Abstract
Deficits in GABAergic inhibitory transmission are a hallmark of temporal lobe epilepsy and have been replicated in animal and tissue culture models of epilepsy. GABAergic inhibition comprises phasic and tonic inhibition that is mediated by synaptic and extrasynaptic GABAA receptors, respectively. We have recently demonstrated that chronic stimulation with cyclothiazide (CTZ) or kainic acid (KA) induces robust epileptiform activity in hippocampal neurons both in vitro and in vivo. Here, we report a downregulation of tonic GABA inhibition after chronic epileptogenic stimulation of rat hippocampal cultures. Chronic pretreatment of hippocampal neurons with CTZ or KA resulted in a marked reduction in GABAergic inhibition, as shown by a significant decrease in whole-cell GABA currents and in the frequency of miniature inhibitory postsynaptic currents (mIPSCs). Interestingly, synaptically localized GABAA receptors remained relatively stable, as evidenced by the unaltered amplitude of mIPSCs, as well as the unchanged punctate immunoreactivity of γ2 subunit-containing postsynaptic GABAA receptors. In contrast, tonic GABA currents, assessed either by a GABAA receptor antagonist bicuculline or a selective extrasynaptic GABAA receptor agonist THIP, were significantly reduced following epileptogenic stimulation. These results reveal a novel form of neural plasticity, that epileptogenic stimulation can selectively downregulate extrasynaptic GABAA receptors while leaving synaptic GABAA receptors unchanged. Thus, in addition to synaptic alteration of GABAergic transmission, regulation of tonic inhibition may also play an important role during epileptogenesis.
GABAA receptors mediate the majority of GABAergic inhibition in the brain (Mody, 2005). Rodent models of temporal lobe epilepsy (TLE) are associated with hippocampal changes in GABAA receptor expression and function (Tsunashima et al. 1997; Brooks-Kayal et al. 1998). Quantitative immunohistochemical studies of brain tissue of human TLE patients have similarly shown GABAA receptor changes (Loup et al. 2000). In addition, mutations in genes encoding GABAA receptor subunits or GABAA receptor trafficking proteins are causal for diverse inherited forms of human epilepsy (Baulac et al. 2001; Wallace et al. 2001; Cossette et al. 2002; Harkin et al. 2002; Dibbens et al. 2004; Harvey et al. 2004). Together, these studies suggest that functional deficits in GABAA receptors play an important role in the aetiology of epilepsy.
GABAA receptors are abundant at both synaptic and extrasynaptic sites. Synaptic GABAA receptors have low affinity for GABA and primarily mediate fast inhibitory transmission also known as phasic inhibition, whereas extrasynaptic GABAA receptors exhibit high affinity for GABA and mediate tonic inhibition (Mody & Pearce, 2004; Semyanov et al. 2004). Comparing to the well-characterized synaptic GABAergic inhibition, much less is understood for the tonic inhibition. Gene knockout studies reveal that deleting extrasynaptic GABAA receptors that contain an α5, α6, or δ subunit results in a significant reduction of tonic but not phasic GABA currents (Brickley et al. 2001; Stell et al. 2003; Caraiscos et al. 2004). Blocking extrasynaptic GABAA receptor-mediated tonic inhibition significantly enhanced neuronal excitability, suggesting an important function of tonic inhibition in the information process in the brain (Hamann et al. 2002; Semyanov et al. 2003, 2004; Mody, 2005).
It is known that alteration of synaptic GABAergic inhibition plays a critical role during epileptogenesis (Sperk et al. 2004), but whether regulation of tonic inhibition plays any role is not clear (Richerson, 2004). Houser & Esclapez (2003) first reported that immunostaining of extrasynaptic α5 subunit-containing GABAA receptors was significantly reduced in hippocampus of the pilocarpine-induced rat model of TLE. Expression of δ subunit-containing extrasynaptic GABAA receptors in the dentate gyrus was similarly reduced in the same model (Peng et al. 2004).
We have recently established a novel epilepsy model in which cyclothiazide (CTZ) induces robust epileptiform activity in hippocampal neurons both in vitro and in vivo (Qi et al. 2006). Here we have further analysed changes in GABAA receptor function associated with epileptiform activity induced by chronic CTZ treatment, and compared them to changes in the well-established chronic kainic acid (KA) model of epilepsy. Irrespective of the stimulation protocol, epileptiform activity of cultured hippocampal neurons was associated with a significant reduction of the whole-cell GABA currents and the frequency of mIPSCs. However, the amplitude of mIPSCs mediated by synaptic GABAA receptors was not altered. Immunocytochemical analysis confirmed that the postsynaptic GABAA receptor puncta and size remained largely unaffected by epileptogenic stimulation. Interestingly, tonic GABA currents revealed by a GABAA receptor antagonist or selective agonist for extrasynaptic GABAA receptors were markedly reduced following epileptogenic stimulation. Therefore, extrasynaptic GABAA receptors exhibit a heightened functional vulnerability to epileptogenic stimulation, and suggest that downregulation of tonic GABA currents may contribute to epileptogenesis.
Methods
Primary hippocampal culture
Primary hippocampal cultures were prepared as previously described (Deng & Chen, 2003; Chen et al. 2004; Qi et al. 2006). In brief, brains from newborn Sprague-Dawley rats were removed and placed in ice-cold modified Hank's balanced salt solution (HBSS). The hippocampal CA1–CA3 region was dissected, cut into ∼1 mm3 cubes and incubated with 0.05% trypsin–EDTA in HBSS for 30 min at 37°C. After trypsin treatment, tissue blocks were triturated with HBSS with 10% horse serum, and dissociated cells were plated onto a monolayer of astrocytes. The culture medium contained 500 ml MEM (Gibco), 5% fetal bovine serum (Hyclone, Logan, Utah, USA), 10 ml B27 (Invitrogen, Carlsbad, California, USA), 100 mg NaHCO3, 20 mmd-glucose, 0.5 mml-glutamine, and 25 u ml−1 penicillin/streptomycin. Culture medium containing 4 μm AraC to stop glial proliferation was replaced three times in the first week and cultures were maintained in a 5% CO2 incubator for 2–3 weeks. The neonatal pups (P0–P1) were decapitated and the adult rats were killed with CO2 according to the animal protocols approved by IACUC committee in Pennsylvania State University and conforming with the federal guidelines.
Whole-cell patch-clamp recordings
Whole-cell recordings of action potentials, mIPSCs and GABA application-induced currents were made in current- or voltage-clamp mode using a MultiClamp 700A amplifier (Molecular Devices Corporation, Sunnyvale, CA, USA). Patch pipettes were pulled from borosilicate glass and fire polished (2–4 MΩ). The recording chamber was continuously perfused with a bath solution consisting of (mm): 128 NaCl, 30 glucose, 25 Hepes, 5 KCl, 2 CaCl2, 1 MgCl2, pH 7.3 adjusted with NaOH. The pipette solution for recording action potentials contained (mm): 125 K-gluconate, 10 KCl, 2 EGTA, 10 Hepes, 10 Tris-phosphocreatine, 4 MgATP, 0.5 Na2GTP, pH 7.3 adjusted with KOH. For mIPSCs, tonic GABA currents and GABA-induced whole-cell currents, patch pipettes were filled with (mm): 135 KCl, 10 Tris-phosphocreatine, 2 EGTA, 10 Hepes, 4 MgATP, 0.5 Na2GTP, pH 7.3 adjusted with KOH. The series resistance was typically 10–20 MΩ and partially compensated by 30–50%. The membrane potential was held around −70 mV in both voltage-clamp and curren-clamp recordings. Data were acquired using pClamp 9 software (Molecular Devices Corporation, Sunnyvale, CA, USA), sampled at 2–10 kHz, and filtered at 1 kHz. Offline analysis was done with Clampfit 9 software. Miniature events were analysed using MiniAnalysis software (Synaptosoft). All data were expressed as mean ± s.e.m. and Student's t test was used for statistical analysis.
Epileptiform activity is defined by at least five consecutive action potentials overlaying a large depolarization shift of minimally 10 mV in amplitude and ≥300 ms in duration. The criteria for quantifying the percentage of neurons showing epileptiform activity is at least four recurrent epileptiform bursts occurring in a 30 min recording period. Pyramidal shape neurons were selected for recordings. For each neuron recorded after KA or CTZ treatment, epileptiform discharges were first verified under current clamp before recording mIPSCs and GABA-evoked currents under voltage clamp.
Immunofluorescent staining and quantification
Immunofluorescent staining of cultured neurons was performed as described (Alldred et al. 2005). Briefly, neurons were washed with PBS, fixed in 4% paraformaldehyde for 12 min and permeabilized for 5 min with 0.2% Triton X-100 in PBS containing 10% donkey serum. The primary antibodies mAb SV2 (1: 2000) and GAD65 (1: 75, Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA, USA) were used to label total and GABAergic nerve terminals, respectively, and developed with CY3-conjugated donkey antimouse secondary antibody (Jackson ImmunoResearch, West Grove, PA, USA; 1: 500). Postsynaptic GABAA receptors were stained with guinea-pig anti-γ2 subunits (1: 1500) (Fritschy & Mohler, 1995) and developed with Alexa Fluor 488-conjugated goat anti-guinea-pig secondary antibodies (1: 1000 Molecular Probes, Eugene, OR, USA). Fluorescence images were captured with an ORCA-100 camera on a Zeiss Axiophot 2 microscope equipped with a ×40, 1.3 NA objective and controlled by Openlab software (Improvision Inc., Lexington, MA, USA). Quantification of postsynaptic GABAA receptors was performed as described (Alldred et al. 2005). For quantification of presynaptic bouton numbers, immunoreactive puncta for glutamic acid decarboxylase (GAD) or the general presynaptic marker SV2 along ∼100 μm dendrite per neuron were analysed using SimplePCI software (Compix Inc., Cranberry, PA, USA), as previously described (Chen et al. 2003). Synaptic density determined by the number of puncta per 100 μm dendrite was compared between different experimental groups.
Drugs
Cyclothiazide, kainic acid, bicuculline, 4,5,6,7-tetrahydroisoxazolo[5,4-c]-pyridin-3-ol (THIP), d(−)-2-amino-5-phosphonopentanoic acid (AP5), and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) were purchased from Tocris (Bristol, UK). TTX and GABA were obtained from Sigma. All of the drugs were freshly diluted in bath solution to final concentrations before experiments. Bath application of drugs was rapidly delivered through a micropipette (400–500 μm tip) positioned about 1000 μm away from recorded neurons at a 45 deg angle. The micropipette was connected to a Warner (Hamden, CT, USA) VC-6 drug delivery system.
Results
Cyclothiazide stimulation induces epileptiform activity in hippocampal cultures
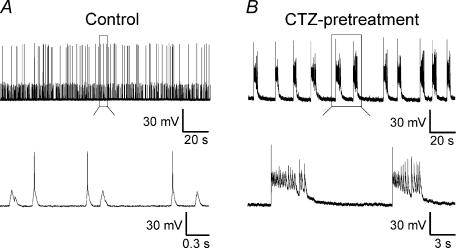
CTZ increases neuronal excitability by synergistically enhancing the excitatory glutamate response and reducing the inhibitory GABA response (Diamond & Jahr, 1995; Bellingham & Walmsley, 1999; Ishikawa & Takahashi, 2001; Deng & Chen, 2003; Qi et al. 2006). Hippocampal CA1–CA3 cultures (>12 days in vitro) were treated with CTZ (5 μm) for 48 h to induce epileptiform activity (Qi et al. 2006). After removal of CTZ, whole-cell patch-clamp recordings revealed periodic bursting activity with large depolarization shift, a hallmark of epileptiform activity (Fig. 1B, n = 21). In contrast, control neurons typically exhibited low-frequency firing of action potentials as well as spontaneous postsynaptic potentials (Fig. 1A, n = 18). Since chronic CTZ pretreatment (5 μm, 48 h) reliably induces epileptiform activity in the majority of hippocampal neurons (Qi et al. 2006), this protocol was used throughout this study to investigate the chronic effects of epileptogenic stimulation on the GABAergic transmission system.
Figure 1. Chronic CTZ treatment induces epileptiform activity in hippocampal cultures.
A, a representative recording from a control neuron showing spontaneous postsynaptic potentials and individual action potential firing. All of the action potentials are clearly separated, as shown in the lower trace for expanded view. B, a typical recording after CTZ pretreatment (5 μm, 48 h), showing recurrent epileptiform bursts. Two of the epileptiform bursts in the upper trace were expanded at the lower trace, with repeated activity overlaying on the top of large depolarization shifts.
Chronic CTZ treatment reduces presynaptic GABA release but not postsynaptic GABAA receptor responses
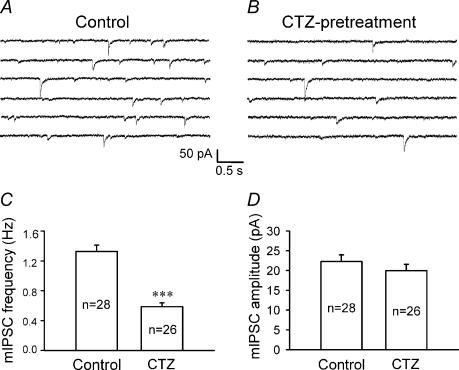
Previous studies suggest that alteration of GABAergic neurotransmission may contribute to epileptogenesis (Gibbs et al. 1997a; Kobayashi & Buckmaster, 2003; Leroy et al. 2004; Macdonald et al. 2004; Dzhala et al. 2005; Mody, 2005). We first examined possible changes in GABAergic synaptic transmission by investigating the frequency and amplitude of miniature inhibitory postsynaptic currents (mIPSCs) after CTZ induction of epileptiform activity. The mIPSCs were recorded in the presence of the action potential blocker TTX (0.5 μm) and the AMPA/kainate receptor antagonist CNQX (20 μm). CTZ-pretreated neurons recorded under current-clamp condition were confirmed to show epileptiform activity before recording mIPSCs. Miniature IPSC traces recorded from CTZ-pretreated neurons showed a clear reduction in the mIPSC frequency compared to untreated controls (Fig. 2A and B). The average mIPSC frequency after CTZ pretreatment was 0.59 ± 0.05 Hz (n = 26), which was significantly lower than the control frequency of 1.33 ± 0.09 Hz (n = 28, P < 0.001; Student's t test) (Fig. 2C). In contrast to the frequency changes, the mIPSC amplitude of CTZ-pretreated neurons (20.0 ± 1.6 pA, n = 26) was the same as that of controls (22.3 ± 1.7 pA, n = 28; P > 0.3) (Fig. 2D). The unaltered mIPSC amplitude suggests that the postsynaptic GABAA receptors are relatively intact, while the significant change of the mIPSC frequency indicates presynaptic alterations in CTZ-pretreated neurons.
Figure 2. Chronic CTZ treatment results in a significant decrease of the frequency but not the amplitude of miniature IPSCs.
A and B, consecutive current traces illustrating mIPSCs recorded in the presence of TTX (0.5 μm) and CNQX (20 μm) in control (A) and CTZ-pretreated (B) neurons. The frequency of mIPSCs was considerably lower in CTZ-pretreated neurons. Throughout the experiments, holding potential =−70 mV. C, bar graphs showing that the average mIPSC frequency was significantly decreased after CTZ pretreatment (control, 1.33 ± 0.09 Hz; CTZ pretreatment, 0.59 ± 0.05 Hz; ***P < 0.001). D, bar graphs showing that the average mIPSC amplitude was similar between control (22.3 ± 1.7 pA) and after CTZ pretreatment (20.0 ± 1.6 pA; P > 0.3).
CTZ-pretreatment does not affect the number of GABAergic synapses
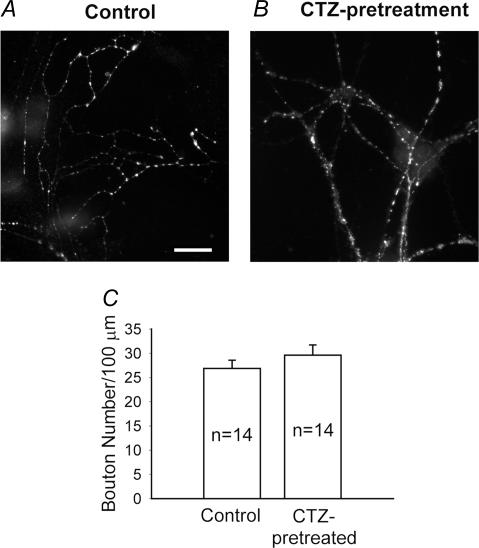
The significant decrease of mIPSC frequency after CTZ pretreatment may be caused by a reduction of the number of GABAergic synapses or by a decrease of the presynaptic release probability at each individual synapse. To assess possible changes in the number of GABAergic synapses, we performed immunofluorescent stainings of CTZ-pretreated and control neurons with GAD, a widely used marker for GABAergic synapses. The number of GAD-immunoreactive puncta of CTZ-pretreated neurons (29.6 ± 2.1 per 100 μm dendrite, n = 14 cells) was indistinguishable from that of controls (26.9 ± 1.7, n = 14; P > 0.3) (Fig. 3A–C). Similarly punctate immunoreactivity for the general presynaptic marker SV2 was unchanged in CTZ-pretreated neurons (42.7 ± 3.2; n = 11) compared to controls (40.8 ± 3.0; n = 13; P > 0.6). These experiments suggest that the number of GABAergic and glutamatergic synapses remained unchanged in CTZ-pretreated neurons. Therefore, the reduction in the frequency of mIPSCs of CTZ-pretreated neurons is probably due to a decrease in the GABA release probability. Our results are consistent with previous findings showing that the quantal release of GABA was reduced in the hippocampal CA1 region of a rodent TLE model (Hirsch et al. 1999).
Figure 3. CTZ treatment does not affect the number of GABAergic synapses.
A and B, fluorescence images of GAD immunostaining showing no obvious changes in the number of GABAergic synapses between control (A) and after CTZ treatment (B). Scale bar, 10 μm. C, quantified data indicating a similar number of GABAergic synapses per 100 μm dendritic length in control (26.9 ± 1.7, n = 14) and after chronic CTZ treatment (29.6 ± 2.1, n = 14) (P > 0.3).
Synaptic GABAA receptor puncta are not altered after epileptogenic stimulation
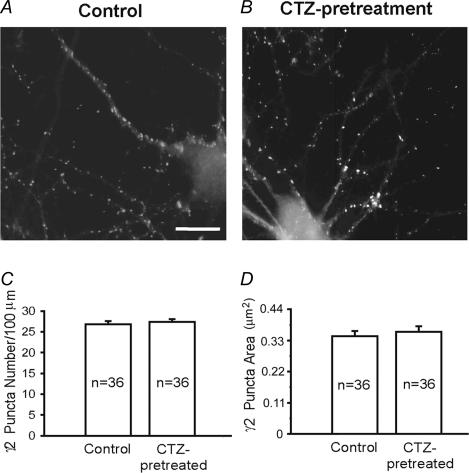
The unchanged amplitude of mIPSCs recorded from CTZ-pretreated neurons suggests that postsynaptic GABAA receptors remain unaffected by CTZ stimulation. To directly assess the expression of postsynaptic GABAA receptors, we used immunofluorescent staining of CTZ-treated and control neurons with an antiserum directed against the γ2 subunit (Fig. 4A and B). Punctate immunofluorescence of the γ2 subunit in cultured neurons and in vivo is representative of the large majority of GABAA receptors that are clustered in the postsynaptic specialization of GABAergic synapses (Essrich et al. 1998; Brunig et al. 2002; Alldred et al. 2005). Quantitative analysis of digitized fluorescence images revealed that neither the number nor the size of γ2 subunit immunoreactive puncta was changed after chronic CTZ treatment (Fig. 4C and D). The number of postsynaptic γ2 puncta in the control group was 26.8 ± 0.8 per 100 μm dendritic length (n = 36) and 27.4 ± 0.7 following CTZ treatment (n = 36; P > 0.5), consistent with the GAD staining result and confirming that the number of GABAergic synapses did not change after CTZ treatment. We also quantified the size of postsynaptic γ2 puncta and found no significant alteration after CTZ treatment (control, 0.35 ± 0.02 μm2 punctum−1, n = 36; and 0.37 ± 0.02 μm2 punctum−1 after CTZ treatment, n = 36; P > 0.5). Thus, the expression and function of postsynaptic GABAA receptors are not affected by chronic epileptogenic stimulation of hippocampal neurons with CTZ.
Figure 4. GABAA receptor γ2 subunit puncta are not changed after CTZ treatment.
A and B, representative images showing the immunoreactive puncta of the GABAA receptor γ2 subunits in control (A) and CTZ-treated (B) neurons. There was no obvious change in γ2 subunit-containing GABAA receptor puncta after CTZ treatment. Scale bar, 12 μm. C, quantified data showing no significant difference in the puncta number between control (26.8 ± 0.8 per 100 μm dendritic length) and CTZ-treated neurons (27.4 ± 0.7 per 100 μm dendritic length; P > 0.5). D, the average size of the γ2 subunit puncta was also similar between the control (0.35 ± 0.02 μm2) and after CTZ treatment (0.37 ± 0.02 μm2; P > 0.5).
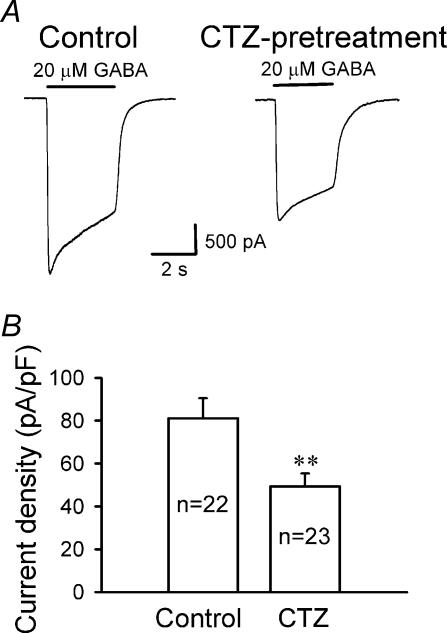
Whole-cell GABAA receptor currents decrease after epileptogenic stimulation
To further study GABAA receptor functional changes after chronic CTZ treatment, we examined whole-cell GABA currents mediated by all GABAA receptors expressed on the cell surface. Rapid bath application of GABA (20 μm) was used to activate both synaptic and extrasynaptic GABAA receptors. GABA-induced whole-cell currents of CTZ-pretreated neurons showed an overt decrease in the peak amplitude compared to controls (Fig. 5A). To normalize whole-cell currents for differences in cell size, we compared the GABAA receptor current density (peak amplitude divided by cell capacitance) of CTZ-pretreated and control neurons. Interestingly, the current density of CTZ-pretreated neurons (49.3 ± 6.0 pA pF−1, n = 23) was dramatically reduced compared to the controls (81.1 ± 9.4 pA pF−1, n = 22, P < 0.01) (Fig. 5B). Importantly, we have verified that the cell size did not change significantly after CTZ or KA pretreatment. The mean cell capacitance in control cells was 40.3 ± 2.3 pF (n = 31), 38.7 ± 2.9 pF after CTZ pretreatment (n = 23, P > 0.67), and 38.3 ± 2.9 pF after KA pretreatment (n = 17, P > 0.58). Our results are consistent with previous reports that GABA-evoked whole-cell currents significantly decreased in a low-Mg2+-induced epilepsy model (Gibbs et al. 1997b).
Figure 5. Whole-cell GABA currents are significantly reduced after CTZ treatment.
A, typical recordings showing whole-cell currents induced by rapid application of GABA (20 μm) in control (left panel) and CTZ-pretreated neurons (right panel) in the presence of TTX (0.5 μm) and CNQX (20 μm). B, summarized data showing a significant decrease in whole-cell GABAA receptor current density (peak amplitude divided by the cell capacitance) after CTZ pretreatment (81.1 ± 9.4 pA pF−1 in control; 49.3 ± 6.0 pA pF−1 after CTZ-pretreatment; **P < 0.01).
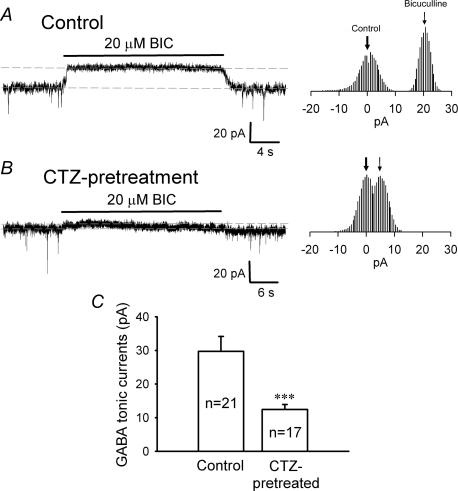
Tonic GABA currents are suppressed after CTZ pretreatment
Given that the number of GABAergic synapses and the postsynaptic GABAA receptors were unaltered in CTZ-pretreated neurons, a significant change in whole-cell GABA currents might reflect changes in extrasynaptic GABAA receptors. In order to test this idea, we investigated changes of tonic GABA currents, which are thought to be mediated primarily by high-affinity extrasynaptic GABAA receptors (Bai et al. 2001; Caraiscos et al. 2004; Semyanov et al. 2004). Tonic GABA currents were revealed by acute application of the GABAA receptor blocker BIC (20 μm), in the continuous presence of TTX (0.5 μm), CNQX (20 μm), and AP5 (40 μm), as previously described (Bai et al. 2001; Caraiscos et al. 2004). BIC treatment of control neurons revealed a constant outward current of approximately 20 pA (Fig. 6A). In addition to the block of mIPSCs, the baseline noise was also greatly reduced in the presence of BIC, indicating the blockade of a tonic GABA conductance. In CTZ-pretreated neurons, the tonic GABA current was greatly reduced (Fig. 6B). The all data point histograms to the right side of the current traces in Fig. 6A and B show an accurate measurement of the BIC-induced baseline shift (Caraiscos et al. 2004). The average amplitude of tonic GABA currents in CTZ-pretreated neurons (12.5 ± 1.5 pA, n = 17) was significantly reduced compared to that of control neurons (29.7 ± 4.4 pA, n = 21, P < 0.001) (Fig. 6C), suggesting that extrasynaptic GABAA receptors are downregulated after chronic CTZ-stimulation.
Figure 6. CTZ treatment suppresses tonic GABA currents.
A, a typical trace of tonic GABA current from a control neuron revealed by application of BIC (20 μm) in the presence of TTX (0.5 μm), CNQX (20 μm) and AP5 (40 μm). Constant outward current appeared after acute application of BIC, while mIPSCs were completely abolished. Holding potential =−70 mV. Histograms on the right side show the all data point plot of the baseline in control and during BIC application. B, a representative trace showing reduced tonic GABA current after CTZ pretreatment. The all data point histogram on the right also shows a significant reduction of the tonic current. C, summarized data demonstrate that the average amplitude of tonic GABA currents was significantly reduced in CTZ-pretreated neurons (12.5 ± 1.5 pA, n = 17), compared to the control (29.7 ± 4.4 pA, n = 21; ***P < 0.001).
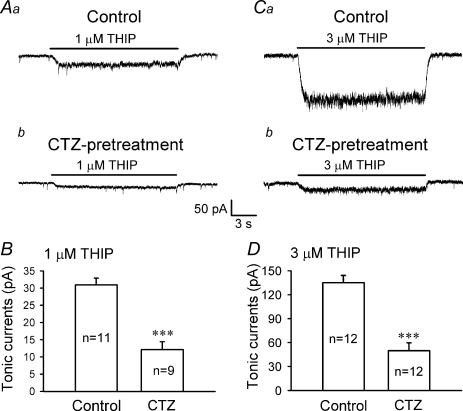
To further investigate the regulation of tonic GABA currents following epileptogenic stimulation, we employed THIP, a selective agonist for extrasynaptic GABAA receptors (Lindquist et al. 2003; Krogsgaard-Larsen et al. 2004; Drasbek & Jensen, 2005). The tonic GABA currents induced by THIP at low concentrations of 1 μm and 3 μm were compared in control and CTZ-pretreated neurons (Fig. 7). THIP (1–3 μm) activated a small, non-desensitizing tonic current in hippocampal pyramidal neurons (Fig. 7Aa and Ca). In CTZ-pretreated neurons, the THIP-induced tonic current was significantly reduced (Fig. 7Ab and Cb). The average tonic current amplitude induced by 1 μm THIP was reduced from 30.9 ± 2.0 pA (n = 11) in control neurons to 12.1 ± 2.3 pA in CTZ-pretreated neurons (n = 9, P < 0.001) (Fig. 7B). Similarly, the tonic current induced by 3 μm THIP was reduced from 135.0 ± 9.1 pA (n = 12) in controls to 49.9 ± 9.8 pA in CTZ-pretreated neurons (n = 12, P < 0.001) (Fig. 7D). Thus, BIC-sensitive and THIP-induced tonic currents are both significantly downregulated in CTZ-pretreated neurons.
Figure 7. Extrasynaptic GABAA receptor agonist THIP-induced tonic currents are significantly decreased after chronic CTZ treatment.
A, tonic currents activated by 1 μm THIP in control (a) and CTZ-pretreated neurons (b). B, summarized data showing a significant reduction of 1 μm THIP-induced tonic currents after CTZ pretreatment (30.9 ± 2.0 pA in control, n = 11; and 12.1 ± 2.3 after CTZ pretreatment, n = 9; ***P < 0.001). C, tonic currents activated by 3 μm THIP in control (a) and CTZ-pretreated neurons (b). D, summarized data showing that tonic currents induced by 3 μm THIP also showed a similar reduction after chronic CTZ stimulation (135.0 ± 9.1 pA in control, n = 12; and 49.9 ± 9.8 pA after CTZ-pretreatment, n = 12; ***P < 0.001).
Chronic KA-stimulation mimics the effect of CTZ on GABAergic responses
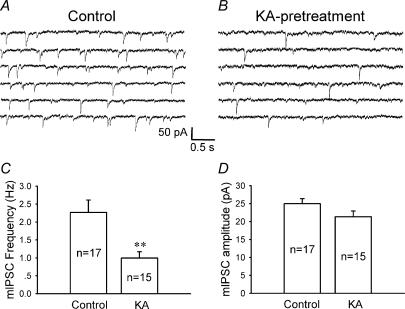
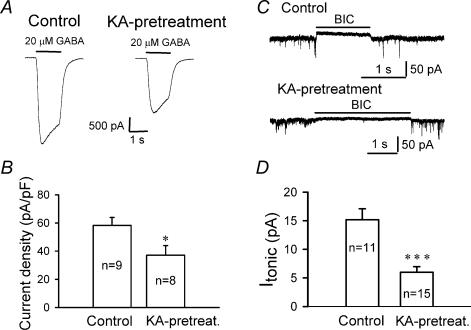
Because CTZ is a novel epileptogenic agent (Qi et al. 2006), the question arises whether downregulation of extrasynaptic GABAA receptors observed with CTZ could be applied to other epilepsy models as well. To address this question, we investigated the effect of chronic KA treatment on GABAergic transmission in hippocampal cultures. Chronic KA treatment (5 μm for 48 h) of hippocampal cultures induces robust epileptiform activity similar to CTZ pretreatment (Qi et al. 2006). We found that KA pretreatment significantly decreased the mIPSC frequency compared to controls (control, 2.3 ± 0.4 Hz, n = 17; KA-treated, 0.99 ± 0.18 Hz, n = 15; P < 0.005), but left the mIPSC amplitude unchanged (25.0 ± 1.3 pA in control; 21.4 ± 1.6 pA following KA treatment; P > 0.09) (Fig. 8). Thus, chronic pretreatment with KA replicates the effect on mIPSCs seen with chronic CTZ stimulation (Fig. 2). Next, we investigated the effect of chronic KA stimulation on whole-cell GABA currents. Similar to the result of CTZ-stimulation (Fig. 5), chronic KA treatment induced a significant decrease in the whole-cell response to bath-applied GABA (20 μm) (Fig. 9A). The average GABA-evoked current density was reduced from 58.3 ± 5.5 pA pF−1 in controls (n = 9) to 37.1 ± 6.8 pA pF−1 following KA pretreatment (n = 8; P < 0.03) (Fig. 9B). To further investigate changes of tonic GABA currents after KA pretreatment, we bath applied BIC (20 μm) similar to the application performed after CTZ pretreatment (Fig. 6). The tonic current in control condition was 15.2 ± 1.9 pA (n = 11), and significantly decreased after KA pretreatment (6.0 ± 0.98 pA, n = 15; P < 0.001) (Fig. 9C and D). In summary, KA pretreatment mimics the effects seen with CTZ and suggests that downregulation of tonic GABA currents is closely associated with chronic epileptogenic stimulation of cultured neurons.
Figure 8. Chronic KA treatment decreases the frequency but not the amplitude of mIPSCs.
A and B, consecutive traces illustrating mIPSCs in control (A) and KA-pretreated (5 μm, 48 h) (B) neurons. Similar to the effect of CTZ, KA pretreatment also resulted in a marked decrease of the frequency of mIPSCs. C, the average frequency of mIPSCs was significantly decreased after chronic KA pretreatment (control, 2.3 ± 0.4 Hz; after KA treatment, 0.99 ± 0.18 Hz; **P < 0.005). D, the average amplitude of mIPSCs did not change significantly after KA pretreatment (25.0 ± 1.3 pA in control; 21.4 ± 1.6 after KA-pretreatment; ***P > 0.09).
Figure 9. Chronic KA treatment significantly decreases whole-cell GABA currents.
A, representative recordings showing whole-cell currents induced by rapid application of GABA (20 μm) in control (left panel) and KA-treated (right panel) neurons in the presence of TTX (0.5 μm) and CNQX (20 μm). B, bar graphs showing a significant decrease in whole-cell GABAA receptor current density after KA treatment (37.1 ± 6.8 pA pF−1), compared to the control (58.3 ± 5.5 pA pF−1; *P < 0.03). C, typical traces illustrating GABA tonic currents induced by BIC (20 μm) in control and KA-pretreated neurons. D, bar graphs showing a significant decrease of the GABA tonic currents after KA pretreatment (***P < 0.001).
Discussion
The present study revealed a novel form of neural plasticity, that chronic epileptogenic stimulation selectively regulates tonic GABA currents mediated by extrasynaptic GABAA receptors. We demonstrated that following chronic stimulation of hippocampal cultures with CTZ or KA, GABAergic transmission was substantially decreased as shown by the significant reduction of mIPSC frequency and whole-cell GABA currents. Interestingly, the number of GABAergic synapses as well as synaptically localized GABAA receptors was unaffected by epileptogenic stimulation. In contrast, tonic GABA currents, as revealed by both GABAA receptor antagonist and selective extrasynaptic GABAA receptor agonist application, were significantly decreased after chronic epileptogenic stimulation. Our results suggest that downregulation of tonic GABA currents, together with reduction of presynaptic GABA release, may contribute to epileptogenesis. A potential contribution of extrasynaptic GABAA receptors to epileptogenesis may provide a new strategy to develop antiepileptic drugs.
GABA tonic inhibition and epileptiform activities
GABAergic inhibition has been found to be substantially altered in epilepsy patients (Lloyd et al. 1986; During et al. 1995; Williamson et al. 1995; Patrylo et al. 2001). Detailed analysis of animal TLE models has revealed breakdown of GABAergic inhibition at multiple levels during epileptogenesis, including loss of inhibitory interneurons, loss of GABAergic nerve terminals, reduction of GABAA receptor subunits and GABA responses, and decrease of GABA release (Gibbs et al. 1997a; Brooks-Kayal et al. 1998; Hirsch et al. 1999; Kobayashi & Buckmaster, 2003; Kobayashi et al. 2003; Leroy et al. 2004; Macdonald et al. 2004; Dzhala et al. 2005; Mody, 2005). In dentate gyrus, GABAergic inhibition was also found to be increased in animal TLE models, suggesting a complexity of changes of the GABAergic system during epileptogenesis (Buhl et al. 1996; Nusser et al. 1998a; Andre et al. 2001; Sperk et al. 2004). Tonic GABA inhibition was more recently identified in cerebellar granule cells and hippocampal neurons (Kaneda et al. 1995; Brickley et al. 1996; Bai et al. 2001; Nusser & Mody, 2002). Compared to fast GABAergic synaptic transmission, the physiological significance of tonic inhibition is much less understood. Whether tonic inhibition plays a role during epileptogenesis is not well understood. Recent studies suggest that tonic GABA inhibition may play a critical function in regulating neuronal excitability in the central nervous system. A slight reduction of tonic GABA currents may result in significant increase in neuronal activity (Mitchell & Silver, 2003; Semyanov et al. 2003). The present study revealed a dramatic reduction of tonic GABA currents in association with epileptiform activity in cultured hippocampal neurons (Figs 6 and 7). Our results suggest that a minimal level of tonic inhibition may be essential for the maintenance of homeostasis of neural networks. A drastic change of tonic inhibition may drive the neural network toward a pathological status such as the induction of epileptiform activity. This conclusion is supported by recent findings that the immunolabelling of GABAA receptor α5 and δ subunits is significantly reduced in the hippocampus of animal TLE models (Houser & Esclapez, 2003; Peng et al. 2004). Mutations in the δ subunit of GABAA receptors have also been mapped in human epilepsy patients (Dibbens et al. 2004). Because GABAA receptors assembled from α5 or δ subunits are probably localized at extrasynaptic sites (Nusser et al. 1998b; Brunig et al. 2002; Crestani et al. 2002; Hamann et al. 2002; Wei et al. 2003), these studies imply that tonic inhibition might be depressed in animal TLE models. Interestingly, like the complexity of changes in GABAergic synaptic inhibition, the tonic inhibition may also be compensated in animal TLE models (Scimemi et al. 2005).
Regulation of synaptic versus extrasynaptic GABAA receptors after epileptogenic stimulation
One of the major findings of this study is the revealing of a preferential regulation of extrasynaptic GABAA receptors after chronic epileptogenic stimulation. In contrast, synaptically localized GABAA receptors are relatively stable (Figs 2 and 4). The preserved synaptic GABAA receptors under epileptogenic stimulation will maintain fast GABAergic transmission during the initial period of epileptogenesis.
Classical epilepsy research has been centred on GABAergic synaptic alterations (Mathern et al. 1995; Longo & Mello, 1997; Buckmaster & Jongen-Relo, 1999; Williamson et al. 1999; Magloczky et al. 2000; Kobayashi et al. 2003). The relationship between synaptic versus extrasynaptic GABAergic regulation during epileptogenesis is largely unknown. One possibility is that the synaptic and extrasynaptic GABAA receptor modulation occurs in different time windows after epileptogenic stimulation. It was found that in dentate granule cells, GABAergic inhibition decreased in 3–7 days after pilocarpine-induced status epilepticus (Kobayashi & Buckmaster, 2003), but increased after 30–45 days in fully kindled epileptic rats (Nusser et al. 1998a). The extrasynaptic GABAA receptor regulation described in the current study occurred within 48 h, whereas many previous studies on synaptic changes of GABAergic inhibition are conducted weeks or months after epileptogenic insult in animal epilepsy models (Nusser et al. 1998a; Buckmaster & Jongen-Relo, 1999; Kobayashi et al. 2003; Sperk et al. 2004). Another possibility is that changes in GABAergic inhibition may be cell type-specific after epileptogenic stimulation. For example, it has been demonstrated that the whole-cell GABAA receptor currents decreased in CA1 pyramidal neurons but increased in dentate gyrus granule cells (Gibbs et al. 1997a).
It is important to point out that our results were obtained in hippocampal cultures and after 48 h chronic epileptogenic stimulation. These in vitro studies may not mimic in vivo epileptogenesis. However, our results revealed an important link between changes of tonic GABA currents and epileptogenesis. The physiological relevance of this study is that regulation of tonic inhibition not only affects normal homeostatic neural network activity, but also may have pathological consequences.
Presynaptic GABA release and epileptogenesis
In addition to the regulation of extrasynaptic GABAA receptors, our data also show a consistent decrease of mIPSC frequency in hippocampal pyramidal neurons after KA and CTZ stimulation, indicating a significant decrease of presynaptic GABA neurotransmitter release. The amplitude of mIPSCs did not change significantly in our hippocampal culture model. These data seem to largely agree with previous reports using experimental epilepsy models. For example, in a pilocarpine-induced TLE model, mIPSC frequency and amplitude did not change in entorhinal cortical neurons (Kobayashi et al. 2003). However, the mIPSC frequency significantly reduced (64%) while the mIPSC amplitude slightly increased in dental granule cells (Kobayashi & Buckmaster, 2003). In a separate study with pilocarpine- and KA-induced TLE models, the frequency of mIPSCs recorded from CA1 pyramidal cells greatly reduced (62%), while the amplitude only slightly decreased (12%) (Hirsch et al. 1999). Thus, an emerging common feature arises from these studies that the mIPSC frequency appears to be significantly reduced to half of the control, while the amplitude of mIPSCs either does not change or shows a slight increase or decrease. Together with our findings in CTZ- and KA-treated hippocampal cultures, we propose that the substantial decrease of presynaptic GABA neurotransmitter release is one of the key contributing factors during epileptogenesis.
Acknowledgments
We are grateful to Dr Matthew Whim for critical reading of the manuscript and invaluable comments. We also thank members of the Chen lab for helpful discussion. Ming Jiang provided valuable help with hippocampal cultures. This work is supported by National Science Foundation (0236429) and a start-up fund provided by Pennsylvania State University to G.C., and an NIH grant (MH62391) to B.L.
References
- Alldred MJ, Mulder-Rosi J, Lingenfelter SE, Chen G, Luscher B. Distinct gamma2 subunit domains mediate clustering and synaptic function of postsynaptic GABAA receptors and gephyrin. J Neurosci. 2005;25:594–603. doi: 10.1523/JNEUROSCI.4011-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre V, Marescaux C, Nehlig A, Fritschy JM. Alterations of hippocampal GABAergic system contribute to development of spontaneous recurrent seizures in the rat lithium-pilocarpine model of temporal lobe epilepsy. Hippocampus. 2001;11:452–468. doi: 10.1002/hipo.1060. [DOI] [PubMed] [Google Scholar]
- Bai D, Zhu G, Pennefather P, Jackson MF, MacDonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by gamma-aminobutyric acid(A) receptors in hippocampal neurons. Mol Pharmacol. 2001;59:814–824. doi: 10.1124/mol.59.4.814. [DOI] [PubMed] [Google Scholar]
- Baulac S, Huberfeld G, Gourfinkel-An I, Mitropoulou G, Beranger A, Prud'homme JF, Baulac M, Brice A, Bruzzone R, LeGuern E. First genetic evidence of GABA(A) receptor dysfunction in epilepsy: a mutation in the gamma2-subunit gene. Nat Genet. 2001;28:46–48. doi: 10.1038/ng0501-46. [DOI] [PubMed] [Google Scholar]
- Bellingham MC, Walmsley B. A novel presynaptic inhibitory mechanism underlies paired pulse depression at a fast central synapse. Neuron. 1999;23:159–170. doi: 10.1016/s0896-6273(00)80762-x. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol. 1996;497:753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABA(A) receptor subunit expression and function in temporal lobe epilepsy. Nat Med. 1998;4:1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- Brunig I, Scotti E, Sidler C, Fritschy JM. Intact sorting, targeting, and clustering of gamma-aminobutyric acid A receptor subtypes in hippocampal neurons in vitro. J Comp Neurol. 2002;443:43–55. doi: 10.1002/cne.10102. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Jongen-Relo AL. Highly specific neuron loss preserves lateral inhibitory circuits in the dentate gyrus of kainate-induced epileptic rats. J Neurosci. 1999;19:9519–9529. doi: 10.1523/JNEUROSCI.19-21-09519.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl EH, Otis TS, Mody I. Zinc-induced collapse of augmented inhibition by GABA in a temporal lobe epilepsy model. Science. 1996;271:369–373. doi: 10.1126/science.271.5247.369. [DOI] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, MacDonald JF, Orser BA. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha5 subunit-containing gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci U S A. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Deng LB, Maeno-Hikichi Y, Lai MZ, Chang SH, Chen G, Zhang JF. Formation of an endophilin-Ca2+ channel complex is critical for clathrin-mediated synaptic vesicle endocytosis. Cell. 2003;115:37–48. doi: 10.1016/s0092-8674(03)00726-8. [DOI] [PubMed] [Google Scholar]
- Chen G, Harata N, Tsien RW. Paired-pulse depression of unitary quantal amplitude at single hippocampal synapses. Proc Natl Acad Sci U S A. 2004;101:1063–1068. doi: 10.1073/pnas.0307149101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossette P, Liu L, Brisebois K, Dong H, Lortie A, Vanasse M, Saint-Hilaire JM, Carmant L, Verner A, Lu WY, Wang YT, Rouleau GA. Mutation of GABRA1 in an autosomal dominant form of juvenile myoclonic epilepsy. Nat Genet. 2002;31:184–189. doi: 10.1038/ng885. [DOI] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Bluthmann H, Mohler H, Rudolph U. Trace fear conditioning involves hippocampal alpha5 GABA(A) receptors. Proc Natl Acad Sci U S A. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng LB, Chen G. Cyclothiazide potently inhibits gamma-aminobutyric acid type A receptors in addition to enhancing glutamate responses. Proc Natl Acad Sci U S A. 2003;100:13025–13029. doi: 10.1073/pnas.2133370100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond JS, Jahr CE. Asynchronous release of synaptic vesicles determines the time course of the AMPA receptor-mediated EPSC. Neuron. 1995;15:1097–1107. doi: 10.1016/0896-6273(95)90098-5. [DOI] [PubMed] [Google Scholar]
- Dibbens LM, Feng HJ, Richards MC, Harkin LA, Hodgson BL, Scott D, Jenkins M, Petrou S, Sutherland GR, Scheffer IE, Berkovic SF, Macdonald RL, Mulley JC. GABRD encoding a protein for extra- or peri-synaptic GABAA receptors is a susceptibility locus for generalized epilepsies. Hum Mol Genet. 2004;13:1315–1319. doi: 10.1093/hmg/ddh146. [DOI] [PubMed] [Google Scholar]
- Drasbek KR, Jensen K. THIP, a hypnotic and antinociceptive drug, enhances an extrasynaptic GABAA receptor-mediated conductance in mouse neocortex. Cereb Cortex. 2006;16:1134–1141. doi: 10.1093/cercor/bhj055. [DOI] [PubMed] [Google Scholar]
- During MJ, Ryder KM, Spencer DD. Hippocampal GABA transporter function in temporal-lobe epilepsy. Nature. 1995;376:174–177. doi: 10.1038/376174a0. [DOI] [PubMed] [Google Scholar]
- Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, Delpire E, Jensen FE, Staley KJ. NKCC1 transporter facilitates seizures in the developing brain. Nat Med. 2005;11:1205–1213. doi: 10.1038/nm1301. [DOI] [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- Gibbs JW, Shumate MD, 3rd, Coulter DA. Differential epilepsy-associated alterations in postsynaptic GABA(A) receptor function in dentate granule and CA1 neurons. J Neurophysiol. 1997a;77:1924–1938. doi: 10.1152/jn.1997.77.4.1924. [DOI] [PubMed] [Google Scholar]
- Gibbs JW, 3rd, Sombati S, DeLorenzo RJ, Coulter DA. Physiological and pharmacological alterations in postsynaptic GABA (A) receptor function in a hippocampal culture model of chronic spontaneous seizures. J Neurophysiol. 1997b;77:2139–2152. doi: 10.1152/jn.1997.77.4.2139. [DOI] [PubMed] [Google Scholar]
- Hamann M, Rossi DJ, Attwell D. Tonic and spillover inhibition of granule cells control information flow through cerebellar cortex. Neuron. 2002;33:625–633. doi: 10.1016/s0896-6273(02)00593-7. [DOI] [PubMed] [Google Scholar]
- Harkin LA, Bowser DN, Dibbens LM, Singh R, Phillips F, Wallace RH, Richards MC, Williams DA, Mulley JC, Berkovic SF, Scheffer IE, Petrou S. Truncation of the GABA(A) -receptor gamma2 subunit in a family with generalized epilepsy with febrile seizures plus. Am J Hum Genet. 2002;70:530–536. doi: 10.1086/338710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey K, Duguid IC, Alldred MJ, Beatty SE, Ward H, Keep NH, Lingenfelter SE, Pearce BR, Lundgren J, Owen MJ, Smart TG, Luscher B, Rees MI, Harvey RJ. The GDP–GTP exchange factor collybistin: an essential determinant of neuronal gephyrin clustering. J Neurosci. 2004;24:5816–5826. doi: 10.1523/JNEUROSCI.1184-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch JC, Agassandian C, Merchan-Perez A, Ben-Ari Y, DeFelipe J, Esclapez M, Bernard C. Deficit of quantal release of GABA in experimental models of temporal lobe epilepsy. Nat Neurosci. 1999;2:499–500. doi: 10.1038/9142. [DOI] [PubMed] [Google Scholar]
- Houser CR, Esclapez M. Downregulation of the alpha5 subunit of the GABA(A) receptor in the pilocarpine model of temporal lobe epilepsy. Hippocampus. 2003;13:633–645. doi: 10.1002/hipo.10108. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Takahashi T. Mechanisms underlying presynaptic facilitatory effect of cyclothiazide at the calyx of Held of juvenile rats. J Physiol. 2001;533:423–431. doi: 10.1111/j.1469-7793.2001.0423a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda M, Farrant M, Cull-Candy SG. Whole-cell and single-channel currents activated by GABA and glycine in granule cells of the rat cerebellum. J Physiol. 1995;485:419–435. doi: 10.1113/jphysiol.1995.sp020739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Buckmaster PS. Reduced inhibition of dentate granule cells in a model of temporal lobe epilepsy. J Neurosci. 2003;23:2440–2452. doi: 10.1523/JNEUROSCI.23-06-02440.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Wen X, Buckmaster PS. Reduced inhibition and increased output of layer II neurons in the medial entorhinal cortex in a model of temporal lobe epilepsy. J Neurosci. 2003;23:8471–8479. doi: 10.1523/JNEUROSCI.23-24-08471.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogsgaard-Larsen P, Frolund B, Liljefors T, Ebert B. GABA(A) agonists and partial agonists: THIP (gaboxadol) as a non-opioid analgesic and a novel type of hypnotic. Biochem Pharmacol. 2004;68:1573–1580. doi: 10.1016/j.bcp.2004.06.040. [DOI] [PubMed] [Google Scholar]
- Leroy C, Poisbeau P, Keller AF, Nehlig A. Pharmacological plasticity of GABA(A) receptors at dentate gyrus synapses in a rat model of temporal lobe epilepsy. J Physiol. 2004;557:473–487. doi: 10.1113/jphysiol.2003.059246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist CE, Ebert B, Birnir B. Extrasynaptic GABA(A) channels activated by THIP are modulated by diazepam in CA1 pyramidal neurons in the rat brain hippocampal slice. Mol Cell Neurosci. 2003;24:250–257. doi: 10.1016/s1044-7431(03)00128-3. [DOI] [PubMed] [Google Scholar]
- Lloyd KG, Bossi L, Morselli PL, Munari C, Rougier M, Loiseau H. Alterations of GABA-mediated synaptic transmission in human epilepsy. Adv Neurol. 1986;44:1033–1044. [PubMed] [Google Scholar]
- Longo BM, Mello LE. Blockade of pilocarpine- or kainate-induced mossy fiber sprouting by cycloheximide does not prevent subsequent epileptogenesis in rats. Neurosci Lett. 1997;226:163–166. doi: 10.1016/s0304-3940(97)00267-x. [DOI] [PubMed] [Google Scholar]
- Loup F, Wieser HG, Yonekawa Y, Aguzzi A, Fritschy JM. Selective alterations in GABAA receptor subtypes in human temporal lobe epilepsy. J Neurosci. 2000;20:5401–5419. doi: 10.1523/JNEUROSCI.20-14-05401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald RL, Gallagher MJ, Feng HJ, Kang J. GABA(A) receptor epilepsy mutations. Biochem Pharmacol. 2004;68:1497–1506. doi: 10.1016/j.bcp.2004.07.029. [DOI] [PubMed] [Google Scholar]
- Magloczky Z, Wittner L, Borhegyi Z, Halasz P, Vajda J, Czirjak S, Freund TF. Changes in the distribution and connectivity of interneurons in the epileptic human dentate gyrus. Neuroscience. 2000;96:7–25. doi: 10.1016/s0306-4522(99)00474-1. [DOI] [PubMed] [Google Scholar]
- Mathern GW, Babb TL, Pretorius JK, Leite JP. Reactive synaptogenesis and neuron densities for neuropeptide Y, somatostatin, and glutamate decarboxylase immunoreactivity in the epileptogenic human fascia dentata. J Neurosci. 1995;15:3990–4004. doi: 10.1523/JNEUROSCI.15-05-03990.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Silver RA. Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron. 2003;38:433–445. doi: 10.1016/s0896-6273(03)00200-9. [DOI] [PubMed] [Google Scholar]
- Mody I. Aspects of the homeostaic plasticity of GABAA receptor-mediated inhibition. J Physiol. 2005;562:37–46. doi: 10.1113/jphysiol.2004.077362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody I, Pearce RA. Diversity of inhibitory neurotransmission through GABA(A) receptors. Trends Neurosci. 2004;27:569–575. doi: 10.1016/j.tins.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Hajos N, Somogyi P, Mody I. Increased number of synaptic GABA (A) receptors underlies potentiation at hippocampal inhibitory synapses. Nature. 1998a;395:172–177. doi: 10.1038/25999. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol. 2002;87:2624–2628. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998b;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrylo PR, Spencer DD, Williamson A. GABA uptake and heterotransport are impaired in the dentate gyrus of epileptic rats and humans with temporal lobe sclerosis. J Neurophysiol. 2001;85:1533–1542. doi: 10.1152/jn.2001.85.4.1533. [DOI] [PubMed] [Google Scholar]
- Peng Z, Huang CS, Stell BM, Mody I, Houser CR. Altered expression of the delta subunit of the GABAA receptor in a mouse model of temporal lobe epilepsy. J Neurosci. 2004;24:8629–8639. doi: 10.1523/JNEUROSCI.2877-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Wang Y, Jiang M, Warren P, Chen G. Cyclothiazide induces robust epileptiform activity in rat hippocampal neurons both in vitro and in vivo. J Physiol. 2006;571:605–618. doi: 10.1113/jphysiol.2005.103812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richerson GB. Looking for GABA in all the wrong places: the relevance of extrasynaptic GABA(A) receptors to epilepsy. Epilepsy Curr. 2004;4:239–242. doi: 10.1111/j.1535-7597.2004.46008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scimemi A, Semyanov A, Sperk G, Kullmann DM, Walker MC. Multiple and plastic receptors mediate tonic GABAA receptor currents in the hippocampus. J Neurosci. 2005;25:10016–10024. doi: 10.1523/JNEUROSCI.2520-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat Neurosci. 2003;6:484–490. doi: 10.1038/nn1043. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Sperk G, Furtinger S, Schwarzer C, Pirker S. GABA and its receptors in epilepsy. Adv Exp Med Biol. 2004;548:92–103. doi: 10.1007/978-1-4757-6376-8_7. [DOI] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc Natl Acad Sci U S A. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunashima K, Schwarzer C, Kirchmair E, Sieghart W, Sperk G. GABA(A) receptor subunits in the rat hippocampus III: altered messenger RNA expression in kainic acid-induced epilepsy. Neuroscience. 1997;80:1019–1032. doi: 10.1016/s0306-4522(97)00144-9. [DOI] [PubMed] [Google Scholar]
- Wallace RH, Marini C, Petrou S, Harkin LA, Bowser DN, Panchal RG, Williams DA, Sutherland GR, Mulley JC, Scheffer IE, Berkovic SF. Mutant GABA(A) receptor gamma2-subunit in childhood absence epilepsy and febrile seizures. Nat Genet. 2001;28:49–52. doi: 10.1038/ng0501-49. [DOI] [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of delta subunit-containing GABA (A) receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson A, Patrylo PR, Spencer DD. Decrease in inhibition in dentate granule cells from patients with medial temporal lobe epilepsy. Ann Neurol. 1999;45:92–99. doi: 10.1002/1531-8249(199901)45:1<92::aid-art15>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Williamson A, Telfeian AE, Spencer DD. Prolonged GABA responses in dentate granule cells in slices isolated from patients with temporal lobe sclerosis. J Neurophysiol. 1995;74:378–387. doi: 10.1152/jn.1995.74.1.378. [DOI] [PubMed] [Google Scholar]