Abstract
Thyrotropin-releasing hormone (TRH) is a tripeptide that is widely distributed in the brain including the hippocampus where TRH receptors are also expressed. TRH has anti-epileptic effects and regulates arousal, sleep, cognition, locomotion and mood. However, the cellular mechanisms underlying such effects remain to be determined. We examined the effects of TRH on GABAergic transmission in the hippocampus and found that TRH increased the frequency of GABAA receptor-mediated spontaneous IPSCs in each region of the hippocampus but had no effects on miniature IPSCs or evoked IPSCs. TRH increased the action potential firing frequency recorded from GABAergic interneurons in CA1 stratum radiatum and induced membrane depolarization suggesting that TRH increases the excitability of interneurons to facilitate GABA release. TRH-induced inward current had a reversal potential close to the K+ reversal potential suggesting that TRH inhibits resting K+ channels. The involved K+ channels were sensitive to Ba2+ but resistant to other classical K+ channel blockers, suggesting that TRH inhibits the two-pore domain K+ channels. Because the effects of TRH were mediated via Gαq/11, but were independent of its known downstream effectors, a direct coupling may exist between Gαq/11 and K+ channels. Inhibition of the function of dynamin slowed the desensitization of TRH responses. TRH inhibited seizure activity induced by Mg2+ deprivation, but not that generated by picrotoxin, suggesting that TRH-mediated increase in GABA release contributes to its anti-epileptic effects. Our results demonstrate a novel mechanism to explain some of the hippocampal actions of TRH.
Thyrotropin-releasing hormone (TRH) was originally isolated from the hypothalamus and shown to act upon the anterior pituitary to evoke the release of thyrotropin-stimulating hormone; however, this tripeptide also functions independently as a paracrine regulatory factor and a neuromodulator (Nillni & Sevarino, 1999). TRH is extensively distributed in the brain (Morley, 1979) where it interacts with two types of G-protein-coupled receptors (TRH-R1 and TRH-R2) (Mantyh & Hunt, 1985; Sun et al. 2003). The amino acid sequences of these two types of receptors are approximately 50% identical (Itadani et al. 1998) and they activate similar signalling pathways (Itadani et al. 1998; O'Dowd et al. 2000). The primary G-proteins coupled to TRH receptors are Gq/11 (Hsieh & Martin, 1992). Activation of TRH receptors results in the activation of phospholipase C (PLC) leading to an increase in intracellular Ca2+ release and the activation of protein kinase C (PKC) (Hsieh & Martin, 1992). TRH receptor activation also stimulates the Ca2+–calmodulin-dependent kinase II (CAMKII) (Cui et al. 1994) and the mitogen-activated protein kinase (MAPK) (Ohmichi et al. 1994). The roles of these second messengers in TRH-mediated physiological functions remain to be determined.
The hippocampus contains high concentration of TRH (Low et al. 1989) and expresses TRH receptors (Mantyh & Hunt, 1985; Manaker et al. 1985; O'Dowd et al. 2000; Heuer et al. 2000; Sun et al. 2003). The TRH receptors expressed in the hippocampus are TRH-R1 (O'Dowd et al. 2000; Heuer et al. 2000; Sun et al. 2003) whereas TRH-R2 is abundant only in the precommissural hippocampus (Heuer et al. 2000). The cells that express TRH-R1 in the hippocampus are scattered in the stratum radiatum of CA1 and CA3 region (Heuer et al. 2000) suggesting that they are GABAergic interneurons. The selective localization of TRH-R1 to GABAergic interneurons suggests that TRH regulates GABAergic function in the hippocampus. Indeed, TRH regulates a variety of physiological functions, including arousal, sleep, cognition, locomotion and mood (Nillni & Sevarino, 1999), which overlap with those of the hippocampus and other limbic structures. In addition, TRH has long been known to have anti-epileptic effects in animal models of seizure (Nillni & Sevarino, 1999). Clinically, TRH treatment has been reported to be efficacious in such intractable epilepsies as infantile spasms, Lennox–Gastaut syndrome, myoclonic seizures and other generalized and refractory partial seizures (Kubek & Garg, 2002). However, the mechanisms underlying TRH-mediated modulation of its physiological function and its anti-epileptic effect have not been determined. In the present study, we examined the effects of TRH on GABAergic transmission in the hippocampus and our results demonstrate that TRH increased GABA release in each region of the hippocampus. We focused on the CA1 region and found that TRH inhibited a resting membrane K+ conductance belonging to the family of two-pore domain K+ channels (K2P) in GABAergic interneurons. TRH-mediated inhibition of the resting K+ conductance increased the excitability of the interneurons and facilitated GABA release. Both with pharmacological approaches and the use of knock-out mice, we also found that the effects of TRH required the function of Gαq/11, but were independent of PLC, CAMKII or MAPK activity suggesting a direct coupling of Gαq/11 to K+ channels. TRH inhibited the seizure activity induced by deprivation of Mg2+ in the extracellular solution in hippocampal slices suggesting that TRH-induced increase in GABA release contributes to its anti-epileptic activity. Our results provide a novel cellular mechanism to explain the functions of TRH in the brain.
Methods
Hippocampal slice preparation
Horizontal hippocampal slices (400 μm) were cut using a Vibratome (Leica VT1000S) usually from 15- to 22-day-old Sprague Dawley rats as previously described (Lei & McBain, 2003; Deng & Lei, 2006). Rats were deeply anaesthetized with isoflurane, and the brain was dissected out in ice-cold saline solution that contained (mm): NaCl 130, NaHCO3 24, KCl 3.5, NaH2PO4 1.25, CaCl2 0.5, MgCl2 5.0 and glucose 10, saturated with 95% O2–5% CO2; pH 7.4. Slices were initially incubated in the above solution at 35°C for 40 min for recovery and then kept at room temperature (∼24°C) until use. All animal procedures conformed to the guidelines approved by the University of North Dakota Animal Care and Use Committee.
Recordings of spontaneous, miniature and evoked GABAA receptor-mediated IPSCs
Whole-cell patch-clamp recordings using an Axopatch 200B or a Multiclamp 700B (Axon Instruments, Union City, CA, USA) in voltage-clamp mode were made usually from CA1 pyramidal neurons visually identified with infrared video microscopy (Olympus BX51WI) and differential interference contrast optics unless stated otherwise. The recording electrodes were filled with the following solution (mm): caesium gluconate 100, EGTA 0.6, MgCl2 5, NaCl 8, ATP2Na 2, GTPNa 0.3, Hepes 40 and QX-314 1; pH 7.3. The extracellular solution contained (mm): NaCl 130, NaHCO3 24, KCl 3.5, NaH2PO4 1.25, MgCl2 1.5, CaCl2 2.5 and glucose 10, saturated with 95% O2–5% CO2; pH 7.4. To record GABAA receptor-mediated spontaneous IPSCs (sIPSCs), the external solution was supplemented with dL-2-amino-5-phosphonovaleric acid (dl-APV) (100 μm) and 6,7-dinitroquinoxaline-2,3(1H, 4H)-dione (DNQX) (10 μm) to block NMDA and AMPA receptor-mediated responses, respectively. Under these conditions, the recorded inhibitory currents had a reversal potential of ∼−30 mV and were completely blocked by bicuculline methobromide (10 μm), confirming that they were mediated by GABAA receptors. Usually sIPSCs were recorded at a holding potential of +30 mV (Lei & McBain, 2003). Miniature IPSCs (mIPSCs) were recorded by including TTX (0.5 μm) in the above external solution to block action potential-dependent responses. Evoked IPSCs were recorded from CA1 pyramidal neurons using the same internal and external solution by placing a stimulation electrode in the stratum radiatum of CA1 region. Data were filtered at 2 kHz, digitized at 10 kHz and acquired on-line using pCLAMP 9 (Clampex) software (Axon Instruments). The recorded sIPSCs and mIPSCs were subsequently analysed by Mini Analysis 6.0.1 (Synaptosoft Inc., Decatur, GA, USA). Each detected event was inspected visually to exclude obvious artifacts before analysis. Mean amplitude, frequency, cumulative amplitude and frequency histograms were calculated by this program. The recorded evoked IPSCs were analysed by pCLAMP 9 (Clampfit). TRH was applied via the bath. To avoid desensitization induced by repeated applications of TRH, one slice was limited to only one application of TRH.
Recordings of action potentials
Action potential firing was recorded from the interneurons in the stratum radiatum of CA1 region with whole-cell patch-clamp recordings in current-clamp mode. Caesium gluconate in the above intracellular solution was replaced by the same concentration of potassium gluconate and QX-314 was omitted. Because dialysis of K+-containing internal solution into cells can change the resting membrane potential and influence action potential firing, we waited for ∼15 min after the formation of whole-cell recordings to allow the resting membrane potential to stabilize. Data were obtained only from those cells displaying resting membrane potential negative to −60 mV. Usually, for most of the cells a positive current injection was needed to elevate the resting membrane potential to ∼−50 mV to induce action potential firing. TRH was applied after the action potential firing had been stable for 5–10 min. The frequency of the action potentials was calculated by Mini Analysis 6.0.1.
Recording of holding current from GABAergic interneurons
Holding current at −55 mV was usually recorded from the interneurons in the stratum radiatum of CA1 region unless stated otherwise. The above K+-containing intracellular solution was used and the extracellular solution contained TTX (0.5 μm) to block action potential firing. Because gradual dialysis of K+ into cells changes the holding current, we began our recordings after waiting for ∼20 min from the formation of whole-cell configuration. Holding currents at −55 mV were recorded every 3 s and then averaged per minute. We subtracted the average of the holding currents recorded for the last minute before the application of TRH from those recorded at different time points to zero the basal level of the holding currents for better comparison. For the experiment involving N-methyl-d-glucamine (NMDG), the extracellular NaCl concentration was replaced by the same concentration of NMDG, and HCl was used to adjust pH to 7.4.
Construction of voltage–current curves
Voltage–current curves were constructed from the interneurons in the stratum radiatum of CA1 region. Potassium gluconate-containing internal solution was used and the extracellular solution was supplemented with (μm): TTX 0.5, CdCl2 100, DNQX 10, dl-APV 50 and bicuculline 10. Voltage–current relationship was obtained by using a ramp protocol from −140 to −40 mV at a speed of 0.07 mV ms−1. Because the maximal effect of TRH usually occurred at ∼3 − 4 min, we compared the voltage–current curves recorded before and during the application of TRH for 3–4 min.
Recordings of the spontaneous seizure activity
Spontaneous seizure activity was induced from in vitro hippocampal slices either by using the extracellular solution containing 0Mg2+ or by applying GABAA receptor blocker picrotoxin (100 μm) in the normal extracellular solution containing 1.5 mm Mg2+. K+ concentration in the extracellular solution was raised to 5 mm for both seizure models. An extracellular recording pipette containing 2 m NaCl was placed in CA3 pyramidal layer to record seizure activity. After spontaneous seizure activity stabilized, TRH (0.5 μm) was applied in the bath. The seizure events were initially recorded by Clampex and subsequently analysed by Mini Analysis 6.0.1.
Breeding and genotyping of mutant mice
Heterozygous mating pairs (F1 hybrid crosses from 129 PLC-β1+/− × C57BL/6J PLC-β1+/−) were obtained from Korea Institute of Science and Technology. The breeders were used to derive wild-type, heterozygous and homozygous pups for experimental analysis. PCR genotyping from purified genomic DNA was performed as previously described (Kim et al. 1997).
Data analysis
Data are presented as the means ± s.e.m. The concentration–response curve of TRH was fitted by the Hill equation:
where Imax is the maximum response, EC50 is the concentration of ligand producing a half-maximal response, and n is the Hill coefficient. Student's paired or unpaired t test or analysis of variance (ANOVA) was used for statistical analysis as appropriate; P values are reported throughout the text and significance was set as P < 0.05. For sIPSC cumulative probability plots, events recorded for 2 min before TRH application and 2 min after the maximal effect of TRH (usually the third and fourth minute from the beginning of TRH application) were selected. Same bin size (100 ms for frequency and 2 pA for amplitude) was used to analyse data from control and TRH treatment. Because the frequency of mIPSCs was lower, the time length was increased to 5 min and the bin size was 300 ms for frequency and 1 pA for amplitude. Kolmogorov-Smirnoff test was used to assess the significance of the cumulative probability plots. n number in the text represents the number of cells examined.
Chemicals
SKF 96365, ruthenium red, anandamide, bupivacaine, GDP-β-S, GTP-γ-S, U73122, calphostin C, KN-62, PD 98059, picrotoxin and staurosporine were purchased from Tocris (Ellisville, MO, USA). The dynamin inhibitory peptide (QVPSRPNRAP) and the scrambled peptide (QPPASNPRVR) were synthesized by American Peptide Company (Sunnyvale, CA, USA). TRH and chlordiazepoxide were purchased from Sigma-Aldrich (St Louis, MO, USA). Antibodies to Gαq/11 or Gβ were products of Biomol (Plymouth Meeting, PA, USA). Other chemicals were products of Sigma-Aldrich or Biomol.
Results
TRH increases sIPSC frequency in the hippocampus
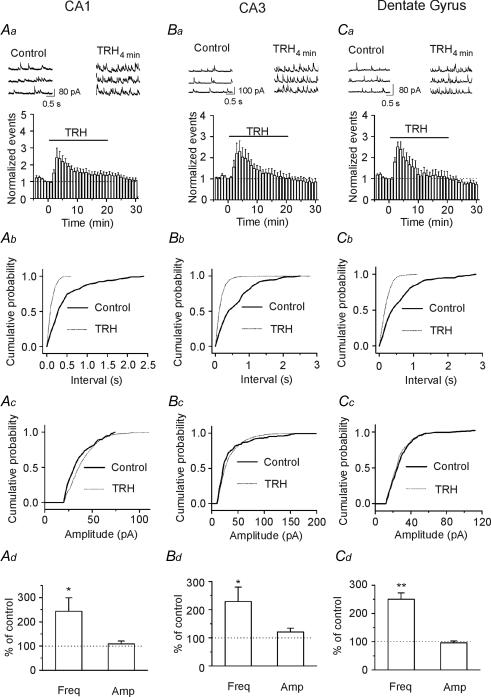
Because TRH-R1 is selectively expressed on the putative hippocampal GABAergic interneurons that form inhibitory synapses onto the pyramidal neurons in the hippocampus (Heuer et al. 2000), we initially examined the effects of TRH on GABAA receptor-mediated spontaneous IPSCs (sIPSCs) recorded in the presence of DNQX (10 μm) and dl-APV (100 μm) to block AMPA and NMDA responses, respectively. Cells were held at +30 mV and in this condition the recorded currents were completely blocked by 10 μm bicuculline (Lei & McBain, 2003) indicating that they are mediated by GABAA receptors. Application of TRH (1 μm) for ∼3–4 min significantly increased the frequency of sIPSCs recorded from CA1 pyramidal neurons to 243 ± 56% of control (n = 11, P = 0.03, Fig. 1Aa, Ab and Ad). Whereas the effects of TRH were observed in each cell examined, the extent to which TRH increased sIPSC frequency varied from 120% to 730% of control. In the continuous presence of TRH, sIPSC frequency was gradually reduced (Fig. 1Aa). This phenomenon occurred possibly because of the desensitization of TRH receptors (Pfleger et al. 2004). There were inconsistent results as to whether TRH modulates sIPSC amplitude varying from increase, no apparent change to slight reduction. The summarized data from 11 cells showed that there was no significant change in sIPSC amplitude (109 ± 12% of control, n = 11, P = 0.48, Fig. 1Ac and Ad).
Figure 1. TRH increases sIPSC frequency in the hippocampus.
A, TRH increased sIPSC frequency in CA1 region. Aa, upper trace, sIPSCs recorded from a CA1 pyramidal neuron before and during the application of TRH (1 μm) for 3–4 min. lower trace, time course of the sIPSC frequency averaged from 11 cells. Note that TRH increased sIPSC frequency. Ab, cumulative frequency distribution from a CA1 pyramidal neuron before and during the application of TRH for 3–4 min. Note that TRH reduced the intervals of the sIPSC (increased sIPSC frequency, P < 0.00001, Kolmogorov-Smirnov test). Ac, cumulative amplitude distribution from the same cell before and during the application of TRH for 3–4 min (P= 0.21, Kolmogorov-Smirnov test). Ad, summarized data from 11 CA1 pyramidal neurons. Note that TRH significantly increased sIPSC frequency without changing sIPSC amplitude. B, TRH increased sIPSC frequency in CA3 pyramidal neurons.. Note that TRH increased sIPSC frequency without altering sIPSC amplitude (n= 11). C, TRH increased sIPSC frequency in dentate gyrus granule cells without significantly changing sIPSC amplitude (n= 11). In B and C, the data were obtained in the same way as in A.
Application of TRH (1 μm) increased the frequency of sIPSCs recorded from CA3 pyramidal neurons to 229 ± 51% of control (n = 11, P = 0.03, Fig. 1Ba, Bb and Bd) without significantly changing sIPSC amplitude (121 ± 13% of control, n = 11, P = 0.15, Fig. 1Bc and Bd). Similarly, application of TRH (1 μm) significantly increased the frequency of sIPSCs recorded from dentate gyrus granule cells to 250 ± 23% of control (n = 11, P < 0.0001, Fig. 1Ca, Cb and Cd) without significantly changing sIPSC amplitude (96 ± 7% of control, n = 11, P = 0.002, Fig. 1Cc and Cd). These results demonstrate that TRH increases GABAergic transmission to a similar extent in all three regions of the hippocampus.
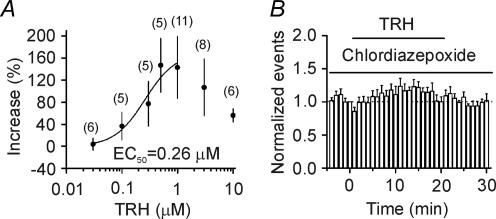
We next used CA1 pyramidal neurons as an example to further determine the underlying mechanisms. TRH dose-dependently increased the frequency of sIPSCs recorded from CA1 pyramidal neurons with an EC50 equal to 0.26 μm (Fig. 2A). The responses were smaller when TRH concentrations were higher than 1 μm (Fig. 2A). This was probably due to the agonist-induced desensitization of TRH receptors (Pfleger et al. 2004). TRH-induced desensitization was related to the applied concentration of the agonist, but it was independent of the application time of the agonists because application of TRH for shorter time (6 min) still increased sIPSC frequency to 355 ± 93% of control (n = 12, p = 0.02) in the first 3 min after the beginning of TRH application, followed by a gradual return to control level (92 ± 16% of control, n = 12, p = 0.6, data not shown). The optimal concentration of TRH was found to be 0.5–1 μm (Fig. 2A). To determine whether the effects of TRH on sIPSC frequency were mediated via the activation of TRH receptors, we attempted to use TRH receptor antagonists. However, the only reliable TRH receptor antagonist currently available is chlordiazepoxide (Simasko & Horita, 1984; Drummond, 1985). Because this compound can interact with the benzodiazepine binding site of GABAA receptors, we initially examined its effects on sIPSCs. Application of chlordiazepoxide (50 μm) slightly increased the amplitude of sIPSCs to 117 ± 5% of control (n = 8, p = 0.012) with no effects on the frequency of sIPSCs (97 ± 4% of control, n = 8, p = 0.63). Because TRH more than doubled sIPSC frequency (Fig. 1), we reasoned that a chlordiazepoxide-induced slight increase in sIPSC amplitude would not significantly affect our results if we pretreated the slices with this compound to elevate the basal activity of the GABAA receptors first. We pretreated the slices with chlordiazepoxide (50 μm) and the extracellular solution contained the same concentration of chlordiazepoxide. Under these conditions, application of TRH (1 μm) failed to significantly increase sIPSC frequency (124 ± 12% of control, n = 8, p = 0.09, Fig. 2B) suggesting that TRH increases sIPSC frequency via the activation of TRH receptors. The involvement of TRH receptors was further confirmed by the result that chlordiazepoxide also blocked TRH-induced inward current recorded from interneurons (see Fig. 4D).
Figure 2. TRH increases sIPSC frequency via the activation of TRH receptors.
A, concentration–response curve of TRH constructed from CA1 pyramidal neurons. Data between 0.03 and 1 μm were fitted by the Hill equation. Numbers in parentheses are the numbers of cells used. EC50 was 0.26 μm. B, TRH-induced increases in sIPSC frequency were blocked by the TRH receptor inhibitor, chlordiazepoxide (50 μm). Slices were pretreated with chlordiazepoxide and the extracellular solution contained the same concentration of chlordiazepoxide (n = 8).
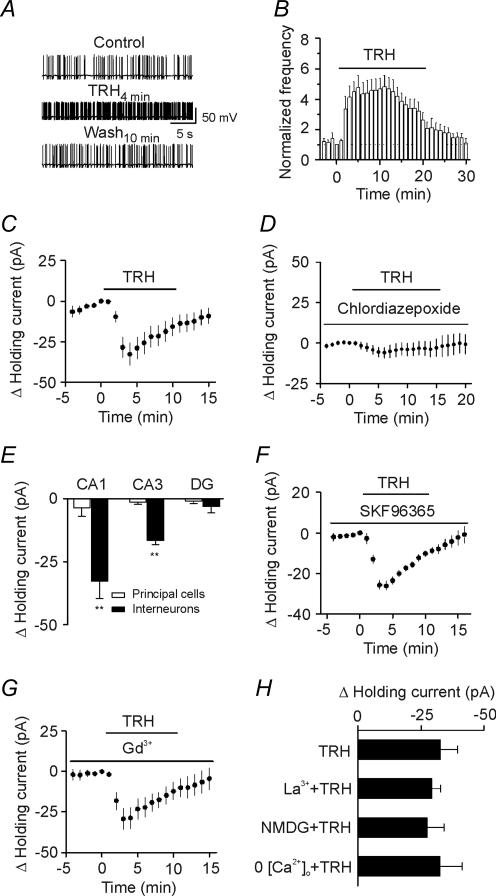
Figure 4. TRH increases the excitability of GABAergic interneurons in CA1 stratum radiatum.
A, spontaneous action potentials recorded from an interneuron before, during and after the application of TRH (0.5 μm). B, time course of the action potential firing frequency averaged from 10 interneurons. Note that TRH increased the action potential firing frequency. C, application of TRH (0.5 μm) produced an inward current at −55 mV (n = 9, p = 0.002). Holding currents at −55 mV were initially recorded every 3 s in the presence of TTX (0.5 μm) and then averaged per minute. The averaged holding current for the last minute before the application of TRH was subtracted to calculate the change in holding current. D, before and during application of the TRH receptor inhibitor, chlordiazepoxide (50 μm), antagonized TRH-induced change in holding currents suggesting the involvement of TRH receptors (n = 7). E, TRH increased inward current at −55 mV in CA1 and CA3 stratum radiatum interneurons with no effects on interneurons in the hilus or any of the principal cells in the hippocampus. DG, dentate gyrus. F, before and during application of SKF96365 (100 μm), a receptor-operated cation channel inhibitor, failed to block TRH-induced change in holding currents (n = 6). G, before and during application of a non-selective cation channel blocker, Gd3+ (10 μm) did not block the effects of TRH (n = 6). H, TRH-induced depolarization was not affected by application of La3+ (10 μm, n = 10), replacement of the extracellular Na+ by NMDG (n = 6) or removal of extracellular Ca2+ (n = 5).
TRH does not change mIPSCs or evoked IPSCs
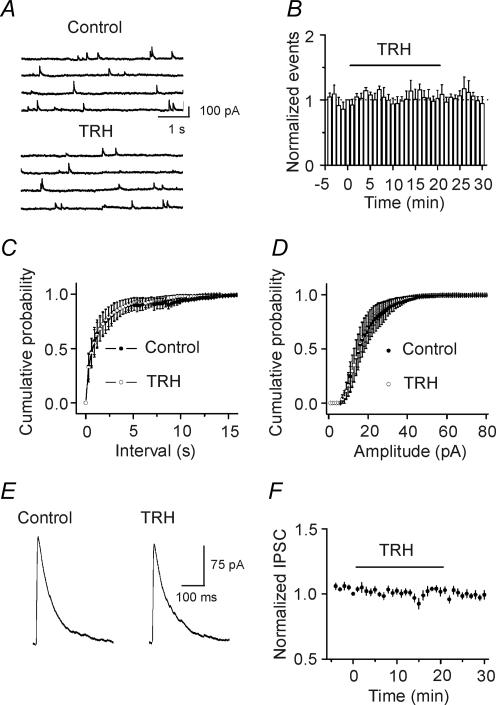
Because sIPSCs recorded in the absence of TTX are action potential- and Ca2+-dependent, we then examined the effects of TRH on miniature IPSCs (mIPSCs) recorded from CA1 pyramidal neurons in the presence of TTX. mIPSCs recorded in the presence of TTX are thought to be action potential- and Ca2+-independent. Application of TRH (0.5 μm) did not change mIPSC frequency (control, 1.7 ± 0.8 Hz, TRH, 2.1 ± 1.2 Hz; n = 5, p = 0.44, Fig. 3A–C) or amplitude (control, 32 ± 7 pA, TRH: 30 ± 5 pA, n = 5, p = 0.27, Fig. 3A and D) suggesting that TRH has no effects on either postsynaptic GABAA receptors or exocytosis downstream of Ca2+ influx. This result also suggests that the effects of TRH are action potential-dependent. We also examined the effects of TRH on GABAA receptor-mediated IPSC recorded from CA1 pyramidal neurons evoked by placing a stimulation electrode in the stratum radiatum. Application of TRH (0.5 μm) did not significantly change the amplitude of evoked IPSCs (101 ± 3% of control, n = 12, p = 0.64, Fig. 3E and F). Because voltage-gated Ca2+ channels have a role in evoked IPSCs, the result that TRH did not modulate the evoked IPSCs suggests that TRH has no direct effects on presynaptic Ca2+ channels. Because action potentials underlying the evoked IPSCs are generated by exogenous stimulation-induced depolarization whereas those responsible for spontaneous IPSCs are determined by the intrinsic excitability of neurons, the results that TRH modulates sIPSC frequency without altering the evoked IPSC amplitude suggest that TRH alters the excitability of GABAergic interneurons to facilitate the generation of action potentials and GABA release.
Figure 3. TRH does not modulate mIPSCs recorded in the presence of TTX and the evoked IPSC amplitude recorded by placing a stimulation electrode in CA1 stratum radiatum.
A, mIPSC current traces recorded from a CA1 pyramidal neuron before and during the application of TRH (0.5 μm). B, time course of mIPSC frequency summarized from five CA1 pyramidal neurons. C, cumulative frequency distribution of mIPSCs before and during the application of TRH (n = 5, p = 0.44, Kolmogorov-Smirnov test). D, cumulative amplitude distribution of mIPSCs before and during the application of TRH (n = 5, p = 0.27, Kolmogorov-Smirnov test). Note that TRH did not change the frequency or the amplitude of mIPSCs. E, evoked IPSC trace averaged from 10 IPSCs before and during the application of TRH. F, summarized time course of the evoked IPSC amplitude from 12 CA1 pyramidal neurons before, during and after the application of TRH (0.5 μm). Note that TRH did not change the amplitude of the evoked IPSCs.
TRH increases the excitability of GABAergic interneurons
We then tested the hypothesis that TRH-mediated increase in sIPSC frequency is due to TRH-induced facilitation of action potential generation in GABAergic interneurons resulting in an increase in GABA release. We recorded action potential firing from the interneurons in the stratum radiatum of CA1 region by using K+-containing internal solution. Caesium gluconate in the internal solution was replaced by the same concentration of potassium gluconate and QX-314 was omitted. Under these conditions, the resting membrane potential of these neurons was −64.3 ± 0.9 mV (n = 10). Application of TRH (0.5 μm) significantly increased the action potential firing frequency to 475 ± 83% of control (n = 10, p = 0.001, Fig. 4A and B) suggesting that TRH increases GABA release by enhancing the excitability of GABAergic interneurons.
Increases in action potential firing could occur if TRH induces membrane depolarization. We next tested this possibility by recording from the interneurons in the stratum radiatum of CA1 region with the holding current at −55 mV, a potential close to the resting membrane potential. The intracellular solution contained potassium gluconate and the extracellular solution contained TTX (0.5 μm) to block action potentials. Application of TRH (0.5 μm) generated an inward holding current (−32.6 ± 6.9 pA, n = 9, p = 0.002, Fig. 4C) with rapid desensitization, suggesting that TRH increases action potential firing rate and GABA release by facilitating membrane depolarization of the interneurons in CA1 region. TRH-induced membrane depolarization was mediated by the activation of TRH receptors because pretreatment with and continuous application of chlordiazepoxide (50 μm), the TRH receptor blocker, significantly inhibited TRH-induced inward current (control, −32.6 ± 6.9 pA, n = 9; chlordiazepoxide, −6.0 ± 3.2 pA, n = 7, p = 0.007, Student's unpaired t test, Fig. 4D). TRH did not change the holding current recorded at −55 mV from CA1 pyramidal neurons (holding current change, −3.6 ± 3.4 pA, n = 7, p = 0.33, Fig. 4E), suggesting that TRH-induced depolarization is limited to the interneurons in CA1 region.
We then examined whether TRH depolarizes interneurons in other regions of the hippocampus. We recorded the holding current at −55 mV from interneurons in the stratum radiatum of CA3 and the hilus of the dentate gyrus. TRH generated an inward holding current in the interneurons of CA3 radiatum (−16.5 ± 1.8 pA, n = 8, P < 0.0001, Fig. 4E) with no significant effects on CA3 pyramidal neurons (−1.5 ± 0.9 pA, n = 6, p = 0.17, Fig. 4E). TRH failed to induce obvious depolarization in either dentate granule cells (−1.1 ± 0.9 pA, n = 6, p = 0.3, Fig. 4E) or hilar interneurons (−3.2 ± 2.5 pA, n = 7, p = 0.25, Fig. 4E). The reason for the preferential effect of TRH on interneurons compared to pyramidal neurons may reflect the selective expression of TRH-R1 on interneurons but not on pyramidal cells (Heuer et al. 2000). Together, these results suggest that TRH increases GABA release by generating an inward current in the interneurons of CA1 and CA3 regions, but not in the dentate gyrus region. This result is consistent with the previous observations that cholecystokinin, another neuropeptide, induces an inward current in CA1 interneurons (Miller et al. 1997), but not in hilar interneurons (Deng & Lei, 2006). In the present study, we focused on CA1 interneurons to determine the underlying mechanisms.
TRH-induced membrane depolarization could be produced by the opening of a cation channel or inhibition of a K+ channel that is available at the resting membrane potential. The following lines of evidence indicate that TRH-induced opening of cation channels is unlikely to be involved. First, we used SKF96365, an inhibitor of receptor-operated cation channels. In the presence of SKF96365 (100 μm), application of TRH (0.5 μm) still produced an inward current (control, −32.6 ± 6.9 pA, n = 9; SKF96365, −25.7 ± 2.2 pA, n = 6, p = 0.44, Student's unpaired t test, Fig. 4F). Second, we applied two non-specific cation channel inhibitors (Gd3+ and La3+). Neither Gd3+ (10 μm) nor La3+ (10 μm) blocked TRH-induced inward currents (Gd3+, −29.4 ± 6.5 pA, n = 6, p = 0.75, Fig. 4G; La3+, −29.2 ± 3.6 pA, n = 10, p = 0.65, Fig. 4H) compared with the change in holding current in control condition. Third, if TRH opens a cation conductance, the influx of extracellular Na+ should be the cause of the inward current. We therefore replaced the NaCl in the extracellular solution with the same concentration of NMDG-Cl. Under these conditions, application of TRH (0.5 μm) still induced an inward holding current (−27.3 ± 6.8 pA, n = 6, p = 0.01, Fig. 4H). Finally, removal of Ca2+ from the extracellular solution did not prevent TRH-induced membrane depolarization (−32.5 ± 8.7 pA, n = 5, p = 0.02, Fig. 4H). Therefore, we concluded that TRH-induced increase in membrane depolarization is unlikely to be due to the opening of a cation conductance.
TRH inhibits a resting membrane K+ conductance
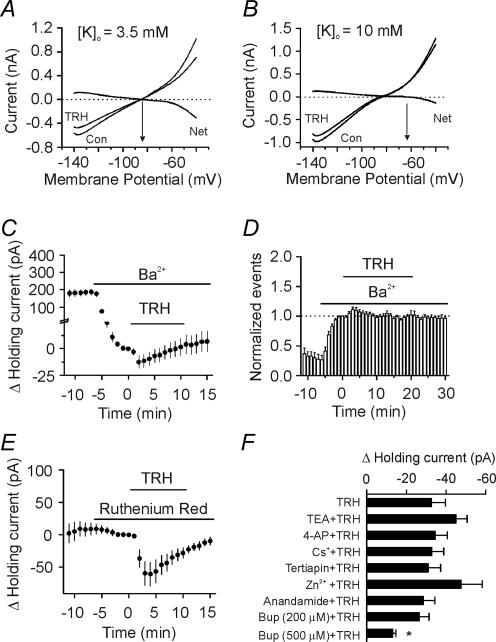
We next examined the roles of K+ channels in TRH-induced membrane depolarization. If TRH induces membrane depolarization by inhibiting a resting K+ conductance, the TRH-induced currents should have a reversal potential close to the K+ reversal potential. We used a ramp protocol (from −140 to −40 mV, at a speed of 0.07 mV ms−1) to construct the voltage–current curve before and during the application of TRH. The intracellular solution contained 100 mm potassium gluconate. Under these conditions, TRH induced a current that had a reversal potential of −84.1 ± 1.3 mV (n = 8), which was close to the calculated K+ reversal potential (−85.4 mV) when the extracellular K+ concentration was 3.5 mm (Fig. 5A). When the extracellular K+ concentration was elevated to 10 mm, the reversal potential of the TRH-induced current changed to–64.3 ± 2.7 mV (n = 9, Fig. 5B) that is close to the calculated K+ reversal potential (−58.7 mV) under these conditions. These results suggest that TRH induces membrane depolarization by inhibiting K+ channels responsible for controlling the resting membrane potentials.
Figure 5. TRH inhibits K+ channels of the interneurons in CA1 stratum radiatum.
A, voltage–current relationship recorded by a ramp protocol (from−140 to−40 mV, at a speed of 0.07 mV ms−1) before and during the application of TRH (0.5 μm) when the extracellular K+ concentration was 3.5 mm. Traces in the figure were averaged traces from eight cells. The TRH-induced net current has a reversal potential at ∼−84 mV, close to the calculated K+ reversal potential (∼−85 mV). B, voltage–current relationship recorded by the same protocol when the extracellular K+ concentration was 10 mm. Note that the reversal potential of the TRH-sensitive net current was ∼−64 mV, close to the calculated K+ reversal potential (∼−59 mV) suggesting that the net current was mediated by K+ ions. C, application of Ba2+ (2 mm) alone suppressed the holding current at −55 mV and attenuated TRH-induced change in current (n = 14). The averaged current 1 min prior to the application of TRH was subtracted from each time point (same for E). D, bath application of Ba2+ (2 mm) increased sIPSC frequency and significantly inhibited TRH-induced increase in sIPSC frequency (n = 8). E, bath application of ruthenium red (10 μm) failed to alter TRH-induced change in holding currents (n = 7). F, summarized results for the effects of K+ blockers on TRH-induced change in holding currents. Note that the TRH-induced changes in holding currents were insensitive to TEA, 4-aminopyridine, Cs+, tertiapin, Zn2+ and anandamide. Application of bupivacaine (Bup) at 500 μm significantly reduced TRH-induced increase in holding currents.
We next characterized the properties of the K+ channels involved. Application of Ba2+ (2 mm) alone generated an inward current and it also significantly inhibited TRH-induced inward holding current (control, −32.6 ± 6.9 pA, n = 9; Ba2+, −11.5 ± 6.3 pA, n = 14, p = 0.04, Fig. 5C). Consistent with its effect on holding current, application of Ba2+ (2 mm) alone significantly increased sIPSC frequency recorded from CA1 pyramidal neurons to 597 ± 120% of control (n = 8, p = 0.004, Fig. 5D). In the presence of Ba2+, TRH only increased sIPSC frequency to 111 ± 4% of control (n = 8, p = 0.02, Fig. 5D). Together, these results suggest that TRH induces membrane depolarization by inhibiting Ba2+-sensitive K+ channels although there was a Ba2+-insensitive component, consistent with the excitatory effects of TRH on motor neurons (Bayliss et al. 1992; Fisher & Nistri, 1993). We next examined the roles of other K+ channel blockers in TRH-induced membrane depolarization. TRH-induced inward currents at −55 mV were not significantly changed by including tetraethylammonium (TEA, 10 mm, −44.9 ± 5.6 pA, n = 10, p = 0.18), 4-aminopyridine (4-AP, 2 mm, −34.5 ± 6.2 pA, n = 11, p = 0.85) or Cs+ (3 mm, −32.8 ± 6.0 pA, n = 5, p = 0.99) in the extracellular solution (Fig. 5F). TRH inhibits the inward rectifier K+ channels (Lei et al. 2001) that are involved in controlling resting membrane potential and are sensitive to Ba2+; however, the TRH-sensitive K+ channels are unlikely to be the inward rectifier K+ channels because these channels are also sensitive to TEA and Cs+. Furthermore, application of the specific inward rectifier K+ channel inhibitor, tertiapin (50 nm), did not significantly change the holding current (−0.2 ± 0.9 pA, n = 6, p = 0.84) nor did it block TRH-induced change in holding current (−31.0 ± 6.3 pA, n = 6, p = 0.87, Fig. 5F) suggesting that the inward rectifier K+ channels are unlikely to be involved in TRH-induced increases in GABA release.
K2P are insensitive to the classical K+ channel blockers (TEA, 4-AP and Cs+), but some of them are sensitive to Ba2+. K2P channels are involved in the control of resting membrane potential. We therefore next examined the roles of K2P channels in TRH-induced membrane depolarization. Seventeen members of this family have been cloned and they can be grouped according to sequence and functional similarities into six subfamilies: TWIK, THIK, TREK, TASK, TALK and TRESK (Bayliss et al. 2003). Because TRH has been shown to inhibit TASK-1 (Talley et al. 2000) or TASK-3 (Talley & Bayliss, 2002) homodimers or TASK-1/TASK-3 heterodimers (Talley & Bayliss, 2002), we probed the roles of TASK channels by using the reported TASK channel blockers. Initially, we used two putative TASK-3 channel blockers, ruthenium red and Zn2+ (Bayliss et al. 2003). Application of ruthenium red (10 μm) or zinc (100 μm) failed to block TRH-induced inward holding current (ruthenium red, −60.5 ± 18.0 pA, n = 7, p = 0.015, Fig. 5E; zinc, −47.5 ± 10.7 pA, n = 9, p = 0.02, Fig. 5F). We then used anandamide, which is thought to inhibit TASK-1 (Bayliss et al. 2003). Application of anandamide (10 μm) did not block TRH-induced change in the holding current (−28.6 ± 5.8 pA, n = 7, p = 0.003, Fig. 5F). These results are not surprising because in native neurons TASK-1 and TASK-3 exist as TASK-1/TASK-3 heterodimers (Berg et al. 2004) which exhibit distinct sensitivities to these blockers (Bayliss et al. 2003).
Bupivacaine is a local anaesthetic known to block TASK channels (Bayliss et al. 2003). Application of bupivacaine at 200 μm did not block TRH-induced inward current (bupivacaine, −26.6 ± 4.8 pA, n = 10, control, −32.6 ± 6.9 pA, n = 9, p = 0.48, Student's unpaired t test, Fig. 5F). Because the IC50 value of bupivacaine is ∼68 μm (Leonoudakis et al. 1998), we used a higher concentration (500 μm) to ensure a complete inhibition of TASK channels. At this concentration, bupivacaine significantly inhibited TRH-generated current (bupivacaine, −13.2 ± 1.6 pA, n = 8, control, −32.6 ± 6.9 pA, n = 9, p = 0.02, Student's unpaired t test, Fig. 5F). These results suggest that TASK channels at least partially contribute to the depolarization generated by TRH. We did not further examine the involvement of other families of K2P channels because of the lack of specific inhibitors for them.
Involvement of G-proteins
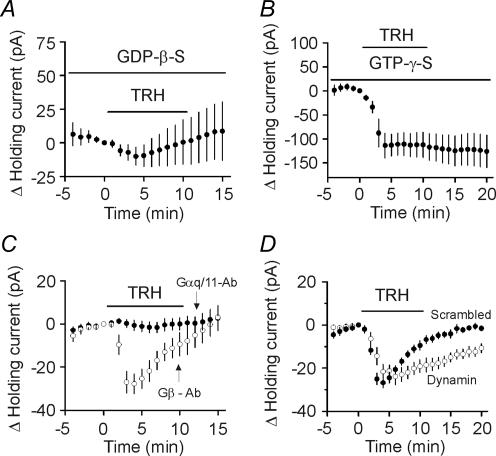
The major G-proteins coupled to TRH receptors are Gαq/11 (Hsieh & Martin, 1992). We next examined the involvement of G-proteins by recording the holding currents at −55 mV from CA1 stratum radiatum interneurons. When GTP in the recording pipettes was replaced by GDP-β-S (4 mm), a G-protein inactivator, TRH did not significantly change the holding currents (−9.6 ± 8.0 pA, n = 12, p = 0.26, Fig. 6A) suggesting that the effects of TRH are mediated by G-proteins. We also used another non-hydrolysable G-protein activator, GTP-γ-S, which is thought to consistently activate G-proteins without being hydrolysed by GTPase. When GTP-γ-S (4 mm) was included in the recording pipettes, TRH induced much larger inward currents (−113.6 ± 25.7 pA, n = 7, p = 0.005, Fig. 6B) and the TRH-induced desensitization of the holding currents disappeared (Fig. 6B). These results are consistent with the effects of TRH in rat motor neurons (Bayliss et al. 1994; Kolaj et al. 1997) suggesting that the effects of TRH on GABA release are mediated by G-proteins.
Figure 6. G-proteins are required for the effects of TRH on holding currents.
A, inclusion of GDP-β-S (4 mm) in the recording pipettes significantly reduced the effects of TRH on holding currents (n = 12). Zero current level was defined as the current flowing prior to TRH application in each panel of this figure. B, TRH produced an irreversible change in current when GTP-γ-S (4 mm) was in the pipette (n = 7). C, intracellular infusion of antibody to Gαq/11 (20 μg ml−1) blocked TRH-induced change in holding currents (n = 11) whereas dialysis of antibody to Gβ (20 μg ml−1) had no effects (n = 8). D, intracellular application of the dynamin inhibitory peptide (QVPSRPNRAP, 50 μm) significantly slowed the desensitization of TRH-induced increase in holding currents (n = 9) compared with that recorded when a scrambled peptide (QPPASNPRVR, 50 μm) was included in the pipettes (n = 7).
We then examined the roles of Gαq/11 in TRH-induced membrane depolarization. We included the Gαq/11 antibody (20 μg ml−1) in the recording pipettes and waited for 20 min after the formation of whole-cell recordings. The Gαq/11 antibody applied via recording pipettes successfully blocked muscarinic stimulation of Ca2+ channel current (Bannister et al. 2004). Application of Gαq/11 antibody blocked TRH-induced increase in holding current (−1.6 ± 2.3 pA, n = 11, p = 0.51, Fig. 6C). However, infusion of Gβ antibody (20 μg ml−1) via the recording pipettes failed to block the effects of TRH (−27.8 ± 4.6 pA, n = 8, p = 0.0005, Fig. 6C) suggesting that the effects of TRH are mediated by Gαq/11.
Dynamin is required for the desensitization of TRH-mediated membrane depolarization
Previous studies demonstrate that TRH receptors undergo endocytosis via clathrin-coated pits (Petrou et al. 1997) and the internalization of TRH receptors is responsible for the desensitization of TRH responses (Yu & Hinkle, 1998). The endocytosis of TRH receptors is controlled by the GTPase, dynamin (Yu & Hinkle, 1998). Amphiphysin, a dynamin GTPase regulator, binds to dynamin via its C-terminal SH3 domain. The interaction between dynamin and amphiphysin is essential for endocytosis (Marks & McMahon, 1998). To test whether dynamin-dependent endocytosis of TRH receptors underlies the desensitization of TRH-induced membrane depolarization, we used a 10 amino acid peptide (QVPSRPNRAP) to selectively interfere with the binding of amphiphysin to dynamin (Marks & McMahon, 1998). We included the dynamin inhibitory peptide (50 μm) in the recording pipettes and waited for ∼20 min after the formation of whole-cell recordings. Under these conditions, application of TRH (0.5 μm) still increased the inward holding current (−21.6 ± 3.3 pA, n = 9, p = 0.0002, Fig. 6D). However, the desensitization of the response was significantly slower than that recorded in the presence of the scrambled peptide (QPPASNPRVR, 50 μm, n = 7, p = 0.03, Fig. 6D). These results suggest that the function of dynamin is required for the desensitization of the TRH-mediated response.
TRH-induced increases in GABA release are independent of intracellular second messengers
We next examined whether intracellular second messengers are required for TRH-induced increase in GABA release. Activation of TRH receptors increases the activity of PLC which hydrolyses phospatidylinositol 4,5-bisphosphate to produce inositol 1,4,5-trisphosphate to facilitate intracellular Ca2+ release and diacylglycerol to activate PKC. We examined the roles of this pathway in TRH-induced increases in GABA release. Application of U73122 (20 μm), a PLC inhibitor, did not block TRH-induced increases in sIPSC frequency (296 ± 70% of control, n = 7, p = 0.03) suggesting that the activity of PLC is not required for the effects of TRH on GABA release. To further confirm this result, we used PLC knock-out mice. TRH receptors are G-protein-coupled receptors which are coupled to PLCβ (Lei et al. 2001). Among the four isoforms of PLCβ (PLCβ1–4), only PLCβ1 is expressed in the hippocampus (Watanabe et al. 1998). We therefore used PLCβ1 knock-out mice to examine the roles of PLC in TRH-induced increases in GABA release. Initially, we tested several different concentrations of TRH on sIPSC frequency recorded from wild-type mice and our results suggested that the optimal concentration was at 0.1 μm in mice. The difference in the optimal concentration of TRH for rats and mice might be due to the structural disparity of TRH receptors in these two species (Gershengorn & Osman, 1996). Application of TRH (0.1 μm) increased sIPSC frequency to 176 ± 22% of control in wild-type mice (n = 8 slices from n = 3 PLCβ1+/+ mice, p = 0.01, Fig. 7A) and 167 ± 10% of control in knock-out mice (n = 19 slices from n = 3 PLCβ1−/− mice, P < 0.0001, Fig. 7B). There was no statistical difference between these two groups (p = 0.66, Student's unpaired t test) demonstrating that the activity of PLC is not required for TRH-induced increase in GABA release.
Figure 7. TRH-induced increases in GABA release are independent of intracellular signals and phosphorylation.
A, application of TRH (0.1 μm) increased sIPSC frequency in eight slices from three wild-type (PLCβ1+/+) mice. B, application of TRH (0.1 μm) increased sIPSC frequency to the same level in 19 slices from three knock-out (PLCβ1−/−) mice. C–E, TRH-induced increases in sIPSC frequency were not changed by the inhibitors of PKC (calphostin C, 0.5 μm, n = 10) (C), MAPK (PD 98059, 50 μm, n = 9) (D) or CAMK II (KN-62, 10 μm, n = 10) (E). F, omission of ATP from the intracellular solution had no effect on TRH-induced inward holding currents (n = 5).
We also explored a potential role for intracellular Ca2+ release. Application of the sarco(endo)plasmic reticulum Ca2+-ATPase inhibitor, thapsigargin (5 μm), did not block TRH-induced increases in sIPSC frequency (319 ± 89% of control, n = 9, p = 0.04, data not shown) suggesting that intracellular Ca2+ release does not underlie TRH-induced increases in GABA release. To further confirm this result, we recorded the holding currents from the interneurons in the stratum radiatum of CA1 region and included BAPTA (10 mm) in the recording pipettes to chelate intracellular Ca2+. We waited for 20 min after the formation of whole-cell recording to allow the dialysis of BAPTA into the cells. Under these conditions, application of TRH still generated a comparable inward holding current (control, −32.6 ± 6.9 pA, n = 9; BAPTA, −31.0 ± 5.2 pA, n = 6, p = 0.87, Student's unpaired t test, data not shown) suggesting that intracellular Ca2+ is not necessary for the effects of TRH on GABA release. Similarly, application of calphostin C (0.5 μm), a broad-spectrum PKC inhibitor, did not block TRH-induced increase in sIPSC frequency (320 ± 64% of control, n = 10, p = 0.007, Fig. 7C) although this compound by itself inhibited sIPSC frequency (75 ± 7% of control, n = 10, p = 0.005, Fig. 7C). Together, these results suggest that TRH-mediated increase in GABA release is independent of PLC pathway.
TRH receptor activation also stimulates MAPK (Ohmichi et al. 1994) and CAMKII (Cui et al. 1994) in some preparations. We then examined the roles of these two intracellular pathways. Application of PD 98059 (50 μm), an MAPK kinase inhibitor, inhibited sIPSC frequency to 58 ± 10% of control (n = 9, p = 0.002, Fig. 7D). However, subsequent application of TRH (0.5 μm) still increased sIPSC frequency to 407 ± 84% of control (n = 9, p = 0.002, Fig. 7D) suggesting that MAPK pathway is not involved. Similarly, application of the CAMKII inhibitor, KN-62 (10 μm) did not block the effects of TRH (343 ± 64% of control, n = 10, p = 0.004, Fig. 7E). To ensure that CAMKII activity was inhibited by KN-62, we tested the effects of KN-62 on induction of long-term potentiation (LTP) in CA1 pyramidal neurons. Application of the pairing protocol (depolarization of the recorded cells from −65 to −10 mV for 6 min) induced robust LTP (166 ± 11% of control, 20 min after the application of the pairing protocol, n = 8, p = 0.006). However, in the presence of KN-62 (10 μm), application of the same pairing protocol failed to induce LTP (97 ± 7% of control, 20 min after the application of the pairing protocol, n = 8, p = 0.68). Taken together, these results indicate that CAMKII activity is not necessary for the effects of TRH on GABA release.
We then used a non-specific protein kinase inhibitor, staurosporine, and tested whether protein phosphorylation is required. Application of staurosporine (100 nm) did not block TRH-induced increases in sIPSC frequency (246 ± 56% of control, n = 7, p = 0.04, data not shown) suggesting that protein phosphorylation is not necessary for the effects of TRH on GABA release. If protein phosphorylation is not required, removal of intracellular ATP should not block the effects of TRH on the holding currents. We therefore used the intracellular solution that did not contain ATP and recorded the holding currents at −55 mV from CA1 stratum radiatum interneurons. We waited for at least 15 min after the formation of whole-cell recordings to allow the replacement of the intracellular solution with the ATP-free solution in the pipettes. Under these conditions, application of TRH (0.5 μm) still generated an inward current (−31.6 ± 2.3 pA, n = 5, Fig. 7F) comparable to control (p = 0.92) suggesting that the effects of TRH are independent of phosphorylation.
TRH inhibits seizure activity
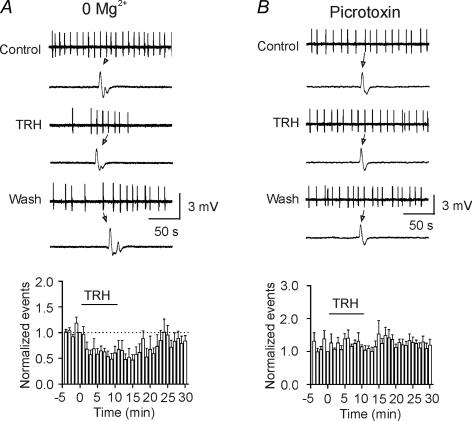
Whereas TRH has been reported to inhibit seizure activity both clinically and in animal models (Nillni & Sevarino, 1999; Kubek & Garg, 2002), the mechanisms underlying its anti-seizure effects are elusive. To test whether TRH-induced GABA release contributes to its anti-seizure effects, we used two seizure models. First, we induced spontaneous seizure activity in hippocampal slices by removal of extracellular Mg2+. GABAergic functions are intact in this seizure model. Application of TRH (0.5 μm) for 10 min significantly inhibited the frequency of the spontaneous seizure activity to 57 ± 9% of control (n = 15, p = 0.0004, Fig. 8A). Second, we recorded the spontaneous seizure activity produced by application of the GABAA receptor antagonist, picrotoxin (100 μm), to the hippocampal slices. However, TRH failed to significantly modulate the seizure activity in this seizure model (114 ± 6% of control, n = 12, P > 0.05, Fig. 8B). These results indicate that TRH-induced GABA release contributes to its anti-seizure activity in the hippocampus.
Figure 8. TRH inhibits seizure activity induced by deprivation of extracellular Mg2+ with no effects on the seizure activity induced by picrotoxin.
A, upper, seizure activity induced by bathing the hippocampal slices in extracellular solution containing 0Mg2+ before, during and after the application of TRH (0.5 μm). A single seizure event was shown in an enlarged scale below each trace. Lower, time course of the frequency of seizure activity averaged from 15 slices before, during and after the application of TRH. The numbers of the events were averaged for every minute and then normalized to the number of the events for the last minute before the application of TRH. B, upper, seizure activity evoked by application of the GABAA receptor blocker, picrotoxin (100 μm) in the hippocampal slices before, during and after the application of TRH (0.5 μm). A single seizure event was shown in an enlarged scale below each trace. Lower, summarized data from 12 slices. Note that TRH failed to alter the seizure activity induced by the picrotoxin seizure model.
Discussion
Our results demonstrate for the first time that TRH increases GABA release and inhibits seizure activity induced by removal of extracellular Mg2+ in hippocampal slices. TRH facilitates GABA release by inhibiting a resting K+ conductance resulting in membrane depolarization and an increase in action potential firing of GABAergic interneurons. The involved resting K+ channels belong to the family of K2P. The effects of TRH are mediated via Gαq/11, but are independent of PLC, PKC, Ca+ and ATP; a result that would be expected if Gαq/11 couples directly to K+ channels. We have also provided evidence demonstrating that dynamin-dependent endocytosis of TRH receptors underlies the desensitization of TRH-mediated depolarization.
Ionic mechanisms of TRH-induced increases in GABA release
Resting K+ channels are the major determinants of neuronal membrane potential and their inhibition is one of the principal mechanisms by which neurotransmitters modulate neuronal excitability. Our results indicate that TRH increases GABA release by inhibiting a resting K+ conductance in CA1 and CA3 interneurons because changing the extracellular K+ concentration shifted the reversal potential and the TRH-induced changes in holding currents were sensitive to Ba2+. Consistent with our results, TRH has been reported to generate membrane depolarization by inhibiting resting K+ channels in GABAergic neurons of the thalamus (Broberger & McCormick, 2005), hypoglossal motoneurons (Bayliss et al. 1992, 2003; Talley et al. 2000) and spinal motoneurons (Travagli et al. 1992; Fisher & Nistri, 1993; Kolaj et al. 1997). In contrast to other types of K+ channels, the resting K+ channels inhibited by TRH in CA1 interneurons are insensitive to the classical K+ channel blockers including TEA, Cs+ and 4-AP. This is consistent with the possible involvement of K2P channels. In mammals, 17 K2P channel genes have been identified, and their mRNA transcripts are expressed in many different cell types and tissues. K2P channels have properties of background or leak K+ channels, and therefore play a crucial role in setting the resting membrane potential and regulating cell excitability. Among these channels, TASK-1, TASK-3, TREK-1, TREK-2, and TWIK-1 have been reported to be sensitive to Ba2+ (Fink et al. 1996; Kim et al. 2000; Lesage & Lazdunski, 2000; Han et al. 2002). Because our results have shown that the effects of TRH are sensitive to Ba2+, it is reasonable to postulate that TRH may interact with one or more of these K2P channels. We made an initial attempt to identity the roles of K2p channels in TRH-induced depolarization. Application of the inhibitors for TASK-1 or TASK-3 homodimeric channels failed to block the effects of TRH. A possible reason for the negative results is that in native neurons TASK-1 and TASK-3 form heterodimeric channels (Berg et al. 2004). The formed heterodimeric TASK-1/TASK-3 channels are insensitive to either ruthenium red or Zn2+ (Bayliss et al. 2003). Because both TASK-1 and TASK-3 are sensitive to bupivacaine, we used bupivacaine to probe the roles of TASK channels in TRH-induced membrane depolarization. Application of bupivacaine (500 μm) significantly inhibited the effects of TRH. These results suggest that TASK channels contribute at least partially to TRH-induced membrane depolarization. Consistent with our results, TASK-like channels are expressed on hippocampal interneurons (Torborg et al. 2006). Whereas the roles of other K2P channels remain to be determined, our results suggest that TREK-1 is unlikely to be the target for TRH based on the following observations. First, substitution of a six-residue sequence at the beginning of the cytoplasmic C terminus of TASK-1 and TASK-3 channels with corresponding residues from TREK-1 in HEK 293 cells abolished the effects of TRH (Talley & Bayliss, 2002) suggesting that TRH does not modulate TREK-1 channels. Second, the currents mediated by TREK-1 were inhibited when the extracellular Na+ was replaced by NMDG (Fink et al. 1996), whereas in our experiments replacing the extracellular Na+ by NMDG did not block the effects of TRH suggesting that TREK-1 is not involved. Our results also exclude the involvement of TRAAK because TRAAK is sensitive to Gd3+ (Lesage & Lazdunski, 2000), but our results show that TRH still induced an inward current in the presence of Gd3+. Whereas TRH-induced membrane depolarization is Ba2+ sensitive, there are still a proportion of currents that are Ba2+ resistant. Therefore, a role of the Ba2+-resistant K2P channels cannot be excluded. Because the hippocampus expresses almost all the K2P channels (Talley et al. 2001) and those K2P channels are likely to assemble as heterogeneous channels, the exact nature of the K2P channels inhibited by TRH remains to be determined after the molecular identities of these K2P channels in native interneurons are fully elucidated.
Whereas TRH induces membrane depolarization in CA1 and CA3 interneurons, it has no effects on hilar interneurons or the principal cells in the hippocampus. This is not surprising because TRH receptors (TRH-R1) are selectively expressed on the interneurons in CA1 and CA3 regions not on the principal neurons (Heuer et al. 2000). Consistent with this observation, cholecystokinin (CCK), another neuropeptide present in the hippocampus, also depolarizes CA1 interneurons (Miller et al. 1997) with no effects on the holding current recorded from hilar interneurons at −55 mV (Deng & Lei, 2006). CCK increases action potential firing and GABA release in the dentate gyrus by inhibiting a Ca2+-dependent K+ channel (Deng & Lei, 2006). Similarly, TRH has been reported to inhibit Ca2+-dependent K+ channels (Haug et al. 2004). The mechanisms underlying TRH-mediated increase in GABA release in the dentate gyrus granule cells will be examined in the future.
Signal transduction mechanisms
Our results indicate that the effects of TRH are mediated by TRH receptors because application of chlordiazepoxide blocked TRH-induced increases in both inward holding currents and sIPSC frequency. Among the two TRH receptors, only TRH-R1 can be detected in the hippocampus, although TRH-R2 is abundant in the precommissural hippocampus (Heuer et al. 2000). Therefore, TRH-induced increase in GABA release is likely to be mediated by TRH-R1. Consistent with this possibility, TRH-R1 is selectively expressed in a subpopulation of neurons scattered in the stratum radiatum (Heuer et al. 2000) that are thought to be GABAergic interneurons. TRH receptors are G-protein coupled. Our results have clearly demonstrated that the function of G-proteins is required for TRH-mediated increase in GABA releases, consistent with previous results (Bayliss et al. 1994; Kolaj et al. 1997). The result that infusion of Gαq/11 antibody into the cells blocked TRH-induced membrane depolarization suggests that Gαq/11 is required for TRH-induced increase in GABA release. However, our results do not support any roles of the intracellular molecules downstream of G-proteins in TRH-mediated increase in GABA release. The major intracellular pathway coupled to TRH-R1 or TRH-R2 receptors is the PLC pathway that leads to an increase in intracellular Ca2+ release and the activation of PKC. However, our results from pharmacological experiments and knock-out mice indicate that this pathway is unlikely to be involved. Neither are the other two pathways (MAP kinase and CAMK II) involved in TRH-induced increases in GABA release. We therefore suggest that TRH receptor activation releases Gαq/11 which directly interacts with K+ channels to increase GABA release. Consistent with our results are the observations showing that TRH-mediated inhibition of background K+ channels is independent of intracellular second messengers in rat hypoglossal motoneurons (Bayliss et al. 1994; Leonoudakis et al. 1998), but occurs via a direct Gαq coupling in a mammalian heterologous expression system (Chen et al. 2006).
Like other G-protein-coupled receptors, TRH receptors undergo endocytosis after the binding of agonists (Petrou et al. 1997), and the internalization of TRH receptors is responsible for the desensitization of TRH responses (Gershengorn & Osman, 1996; Yu & Hinkle, 1998). Because we observed pronounced desensitization of TRH-mediated depolarization in our system, we probed the mechanism underlying this phenomenon. Our results demonstrate that dynamin-dependent endocytosis of TRH receptors contributes to the desensitization of the TRH response. Functional inhibition of dynamin can potentially reduce the desensitization of TRH receptors to generate a persistent increase in GABA release. This novel finding may have significant impact on the possibility that TRH receptor modulators serve as effective anti-epileptic agents (see below).
Physiological significance
The anti-epileptic effect of TRH has been known for decades. However, the underlying mechanisms are unclear. We provide evidence that TRH inhibits seizure activity in the hippocampus at least by inhibiting the background K2P channels of the interneurons to facilitate GABA release. Consistent with our results, alteration in K2P channel function promotes pro-convulsant effects (Heurteaux et al. 2004). In addition, TRH is involved in modulating a variety of physiological functions including arousal, sleep, cognition, locomotion and mood (Nillni & Sevarino, 1999). All these functions are related to the limbic structures including the hippocampus. Changes in the function of GABAergic transmission underlie almost all those physiological functions. Therefore, understanding how TRH modulates GABAergic function would facilitate the elucidation of the roles of TRH in these physiological functions. Taken together, our results provide a fundamental mechanism to explain multifaceted physiological and pathological roles of TRH in the brain.
Acknowledgments
This work was supported by National Institutes of Health, National Center for Research Resources Grant 5P20RR017699-02 (S.L.) and National Science Foundation Grant 0235146 (J.E.P.).
References
- Bannister RA, Melliti K, Adams BA. Differential modulation of CaV2.3 Ca2+ channels by Gαq/11-coupled muscarinic receptors. Mol Pharmacol. 2004;65:381–388. doi: 10.1124/mol.65.2.381. [DOI] [PubMed] [Google Scholar]
- Bayliss DA, Sirois JE, Talley EM. The TASK family: two-pore domain background K+ channels. Mol Interv. 2003;3:205–219. doi: 10.1124/mi.3.4.205. [DOI] [PubMed] [Google Scholar]
- Bayliss DA, Viana F, Berger AJ. Mechanisms underlying excitatory effects of thyrotropin-releasing hormone on rat hypoglossal motoneurons in vitro. J Neurophysiol. 1992;68:1733–1745. doi: 10.1152/jn.1992.68.5.1733. [DOI] [PubMed] [Google Scholar]
- Bayliss DA, Viana F, Berger AJ. Effects of thyrotropin-releasing hormone on rat motoneurons are mediated by G proteins. Brain Res. 1994;668:220–229. doi: 10.1016/0006-8993(94)90527-4. [DOI] [PubMed] [Google Scholar]
- Berg AP, Talley EM, Manger JP, Bayliss DA. Motoneurons express heteromeric TWIK-related acid-sensitive K+ (TASK) channels containing TASK-1 (KCNK3) and TASK-3 (KCNK9) subunits. J Neurosci. 2004;24:6693–6702. doi: 10.1523/JNEUROSCI.1408-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberger C, McCormick DA. Excitatory effects of thyrotropin-releasing hormone in the thalamus. J Neurosci. 2005;25:1664–1673. doi: 10.1523/JNEUROSCI.3198-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Talley EM, Patel N, Gomis A, McIntire WE, Dong B, et al. Inhibition of a background potassium channel by Gq protein alpha-subunits. Proc Natl Acad Sci U S A. 2006;103:3422–3427. doi: 10.1073/pnas.0507710103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui ZJ, Gorelick FS, Dannies PS. Calcium/calmodulin-dependent protein kinase-II activation in rat pituitary cells in the presence of thyrotropin-releasing hormone and dopamine. Endocrinology. 1994;134:2245–2250. doi: 10.1210/endo.134.5.8156928. [DOI] [PubMed] [Google Scholar]
- Deng PY, Lei S. Bidirectional modulation of GABAergic transmission by cholecystokinin in hippocampal dentate gyrus granule cells of juvenile rats. J Physiol. 2006;572:425–442. doi: 10.1113/jphysiol.2005.104463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond AH. Chlordiazepoxide is a competitive thyrotropin-releasing hormone receptor antagonist in GH3 pituitary tumour cells. Biochem Biophys Res Commun. 1985;127:63–70. doi: 10.1016/s0006-291x(85)80126-1. [DOI] [PubMed] [Google Scholar]
- Fink M, Duprat F, Lesage F, Reyes R, Romey G, Heurteaux C, et al. Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO J. 1996;15:6854–6862. [PMC free article] [PubMed] [Google Scholar]
- Fisher ND, Nistri A. A study of the barium-sensitive and -insensitive components of the action of thyrotropin-releasing hormone on lumbar motoneurons of the rat isolated spinal cord. Eur J Neurosci. 1993;5:1360–1369. doi: 10.1111/j.1460-9568.1993.tb00922.x. [DOI] [PubMed] [Google Scholar]
- Gershengorn MC, Osman R. Molecular and cellular biology of thyrotropin-releasing hormone receptors. Physiol Rev. 1996;76:175–191. doi: 10.1152/physrev.1996.76.1.175. [DOI] [PubMed] [Google Scholar]
- Han J, Truell J, Gnatenco C, Kim D. Characterization of four types of background potassium channels in rat cerebellar granule neurons. J Physiol. 2002;542:431–444. doi: 10.1113/jphysiol.2002.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug TM, Hafting T, Sand O. Inhibition of BK channels contributes to the second phase of the response to TRH in clonal rat anterior pituitary cells. Acta Physiol Scand. 2004;180:347–357. doi: 10.1111/j.1365-201X.2004.01266.x. [DOI] [PubMed] [Google Scholar]
- Heuer H, Schafer MK, O'Donnell D, Walker P, Bauer K. Expression of thyrotropin-releasing hormone receptor 2 (TRH-R2) in the central nervous system of rats. J Comp Neurol. 2000;428:319–336. [PubMed] [Google Scholar]
- Heurteaux C, Guy N, Laigle C, Blondeau N, Duprat F, Mazzuca M, et al. TREK-1, a K+ channel involved in neuroprotection and general anesthesia. EMBO J. 2004;23:2684–2695. doi: 10.1038/sj.emboj.7600234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh KP, Martin TF. Thyrotropin-releasing hormone and gonadotropin-releasing hormone receptors activate phospholipase C by coupling to the guanosine triphosphate-binding proteins Gq and G11. Mol Endocrinol. 1992;6:1673–1681. doi: 10.1210/mend.6.10.1333052. [DOI] [PubMed] [Google Scholar]
- Itadani H, Nakamura T, Itoh J, Iwaasa H, Kanatani A, Borkowski J, et al. Cloning and characterization of a new subtype of thyrotropin-releasing hormone receptors. Biochem Biophys Res Commun. 1998;250:68–71. doi: 10.1006/bbrc.1998.9268. [DOI] [PubMed] [Google Scholar]
- Kim D, Jun KS, Lee SB, Kang NG, Min DS, Kim YH, et al. Phospholipase C isozymes selectively couple to specific neurotransmitter receptors. Nature. 1997;389:290–293. doi: 10.1038/38508. [DOI] [PubMed] [Google Scholar]
- Kim Y, Bang H, Kim D. TASK-3, a new member of the tandem pore K+ channel family. J Biol Chem. 2000;275:9340–9347. doi: 10.1074/jbc.275.13.9340. [DOI] [PubMed] [Google Scholar]
- Kolaj M, Shefchyk SJ, Renaud LP. Two conductances mediate thyrotropin-releasing-hormone-induced depolarization of neonatal rat spinal preganglionic and lateral horn neurons. J Neurophysiol. 1997;78:1726–1729. doi: 10.1152/jn.1997.78.3.1726. [DOI] [PubMed] [Google Scholar]
- Kubek MJ, Garg BP. Thyrotropin-releasing hormone in the treatment of intractable epilepsy. Pediatr Neurol. 2002;26:9–17. doi: 10.1016/s0887-8994(01)00321-6. [DOI] [PubMed] [Google Scholar]
- Lei Q, Talley EM, Bayliss DA. Receptor-mediated inhibition of G protein-coupled inwardly rectifying potassium channels involves Gαq family subunits, phospholipase C, and a readily diffusible messenger. J Biol Chem. 2001;276:16720–16730. doi: 10.1074/jbc.M100207200. [DOI] [PubMed] [Google Scholar]
- Lei S, McBain CJ. GABAB receptor modulation of excitatory and inhibitory synaptic transmission onto rat CA3 hippocampal interneurons. J Physiol. 2003;546:439–453. doi: 10.1113/jphysiol.2002.034017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonoudakis D, Gray AT, Winegar BD, Kindler CH, Harada M, Taylor DM, et al. An open rectifier potassium channel with two pore domains in tandem cloned from rat cerebellum. J Neurosci. 1998;18:868–877. doi: 10.1523/JNEUROSCI.18-03-00868.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage F, Lazdunski M. Molecular and functional properties of two-pore-domain potassium channels. Am J Physiol Renal Physiol. 2000;279:F793–F801. doi: 10.1152/ajprenal.2000.279.5.F793. [DOI] [PubMed] [Google Scholar]
- Low WC, Roepke J, Farber SD, Hill TG, Sattin A, Kubek MJ. Distribution of thyrotropin-releasing hormone (TRH) in the hippocampal formation as determined by radioimmunoassay. Neurosci Lett. 1989;103:314–319. doi: 10.1016/0304-3940(89)90119-5. [DOI] [PubMed] [Google Scholar]
- Manaker S, Winokur A, Rostene WH, Rainbow TC. Autoradiographic localization of thyrotropin-releasing hormone receptors in the rat central nervous system. J Neurosci. 1985;5:167–174. doi: 10.1523/JNEUROSCI.05-01-00167.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyh PW, Hunt SP. Thyrotropin-releasing hormone (TRH) receptors. Localization by light microscopic autoradiography in rat brain using [3H][3-Me-His2]TRH as the radioligand. J Neurosci. 1985;5:551–561. doi: 10.1523/JNEUROSCI.05-02-00551.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks B, McMahon HT. Calcium triggers calcineurin-dependent synaptic vesicle recycling in mammalian nerve terminals. Curr Biol. 1998;8:740–749. doi: 10.1016/s0960-9822(98)70297-0. [DOI] [PubMed] [Google Scholar]
- Miller KK, Hoffer A, Svoboda KR, Lupica CR. Cholecystokinin increases GABA release by inhibiting a resting K+ conductance in hippocampal interneurons. J Neurosci. 1997;17:4994–5003. doi: 10.1523/JNEUROSCI.17-13-04994.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley JE. Extrahypothalamic thyrotropin releasing hormone (TRH) – its distribution and its functions. Life Sci. 1979;25:1539–1550. doi: 10.1016/0024-3205(79)90435-1. [DOI] [PubMed] [Google Scholar]
- Nillni EA, Sevarino KA. The biology of pro-thyrotropin-releasing hormone-derived peptides. Endocr Rev. 1999;20:599–648. doi: 10.1210/edrv.20.5.0379. [DOI] [PubMed] [Google Scholar]
- O'Dowd BF, Lee DK, Huang W, Nguyen T, Cheng R, Liu Y, et al. TRH-R2 exhibits similar binding and acute signaling but distinct regulation and anatomic distribution compared with TRH-R1. Mol Endocrinol. 2000;14:183–193. doi: 10.1210/mend.14.1.0407. [DOI] [PubMed] [Google Scholar]
- Ohmichi M, Sawada T, Kanda Y, Koike K, Hirota K, Miyake A, et al. Thyrotropin-releasing hormone stimulates MAP kinase activity in GH3 cells by divergent pathways. Evidence of a role for early tyrosine phosphorylation. J Biol Chem. 1994;269:3783–3788. [PubMed] [Google Scholar]
- Petrou C, Chen L, Tashjian AH., Jr A receptor-G protein coupling-independent step in the internalization of the thyrotropin-releasing hormone receptor. J Biol Chem. 1997;272:2326–2333. doi: 10.1074/jbc.272.4.2326. [DOI] [PubMed] [Google Scholar]
- Pfleger KD, Kroeger KM, Eidne KA. Receptors for hypothalamic releasing hormones TRH and GnRH: oligomerization and interactions with intracellular proteins. Semin Cell Dev Biol. 2004;15:269–280. doi: 10.1016/j.semcdb.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Simasko S, Horita A. Chlordiazepoxide displaces thyrotropin-releasing hormone (TRH) binding. Eur J Pharmacol. 1984;98:419–423. doi: 10.1016/0014-2999(84)90291-7. [DOI] [PubMed] [Google Scholar]
- Sun Y, Lu X, Gershengorn MC. Thyrotropin-releasing hormone receptors – similarities and differences. J Mol Endocrinol. 2003;30:87–97. doi: 10.1677/jme.0.0300087. [DOI] [PubMed] [Google Scholar]
- Talley EM, Bayliss DA. Modulation of TASK-1 (KCNK3) and TASK-3 (KCNK9) potassium channels: volatile anesthetics and neurotransmitters share a molecular site of action. J Biol Chem. 2002;277:17733–17742. doi: 10.1074/jbc.M200502200. [DOI] [PubMed] [Google Scholar]
- Talley EM, Lei Q, Sirois JE, Bayliss DA. TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron. 2000;25:399–410. doi: 10.1016/s0896-6273(00)80903-4. [DOI] [PubMed] [Google Scholar]
- Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA. CNS distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci. 2001;21:7491–7505. doi: 10.1523/JNEUROSCI.21-19-07491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torborg CL, Berg AP, Jeffries BW, Bayliss DA, McBain CJ. TASK-like conductances are present within hippocampal CA1 stratum oriens interneuron subpopulations. J Neurosci. 2006;26:7362–7367. doi: 10.1523/JNEUROSCI.1257-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travagli RA, Gillis RA, Vicini S. Effects of thyrotropin-releasing hormone on neurons in rat dorsal motor nucleus of the vagus, in vitro. Am J Physiol Gastrointest Liver Physiol. 1992;263:G508–G517. doi: 10.1152/ajpgi.1992.263.4.G508. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Nakamura M, Sato K, Kano M, Simon MI, Inoue Y. Patterns of expression for the mRNA corresponding to the four isoforms of phospholipase Cβ in mouse brain. Eur J Neurosci. 1998;10:2016–2025. doi: 10.1046/j.1460-9568.1998.00213.x. [DOI] [PubMed] [Google Scholar]
- Yu R, Hinkle PM. Signal transduction, desensitization, and recovery of responses to thyrotropin-releasing hormone after inhibition of receptor internalization. Mol Endocrinol. 1998;12:737–749. doi: 10.1210/mend.12.5.0110. [DOI] [PubMed] [Google Scholar]