Abstract
Desensitization of the β-adrenoreceptors (β-AR) may contribute to a post-exercise reduction in left ventricular (LV) function. However, attenuation of the chronotropic and inotropic responses to a β-AR agonist may depend upon alterations in parasympathetic tone. Furthermore, changes in cardiac output Q˙ and LV diastolic function in response to a β-AR agonist, pre- to post-prolonged exercise, remain unclear. Seven trained males (mean ± s.d., age 27 ± 6 years) performed 4 h of ergometer rowing. Peak heart rate (HR) and LV systolic and diastolic functional responses to incremental isoproterenol (isoprenaline) infusion (2, 4 and 6 μg kg min−1) were assessed after vagal blockade (glycopyrrolate, 1.2 mg). LV systolic function was assessed by the pressure/volume ratio (systolic blood pressure/end systolic volume) and Q˙, whilst diastolic function was evaluated as peak early and late transmitral filling velocities. Following exercise, the pressure/volume ratio decreased by 25% (P < 0.05), whereas Q˙ was unchanged (P > 0.05). The early/late filling ratio was reduced by 36% after exercise, due to an elevation in late LV filling (P < 0.01). The increase in HR response to isoproterenol infusion was blunted post-exercise at both 4 and 6 μg kg min−1 (127 ± 7 and 132 ± 6 beats min−1) compared with pre-exercise (138 ± 8 and 141 ± 12 beats min−1, P < 0.05). Additionally, the pressure/volume ratio and Q˙ were blunted post-exercise in response to isoproterenol (P < 0.05). In contrast, diastolic function was similar before and after exercise during isoproterenol infusion (P > 0.05). Desensitization of the β-AR contributes to an attenuated left ventricular systolic but not diastolic function following prolonged exercise.
Left ventricular (LV) systolic and diastolic function are reduced following prolonged exercise (Seals et al. 1988; Rifai et al. 1999; Haykowsky et al. 2001; Shave et al. 2004; Whyte et al. 2005; George et al. 2005; Middleton et al. 2006; Neilan et al. 2006); however, the underlying mechanism(s) remain elusive. Proposed mechanisms include elevated circulating free fatty acids resulting in a decreased inotropic state (Dawson et al. 2003), myocyte injury due to increased free radical production (Starnes & Bowles, 1995), transient myocardial ischaemia (Siegel, 1997) and β-adrenergic receptor (β-AR) desensitization (Eysmann et al. 1996).
Following strenuous exercise β-AR desensitization may be provoked consequent to the prolonged elevation in circulating catecholamines (Malarkey et al. 1993). A reduced chronotropic response to the β-AR agonist isoproterenol has been demonstrated following prolonged exercise in both untrained (Eysmann et al. 1996) and trained subjects (Douglas et al. 1998). In addition, Welsh et al. (2005) demonstrated a reduction in chronotropic response and LV contractility in response to continuous dobutamine infusion subsequent to a half ironman triathlon. However, the response of cardiac output (Q˙) to a β-AR agonist before and after prolonged exercise has not been measured; moreover, the potential post-exercise alteration in parasympathetic tone (Savin et al. 1982) has not been addressed. Thus, post-exercise differences in heart rate (HR) response to a β-AR agonist could result from altered vagal tone rather than reduced β-AR sensitivity. Furthermore, the lusitopic response of the LV to a β-AR agonist, after prolonged exercise, remains unknown.
Rowing poses a substantial haemodynamic stress on the LV as it is associated with volume overload and large acute increases in arterial pressure, due to the performance of a valsalva-like manoeuvre at the catch of each stroke and the large upper body muscle mass involved (Clifford et al. 1994; Pott et al. 1997; Nielsen et al. 1999). Consequently, this study examined (a) changes in LV function following a 4 h rowing bout and (b) the cardiovascular response to isoproterenol infusion, after parasympathetic inhibition, prior to and following prolonged ergometer rowing. We hypothesized that LV diastolic and systolic function would be attenuated following a prolonged bout of rowing at rest and in response to β-AR stimulation, after vagal blockade.
Methods
After the study had been approved by the local ethics committee, seven healthy trained male rowers (mean ± s.d., age 27 ± 6 years, height 1.88 ± 0.08 m, body mass 84.3 ± 9.0 kg and maximal oxygen uptake (V˙O2max) 57.3 ± 6.3 ml kg min−1) provided written informed consent to participate in the study. All procedures employed within the study conformed to the standards set by the Declaration of Helsinki.
Design
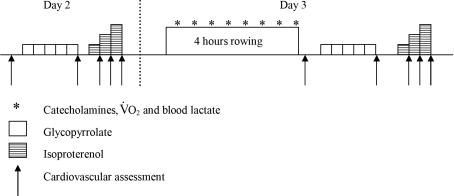
Each participant visited the laboratory on three occasions. On day 1, participants performed incremental exercise to volitional exhaustion on a wind braked rowing ergometer (Concept II, Morrisville, VT, USA) to determine V˙O2max and the workload associated with lactate threshold, defined as the first inflection in blood lactate. Baseline cardiovascular assessment was completed on day 2, including an evaluation of the cardiovascular response to complete parasympathetic blockade (glycopyrrolate; Robinul, Meda, Denmark) and β-AR stimulation (isoproterenol; Danish Pharmaceutical Company) (Fig. 1). On day 3 subjects performed a 4 h bout of ergometer rowing followed by a post-exercise cardiovascular assessment within 30 min of cessation of exercise, immediately followed by a repeat evaluation of the cardiovascular response to both glycopyrrolate and isoproterenol. Pre- and post-exercise evaluations were not made on the same day, as glycopyrrolate stimulates an inhibited sweat response.
Figure 1.
Outline of procedures for experimental days 2 and 3
Prolonged exercise
Four hours of continuous exercise was performed on the rowing ergometer at a work rate corresponding to 10–15% below the individuals lactate threshold, at a cadence of 22–24 strokes min−1. Oxygen uptake (V˙O2) was measured before and at 30 min intervals throughout exercise via an online gas analysis system (CPX/D; MedGraphics, St Paul, MN, USA). HR was monitored via telemetry (Polar Team system, Kemple, Finland), and blood samples were obtained before, during and after exercise (Fig. 1).
Blood sampling
Venous blood samples were obtained before, and at 30 min intervals during and immediately after exercise, for the analyses of lactate, adrenaline and noradrenaline. Blood samples for the analysis of lactate were obtained via a catheter situated in the superior vena cava, using heparinized syringes (QS50; Radiometer, Copenhagen, Denmark), stored in ice-water and analysed within 15 min (ABL725; Radiometer). Blood samples for catecholamine analyses were drawn into EDTA vacutainers (BD Diagnostics, Oxford, UK) and centrifuged; plasma was subsequently drawn off and stored at −80°C. Plasma content of catecholamines was determined using an ELISA (Catcombi; IBL, Hamburg, Germany). Intra-assay coefficients of variation (CVs), for the measurement of adrenaline and noradrenaline, were <7% and 7% at ranges of 51–837 and 560–12380 pg ml−1, respectively. Inter-assay CVs for adrenaline and noradrenaline were <4% and 13% at ranges of 106–1064 and 569–1945 pg ml−1, respectively. The limits of detection for adrenaline and noradrenaline were 10 and 20 pg ml−1, respectively.
Cardiovascular assessment
On day 2, cardiovascular assessments were conducted at rest, following bolus administration of glycopyrrolate, and following each incremental infusion of isoproterenol. On day 3, cardiovascular assessments were completed immediately post-exercise, following glycopyrrolate infusion, and following each incremental infusion of isoproterenol. Cardiovascular assessments included an echocardiographic examination with continuous blood pressure recordings and HR measurement.
Echocardiographic analysis was completed using an Esoate GPX (Italy) with a 2.5 MHz phased array transducer and simultaneous ECG recordings. A single experienced sonographer obtained all measurements with participants positioned in the left lateral decubitus position. Images were obtained in accordance to the guidelines established by the American Society of Echocardiography for M mode and two-dimensional (2D) echocardiography (Lang et al. 2005).
Systolic function was assessed via the systolic pressure/volume ratio (systolic blood pressure/end systolic volume; Robotham et al. 1991), ejection fraction (EF), stroke volume (SV) and Q˙. Diastolic function was assessed via pulsed-wave Doppler recordings of transmitral inflow obtained from the apical four-chamber view. Peak velocities of early (E) and late (A) LV filling were measured and the E/A ratio was calculated. In addition, the flow propagation velocity (Vp) was calculated via colour M-mode echocardiography in the apical four-chamber view.
Echocardiographic indicators of LV preload (LV internal diameter during diastole; LVIDd) and afterload (LV meridonial wall stress; LVMWS) were obtained during each assessment. Preload to the heart, pre- and post-exercise, was also evaluated by central venous pressure (CVP) whilst the subjects were sitting on the rowing ergometer. A catheter (1.7 mm i.d.) was inserted into the brachial vein and advanced to the superior vena cava. The catheter was connected to a Bently pressure transducer (Uden, The Netherlands) positioned at the level of the heart, monitored on a personal computer, and expressed as an average over 5 min. In addition, arterial pressure (BP) was measured throughout each assessment using Finapres model 5 (TNO-BMI, Amsterdam, The Netherlands) prior to exercise, and via a catheter in the radial artery of the non-dominant arm (1.1 mm, 20 gauge) during the post-exercise examination. The two BP systems were employed so as to minimize trauma to the participants. When using the Finapres, the cuff was applied to the midphalanx line of the middle finger of the nondominant arm and placed at the level of the heart (Harms et al. 1999). The radial artery catheter was connected to the Bently pressure transducer and connected to a pressure monitoring system (model M12755A; Hewlett-Packard). The radial pressure wave was downloaded onto a personal computer and analysed offline using data acquisition software (WinDaq194; DATAQ instruments, Ohio, USA). Beat-to-beat mean arterial pressure (MAP) was calculated from the radial and finger pulse pressure waves by model flow analysis and systemic vascular resistance (SVR) was calculated as MAP/Q˙. Model flow computes an aortic waveform based on nonlinear pressure–volume, pressure–compliance and pressure–characteristic impedance equations, incorporating age, sex, height and body mass (Wessling et al. 1993). Although arterial pressures were derived via Finapres and intra-arterial methods, the measurement error between these methods is small and both detect BP accurately, e.g. during orthostatic stress (Harms et al. 1999). The relationship between these methods in estimating BP ranges from a correlation coefficient of 0.82–0.97 (Stokes et al. 1991; Gabriel et al. 1992).
Parasympathetic inhibition
The cardiovascular response to parasympathetic inhibition was assessed at rest on day 2 and immediately post-exercise on day 3. Vagal blockade was established by intravenous bolus administration of glycopyrrolate via the indwelling cannula in the brachial vein. Before glycopyrrolate was administered, the subjects were encouraged to micturate, in order to avoid an increased sympathetic outflow associated with a full bladder (Weaver, 1985). Participants received six boluses of 0.2 mg of glycopyrrolate, infused at 5 min intervals. Complete parasympathetic blockade was taken at the point where HR increased to 100 ± 5 beats min−1, or would increase no further (Katona et al. 1982). Glycopyrrolate was chosen over atropine because the highly polar quaternary group limits the passage of glycopyrrolate through the blood–brain barrier and it is therefore not related to hallucinations or other central nervous complaints (Simpson et al. 1987).
β-AR stimulation
Immediately following parasympathetic blockade, the cardiovascular response to incremental isoproterenol infusion was evaluated. Isoproterenol was administered continuously via an infusion pump in increasing doses of 2, 4 and 6 μg kg min−1 at 7 min intervals. Repeat echocardiographic assessments were completed 3.5 min into each stage of isoproterenol infusion. Peak HR was calculated offline for the last 3.5 min of each stage of the infusion.
Data analysis
Pre- and post-exercise HR, body mass, arterial pressure, CVP and echocardiographic indices of LV function were analysed using a one-way repeated measures analysis of variance (ANOVA). Differences in arterial pressure, HR and LV systolic and diastolic functional responses to glycopyrrolate and isoproterenol infusions were analysed using two-way repeated measures ANOVA with a Bonferroni adjustment for pairwise comparisons. Relationships between changes in LV systolic and diastolic function to changes in LV loading parameters were determined using Pearson's product-moment analysis. The critical alpha level was set at 0.05 and data were analysed using SPSS software (version 13.0; Illinois, USA). Data are reported as means ± s.d.
Results
In-exercise data
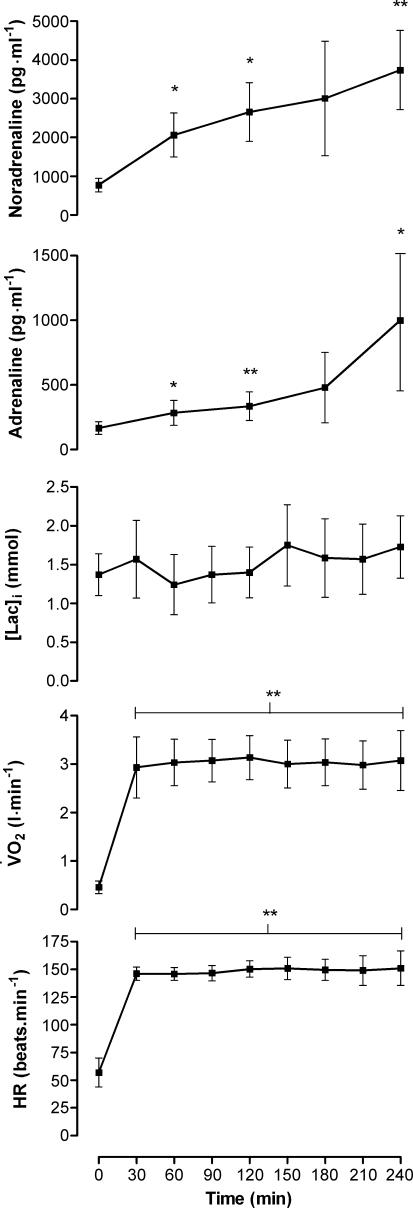
All participants completed 4 h of ergometer rowing. Mean work rate and HR were 160 ± 38 W and 143 ± 8 beats · min−1, respectively. Only a small and non-significant increase in HR was demonstrated during exercise, and blood lactate remained stable throughout exercise (Fig. 2). In contrast, during exercise, circulating catecholamines continued to increase throughout exercise (P < 0.05).
Figure 2. Heart rate (HR), oxygen uptakeV˙O2, blood lactate [Lac]i, adrenaline and noradrenaline during exercise.
Data are means ±s.d. Different from baseline, *P < 0.05, **P < 0.01.
Cardiovascular function
Echocardiographic assessment of LV function pre- to post-exercise demonstrated a reduction in both systolic and diastolic function (Table 1). Systolic function, measured via the pressure/volume ratio, EF and SV decreased by 25, 17 and 15%, respectively (P < 0.01), whereas Q˙ remained similar pre- to post-exercise. Global LV diastolic function, expressed as the E/A ratio, was reduced by 36% following exercise, primarily due to an elevation in late LV filling (P < 0.01). There was also a trend towards a decrease in early Vp (P = 0.09).
Table 1.
Indices of left ventricular loading and function pre- to post-exercise, prior to and following vagal blockade
| Without vagal blockade | With vagal blockade | |||
|---|---|---|---|---|
| Parameter | Pre-exercise | Post-exercise | Pre-exercise | Post-exercise |
| SBP/ESV | 2.8 ± 0.6 | 2.1 ± 0.5* | 3.5 ± 0.6 | 2.5 ± 0.7† |
| EF (%) | 65 ± 4 | 58 ± 2** | 64 ± 4 | 60 ± 4 |
| Q˙ (l min−1) | 5.1 ± 0.6 | 5.2 ± 0.6 | 7.5 ± 1.2 | 6.7 ± 1.1 |
| SV (ml) | 93 ± 16 | 79 ± 13* | 77 ± 9 | 71 ± 10 |
| E/A | 1.9 ± 0.4 | 1.2 ± 0.3** | 1.1 ± 0.3 | 1.0 ± 0.3 |
| E (m s−1) | 0.79 ± 0.18 | 0.72 ± 0.14 | 0.70 ± 0.18 | 0.64 ± 0.16 |
| A (m s−1) | 0.42 ± 0.06 | 0.60 ± 0.10** | 0.69 ± 0.10 | 0.68 ± 0.06 |
| Vp (cm s−1) | 52.1 ± 4.2 | 46.1 ± 7.8 | 53.5 ± 3.9 | 43.2 ± 4.0† |
| HR (beats min−1) | 57 ± 13 | 66 ± 10* | 97 ± 6 | 95 ± 7 |
| LVIDd (cm) | 5.27 ± 0.58 | 5.32 ± 0.59 | 5.08 ± 0.56 | 5.18 ± 0.65 |
| MAP (mmHg) | 84 ± 21 | 87 ± 10 | 93 ± 14 | 93 ± 9 |
| SVR (mmHg l min−1) | 17 ± 5 | 17 ± 2 | 13 ± 2 | 14 ± 2 |
| LVMWS (g cm−1) | 126 ± 27 | 118 ± 23 | 147 ± 40 | 126 ± 14 |
SBP/ESV, systolic blood pressure to end systolic volume ratio; EF, ejection fraction; Q˙, cardiac output; SV, stroke volume; E/A, ratio of early to late LV filling; E, peak velocity of early transmitral filling; A, peak velocity of late transmitral filling; Vp, flow propagation velocity of early transmitral filling; HR, heart rate; LVIDd, LV internal diameter in diastole; MAP, mean arterial pressure; SVR, systemic vascular resistance; LVMWS, LV meridonial wall stress. Data are means ±s.d. Different from pre-exercise without vagal blockade
P < 0.05
P < 0.01; different from pre-exercise with vagal blockade
P < 0.05.
Preload to the heart was similar pre- to post-exercise as assessed via LVIDd and CVP (3.2 ± 0.9 versus 2.9 ± 1.2 mmHg) as was LVMWS (Table 1). Also MAP and SVR remained unchanged pre- to post-exercise, whereas HR was elevated following the rowing (P < 0.05). Changes in the pressure/volume ratio, EF and SV were unrelated to the minor alterations in CVP and LVIDd (Table 2). Alterations in EF were unrelated to changes LVMWS, whereas the pressure/volume ratio correlated with LVMWS (r2 = 0.87 P < 0.05). Attenuation of EF, SV and the pressure/volume ratio were unrelated to changes in pre- to post-exercise HR. The decline in post-exercise EF was related to the increase in adrenaline from rest to the end of exercise (r2 = 0.96, P < 0.05). Alterations in diastolic function were independent of changes in pre- to post-exercise preload, afterload or HR.
Table 2.
Correlation coefficients between altered left ventricular loading parameters and indices of left ventricular function before and after exercise, with and without vagal blockade
| Pre- vs post-exercise without vagal blockade | Pre- vs post-exercise with vagal blockade | ||||||
|---|---|---|---|---|---|---|---|
| Parameter | LVIDd | CVP | LVMWS | HR | LVIDd | LVMWS | HR |
| SBP/ESV | 0.13 | 0.21 | 0.94* | −0.20 | −0.35 | 0.81* | 0.13 |
| EF | −0.43 | −0.68 | 0.28 | −0.69 | 0.68 | 0.28 | −0.86 |
| Q˙ | −0.10 | 0.15 | −0.57 | 0.41 | 0.39 | 0.55 | −0.82 |
| SV | 0.07 | −0.30 | 0.51 | −0.72 | 0.35 | 0.87* | 0.35 |
| E/A | −0.49 | −0.56 | −0.61 | −0.02 | −0.01 | 0.21 | −0.69 |
| Vp | 0.43 | 0.27 | −0.09 | −0.67 | 0.17 | 0.47 | −0.50 |
Abbreviations are as in Table 1.
P < 0.05.
Parasympathetic inhibition
Following vagal blockade, HR increased and was not different pre- to post-exercise (Table 1). Glycopyrrolate infusion led to a decrease in SV both prior to and after exercise (17 and 10%, respectively, both P < 0.01). Conversely, Q˙ increased by 44% (pre-exercise) and 29% (post-exercise, P < 0.01). Following parasympathetic inhibition, the impact of prolonged exercise on LV function was blunted. Exercise-induced reductions in EF, SV and Q˙ were reduced, with only the pre- to post-exercise difference in the pressure/volume ratio remaining statistically significant following vagal blockade. Prior to exercise, the E/A ratio was reduced (1.9–1.2, P < 0.05) with parasympathetic inhibition, whereas the decrease observed post-exercise (1.1–1.0) was not statistically significant. In contrast, Vp was not altered by vagal blockade before exercise (52–46 cm s−1), but was reduced with glycopyrrolate infusion after exercise (54–43 cm s−1, P < 0.05). Preload, afterload and MAP were similar pre- to post-exercise, following vagal blockade. However, alterations in the pressure/volume ratio and SV were related to the non-significant changes in LVMWS (Table 2).
β-AR stimulation
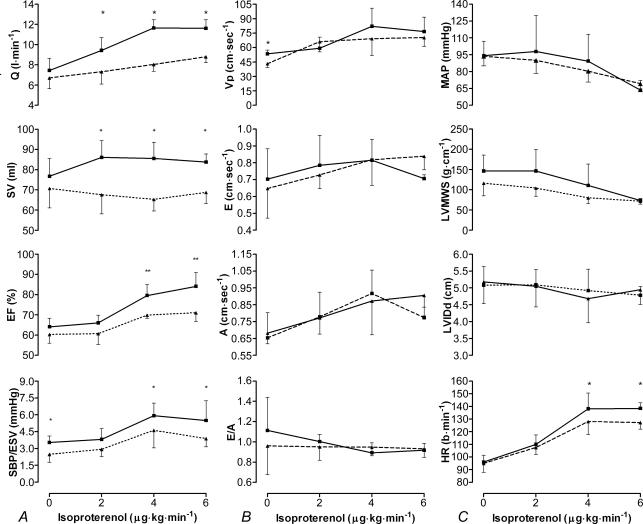
Following parasympathetic inhibition, the HR response to incremental isoproterenol infusion was blunted post-exercise at both 4 and 6 μg kg min−1 by 11 and 9 beats · min−1, respectively (P < 0.05, Fig. 3C). The left ventricular inotropic response to isoproterenol was also blunted following prolonged exercise (Fig. 3A): the pressure/volume ratio and EF were reduced post-exercise at 4 and 6 μg kg min−1 of isoproterenol. Similarly SV and Q˙ were diminished following exercise at all levels of infusion (P < 0.05). The lusitropic response of the LV to isoproterenol was, however, similar before and after exercise: E, A, E/A and Vp followed a similar pattern of change pre- to post-exercise (Fig. 3B).
Figure 3. Left ventricular response to incremental isoproterenol infusion pre- and post-exercise.
Inotropic (A), lusitropic (B) and chronotropic/loading (C) response to isoproterenol pre- (continuous line) and post-exercise (dashed line). Data are means ±s.d., see Table 1 for abbreviations. Different from pre-exercise *P < 0.05, **P < 0.01.
Preload, afterload and MAP before and after exercise decreased at a similar rate throughout the infusion (Fig. 3C). Alterations in pre- to post-exercise EF, SV and Q˙ were independent of differences in HR, preload and afterload at all levels of infusion.
Discussion
The findings of this study provide unique evidence of (1) reduced left ventricular systolic and diastolic function immediately following a 4 h bout of rowing, and (2) blunted post-exercise left ventricular chronotropic and inotropic response to isoproterenol infusion following vagal blockade, with no significant alterations in diastolic function.
Pre- and post-exercise LV function
The present study demonstrates a decrease in both systolic and diastolic function after 4 h continuous ergometer rowing. Studies demonstrating reductions in LV function subsequent to 4 h exercise, report alterations in diastolic function only (Lucia et al. 1999; McGavock et al. 2002; George et al. 2004; Dawson et al. 2005). Four hours of rowing appears to present a haemodynamic stress on the LV that leads to a reduction in both diastolic and systolic function, similar to that demonstrated following an ironman triathlon (Rifai et al. 1999). The high arterial pressures generated at the catch of each stroke (Clifford et al. 1994; Pott et al. 1997) impose a cardiac workload that results in a reduced systolic function following shorter duration exercise than reported previously (McGavock et al. 2002).
Alterations in systolic function were characterized by a reduction in the pressure/volume ratio representing a decline in contractility. Moreover, EF and SV were decreased following exercise. Reduced diastolic function, following prolonged exercise, was evident by a decrease in the E/A ratio, independent of changes in pre- to post-exercise HR. The current data support findings that the E/A ratio is reduced largely due to an increase in peak late LV filling rather than alterations in early LV filling (Dawson et al. 2005; George et al. 2005). The minor alteration in peak early LV filling velocity was mirrored by a 12% decrease in Vp of early transmitral flow, which is comparable to the 11% decrease in Vp following a marathon (Middleton et al. 2006).
The dependence of a reduction in LV function, following prolonged exercise, upon alterations in LV loading remains unclear. The CVP and LVIDd were unchanged pre- to post-exercise, implying that preload was unaltered. However, alterations in the pre- to post-exercise pressure/volume ratio were related to the small decline in LVMWS, which may be due to the shared use of SBP in the calculation of each variable. Therefore alterations in LV function appear not to be related to minor changes in preload and afterload following exercise. A limitation of the present study was the use of a single end-systolic measure of elastance, future studies may wish to assess elastance across various ventricular volumes, in order to further understand the influence of prolonged exercise upon cardiac function.
Parasympathetic inhibition
Vagal inhibition caused pre- and post-exercise HR to increase to 97 and 95 beats · min−1, respectively. Katona et al. (1982) report a similar increase in HR following complete vagal inhibition in trained participants. Vagal blockade had little effect on the pre- and post-exercise pressure/volume ratio and EF. The SV decreased, whilst Q˙ increased both before and after exercise in response to glycopyrrolate; this may be attributed to the increase in HR. Conversely, Boushel et al. (2001) demonstrated a 50% decrease in SV and an unchanged Q˙ in untrained individuals at rest following glycopyrrolate administration. Vagal blockade resulted in a reduction in pre-exercise E/A, supporting data from Spina et al. (1998); this may be attributed, in part, to the increase in HR and consequently elevation in late LV filling (Solomon et al. 1999). Flow propagation velocity of early LV filling was unaffected by vagal blockade both before and after exercise, suggesting that Vp may be less influenced by HR than E/A, although this is controversial (Ogawa et al. 2005).
Echocardiography following prolonged exercise has not been able to discount whether differences in pre- to post-exercise systolic and diastolic function are due to an increase HR (George et al. 2005; Middleton et al. 2006). The current data demonstrate that whilst some of the exercise-induced alteration in LV function may be related to an elevated HR post-exercise, these changes cannot account for all perturbations in LV systolic and diastolic function. Specifically, the pressure/volume ratio was persistently reduced post-exercise, irrespective of vagal inhibition. Following parasympathetic blockade, the difference in pre- to post-exercise E/A disappeared, whilst Vp became reduced, which suggests impaired passive filling, regardless of changes in HR.
β-AR stimulation
Elevated circulating catecholamines result in a desensitization and down regulation of the β-AR, forming a basis for the pathophysiology of chronic heart failure (Dzirmi, 1999). Following complete vagal blockade, the cardiac chronotropic response to incremental isoproterenol infusion was blunted after prolonged exercise. The reduced chronotropic response may therefore be a function of decreased sensitivity of the β-AR, rather than an alteration in post-exercise parasympathetic tone. Clinical and pathological studies demonstrate that following withdrawal of prolonged administration of pharmacological inotropic agonists, cardiac dysfunction may occur (Xiao et al. 2004). A similar mechanism may be apparent following prolonged exercise resulting in β-AR desensitization and a subsequent reduction in LV function.
During isoproterenol infusion, there was a reduction in the inotropic response of the LV. Ejection fraction, SV and, more importantly, Q˙ were reduced at all levels of isoproterenol infusion. Contractility, as assessed by the pressure/volume ratio, was also reduced post-exercise at the highest two doses of isoproterenol. This concurs with Welsh et al. (2005), who demonstrated a reduction in pressure/volume ratio during dobutamine infusion after a half ironman triathlon. The elevation in circulating adrenaline at the end of exercise was related to the alteration in pre- to post-exercise EF, before glycopyrrolate infusion. Although this relationship does not prove cause and effect, it does support a role of increased circulating catecholamines in the genesis of altered LV systolic function following prolonged exercise.
The LV diastolic function was not altered in response to incremental isoproterenol infusion pre- to post-exercise. The E/A ratio and Vp were similar before and after exercise at all levels of isoproterenol infusion, suggesting that the reduction in diastolic function evident immediately following prolonged exercise is not related to β-AR desensitization. Wang et al. (2004) demonstrated that alterations in myocyte relaxation rate in response to sustained β1-AR stimulation were independent of calcium/calmodulin-dependent protein kinase II, an enzyme involved in β-AR desensitization and cell apoptosis. Diastolic dysfunction is associated with accumulation of intracellular calcium (Ca2+) due to a delay in Ca2+ resequestration (Kass et al. 2004). Immediately following prolonged exercise, alterations in intracellular pH and inorganic phosphates may cause a transient delay in Ca2+ re-uptake by the sacroplasmic reticulum (Dawson et al. 2003) and interfere with cross-bridge uncoupling (Kass et al. 2004) and, consequently, impaired diastolic function. Alterations in right ventricular pressure or pericardial constraint may also have impacted upon the E/A ratio post-exercise, but are presently not supported with empirical data.
β-AR desensitization following prolonged exercise
Cardiac β-AR subtypes, β1 and β2, activate different signalling pathways within the myocyte (Xiao et al. 2004). Over stimulation of the β1-AR, coupled to the stimulatory G protein coupled receptor (Gs), increases cAMP mediated phosphorylation of L-type Ca2+ channels and may lead to apoptosis of the myocyte (Rohrer et al. 1999; Xiao et al. 2004). Conversely, stimulation of the β2-AR, coupled to both the Gs and the inhibitory G protein coupled receptor (Gi), has an antiapoptotic effect and protects the myocyte against injury (Xiao et al. 2004; Patterson et al. 2004). Desensitization of the β1-AR following prolonged exercise could therefore limit breakdown of the myocyte. However, the present study cannot distinguish between β-AR subtypes, as isoproterenol is both a β1 and β2 agonist. Concomitant to a reduction in LV function following prolonged exercise, a transient elevation in circulating markers of cardiac damage (cardiac troponin T and I; cTnT and cTnI) has been demonstrated (Rifai et al. 1999; Shave et al. 2005; Whyte et al. 2005) likely to be indicative of reversible cardiomyocyte injury. The mechanism underlying increases in circulating cardiac-specific proteins is unknown; however, stimulation of an apoptotic pathway due to elevated catecholamines may be involved in the increase in cTnT and cTnI during prolonged exercise and β-AR desensitization may limit the extent to which these contractile proteins are released.
Conclusion
Prolonged ergometer rowing resulted in a reduction in both LV systolic and diastolic function. Moreover, LV chronotropic and inotropic responses to isoproterenol were blunted as a result of prolonged exercise. Consequently, increases in Q˙ response to combined β1 and β2 stimulation were limited following exercise even when vagal tone was blocked. Thus, β-AR desensitization may explain attenuated LV systolic but not diastolic function following prolonged exercise.
References
- Boushel R, Calbet JAL, Rådegran G, Søndergaard H, Wagner PD, Saltin B. Parasympathetic neural activity accounts for lowering of exercise heart rate at high altitude. Circulation. 2001;104:1785–1791. doi: 10.1161/hc4001.097040. [DOI] [PubMed] [Google Scholar]
- Clifford P, Hanel B, Secher NH. Arterial blood pressure response to rowing. Med Sci Sports Exerc. 1994;26:715–719. doi: 10.1249/00005768-199406000-00010. [DOI] [PubMed] [Google Scholar]
- Dawson E, George K, Shave R, Whyte G, Ball D. Does the human heart fatigue subsequent to prolonged exercise? Sports Med. 2003;33:365–380. doi: 10.2165/00007256-200333050-00003. [DOI] [PubMed] [Google Scholar]
- Dawson E, Shave R, George K, Whyte G, Ball D, Gaze D, Collinson P. Cardiac drift during prolonged exercise with echocardiographic evidence of reduced diastolic function of heart. Eur J Appl Physiol. 2005;94:305–309. doi: 10.1007/s00421-005-1318-3. [DOI] [PubMed] [Google Scholar]
- Douglas PS, O'Toole ML, Katz SE. Prolonged exercise alters cardiac chronotropic responsiveness in endurance athletes. J Sports Med Phys Fitness. 1998;38:158–163. [PubMed] [Google Scholar]
- Dzirmi N. Regulation of β-adrenoreceptor signalling in cardiac function and disease. Pharmacol Rev. 1999;51:465–501. [PubMed] [Google Scholar]
- Eysmann SB, Gervino E, Vatner DE, Katz SE, Decker L, Douglas PS. Prolonged exercise alters β-adrenergic responsiveness in healthy sedentary humans. J Appl Physiol. 1996;80:616–622. doi: 10.1152/jappl.1996.80.2.616. [DOI] [PubMed] [Google Scholar]
- Gabriel A, Lindblad L, Angleryd C. Non-invasive vs. invasive beat-to-beat monitoring of blood pressure. Clin Physiol. 1992;12:229–235. doi: 10.1111/j.1475-097x.1992.tb00309.x. [DOI] [PubMed] [Google Scholar]
- George K, Oxborough D, Forster J, Whyte G, Shave R, Dawson E, Stephenson C, Dugdill L, Edwards B, Gaze D. Mitral annular myocardial velocity assessment of segmental left ventricular diastolic function after prolonged exercise in humans. J Physiol. 2005;569:305–313. doi: 10.1113/jphysiol.2005.095588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George K, Whyte G, Stephenson C, Shave R, Dawson E, Edwards B, Gaze D, Collinson P. Postexercise left ventricular function and cTnT in recreational marathon runners. Med Sci Sports Exerc. 2004;36:1709–1715. doi: 10.1249/01.mss.0000142408.05337.49. [DOI] [PubMed] [Google Scholar]
- Harms PM, Wesseling KH, Pott F, Jenstrup M, Van Goudoever J, Secher NH, Van Leishout JJ. Continuous stroke volume monitoring by modelling flow from non-invasive measurement of arterial pressure in humans under orthostatic stress. Clin Sci. 1999;97:291–301. [PubMed] [Google Scholar]
- Haykowsky M, Welsh R, Humen D, Waburton D, Taylor D. Impaired left ventricular systolic function after a half-ironman race. Can J Cardiol. 2001;17:687–690. [PubMed] [Google Scholar]
- Kass DA, Bronzwaer JGF, Paulus WJ. What mechanisms underlie diastolic function in heart failure? Circ Res. 2004;94:1533–1542. doi: 10.1161/01.RES.0000129254.25507.d6. [DOI] [PubMed] [Google Scholar]
- Katona PG, McLean M, Dighto D, Guz A. Sympathetic and parasympathetic cardiac control in athletes and non-athletes at rest. J Appl Physiol. 1982;52:1652–1657. doi: 10.1152/jappl.1982.52.6.1652. [DOI] [PubMed] [Google Scholar]
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Lucia A, Serratosa L, Sabrodio A, Pardo J, Boraita A, Moran M, Bandres F, Mefias A, Chicharro JL. Short-term effects of marathon running: no evidence of cardiac dysfunction. Med Sci Sports Exerc. 1999;31:1414–1421. doi: 10.1097/00005768-199910000-00009. [DOI] [PubMed] [Google Scholar]
- McGavock JM, Warburton D, Taylor D, Welsh R, Quinney H, Haykowsky MJ. The effects of prolonged strenuous exercise on left ventricular function: a brief review. Heart Lung. 2002;31:279–292. doi: 10.1067/mhl.2002.126106. [DOI] [PubMed] [Google Scholar]
- Malarkey WB, Hall JC, Rice RR, Kotur MS, O'Toole ML, Douglas PS. The influence of age on endocrine responses to ulra-endurance stress. J Gerontol. 1993;48:M134–M139. doi: 10.1093/geronj/48.4.m134. [DOI] [PubMed] [Google Scholar]
- Middleton N, Shave R, George K, Whyte G, Forster J, Oxborough D, Gaze D, Collinson P. Novel application of flow propagation velocity and ischaemia modified albumin in analysis of post-exercise cardiac function. Exp Physiol. 2006;91:511–519. doi: 10.1113/expphysiol.2005.032631. [DOI] [PubMed] [Google Scholar]
- Neilan TG, Yoerger DM, Douglas PS, Marshall JE, Hapern EF, Lawlor D, Picard MH, Wood MJ. Persistant and reversible cardiac dysfunction among amateur marathon runners. Eur Heart J. 2006;27:1079–1084. doi: 10.1093/eurheartj/ehi813. [DOI] [PubMed] [Google Scholar]
- Nielsen HB, Boushel R, Madsen P, Secher NH. Cerebral desaturation during exercise reversed by O2 supplementation. Am J Physiol Heart Circ Physiol. 1999;277:H1045–H1052. doi: 10.1152/ajpheart.1999.277.3.H1045. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Scotten LN, Walker DK, Yoganathan AP, Bess RL, Nordstrom CH, Gardin JM. What parameters affect left ventricular diastolic flow propagation velocity? In vitro studies using colour m-mode Doppler echocardiography. Cardiovasc Ultrasound. 2005;3:24. doi: 10.1186/1476-7120-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson AJ, Zhu W, Chow A, Agrawal R, Kosek J, Xiao RP, Kobilka B. Protecting the myocardium: a role for the β2 adrenergic receptor in the heart. Crit Care Med. 2004;32:1041–1048. doi: 10.1097/01.ccm.0000120049.43113.90. [DOI] [PubMed] [Google Scholar]
- Pott F, Knudsen L, Nowak M, Nielsen HB, Hanel B, Secher NH. Middle cerebral artery blood velocity during rowing. Acta Physiol Scand. 1997;160:251–255. doi: 10.1046/j.1365-201X.1997.00144.x. [DOI] [PubMed] [Google Scholar]
- Rifai N, Douglas PS, O'Toole M, Rimm E, Ginsburg G. Cardiac troponin T and I, electrocardiographic wall motion analyses, and ejection fractions in athletes participating in the Hawaii Ironman Triathlon. Am J Cardiol. 1999;83:1085–1089. doi: 10.1016/s0002-9149(99)00020-x. [DOI] [PubMed] [Google Scholar]
- Robotham JL, Takata M, Berman M. Ejection fraction revisited. Anesthesiology. 1991;74:172–183. doi: 10.1097/00000542-199101000-00026. [DOI] [PubMed] [Google Scholar]
- Rohrer DK, Chruscinski A, Schauble EH, Bernstein D, Kobilka BK. Cardiovascular and metabolic alterations in mice lacking both β1- and β2-adrenergic receptor. J Biol Chem. 1999;274:16701–16708. doi: 10.1074/jbc.274.24.16701. [DOI] [PubMed] [Google Scholar]
- Savin WM, Davidson DM, Haskell WL. Autonomic contribution to heart rate recovery from exercise in humans. J Appl Physiol. 1982;53:1572–1575. doi: 10.1152/jappl.1982.53.6.1572. [DOI] [PubMed] [Google Scholar]
- Seals DJ, Rogers MA, Hagberg JM, Yamamoto C, Cryer PE, Ehansi AA. Left ventricular dysfunction after prolonged strenuous exercise in healthy subjects. Am J Cardiol. 1988;61:875–879. doi: 10.1016/0002-9149(88)90362-1. [DOI] [PubMed] [Google Scholar]
- Shave R, Dawson E, Whyte G, George K, Gaze D, Collinson P. Altered cardiac function and minimal cardiac damage during prolonged exercise. Med Sci Sport Exerc. 2004;36:1098–1103. doi: 10.1249/01.mss.0000131958.18154.1e. [DOI] [PubMed] [Google Scholar]
- Shave RE, Whyte GP, George K, Gaze DC, Collinson PO. Prolonged exercise should be considered alongside typical symptoms of acute myocardial infarction when evaluating increases in cardiac troponin T. Heart. 2005;91:1219–1220. doi: 10.1136/hrt.2004.046052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel AJ. Relative risk of sudden cardiac death during marathon running. Arch Intern Med. 1997;157:1269–1270. [PubMed] [Google Scholar]
- Simpson KH, Smith RJ, Davies LF. Comparison of the effects of atropine and glycopyrrolate on cognitive function following general anaesthesia. Br J Anaesth. 1987;59:966–969. doi: 10.1093/bja/59.8.966. [DOI] [PubMed] [Google Scholar]
- Solomon SB, Barbier P, Glantz SA. Changes in porcine transmitral flow velocity pattern and its diastolic determinants during partial coronary occlusion. J Am Coll Cardiol. 1999;33:854–866. doi: 10.1016/s0735-1097(98)00638-x. [DOI] [PubMed] [Google Scholar]
- Spina RJ, Turner MJ, Ehansi AA. β-Adrenergic-mediated improvement in left ventricular function by exercise training in older men. Am J Physiol Heart Circ Physiol. 1998;274:H397–H404. doi: 10.1152/ajpheart.1998.274.2.H397. [DOI] [PubMed] [Google Scholar]
- Starnes JW, Bowles DK. Role of exercise in the cause and prevention of cardiac dysfunction. Exerc Sport Sci Rev. 1995;23:349–373. [PubMed] [Google Scholar]
- Stokes DN, Clutton-Brock T, Patil C, Thompson JM, Hutton P. Comparison of invasive and non-invasive measurement of continuous arterial pressure using the finapres. Br J Anaesth. 1991;67:26–35. doi: 10.1093/bja/67.1.26. [DOI] [PubMed] [Google Scholar]
- Wang W, Zhu W, Wang S, Yang D, Crow MT, Xiao RP, Cheng H. Sustaine β1-adrenergic receptor stimulation modulates cardiac contractility by Ca2+/calmodulin kinase signalling pathway. Circ Res. 2004;95:798–806. doi: 10.1161/01.RES.0000145361.50017.aa. [DOI] [PubMed] [Google Scholar]
- Weaver LC. Organisation of sympathetic responses to distension of the urinary bladder. Am J Physiol Regul Integr Comp Physiol. 1985;248:R236–R240. doi: 10.1152/ajpregu.1985.248.2.R236. [DOI] [PubMed] [Google Scholar]
- Welsh RC, Warburton DE, Humen DP, Taylor DA, McGovarck J, Haykosky MJ. Prolonged strenuous exercise alters cardiovascular response to dobutamine stimulation in male athletes. J Physiol. 2005;569:325–330. doi: 10.1113/jphysiol.2005.096412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessling KH, Jansen JR, Settels JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using a nonlinear, three-linear element model. J Appl Physiol. 1993;74:2566–2573. doi: 10.1152/jappl.1993.74.5.2566. [DOI] [PubMed] [Google Scholar]
- Whyte G, George K, Shave R, Dawson E, Stephenson C, Edwards B, Gaze D, Oxborough D, Forster J, Simpson R. Impact of marathon running on cardiac structure and function in recreational runners. Clin Sci. 2005;108:1–8. doi: 10.1042/CS20040186. [DOI] [PubMed] [Google Scholar]
- Xiao RP, Zhu W, Zheng M, Chakir K, Bond R, Lakatta EG, Cheng H. Sub-type specific β-adrenoreceptor signalling pathways in the heart and their potential clinical implications. Trends Pharmacol Sci. 2004;25:358–356. doi: 10.1016/j.tips.2004.05.007. [DOI] [PubMed] [Google Scholar]