Abstract
Previous work has indicated that metabotropic glutamate receptors (mGluRs) modulate visual responses of superior colliculus (SC) neurones in vivo in a variety of ways, in a manner that can be dependent upon visual stimulus properties. How this occurs remains unclear. In this study we aimed to determine how activation of mGluR2 and mGluR3 receptors (Group II) might modulate visual responses, by using field potential and whole-cell patch clamp recording techniques in rat SC slice. Stimulation within the superficial layers of the SC, in the presence of ionotropic glutamate receptor antagonists, evoked IPSCs that were blocked by bicuculline indicating that they are mediated via GABAA receptors. It is likely that these IPSCs were of heterogeneous origin as they showed substantial variation in paired-pulse behaviour. Nevertheless, activation of Group II mGluRs with the group-selective agonist LY354740 (300 nm, bath application) resulted in a reduction of these IPSCs (to 56% of control amplitude), and this was associated with a decrease in paired-pulse depression. At the same concentration, LY354740 did not reduce the EPSC or field-EPSP evoked by stimulation of the retinal input to the SC. The effects of LY354740 on IPSCs were not mimicked by the mGluR3-selective agonist N-acetyl-aspartyl-glutamate (NAAG, 200–500 μm). Stimulation of IPSCs with trains of impulses (10 at 20 Hz) in order to mimic natural activation patterns resulted in sequences of IPSCs that were reduced in amplitude towards the end of the stimulus train. Application of the Group II antagonist LY341495 (100 nm) under these conditions resulted in an increase in later IPSCs in a third of neurones tested. These findings indicate that mGluR2 (but not mGluR3) can selectively modulate GABAergic inhibition in SC, probably via a presynaptic mechanism. Furthermore, these receptors may be activated by synaptically released transmitter during patterns of activation similar to those seen during visual processing. Thus mGluR2 receptors could have a function in activity-dependent modulation of inhibitory processing during visual responses.
The metabotropic glutamate receptors (mGluRs) are a family of eight receptors divided into three groups according to sequence homology, pharmacology and signal transduction mechanisms (Conn & Pin, 1997). The principal action of mGluRs is to alter neuronal excitability, and this can be achieved through a variety of different mechanisms including presynaptic effects on neurotransmitter release, and postsynaptic modulation of ionic conductances (Conn & Pin, 1997; Anwyl, 1999; Schoepp, 2001). Activation of these receptors can influence neuronal activity over a range of time scales from a few seconds through to the possibly permanent changes in synaptic efficacy underlying long-term potentiation and depression (Anwyl, 1999; Bortolotto et al. 1999).
The mammalian superior colliculus (SC) is a subcortical structure that integrates sensory input and plays a major role in controlling eye, head, trunk and limb movements for the generation of behavioural responses to novel sensory inputs (Stein & Meredith, 1993). The SC is formed of seven alternating cellular and fibrous layers and, on the basis of function and anatomy, is divided into the superficial SC (SSC; the outermost layers 1–3) and the deep SC (DSC; layers 4–7). The SSC is concerned exclusively with processing visual inputs; the DSC, in addition to responding to visual stimuli, also receives auditory, somatosensory and nociceptive inputs (Edwards, 1980; Stein & Meredith, 1993; Binns, 1999).
Expression of mGluR subtypes from each of the three groups has been reported in the rat SC (Martin et al. 1992; Ohishi et al. 1993b, 1995; Cirone et al. 2002b). Using group-selective ligands we have demonstrated that receptors from all three groups are functional in the SSC: local iontophoretic application of exogenous agonists for group I (mGluR1 and mGluR5) and group III (mGluR4, 6, 7 and 8) receptors depressed the firing rate in the majority of SSC cells studied in vivo (Cirone & Salt, 2001; Cirone et al. 2002a). This depression is possibly due to presynaptic depression of glutamate release (Cirone et al. 2002a; Pothecary et al. 2002; White et al. 2003). The group II mGluRs (mGluR2 and mGluR3) are selectively activated by low nanomolar concentrations of the exogenous agonist LY354740 and antagonized by LY341495 (Schoepp et al. 1999). Iontophoretic application of LY354740 either enhanced or reduced visual responses of individual neurons in the rat SSC in vivo, and these effects were occluded when LY341495 was coapplied with LY354740 (Cirone & Salt, 2001). Furthermore, LY341495 when applied alone affected visual responses, producing effects opposite to the agonist. This effect of the antagonist was greater when visual stimuli that resulted in larger visual responses were used. This stimulus dependence led to the suggestion that Group II mGluRs are activated by an endogenous agonist released during visual response processing (Cirone & Salt, 2001).
The mechanism(s) underlying these actions of the group II mGluRs are not known, but it is conceivable that the net effect of Group II receptor modulation in vivo is a result of actions at different targets, for example direct effects on excitatory transmission or modulation of GABAergic inhibitory circuits (Cirone & Salt, 2001). To address this issue we have utilized an in vitro slice preparation of the SC and electrophysiological techniques to investigate the effect that activation of the receptors has on synaptic transmission.
Some of these results have been published previously in abstract form (Neale & Salt, 2003, 2004).
Methods
All experiments were conducted on tissue from adult (> P28) Lister Hooded rats. Animals were bred in-house or purchased from Harlan Olac and were housed on a 12 h light/dark cycle with unlimited access to food and water. All experimental conditions and procedures were in accordance with the UK Animals (Scientific Procedures) Act 1986 and associated guidelines.
Rats were anaesthetized with halothane and decapitated. Their brains were then removed rapidly and placed in ice-cold, oxygenated sucrose Krebs' medium containing (mm): sucrose 202, KCl 2, KH2PO4 1.25, MgSO4 10, CaCl2 0.5, NaHCO3 26, glucose 10. The cerebellum was removed and an angled (40–50 deg to the midline) cut made coronally across the frontal cortex. To produce slices for field recordings, the block of brain was glued to the cutting stage of an oscillating microtome from which 300 μm slices of the SC were prepared. For whole cell recording 250–300 μm slices were prepared using an Integraslice oscillating microtome (Campden Instruments, Loughborough, UK). This angle of cutting maintains the integrity of retinal input to the superficial layers of the SC, although it is likely that other afferents are also present. Nevertheless the majority (∼90%) of excitatory afferents are indeed of retinal rather than cortical origin based on anatomical (Lund & Lund, 1971; Harvey & Worthington, 1990) and physiological studies (Miyamoto et al. 1990; Turner et al. 2005).
The slices were transferred to oxygenated recovery Krebs' medium at room temperature containing (mm): NaCl 124, KCl 2, KH2PO4 1.25, MgSO4 5, CaCl2 1, NaHCO3 26, glucose 10. After 1 h, a slice was transferred to a recording chamber (vol. 0.8 ml) where it was held submerged and perfused with oxygenated Krebs' medium containing (mm): NaCl 124, KCl 2, KH2PO4 1.25, MgSO4 1, CaCl2 2, NaHCO3 26, glucose 10.
Extracellular field recordings were made via Krebs' medium-filled, glass patch pipettes (3–5 MΩ) positioned in the superficial grey layer of the SC. Intracellular voltage clamp recordings were made using the blind whole cell patch clamp technique. Recording pipettes contained (mm) K-gluconate 130, EGTA 0.5, Hepes 10, NaCl 5, MgATP 4, NaGTP 0.4 and QX314 10. The pH was adjusted to 7.33–7.39 with KOH and the osmolarity was adjusted to 293–299 mosmol l−1. The open pipette resistance was 7–12 MΩ and after break through, the uncompensated access resistance was 14–32 MΩ; this was compensated by 50–60%. The term ‘holding potential’ refers to the potential uncorrected for the liquid-junction potential; other references to potential relate to the holding potential corrected for the calculated junction potential (pCLAMP, Axon Instruments, Union City, CA, USA).
To stimulate the retinal input (0.1 ms pulses, 4–15 V, 0.067 Hz) a bipolar tungsten-in-glass electrode was positioned in the optic tract outside of the body of the SC. To stimulate the inhibitory interneurones the stimulating electrode was positioned with both poles entering the superficial grey layer. In this configuration it was possible to evoke an EPSC followed by an IPSC (stimulation pulses 0.02–0.1 ms; 3–20 V). Application of a combination of ionotropic glutamate antagonists (CNQX (10 μm) and either d-AP5 (100 μm) or MK-801 (3 μm)) abolished the EPSC; thereby allowing the IPSC to be studied in isolation.
Responses were recorded using an Axoclamp 2B amplifier (Axon Instruments), digitized at 10 kHz and recorded to a PC running Spike2 software (Cambridge Electronic Design, Cambridge, UK). Data were analysed by measuring the average amplitude of EPSPs, EPSCs or IPSCs collected over 60 s and are expressed as means ± s.e.m.; ‘n’ values refer to the number of cells tested, each cell being from a different slice. In all illustrations of sample traces the stimulation artifact has been truncated. Paired-pulse ratio for IPSCs was calculated by dividing the amplitude of the second IPSC by the amplitude of the first IPSC in response to a pair of stimuli (100 ms separation). To quantify changes in the late phase of the fEPSP in some experiments, the area under the curve between the point where the fEPSP had decayed to 50% of its peak and the point where it had returned to baseline was computed.
Agonists and antagonists were applied to the tissue by addition to the bathing medium. LY354740 and LY341495 were donated by Lilly Research and CGP55845A was a donation from Novartis, Basel. Other compounds were obtained from either Tocris or Sigma.
Statistical significance was tested using Wilcoxon's matched-pairs signed-ranks test. P < 0.05 was considered statistically significant.
Results
Group II receptor modulation of SSC responses to retinal input
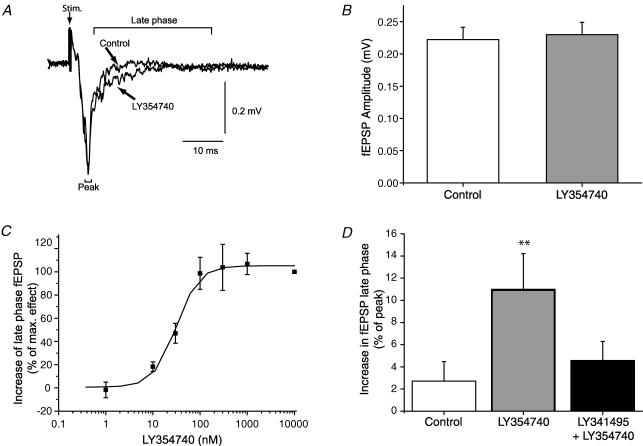
Stimulation of the retinal input to the SC resulted in a characteristic, ionotropic glutamate receptor-mediated (Binns & Salt, 1994; Lo & Mize, 1999) field EPSP (fEPSP; Fig. 1A). In contrast to the reported effects of group I and III mGluR agonists (Cirone et al. 2002a; Pothecary et al. 2002; White et al. 2003; Thompson et al. 2004), application of LY354740 (100 nm) had no significant effect on the peak amplitude of the fEPSP (control 0.26 ± 0.02 mV, LY3534740 0.27 ± 0.03 mV; P > 0.05; n = 15; Fig. 1). There was, however, a change to the decay phase of the fEPSP. In the presence of LY354740 (100 nm) the magnitude of the fEPSP decay phase changed from 4 ± 1.5% to 13 ± 1.5% (P < 0.01; n = 15; expressed as the difference between fEPSP peak and the late phase, normalized to the fEPSP peak). This effect was concentration dependent and reversed on washout; the EC50 was 33 nm and the maximal effect was observed at 300 nm (Fig. 1C).
Figure 1. The effect of the group II mGluR agonist LY354740 on synaptic transmission.
A, effect of LY354740 (100 nm) on a typical fEPSP evoked by optic tract stimulation. Each trace is an average of 6 consecutive sweeps. Control and drug traces are superimposed. Note the lack of effect of the agonist on the fEPSP peak, but the small effect on the late phase of the fEPSP. B, LY354740 (100 nm) exerts no significant effect on the peak amplitude (n = 15). C, LY354740 produces a concentration-dependent increase in the decay phase of the fEPSP (n = 3–6). D, the effect of LY354740 (100 nm) on the fEPSP late phase is reversed by the antagonist LY341495 (50 nm; n = 7; **P < 0.01).
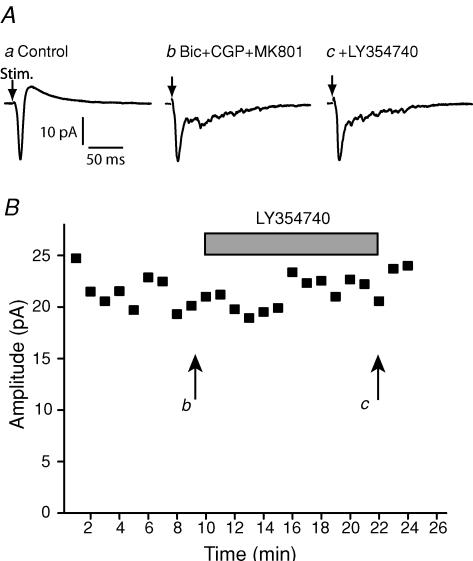
To verify that LY354740 was acting via group II mGluRs, we examined whether the antagonist LY341495, at a concentration selective for group II mGluRs, affected the action of LY354740. LY354740 (100 nm) produced a statistically significant change to the fEPSP late phase from 2 ± 6% to 12 ± 6% (P < 0.05; n = 7). In the continued presence of LY354740, application of LY341495 (100 nm) reversed this fEPSP component to levels not statistically different from control (4 ± 4%; P > 0.05; n = 7; Fig. 1D). In order to confirm the apparent lack of effect of LY354740 on monosynaptic retinal input onto SC neurones, we also made whole-cell recordings from single neurones in response to optic tract stimulation (Fig. 2). Optic tract stimulation evoked an inward current, followed by an outward current. The outward current was blocked by GABA receptor antagonists (50 μm bicuculline methochloride and 5 μm CGP55845). Addition of the group II agonist LY354740 (100 nm) did not have any effect on the optic-tract-evoked EPSC under these conditions (from 22 ± 3.1 pA to 23 ± 6.1 pA, n = 3; Fig. 2).
Figure 2. The effect of LY354740 on optic-tract-evoked EPSCs.
A, whole cell recordings showing EPSCs in response to optic tract stimulation. a, control; b, in the presence of GABA antagonists and the NMDA antagonist MK801; c, after the further addition of the agonist LY354740 (100 nm). B, the time course of EPSC peak amplitudes is plotted, and it can be seen that LY354740 has no effect.
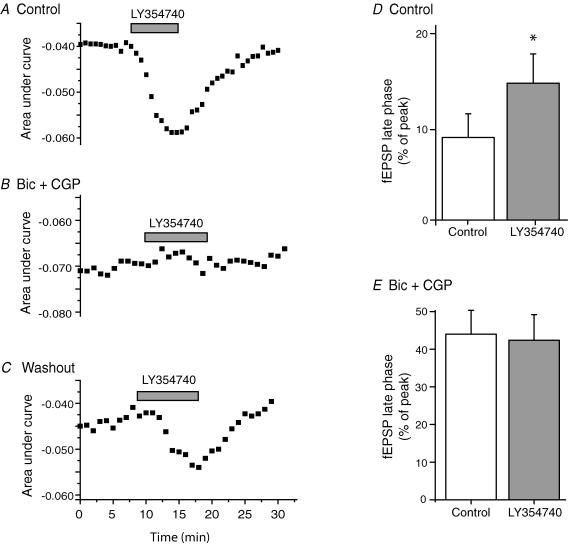
The effect of LY354740 on the late phase of the fEPSP bears similarity to the effect of blockade of GABA receptors in this preparation (Cirone et al. 2002a). We therefore hypothesized that the action of LY354740 might be due to inhibition of GABAergic transmission. Application of the GABA receptor antagonists (50 μm bicuculline methochloride and 5 μm CGP55845) increased the decay phase (9.1 ± 2.6% to 44 ± 6.3%), but did not affect the fEPSP peak (from 0.25 ± 0.04 mV to 0.24 ± 0.05 mV). Under control conditions LY354740 (50 nm) significantly increased the decay phase (from 9.1 ± 2.6% to 15 ± 3.2%, P < 0.05), but when applied in the presence of the GABA receptor antagonists produced no increase (44 ± 6.3% to 42 ± 6.8%; n = 5; Fig. 3).
Figure 3. The effect of the group II agonist LY354740 on the fEPSP late-phase is occluded in the presence of GABA receptor antagonists.
A–C, each plot shows time course of the effect of repeated applications of LY354740 (50 nm; grey bar represents duration of compound application) to a single slice under the following conditions. A, control; B, in the presence of the GABAA+B receptor antagonists bicuculline (50 μm) and CGP55845 (5 μm); C, under control conditions following washout of GABA receptor antagonists. D and E, mean data showing action of LY354740 on the fEPSP late phase. D, control conditions (n = 9; *P < 0.05); E, in the presence of GABA receptor antagonists (n = 5).
Modulation of inhibitory responses by group II mGluRs
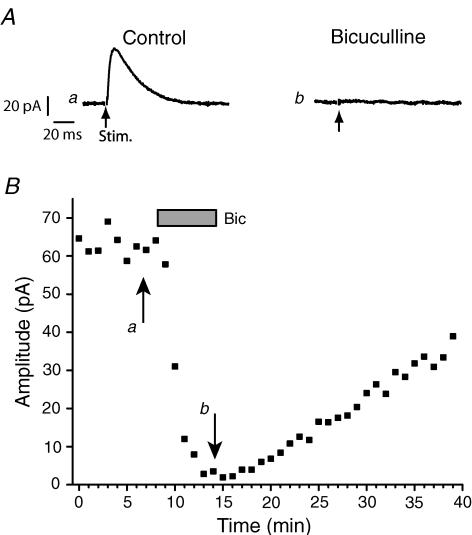
To directly test the effect of LY354740 on inhibitory activity we examined the effect of the agonist on IPSCs recorded using the whole cell patch clamp technique. At a holding potential of −40 to −45 mV, and in the presence of ionotropic glutamate receptor antagonists, outward currents could be recorded from SSC neurons upon local stimulation. These currents were attenuated in the presence of bicuculline (10 μm; Fig. 4) from a mean amplitude of 51 ± 9 pA under control conditions to 1 ± 2 pA (n = 9) in the presence of bicuculline, consistent with their being GABAA receptor mediated IPSCs.
Figure 4. GABAergic IPSC evoked by local stimulation within the slice.
A, whole cell recordings in response to local stimulation within the slice in the presence of CNQX (10 μm) and MK-801 (3 μm) alone (a) or in the presence of additional bicuculline (b). B, the time course of the bicuculline effect.
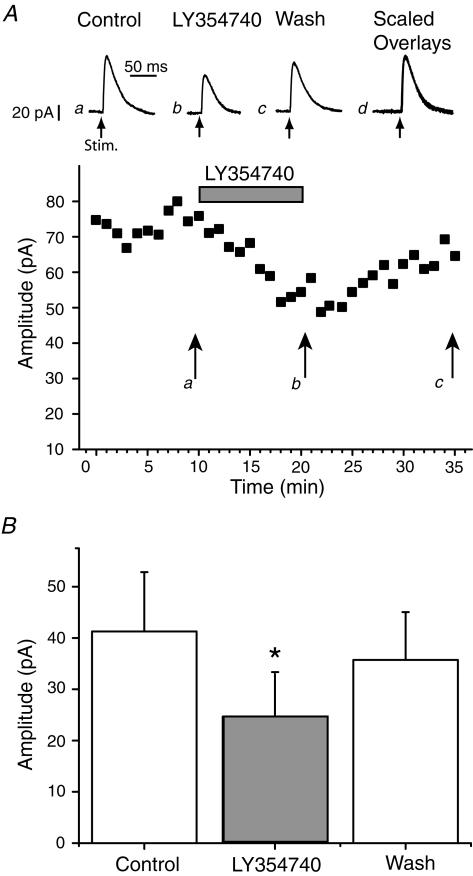
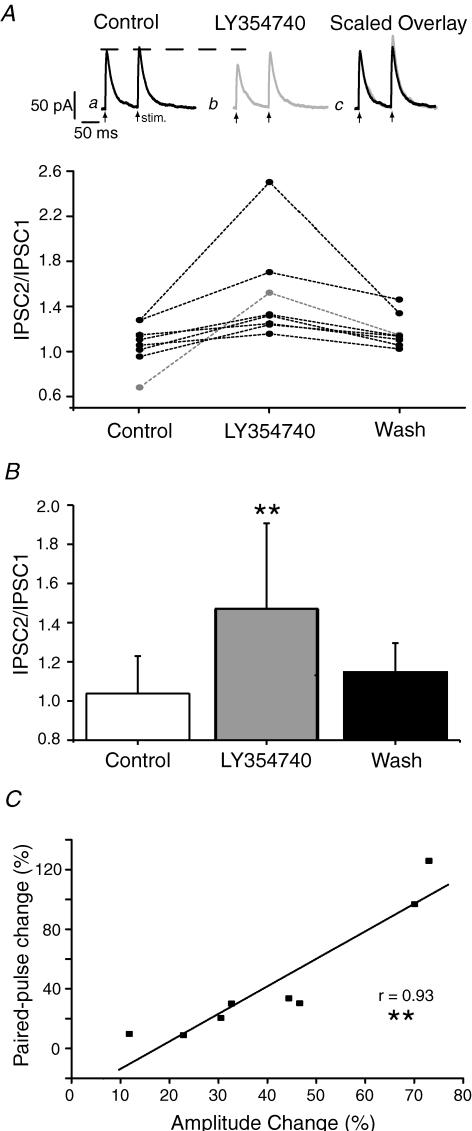
LY354740, at a concentration that was maximally effective in the field recording (300 nm), reduced the IPSCs to 56 ± 7% (P < 0.01; n = 9; Fig. 5) of control. On washout the IPSC amplitude recovered to control levels (94 ± 6%; P > 0.05; n = 9). To investigate whether LY354740 was acting presynaptically, we measured the effect of the agonist on the paired-pulse ratio of IPSCs. Pairs of stimuli, separated by 100 ms, were applied (repetition rate 0.1 Hz). Under control conditions there was some variation between cells ranging from a paired-pulse depression to paired-pulse facilitation (Fig. 6A). In all cells the ratio increased in the presence of LY354740 (300 nm) from a mean of 1.1 ± 0.1 to 1.5 ± 0.1 (P < 0.01; n = 8), and these effects reversed upon washout (Fig. 6B). Furthermore, there was a significant correlation (P < 0.01) between the effect of the agonist on IPSC amplitude and the effect of the agonist on paired-pulse ratio (Fig. 6C).
Figure 5. The group II agonist LY354740 depresses inhibitory synaptic transmission.
A, whole cell recordings in response to local stimulation within the slice in the presence of ionotropic glutamate receptor antagonists. a, control; b, in the presence of LY354740 (300 nm); c, upon washout of the agonist; d, scaled overlay of traces a–c. The time course of the effects is shown below. B, accumulated data from 9 cells (P < 0.05).
Figure 6. Depression of the IPSC by LY354740 is associated with increases in the paired-pulse ratio.
A, the upper section shows data recorded from a single cell where LY354740 (300 nm) depresses the amplitude of IPSC1. Each trace is an average of 6 consecutive sweeps. a, control; b, in the presence of LY354740 (300 nm); c, scaled overlay of traces a and b. Below is shown the individual data from 8 experiments. B, mean data from 8 experiments. C, correlation of the agonist effect on amplitude of the first IPSC of a pair with change in paired-pulse ratio.
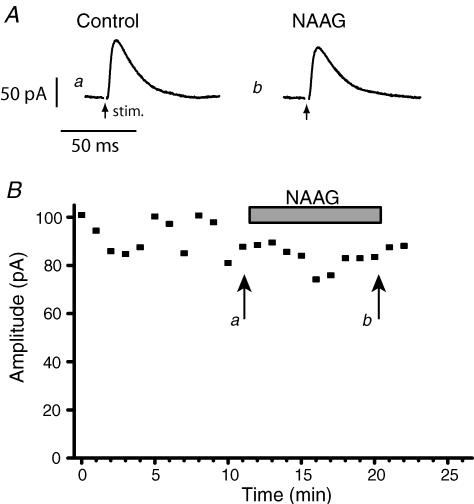
LY354740 activates both of the group II receptors, mGluR2 and mGluR3. In contrast, the endogenous dipeptide N-acetyl-aspartyl-glutamate (NAAG) selectively activates mGluR3 with an EC50 of less than 100 μm whilst producing no significant activation of mGluR2 receptors at 1 mm (Wroblewska et al. 1997). Furthermore, NAAG is expressed strongly in retinal ganglion cells (Moffett, 2003), is released in the SC following optic tract stimulation (Tsai et al. 1990) and has been proposed to function as a neurotransmitter (Neale et al. 2000; Gafurov et al. 2001). It was thus of significant interest to see if NAAG could act in the same way as LY354740. However, as illustrated in Fig. 7, application of NAAG (200 μm or 500 μm) did not depress the IPSC amplitude in the same manner as LY354740 (Control 56 ± 15 pA, NAAG 53 ± 12 pA; n = 5).
Figure 7. NAAG exerts no effect on inhibitory neurotransmission.
A, whole cell recording in response to local stimulation within the slice in the presence of ionotropic glutamate receptor antagonists. a, control; b in the presence of NAAG (200 μm). B, time course of the experiment.
Are Group II receptors activated synaptically?
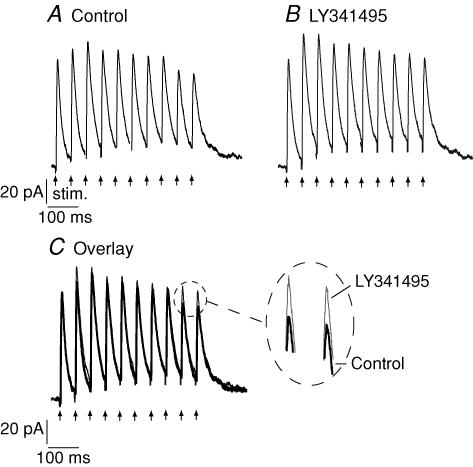
In order to test whether the effects of the exogenous agonist could be reproduced by endogenous, synaptically released agonist, trains of 10 stimuli were applied at frequencies of 20 Hz, repeated every 30 s. These parameters were chosen in an attempt to mimic the type of activity that might be encountered in the SC in vivo during visual stimulation (Cirone & Salt, 2001). Under control conditions, such protocols evoked a train of IPSC responses where responses to the later stimuli in the train tended to be reduced in amplitude compared to the IPSC evoked by the first stimulus of the train (Fig. 8). Application of the antagonist LY341495 (100 nm) resulted in an enhancement of the amplitude of the second and subsequent IPSCs of the train in three of nine neurones tested in this way (Fig. 8). In the remaining six neurones, no clear effect of the antagonist was discernable. There appeared to be no difference between the initial paired-pulse ratios of those neurones whose responses were enhanced by the antagonist and those that were not enhanced. Three of the neurones that were unaffected by application of the antagonist were also investigated with higher frequency stimulus trains (50Hz): however, in these cases responses were also not enhanced.
Figure 8. Stimulation of IPSC trains.
A, whole cell recordings in response to local stimulation (10 stimuli at 20 Hz) within the slice in the presence of ionotropic glutamate receptor antagonists (Control). B, responses in the presence of the group II antagonist LY341495 (15 min). C, overlay of traces from A and B; zoomed area indicates effect of the antagonist on the latter portions of the train response.
Discussion
We have previously demonstrated that the group II mGluRs modulate the response of collicular neurons to visual stimulation in vivo (Cirone & Salt, 2001), although it was unclear by what mechanism(s) this might occur. In the present study we now provide evidence that group II (probably mGluR2) receptors act presynaptically to suppress GABA release, whilst having little direct effect on retino-collicular transmission.
Group II receptors and retino-collicular transmission
The group II mGluR agonist LY354740 did not significantly depress the peak amplitude of the fEPSP evoked by stimulation of the optic tract. Consistent with this, the agonist also had no significant effect on the peak amplitudes of EPSCs in single cell recordings. This observation suggests that group II mGluRs do not directly modulate glutamatergic retino-collicular neurotransmission in the SC; this contrasts with the depressant actions of group I and III agonists on the fEPSP (Cirone et al. 2002a; Pothecary et al. 2002; Thompson et al. 2004). Immunocytochemical and in situ hybridization evidence indicates that there is relatively little mGluR2 receptor protein or mRNA in the SC (Ohishi et al. 1993a, 1998), although this receptor is found in cortical pyramidal cells but not retinal ganglion cells (Koulen et al. 1996; Ohishi et al. 1998). By contrast there are moderate levels of expression of mGluR3 mRNA in both the SC and visual cortex but not the retina (Ohishi et al. 1993b; Koulen et al. 1996). This indicates that the source of the mGluR2 and/or mGluR3 in the SC could be either from cortical afferents or intrinsic SC cells, but is unlikely to be due to receptors located on retinal axon terminals in the SC. Our finding that LY354740 had no apparent effect on the fast component of retino-collicular transmission is consistent with these findings.
Modulation of GABAergic transmission by Group II receptors
Although LY354740 did not reduce the peak of the fEPSP response, it did produce an increase in the late phase of the fEPSP. In the presence of GABA receptor antagonists the effect of LY354740 on the fEPSP late-phase was occluded. This suggests the effect of LY354740 was via an effect on inhibitory neurotransmission. More direct evidence for this was obtained using whole-cell recording: under voltage-clamp conditions LY354740 produced a significant depression of GABAA receptor-mediated IPSCs.
In response to pairs of stimuli given under control conditions, either paired-pulse depression or facilitation of IPSCs was observed, but in all cells tested LY354740 increased the paired-pulse ratio. This increase occurred in all cells to the extent that in all cases facilitation was observed, irrespective of whether the synapses initially facilitated or depressed. This change in the paired-pulse ratio, correlated with the change in IPSC amplitude, is consistent with LY354740 acting presynaptically to suppress transmitter release. Such a conclusion would be consistent with previous studies into the regulation of GABAergic neurotransmission by mGluRs (Mitchell & Silver, 2000; Semyanov & Kullmann, 2000).
The mammalian SC contains at least three types of GABA immunoreactive neurone: horizontal, piriform and stellate neurones. These cells represent about 40% of the neurones in the SSC and are distributed throughout the SC (Mize, 1992). The three classes of GABA receptor are also relatively abundant and each plays distinct roles in collicular neuronal processing (Binns & Salt, 1997; Schmidt et al. 2001). The SC also receives GABAergic input from the substantia nigra, zona incerta, pretectum and the contralateral colliculus (Mize, 1992; Baldauf et al. 2003; Born & Schmidt, 2004). The variability of the IPSC paired-pulse behaviour under control conditions (prior to Group II agonist application) is consistent with the notion that there is a substantial variation of inhibitory circuits, but it is nevertheless noteworthy that in all cases IPSCs were affected in the same way by Group II receptor activation. Although detailed information on the subcellular localization of Group II mGluRs in the SSC is not at present available, immunohistochemical studies do indicate that mGluR2 and/or mGluR3 are located in the SSC on neuronal processes and astrocytes (Petralia et al. 1996; Cirone et al., 2002b). This is consistent with the idea of mGluR2/mGluR3 modulation of GABAergic inhibition, but it is also conceivable that there is a more complex arrangement involving interactions between glia and neurones (Parri & Crunelli, 2003).
Identity of the Group II receptor that modulates inhibition
At the concentrations tested in the present study both LY354740 and LY341495 would be expected to select for the group II receptors over other mGluR subtypes. LY354740 activates group II receptors with EC50 values in the range of 4–100 nm (Schoepp et al. 1999). These figures are comparable with the value of 33 nm determined in the present study. At much higher concentrations, not used in our study, LY354740 also has some effects at group I and group III receptors (Schoepp et al. 1999). LY341495 antagonizes the group II receptors at low nanomolar concentrations with ∼16-fold greater selectivity for group II over mGluR8 and greater selectivity over the other mGluR subtypes. LY354740 and LY341495, in common with most other currently available pharmacological agents, do not distinguish between mGluR2 and mGluR3 to any great degree, and thus do not provide information concerning which group II receptor(s) mediate the responses seen. Immunocytochemical and in situ hybridization evidence indicates a predominance of mGluR3 (Ohishi et al. 1993b) over mGluR2 (Ohishi et al. 1993a, 1998) in SC, and thus it might be expected that the actions of LY354740 reported here are mediated via the mGluR3. However, the endogenous selective mGluR3 agonist NAAG, at concentrations expected to activate mGluR3 (Wroblewska et al. 1997; Lea et al. 2001), did not affect the IPSC, thus suggesting that the depression of inhibition is via activation of a presynaptic mGluR2. Interestingly, recent work indicates that the majority of c-fos activation via group II receptors in the SC can be accounted for by activation of mGluR2 (Linden et al. 2006), and this is consistent with the lack of effect of NAAG seen in our experiments. Nevertheless, the function of mGluR3 still remains obscure and warrants further investigation.
Physiological role for group II modulation of inhibition
The superficial SC receives direct visual input from retinal axons, and this input feeds onto both excitatory and inhibitory neurons in these superficial layers. It is well known that there is a diversity of GABA interneurones in these layers (Mize, 1992; Binns, 1999), and it is likely that these serve different functions in integration of visual responses in vivo, such as centre–surround antagonism and response habituation (Binns & Salt, 1997). In this light, it is interesting that we found that mGluR2 activation affected only inhibitory responses but not excitatory optic tract input, as this allows for the possibility of selective modulation of local integrative processes.
Previous work has shown that application of LY354740 in vivo resulted in two distinct effects: either enhancing or depressing neuronal responses to visual stimuli (Cirone & Salt, 2001). The enhancement of visual responses observed in some cells can be explained by the present findings: glutamate released from retinal axon terminals upon visual stimulation could activate group II receptors and depress GABA release, thereby increasing excitability. By contrast, the mechanism underlying the decrease in firing observed in some SSC cells is less easily explained, especially as the present study shows that LY354740 produced no significant change to the fEPSP peak, suggesting that group II receptors do not directly affect excitatory transmission between retinal afferents and SSC neurones. However, it has been demonstrated that inhibitory interneurones in the SC are themselves subject to GABAergic inhibition (Schmidt et al. 2001). Activation of the mGluR2 receptor at these synapses might then be expected to disinhibit these cells leading to increased levels of inhibition experienced by other cells; thereby accounting for the decreased activity reported in vivo. It could be that the expression of mGluR varies between synapses, and that this could account for the final balance of effect.
The actions of group II mGluRs in vivo appear to be dependent on the stimulus intensity, as responses are modulated by a group II antagonist to a greater extent under conditions of high-intensity stimulation, which results in higher frequency activity of retinal axons and SSC neurones (Cirone & Salt, 2001). That mGluR activation is dependent on stimulus frequency/intensity has been observed in vitro in other brain areas where short bursts of high-frequency stimulation are necessary in order for the receptors to be activated by the synaptically released endogenous agonist (Scanziani et al. 1997; Dube & Marshall, 2000; Mitchell & Silver, 2000; Semyanov & Kullmann, 2000; Neale et al. 2001). We attempted to mimic patterns of visual responses with trains of stimuli applied at 20 and 50 Hz, in order to see if this could produce effects which were affected by the group II antagonist LY341495. However, although such protocols did reveal evidence for modulation of group II receptors by an endogenous ligand in some cells, they did not do so reliably under our experimental conditions. A possible explanation for this finding is that it is not glutamate released from retinal terminals that activates the group II receptors, but rather that the glutamate comes from another source which may be more reliably recruited under in vivo visual stimulation conditions. Nevertheless, these data are in concordance with the idea that activity-dependent activation of mGluR2 has a function in visual response processing.
Conclusions
In conclusion, the data presented here indicate that inhibitory neurotransmission in the rat superficial SC is subject to modulation by presynaptic mGluR2. The level of GABA in the SC is amongst the highest in the CNS (Mize, 1992). Thus, the action of group II mGluR described here may be an important feature of visual processing regulating the complex interplay between excitatory and inhibitory systems.
Acknowledgments
Thanks to Carina Pothecary and Hannah Thomson for technical assistance. We thank Ann Kingston (Lilly Research Laboratories, Indianapolis) for gifts of LY354740 and LY341495. This work was supported by the Wellcome Trust.
References
- Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Rev. 1999;29:83–120. doi: 10.1016/s0165-0173(98)00050-2. [DOI] [PubMed] [Google Scholar]
- Baldauf ZB, Wang XP, Wang S, Bickford ME. Pretectotectal pathway: an ultrastructural quantitative analysis in cats. J Comp Neurol. 2003;464:141–158. doi: 10.1002/cne.10792. [DOI] [PubMed] [Google Scholar]
- Binns KE. The synaptic pharmacology underlying sensory processing in the superior colliculus. Prog Neurobiol. 1999;59:129–159. doi: 10.1016/s0301-0082(98)00099-9. [DOI] [PubMed] [Google Scholar]
- Binns KE, Salt TE. Excitatory amino acid receptors participate in synaptic transmission of visual responses in the superficial layers of the cat superior colliculus. Eur J Neurosci. 1994;6:161–169. doi: 10.1111/j.1460-9568.1994.tb00257.x. [DOI] [PubMed] [Google Scholar]
- Binns KE, Salt TE. Different roles for GABAA and GABAB receptors in visual processing in the rat superior colliculus. J Physiol. 1997;504:629–639. doi: 10.1111/j.1469-7793.1997.629bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born G, Schmidt M. Inhibition of superior colliculus neurons by a GABAergic input from the pretectal nuclear complex in the rat. Eur J Neurosci. 2004;20:3404–3412. doi: 10.1111/j.1460-9568.2004.03820.x. [DOI] [PubMed] [Google Scholar]
- Bortolotto ZA, Fitzjohn SM, Collingridge GL. Roles of metabotropic glutamate receptors in LTP and LTD in the hippocampus. Curr Opin Neurobiol. 1999;9:299–304. doi: 10.1016/s0959-4388(99)80044-0. [DOI] [PubMed] [Google Scholar]
- Cirone J, Pothecary CA, Turner JP, Salt TE. Group I metabotropic glutamate receptors (mGluRs) modulate visual responses in the superficial superior colliculus of the rat. J Physiol. 2002a;541:895–903. doi: 10.1113/jphysiol.2002.016618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirone J, Salt TE. Group II and III metabotropic glutamate (mGlu) receptors contribute to different aspects of visual response processing in the superior colliculus. J Physiol. 2001;534:169–178. doi: 10.1111/j.1469-7793.2001.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirone J, Sharp C, Jeffery G, Salt TE. Distribution of metabotropic glutamate receptors in the superior colliculus of the adult rat, ferret and cat. Neuroscience. 2002b;109:779–786. doi: 10.1016/s0306-4522(01)00485-7. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:207–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Dube GR, Marshall KC. Activity-dependent activation of presynaptic metabotropic glutamate receptors in locus coeruleus. J Neurophysiol. 2000;83:1141–1149. doi: 10.1152/jn.2000.83.3.1141. [DOI] [PubMed] [Google Scholar]
- Edwards SB. The deep layers of the superior colliculus: their reticular characteristics and structural organization. In: Hobson, Brazier, editors. The Reticular Formation Revisited. New York: Raven Press; 1980. pp. 193–209. [Google Scholar]
- Gafurov B, Urazaev AK, Grossfeld RM, Lieberman EM. N-Acetylaspartylglutamate (NAAG) is the probable mediator of axon-to-glia signaling in the crayfish medial giant nerve fiber. Neuroscience. 2001;106:227–235. doi: 10.1016/s0306-4522(01)00271-8. [DOI] [PubMed] [Google Scholar]
- Harvey AR, Worthington DR. The projection from different visual cortical areas to the rat superior colliculus. J Comparative Neurol. 1990;298:281–292. doi: 10.1002/cne.902980303. [DOI] [PubMed] [Google Scholar]
- Koulen P, Malitschek B, Kuhn R, Wassle H, Brandstatter JH. Group II and Group III metabotropic glutamate receptors in the rat retina: distributions and developmental expression patterns. Eur J Neurosci. 1996;8:2177–2187. doi: 10.1111/j.1460-9568.1996.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Lea PM, Wroblewska B, Sarvey JM, Neale JH. β-NAAG rescues LTP from blockade by NAAG in rat dentate gyrus via the Type 3 metabotropic glutamate receptor. J Neurophysiol. 2001;85:1097–1106. doi: 10.1152/jn.2001.85.3.1097. [DOI] [PubMed] [Google Scholar]
- Linden AM, Baez M, Bergeron M, Schoepp DD. Effects of mGlu2 or mGlu3 receptor deletions on mGlu2/3 receptor agonist ( LY354740)-induced brain c-Fos expression: Specific roles for mGlu2 in the amygdala and subcortical nuclei, and mGlu3 in the hippocampus. Neuropharmacology. 2006;51:213–228. doi: 10.1016/j.neuropharm.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Lo FS, Mize RR. Retinal input induces three firing patterns in neurons of the superficial superior colliculus of neonatal rats. J Neurophysiol. 1999;81:954–958. doi: 10.1152/jn.1999.81.2.954. [DOI] [PubMed] [Google Scholar]
- Lund RD, Lund JS. Synaptic adjustment after deafferentation of the superior colliculus of the rat. Science. 1971;171:804–807. doi: 10.1126/science.171.3973.804. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Blackstone CD, Huganir RL, Price DL. Cellular localization of a metabotropic glutamate receptor in rat brain. Neuron. 1992;9:259–270. doi: 10.1016/0896-6273(92)90165-a. [DOI] [PubMed] [Google Scholar]
- Mitchell SJ, Silver RA. Glutamate spillover suppresses inhibition by activating presynaptic mGluRs. Nature. 2000;404:498–502. doi: 10.1038/35006649. [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Sakurai T, Okada Y. Masking effect of NMDA receptor antagonists on the formation of long-term potentiation (LTP) in superior colliculus slices from the guinea pig. Brain Res. 1990;518:166–172. doi: 10.1016/0006-8993(90)90968-h. [DOI] [PubMed] [Google Scholar]
- Mize RR. The organization of GABAergic neurons in the mammalian superior colliculus. Prog Brain Res. 1992;90:219–248. doi: 10.1016/s0079-6123(08)63616-x. [DOI] [PubMed] [Google Scholar]
- Moffett JR. Reductions in N-acetylaspartylglutamate and the 67 kDa form of glutamic acid decarboxylase immunoreactivities in the visual system of albino and pigmented rats after optic nerve transections. J Comp Neurol. 2003;458:221–239. doi: 10.1002/cne.10570. [DOI] [PubMed] [Google Scholar]
- Neale JH, Bzdega T, Wroblewska B. N-Acetylaspartylglutamate: the most abundant peptide neurotransmitter in the mammalian central nervous system. J Neurochem. 2000;75:443–452. doi: 10.1046/j.1471-4159.2000.0750443.x. [DOI] [PubMed] [Google Scholar]
- Neale SA, Garthwaite J, Batchelor AM. mGlu1 receptors mediate a post-tetanic depression at parallel fibre-Purkinje cell synapses in rat cerebellum. Eur J Neurosci. 2001;14:1313–1319. doi: 10.1046/j.0953-816x.2001.01769.x. [DOI] [PubMed] [Google Scholar]
- Neale SA, Salt TE. Group II metabotropic glutamate receptors modulate synaptic transmission in the rat superior colliculus (SC) Soc Neurosci Abs. 2003;29:700.11. Abstract. [Google Scholar]
- Neale SA, Salt TE. Modulation of GABAergic neurotransmission by a presynaptic group II metabotropic glutamate receptor (mGluR) in the rat superior colliculus (SC) Soc Neurosci Abs. 2004;30:647.7. Abstract. [Google Scholar]
- Ohishi H, Akazawa C, Shigemoto R, Nakanishi S, Mizuno N. Distributions of the mRNAs for L-2-amino-4-phosphonobutyrate-sensitive metabotropic glutamate receptors, mGluR4 and mGluR7, in the rat brain. J Comp Neurol. 1995;360:555–570. doi: 10.1002/cne.903600402. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Neki A, Mizuno N. Distribution of a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat and mouse: an immunohistochemical study with a monoclonal antibody. Neurosci Res. 1998;30:65–82. doi: 10.1016/s0168-0102(97)00120-x. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Shigemoto R, Nakanishi S, Mizuno N. Distribution of the messenger RNA for a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat. Neuroscience. 1993a;53:1009–1018. doi: 10.1016/0306-4522(93)90485-x. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR3) in the rat brain: An in situ hybridization study. J Comp Neurol. 1993b;335:252–266. doi: 10.1002/cne.903350209. [DOI] [PubMed] [Google Scholar]
- Parri HR, Crunelli V. The role of Ca2+ in the generation of spontaneous astrocytic Ca2+ oscillations. Neuroscience. 2003;120:979–992. doi: 10.1016/s0306-4522(03)00379-8. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Niedzielski AS, Wenthold RJ. The metabotropic glutamate receptors, mGluR2 and mGluR3, show unique postsynaptic, presynaptic and glial localizations. Neuroscience. 1996;71:949–976. doi: 10.1016/0306-4522(95)00533-1. [DOI] [PubMed] [Google Scholar]
- Pothecary CA, Jane DE, Salt TE. Reduction of excitatory transmission in the retino-collicular pathway via selective activation of mGlu8 receptors by DCPG. Neuropharmacology. 2002;43:231–234. doi: 10.1016/s0028-3908(02)00077-1. [DOI] [PubMed] [Google Scholar]
- Scanziani M, Salin PA, Vogt KE, Malenka RC, Nicoll RA. Use-dependent increases in glutamate concentration activate presynaptic metabotropic glutamate receptors. Nature. 1997;385:630–634. doi: 10.1038/385630a0. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Boller M, Ozen G, Hall WC. Disinhibition in rat superior colliculus mediated by GABAC receptors. J Neurosci. 2001;21:691–699. doi: 10.1523/JNEUROSCI.21-02-00691.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther. 2001;299:12–20. [PubMed] [Google Scholar]
- Schoepp DD, Jane DE, Monn JA. Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology. 1999;38:1431–1476. doi: 10.1016/s0028-3908(99)00092-1. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Kullmann DM. Modulation of GABAergic signaling among interneurons by metabotropic glutamate receptors. Neuron. 2000;25:663–672. doi: 10.1016/s0896-6273(00)81068-5. [DOI] [PubMed] [Google Scholar]
- Stein BE, Meredith MA. The Merging of the Senses. Cambridge, MA, USA: MIT Press; 1993. [Google Scholar]
- Thompson H, Neale SA, Salt TE. Activation of Group II and Group III metabotropic glutamate receptors by endogenous ligand(s) and the modulation of synaptic transmission in the superficial superior colliculus. Neuropharmacology. 2004;47:822–832. doi: 10.1016/j.neuropharm.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Tsai G, Stauch BL, Vornov JJ, Deshpande JK, Coyle JT. Selective release of N-acetylaspartylglutamate from rat optic nerve terminals in vivo. Brain Res. 1990;518:313–316. doi: 10.1016/0006-8993(90)90989-o. [DOI] [PubMed] [Google Scholar]
- Turner JP, Sauve Y, Varela-Rodriguez C, Lund RD, Salt TE. Recruitment of local excitatory circuits in the superior colliculus following deafferentation and the regeneration of retinocollicular inputs. Eur J Neurosci. 2005;22:1643–1654. doi: 10.1111/j.1460-9568.2005.04359.x. [DOI] [PubMed] [Google Scholar]
- White A-M, Kylanpaa RA, Christie LA, McIntosh SJ, Irving AJ, Platt B. Presynaptic group I metabotropic glutamate receptors modulate synaptic transmission in the rat superior colliculus via 4-AP sensitive K+ channels. Br J Pharmacol. 2003;140:1421–1433. doi: 10.1038/sj.bjp.0705570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wroblewska B, Wroblewski JT, Pshenichkin S, Surin A, Sullivan SE, Neale JH. N-Acetylaspartylglutamate selectively activates mGluR3 receptors in transfected cells. J Neurochem. 1997;69:174–181. doi: 10.1046/j.1471-4159.1997.69010174.x. [DOI] [PubMed] [Google Scholar]