanchnic vasculature, thus having a profound effect on peripheral resistance and overall MAP. The problem is worsened by the loss of descending control from the baroreflex that would usually serve to dampen such increases in sympathetic nerve activity.
High spinal cord injury also impairs thermoregulation because it disconnects the thoracic cord from central thermoregulatory centres thus preventing central control of cutaneous blood flow, sweating, piloerection and shivering (Downey et al. 1967, 1973). This disruption leads to spinal patients having a greater susceptibility to changes in core temperature resulting from environmental temperature changes, exercise or infection (Sugarman et al. 1982; Montgomerie, 1997; McLean et al. 1999; Price & Campbell, 1999). The time course of the development of thermoregulatory dysfunction following spinal cord injury has not been investigated previously. In addition to this, episodes of autonomic dysreflexia are known to challenge thermoregulation and can lead to severe hyperthermia (Colachis, 2002). Although cold surface temperature and pale lower body extremities are commonly reported as symptoms of autonomic dysreflexia (Karlsson, 2006), the time course of the development of these abnormal temperature profiles and their relationship to other parameters is not known.
In the present study we have used infrared thermography and radiotelemetry after complete cord transection to follow the development of altered baseline autonomic parameters, autonomic dysreflexia and the temperature changes that accompany it.
Methods
Experiments were performed on five adult male Australian inbred Wistar (AAW) rats (Biological Resource Centre, Sydney, New South Wales) (300–350 g). From each rat, recordings were made 1 day prior to spinal cord transection (intact values) as well as 1, 3 and 5 days (acute phase) and then weekly for 1–6 weeks (chronic phase) post-transection. Although the chronic phase referred to in this study would be considered too early to be chronic in a clinical setting, this term has been used for easy reference to the later stage of our study. All experimental procedures were approved by the Animal Care and Ethics Committee of the University of New South Wales in accordance with guidelines of the National Health and Medical Research Council of Australia for animal research. All animals were killed using an overdose of sodium pentobarbitone (200 mg kg−1i.p.) following completion of the study (7 weeks following transection).
Radiotelemetric probe implantation
Implantation of radiotelemetric probes (PA-C40, Data Sciences International St. Paul, MN, USA.) was the same as previously reported (Choi et al. 2005). The probes were prepared for implantation by sterilization with a 2% glutaldehyde solution for 24 h prior to use, as well as a final rinse in saline solution for 30 min prior to implantation. Rats were then anaesthetized with an intraperitoneal injection of ketamine (120 mg kg−1) and xylazine (6.5 mg kg−1). The abdomen was shaved and swabbed with iodine, and a 6 cm incision was made on the midline. The abdominal aorta was isolated and retracted with 3.0 silk to allow the bifurcation of the abdominal aorta to be pierced and the probe catheter inserted. Tissue adhesive (VetBond, 3M) was used to secure the catheter and cellulose strips (3M) were added to better anchor the catheter to the aorta. Antibiotic (Cephalothin, 30 mg kg−1i.p.) was administered and pain management was accomplished with injections of the non-steriodal anti-inflammatory Carprofen (50 mg kg−1s.c.) post-operatively and at any stage that pain was observed during recovery. Post-operative observations were made for 2 weeks and any rat exhibiting poor hindlimb vascular perfusion (observed as a cyanotic hindpaw with limited mobility and sensation) was killed.
Spinal cord transection
Two weeks after probe implantation, rats were rendered paraplegic via a complete transection of the spinal cord at the fourth thoracic spinal level under anaesthesia (ketamine and xylazine, 120 mg + 6.5 mg kg−1i.p.). Following dissection of the superficial and deep muscles of the back, a laminectomy of the T3 vertebra was performed to reveal the T4 spinal segment for transection with microscissors. Complete transection was confirmed with a scalpel blade and observation under the dissecting microscope. Following transection, a gap of approximately 1–2 mm was present between the rostral and caudal ends of the cord and a piece of gelfoam was inserted to fill the gap and reduce bleeding. Gelfoam was also placed on the dorsal surface of the cord and the muscle tissue and skin were sutured in layers.
Postoperative care
Rats received an antibiotic (Cephalothin, 10 mg kg−1s.c.) twice daily until no sign of urinary infection (noted by cloudiness of expressed urine) was seen for three consecutive days. Animals also received analgesic (Carprofen, 50 mg kg−1s.c.) daily for 2–3 days and saline solution (5 ml of 0.9% saline s.c.) to provide hydration until the rat could drink following the surgery. Manual emptying of the urinary bladder was carried out 3 times a day until the rats developed automatic bladder emptying (usually 2 weeks post-transection). For the first three days following transection heat mats were placed under the rats' cages in order to help keep their body temperature within the normal range. The room temperature was maintained at 27–28°C and rat core temperature was measured regularly until a normal temperature was maintained and then the room temperature was reduced to approximately 23°C. Other interventions included administrating laxative (Catlax, Novartis Australasia Pty Ltd, North Ryde, Australia) if bowel impaction occurred and dietary supplements (Nutrigel, Troy Laboratories Pty Ltd, Smith field, Australia) were given to treat weight loss. Rats were weighed daily and monitored for skin lesions or swelling.
Infrared thermography and core temperature recording
Infrared thermographic images were taken with an infrared camera (ThermaCam P45, FLIR Systems Inc, Boston, MA, USA) as previously described (Vianna & Carrive, 2005). The camera has a sensitivity of 0.1°C and an accuracy of 0.6°C. Emissivity was set at 0.98 (the emissivity of skin and fur) and room temperature was approximately 23°C. Images of the rats' whole body at rest in their home cage were taken from a distance of 0.7 m at pre- and post-operative time points. The infrared images were analysed with the program ThermaCAM QuickView which extracts the temperature value of pixels on saved jpeg images. Single pixel temperature values were extracted for the tail tip, mid-tail and feet. In addition core body temperature was recorded with a digital thermometer inserted into the rectum approximately 1 min after the capture of the IR image.
Colorectal distension
The responses to colorectal distension (CRD) were recorded in two different manners, in the restrained and unrestrained animal. Both techniques involved the 1 min inflation of a colorectal balloon (1.5 cm long × 8 mm wide PTA dilatation catheters, Cordis Endovascular, North Ryde, Australia) with 1.3 ml water (exerting a pressure of approximately 25 mmHg) to trigger autonomic dysreflexia.
Restrained CRD for cardiovascular recordings
Weekly MAP and HR responses to colorectal distension were recorded in the restrained rat. During this recording it was important that rats (intact and SCI) be restrained in a Perspex tube to prevent excessive movement and to prevent the rats from biting or pulling out the colorectal balloon. This procedure involved a 2 h prerecording period to allow rats to acclimatize to the restraint and colorectal balloon. After this period, when rats were more relaxed in their environment, as indicated by a stable HR and MAP, the balloon was inflated for 1 min. The average response was calculated from three distensions made during the recording period. The entire cardiovascular response to colorectal distension, including a 10 min period following the distension, was recorded and the average peak changes in MAP and HR were calculated.
Unrestrained CRD for temperature recordings
A different procedure had to be used to allow weekly recording of core and body surface temperature during CRD. This was necessary because (i) it was not possible for the rectal thermometer and colorectal balloon to be in place at the same time, and (ii) infrared light does not pass through Perspex, which was the material used for the restraint tubes. To overcome these problems colorectal distension was performed in unrestrained animals (to provide an unobstructed view of the rats' surface) and the temperature measurements (core and surface) were made 1 min prior to inserting the balloon and again 1 min after removing the balloon following colorectal distension. The short period that the rectal balloon was in place resolved the issue of rats pulling out and biting the balloon.
The same set-up was used for continuous recording of the rat's surface temperature during autonomic dysreflexia 6 weeks post-transection. In this case, because of the presence of the rectal balloon, rectal temperature was not measured at the same time. The recording lasted 16 min, Consisting of 8 min baseline period prior to inflation followed by an 8 min recovery period following the distension. Continuous recording of the surface temperature during CRD in intact animals was not possible because they needed to be restrained in the Perspex tubing.
Radiotelemetric data collection
At least half an hour prior to recording cardiovascular baselines and CRD responses, radiotelemetric probes were turned on. MAP and HR were collected for 3 s every 10 s via a data acquisition package (Dataquest ART, Data Sciences International St. Paul, MN, USA). Baseline cardiovascular recordings were made over a 30 min period whilst rats were at rest in their home cage. The average MAP and HR for each rat was then calculated over the entire 30 min period. CRD response recordings were made with the same settings.
Data analysis
The software package StatView 5.0 (SAS Institute, Cary, NC, USA) was used for all statistical comparisons. Cardiovascular and temperature parameters of rats before and after spinal transection, both at rest and in response to CRD, were compared statistically using a repeated-measure analysis of variance (ANOVA) where the repeated measure was time measured in days (for the acute data) or weeks (for the chronic data). If the repeated measure analysis identified that there was a significant effect of time, i.e. that the response changed over the time period measured, the data was analysed further with Fisher's post hoc test. This test identified at which time points the results were significantly different. All results were considered significant if P < 0.05.
Results
Basal cardiovascular and temperature parameters before and after SCI
The cardiovascular and temperature baseline values before and after SCI are displayed in Fig. 1A. Two post-transection phases will be considered: the acute injury phase (days 1, 3 and 5) and the chronic phase (weeks 1–6).
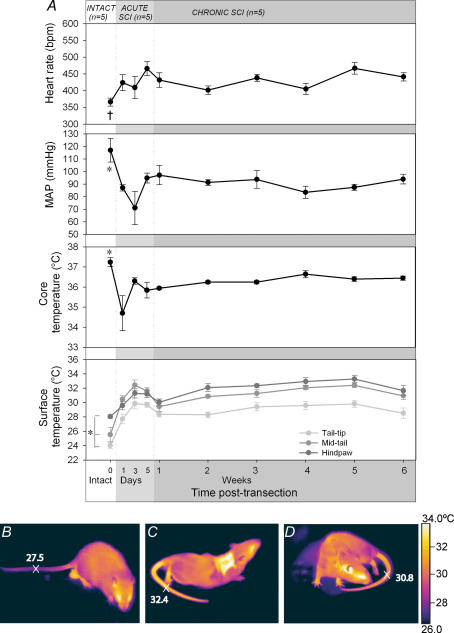
Figure 1. The baseline cardiovascular and temperature parameters of rats prior to SCI (intact) and during the acute and chronic SCI phases.
A, baseline HR, MAP, core (rectal) temperature and surface temperature (tail tip, mid-tail and hind paw) are shown (means ±s.e.m.). Intact points marked with an asterisk (*) are significantly different from all weeks during the chronic SCI phase. † indicates that the intact value is significantly different from weeks 1, 3, 5 and 6. All differences displayed are P < 0.05. Thermographic images showing the surface temperature of intact (B), acute SCI (C) and chronic SCI (D) animals at rest. The surface temperature of the mid-tail region of each animal is indicated. The scar region of acute SCI rat in C is warmer due to inflammation and the shorter hair in this shaved region.
Changes in basal MAP
During the acute recovery phase following SCI (defined here as days 1–5) basal MAP was markedly reduced before it settled at a new baseline level, lower than the previous intact MAP. A repeated-measure ANOVA confirmed that there was a significant effect of time on the baseline MAP during these first five days (F4,3 = 4.9, P = 0.019). Post hoc analysis revealed that the difference occurred between the intact MAP levels and days 1 and 3 post-transection (P < 0.032; see Fig. 1A). MAP was lowest 3 days post-transection (71 ± 13.3 mmHg compared with the intact level of 117 ± 9.5 mmHg) but by 5 days post-transection it had risen to 95 mmHg where it remained reasonably stable for the 6 weeks to follow. The lower baseline MAP present throughout the chronic SCI phase was confirmed by a significant effect of time in a repeated-measure ANOVA (F4,6 = 3.0, P = 0.027). Post hoc analysis revealed that the difference with pretransection level was present for each week of the chronic SCI phase (weeks 1–6; P < 0.035).
Changes in basal HR
Although Fig. 1A displays an increased basal HR during the acute phase following SCI, a repeated-measure ANOVA did not find this difference to be statistically significant (F4,3 = 2.4, P = 0.12; Fig. 1A). An increased basal HR can also be seen during the chronic SCI phase and in this case there was a significant time effect (F4,6 = 6.2, P = 0.0005). The increased HR was variable with significant differences occurring between the intact HR level and those of 1, 3, 5 and 6 weeks post-transection (P < 0.016). Overall, the average HR of rats during the chronic SCI phase was elevated compared to their intact HR level (increased from 366 ± 11.8 bpm to 431 ± 7.2 bpm).
Changes in basal core temperature
Following SCI the rats' core temperatures fell dramatically for the first day (from 37.2°C to 34.7°C), after which point the temperature increased but did not return to pretransection levels (Fig. 1A). Statistical analysis confirmed that the basal core temperature changed over the acute injury period (F4,3 = 5.3, P = 0.014) despite the use of raised room temperature (27.0°C) and heating mats. Post hoc analysis further confirmed that whilst the core temperature of rats was markedly reduced 1 day following injury (P = 0.002), by the third day post-transection it had returned to an almost normal level (36.3°C, P = 0.17). Core temperature improved enough after 1 week post-transection to allow the cessation of use of heating mats and lowering of room temperature to 23°C. However core temperature levels remained significantly lower than the pretransection for all 6 weeks that followed injury. There was a significant time effect (F4,6 = 9.8, P < 0.0001) and post hoc analysis revealed that core temperature was lower than pretransection levels each week post-SCI (P < 0.0035).
Changes in basal surface temperature
As core temperatures decreased during the acute SCI period, surface temperatures increased in the tail tip, mid-tail (+6.0°C) and hindpaw (+2.7°C) regions (F4,3 > 8.514, P < 0.0027; Fig. 1A). The tail regions were significantly warmer for each day of the acute SCI phase compared to intact temperatures (P < 0.001). The hindpaw surface temperature was significantly warmer 3 and 5 days post-transection than before injury (P < 0.0013), with the temperature 3 days following injury significantly higher than on the first day post-transection (P = 0.039). These differences are visible in the comparison of infrared thermal images of the intact (Fig. 1B) and acute SCI rats (Fig. 1C). The tail and hindpaw surface temperatures remained elevated during the chronic SCI period (F4,6 > 13.7, P < 0.0001; Fig. 1A) with each week post-transection showing higher surface temperatures than intact levels (P < 0.003). The warm surface temperature of rats with chronic SCI can be seen in Fig. 1D compared to the lower surface temperature of intact animals (Fig. 1B).
Development of autonomic dysreflexia
The time course of development of hypertensive responses to CRD, as well as the HR and temperature responses associated with it, is displayed in Fig. 2.
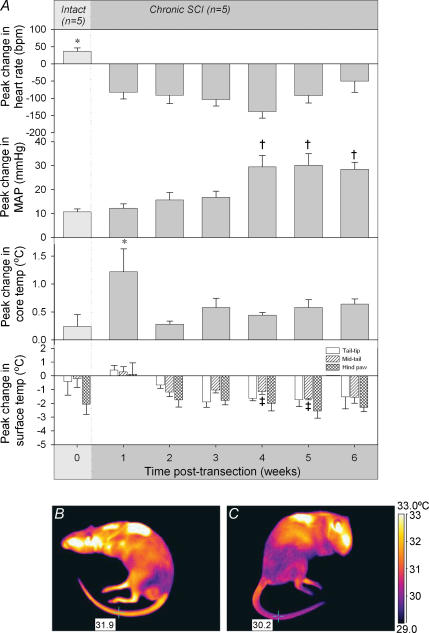
Figure 2. The cardiovascular and temperature changes that occur in responses to CRD during the chronic SCI period.
A, the peak change in heart rate, MAP, core (rectal) temperature and surface temperature (tail tip, mid-tail and hindpaw) in response to CRD were measured weekly. Average responses marked with an asterisk (*) were significantly different from the equivalent response of all other weeks; † indicates that the response is significantly different from responses from weeks 0–3; and ‡ indicates that the response was significantly different from week 0. All differences displayed are P < 0.05. B and C, thermographic images showing the surface temperature of a chronic SCI rat (5 weeks post-transection) before (B) and during (C) an episode of autonomic dysreflexia induced by CRD. Comparison of these images shows that the mid-tail temperature decreased by 1.7°C during CRD.
Changes in MAP in response to CRD
Whilst they were intact, animals exhibited a small increase in MAP (10 mmHg) in response to CRD (Fig. 2A). After SCI this response was clearly enhanced, especially in the final 3 weeks tested. A repeated measure ANOVA confirmed that there was an effect of time on the peak change in MAP response to CRD (F4,6 = 9.4, P < 0.0001). Post hoc analysis revealed that during the first 3 weeks following SCI, rats exhibited MAP responses similar to that of intact animals (average increase of 15 mmHg, P > 0.16; Fig. 2A). In contrast, during weeks 4–6 following transection, the MAP response was significantly larger than that of intact rats (P < 0.0003) with hypertensive responses indicative of full autonomic dysreflexia (increases in MAP larger than 20 mmHg).
Changes in HR in response to CRD
There were marked differences between the pre- and post-transection peak changes in HR in response to CRD (F4,6 = 9.7, P < 0.0001). Prior to SCI rats exhibited a tachycardia (+36 bpm) in response to the distension, compared to a bradycardia (−93 bpm) recorded following SCI (Fig. 2A). Post hoc analysis revealed that significantly different HR responses were present each week following SCI compared to prior to injury (P < 0.0023).
Changes in core temperature in response to CRD
Prior to SCI, as well as in the chronic phase following injury, animals exhibited small increases in core temperature in response to CRD (Fig. 2A). A repeated measure ANOVA revealed that core temperature did vary from intact levels through the SCI period (F4,6 = 3.5, P = 0.012). However post hoc analysis revealed that the only responses different from that prior to SCI were those during the first week post-transection (increases in temperature more than 5 times that seen in intact animals, P = 0.0005) and that this acute response was significantly different from all other weeks post-transection (P < 0.027).
Changes in surface temperature in response to CRD
The surface temperature of the rats' tail did not change in response to CRD before SCI but that of the hindpaw decreased (Fig. 2A). After SCI decreases in surface temperature were observed in the tail but no further decrease occurred in the hindpaw. The effect reached statistical significance in the mid-tail (F4,6 = 4.1, P = 0.0058). The decrease in surface temperature seen in the mid-tail region was significantly greater 5 and 6 weeks post-transection (−1.7 ± 0.1°C and −1.6 ± 0.4°C) compared to the change seen before SCI (−0.2 ± 0.6°C, P < 0.012). The tail tip also decreased by varying amounts between 2 and 6 weeks after SCI, but this decrease was not significant (F4,6 = 2.1, P = 0.0975). Figure 2 displays the typical infrared images observed in SCI rats when imaged before (Fig. 2B) and during an episode of autonomic dysreflexia (Fig. 2C).
Cardiovascular and temperature changes during colorectal distension
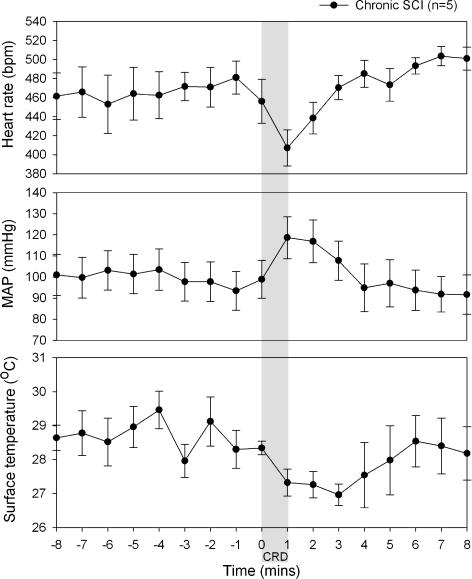
Figure 3 displays the time course of cardiovascular and temperature changes before, during and after CRD recorded 6 weeks after SCI. During the CRD the SCI rats exhibited bradycardia (−60 bpm) and large increases in MAP (+25 mmHg). These changes were maintained for 4 min following the start of distension. CRD also caused the surface temperature of the rats' mid-tail region to decrease (peak decrease of −1.5°C), for a period of 5 min.
Figure 3. Changes in MAP, HR and mid-tail surface temperature during an episode of autonomic dysreflexia 6 weeks following transection.
The shaded area corresponds to the 1 min period during which CRD was performed.
Discussion
This is the first study to use radiotelemetric recording of MAP and HR in spinally transected animals to allow recordings of basal cardiovascular parameters as well as the time course of development of autonomic dysreflexia. It is also the first time that infrared thermography has been used to study the spinally injured rat. This technique allowed analysis of the surface temperature of various body parts and thus provided an indication of heat dissipation and cutaneous vasoconstriction (Cena & Clark, 1973), both at rest and during episodes of autonomic dysreflexia.
Basal parameters
This study confirms that following spinal cord injury, basal MAP is approximately 20 mmHg lower than prior to injury. An interesting finding is that prior to the stabilization of MAP at this lower level, MAP initially falls by over 40 mmHg for several days following spinal cord injury. This observation suggests that in the acute phase following SCI, rats experience a low level of sympathetic activity, perhaps due to the generalized depression of spinal reflexes, known as spinal shock, which occurs during this period (Meshkinpour et al. 1985). The prolonged hypotension that continues throughout the experiment, even after this period of spinal shock has subsided, is ultimately due to the loss of tonic vasoconstrictor tone (below the level of the lesion) usually maintained by supraspinal structures (Krassioukov & Claydon, 2006).
One parameter that can potentially assist in normalizing low levels of MAP is HR, since the autonomic control of the heart is still intact (both the vagal control and the sympathetic component that exits the spinal cord from T1–3). Indeed, basal HR levels are elevated following spinal cord injury. This tachycardia serves to counter the hypotension that has developed in these animals. It may be mediated in part by a decrease in vagal drive as well as through an increase in sympathetic excitation.
Another consequence of the injury was the fall in core temperature that occurred following SCI, particularly during the first week post-transection. The initial marked fall in core temperature was probably caused by a decreased level of sympathetic activity due to the spinal shock condition of acute SCI. This decreased sympathetic activity would have led to a loss of vasoconstrictor tone in cutaneous blood vessels and thus increased skin blood flow, causing heat loss through dissipation to the colder ambient air. Indeed the tail and the hindpaws were warmer after SCI, indicating that these areas were dissipating more heat than before SCI. The heat lost from the tail would have been quite substantial because of its large surface area. This large surface area makes the tail one of the most important thermoregulatory organs in the rat (Rand et al. 1965), just as the skin surface is important in humans.
The hypothermia was particularly marked on the first day after transection but improved, to some degree, within 3 days. The partial recovery of core temperature that followed may have been due to the resolution of spinal shock. Another explanation for the partial recovery of core temperature is a compensatory increase in thermogenesis, perhaps mediated by the interscapular brown adipose tissue in a similar manner to the increased HR present for recovery of MAP. Like the heart, interscapular brown adipose tissue is controlled by sympathetic preganglionic neurons located in the thoracic spinal cord above the level of injury (Morrison, 2004). Thus interscapular brown adipose tissue up-regulation may have played an important role in compensating for the heat lost from cutaneous territories located below the lesion.
Autonomic dysreflexia
Analysis of the time course of development of autonomic dysreflexia revealed that it took 4 weeks for full hypertension, indicative of autonomic dysreflexia, to occur. The only other investigation of the development of autonomic dysreflexia conducted with radiotelemetry was completed by Mayorov et al. (2001) who used the clip compression model of spinal cord injury rather than complete transection. This compression model produces a contusion of the spinal cord similar to that produced by many human spinal trauma injuries (Noble & Wrathall, 1985). However a contusion injury can spare some descending pathways, which may contribute to variable amounts of recovery (Noble & Wrathall, 1985). The sparing of fibres in the compression model may account for the recovery of basal MAP seen within 2 days post-injury in the study of Mayorov et al. (2001), which contrasts with the persistent hypotension seen here and in spinal patients (Bravo et al. 2004). Maiorov et al. (1997) have also recorded autonomic dysreflexia following complete transection; however, in that study they used acute blood pressure recordings which limited recording of the development of altered cardiovascular parameters and autonomic dysreflexia at multiple time points over an extended period.
Marsh et al. (2002) and Marsh & Weaver (2004) reported that autonomic dysreflexia was present after 2 weeks following a clip compression injury at T4. However, the balloon volume was critical, with 1 ml distension failing to cause dysreflexia (Marsh & Weaver, 2004) while 2 ml distension caused MAP to increase gradually over 2–6 weeks. As might be expected, our use of 1.3 ml was found to give intermediary results between these, taking 4 weeks to develop. However, balloon volume alone is not the only factor that might account for these variations. The studies also differed in the type of injury (clip compression versus transection), method of recording MAP (acute cannulation versus telemetry), balloon size (inflated diameter 1.5 cm versus 0.8 cm), pressure (balloon, 25 mmHg, Marsh et al. (2002) and this paper; colonic transmural, 70–95 mmHg, Marsh & Weaver (2004)) and location (inserted 4 cm beyond the anus into the colon, Marsh & Weaver versus mainly rectal in the current study) and any of these might affect the development of the dysreflexic responses. Thus any studies seeking to record and compare fully developed autonomic dysreflexia need to be aware of these variables and allow sufficient time after cord injury.
The rise in MAP during autonomic dysreflexia is caused by an exaggerated increase in sympathetic outflow which increases peripheral vascular resistance below the level of injury. The vascular bed responsible for this effect is likely to involve that of the splanchnic area, but other areas are likely to have made a contribution. In particular we observed a drop in surface temperature in the mid-tail, indicating that vasoconstriction also occurs in the cutaneous vascular bed of this area. This decreased surface temperature corresponds to the cold and pale lower body extremities seen during autonomic dysreflexia in spinal patients (Karlsson, 2006). The fall in mid-tail temperature was only significant at weeks 5 and 6 when the MAP responses to CRD are maximal. Although the change in surface temperature of the tail-tip during CRD was not significantly greater than intact changes it followed a similar trend to the mid-tail region. In this case, and further cases where results approached significance, a type II (β) statistical error may be present due to the small sample size. The fall in tail temperature during episodes of fully developed autonomic dysreflexia suggests widespread increases in cutaneous vascular resistance contribute to the raised MAP seen. Nevertheless the effect remained small (and short) and was not observed in the hindpaws. This may be the reason that no significant increases in core temperature developed in response to CRD.
By comparing the observations of cardiovascular and temperature responses following SCI, this study has presented for the first time the extent of autonomic dysfunction that occurs following full spinal cord transection as well as the relationship between the two parameters. Cardiovascular and thermal dysfunction in the rat after SCI can provide a useful model for understanding autonomic changes in spinal patients, although the time course of development is faster in rats. Recording the dynamic changes in surface temperature of spinal patients, such as through the monitoring of skin temperature in the toe, might allow the detection of episodes of autonomic dysreflexia before they manifest as more severe symptoms such as headache. The early detection of autonomic dysreflexia during the presymptomatic stage would aid the care of spinal patients by allowing earlier elimination of the triggering stimuli, thus preventing the need for vasoactive drugs.
Acknowledgments
This work was supported by University of New South Wales Goldstar grant and the International Spinal Research Trust.
References
- Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21:1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- Bravo G, Guizar-Sahagun G, Ibarra A, Centurion D, Villalon CM. Cardiovascular alterations after spinal cord injury: an overview. Curr Med Chem Cardiovasc Hematol Agents. 2004;2:133–148. doi: 10.2174/1568016043477242. [DOI] [PubMed] [Google Scholar]
- Cena K, Clark JA. Thermographic measurements of the surface temperatures of animals. J Mammal. 1973;54:1003–1007. [PubMed] [Google Scholar]
- Choi EA, Leman S, Vianna DM, Waite PM, Carrive P. Expression of cardiovascular and behavioural components of conditioned fear to context in T4 spinally transected rats. Auton Neurosci. 2005;120:26–34. doi: 10.1016/j.autneu.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Colachis SC., 3rd Hypothermia associated with autonomic dysreflexia after traumatic spinal cord injury. Am J Phys Med Rehabil. 2002;81:232–235. doi: 10.1097/00002060-200203000-00013. [DOI] [PubMed] [Google Scholar]
- Collins HL, Rodenbaugh DW, DiCarlo SE. Spinal cord injury alters cardiac electrophysiology and increases the susceptibility to ventricular arrhythmias. Prog Brain Res. 2006;152:275–288. doi: 10.1016/S0079-6123(05)52018-1. [DOI] [PubMed] [Google Scholar]
- Downey JA, Chiodi HP, Darling RC. Central temperature regulation in the spinal man. J Appl Physiol. 1967;22:91–94. doi: 10.1152/jappl.1967.22.1.91. [DOI] [PubMed] [Google Scholar]
- Downey JA, Huckaba CE, Myers SJ, Darling RC. Thermoregulation in the spinal man. J Appl Physiol. 1973;34:790–794. doi: 10.1152/jappl.1973.34.6.790. [DOI] [PubMed] [Google Scholar]
- Erickson RP. Autonomic hyperreflexia: pathophysiology and medical management. Arch Phys Med Rehabil. 1980;61:431–440. [PubMed] [Google Scholar]
- Gao SA, Ambring A, Lambert G, Karlsson AK. Autonomic control of the heart and renal vascular bed during autonomic dysreflexia in high spinal cord injury. Clin Auton Res. 2002;12:457–464. doi: 10.1007/s10286-002-0068-0. [DOI] [PubMed] [Google Scholar]
- Karlsson AK. Autonomic dysfunction in spinal cord injury: clinical presentation of symptoms and signs. Prog Brain Res. 2006;152:1–8. doi: 10.1016/S0079-6123(05)52034-X. [DOI] [PubMed] [Google Scholar]
- Krassioukov A, Claydon VE. The clinical problems in cardiovascular control following spinal cord injury: an overview. Prog Brain Res. 2006;152:223–229. doi: 10.1016/S0079-6123(05)52014-4. [DOI] [PubMed] [Google Scholar]
- Krassioukov AV, Furlan JC, Fehlings MG. Autonomic dysreflexia in acute spinal cord injury: an under-recognized clinical entity. J Neurotrauma. 2003;20:707–718. doi: 10.1089/089771503767869944. [DOI] [PubMed] [Google Scholar]
- Krenz NR, Meakin SO, Krassioukov AV, Weaver LC. Neutralizing intraspinal nerve growth factor blocks autonomic dysreflexia caused by spinal cord injury. J Neurosci. 1999;19:7405–7414. doi: 10.1523/JNEUROSCI.19-17-07405.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenz NR, Weaver LC. Changes in the morphology of sympathetic preganglionic neurons parallel the development of autonomic dysreflexia after spinal cord injury in rats. Neurosci Lett. 1998;243:61–64. doi: 10.1016/s0304-3940(98)00101-3. [DOI] [PubMed] [Google Scholar]
- Maiorov DN, Krenz NR, Krassioukov AV, Weaver LC. Role of spinal NMDA and AMPA receptors in episodic hypertension in conscious spinal rats. Am J Physiol. 1997;273:H1266–H1274. doi: 10.1152/ajpheart.1997.273.3.H1266. [DOI] [PubMed] [Google Scholar]
- Marsh DR, Weaver LC. Autonomic dysreflexia, induced by noxious or innocuous stimulation, does not depend on changes in dorsal horn substance P. J Neurotrauma. 2004;21:817–828. doi: 10.1089/0897715041269605. [DOI] [PubMed] [Google Scholar]
- Marsh DR, Wong ST, Meakin SO, MacDonald JI, Hamilton EF, Weaver LC. Neutralizing intraspinal nerve growth factor with a trkA-IgG fusion protein blocks the development of autonomic dysreflexia in a clip-compression model of spinal cord injury. J Neurotrauma. 2002;19:1531–1541. doi: 10.1089/089771502762300201. [DOI] [PubMed] [Google Scholar]
- Mathias CJ, Frankel HL. Autonomic disturbances in spinal cord lesions. In: Mathias CJ, Bannister R, editors. Autonomic Failure: A Textbook of Clinical Disorders of the Autonomic Nervous System. Oxford: Oxford University Press; 1999. pp. 494–513. [Google Scholar]
- Mayorov DN, Adams MA, Krassioukov AV. Telemetric blood pressure monitoring in conscious rats before and after compression injury of spinal cord. J Neurotrauma. 2001;18:727–736. doi: 10.1089/089771501750357663. [DOI] [PubMed] [Google Scholar]
- McLean DE, Kearney J, Cawley MF. Environmentally responsive temperature instability in pediatric spinal cord injury. Spinal Cord. 1999;37:705–709. doi: 10.1038/sj.sc.3100888. [DOI] [PubMed] [Google Scholar]
- Meshkinpour H, Harmon D, Thompson R, Yu J. Effects of thoracic spinal cord transection on colonic motor activity in rats. Paraplegia. 1985;23:272–276. doi: 10.1038/sc.1985.44. [DOI] [PubMed] [Google Scholar]
- Montgomerie JZ. Infections in patients with spinal cord injuries. Clin Infect Dis. 1997;25:1285–1290. doi: 10.1086/516144. quiz 1291–1292. [DOI] [PubMed] [Google Scholar]
- Morrison SF. Central pathways controlling brown adipose tissue thermogenesis. News Physiol Sci. 2004;19:67–74. doi: 10.1152/nips.01502.2003. [DOI] [PubMed] [Google Scholar]
- Noble LJ, Wrathall JR. Spinal cord contusion in the rat: morphometric analyses of alterations in the spinal cord. Exp Neurol. 1985;88:135–149. doi: 10.1016/0014-4886(85)90119-0. [DOI] [PubMed] [Google Scholar]
- Price MJ, Campbell IG. Thermoregulatory responses of spinal cord injured and able-bodied athletes to prolonged upper body exercise and recovery. Spinal Cord. 1999;37:772–779. doi: 10.1038/sj.sc.3100907. [DOI] [PubMed] [Google Scholar]
- Rand RP, Burton AC, Ing T. The tail of the rat, in temperature regulation and acclimatization. Can J Physiol Pharmacol. 1965;43:257–267. doi: 10.1139/y65-025. [DOI] [PubMed] [Google Scholar]
- Shergill IS, Arya M, Hamid R, Khastgir J, Patel HR, Shah PJ. The importance of autonomic dysreflexia to the urologist. BJU Int. 2004;93:923–926. doi: 10.1111/j.1464-410X.2003.04756.x. [DOI] [PubMed] [Google Scholar]
- Stjernberg L, Blumberg H, Wallin BG. Sympathetic activity in man after spinal cord injury. Outflow to muscle below the lesion. Brain. 1986;109:695–715. doi: 10.1093/brain/109.4.695. [DOI] [PubMed] [Google Scholar]
- Sugarman B, Brown D, Musher D. Fever and infection in spinal cord injury patients. JAMA. 1982;248:66–70. [PubMed] [Google Scholar]
- Teasell RW, Arnold JM, Krassioukov A, Delaney GA. Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch Phys Med Rehabil. 2000;81:506–516. doi: 10.1053/mr.2000.3848. [DOI] [PubMed] [Google Scholar]
- Vianna DM, Carrive P. Changes in cutaneous and body temperature during and after conditioned fear to context in the rat. Eur J Neurosci. 2005;21:2505–2512. doi: 10.1111/j.1460-9568.2005.04073.x. [DOI] [PubMed] [Google Scholar]