Abstract
In urethane–chloralose anaesthetized, neuromuscularly blocked, ventilated rats, we examined the effects on sympathetic outflow to brown adipose tissue (BAT) of separate and simultaneous spinal microinjections of NMDA and serotonin. Microinjection of NMDA (12 pmol) into the right T4 spinal intermediolateral nucleus (IML) immediately increased ipsilateral brown adipose tissue (BAT) sympathetic nerve activity (SNA; peak: +546% of control), BAT thermogenesis (+0.8°C) and heart rate (+53 beats min−1), whereas microinjection of a lower dose of NMDA (1.2 pmol) did not change any of the recorded variables. Microinjection of 5-hydroxytryptamine (5-HT, 2 nmol) into the T4 IML increased BAT SNA (peak: +342% of control) at a long latency (mean onset: 23min). The long latency 5-HT-evoked increase in BAT SNA was prevented by microinjection of methysergide (600 pmol) into the T4 IML. The increases in BAT SNA evoked by T4 IML microinjections of NMDA (12 pmol) were significantly potentiated (two to three times larger than the response to NMDA alone) following T4 IML microinjections of 5-HT (100 pmol to 2 nmol, but not 20 pmol). Also, microinjection of 5-HT (200 pmol) converted the subthreshold dose of NMDA (1.2 pmol) into an effective dose for increasing BAT SNA and heart rate. The 5-HT-mediated potentiation of the increase in BAT SNA evoked by microinjection of NMDA into the T4 IML was reversed by microinjection of methysergide (600 pmol) into the T4 IML. These results demonstrate that BAT SNA and thermogenesis can be driven by activation of spinal excitatory amino acid or 5-HT receptors and that concomitant activation of spinal NMDA and 5-HT receptors can act synergistically to markedly increase BAT SNA and thermogenesis.
Increased metabolism in brown adipose tissue (BAT) is an important homeostatic mechanism involved in several functions such as adaptive non-shivering thermogenesis, pathogen-evoked febrile responses, and acute thermal effects of eating, as well as the increase in energy expenditure in response to excess fuel storage (see Cannon & Nedergaard, 2004); dysregulation of metabolism in BAT may play a role in pathological conditions related to these homeostatic processes. For example, it has been demonstrated in mice that destruction of BAT leads to susceptibility to obesity (Hamann et al. 1996). Interestingly, obese humans have been found to have decreased levels of uncoupling protein 1 mRNA, a marker for BAT, compared to lean humans (Oberkofler et al. 1997). Since sympathetic activation of BAT is the primary determinant of metabolism in this tissue and given the obvious importance of understanding the regulation of metabolism in BAT it is not surprising that many studies have begun to define the neural circuits involved in the regulation of this effector output (Morrison et al. 1999; Zaretskaia et al. 2002, 2003; Madden & Morrison, 2003, 2004, 2005; Morrison, 2004; Nakamura et al. 2004; Cerri & Morrison, 2005; Rathner & Morrison, 2006). However, much of the detailed neurocircuitry and functional interactions within central regions implicated in the regulation of sympathetic outflow to BAT remain unknown.
Neurones of the rostral ventromedial medulla including the raphe pallidus area (RPa) play an essential role in the regulation of sympathetically regulated metabolism in BAT (Morrison et al. 1999). Activation of excitatory amino acid receptors within the intermediolateral cell column (IML) of the spinal cord is necessary for the RPa-evoked increase in BAT temperature (Nakamura et al. 2004). However, the possibility that serotonergic innervation of the IML also plays a role in sympathetically regulated metabolism in BAT is suggested by the presence of bulbospinal tryptophan hydroxylase (a biosynthetic enzyme involved in the production of 5-hydroxytryptamine)-containing or 5-hydroxytryptamine (5-HT)-containing neurones in the RPa that are retrogradely labelled by injection of trans-synaptic viral tracers into BAT (Cano et al. 2003; Nakamura et al. 2004). Further evidence for a role of 5-HT in BAT activation is that the depletion of central 5-HT inhibits BAT thermogenesis (Fuller et al. 1987), though the relative roles of bulbospinal 5-HT versus ascending forebrain 5-HT projections have not been well defined. In addition, cold exposure activates bulbospinal 5-HT neurones (Passerin & Henley, 1994; Passerin et al. 1999; Martin-Cora et al. 2000). 5-HT receptors are located within the IML (Marlier et al. 1991; Thor et al. 1993; Maeshima et al. 1998; Cornea-Hebert et al. 1999). Furthermore, in the cat (De Groat & Ryall, 1967; Coote et al. 1981; Gilbey & Stein, 1991) and the rat (Lewis & Coote, 1990; Pickering et al. 1994) the majority of sympathetic preganglionic neurones are activated by 5-HT. Therefore we sought to test the hypothesis that activation of 5-HT receptors within the IML of the spinal cord would increase BAT sympathetic nerve activity (SNA) and thermogenesis. In addition, since 5-HT facilitates the effects of excitatory amino acids on several motoneurone pools (McCall & Aghajanian, 1979; White & Neuman, 1980; Fuller et al. 2000), we hypothesized that a similar interaction within the intermediolateral cell column would result in synergistic effects of 5-HT and excitatory amino acids to enhance BAT SNA responses.
Methods
All procedures conform to the regulations detailed in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of the Oregon Health and Science University. Male Sprague-Dawley rats (Charles River, Indianapolis, IN, USA, n = 34) weighing 250–450 g were given ad libitum access to standard rat chow and water in a colony room maintained at 22–23°C and kept on a 12: 12 h light–dark cycle. Rats were anaesthetized with isoflurane (2–3% in oxygen) and implanted with femoral arterial and venous catheters and transitioned to intravenous (i.v.) urethane and chloralose anaesthesia (750 mg kg−1 and 60 mg kg−1, respectively) over a 10 min period. All physiological variables were digitized (Micro 1401 MKII; Cambridge Electronic Design (CED), Cambridge, UK) and recorded onto a computer hard drive. Arterial blood pressure was recorded from the arterial catheter attached to a pressure transducer and heart rate (HR) was derived from the arterial pressure signal. The trachea was cannulated, and the animals were ventilated (tidal volume: ∼1 ml per 100 g body weight, 60 cycles min−1) with 100% oxygen. Neuromuscular blockade was achieved by administration of d-tubocurarine (0.5 mg i.v., supplemented with 0.1 mg as needed to suppress spontaneous diaphragmatic contractions). Adequacy of anaesthesia was verified prior to neuromuscular blockade by absence of withdrawal reflex, and pressor response to foot pinch as well as absence of eye blink response to gentle probing of the cornea. In addition, prior to supplementation of neuromuscular blockade, adequacy of anaesthesia was re-assessed and anaesthetic was supplemented (10% of initial dose) as necessary. A capnometer (Dynatech Electro-optics, Saline MI, USA, model 2200) was used to measure end-expiratory CO2, which reflects increased metabolism (in the current study, mainly produced by activation of brown adipose tissue and/or the heart). Colonic (core) temperature was monitored using a copper–constantan thermocouple inserted 6 cm into the rectum and was maintained between 36.5 and 37.5°C with a heating plate or a water perfused heating/cooling blanket and a heat lamp. Animals were placed in a stereotaxic instrument with the incisor bar positioned 4 mm below the interaural line. To stabilize the spinal cord a clamp was placed on the caudal thoracic spinal vertebrae. The third thoracic vertebra was removed to provide access to the underlying fourth thoracic spinal segment (T4), (see Gelderd & Chopin, 1977).
Recording BAT SNA and temperature
The BAT temperature was monitored using a thermocouple meter (TC-1000, Sable Systems International, Las Vegas, NV, USA) with a copper–constantan thermocouple (Physitemp, Clifton, NJ, USA) inserted into the intact, left interscapular fat pad. Postganglionic BAT SNA was recorded under mineral oil with a bipolar hook electrode from the central cut end of a small diameter (∼100 μm) nerve bundle isolated from the ventral surface of the right interscapular fat pad. BAT temperature was recorded in the BAT pad contralateral to the nerve recording because the recording of BAT SNA partially denervates the right BAT pad and the exposure of the ventral surface of the right BAT pad during nerve dissection limits its utility for BAT temperature recording. Nerve activity was filtered (1–300 Hz) and amplified (10 000 − 50 000×) with a Cyberamp 380 (Axon Instruments, Union City, CA, USA). Spike2 software (CED) was used to obtain a continuous measure (4 s bins) of BAT SNA amplitude by calculating the root mean square (r.m.s.) amplitude of the BAT SNA (square root of the total power in the 0.1–20 Hz band, this power is eliminated by ganglionic blockade) from the autospectra of sequential 4 s segments of BAT SNA. Control values of BAT SNA were the averages of the BAT SNA amplitudes during the 32 s periods immediately prior to treatments. Peak BAT SNA was defined as the average value during the 32 s period of maximal change in BAT SNA evoked by a treatment. The onset of the evoked response was defined as the time point at which the measured variable exceeded (for at least 12 s) the maximal value recorded during the control period. The offset was defined as the time point at which the measured variable returned to the maximal value recorded during the control period. The area under the curve (AUC) was taken between the times of onset and offset, except when the AUC of one response was expressed as a function of another response (such as, the response to NMDA after 5-HT as a percentage of the NMDA-only response) the AUC of the shorter response was taken over the same time period as the longer response.
Microinjections into the spinal cord
Microinjection coordinates for the IML were 0.5 mm lateral to the midline (just medial to the area at which the dorsal roots enter the spinal cord), and 0.8 mm ventral to the dorsal surface of the spinal cord; and for the dorsal horn: 0.5 mm lateral to the midline and 0.15 mm ventral to the dorsal surface of the spinal cord. Glass micropipettes (outer tip diameter, 20–30 μm) were used for all microinjections which were given in a volume of 60 nl over a 10–20 s period using a pressure injection system (model IIe, Toohey Company) and a reticule to watch the movement of the meniscus in the micropipette. Multiple microinjections into the same IML coordinates were made by retracting the micropipette vertically, emptying, rinsing with distilled water and refilling the micropipette, and then lowering the micropipette tip into the IML.
At the conclusion of each experiment, the IML and dorsal horn microinjection sites were marked by electrophoretic (15 μA, 10 min) application of fast green, as previously described (Madden & Morrison, 2005). In some cases microinjection sites were marked by inclusion of fluorescent polystyrene microspheres (FluoSpheres, F8801 or F8803, Molecular Probes, Eugene, OR, USA) in the injectate (1: 200 dilution of Fluospheres in the injectate). Rats were perfused (10% paraformaldehyde) transcardially, spinal cords were removed, post-fixed (12–24 h) and sectioned (60 μm coronal sections) on a vibratome. Spinal cord sections were mounted on slides and microinjection sites were localized and photographed using brightfield or appropriate fluorescence filters (excitation: 533–588 nm, emission: 608–683 nm; or excitation: 460–490 nm, emission: 510–550 nm).
Drugs and solutions
All drugs were obtained from Sigma (St Louis, MO, USA) except isofluorane, which was obtained from Abbott Laboratories (North Chicago, IL, USA). All drugs were dissolved in saline, except 5-HT which was dissolved in saline containing 0.1% ascorbic acid.
All statistics were performed using Systat software (version 10, Systat Software Inc., Richmond, CA, USA). Data are expressed as means ± s.e.m. Statistical significance was assessed using Student's t test for paired data, ANOVA or ANOVA with repeated measures, as appropriate. Following a significant F value, post hoc testing was performed using layered Bonferroni's analysis. The significance level was P < 0.05.
Protocols
The basic protocol to test for a potentiation of the N-methyl-d-aspartate (NMDA)-evoked increase in BAT SNA consisted of a series of three microinjections into the T4 IML: an initial (control) microinjection of NMDA, microinjection of a test compound, followed by a repeated microinjection of NMDA. All rats (n = 34) received a microinjection of NMDA (12 pmol) into the right T4 IML (ipsilateral to the nerve recording). After the responses to the first microinjection of NMDA returned to control levels, one of the following drugs was microinjected at the same IML coordinates: ascorbic acid vehicle (0.1% in saline, n = 7); 5-hydroxytryptamine creatinine sulphate (5-HT; 20 pmol, 100 pmol, 200 pmol, 1 nmol or 2 nmol in 0.1% ascorbic acid, n = 5, 5, 9, 5 and 15, respectively); or the broad spectrum 5-HT receptor antagonist, methysergide maleate (600 pmol, n = 5). Within 5 min of the microinjection of one of the preceding compounds a second microinjection of NMDA (12 pmol) was made at the same coordinates within the T4 IML. To determine whether serotonin could convert a subthreshold dose of NMDA into an effective dose, we also tested a subset of rats (n = 4) for potentiation of a 1.2 pmol dose of NMDA by 5-HT (200 pmol). The effect on NMDA-evoked responses in BAT SNA of more than one compound was determined in several rats. In these cases two criteria were met prior to the testing of a subsequent drug: basal BAT SNA returned to the control level and the magnitude of the increase in BAT SNA evoked by a microinjection of NMDA into the T4 IML returned to that observed during the original control microinjection of NMDA. A subset of rats (n = 13) also received a microinjection into the IML of 5-HT (2 nmol) alone followed by a period of 2 h during which no further microinjections were made.
To verify that the 5-HT potentiation of the NMDA-evoked increase in BAT SNA was mediated by the selective activation of 5-HT receptors, a subset of the rats that underwent the basic protocol for testing potentiation of the NMDA response by 5-HT (2 nmol) also underwent the following protocol: the second NMDA microinjection of the basic protocol was followed within 5 min by a microinjection of the broad spectrum 5-HT receptor antagonist methysergide (600 pmol, n = 7) or saline (n = 2) or no injection (n = 2) into the T4 IML and after a 20–60 min delay a subsequent (third) microinjection of NMDA (12 pmol) into the same site.
To control for the possibility that the responses evoked by microinjection of agents into the IML were mediated by diffusion to sites outside of the IML, a subset of rats (n = 6) also received a microinjection of NMDA (12 pmol) into the dorsal horn. Four of these six rats also received a microinjection of NMDA into the IML followed by a microinjection of 5-HT (100 pmol) into the dorsal horn and a subsequent microinjection of NMDA into the IML.
To verify that the BAT SNA responses were indicative of changes in thermogenesis in the adipose tissue a subset of rats (n = 7) received a microinjection of NMDA (12 pmol) into the left T4 IML (ipsilateral to the BAT pad from which temperature was recorded; contralateral to the nerve recording). After the responses to the first microinjection of NMDA returned to resting levels a microinjection of 5-HT (2 nmol) was made into the left T4 IML followed by a microinjection of NMDA (12 pmol) at the same coordinates.
Results
NMDA in T4 IML
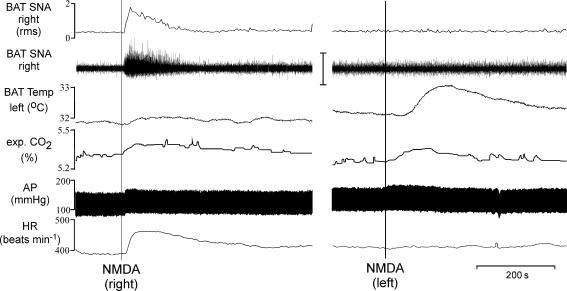
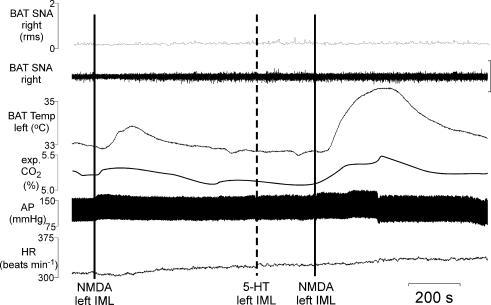
To test the hypothesis that activation of excitatory amino acid receptors within the IML is capable of driving BAT SNA and thermogenesis, NMDA was microinjected into the T4 IML. Consistent with our previous reports (Madden & Morrison, 2003) and as illustrated in Fig. 1, in untreated rats whose core temperature was maintained at 37.0 ± 0.5°C, BAT SNA was nearly quiescent, exhibiting only low-amplitude discharge. Microinjection of NMDA (12 pmol) into the right T4 IML (ipsilateral to the BAT sympathetic nerve recording) immediately increased BAT SNA, expired CO2, arterial pressure and HR but did not increase BAT temperature (recorded from the contralateral BAT) (Fig. 1; Table 1); whereas microinjection of a smaller dose of NMDA (1.2 pmol) into the right T4 IML did not change any of the recorded variables. Microinjection of NMDA (12 pmol) into the right dorsal horn slightly increased BAT SNA (peak increase: +32 ± 12% of control); for comparison microinjection of NMDA into the right IML markedly increased BAT SNA (peak increase: +546 ± 75% of control). Microinjection of NMDA into the left T4 IML (ipsilateral to the BAT pad from which temperature was recorded) increased BAT temperature and expired CO2 but did not increase BAT SNA (recorded contralateral to the microinjection), arterial pressure, or HR (Fig. 1; Table 1).
Figure 1. Microinjection of NMDA into T4 IML elicited thermogenic, respiratory and cardiovascular responses.
Microinjection of NMDA into the right T4 IML immediately increased brown adipose tissue (BAT) sympathetic nerve activity (SNA; peak: +338% of control), expired CO2 (peak: +0.1%), mean arterial pressure (AP) (peak: +10 mmHg), and heart rate (HR, peak: +72 beats min−1). BAT temperature was increased (+0.9°C) when NMDA was microinjected into the left IML (ipsilateral to the BAT from which temperature was recorded). Vertical scale bar in BAT SNA tracing represents 100 μV.
Table 1.
Effects on cardiovascular, metabolic and thermogenic variables elicited by microinjection of NMDA into the intermediolateral cell column at the fourth thoracic segment (T4 IML)
| NMDA into T4 IML | |||
|---|---|---|---|
| Control | Right | Left | |
| BAT SNA, right (% control) | 100 | +546 ± 75* | −2 ± 5 |
| BAT Temp, left (°C) | 33.0 ± 0.2 | +0.0 ± 0.0 | +0.8 ± 0.1* |
| Expired CO2 (%) | 4.8 ± 0.1 | +0.1 ± 0.0* | +0.1 ± 0.0* |
| HR (beats min−1) | 376 ± 7 | +53 ± 5* | +7 ± 5 |
| MAP (mmHg) | 97 ± 3 | + 8 ± 2* | +5 ± 2 |
Values are mean ±s.e.m. for physiological variables in the control condition (Control, n = 34) and the peak changes within 10 min after the microinjection of NMDA (12 pmol) into the intermediolateral cell column at the fourth thoracic segment either on the right side (ipsilateral to the recorded nerve and contralateral to the recorded BAT temperature, n = 34) or on the left side (contralateral to the recorded nerve and ipsilateral to the recorded BAT temperature, n = 7)
P < 0.05, compared to the control condition.
5-HT in T4 IML
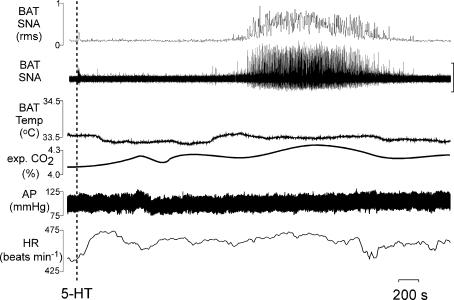
The amplitude of BAT SNA was low in rats under control conditions with core temperature maintained at 37.0 ± 0.5°C. Microinjection of 5-HT (2 nmol) into the T4 IML produced a significant increase in BAT SNA (area under the curve: 292 ± 59% of control; peak: +342 ± 85% of control). The 5-HT-evoked activation of BAT SNA occurred at a long latency (onset: 23 ± 3 min post-injection; peak: 35 ± 2 min post-injection) and lasted for 30 ± 4 min (Fig. 2). Microinjection of 5-HT (1 nmol) into the T4 IML produced variable results with a long latency increase in BAT SNA occurring in two of five rats; microinjections into the IML of 5-HT in doses of 20 pmol, 100 pmol or 200 pmol did not increase BAT SNA over a 1 h period of observation. Microinjection of 5-HT (2 nmol) into the T4 IML typically (10 of 13 cases) produced an immediate increase in HR (+30 ± 5 beats min−1). Microinjection of 5-HT (2 nmol) into the T4 IML also produced significant long latency increases in expired CO2 (peak change: +0.2 ± 0.0% at 35 ± 2 min postinjection from a control level of 4.7 ± 0.2%), and HR (peak change: +36 ± 8 beats min−1 at 31 ± 2 min post-injection from a control value of 385 ± 13 beats min−1).
Figure 2. Microinjection of 5-hydroxytryptamine (5-HT) into T4 IML elicited thermogenic, respiratory and cardiovascular responses.
Microinjection of 5-HT into the right T4 IML (ipsilateral to the recorded nerve) increased brown adipose tissue (BAT) sympathetic nerve activity (SNA; peak: +381% of control), expired CO2 (peak: +0.2%), and heart rate (HR, peak: +34 beats min−1). Due to the long latency to onset of the 5-HT response and the variability in BAT temperature over long periods of time, responses to microinjection of 5-HT ipsilateral to the BAT from which temperature was recorded were not performed. Vertical scale bar in BAT SNA tracing represents 100 μV.
Synergism between 5-HT and NMDA in the IML
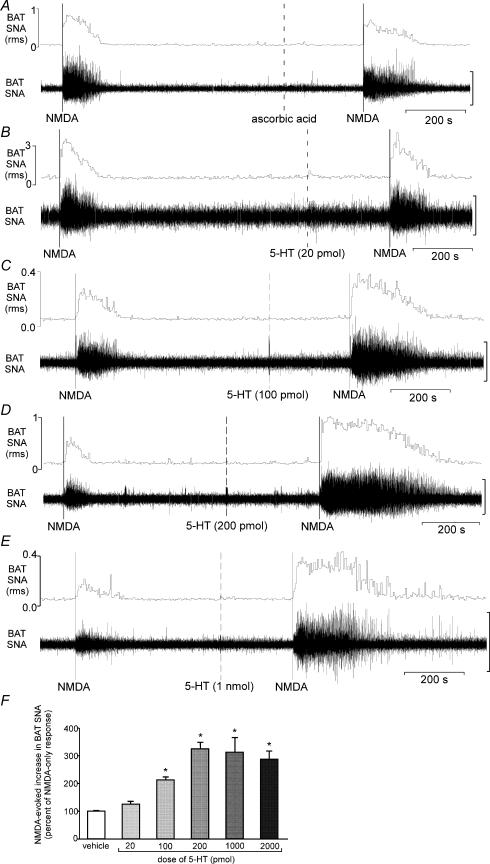
Responses to microinjection of NMDA into the T4 IML following microinjection of either 0.1% ascorbic acid vehicle or the lowest dose of 5-HT (20 pmol) into the T4 IML did not differ from those evoked by microinjection of NMDA alone (Fig. 3A, B and F). In contrast, microinjection into the T4 IML of 5-HT at doses of at least 100 pmol potentiated the NMDA-evoked increase in BAT SNA (Figs 3C–F and 4). The 5-HT-mediated potentiation of the NMDA-evoked increase in BAT SNA was manifested as an increase in both the peak magnitude and the response duration (Figs 3C–E and 4). In addition microinjection of NMDA (1.2 pmol) into the T4 IML, which alone did not increase BAT SNA (peak increase: +5 ± 3%), increased BAT SNA following microinjection of 5-HT (200 pmol) into the T4 IML (peak increase: +210 ± 38%). Furthermore, the potentiation of the NMDA-evoked response by the highest dose of 5-HT (2 nmol) was observed as long as an hour after the T4 IML microinjection of 5-HT (Fig. 4). The example in Fig. 4A shows a 104% potentiation (doubling) of the BAT SNA excitation elicited by NMDA microinjected into the T4 IML when the NMDA was microinjected 4 min after the microinjection of 5-HT (2 nmol) into the T4 IML and this was comparable to the 155% and 122% potentiations observed in the BAT SNA responses evoked when NMDA was microinjected at 35 min and 42 min after 5-HT. The change in BAT temperature evoked by microinjection of NMDA into the left T4 IML (ipsilateral to the BAT temperature recording; n = 7), was also potentiated by microinjection of 5-HT (2 nmol) into the same site (+0.8 ± 0.1°C for NMDA alone versus +2.0 ± 0.2°C, for NMDA after 5-HT) (Fig. 5).
Figure 3. Effect of vehicle or several doses of 5-hydroxytryptamine (5-HT) on the increase in BAT SNA evoked by microinjection of NMDA into the T4 IML.
A, microinjection of ascorbic acid vehicle into the T4 IML did not alter the NMDA-evoked increase in BAT SNA (the area under the curve, AUC, after ascorbic acid was 103% of the AUC for NMDA only). B, microinjection of 5-HT (20 pmol) into the T4 IML did not alter the NMDA-evoked increase in BAT SNA (AUC after 5-HT was 98% of the AUC for NMDA only). C, microinjection of 5-HT (100 pmol) into the T4 IML potentiated the NMDA-evoked increase in BAT SNA (AUC after 5-HT was 191% of the AUC for NMDA only). D, microinjection of 5-HT (200 pmol) into the T4 IML potentiated the NMDA-evoked increase in BAT SNA (AUC after 5-HT was 385% of the AUC for NMDA only). E, microinjection of 5-HT (1 nmol) into the T4 IML potentiated the NMDA-evoked increase in BAT SNA (AUC after 5-HT was 282% of the AUC for NMDA only). F, the group data for the area under the curve of the NMDA (12 pmol)-evoked BAT SNA following ascorbic acid or 5-HT (20 pmol, 100 pmol, 200 pmol, 1 nmol or 2 nmol) are illustrated as a percentage of the paired NMDA-only response. Note a representative example of the data for the 2 nmol dose of 5-HT is presented in Fig. 4. Vertical scale bar for BAT SNA tracing in panels A–E represents 100 μV, 150 μV, 50 μV, 20 μV and 50 μV, respectively. P < 0.05 compared to the NMDA response following ascorbic acid.
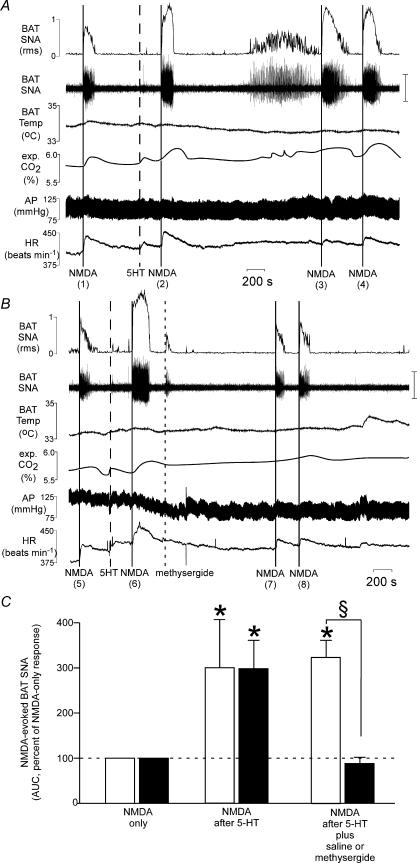
Figure 4. The potentiation of the NMDA-evoked increase in brown adipose tissue sympathetic nerve activity (BAT SNA) by prior microinjection of 5-hyroxytryptamine (5-HT) is prevented by the broad spectrum 5-HT receptor antagonist, methysergide.
A, microinjection of NMDA into the right T4 IML immediately increased BAT SNA (NMDA (1)) and microinjection of 5-HT (2 nmol) into the same site potentiated the NMDA-evoked increases in BAT SNA (NMDA (2), (3) and (4)). The potentiation of the NMDA-evoked response was maintained for at least 45 min. B, microinjection of NMDA into the right T4 IML immediately increased BAT SNA (NMDA (5)), and microinjection of 5-HT (2 nmol) into the same site potentiated the NMDA-evoked increase in BAT SNA (NMDA (6)). Subsequent microinjection of methysergide reversed the 5-HT-induced potentiation of the NMDA-evoked response (NMDA (7) and (8)). Note the long-latency activation of BAT SNA evoked by 5-HT was also prevented by microinjection of methysergide. C, bar graph depicting the area under the curve (AUC) of the NMDA-evoked BAT SNA presented as a percentage of the NMDA-only response in rats receiving injections of NMDA alone, NMDA following 5-HT, and NMDA following 5-HT plus saline (open bars, n = 4) or NMDA following 5-HT plus methysergide (black bars, n = 7). Vertical scale bar in BAT SNA tracing represents 100 μV *P < 0.05, compared to the corresponding NMDA-only response. §P < 0.05, between the saline and the methysergide groups.
Figure 5. NMDA-evoked thermogenesis in BAT was potentiated by prior administration of 5-hydroxytryptamine (5-HT).
Microinjection of NMDA into the T4 IML increased BAT temperature (+0.9°C) ipsilateral to the injection site. Following microinjection of 5-HT (2 nmol) into the T4 IML, microinjection of NMDA into the same site resulted in a larger increase in BAT temperature (+2.9°C). BAT SNA contralateral to the injection site was not altered. Vertical scale bar in BAT SNA tracing represents 60 μV.
Expired CO2 and arterial pressure were not changed by microinjection of a low dose of NMDA (1.2 pmol) into the IML either before or following microinjection of 5-HT into the T4 IML, whereas the NMDA (1.2 pmol)-evoked change in HR following 5-HT (200 pmol) was larger than that evoked by NMDA (1.2 pmol) alone (+29 ± 9 beats min−1versus +4 ± 2 beats min−1, P < 0.05). For the larger dose of NMDA (12 pmol) the changes in expired CO2, arterial pressure, and HR following microinjection of 5-HT into the T4 IML did not differ from those following microinjection of ascorbic acid vehicle (Table 2).
Table 2.
Effects on cardiovascular, metabolic and thermogenic variables of microinjection into the intermediolateral cell column at the fourth thoracic segment (T4 IML) of NMDA after ascorbic acid vehicle, or NMDA after five doses of 5-HT
| NMDA after 5-HT (pmol) | ||||||
|---|---|---|---|---|---|---|
| NMDA after vehicle | 20 pmol | 100 pmol | 200 pmol | 1000 pmol | 2000 pmol | |
| n | 7 | 5 | 5 | 9 | 5 | 15 |
| BAT temp. (°C) | +0.1 ± 0.0 | 0.0 ± 0.0 | +0.1 ± 0.1 | +0.1 ± 0.0 | +0.1 ± 0.0 | +0.1 ± 0.0 |
| Expired CO2 (%) | +0.2 ± 0.0 | +0.1 ± 0.0 | +0.0 ± 0.0 | +0.1 ± 0.0 | +0.0 ± 0.0 | +0.2 ± 0.0 |
| HR (beats min−1) | +79 ± 9 | +47 ± 13 | +34 ± 5 | +54 ± 10 | +49 ± 5 | +59 ± 9 |
| MAP(mmHg) | +10 ± 6 | +12 ± 8 | +3 ± 1 | +9 ± 4 | +2 ± 1 | +8 ± 2 |
Values are mean ±s.e.m. for peak changes from control values within 5 min of the microinjection of NMDA (12 pmol) into the right T4 IML (contralateral to the recorded BAT temperature). NMDA-evoked responses following microinjections of ascorbic acid or 5-HT did not differ from those evoked by NMDA only. Also the NMDA-evoked changes following 5-HT did not differ from those following ascorbic acid vehicle.
Effect of methysergide in the IML on evoked responses
The 5-HT-mediated potentiation of the NMDA-evoked increase in BAT SNA was blocked by the 5-HT receptor antagonist methysergide microinjected into T4 IML subsequent to the microinjection of 5-HT. The individual example in Fig. 4B shows a 244% potentiation (more than tripling) of the BAT SNA excitation elicited by NMDA microinjected into the T4 IML when the NMDA was microinjected 4 min after the microinjection of 5-HT (2 nmol) into the T4 IML and this potentiation was completely abolished (75% and 95% of the original NMDA-evoked response when NMDA was microinjected at 30 min and 35 min after 5-HT) following microinjection of methysergide after 5-HT. In rats (n = 4) receiving only NMDA microinjections 30–70 min after the microinjection of 5-HT, 5-HT produced an approximate tripling of the NMDA response (Fig. 4A compare NMDA (1) to NMDA (3), and Fig. 4C). When methysergide was microinjected into the T4 IML (n = 7) following the 5-HT (Fig. 4B), the subsequent (30–70 min after the microinjection of 5-HT) NMDA-evoked responses were not different from those evoked prior to microinjection of 5-HT (Fig. 4B compare NMDA (5) to NMDA (7), and Fig. 4C. Microinjection of methysergide alone (without prior administration of 5-HT) did not alter the NMDA-evoked changes in any of the recorded variables.
Methysergide microinjection also prevented the long-latency increase in spontaneous BAT SNA elicited by T4 IML microinjection of 5-HT (compare Figs 2 and 4A with Fig. 4B). In rats (n = 4) receiving a microinjection of 5-HT without subsequent administration of methysergide, during the interval between 20 and 35 min following microinjection of 5-HT into the T4 IML, the mean peak BAT SNA was 435 ± 160% of the control level prior to 5-HT microinjection, whereas during this same interval in rats receiving a microinjection of methysergide following the 5-HT, the mean peak BAT SNA (90 ± 6% control) was not different from the control level prior to 5-HT microinjection.
Histological localization of microinjection sites
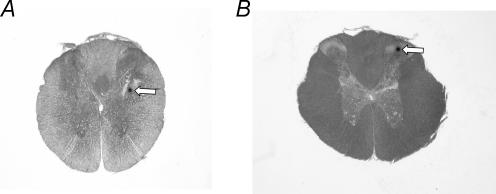
Representative examples illustrating the locations of the microinjection sites in the T4 IML and the dorsal horn are shown in Fig. 6. All microinjection sites targeting the T4 IML were found in the IML or just lateral to the IML in the white matter within 100 μm of the IML. All microinjection sites targeting the dorsal horn were found in laminae 1–3. In all cases multiple microinjections of distinct Fluospheres resulted in overlapping areas of labelling within the IML and the immediately surrounding area, indicating that multiple microinjections into the IML were localized to very similar areas.
Figure 6. Representative examples of transverse sections through the spinal cord illustrating the histological localization of the position of the micropipette tip targeting the intermediolateral cell column (A) and the dorsal horn within the fourth thoracic segment (B).
Discussion
The results of the present study demonstrate that activation of excitatory amino acid receptors or 5-HT receptors within the spinal cord can drive sympathetic activation of BAT and that combined activation of NMDA and 5-HT receptors within the IML, but not the dorsal horn, synergistically increases BAT SNA and thermogenesis. These data support and extend the previous observation that activation of excitatory amino acid receptors within the IML increases BAT temperature (Nakamura et al. 2004). Based on the presence of sympathetic premotor neurones within the RPa and the observation that disinhibition of neurones within this area activates BAT SNA, it has been suggested that neurones in the RPa provide the excitatory drive to sympathetic preganglionic neurones that regulate BAT thermogenesis (Morrison et al. 1999). Furthermore, the sympathetic premotor cells mediating BAT activation may be glutamatergic (Nakamura et al. 2004) since vesicular glutamate transporter-3 (VGLUT-3) is present in RPa neurones with putative projections to sympathetic preganglionic neurones (SPNs) and blockade of excitatory amino acid receptors within the IML blocks the increase in BAT temperature evoked by disinhibition of neurones within the RPa. The results of the present study suggest that, in addition to spinally projecting glutamatergic neurones in the RPa, spinally projecting serotonergic neurones in the RPa also play an important role in regulating BAT SNA.
Following injection of a trans-synaptic retrograde viral tracer into the interscapular BAT, both serotonergic and VGLUT-3 containing neurones in the rostral ventromedial medulla are labelled (Cano et al. 2003; Nakamura et al. 2004). Although the most straightforward interpretation of these data is that VGLUT-3 positive neurones and serotonergic neurones of the rostral ventromedial medulla directly influence the activity of BAT SPNs by the release of glutamate and serotonin, the detailed spinal neurocircuitry of serotonergic and glutamatergic inputs to the IML may be more complicated. Of particular interest is the demonstration that some VGLUT-3 mRNA positive cells of the rostral ventromedial medulla also contain tryptophan hydroxylase (Stornetta et al. 2005), suggesting that alterations in the firing of such RPa neurons could simultaneously influence the release of both glutamate and serotonin in the IML. In addition, within the IML, terminals that contain both VGLUT-3 and GABA form symmetric (putative inhibitory) synapses on GABA neurons, providing an anatomical substrate for a descending pathway that increases the activity of SPNs through disinhibition (Stornetta et al. 2005).
The present study has demonstrated that microinjection of 5-HT into the IML results in a long latency (onset ∼23 min post injection) sympathetic activation of BAT. Similar long latency 5-HT-mediated responses have been reported with respect to long-term facilitation of phrenic nerve activity (Fuller et al. 2000). Activation of spinal 5-HT2A receptors and a resulting synthesis of BDNF have been implicated in the long term facilitation of phrenic nerve activity (Baker-Herman & Mitchell, 2002; Baker-Herman et al. 2004). Given that 5-HT2 receptors are located on cell bodies within the IML (Maeshima et al. 1998) it is tempting to speculate that mechanisms similar to those reported for long-term facilitation of phrenic nerve activity are involved in the delayed increase in BAT SNA in response to spinally administered serotonin. Alternatively, Pickering et al. (1994) have described a slow onset, long lasting depolarization of SPNs evoked by 5-HT acting via closure of an outwardly rectifying potassium conductance, which could contribute to the long latency activation seen in the current study. A third possibility is that other mechanisms such as down-regulation of serotonergic receptors caused by a high dose of 5-HT (only the highest dose of 5-HT, 2 nmol, consistently produced the long latency activation of BAT SNA) could be involved.
Previous studies have demonstrated that systemic administration of a 5-HT2A receptor agonist increases BAT temperature (Ootsuka & Blessing, 2006) and results in hyperthermia (Gudelsky et al. 1986; Ootsuka & Blessing, 2006). In the present study, BAT SNA and thermogenesis were enhanced by spinal administration of 5-HT. Together, these data are consistent with the possibility that the hyperthermia seen following systemic administration of a 5-HT2A receptor agonist is mediated by activation of spinal 5-HT2A receptors and a resulting enhancement of BAT SNA and thermogenesis. However, the systemically administered 5-HT2A agonist could also be exerting its effects on body temperature and BAT temperature by acting at a supraspinal site, such as the RPa or areas of the forebrain that have been implicated in thermoregulation. In addition, 5-HT2A receptor activation following systemic administration of a 5-HT2A receptor agonist could increase body temperature by activating spinal 5-HT2A receptors involved in cutaneous vasoconstriction (Ootsuka & Blessing, 2005).
Previous in vivo electrophysiological experiments have demonstrated that the majority of SPNs are excited with a short latency by serotonin (De Groat & Ryall, 1967; Coote et al. 1981; Lewis & Coote, 1990). In the present study, stimulation of 5-HT receptors alone did not consistently increase BAT SNA with a short latency; however, activation of these receptors did immediately increase HR and did elicit a potent and immediate facilitation of the NMDA-evoked increase in BAT SNA. These data are most easily explained if the majority of SPNs recorded in previous experiments were cardiac SPNs. Alternatively, previous studies may have recorded BAT SPNs and the short latency serotonin evoked excitation in those studies could be related to the degree of endogenous excitatory amino acid release under their experimental conditions.
A general limitation of microinjection studies is the inability to determine the precise site of action of the injectate. Thus the current study does not address whether the actions of NMDA and 5-HT are mediated at presynaptic or postsynaptic sites, by direct activation of SPNs or by indirect actions through spinal interneurones. However, since control microinjections into the dorsal horn did not increase BAT SNA to the same extent as microinjections into the IML, the BAT SNA responses we observed likely resulted from the effects of NMDA and 5-HT on neurones within the IML. One possibility is that NMDA and 5-HT activate postsynaptic receptor populations on the same SPN, in which case the 5-HT receptor activation may result in alterations in NMDA receptor trafficking as has been reported previously (Yuen et al. 2005), or in a subthreshold change in the membrane potential as reported previously for SPNs (Pickering et al. 1994). Another possibility is that spinal 5-HT reduces a tonic inhibitory input to SPNs through activation of receptors (probably of the 5-HT1A subtype) located on GABA interneurones. In this case, selective activation of 5-HT1A receptors or blockade of GABA receptors within the IML should have a potentiating effect on the NMDA-evoked increase in BAT SNA, similar to that seen with 5-HT.
The present study confirms previous observations that microinjection of excitatory amino acids into the right IML at rostral thoracic segments produces tachycardia, whereas microinjection of excitatory amino acids into the left IML at rostral thoracic segments does not increase heart rate (Sundaram et al. 1989). In the present study, microinjection of 5-HT potentiated the chronotropic effect of a low dose of NMDA (1.2 pmol) but not that of a higher dose of NMDA (12 pmol). These data suggest that the potentiating effect of 5-HT on cardiac SPNs is similar to that on BAT SPNs, but that the higher dose of NMDA used in our experiments produced a maximal activation of cardiac SPNs above which no potentiation could be elicited due to a ceiling effect. It is of interest that under our experimental conditions, cardiac SNA exhibits a markedly higher level of tonic discharge than does BAT SNA (Cao et al. 2004), a situation that could arise in part from a greater level of tonic GABAergic inhibition of BAT SPNs and the potential for a greater potentiating effect of 5-HT due to disinhibition of SPNs (see above).
Synergistic interactions between 5-HT and excitatory amino acids have been reported for several motoneurone pools, including facial motor neurones, and both alpha and gamma spinal motor neurones (Myslinski & Anderson, 1978; McCall & Aghajanian, 1979; White & Neuman, 1980). It is interesting to note that skin cooling can produce an activation of fusimotor fibres that is dependent on neurones in the medullary raphe (Tanaka et al. 2006). Since the activity of gamma motor neurones is facilitated by serotonin (Myslinski & Anderson, 1978) and serotonin neurons of the medullary raphe are activated by cold exposure (Martin-Cora et al. 2000), it seems reasonable to suggest that bulbospinal serotonergic inputs could amplifying shivering by potentiating the cold-evoked activation of fusimotor fibres. Thus, within the spinal cord, serotonin-induced facilitation of excitatory amino acid-evoked excitation may represent a general mechanism for plasticity in diverse effector systems, including somatic motor output, and autonomic efferents involved in thermoregulation.
Further, the branching of individual serotonergic neurons within the spinal cord could provide a basis for simultaneously influencing the outputs to multiple, functionally distinct effectors. Within this framework, the results of the current study provide a model of spinal integration that could contribute to the coordinated responses of thermoregulatory efferents, but may also apply more generally to other sympathetic and motor outflows, as suggested previously (Jacobs et al. 2002). Indeed, both spinal serotonin and excitatory amino acids can increase cutaneous sympathetic outflow (Ootsuka & Blessing, 2005; Marina et al. 2006), can enhance gamma motor neurone activity (Myslinski & Anderson, 1978) contributing to shivering and can stimulate BAT thermogenesis (present study). A common synergistic mechanism for the effect of spinal serotonin similar to that observed in the current study could therefore optimize thermoregulatory responses to cold through a coordinated facilitation of sympathetic cutaneous vasomotor outflow, fusimotor output and BAT thermogenesis.
Finally, since drugs that affect the activation of 5-HT receptors are capable of altering energy homeostasis by affecting energy intake and/or energy expenditure (Breisch et al. 1976; Levitsky & Troiano, 1992), the results of the current study also suggest a potential mechanism contributing to the therapeutic effects of 5-HT-related compounds on energy homeostasis. Thus, activation of 5-HT receptors within the IML may contribute to the increased energy expenditure elicited by drugs such as fenfluramine (Levitsky & Troiano, 1992), previously used in weight-reduction programmes for the overweight and obese. Further studies will be required to investigate the detailed neurocircuitry and the specific pharmacology underlying the interactions between excitatory amino acid and serotonergic mechanisms within the IML and to determine the physiological conditions under which these mechanisms play a role.
In conclusion, the findings of the present study demonstrate that activation of NMDA or 5-HT receptors within the IML can drive BAT SNA and BAT thermogenesis and that combined activation of NMDA and 5-HT receptors within the IML can act synergistically to markedly increase metabolism in BAT. There has been controversy over the neurochemical phenotype of sympathetic premotor neurones responsible for driving sympathetic outflow to BAT. Several lines of evidence suggest that glutamatergic inputs to preganglionic neurones are responsible for driving sympathetic outflow to BAT; however, other data suggest that serotonergic inputs to preganglionic neurones are involved. The findings of the current study provide a novel conceptual framework for resolving this controversy: both glutamatergic and serotonergic inputs to SPNs may be important for BAT thermogenesis and concurrent activation of these inputs may serve as a mechanism for enhancing thermogenesis.
Acknowledgments
This work was supported by NIH grants NS40987 (S.F.M.) and DK065401 (C.J.M.). We are grateful to Joseph Rathner and Kazuhiro Nakamura for their comments on this manuscript and to Brad Sugden for histological assistance.
References
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci. 2004;7:48–55. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci. 2002;22:6239–6246. doi: 10.1523/JNEUROSCI.22-14-06239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breisch ST, Zemlan FP, Hoebel BG. Hyperphagia and obesity following serotonin depletion by intraventricular p-chlorophenylalanine. Science. 1976;192:382–385. doi: 10.1126/science.130678. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J Comp Neurol. 2003;460:303–326. doi: 10.1002/cne.10643. [DOI] [PubMed] [Google Scholar]
- Cao WH, Fan W, Morrison SF. Medullary pathways mediating specific sympathetic responses to activation of dorsomedial hypothalamus. Neuroscience. 2004;126:229–240. doi: 10.1016/j.neuroscience.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Cerri M, Morrison SF. Activation of lateral hypothalamic neurons stimulates brown adipose tissue thermogenesis. Neuroscience. 2005;135:627–638. doi: 10.1016/j.neuroscience.2005.06.039. [DOI] [PubMed] [Google Scholar]
- Coote JH, Macleod VH, Fleetwood-Walker S, Gilbey MP. The response of individual sympathetic preganglionic neurones to microelectrophoretically applied endogenous monoamines. Brain Res. 1981;215:135–145. doi: 10.1016/0006-8993(81)90497-2. [DOI] [PubMed] [Google Scholar]
- Cornea-Hebert V, Riad M, Wu C, Singh SK, Descarries L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol. 1999;409:187–209. doi: 10.1002/(sici)1096-9861(19990628)409:2<187::aid-cne2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- De Groat WC, Ryall RW. An excitatory action of 5-hydroxytryptamine on sympathetic preganglionic neurons. Exp Brain Res. 1967;3:299–305. doi: 10.1007/BF00237556. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Bach KB, Baker TL, Kinkead R, Mitchell GS. Long term facilitation of phrenic motor output. Respir Physiol. 2000;121:135–146. doi: 10.1016/s0034-5687(00)00124-9. [DOI] [PubMed] [Google Scholar]
- Fuller NJ, Stirling DM, Dunnett S, Reynolds GP, Ashwell M. Decreased brown adipose tissue thermogenic activity following a reduction in brain serotonin by intraventricular p-chlorophenylalanine. Biosci Rep. 1987;7:121–127. doi: 10.1007/BF01121875. [DOI] [PubMed] [Google Scholar]
- Gelderd JB, Chopin SF. The vertebral level of origin of spinal nerves in the rat. Anat Rec. 1977;188:45–47. doi: 10.1002/ar.1091880106. [DOI] [PubMed] [Google Scholar]
- Gilbey MP, Stein RD. Characteristics of sympathetic preganglionic neurones in the lumbar spinal cord of the cat. J Physiol. 1991;432:427–443. doi: 10.1113/jphysiol.1991.sp018392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudelsky GA, Koenig JI, Meltzer HY. Thermoregulatory responses to serotonin (5-HT) receptor stimulation in the rat. Evidence for opposing roles of 5-HT2 and 5-HT1A receptors. Neuropharmacology. 1986;25:1307–1313. doi: 10.1016/0028-3908(86)90101-2. [DOI] [PubMed] [Google Scholar]
- Hamann A, Flier JS, Lowell BB. Decreased brown fat markedly enhances susceptibility to diet-induced obesity, diabetes, and hyperlipidemia. Endocrinology. 1996;137:21–29. doi: 10.1210/endo.137.1.8536614. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Martin-Cora FJ, Fornal CA. Activity of medullary serotonergic neurons in freely moving animals. Brain Res Brain Res Rev. 2002;40:45–52. doi: 10.1016/s0165-0173(02)00187-x. [DOI] [PubMed] [Google Scholar]
- Levitsky DA, Troiano R. Metabolic consequences of fenfluramine for the control of body weight. Am J Clin Nutr. 1992;55:167S–172S. doi: 10.1093/ajcn/55.1.167s. [DOI] [PubMed] [Google Scholar]
- Lewis DI, Coote JH. The influence of 5-hydroxytryptamine agonists and antagonists on identified sympathetic preganglionic neurones in the rat, in vivo. Br J Pharmacol. 1990;99:667–672. doi: 10.1111/j.1476-5381.1990.tb12987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Excitatory amino acid receptor activation in the raphe pallidus area mediates prostaglandin-evoked thermogenesis. Neuroscience. 2003;122:5–15. doi: 10.1016/s0306-4522(03)00527-x. [DOI] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Excitatory amino acid receptors in the dorsomedial hypothalamus mediate prostaglandin-evoked thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2004;286:R320–R325. doi: 10.1152/ajpregu.00515.2003. [DOI] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Hypoxic activation of arterial chemoreceptors inhibits sympathetic outflow to brown adipose tissue in rats. J Physiol. 2005;566:559–573. doi: 10.1113/jphysiol.2005.086322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeshima T, Ito R, Hamada S, Senzaki K, Hamaguchi-Hamada K, Shutoh F, Okado N. The cellular localization of 5-HT2A receptors in the spinal cord and spinal ganglia of the adult rat. Brain Res. 1998;797:118–124. doi: 10.1016/s0006-8993(98)00360-6. [DOI] [PubMed] [Google Scholar]
- Marina N, Taheri M, Gilbey MP. Generation of a physiological sympathetic motor rhythm in the rat following spinal application of 5-HT. J Physiol. 2006;571:441–450. doi: 10.1113/jphysiol.2005.100677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlier L, Teilhac JR, Cerruti C, Privat A. Autoradiographic mapping of 5-HT1, 5-HT1A, 5-HT1B and 5-HT2 receptors in the rat spinal cord. Brain Res. 1991;550:15–23. doi: 10.1016/0006-8993(91)90400-p. [DOI] [PubMed] [Google Scholar]
- Martin-Cora FJ, Fornal CA, Metzler CW, Jacobs BL. Single-unit responses of serotonergic medullary and pontine raphe neurons to environmental cooling in freely moving cats. Neuroscience. 2000;98:301–309. doi: 10.1016/s0306-4522(00)00133-0. [DOI] [PubMed] [Google Scholar]
- McCall RB, Aghajanian GK. Serotonergic facilitation of facial motoneuron excitation. Brain Res. 1979;169:11–27. doi: 10.1016/0006-8993(79)90370-6. [DOI] [PubMed] [Google Scholar]
- Morrison SF. Central pathways controlling brown adipose tissue thermogenesis. News Physiol Sci. 2004;19:67–74. doi: 10.1152/nips.01502.2003. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Sved AF, Passerin AM. GABA-mediated inhibition of raphe pallidus neurons regulates sympathetic outflow to brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 1999;276:R290–R297. doi: 10.1152/ajpregu.1999.276.2.R290. [DOI] [PubMed] [Google Scholar]
- Myslinski NR, Anderson EG. The effect of serotonin precursors on alpha- and gamma-motoneuron activity. J Pharmacol Exp Ther. 1978;204:19–26. [PubMed] [Google Scholar]
- Nakamura K, Matsumura K, Hubschle T, Nakamura Y, Hioki H, Fujiyama F, Boldogkoi Z, Konig M, Thiel HJ, Gerstberger R, Kobayashi S, Kaneko T. Identification of sympathetic premotor neurons in medullary raphe regions mediating fever and other thermoregulatory functions. J Neurosci. 2004;24:5370–5380. doi: 10.1523/JNEUROSCI.1219-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberkofler H, Dallinger G, Liu YM, Hell E, Krempler F, Patsch W. Uncoupling protein gene: quantification of expression levels in adipose tissues of obese and non-obese humans. J Lipid Res. 1997;38:2125–2133. [PubMed] [Google Scholar]
- Ootsuka Y, Blessing WW. Activation of slowly conducting medullary raphe-spinal neurons, including serotonergic neurons, increases cutaneous sympathetic vasomotor discharge in rabbit. Am J Physiol Regul Integr Comp Physiol. 2005;288:R909–R918. doi: 10.1152/ajpregu.00564.2004. [DOI] [PubMed] [Google Scholar]
- Ootsuka Y, Blessing WW. Thermogenesis in brown adipose tissue: Increase by 5-HT2A receptor activation and decrease by 5-HT1A receptor activation in conscious rats. Neurosci Lett. 2006;395:170–174. doi: 10.1016/j.neulet.2005.10.062. [DOI] [PubMed] [Google Scholar]
- Passerin AM, Bellush LL, Henley WN. Activation of bulbospinal serotonergic neurons during cold exposure. Can J Physiol Pharmacol. 1999;77:250–258. [PubMed] [Google Scholar]
- Passerin AM, Henley WN. Activation of spinal cord serotonergic neurons accompanies cold-induced sympathoexcitation. Can J Physiol Pharmacol. 1994;72:884–892. doi: 10.1139/y94-125. [DOI] [PubMed] [Google Scholar]
- Pickering AE, Spanswick D, Logan SD. 5-Hydoxytryptamine evokes depolarizations and membrane potential oscillations in rat sympathetic preganglionic neurones. J Physiol. 1994;480:109–121. doi: 10.1113/jphysiol.1994.sp020345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathner JA, Morrison SF. Rostral ventromedial periaqueductal gray: a source of inhibition of the sympathetic outflow to brown adipose tissue. Brain Res. 2006;1077:99–107. doi: 10.1016/j.brainres.2006.01.035. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Rosin DL, Simmons JR, McQuiston TJ, Vujovic N, Weston MC, Guyenet PG. Coexpression of vesicular glutamate transporter-3 and γ-aminobutyric acidergic markers in rat rostral medullary raphe and intermediolateral cell column. J Comp Neurol. 2005;492:477–494. doi: 10.1002/cne.20742. [DOI] [PubMed] [Google Scholar]
- Sundaram K, Murugaian J, Sapru H. Cardiac responses to the microinjections of excitatory amino acids into the intermediolateral cell column of the rat spinal cord. Brain Res. 1989;482:12–22. doi: 10.1016/0006-8993(89)90537-4. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Owens NC, Nagashima K, Kanosue K, McAllen RM. Reflex activation of rat fusimotor neurons by body surface cooling, and its dependence on the medullary raphe. J Physiol. 2006;572:569–583. doi: 10.1113/jphysiol.2005.102400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor KB, Nickolaus S, Helke CJ. Autoradiographic localization of 5-hydroxytryptamine1A, 5-hydroxytryptamine1B and 5-hydroxytryptamine1C/2 binding sites in the rat spinal cord. Neuroscience. 1993;55:235–252. doi: 10.1016/0306-4522(93)90469-v. [DOI] [PubMed] [Google Scholar]
- White SR, Neuman RS. Facilitation of spinal motoneurone excitability by 5-hydroxytryptamine and noradrenaline. Brain Res. 1980;188:119–127. doi: 10.1016/0006-8993(80)90561-2. [DOI] [PubMed] [Google Scholar]
- Yuen EY, Jiang Q, Chen P, Gu Z, Feng J, Yan Z. Serotonin 5-HT1A receptors regulate NMDA receptor channels through a microtubule-dependent mechanism. J Neurosci. 2005;25:5488–5501. doi: 10.1523/JNEUROSCI.1187-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaretskaia MV, Zaretsky DV, DiMicco JA. Role of the dorsomedial hypothalamus in thermogenesis and tachycardia caused by microinjection of prostaglandin E2 into the preoptic area in anesthetized rats. Neurosci Lett. 2003;340:1–4. doi: 10.1016/s0304-3940(03)00047-8. [DOI] [PubMed] [Google Scholar]
- Zaretskaia MV, Zaretsky DV, Shekhar A, DiMicco JA. Chemical stimulation of the dorsomedial hypothalamus evokes non-shivering thermogenesis in anesthetized rats. Brain Res. 2002;928:113–125. doi: 10.1016/s0006-8993(01)03369-8. [DOI] [PubMed] [Google Scholar]