Abstract
Enterochromaffin (EC) cells are sensors that detect chemical or mechanical stimuli and respond with release of serotonin (5-HT). 5-HT activates local motor reflexes, but whether local motor reflexes also evoke 5-HT release is unknown. The aim of the present study was to establish the relationship between the release of 5-HT and the enteric neural circuits controlling the movements of the intestine. Recordings were made from full-thickness preparations of guinea pig ileum using electrochemical techniques with carbon fibre electrodes to measure local concentrations of 5-HT. The tension in the circular muscle (CM) and longitudinal muscle (LM) was recorded with force transducers. The release of 5-HT from the EC cells was detected selectively and the timing of the events quantified. Pressure-evoked peristalsis caused detectable 5-HT release only when the recording site was invaded by a ring of CM contraction. Spontaneous and stretch-evoked reflex contraction of the CM and LM occurred simultaneously with 5-HT release. Paralysis of the smooth muscle significantly reduced the stretch-evoked release. Muscarinic agonists evoked reflexes that were associated with increases in tension in CM and LM simultaneous with 5-HT release. Tetrodotoxin abolished the coordination between the CM contraction and 5-HT release but not the direct activation of the CM and EC cells by the agonists. In conclusion, the correlation between local motor reflexes and 5-HT release observed in the present study is caused primarily by the contraction of the smooth muscle and subsequent deformation of the mucosa. The EC cell is, thus, a site of convergence for mechanical forces that contribute to the release of 5-HT during motor reflexes.
Serotonin (5-HT) is found in the enterochromaffin (EC) cells of the intestinal epithelium (Erspamer, 1954). The release of 5-HT from the EC cell is one of the critical steps involved in the transduction of sensory information from the lumen of the gut to the intrinsic and extrinsic sensory neurons that innervate the small and large bowel (Furness et al. 1998; Kirkup et al. 2001; Bertrand, 2003; Grundy, 2005). One consequence of 5-HT release is the modulation of motor reflexes, but whether an ongoing motor reflex causes 5-HT release is unknown.
It was Bülbring who originally showed that pressure applied to the mucosal epithelium could release 5-HT, and who put forward the idea that 5-HT acts as a local hormone (Bülbring & Lin, 1958; Bülbring & Crema, 1959). Bülbring's idea has since been extended by others who have shown that 5-HT receptor blockade can, in animal models, reduce or prevent motor reflexes (Kirchgessner et al. 1992; Foxx-Orenstein et al. 1996; Grider et al. 1996; Kadowaki et al. 1996; Bush et al. 2001) or secretory reflexes (Sidhu & Cooke, 1995; Cooke et al. 1997). More recent studies in humans have shown that compounds that block or activate 5-HT receptors are therapeutic in some functional bowel disorders (Camilleri et al. 2000; Beglinger, 2002; Galligan & Vanner, 2005; Tonini, 2005). There has also been a renewed interested in the role 5-HT may play in inflammatory bowel diseases (Gershon, 2004). Despite this interest, how 5-HT is released from the EC cells, and how release is regulated is largely unknown (Galligan, 2004; Grundy & Schemann, 2004).
What is known about the regulation of 5-HT release is primarily at the organ level and is due to the extensive work by Racké, Schwörer and colleagues who described the overflow of 5-HT from in vitro segments of small intestine from dog, pig, guinea pig and human (Racké & Schwörer, 1991; Racké et al. 1996). These experiments were extended by Minami and colleagues to ferret, rat and mouse (Minami et al. 1995; Hirafuji et al. 2001). In general, 5-HT release is via an external calcium-dependent process that can be evoked by agonists at β-adrenoceptors as well as agonists at muscarinic, nicotinic and 5-HT3 receptors. In addition, muscarinic receptor activation induces 5-HT release in the absence of external calcium ions. Recently Itoh and colleagues extended the overflow technique and showed that the migrating motor complex was associated with 5-HT release (Tanaka et al. 2004). At the cellular level, the regulation of 5-HT release has been studied using BON cells, which are derived from a human carcinoid tumour of the pancreas. BON cells have been used by Christofi and others as a model system for studying 5-HT release (Kim et al. 2001a,b; Christofi et al. 2004; Tran et al. 2004). Similarly, there have been successes at purifying EC cells from the intestine (Schafermeyer et al. 2004; Modlin et al. 2006) and in studying the EC cells in acutely dissociated intact crypts (Satoh et al. 1995; Lomax et al. 1999; Satoh et al. 1999).
A molecular approach to measuring 5-HT was used in an elegant series of studies showing that when histochemical markers for 5-HT production, storage or uptake are depressed, a simple measure of 5-HT release can still appear normal (Linden et al. 2003; Coates et al. 2004; O'Hara et al. 2004). These paradoxical findings suggest that 5-HT release is more complex than previously thought. Ideally, 5-HT release should be measured at the site of action and with a time course that captures the dynamics of its release and subsequent diffusion to the receptor. Recently an electrochemical technique was pioneered in the guinea pig small intestine which allows 5-HT release to be recorded specifically and with a high spatio-temporal resolution (Bertrand, 2004b). In this previous study, direct measurements of 5-HT concentrations were made from a small number of EC cells in real-time and at the site of action demonstrating that high concentrations of 5-HT release can occur rapidly and as distinct events.
The aim of the present study was to investigate the relationship between 5-HT release and the enteric neural circuits controlling the movements of the intestine. The real-time release of 5-HT was recorded from only a few EC cells at a time while the reflex activity of the longitudinal muscle (LM) and circular muscle (CM) was assessed. The main finding was that local reflex contractions of the circular muscle were strongly correlated with 5-HT release regardless of how the reflexes were evoked. The release of 5-HT was caused primarily by the contraction of the smooth muscle and subsequent deformation of the mucosa. These data support the idea that the EC cell is a site of convergence for mechanical forces that contribute to the release of 5-HT during motor reflexes.
Methods
Tissue preparation
All experiments were performed using guinea pigs of either sex (160–280 g, Hartley strain, from the University of Nevada) which were fed a standard lab diet until the day of the experiment. Animals were stunned by a blow to the head and killed by severing the carotid arteries and spinal cord in accordance with the guidelines of the University of Nevada Animal Experimentation Ethics Committee. A segment of ileum, full width (∼1.5 cm) and 2–5 cm in length, was taken from 10 to 20 cm from the ileocecal junction. The segment was removed and placed in oxygenated (95% O2–5% CO2) physiological saline of the following composition (mm): NaCl, 117; NaH2PO4, 1.2; MgSO4, 1.2; CaCl2, 2.5; KCl, 4.7; NaHCO3, 25; and glucose, 11. No L-type calcium channel antagonists were used in these experiments as even low concentrations were found to suppress 5-HT release (Bertrand, 2004b).
The segment was pinned loosely in a Petri dish lined with a silicone elastomer (Sylgard 184, Dow Corning, MI, USA). Some preparations were left intact (tube preparations) while the remaining preparations were cut open along the line of the mesenteric attachment (flat preparations). The preparation was transferred to a small recording chamber (volume approximately 3 ml) and superfused with warmed (35°C) physiological saline at a flow rate of approximately 5 ml min−1. If left intact, the lumen was perfused with physiological saline from the oral end with a separate cannula. Flat preparations were pinned loosely with the mucosa facing up, with 80 μm and 150 μm pins.
Electrochemistry
Measurements of 5-HT oxidation (and therefore 5-HT release) commenced after 60 min or more of tissue equilibration. Preparations were visualized at ×20 magnification using an upright dissecting microscope. 5-HT oxidation currents appear as a positive current deflection and were measured using carbon fibre electrodes insulated with glass micropipettes (1.5 mm outer diameter, 0.8 mm inner diameter, Clark Electromedical Instruments, Edenbridge, UK). The electrodes were placed above or touching the mucosa using a precision mechanical micromanipulator (MP-1, Narishige Scientific Instruments, Tokyo, Japan). Current recordings were made using a VA-10 amplifier (npi Electronic, Tamm, Germany), digitized at 5–20 kHz (Digidata 1200A) with a Ag–AgCl ground, recorded on a personal computer and filtered with a 60 Hz notch filter using pCLAMP 9.0 (all from Axon Instruments, Union City, CA, USA) and then analysed with Origin 7.5 (OriginLab Corp., Northampton, MA, USA).
Validation of 5-HT release
The mucosa of the intestine contains large amounts of 5-HT but could also contain small amounts of adrenaline, noradrenaline, dopamine and their metabolites. In the present study, the specificity of the electrodes for 5-HT was increased by coating each with 2.5% Nafion (from a 5% solution, Sigma-Aldrich, St Louis, MO, USA). Nafion is an anionic exchange resin that repels ascorbic acid, uric acid and 5-hydroxindole acetic acid (5-HIAA; a metabolite of 5-HT) and attracts cationic species like the catecholamines and 5-HT (Gerhardt et al. 1984). Further, the identity of the oxidation currents were investigated using cyclic voltammetry (ramp from −0.4 V to +1 V and back, ∼0.47 V s−1) (Stamford, 1986). The oxidation peaks from control solutions of 5-HT, melatonin and epinephrine were compared to those observed in the mucosa. The peaks for the 5-HT control solution and Peak 1 from the mucosa oxidized at ∼+350 mV and were clearly separated from the peaks seen in the control solutions. These precautions and control data make it probable that the oxidation currents investigated in the present study were due to 5-HT.
General characteristics of 5-HT release
The carbon fibre was voltage-clamped at +300 to +400 mV where the first peak from the mucosa and 5-HT both have an oxidation maximum. 5-HT (1–10 μm) was used to calibrate electrodes before and after recording in the mucosa. All electrodes showed a gradual and linear decrease in sensitivity over time, an effect that was minimized by exchanging carbon fibre electrodes after ∼60 min of recording.
Two types of release from the mucosa were noted that have been previously described (Bertrand, 2004b). Initial contact with the mucosa produced a sharp, transient increase in the release of 5-HT equivalent to > 10 μm 5-HT (Fig. 1A). This release was probably due to the direct compression of the mucosa and activation of the EC cells. In the present study, using a Mettler balance, the force applied by the carbon fibre was estimated as 0.82 ± 0.27 mg spread over the 100 μm length of the fibre (7 μm diameter). The same region of mucosa could respond reliably to repeated compression, suggesting that damage was not occurring at the site of recording. Following the transient release was a sustained release of 5-HT that was composed of a constant background equivalent to ∼3 μm 5-HT with many distinct current peaks superimposed on it. These current peaks are the ‘individual release events’ previously described (Bertrand, 2004b). Given the large amplitude of these events, it is likely they represent the coordinated release of many granules from one or more EC cells that were near to the electrode.
Figure 1. Compression of the mucosa evokes 5-HT release.
A, an amperometry trace from a single carbon fibre in one preparation. The carbon fibre electrode was held at a potential of +350 mV and brought into contact with the mucosa during the time indicated by the grey bar. During this time, a large increase in current was observed consistent with the sustained release of 5-HT from the mucosa. The small white section of bar at the start indicates a ∼20 s period of time during which the electrode was returned to 0 mV (see B). B, an expanded trace from A showing the initial contact between the carbon fibre electrode and the mucosa. Note that the oxidation current and individual release events are absent before the electrode contacts the mucosa despite the fact that the electrode is being held at +350 mV (grey bar underneath, on left). Approximately 20 s after contact, the potential of the electrode was briefly returned to 0 mV (white bar underneath). Note that during this time, the current and release events are absent. These events both appear again once the electrode is returned to +350 mV (grey bar underneath, on right). This indicates that the recorded signal is due to an oxidizable compound released from the mucosa which is consistent with the sustained release of 5-HT. See Methods for the other precautions and controls used to verify that the oxidizable compound was 5-HT.
5-HT concentrations measured from the lumen
In the present study, 5-HT was measured from the lumenal surface of the mucosa. Physiologically, 5-HT release is likely to be from a combination of 5-HT released from granules stored near the basal border of the EC cell, and from granules near the apical membrane (Ahlman et al. 1981; Nilsson et al. 1987). That both lumenal and basal release is important has been shown by Fujimiya et al. (1997) in a vascularly and luminally perfused preparation and by Forsberg & Miller (1982, 1983), who used an Ussing-style preparation. The in vitro preparation used here does not have an intact vascular system to transport basally released 5-HT away, so any 5-HT that escapes the serotonin transporter will overflow into the lumen. Thus, the recordings made here from the lumen are likely to include both lumenally released 5-HT and the overflow of basally released 5-HT.
Measurement of muscle excitation
Electrophysiology
A short (< 50 μm) carbon fibre electrode was used to measure local action currents (ACs – i.e. an action potential recorded under voltage clamp conditions) from the CM. ACs could be recorded with or without holding current (n = 6) and, thus, were not due to the oxidation of 5-HT or any other compound. To confirm the identity of the AC, a suction electrode was used to record action potentials (APs) from the serosal surface. APs and ACs were both correlated in time to the contractions of the CM and to each other when dual recordings were made from the same ring of CM (see Fig. 2B). Preparations in which the CM had been removed gave small or no ACs and also showed poor release of 5-HT. ACh-evoked contractions, ACs and 5-HT release were abolished by the L-type channel blocker nicardipine (n = 4, P < 0.05) while TTX reduced ACh-evoked 5-HT release (n = 3, P < 0.05) but did not reduce the contractions or ACs (n = 3, P > 0.05).
Figure 2. Peristalsis evoked 5-HT release and circular muscle excitation.
A, schematic of the tubular preparation used for these experiments. A cannula was connected to the oral end to perfuse physiological saline. Local 5-HT measurements (carbon fibre) and electrical recordings from circular smooth muscle (suction electrode) were made at the anal end. B, in some experiments, a conventional suction electrode was used to detect action potentials in the CM. These recordings confirmed that electrical excitation of the CM occurred at the carbon fibre recording site at the same time as the ACs were recorded. C, when a peristaltic reflex was evoked by a bolus of solution applied through the cannula, a lumen occluding wave of contraction spread from the oral end to the anal end of the preparation. When the carbon fibre electrode was placed into contact with the mucosa (grey bar underneath) at the anal end, a sharp increase in oxidation current could be detected when the wave of contraction reach the anal end (white bar underneath). Inset, an expanded view of the time that the peristalsis-like wave reached the recording site. The carbon fibre electrode recorded a clear burst of action currents (ACs) in the circular smooth muscle (CM) that is indicative of contraction local to the recording site. These data confirm that local reflex contraction of the circular muscle was taking place at the same time as the release of 5-HT.
Force transducers
The LM and CM were connected to isometric force transducers (FT-03C, no springs, Grass, Quincy, MA, USA). The smooth muscle was put under 0.5–1 g of resting tension and allowed to equilibrate for 1 h. The CM and LM were each attached near the anal end of the preparation. These preparations were opened and pinned loosely with the anal end and one side left unpinned but attached to the force transducers (see Fig. 3A). The preparations were about 15 mm wide and 25 mm in length. The carbon fibre electrode was placed on the opposite side of the preparation away from the attachment site of the CM force transducer.
Figure 3. Mucosal compression evoked reflex of circular muscle and longitudinal muscle.
A, schematic diagram of the preparation used for these, and subsequent experiments. Oral is to the top, and the mucosa was facing up into the bath. The preparation measured 5 cm in length with a full width (∼1.5 cm). The longitudinal muscle (LM) and circular muscle (CM) were attached to force transducers to measure global tension changes in the width and length of the preparation, respectively. The carbon fibre electrode was place directly across from the CM recording site and near to the LM recording site. Small pins (80 μm) were used to stabilize the carbon fibre recording area. B, from top to bottom, representative traces showing the tension in the LM, oxidation current (5-HT), and the tension in the CM. The horizontal dashed lines indicate baseline. Compression of the mucosa with the carbon fibre electrode (during the grey bar – middle trace) caused a transient increase in oxidation current (first vertical line). Approximately 2 s later (second vertical line), a reflex increase in tension of both the LM and CM occurred. This coincided with another sharp increase in oxidation current and generation of action currents (ACs).
Comparison across different measurements
Measurements of reflex-evoked 5-HT release, CM excitation (i.e. ACs), CM tension and LM tension were compared by looking at the time at which the events just left baseline (i.e. started). When the methods for evoking a reflex provided a clear point of reference (e.g. ACh application, start of stretch, or primary release of 5-HT evoked by compression) then the start times of the events were measured from that. When there was no clear starting time (e.g. peristalsis, spontaneously generated reflexes or a compression-evoked reflex without a primary release of 5-HT) then the start of the 5-HT release event was taken as the point of reference.
Electrical stimulation of the mucosa
A bipolar, stainless steel electrode (114 μm stainless steel insulated with Teflon, MedWire, Mt Vernon, VT, U.S.A) was used to stimulate an area of mucosa of about 0.25 mm2 (an area covering several circumferentially orientated rows of villi; Bertrand et al. 1998). Stimulus pulses used were from 0.5 to 5.0 mA (typically 1.0 mA) and of 0.5 ms duration (Master-8 stimulator, ISO-Flex stimulus isolation unit, both from AMPI, Jerusalem, Israel), a duration that is long enough to activate nerve fibres but too short to activate smooth muscle directly.
Solutions
Receptor antagonists were applied by addition to the superfusing physiological saline solution in known concentrations. They were allowed to equilibrate with the tissue for 10–15 min before further measurements were taken. ACh (100 μm to 1 m) was applied in a HEPES (10 mm) buffered physiological saline by pressure ejection (10 p.s.i. Picospritzer II, General Valve Corp., USA) from a micropipette (10–20 μm tip diameter) positioned over the intact mucosa (Bertrand & Bornstein, 2002), just under the serosal surface or by addition of ACh (1 mm) to the superfusing physiological saline solution. Drugs were purchased as follows: TTX was from Alomone Laboratories (Jerusalem, Israel) and all other drugs were from Sigma (St Louis, MO, USA).
Statistics
Unless otherwise noted, data are given as means ±standard error of the mean (s.e.m.) with the range and/or median given where appropriate. In all cases, the n-value refers to the number of animals used. Student's t test was used to compare data for significant differences, with an α of 0.05 taken as the cutoff for significance; Wilcoxon's signed rank test was used for non-parametric data. All tests were two-tailed and paired unless noted; Bonferroni's correction for multiple comparisons was used when appropriate. A χ2 test was used to compare the frequency of occurrence of spontaneously occurring events. Linear regression analysis was done using Origin 7.5 and was not constrained to pass through zero.
Results
Peristalsis evoked 5-HT release
The original experiments of Bülbring & Lin (1958) showed that each wave of peristaltic contraction was followed by expulsion of 5-HT laden fluid, though the absolute amounts of 5-HT expelled varied considerably. In the present study, a similar preparation was used to examine, in finer detail, the relationship between peristalsis and 5-HT release. 5-HT release was recorded from a small region of mucosa at the anal end of the preparation. In 16 preparations, the segment of ileum was left as an intact tube with a cannula attached to the oral end (Fig. 2A). Local excitation of the CM was examined by using the carbon fibre electrode to record action currents (ACs – action potentials recorded under voltage clamp) from the CM (see Methods).
In 10 of the 16 tube preparations, a stereotypical peristaltic motor pattern could be evoked by stimulation of the oral end with either a bolus of saline (∼5 ml in less then 3 s; n = 7) or ACh (1 mm; 5 μl) transiently applied to the oral end (n = 3). When the contraction reached the ring of CM containing the carbon fibre recording site, an increase in the release of 5-HT was observed (Fig. 2C), and in 9 of 10 preparations ACs were observed. Basal 5-HT release from these preparations was 4.5 ± 1.6 μm (n = 10) compared to evoked 5-HT release of 3.7 ± 1.2 μm (83% of basal release). Evoked release consisted of 4.8 ± 0.6 events with a time to peak of 1.1 ± 0.4 s and a time taken to reach 80% return to baseline (t80) of 33 ± 21 s (n = 10). In the inset of Fig. 2C, it is clear that ACs originating in the CM were recorded during the release of 5-HT at a time when visually, a wave of peristalsis swept through the area. There was a burst of 2.7 ± 0.4 ACs (6.9 ± 1.1 Hz) that on average started at the same time as the start of 5-HT release (0.40 ± 0.46 s; n = 9; e.g. Fig. 2B; preceding 5-HT release: −0.34 ± 0.04 s; n = 5; following 5-HT release: 1.32 ± 0.91; n = 4). In 3 of the 10 tube preparations, there was an ongoing occurrence of spontaneous 5-HT release events.
Muscle activity and 5-HT release
It was clear from the preceding experiments that neurally evoked contractions of the CM were occurring at the same times as 5-HT release. To more clearly identify the interaction between reflex-evoked smooth muscle contraction and 5-HT release, recordings of force from LM and CM were made in 11 flat preparations at the same time as recordings of release of 5-HT (Fig. 3A).
Compression-induced reflexes
The carbon fibre electrode was used to evoked release of 5-HT via compression of the mucosa in all 11 flat preparations with force transducers and in a further 10 preparations without force transducers but with measurable CM excitation (ACs). In 13 of 21 preparations, compression of the mucosa caused a transient increase in 5-HT release as described above. In 5 of 21 preparations, the first 5-HT release event (peak = 15.0 ± 6.7 μm) was followed ∼3 s later by a second 5-HT release event (peak = 3.8 ± 1.3 μm; 29 ± 14% of initial peak; n = 6) associated with an increase in CM tension (5-HT start time: 2.9 ± 0.8 s; CM start time: 2.0 ± 0.7 s; n = 2; Fig. 3B) or an increase in CM excitation (5-HT start time: 1.8 ± 0.3 s; AC start time: 0.8 ± 0.4 s; n = 4, including 1 of the previous). In contrast, the remaining three preparations lacked an initial release of 5-HT (peak = 5.3 ± 3.16 μm) but showed clear evidence of an increase in CM tension occurring at the same time as a delayed 5-HT release (CM start time measured from the start of 5-HT release: 0.1 ± 0.1 s; n = 2) and an increase in CM excitation preceding 5-HT release (−0.4 ± 0.1 s; P < 0.05; n = 3, including the previous 2).
Spontaneously occurring increases in tension and 5-HT release
Spontaneously occurring increases in tension in CM and LM were recorded simultaneously with 5-HT release (Fig. 4). In 4 of 11 flat preparations with force transducers, there was ongoing (n = 2) or sporadic (n = 2) spontaneous increases in tension of the LM and CM that were synchronized with the release of 5-HT.
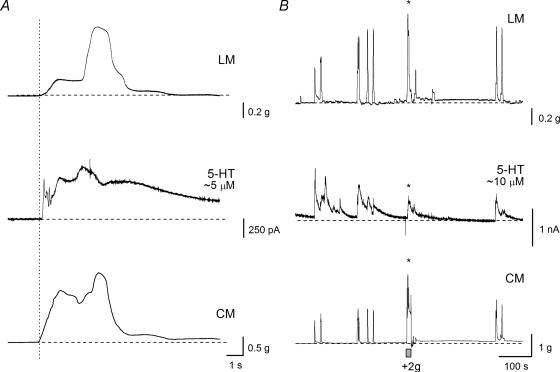
Figure 4. Spontaneous increases in tension and oxidation current.
A and B, representative traces showing the tension in the longitudinal muscle (LM), the oxidation current (5-HT) and the tension in the circular muscle (CM) from two preparations. The horizontal dashed lines indicate baseline. The carbon fibre electrode was at a +350 mV potential and in contact with the mucosa for the duration of these traces. A, this preparation showed occasional spontaneous increases in tension and oxidation. The vertical dotted line shows the time at which tension was first detected in the CM. The approximate peak 5-HT concentration detected was 5 μm. Note the complex data, with multiple smaller events making up each increase in tension and in oxidation current. B, this preparation showed more robust transient increases in tension and oxidation current. The approximate peak 5-HT concentration detected during the trace was 10 μm. The asterisk indicates the point at which an additional 2 g of tension was applied to the CM. See Fig. 5 for an expanded trace and details.
On average, in all four preparations, the start of CM contraction was not significantly different from the start of 5-HT release (CM start measured from start of 5-HT release: 350 ± 300 ms; P > 0.05; n = 4) and both peaked at ∼1 s after starting (CM time to peak: 1.2 ± 0.2 s; 5-HT time to peak: 0.7 ± 0.3; P > 0.05; n = 4); the small level of LM tension in three of these preparations prevented its accurate assessment. These data were combined with a further 10 preparations which did not have force transducers, but did have clear ACs associated with 5-HT release events. Of these, five of ten preparations showed ongoing spontaneous 5-HT release coupled with ACs as did a further two of four preparations from above. Taking all seven preparations together, the 5-HT release was composed of 2.6 ± 0.6 events and was equivalent to a peak concentration of 2.0 ± 0.7 μm (t80 = 6.7 ± 2.8 s). The AC start times were not significantly different from the start of 5-HT release (AC start measured from start of 5-HT release: 0.08 ± 0.45 s; 5 preceded and 2 followed; number of ACs = 3.0 ± 0.5; P > 0.05; n = 7).
Stretch-evoked reflexes
Circumferential stretch of the ileum activates motor patterns such as peristalsis as well as simpler motor reflexes like ascending excitation of the CM (Bian et al. 2004). The idea was tested that circumferential stretch also evokes 5-HT release in the area of stretch. Twenty-one preparations that showed release of 5-HT to compression or ACh were tested for CM stretch-evoked local reflex contraction of the CM. Stretch evoked a fast increase in muscle tension of 1–5 g in 0.5–0.7 s (Fig. 5A) (average stretch duration: 7.3 ± 0.7 s). In all preparations, stretch was followed by a myogenic relaxation (from 2.1 ± 0.3 g to 1.5 ± 0.2 g; n = 21).
Figure 5. Effect of TTX on circular muscle-evoked reflexes.
Representative traces showing the tension in the longitudinal muscle (LM), the oxidation current (5-HT) and the tension in the circular muscle (CM) from one preparation. The horizontal dashed lines indicate baseline. The carbon fibre electrode was at a +350 mV potential and in contact with the mucosa for the duration of these traces. A, starting at the first vertical dotted line, an additional 2 g of tension was applied to the CM (duration indicated by the grey bar) and was released back to baseline tension of 1 g approximately 7 s later. See Fig. 4B for a longer time base. At the second vertical dotted line, approximately 2 s later, there is an active increase in tension in the CM which is coincident with a sharp increase in oxidation current and generation of action currents (ACs) and a further increase in tension of the LM. The approximate peak 5-HT concentration detected was 5 μm. Note, during the initial increase in CM tension, there is a myogenic relaxation. Also, the LM begins generating tension after only a second, but it is not clear that this was reflex evoked. B, tetrodotoxin (TTX) was used to block neural conduction in the enteric reflex pathways. TTX (1 μm) was allowed to equilibrate with the preparation for 10 min before a reflex was again evoked. In this case, an additional 2 g of tension applied to the CM (during the grey bar) failed to evoked active increases in LM or CM tension or in oxidation current. Note, there is still a small myogenic relaxation of the CM during the initial increase in tension. Also, there is a small amount of cross-talk between the CM and the LM transducers which accounts for the very small increase in tension (< 0.1 g).
Circumferential tension
In 15 preparations, CM was recorded and 5-HT release assessed. CM stretch evoked a reflex in 14 of these 15 preparations. Measuring from the onset of the stretch, there was a near simultaneous increase in tension of the CM and of 5-HT release (CM start time: 1.31 ± 0.19 s; 5-HT start time: 1.53 ± 0.27 s; P > 0.05; n = 14) The CM generated 2.20 ± 0.24 g of force (time to peak: 2.19 ±0.25 s; n = 14). 5-HT release was composed of 2.6 ± 0.3 events with a time to peak of 2.27 ± 0.32 s (n = 14).
Circumferential and longitudinal tension
In a further six preparations, both CM and LM were recorded along with 5-HT release. CM stretch evoked a reflex in three of these six preparations. Measuring from the onset of the stretch, there was a near simultaneous increase in tension of the CM and LM, and of 5-HT release (CM start time: 2.42 ± 0.87 s; LM start time: 2.65 ± 1.08 s; 5-HT start time: 2.56 ± 1.13 s; P > 0.05; n = 3) The CM generated 1.34 ± 0.46 g of force (time to peak: 0.99 ± 0.25 s; t80: 1.52 ± 0.71 s; n = 3) while, over a similar period, the LM generated only 0.14 ± 0.06 g of force (time to peak: 0.64 ± 0.26; t80: 1.50 ± 0.56; n = 3). 5-HT release was composed of 3.2 ± 1.2 events and was equivalent to a peak concentration of 3.5 ± 2.2 μm (2.42 ± 1.40 nA of oxidation current; n = 3), and as with spontaneously occurring reflexes, this took place over a longer time course than the increase in muscle tension (time to peak: 1.14 ± 0.44 s; t80: 9.40 ± 4.04 s; P < 0.05; n = 3). Blockade of neural conduction with TTX (1 μm; n = 2) abolished both the reflex contractions and 5-HT release and revealed the myogenic changes in tension of the CM associated with the stretch (Fig. 5B).
Effect of muscle relaxants on 5-HT release
The movement of the smooth muscle during recordings of 5-HT release makes it difficult to separate out the neural control of EC cell function and the intrinsic mechanosensitivity of the EC cell. Traditionally, L-type calcium channel blockers have been used to reduced the movement of the smooth muscle; however, these channels are also responsible for sustaining release of 5-HT from EC cells. To work around this problem, several other smooth muscle relaxants with different mechanisms of action were used.
Effect of atropine, nitroprusside, isoproternol or papaverine
Activation of muscarinic receptors is the primary excitatory input to the smooth muscle. Atropine, the muscarinic receptor antagonist, was used to block this input during activation of stretch-evoked motility reflexes. Superfusion of atropine (1 μm) alone caused no significant change in either circular muscle contraction or 5-HT release (CM: 190 ± 60% of control; 5-HT: 130 ± 80% of control; n = 3). In contrast, release was significantly reduced, but not abolished, by the smooth muscle relaxants sodium nitroprusside (100 μm), papaverine (100 μm) or isoproterenol (1 μm) (n = 5 for all; Fig. 6C for summary). The residual 5-HT release appeared to be correlated with a small amount of residual muscle movement, so atropine was used in an attempt to completely quiet the muscle. Addition of atropine to any of the relaxants caused a complete paralysis of the smooth muscle and block of stretch-evoked 5-HT release (n = 5 for all; Fig. 6C). Compression-evoked 5-HT release was unaffected by the majority of these treatments; the exception was isoproterenol which reduced the amplitude to 37 ± 12% of control. In contrast, isoproternol with atropine did not cause a significant change in the compression response. These data suggest that the stretch-evoked 5-HT release is primarily the result of mechanical stimulation of the EC cells by the movement of the underlying musculature.
Figure 6. Effect of smooth muscle relaxants on stretch-evoked reflexes.
Representative traces from guinea pig ileum showing the oxidation current (5-HT; upper, holding potential: + 450 mV) and the tension in the circular muscle (CM; lower). The carbon fibre electrode was in contact with the mucosa for the duration of these traces. A, starting at the first vertical dotted line, an additional 1.3 g of tension was applied to the CM (duration indicated by the grey bar). Approximately 0.5 s later, there is an active increase in tension in the CM which is coincident with a sharp increase in oxidation current and generation of action currents (ACs). B, in the presence of the phosphodiesterase blocker, papaverine (100 μm superfused into the bath), both the active increase in tension in the CM and the increases is oxidation current are significantly reduced. C, summary of experiments in A and B. using the soluble guanylyl cyclase activator sodium nitroprusside (SNP; 100 μm), papaverine (PAP) and the β agonist isoproterenol (ISO; 1 μm), all either alone or in the presence of the muscarinic receptor antagonist atropine (ATR; 1 μm). Neither CM tension nor 5-HT release were reduced by atropine alone (1 μm; n= 3; see text). In contrast, release was significantly reduced by nitroprusside, papaverine or isoproterenol (n= 5 for all). Addition of atropine to any of these relaxants caused complete quieting of the smooth muscle and block of stretch-evoked 5-HT release (n= 5 for all). Compression-evoked 5-HT release was unaffected by these treatments (see text).
Electrical stimulation in paralysed preparations
In order to test whether enteric nerve circuits could increase 5-HT release, focal electrical stimulation near to the carbon fibre electrode was used. Stimulation failed to evoke 5-HT release unless it also caused a local contraction in the ring of CM where the recording site was located. When the stimulating electrode was moved circumferentially 5 mm, the same ring of CM contracted and 5-HT release could be detected. In contrast, when the stimulating electrode was moved oral 5 mm, the CM at the site of the carbon fibre stopped contracting and 5-HT release ceased, though there was a contraction of the ring of CM at the new stimulation site.
In the presence of isoproterenol (1 μm; n = 4) or sodium nitroprusside (100 μm; n = 3), the contraction associated with electrical stimulation was reduced (18 ± 13% of control; 50 ± 38% of control, respectively). At the same time, 5-HT release was significantly reduced (3 ± 3% of control; 7 ± 6% of control; P < 0.05) suggesting the contraction of the smooth muscle rather than an activated nerve circuit, was driving 5-HT release.
Acetylcholine induced reflexes and 5-HT release
Acetylcholine (ACh) is the primary excitatory transmitter in the ENS, and many enteric cholinergic fibres terminate near epithelial cells to mediate secretion (Cooke, 2000). My previous work has shown that ACh evokes robust release of 5-HT (Bertrand, 2004b), and so ACh was used here as a tool to investigate the activation of nerve circuits and reflexes.
Effect of acetylcholine on muscle excitation
The correlation between the ACh-evoked 5-HT release events and the ACs was analysed in detail. ACh was added to either the serosal surface or the mucosal surface and the times at which ACs and 5-HT release occurred were compared. On average, ACh-evoked 5-HT release was composed of 6.8 ± 1.3 events that were analysed and paired with bursts of ACs (n = 6). The average interval between 5-HT release events was 5.4 ± 3.1 s, while for ACs it was 8.1 ± 3.8 s (n = 6). A linear regression (unconstrained) showed that there was a good correlation between the two events (R = 0.99 ± 0.01) with a slope close to unity (slope = 0.84 ± 0.18; n = 6). The ACs could appear before, after or during the peak of the 5-HT release events, though for individual preparations the ACs appeared at approximately the same time in relation to 5-HT release.
Effect of acetylcholine on muscle tension
The interaction between acetylcholine-evoked muscle contraction and 5-HT release was assessed with recordings of tension from LM and CM. When ACh was applied to the mucosa near the carbon fibre recording site (Fig. 7A) or applied to the serosal surface in the same circumferential ring (not illustrated), it caused a rapid increase in tension in the LM and the CM, and in 5-HT release (n = 7). Despite a relatively uncoordinated start from the time of ACh application (CM = 4.2 ± 1.2 s; 5-HT = 3.7 ± 1.5 s; LM = 3.2 ± 1.9 s; n = 7), in five of the seven preparations, the three measures fell into a pattern of synchronized increases in tension and oxidation current. In the remaining two preparations, the responses were mainly smooth increases in tension or 5-HT release that did not have clearly delineated peaks that could be analysed.
Figure 7. ACh evoked reflex with the electrode in contact with the mucosa.
Representative traces showing the tension in the longitudinal muscle (LM), the oxidation current (5-HT) and the tension in the circular muscle (CM) from one preparation. The horizontal dashed lines indicate baseline. The carbon fibre electrode was at a +350 mV potential and in contact with the mucosa for the duration of these traces. Acetylcholine (ACh; 1 m) was applied to the mucosa, near the carbon fibre recording electrode, at the time marked by the filled triangle (bottom traces A and B). Between 1 and 2 μl of ACh was pressure ejected into a bath volume of ∼5 ml. The concentrations close to the recording electrode were initially high, but rapidly decreased to ∼100 μm during the time measurements were taken. A, ACh caused a rapid increase in tension in the LM and later the CM. The oxidation current also increased during this time. Despite the relatively uncoordinated start, all three measures fall into a pattern of synchronized increases in tension and oxidation current (vertical dotted lines). B, tetrodotoxin (TTX) was used to block neural conduction. TTX (1 μm) was equilibrated with the preparation for 10 min before ACh was re-applied. The direct versus the indirect (i.e. reflex) effects of ACh are revealed. Upon application of ACh, the LM fails to respond, indicating that it was activated by enteric nerves, probably as a result of stimulation of intrinsic sensory nerves in the mucosa or as an indirect consequence of release of 5-HT or other sensory mediators. The CM and oxidation current are still both increased, indicating a direct action of ACh on the CM (via diffusion) and the EC cells in the mucosa (in the case of 5-HT release). Note, the lack of coordination between increases in CM tension and oxidation current.
In the five of seven preparations showing synchronization, ACh-evoked 5-HT release was composed of 18.6 ± 3.5 events (n = 5) that occurred during the same period as increases in CM tension (number of events = 12.8 ± 2.2) and LM tension (number of events = 11.1 ± 1.7). The average interval between 5-HT release events was 2.4 ± 0.5 s, which was significantly shorter than either CM (3.7 ± 0.9 s) or LM (4.1 ± 0.6 s); the CM and LM intervals were not significantly different from each other (P > 0.05; repetitions = 7; n = 5). As would be expected with serosal ACh application, the LM contraction started first (LM: 1.7 ± 0.5 s; CM: 2.1 ± 0.3 s; 5-HT: 2.6 ± 0.7 s; n = 4) while with mucosal ACh application, 5-HT release started first (5-HT: 5.2 ± 2.7 s: CM: 6.1 ± 1.6 s; LM: 6.2 ± 2.8 s; n = 4).
In three preparations, TTX (1 μm) was used to block neural conduction in an effort to reveal the direct effects of ACh on the smooth muscle and EC cells. With the highest concentrations of ACh (e.g. 1 m) direct activation of the muscle or EC cells was observed (Fig. 7B) while with the lower concentrations (e.g. 100 μm), all activity was prevented by TTX. Importantly, when there was a direct activation of smooth muscle and 5-HT release, TTX prevented the coordination between them (e.g. Fig. 7B).
Effect of electrode position on concentration and time course of 5-HT release
In order to exclude the possibility that contact with the mucosa by the electrode contributed to the coordination between motility and 5-HT release seen above, the electrode was positioned 100–200 μm above the mucosa. In three preparations, the muscarinic agonist bethanechol (1 mm; 5 μl) was microapplied to the mucosal surface near the recording electrode. The general characteristics of 5-HT release in relation to CM contraction were identical to recordings made with the electrode touching the mucosa, although the maximal concentration of 5-HT was 10-fold less (n = 3; Fig. 8). In these same preparations, stretch of the CM was used to evoke a motor reflex. In all three preparations, the CM contractions evoked were correlated with 5-HT release (not illustrated). In two of the three preparations, spontaneously occurring CM contraction and 5-HT release were recorded and seen to be associated in time (not illustrated). Thus, the carbon fibre recording electrode does not need to touch the epithelium to record the release of 5-HT, but under these conditions the concentration of 5-HT detected was ∼10-fold less.
Figure 8. Muscarinic agonist evoked 5-HT release without contact with the electrode.
Representative traces showing the oxidation current (5-HT) and the tension in the circular muscle (CM) from one preparation. The horizontal dashed lines indicate baseline. The carbon fibre electrode was not touching the mucosa, but was approximately 100 μm above it for the duration of these traces. Bethanechol (1 mm, 5 μl) was applied to the mucosa, near the carbon fibre recording electrode, at the time marked by the filled triangle (upper and lower traces). Bethanechol caused a rapid increase in tension in the CM. The oxidation current also increased during this time; the approximate peak 5-HT concentration detected was 1 μm. 5-HT release starts first, then it and CM tension fall into a pattern of synchronized increases in oxidation current and tension (vertical dotted lines). Inset, the raw oxidation current trace is shown here without the filtering that was necessary at the higher amplifications used to generate these data. The general characteristics of 5-HT release in relation to CM contraction for the muscarinic agonist, stretch-evoked reflexes (see text) and spontaneously occurring reflexes (see text) were identical to recordings made with the electrode touching the mucosa, although notably, the maximal concentrations of 5-HT recorded are approximately 10-fold less.
Discussion
The main finding of the present study is that 5-HT release from EC cells is strongly correlated with the local motor reflex of the smooth muscle regardless of how the reflex was evoked. Additional evidence suggests that contraction of the smooth muscle and subsequent deformation of the mucosa is the primary stimulus for EC cell activation and 5-HT release. Thus, the EC cell is a site of convergence for mechanical forces which contribute to the excitation of the EC cell and to substantial 5-HT release during motor reflexes.
Released 5-HT reaches high concentrations locally
When the electrode was in direct contact with the mucosa, the concentration of 5-HT detected at the tip of the carbon fibre during a motor reflex peaked at 3 μm or more above basal release levels. When the electrode was ∼100 μm from the mucosal surface, the concentration of 5-HT detected at the carbon fibre was around 300 nm indicating that there is a steep concentration gradient around the EC cell. This is not surprising given the abundance of the serotonin reuptake system in the epithelium (Wade et al. 1996). Physiologically, this concentration gradient controls the type of 5-HT receptor activated. The 5-HT3 receptor is activated by > 100 nm of 5-HT while G-protein-coupled 5-HT receptors respond to as little as 1 nm 5-HT but become desensitized at higher than 100 nm. Thus, far from the EC cell, the lower concentrations would activate G-protein-coupled receptors without desensitization while closer to the EC cell, higher concentrations of 5-HT could act on 5-HT3 receptors located on, for example, the intrinsic and extrinsic sensory nerve terminals (Bertrand, 2003; Grundy, 2005). G-protein-coupled receptors such as the 5-HT4 receptors could also be activated, but they would then likely become desensitized (Chen et al. 2001). The data presented here are the first direct evidence that reflex-evoked 5-HT release can reach the high concentrations required to effectively activate 5-HT3 receptors and potentially desensitize G-protein-coupled 5-HT receptors close to the EC cell.
5-HT release is composed of many events
There were a number of distinct peaks (i.e. increases in 5-HT concentration) that made up the total release of 5-HT during a motor reflex. For example, each peristaltic wave was associated with five or more distinct 5-HT release events over 30 s with a summated peak at ∼1 s. Many 5-HT release events occurred too closely together to be analysed, and thus, for each event counted, many other events probably contributed. Whether the release from a single EC cell can account for multiple, concurrent 5-HT release events seen here is unclear. Future experiments studying the release of 5-HT from partially isolated EC cells would be one way to answer this question.
The pattern of peak 5-HT release is important because it can control the activation of the 5-HT3 receptor on the enteric sensory nerve terminals which can respond to the high concentrations and fast time course of the release events (Bertrand et al. 2000). The activated 5-HT3 receptor evokes bursts of action potentials, the pattern of which can control the types of transmitter released in the circuitry downstream (Bertrand, 2004a; Bertrand & Thomas, 2004).
5-HT release is coupled to enteric reflexes
Bülbring and colleagues originally investigated the role of 5-HT in intestinal reflexes and showed that there was an increased outflow of 5-HT with each peristaltic wave (Bülbring & Crema, 1958, 1959; Bülbring & Lin, 1958). More recently, the migrating motor complex has been shown to be associated with increased outflow of 5-HT (Tanaka et al. 2004). These experiments were extended in the present study where a variety of motility reflexes were evoked and the local release of 5-HT, and the local activation and the global contraction of the smooth muscle was recorded.
A major finding of the present study was that all motor reflex protocols were associated with 5-HT release and in most cases there was no differentiation between the start of CM activation and the start of 5-HT release. This correlation held true across different preparations and over time in the same preparation, but there were exceptions. For example, detection of 5-HT release regularly continued after the global contraction of the CM had stopped (e.g. see Fig. 4B) while contractions evoked by muscarinic agonists continued after 5-HT release had stopped (not illustrated). In the latter case, it seems there was a temporary depletion of 5-HT as recovery of release could occur within 10 min. Future studies are needed to identify the properties of depletion or rundown by, for instance, manipulating 5-HT production, storage, uptake or degradation.
Together, these data show that 5-HT release and reflex-evoked contraction are far more closely related in time and space than has previously been shown by other methods. In the present study, the two events have been shown to occur within a few hundred milliseconds and to be localized to within 5–10 mm. Thus, whenever a motor reflex is evoked it is likely that 5-HT release will also be evoked. An important question to ask is what provides the link between these two events?
Do enteric circuits contribute directly to 5-HT release?
Circumstantial evidence from the present study suggests that enteric nerve circuits may play a role in some EC cell 5-HT release (Fig. 9C). For example, in some cases, 5-HT release was seen to precede CM contraction suggesting that a mechanism other than contraction was driving 5-HT release. In addition, exogenous muscarinic agonists could evoke 5-HT release and this could be blocked by muscarinic antagonists. Given that many cholinergic nerve fibres terminate near the EC cells, it is reasonable to expect some input. This is supported by the observation that tetrodotoxin abolished the coordination between CM contraction and 5-HT release. These data are not, however, conclusive.
Figure 9. Regulation of EC cells by nerves and motility.
Schematic diagram showing the hypothesized interactions between 5-HT released from EC cells (A), the smooth muscle (B) and the nerves (C). Asterisks (*) indicates interactions for which data from the present study is applicable. Dotted lines indicate weak or non-physiological evidence exists to support the interaction while solid lines suggest strong data exist. In A the EC cells release 5-HT which, under conditions that promote diffusion of 5-HT, may reach the circular smooth muscle (B) and act on excitatory (+) 5-HT2 receptors and on inhibitory (−) 5-HT4/7 receptors (Tonini, 2005). The present study supports the idea that 5-HT can activate excitatory (+) 5-HT1P,3,4 receptors on the nerve terminals of the enteric intrinsic sensory neurons (C) (Kirchgessner et al. 1992; Grider et al. 1996; Bertrand et al. 2000). B, data from the present study also support the idea that contraction of the smooth muscle causes distortion of the epithelium which excites the EC cells causing 5-HT release. C, although not supported by the present study, EC cells have been hypothesized to be excited by acetylcholine (ACh) released by enteric nerves which acts at excitatory muscarinic (M3) receptors (Rackéet al. 1996; Schafermeyer et al. 2004). There is also evidence for the release of an unknown inhibitory neurotransmitter (NT) (Rackéet al. 1996). Interactions not illustrated. Enteric nerve terminals (C) release excitatory transmitters such as ACh and tachykinins onto the smooth muscle (B) and activate excitatory muscarinic (M2/3) neurokinin (NK1/2) receptors which cause contraction. Contraction (length or tension changes) can, in turn, excite enteric intrinsic sensory neurons (Kunze et al. 1999; Spencer & Smith, 2004). Finally, the 5-HT released from the EC cells (A) may act back on the EC cells in an autocrine fashion (Rackéet al. 1996).
On the other hand, the present study provides direct evidence that activated enteric nerves are not responsible for the 5-HT release seen during motor reflexes. First, there was little residual 5-HT release in response to stretch in paralysed preparations. Second, electrical stimulation of the nerves only activated 5-HT release when there was also a neurogenic contraction; when the preparation was paralysed, both the 5-HT release and contraction were blocked. Finally, atropine, which would be expected to block muscarinic receptors on the EC cell, did not reduce stretch-evoked 5-HT release.
One solution to this puzzle comes from the work of Racké & Schwörer (1992) who found that cholinergic agonists also induced the release of a transmitter that inhibited 5-HT release suggesting that some neural input to the EC cell is inhibitory. If true, then this would account for the failure of electrical stimulation to evoke 5-HT release, as both inhibitory and excitatory fibres would be activated. Future experiments that would help clarify the role for excitatory neural input to the EC cell could explore the actions of other transmitters, and utilize divided organ bath techniques, where reflexes evoked in unparalysed regions are allowed to invade paralysed regions.
Does movement or contraction cause 5-HT release?
In 1958, Bülbring and Lin concluded that intraluminal pressure could increase 5-HT release independent of peristalsis, presumably by exerting a uniform pressure which deformed the mucosal surface (Bülbring & Lin, 1958; Grundy, 2006). The EC cells and surrounding enterocytes are both believed to be sensitive to mechanical stimulation (Cooke et al. 2003), and thus deformation of the mucosal surface would be expected to cause 5-HT release (Fig. 9B). The present study provides support for this idea. Whenever the carbon fibre electrode made contact with the mucosa, it evoked a transient increase in 5-HT release, referred to as compression-evoked release.
This cannot, however, account for the data in the present study showing that, in the absence of a lumen to contain the pressure, 5-HT release from the EC cells is strongly correlated with the local reflex contractions of smooth muscle. In addition, when the smooth muscle was relaxed, stretch-evoked 5-HT release was reduced, but compression-evoked release was not. These data suggest that contraction of the underlying smooth muscle deforms the mucosa and causes release of 5-HT. It also suggests that passive lengthening of the muscle or movement of the preparation, does not activate the EC cells. This is supported by the finding that there were no mechanical artifacts due to interactions between the carbon fibre electrode and the mucosa. Taken together, these data support the idea that, during a motor reflex, 5-HT can be released as an indirect consequence of the activation of enteric motor circuits that excite the smooth muscle. This relationship would ensure that motility and 5-HT release are tightly coupled in time. It would be predicted that this tight coupling would be most relevant for 5-HT in a role as a paracrine substance acting, as discussed above, through the 5-HT3 receptor.
It is interesting to note that the study by Bülbring & Lin (1958) supports this relationship between contraction and 5-HT release. They reported that, in the presence of procaine or hexamethonium, the pressure-induced 5-HT release was associated with high amplitude, high frequency contractions of the intestine. That the contractions did not propel contents meant that they were not peristaltic in nature. Thus, their conclusion was that when peristalsis was blocked pressure still induced 5-HT release. It is tempting to speculate from the data presented here that some of the pressure-evoked 5-HT release that Bülbring & Lin (1958) observed was, in fact, due to the myogenic contractions of the intestine. A later paper, Bülbring & Crema (1959) investigated and supported this idea, but concluded that it was the increase in pressure during a lumen-occluding peristaltic wave that was the major cause of 5-HT release and that contraction per se had only a minor contribution. I would argue that any contraction, peristaltic or otherwise, can cause an active pinching or squeezing of the mucosa epithelium and that this is an important mechanism for the release of a large proportion of the 5-HT by the EC cells.
The physiology of 5-HT and the diagnostic relevance of 5-HT levels
The role played by the 5-HT released from EC cells in normal physiology is unclear. Gershon (1999) calls this ‘the enteric 5-HT conundrum’. 5-HT is thought to initiate some reflexes, but more generally, it is thought to increase the overall excitability of the intestine. For example, 5-HT in the lumen will lower the threshold for peristalsis (Bülbring & Lin, 1958). Part of the problem in understanding the actions of 5-HT comes from the central position of the EC cell in a variety of mechanically and chemically evoked motor and secretory reflexes (Fig. 9). 5-HT from the EC cell actively modulates the excitability of the nerves and muscle and they in turn can affect the excitability of the EC cell.
The role of 5-HT in diseased states is also unclear (Galligan, 2004). In some diseases such as ulcerative colitis (UC), the 5-HT content of EC cells goes down (Verity et al. 1962), while in other studies the content goes up (El-Salhy et al. 1997). Unfortunately, the histological methods normally employed to study biopsy samples provide only a snapshot of the 5-HT content at a single point in time, making interpretation difficult. One improvement has been to look at a snapshot of many parameters as was done recently in a very elegant study (Coates et al. 2004). It was found that patients with severe UC had a reduced content and production of 5-HT while a simple measure of 5-HT release from the biopsy samples (overflow after 3 min of mild agitation) appeared normal. This argues that overflow of 5-HT will give only a poor prediction of disease state. The challenge, then, is to find parameters of 5-HT release that are predictive of disease state. One way forward is to find and record more complex parameters of 5-HT release in the hope that they will yield better predictions. The present study has taken the first step in solving this problem by looking at the release of 5-HT in real time and at the site of action. Importantly, support has been provided for the role of enteric motor reflexes in the control of 5-HT release. If the levels of 5-HT release could be calibrated versus known gastrointestinal reflexes it could provide a better way to correlate 5-HT levels with disease state. For the diagnostic detection of 5-HT to become routine, however, the dual role that 5-HT plays in evoking some reflexes while being released by other reflexes must be appreciated.
Conclusions
The present study has shown that local reflex contractions of the smooth muscle are a primary driver of 5-HT release. These data support the idea that the EC cell is a site of convergence for mechanical forces that contribute to the excitation of the EC cell and the 5-HT release that occurs during a motor reflex. For the clinical detection of 5-HT to be meaningful, this motor reflex-evoked 5-HT release must also be taken into account.
Acknowledgments
Kind thanks to Drs Joel C Bornstein and Rebecca L Monro for providing helpful comments and Drs Nicolas Spencer, Terence Smith and Kenton Sanders for contributing resources. This work was upported by the NH & MRC (Australia) grant no. 299803 and by the Department of Physiology and Cell Biology, University of Nevada, School of Medicine.
References
- Ahlman H, Bhargava HN, Dahlstrom A, et al. On the presence of serotonin in the gut lumen and possible release mechanisms. Acta Physiol Scand. 1981;112:263–269. doi: 10.1111/j.1748-1716.1981.tb06815.x. [DOI] [PubMed] [Google Scholar]
- Beglinger C. Tegaserod: a novel, selective 5-HT4 receptor partial agonist for irritable bowel syndrome. Int J Clin Pract. 2002;56:47–51. [PubMed] [Google Scholar]
- Bertrand PP. ATP and sensory transduction in the enteric nervous system. Neuroscientist. 2003;9:243–260. doi: 10.1177/1073858403253768. [DOI] [PubMed] [Google Scholar]
- Bertrand PP. Bursts of recurrent excitation in the activation of intrinsic sensory neurons of the intestine. Neuroscience. 2004a;128:51–63. doi: 10.1016/j.neuroscience.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Bertrand PP. Real-time detection of serotonin release from enterochromaffin cells of the guinea pig ileum. Neurogastroenterol Motil. 2004b;16:511–514. doi: 10.1111/j.1365-2982.2004.00572.x. [DOI] [PubMed] [Google Scholar]
- Bertrand PP, Bornstein JC. ATP as a putative sensory mediator: activation of intrinsic sensory neurons of the myenteric plexus via P2X receptors. J Neurosci. 2002;22:4767–4775. doi: 10.1523/JNEUROSCI.22-12-04767.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand PP, Kunze WA, Bornstein JC, et al. Electrical mapping of the projections of intrinsic primary afferent neurons to the mucosa of the guinea-pig small intestine. Neurogastroenterol Motil. 1998;10:533–541. doi: 10.1046/j.1365-2982.1998.00128.x. [DOI] [PubMed] [Google Scholar]
- Bertrand PP, Kunze WA, Furness JB, et al. The terminals of myenteric intrinsic primary afferent neurons of the guinea-pig ileum are excited by 5-hydroxytryptamine acting at 5-hydroxytryptamine-3 receptors. Neuroscience. 2000;101:459–469. doi: 10.1016/s0306-4522(00)00363-8. [DOI] [PubMed] [Google Scholar]
- Bertrand PP, Thomas EA. Multiple levels of sensory integration in the intrinsic sensory neurons of the enteric nervous system. Clin Exp Pharmacol Physiol. 2004;31:745–755. doi: 10.1111/j.1440-1681.2004.04092.x. [DOI] [PubMed] [Google Scholar]
- Bian XC, Heffer LF, Gwynne RM, et al. Synaptic transmission in simple motility reflex pathways excited by distension in guinea pig distal colon. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1017–G1027. doi: 10.1152/ajpgi.00039.2004. [DOI] [PubMed] [Google Scholar]
- Bülbring E, Crema A. Observations concerning the action of 5-hydroxytryptamine on the peristaltic reflex. Br J Pharmacol. 1958;13:444–457. doi: 10.1111/j.1476-5381.1958.tb00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E, Crema A. The release of 5-hydroxytryptamine in relation to pressure exerted on the intestinal mucosa. J Physiol. 1959;146:18–28. doi: 10.1113/jphysiol.1959.sp006175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E, Lin RC. The effect of intraluminal application of 5-hydroxytryptamine and 5-hydroxytryptophan on peristalsis; the local production of 5-HT and its release in relation to intraluminal pressure and propulsive activity. J Physiol. 1958;140:381–407. [PMC free article] [PubMed] [Google Scholar]
- Bush TG, Spencer NJ, Watters N, et al. Effects of alosetron on spontaneous migrating motor complexes in murine small and large bowel in vitro. Am J Physiol Gastrointest Liver Physiol. 2001;281:G974–G983. doi: 10.1152/ajpgi.2001.281.4.G974. [DOI] [PubMed] [Google Scholar]
- Camilleri M, Northcutt AR, Kong S, et al. Efficacy and safety of alosetron in women with irritable bowel syndrome: a randomised, placebo-controlled trial. Lancet. 2000;355:1035–1040. doi: 10.1016/S0140-6736(00)02033-X. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Li Z, Pan H, et al. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: Abnormal intestinal motility and the expression of cation transporters. J Neurosci. 2001;21:6348–6361. doi: 10.1523/JNEUROSCI.21-16-06348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christofi FL, Kim M, Wunderlich JE, et al. Endogenous adenosine differentially modulates 5-hydroxytryptamine release from a human enterochromaffin cell model. Gastroenterology. 2004;127:188–202. doi: 10.1053/j.gastro.2004.04.070. [DOI] [PubMed] [Google Scholar]
- Coates MD, Mahoney CR, Linden DR, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657–1664. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Cooke HJ. Neurotransmitters in neuronal reflexes regulating intestinal secretion. Ann N Y Acad Sci. 2000;915:77–80. doi: 10.1111/j.1749-6632.2000.tb05225.x. [DOI] [PubMed] [Google Scholar]
- Cooke HJ, Sidhu M, Wang YZ. 5-HT activates neural reflexes regulating secretion in the guinea-pig colon. Neurogastroenterol Motil. 1997;9:181–186. doi: 10.1046/j.1365-2982.1997.d01-41.x. [DOI] [PubMed] [Google Scholar]
- Cooke HJ, Wunderlich J, Christofi FL. ‘The force be with you’: ATP in gut mechanosensory transduction. News Physiol Sci. 2003;18:43–49. doi: 10.1152/nips.01411.2002. [DOI] [PubMed] [Google Scholar]
- El-Salhy M, Danielsson A, Stenling R, et al. Colonic endocrine cells in inflammatory bowel disease. J Intern Med. 1997;242:413–419. doi: 10.1046/j.1365-2796.1997.00237.x. [DOI] [PubMed] [Google Scholar]
- Erspamer V. The pharmacology of indolealkylamines. Pharmacol Rev. 1954;6:425–487. [PubMed] [Google Scholar]
- Forsberg EJ, Miller RJ. Cholinergic agonists induce vectorial release of serotonin from duodenal enterochromaffin cells. Science. 1982;217:355–356. doi: 10.1126/science.7089569. [DOI] [PubMed] [Google Scholar]
- Forsberg EJ, Miller RJ. Regulation of serotonin release from rabbit intestinal enterochromaffin cells. J Pharmacol Exp Ther. 1983;227:755–766. [PubMed] [Google Scholar]
- Foxx-Orenstein AE, Kuemmerle JF, Grider JR. Distinct 5-HT receptors mediate the peristaltic reflex induced by mucosal stimuli in human and guinea pig intestine. Gastroenterology. 1996;111:1281–1290. doi: 10.1053/gast.1996.v111.pm8898642. [DOI] [PubMed] [Google Scholar]
- Fujimiya M, Okumiya K, Kuwahara A. Immunoelectron microscopic study of the luminal release of serotonin from rat enterochromaffin cells induced by high intraluminal pressure. Histochem Cell Biol. 1997;108:105–113. doi: 10.1007/s004180050151. [DOI] [PubMed] [Google Scholar]
- Furness JB, Kunze WA, Bertrand PP, et al. Intrinsic primary afferent neurons of the intestine. Prog Neurobiol. 1998;54:1–18. doi: 10.1016/s0301-0082(97)00051-8. [DOI] [PubMed] [Google Scholar]
- Galligan JJ. 5-Hydroxytryptamine, ulcerative colitis, and irritable bowel syndrome: molecular connections. Gastroenterology. 2004;126:1897–1899. doi: 10.1053/j.gastro.2004.04.028. [DOI] [PubMed] [Google Scholar]
- Galligan JJ, Vanner S. Basic and clinical pharmacology of new motility promoting agents. Neurogastroenterol Motil. 2005;17:643–653. doi: 10.1111/j.1365-2982.2005.00675.x. [DOI] [PubMed] [Google Scholar]
- Gerhardt GA, Oke AF, Nagy G, et al. Nafion-coated electrodes with high selectivity for CNS electrochemistry. Brain Res. 1984;290:390–395. doi: 10.1016/0006-8993(84)90963-6. [DOI] [PubMed] [Google Scholar]
- Gershon MD. Roles played by 5-hydroxytryptamine in the physiology of the bowel. Aliment Pharmacol Ther. 1999;13(Suppl. 2):15–30. [PubMed] [Google Scholar]
- Gershon MD. Serotonin receptors and transporters – roles in normal and abnormal gastrointestinal motility. Aliment Pharmacol Ther. 2004;20(Suppl. 7):3–14. doi: 10.1111/j.1365-2036.2004.02180.x. [DOI] [PubMed] [Google Scholar]
- Grider JR, Kuemmerle JF, Jin JG. 5-HT released by mucosal stimuli initiates peristalsis by activating 5-HT4/5-HT1p receptors on sensory CGRP neurons. Am J Physiol. 1996;270:G778–G782. doi: 10.1152/ajpgi.1996.270.5.G778. [DOI] [PubMed] [Google Scholar]
- Grundy D. Sensory signals from the gastrointestinal tract. J Pediatr Gastroenterol Nutr. 2005;41(Suppl. 1):S7–S9. doi: 10.1097/01.scs.0000180286.58988.cf. [DOI] [PubMed] [Google Scholar]
- Grundy D. Serotonin and sensory signalling from the gastrointestinal lumen. J Physiol. 2006;575:1–2. doi: 10.1113/jphysiol.2006.113472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy D, Schemann M. Serotonin in the gut: pretty when it gets down to the nitty gritty. Neurogastroenterol Motil. 2004;16:507–509. doi: 10.1111/j.1365-2982.2004.00573.x. [DOI] [PubMed] [Google Scholar]
- Hirafuji M, Ogawa T, Kato K, et al. Noradrenaline stimulates 5-hydroxytryptamine release from mouse ileal tissues via α2-adrenoceptors. Eur J Pharmacol. 2001;432:149–152. doi: 10.1016/s0014-2999(01)01474-1. [DOI] [PubMed] [Google Scholar]
- Kadowaki M, Wade PR, Gershon MD. Participation of 5-HT3, 5-HT4, and nicotinic receptors in the peristaltic reflex of guinea pig distal colon. Am J Physiol. 1996;271:G849–G857. doi: 10.1152/ajpgi.1996.271.5.G849. [DOI] [PubMed] [Google Scholar]
- Kim M, Cooke HJ, Javed NH, et al. d-Glucose releases 5-hydroxytryptamine from human BON cells as a model of enterochromaffin cells. Gastroenterology. 2001a;121:1400–1406. doi: 10.1053/gast.2001.29567. [DOI] [PubMed] [Google Scholar]
- Kim M, Javed NH, Yu JG, et al. Mechanical stimulation activates Gαq signaling pathways and 5-hydroxytryptamine release from human carcinoid BON cells. J Clin Invest. 2001b;108:1051–1059. doi: 10.1172/JCI12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchgessner AL, Tamir H, Gershon MD. Identification and stimulation by serotonin of intrinsic sensory neurons of the submucosal plexus of the guinea pig gut: activity-induced expression of Fos immunoreactivity. J Neuroscience. 1992;12:235–248. doi: 10.1523/JNEUROSCI.12-01-00235.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkup AJ, Brunsden AM, Grundy D. Receptors and transmission in the brain-gut axis: potential for novel therapies. I. Receptors on visceral afferents. Am J Physiol Gastrointest Liver Physiol. 2001;280:G787–G794. doi: 10.1152/ajpgi.2001.280.5.G787. [DOI] [PubMed] [Google Scholar]
- Kunze WA, Clerc N, Bertrand PP, et al. Contractile activity in intestinal muscle evokes action potential discharge in guinea-pig myenteric neurons. J Physiol. 1999;517:547–561. doi: 10.1111/j.1469-7793.1999.0547t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DR, Chen JX, Gershon MD, et al. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2003;285:G207–G216. doi: 10.1152/ajpgi.00488.2002. [DOI] [PubMed] [Google Scholar]
- Lomax RB, Gallego S, Novalbos J, et al. L-Type calcium channels in enterochromaffin cells from guinea pig and human duodenal crypts: an in situ study. Gastroenterology. 1999;117:1363–1369. doi: 10.1016/s0016-5085(99)70286-6. [DOI] [PubMed] [Google Scholar]
- Minami M, Tamakai H, Ogawa T, et al. Chemical modulation of 5-HT3 and 5-HT4 receptors affects the release of 5-hydroxytryptamine from the ferret and rat intestine. Res Commun Mol Pathol Pharmacol. 1995;89:131–142. [PubMed] [Google Scholar]
- Modlin IM, Kidd M, Eick GN, et al. The transcriptome and function of normal and neoplastic human EC cells. Gastroenterology. 2006;130(Suppl. 2):A704. [Google Scholar]
- Nilsson O, Ahlman H, Geffard M, et al. Bipolarity of duodenal enterochromaffin cells in the rat. Cell Tissue Res. 1987;248:49–54. doi: 10.1007/BF01239961. [DOI] [PubMed] [Google Scholar]
- O'Hara JR, Ho W, Linden DR, et al. Enteroendocrine cells and 5-HT availability are altered in mucosa of guinea pigs with TNBS ileitis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G998–G1007. doi: 10.1152/ajpgi.00090.2004. [DOI] [PubMed] [Google Scholar]
- Racké K, Reimann A, Schwörer H, et al. Regulation of 5-HT release from enterochromaffin cells. Behav Brain Res. 1996;73:83–87. doi: 10.1016/0166-4328(96)00075-7. [DOI] [PubMed] [Google Scholar]
- Racké K, Schwörer H. Regulation of serotonin release from the intestinal mucosa. Pharmacol Res. 1991;23:13–25. doi: 10.1016/s1043-6618(05)80101-x. [DOI] [PubMed] [Google Scholar]
- Racké K, Schwörer H. Nicotinic and muscarinic modulation of 5-hydroxytryptamine (5-HT) release from porcine and canine small intestine. Clin Invest. 1992;70:190–200. doi: 10.1007/BF00184650. [DOI] [PubMed] [Google Scholar]
- Satoh Y, Habara Y, Ono K, et al. Carbamylcholine- and catecholamine-induced intracellular calcium dynamics of epithelial cells in mouse ileal crypts. Gastroenterology. 1995;108:1345–1356. doi: 10.1016/0016-5085(95)90681-9. [DOI] [PubMed] [Google Scholar]
- Satoh Y, Williams MR, Habara Y. Effects of AIF4- and ATP on intracellular calcium dynamics of crypt epithelial cells in mouse small intestine. Cell Tissue Res. 1999;298:295–305. doi: 10.1007/s004419900069. [DOI] [PubMed] [Google Scholar]
- Schafermeyer A, Gratzl M, Rad R, et al. Isolation and receptor profiling of ileal enterochromaffin cells. Acta Physiol Scand. 2004;182:53–62. doi: 10.1111/j.1365-201X.2004.01299.x. [DOI] [PubMed] [Google Scholar]
- Sidhu M, Cooke HJ. Role for 5-HT and ACh in submucosal reflexes mediating colonic secretion. Am J Physiol. 1995;269:G346–G351. doi: 10.1152/ajpgi.1995.269.3.G346. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Smith TK. Mechanosensory S-neurons rather than AH-neurons appear to generate a rhythmic motor pattern in guinea-pig distal colon. J Physiol. 2004;558:577–596. doi: 10.1113/jphysiol.2004.063586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamford JA. In vivo voltammetry: some methodological considerations. J Neurosci Meth. 1986;17:1–29. doi: 10.1016/0165-0270(86)90031-2. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Mizumoto A, Mochiki E, et al. Relationship between intraduodenal 5-hydroxytryptamine release and interdigestive contractions in dogs. J Smooth Muscle Res. 2004;40:75–84. doi: 10.1540/jsmr.40.75. [DOI] [PubMed] [Google Scholar]
- Tonini M. 5-Hydroxytryptamine effects in the gut: the 3, 4, and 7 receptors. Neurogastroenterol Motil. 2005;17:637–642. doi: 10.1111/j.1365-2982.2005.00716.x. [DOI] [PubMed] [Google Scholar]
- Tran VS, Marion-Audibert AM, Karatekin E, et al. Serotonin secretion by human carcinoid BON cells. Ann N Y Acad Sci. 2004;1014:179–188. doi: 10.1196/annals.1294.019. [DOI] [PubMed] [Google Scholar]
- Verity MA, Mellinkoff SM, Frankland M, et al. Serotonin content and argentaffin and Paneth cell changes in ulcerative colitis. Gastroenterology. 1962;43:24–31. [PubMed] [Google Scholar]
- Wade PR, Chen J, Jaffe B, et al. Localization and function of a 5-HT transporter in crypt epithelia of the gastrointestinal tract. J Neuroscience. 1996;16:2352–2364. doi: 10.1523/JNEUROSCI.16-07-02352.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]