Abstract
Acute behavioural stress has been recognized as a strong influence on the inducibility of hippocampal long-term synaptic plasticity. We have reported previously that in adult male rats, acute behavioural stress impairs long-term potentiation (LTP) but enhances long-term depression (LTD) in the hippocampal CA1 region. In this study we report that the effects of stress on LTP and LTD were reversed when animals were introduced into a novel ‘stimulus-rich’ environment immediately after the stress. Novelty exploration-induced reversal of stress effects was prevented when the animals were given the NMDA receptor antagonist d-(−)-2-amino-5-phosphonopentanoic acid, the cholinergic antagonist atropine and the protein phosphatase (PP) 2B inhibitors cyclosporin A and cypermethrin, but not the α1-adrenergic antagonist prazosin, the β-adrenergic antagonist propranolol or the PP1/2A inhibitor okadaic acid, respectively before being subjected to the novel environment. In addition, the ability of novelty exploration to reverse the stress effects was mimicked by a direct application of the cholinergic agonist carbachol. Exposure to the novel environment following stress was accompanied by the activation of both PP2B and striatal-enriched tyrosine phosphatase (STEP). Taken together, these findings suggest that the activation of the cholinergic system and, in turn, the triggering of an NMDA receptor-mediated activation of PP2B to increase STEP activity appear to mediate the novelty exploration-induced reversal of stress-related modulation of hippocampal long-term synaptic plasticity.
Stress has long been recognized as a strong influence on hippocampal long-term synaptic plasticity (Kim & Yoon, 1998; McEwen, 1999; Garcia, 2001; Kim & Diamond, 2002; Diamond et al. 2004; Huang et al. 2005). For example, both in vitro and in vivo electrophysiological studies indicate that a brief experience of acute inescapable stress can impair high-frequency stimulation (HFS)-induced long-term potentiation (LTP; Shors et al. 1989; Diamond et al. 1990; Kim et al. 1996; Xu et al. 1997; Yang et al. 2004), whereas low-frequency stimulation (LFS)-induced long-term depression (LTD) can be facilitated in the CA1 region of the hippocampus (Kim et al. 1996; Xu et al. 1997; Yang et al. 2004, 2005). Although these effects of stress on subsequent LTP and LTD are persistent (Shors et al. 1997; Yamada et al. 2003; Yang et al. 2004) and depend on new protein synthesis (Xu et al. 1998), they are not permanent and decay within several hours after cessation of the stressor. These findings reflect a passive ‘run-down’ of processes that maintain the effects of stress. In addition to a passive decay, the brain has an active process that can alter the longevity of stress effects on LTP and LTD. It has been shown that such stress effects are greatly prolonged by the induction of anaesthesia immediately after the stress, whereas they are rapidly reversed in awake animals that are allowed to acclimate behaviourally to aversive conditions to or remove themselves from the stressor (Xu et al. 1997).
The extracellular signal-regulated kinase/mitogen-activated protein kinase (ERK)1/2 signalling is a highly conserved kinase cascade linking the transmembrane receptors to downstream effector mechanisms (Chang & Karin, 2001). It has been reported that neuronal ERK1/2 activation plays a critical role in mediating a wide variety of forms of synaptic plasticity and memory formation (Sweatt, 2004). In the hippocampal CA1 region, activation of ERK1/2 signalling is necessary for the expression of a late phase of LTP (English & Sweatt, 1996; Impey et al. 1996) and is an important process through which neurotransmitters modulate LTP induction (Roberson et al. 1999; Watabe et al. 2000). Furthermore, ERK1/2 activation is necessary for both contextual fear conditioning and escape training in the Morris water maze, both of which are hippocampus-dependent associative learning paradigms (Atkins et al. 1998; Blum et al. 1999; Selcher et al. 1999). Recent findings from our laboratory demonstrated a parallel in time course of increased ERK1/2 activation to that of the effects of stress on LTP and LTD; additionally, a pharmacological blockade of the ERK1/2 signalling pathway completely prevented the stress effects, strongly suggesting a critical role of sustained ERK1/2 activation in mediating the blockade of LTP and the facilitation of LTD induced by the stress (Yang et al. 2004; Huang et al. 2005). Thus, in addition to its role in mediating synaptic plasticity and various forms of memory formation, ERK1/2 also plays a key role in the metaplastic determination of the polarity of subsequent synaptic plasticity.
The molecular mechanisms underlying the alterations of the inducibility of LTP and LTD by stress have received significant attention recently, and it has been shown that stress affects subsequent hippocampal synaptic plasticity, perhaps by sharing similar molecular machinery to that required to support LTP (Diamond et al. 1990, 2004; Shors & Thompson, 1992; Shors & Dryver, 1994; Kim & Yoon, 1998; Huang et al. 2005). Our recent work has provided empirical support for this prediction by showing that hippocampal CA1 LTP can normally be elicited by HFS in slices from stressed rats that have previously received depotentiating stimulation (Yang et al. 2004). These observations provoked an intriguing question about whether pharmacological agents or behavioural handlings that can induce LTD or depotentiation may have clinical applications in the treatment of individuals whose cognitive performances are impaired following stressful events. Since exposure of rats to a novel ‘stimulus-rich’ environment has been reported to effectively induce forms of endogenous LTD (Manahan-Vaughan & Braunewell, 1999) or depotentiation (Xu et al. 1998) in the hippocampal CA1 region, this motivated us to investigate whether the effects of stress on hippocampal synaptic plasticity can be reversed by exposure to spatial novelty. Here, we show that a behavioural encounter with a non-stressful novel environmental context immediately after the stress induces a complete reversal of stress-induced modulation of LTP and LTD in the hippocampal CA1 region. Moreover, these effects are apparently mediated by the activation of the cholinergic system.
Methods
Animals
Healthy adult male Sprague–Dawley rats weighing 250–300 g were used in these experiments. Animal care was consistent with the guidelines set by the Laboratory Animal Center of National Cheng Kung University. All experimental procedures were approved by the National Cheng Kung University Institutional Animal Care and Use Committee governing the participating laboratories. Animals were housed in groups of four in a vivarium with a 12 h light–12 h dark cycle (light on at 7.00 am), 50–60% humidity, and free access to food and water. All experiments were conducted during the light phase of the cycle. All efforts were made to minimize animal suffering and to use only the number of animals necessary to produce reliable scientific data.
Stress protocol
Animals were allowed to acclimate to the laboratory for 5–7 days before any experimental manipulation. Behavioural stress was evoked by 60 tail shocks (1 mA for 1 s, 30–90 s apart) while restrained in a Plexiglass tube as previously described (Yang et al. 2004). Control animals remained in their home cages. Control and stressed animals did not have available food and water during the experiments.
Novel and familiar environments
The novel cage (NC) was identical to the home cage (47 cm × 25 cm × 20 cm), apart from the addition of three to four plastic and rubber objects, such as a bottle cap, box and a ball with non-food odours. Objects were mounted permanently on the cage to prevent displacement by the animals, which may confound measures of total activity, and to maintain specific configurations of objects within the cage. Familiarity was promoted by previous daily exposure (habituation) of animals into the novel cage. During habituation, the animals remained in the cage for 15 min, after which they were returned to the home cage (HC) in the colony room. Animals were given a total of three pre-exposures.
Locomotor activity
The horizontal locomotor activity during the 15 min period of exploration was monitored with a video tracking system (Ethovision; Noldus, Wagenlngen, The Netherlands). Activity during exploration of the home or novel environments is expressed as the total distance travelled over the 15 min period.
Cannulation
In some experiments, to implant cannulae, rats were deeply anaesthetized with pentobarbitone (50 mg kg−1, i.p.), and 24 gauge cannulae were stereotaxically placed into both lateral brain ventricles (co-ordinates: anterior, −0.5 mm; lateral, ±1; ventral, 3) or directed towards 1.0 mm above the stratum pyramidale of the dorsal CA1 region of the hippocampi (co-ordinates: anterior, −4.0 mm; lateral, ±4.0 mm; ventral, 2.6 mm), in accordance with the description by Paxinos & Watson (1997). The cannulae were fixed to the skull with dental cement. The animals were allowed to recover from surgery for 7 days before the experiments started. To deliver the various drugs at the predetermined sites, we used a 28 gauge infusion cannula connected by a polyethylene tube to a 5 μl microsyringe. Bilateral injections were performed using an infusion pump (CAM/100; CAM Microdialysis, Solna, Sweden). A total volume of 1 μl was infused into each side over 1 min, and the infusion cannula was kept in place for an additional 2 min to minimize backflow of the injectant. Rats with placements of injection cannualae outside the hippocampus or with extensive tissue damage at the injection needle site were excluded from analyses. For electrophysiological experiments, the double-guide cannulae placement was verified by unilateral Methylene Blue injection. We never observed any effects of cannulation itself or vehicle ACSF (10% DMSO) injection.
Drug treatment
Carbachol (0.2 mg kg−1), atropine hydrochloride (50 mg kg−1), prazosin hydrochloride (2 mg kg−1) and propranolol hydrochloride (10 mg kg−1) were each dissolved in 0.9% NaCl and separately administered intraperitoneally 20 min before placement in a NC. Drug doses were selected on the basis of published studies (Davis et al. 2004; Kitchigina et al. 1997; Tiesinga et al. 2001; Straube et al. 2003) and pilot experiments in our laboratory. Okadaic acid, cyclosporin A and cypermethrin were stored as 1 mm stock solutions in DMSO. For injection, the solution was diluted with ACSF to a final concentration of 1 μm. d-(−)-2-Amino-5-phosphonopentanoic acid (d-APV) was stored as a 25 mm stock solution. For injection, the solution was diluted with ACSF to a final concentration of 25 μm, which has been shown to be effective to completely abolish the novelty-induced retrograde amnesia (Izquierdo et al. 1999). There were no observed effects of drug application on exploratory behaviours. Atropine hydrochloride, d-APV, okadaic acid, cyclosporin A and cypermethrin were purchased from Tocris Cookson (Bristol, UK); urethane, carbachol, prazosin hydrochloride and propranolol hydrochloride were obtained from Sigma (St Louis, MO, USA).
Plasma adrenocorticotrophic hormone (ACTH) and corticosterone assays
Blood samples were obtained from the tail before and at different times after stress, immediately centrifuged at 1000 g, and plasma was separated and stored at −20°C. Plasma ACTH and corticosterone levels were determined by radioimmunoassay (RIA) as previously described (Yang et al. 2004).
Electrophysiology
Animals were anaesthetized with halothane and decapitated. The hippocampal slices (400 μm thick) were prepared using standard procedures, allowed to recover for a minimum of 1 h, and then transferred to a submersion-type recording chamber continuously perfused at 30–32°C with oxygenated artificial CSF (ACSF) solution (mm: NaCl, 117; KCl, 4.7; CaCl2, 2.5; MgCl2, 1.2; NaHCO3, 25; NaH2PO4, 1.2; and glucose, 11, pH 7.4). Area CA3 was surgically removed after sectioning. Extracellular field potential recordings were carried out using an Axoclamp-2B amplifier (Axon Instruments, Union City, CA, USA). The responses were low-pass filtered at 2 kHz, digitally sampled at 5–10 kHz, and analysed using pCLAMP software (version 8.0; Axon Instruments). The evoked postsynaptic responses were induced in CA1 stratum radiatum by stimulation (0.02 ms duration) of Schaffer collateral/commissural afferents at 0.033 Hz with a bipolar stainless-steel stimulating electrode. The stimulation strength was set to elicit a response having an amplitude that was 30–40% of the maximum spike-free response. Field excitatory postsynaptic potentials (fEPSPs) were recorded with a glass pipette filled with 1 m NaCl (2–3 MΩ resistance), and the fEPSP slope was measured from approximately 20–70% of the rising phase using a least-squares regression. The LTP was induced by HFS, at the test pulse intensity, consisting of two 1 s trains of stimuli separated by an intertrain interval of 20 s at 100 Hz. Long-term depression was induced using a standard protocol of 900 stimuli at 1 Hz (low-frequency stimulation, LFS). The stimulation intensity during LFS application was the same as the test pulse intensity.
Western blotting
For each experimental group, homogenates from at least three slices were pooled. The microdissected subregions were lysed in ice-cold Tris-HCl buffer solution (TBS; pH 7.4) containing a cocktail of protein phosphatase and proteinase inhibitors (50 mm Tris-HCl, 100 mm NaCl, 15 mm sodium pyrophosphate, 50 mm sodium fluoride, 1 mm sodium orthovanadate, 5 mm EGTA, 5 mm EDTA, 1 mm phenylmethylsulphonyl fluoride, 1 μm microcystin-LR, 1 μm okadaic acid, 0.5% Triton X-100, 2 mm benzamidine, 60 μg ml−1 aprotinin and 60 μg ml−1 leupeptin) to avoid dephosphorylation and degradation of proteins, and ground with a pellet pestle (Kontes glassware, Vineland, NJ, USA). Samples were sonicated and spun down at 15 000 g at 4°C for 10 min. The supernatant was then assayed for total protein concentration using Bio-Rad Bradford Protein Assay Kit (Hercules, CA, USA). Each sample of tissue homogenate was separated in 10% SDS-PAGE gel. Following the transfer to nitrocellulose membranes, blots were blocked in TBS containing 3% bovine serum albumin and 0.01% Tween 20 for 1 h and then blotted for 2 h at room temperature with antibodies that recognize phosphorylated ERK1/2 mitogen-activated protein kinase (MAPK) at Thr202 and Tyr204 (1:1000; Cell Signaling Technology, Beverly, MA, USA), phosphorylated MEK1/2 at Thr217 and Thr221 (1:1000; Cell Signaling Technology) striatal-enriched tyrosine phosphatase (STEP; 1:1000; Upstate Biotechnology, Lake Placid, NY, USA). It was then probed with HRP-conjugated secondary antibody for 1 h and developed using the Ezymatic Chemiluminescence immunoblotting detection system. The immunoblots using phosphorylation site-specific antibodies were subsequently stripped and reprobed with the anti-ERK1/2 MAPK antibody (1:1000; Cell Signaling Technology) or anti-MEK1/2 antibody (1:1000; Cell Signaling Technology). Phosphorylation site-specific antibodies and the phosphorylation-independent antibodies were used to determine the relative amount of ERK1/2 MAPK or MEK1/2 phosphorylation (i.e. the ratio of the signals). Immunoblots were analysed by densitometry using Bio-Profil BioLight PC software.
Protein phosphatase 2B (PP2B) activity assay
Protein phosphatase 2B activity was measured using the Serine/Threonine Phosphatase Assay System (Promega, Madison, WI, USA), according to the manufacturer's instructions. Hippocampal slices were treated and dissected as described above. A single CA1 subregion was homogenized in ice-cold buffer (50 mm Tris-HCl, pH 7.5, 50 mm NaCl, 10 mm EGTA, 5 mm EDTA, 1 mm sodium orthovanadate, 1 mm phenylmethylsulphonyl fluoride, 20 μg ml−1 leupeptin and 4 μg ml−1 aprotinin) and centrifuged at 15 000 g for 1 h at 4°C to remove particular matter. Supernatants were added to the reaction buffer from the kit, and the reaction was incubated at 30°C for 10 min. The reaction buffer contained 50 mm imidazole, 0.2 mm EGTA, 10 mm MgCl2, 1 mm NiCl2, 50 μg ml−1 calmodulin and 0.02% β-mercaptoethanol, adjusted to pH 7.2 with HCI. Protein phosphatase 2B activity was calculated as a function of the dephosphorylation of a synthetic phosphopeptide substrate, RRV(pS)VAA. The amount of phosphate released was determined colorimetrically with a microtitre-platereader at an optical density of 620 nm. Cyclosporin A (1 μm) in the supernatant completely blocked phosphate release, indicating that the measured phosphatase activity reflects PP2B function.
Striatal-enriched tyrosine phosphatase (STEP) activity assay
Striatal-enriched tyrosine phosphatase activity was measured using the Universal Tyrosine Kinase Assay Kit (Takara Shuzo, Otsu, Shiga, Japan) according to the manufacturer's instructions. Hippocampal CA1 lysates were prepared as described above. Protein lysates (∼100 μg protein) were immunoprecipitated with anti-STEP antibody (1:1000; Upstate Biotechnology) at 37°C for 1 h, and the resulting immune complex was incubated with a substrate poly(Glu-Tyr) immobilized on microplates and unlabelled ATP in the presence of 1 mm sodium orthovanadate and 50 mm NaF. After termination of the reaction, the extent of tyrosine phosphorylation was measured by ELISA at an optical density of 450 nm with antiphosphotyrosine (PY20-HRP) and HRP substrate, 3,3′,5,5′-tetramethylbenzidine.
Data analysis
All data are expressed as means ± s.e.m. and, unless stated otherwise, the statistical significance was determined using a Mann-Whitney U test. Number of animals used is indicated by n. Probability values of P < 0.05 were considered to represent significant differences.
Results
Novelty exploration induces a rapid loss of stress effects
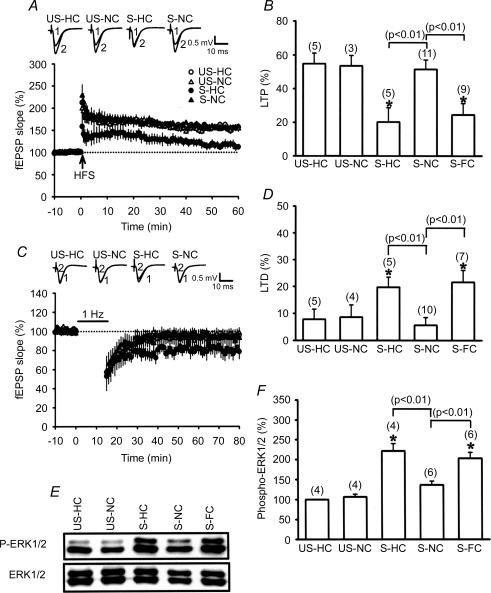
In response to stress, significantly higher corticosterone concentrations (126.9 ± 6.7 versus 8.7 ± 1.9 ng ml−1 in basal conditions, n = 12; P < 0.05) were observed, confirming that our experimental restraint–tailshock stressor activates hypothalamus–pituitary–adrenal responses as previously described (Kim et al. 1996; Yang et al. 2004). We next examined the effects of acute stress on subsequent LTP and LTD induction in hippocampal CA1 neurons. In agreement with previous observations on the effects of stress (Kim et al. 1996; Xu et al. 1997; Yang et al. 2004), the hippocampal slices from stressed rats that had returned to the home cage (HC) exhibited impaired LTP (20.2 ± 8.5%, n = 5) but facilitated LTD (19.7 ± 3.8%, n = 5) in comparison with slices from unstressed control rats (Fig. 1A and B). To test whether novelty exploration may have an ability to modify the stress effects, the animals were allowed to explore a NC immediately after the stress for 1 h, and subsequently the inducibility of the hippocampal CA1 LTP and LTD was examined. One hour of exploration was chosen to minimize any potential confounding effect of concurrent transient activity-related changes in excitability (Leung, 1980; Moser et al. 1993) on subsequent LTP and LTD induction. As expected, novelty exploration completely reversed the stress effects, as indicated by normal LTP (51.5 ± 5.6%, n = 11) and LTD (5.8 ± 2.9%, n = 10) induction in stressed rats. In unstressed rats receiving an exploration in the NC for 1 h, LTP was reliably induced by HFS (52.9 ± 6.2%, n = 3), whereas LFS failed to induce LTD (8.6 ± 4.6%, n = 4). We also assessed whether the novelty exploration-induced reversal of stress effects on LTP and LTD habituates with familiarity of the novel environment. In these experiments, animals were placed in a NC and allowed to explore this novel environment for a 15 min period over 3 consecutive days. On the fourth day, the animals received restraint–tailshock stress and were allowed to explore the same novel environment (familiar cage, FC) for 1 h immediately after the stress. In contrast to the earlier observation comparing LTP and LTD induction in the NC, hippocampal slices from stressed rats that had returned to FC immediately after the stress exhibited impaired LTP (24.5 ± 6.8%, n = 9) but facilitated LTD (21.6 ± 4.5%, n = 7), which were not significantly different from those observed in the HC (Fig. 1A–D). Likewise, an exploration in the NC also reversed the stress-induced increase of ERK1/2 phosphorylation, whereas exploration in the HC or FC immediately after stress had no effect (Fig. 1E and F). In addition, the levels of corticosterone were not significantly changed in animals simply exposed to novel or familiar environments (data not shown). These results suggest that the behavioural state elicited during exposure to spatial novelty can elicit a rapid loss of stress effects and that such action is attenuated by familiarity with the environment.
Figure 1. Novelty exploration induces a reversal of stress effects.
A, hippocampal slices obtained from stressed rats that were allowed to explore a novel cage (S-NC) immediately after the stress showed a stable LTP following HFS, whereas slices from stressed rats immediately placed into the home cage (S-HC) did not. US-HC, unstressed rats in home cage; US-NC, unstressed rats in a novel cage. B, comparison of the magnitude of LTP 50 min after HFS. C, animals that received stress plus novelty exploration were impaired in LFS-induced LTD in comparison with animals that received stress plus home cage. D, comparison of the magnitude of LTD 50 min after LFS. E, representative immunoblots show ERK1/2 phosphorylation after stress plus NC, HC or familiar cage (FC) treatment. The corresponding densitometric analysis is shown in F. The number of experiments per group is indicated by n. Asterisks indicate significant difference (P < 0.05) in comparison to the slices from US-HC rats.
We also investigated whether locomotor activity was affected by introduction into a novel environment. Confirming recent findings (Davis et al. 2004), we found no significant differences in overall locomotor activity among animals exploring in either HC or NC over the 15 min of exploration (see Fig. S1 in online Supplemental material). This finding argues against the view that increased locomotor activity underlies the novelty exploration-induced reversal of stress effects.
Requirement of cholinergic receptor activation for novelty exploration-induced reversal of stress effects
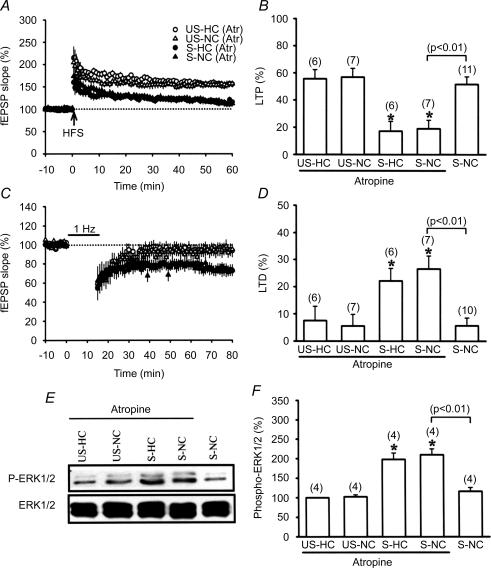
Having confirmed the role of novelty exploration in mediating a reversal of stress effects, we subsequently investigated the underlying molecular mechanism of novelty exploration. Among the mechanisms that have been implicated in detection of a novel environment, activation of the hippocampal cholinergic system plays a prominent role (Acquas et al. 1996; Aloisi et al. 1997). We therefore examined the possible contribution of cholinergic receptor activation to the novelty exploration-induced reversal of stress effects by using a systemic administration of cholinergic antagonist, atropine (30 mg kg−1, i.p.). Injection of atropine 20 min before placement into the NC completely prevented the effect of novelty exploration, as indicated by the presence of stress-induced impairment of LTP (18.9 ± 6.3%, n = 7) and facilitation of LTD (26.5 ± 4.8%, n = 7), which were not significantly different from those observed in the HC (Fig. 2A–D). This action of atropine was did not result from a direct interference with the LTP induction mechanism because LTP was induced reliably in slices from atropine-treated unstressed rats in the HC (55.2 ± 6.8%, n = 6) or the NC (57.8 ± 6.5%, n = 7). In addition, atropine-treated unstressed rats in neither the HC nor the NC showed significant LFS-induced LTD (HC, 7.5 ± 3.3%, n = 6; NC, 5.5 ± 3.7%, n = 7). A similar blockade of the effect of novelty exploration on stress-induced increase of ERK1/2 phosphorylation was also seen in atropine-treated rats (Fig. 2E and F). Thus, an increase in cholinergic activity seems to be one important factor for the establishment of the novelty exploration-induced reversal of stress effects.
Figure 2. Involvement of cholinergic receptor activation in the novelty exploration-induced reversal of stress effects.
A, the ability of novelty exploration to reverse the stress-induced LTP impairment was prevented when rats were injected with the cholinergic receptor antagonist atropine (30 mg kg−1, i.p.) 20 min before being allowed explore a novel cage (NC). Atropine did not significantly affect LTP in slices from unstressed-home cage (US-HC), unstressed-novel cage (US-NC) and stressed-home cage (S-HC) rats. B, comparison of the magnitude of LTP 50 min after HFS. C, atropine blocked novelty exploration-induced reversal of stress-induced facilitation of LTD. Atropine did not significantly affect LTD in slices from US-HC, US-NC and S-HC rats. D, comparison of the magnitude of LTD 50 min after LFS. E, representative immunoblots showing ERK1/2 phosphorylation after stress plus NC and HC among atropine-treated rats. The corresponding densitometric analysis is shown in F.
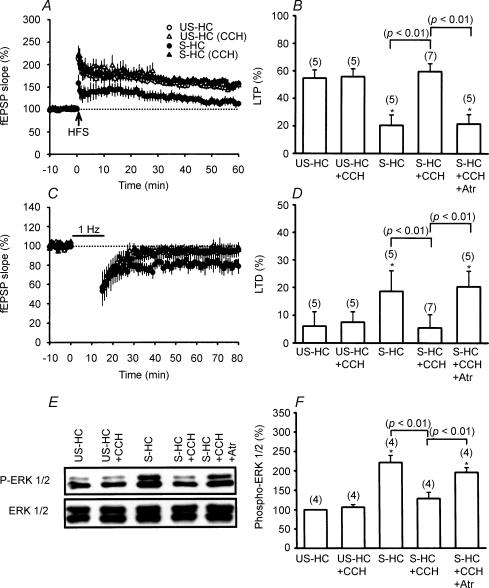
To further establish that the novelty exploration-induced reversal of stress effects is mediated through the activation of the cholinergic system, it is essential to demonstrate that such an effect could be mimicked by a direct application of cholinergic agonists. For this purpose, the effect of the cholinergic agonist carbachol on stress-induced alterations of LTP and LTD induction was investigated. As shown in Fig. 3A–D, application of carbachol (0.2 mg kg−1, i.p.) immediately after the stress was sufficient to completely prevent the impairment of LTP (59.8 ± 5.8%, n = 7) and facilitation of LTD (5.5 ± 4.2%, n = 7) by the stress in the HC. Consistent with a role for cholinergic receptor activation in mediating the effect of carbachol, carbachol-induced reversal of stress effects was also prevented by atropine pretreatment (30 mg kg−1, i.p. 20 min before carbachol application). Injection of carbachol in unstressed rats had no effect on the induction of both LTP (55.9 ± 5.7%, n = 5) and LTD (7.5 ± 3.8%, n = 5). In addition, application of carbachol immediately after the stress also reversed the stress-induced increase of ERK1/2 phosphorylation (Fig. 3E and F).
Figure 3. Pharmacological activation of cholinergic receptors mimics the effect of novelty exploration.
A, hippocampal slices obtained from stressed rats that received the cholinergic agonist carbachol (CCH; 0.2 mg kg−1, i.p.), before introduction into the home cage (S-HC rats) showed a stable LTP following HFS. Carbachol did not significantly affect LTP in slices from unstressed-home cage (US-HC) rats. B, comparison of the magnitude of LTP 50 min after HFS. C, animals that received stress plus carbachol were impaired in LFS-induced LTD in comparison with animals that received stress plus vehicle. D, comparison of the magnitude of LTD 50 min after LFS. E, representative immunoblots showing ERK1/2 phosphorylation after stress plus carbachol or vehicle treatment. The corresponding densitometric analysis is shown in F.
Previous studies have reported that novelty detection is also accompanied by increased hippocampal noradrenergic activity (Klukowski & Harley, 1994; Straube et al. 2003). To assess the role of noradrenergic activation in the novelty exploration-induced reversal of stress effects, the α1-adrenergic receptor antagonist prazosin or the β-adrenergic antagonist propranolol was delivered to stressed rats 20 min before placement in the NC. However, neither prazosin (10 mg kg−1, i.p.) nor propranolol treatment (10 mg kg−1, i.p.) had any influence on the effects of novelty exploration on the stress-induced impairment of LTP and facilitated LTD (data not shown). Likewise, the ability of novelty exploration to reverse the stress-induced increase of ERK1/2 phosphorylation was not significantly affected by either prazosin or propranolol pretreatment.
Putative mechanisms underling novelty exploration-induced reversal of stress effects
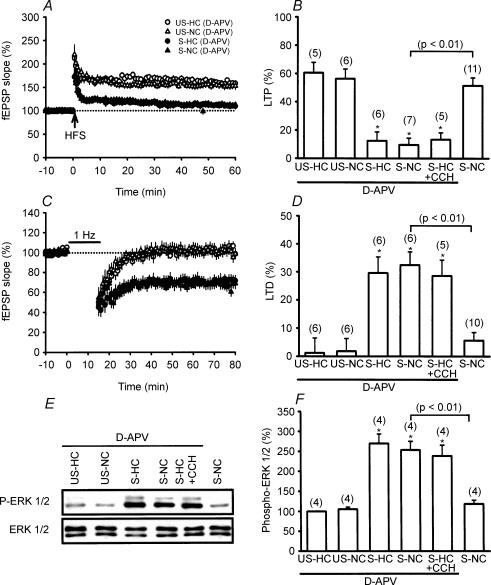
It has been shown that the amnesic effect of exposure to novelty on the retention of inhibitory avoidance task requires the activation of NMDA receptors (Izquierdo et al. 1999). It has also been shown that hippocampal theta rhythm may enhance the release of glutamate to activate NMDA receptors (Vertes, 2005). We therefore investigated whether the novelty exploration-induced reversal of stress effects depends on the activation of NMDA receptors by using bilateral intrahippocampal injection of a competitive NMDA receptor antagonist, d-APV. As shown in Fig. 4A–D, when administered 20 min before placement in the NC, d-APV (25 μm) almost completely prevented the effects of novelty exploration on the stress-induced impairment of LTP (9.5 ± 4.8%, n = 7) and facilitation of LTD (32.5 ± 4.7%, n = 6). Likewise, d-APV also prevented the carbachol-induced reversal of stress effects. Thus, NMDA receptor activation may act as a biochemical step downstream from cholinergic receptor activation to promote the reversal of stress effects. However, slices obtained from d-APV-treated stressed rats that were returned to their HC immediately after stress showed the usual stress-induced impairment of LTP (12.5 ± 6.3%, n = 6) and facilitation of LTD (29.6 ± 4.7%, n = 6). In contrast, slices obtained from d-APV-treated unstressed rats in the HC and the NC showed robust LTP (HC group, 61.6 ± 7.2%, n = 5; NC group, 56.4 ± 6.8%, n = 6) but no LTD (HC group, 2.5 ± 5.1%, n = 6; NC group, 2.8 ± 4.5%, n = 6). A similar blockade of the effect of novelty exploration or carbachol on stress-induced increase of ERK1/2 phosphorylation was also observed in d-APV-treated stressed rats (Fig. 4E and F). These experiments indicate that NMDA receptor activation is also required for the novelty exploration- and carbachol-induced reversal of stress effects.
Figure 4. Involvement of NMDA receptor activation in novelty exploration-induced reversal of the stress effects.
A, the ability of novelty exploration to reverse the stress-induced LTP impairment was prevented when rats were given the NMDA receptor antagonist d-APV (25 μm, bilateral intrahippocampal injection) 20 min before being allowed explore a NC. d-APV did not significantly affect LTP in slices from US-HC, US-NC and S-HC rats. B, comparison of the magnitude of LTP 50 min after HFS. C, d-APV blocks novelty exploration-induced reversal of stress-induced facilitation of LTD. d-APV did not significantly affect LTD in slices from US-HC, US-NC and S-HC rats. D, comparison of the magnitude of LTD 50 min after LFS. E, representative immunoblots show ERK1/2 phosphorylation after stress plus NC and HC among d-APV-treated rats. The corresponding densitometric analysis is shown in F.
We next examined the mechanism by which novelty exploration reverses the stress-induced increase of ERK1/2 phosphorylation, taking into account the fact that the extent and duration of ERK1/2 phosphorylation play a key role in controlling the inducibility of LTP and LTD in response to the stress (Yang et al. 2004; Huang et al. 2005). We addressed this question first by testing whether the reversal of stress-induced ERK1/2 phosphorylation by novelty exploration results from a reduction of its upstream kinase activity. In vivo, ERK1/2 can be activated by upstream MEK1/2 (Hagemann & Blank, 2001). Thus, any changes in MEK1/2 phosphorylation should result in changes in ERK1/2 activity. Consistent with our previous report (Yang et al. 2004), behavioural stress leads to a significant increase in the levels of phosphorylated MEK1/2 (Supplemental Fig. S2A). However, placement of animals in the NC, HC or FC immediately after the stress had no significant effect on the stress-induced increase in hippocampal CA1 MEK1/2 phosphorylation.
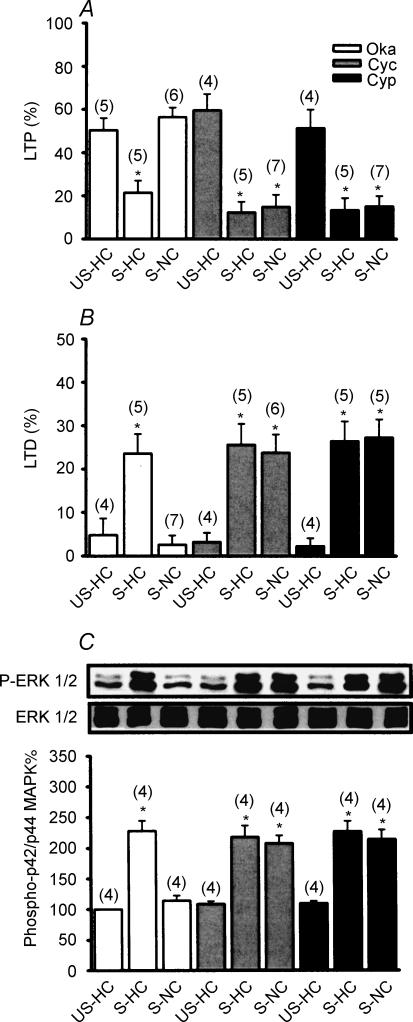
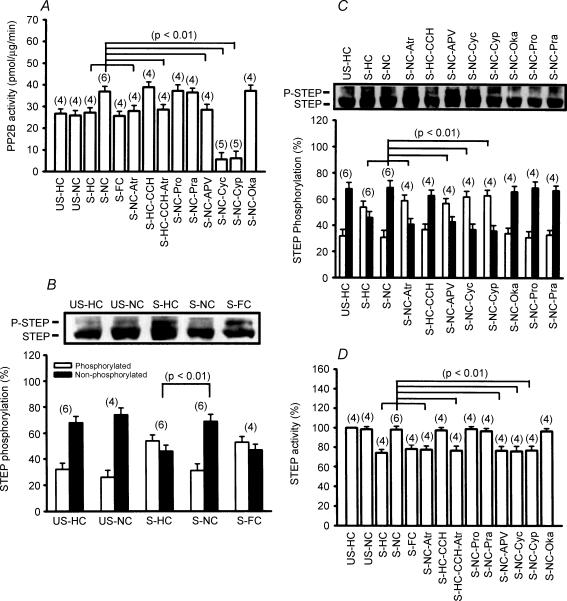
We next examined whether the observed reversal of stress-induced increase in ERK1/2 phosphorylation by novelty exploration is the result of an increase in phosphatase activity. To test this possibility, multiple subtypes of serine/threonine PP inhibitors were administered via bilateral intrahippocampal injection into stressed rats 20 min before placement in the NC. As shown in Fig. 5A and B, treatment with a potent serine/threonine PP1/2A inhibitor, okadaic acid (1 μm), did not significantly modify the effects of novelty exploration on the stress effects, as indicated by the absence of stress-induced impairment of LTP (56.4 ± 4.5%, n = 6) and facilitation of LTD in the NC (2.6 ± 2.1%, n = 6), which were not significantly different from those observed in vehicle-treated stressed rats placed in the NC. In contrast, treatment with the PP2B inhibitors cyclosporin A (1 μm) or cypermethrin (1 μm) completely prevented the effect of novelty exploration on the stress, as indicated by the presence of stress-induced impairment of LTP (cyclosporin A, 14.8 ± 5.7%, n = 7; cypermethrin, 15.2 ± 4.8%, n = 7) and facilitation of LTD (cyclosporin A, 23.8 ± 4.2%, n = 6; cypermethrin, 27.3 ± 4.2%, n = 5). A similar blockade of the effect of novelty exploration on stress-induced increase of ERK1/2 phosphorylation was also observed in cyclosporin A- and cypermethrin-treated stressed rats but not in okadaic acid-treated stressed rats (Fig. 5C), suggesting a contributory role for PP2B in the dephosphorylation of ERK1/2 following a novelty exploration. To test this hypothesis directly, we then measured PP2B activity in the hippocampal CA1 region collected from all experimental groups. As seen in Fig. 6A, exploration in the NC immediately after stress caused a significant increase in PP2B activity. This effect was completely blocked by pretreatment with atropine, d-APV, cyclosporin A or cypermethrin. Note that stress alone had no effect on PP2B activity. Application of carbachol mimicked the action of novelty exploration to augment PP2B activity in stressed rats, which was also prevented by pretreatment with atropine.
Figure 5. Serine/threonine protein phosphatase 2B signalling mediates the reversal of stress effects by novelty exploration.
A, comparison of the magnitude of LTP 50 min after HFS. The LTP observed in slices from S-NC rats is PP2B dependent, as evidenced by blockade using the PP2B inhibitors cyclosporin A (Cys) and cypermethrin (Cyp). The PP1/2A inhibitor okadaic acid (Oka; 1 μm) did not significantly affect LTP in slices from S-NC rats. B, comparison of the magnitude of LTD 50 min after LFS. Cyclosporin A (1 μm) and cypermethrin (1 μm) blocked novelty exploration-induced reversal of stress-induced facilitation of LTD. Okadaic acid did not significantly affect LTD in slices from S-NC rats. C, representative immunoblots and corresponding densitometric analysis show ERK1/2 phosphorylation after stress plus NC and HC among okadaic acid-, cyclosporin A- or cypermethrin-treated rats.
Figure 6. Regulation of PP2B and STEP activity in the hippocampal CA1 region by novelty exploration.
A, a summary of different experimental conditions on PP2B activity. B, representative immunoblots and corresponding densitometric analysis showing STEP phosphorylation after stress plus home cage (HC), novel cage (NC) and familiar cage (FC). C, representative immunoblots and corresponding densitometric analysis showing STEP phosphorylation in rats after stress plus HC (S-HC), NC (S-NC), NC-atropine (S-NC-Atr), HC-carbachol (S-HC-CCH), NC-d-APV (S-NC-APV), NC-cyclosporin A (S-NC-Cyc), NC-cypermethrin (S-NC-Cyp), NC-okadaic acid (S-NC-Oka), NC-propanolol (S-NC-Pro) and NC-prazosin (S-NC-Pra). D, a summary of different experimental conditions on STEP activity.
Given the fact that ERK1/2 is probably not a substrate for PP2B (Paul et al. 2003), it is therefore possible that another class of PPs act as a downstream mediator of PP2B to mediate the reversal of phosphorylated ERK1/2 to ERK1/2 in response to novelty exploration. One potential candidate that might mediate the direct dephosphorylation of ERK1/2 is the STEP, which is preferentially expressed in hippocampal neurons and inactivates ERK1/2 by dephosphorylating the regulatory residues of the kinases (Paul et al. 2003). Previous studies have indicated that STEP loses its ability to dephosphorylate ERK1/2 when it is phosphorylated by protein kinase A (PKA) on a regulatory serine residue within its ERK1/2-binding domain (Pulido et al. 1998). Dephosphorylation of this site, a reaction catalysed by several PPs, including PP1 (Nika et al. 2004) and PP2B (Paul et al. 2003), permits STEP to bind to and dephosphorylate ERK1/2. This raises the possibility that novelty exploration may cause an increase in dephosphorylation and activation of STEP to dephosphorylate ERK1/2. To test this notion, we compared the levels of phosphorylated STEP in the hippocampal CA1 region collected from all experimental groups. As previously reported, 61 kDa STEP, the predominant isoform expressed in the hippocampus, was presented as a doublet (Paul et al. 2003). The upper band was expected to be decreased after λ-phosphatase treatment, defining it as the phosphorylated form of STEP (Supplemental Fig. S2B). As shown in Fig. 6B, the levels of phosphorylated STEP were significantly upregulated in slices from stressed rats, supporting the regulation of STEP by behavioural stress in vivo. In addition, an exploration in the NC effectively reversed the stress-induced increase of STEP phosphorylation, whereas exploration in the HC or FC immediately after stress had no effect. The reversal effect of novelty exploration on the stress-induced increase of STEP phosphorylation was prevented in stressed rats that were injected with atropine or d-APV (Fig. 6C), 20 min before placement in the NC. Application of the PP2B inhibitors cyclosporin A (1 μm) or cypermethrin (1 μm), but not the PP1/2A inhibitor okadaic acid (1 μm) 20 min before placement in the NC also prevented the effect of novelty exploration on the stress-induced increase of STEP phosphorylation (Fig. 6C). In parallel studies, analysis of tyrosine phosphatase activity also showed that behavioural stress induced a marked decrease in STEP activity. This effect was completely reversed by novelty exploration, whereas exploration in the HC or the FC had no effect. The reversal effect of novelty exploration on the stress-induced decrease of STEP activity was also mimicked by carbachol administration and was prevented by pretreatment with atropine, d-APV, cyclosporin A or cypermethrin (Fig. 6D). These findings strongly support the conclusion that novelty exploration induces dephosphorylation and activation of STEP, at least in part, by an increase in PP2B activity to cause a reversal of stress-induced ERK1/2 hyperphosphorylation.
Discussion
A previous study from this laboratory has implicated a passive run-down of processes that maintain the effects of acute inescapable stress on subsequent hippocampal CA1 LTP and LTD induction (Yang et al. 2004). In the present study, we confirm and extend these original findings by specifically demonstrating that such stress effects can be completely reversed when animals are introduced into a novel environment immediately after the stress. Our results also provide a plausible mechanism which may underlie the novelty exploration-induced reversal of stress effects. Behavioural encounter with a non-stressful novel stimulus can increase hippocampal cholinergic activity, which in turn facilitates the NMDA receptor activation of the hippocampal CA1 neurons. Activation of NMDA receptors causes a rapid increase in the intracellular Ca2+ concentration, leading to stimulation of a PP2B-dependent dephosphorylation and activation of STEP and eventually causing a reversal of stress-induced ERK1/2 hyperphosphorylation.
In the present study, the reversal of stress effects on LTP and LTD was blocked by application of atropine. Thus, an increased hippocampal cholinergic activity seems to be one important factor for the novelty exploration-induced reversal of stress effects. This assumption is supported by the fact that activation of cholinergic receptors with carbachol before introduction of stressed rats into the HC mimicked the effect of novelty exploration (Fig. 3). Furthermore, it is well documented that novelty exploratory behaviour may enhance the activity of cholinergic neurons of the medial septum (Acquas et al. 1996; Aloisi et al. 1997), whose fibres densely innervate the hippocampus (Fibiger, 1982). Consistent with a key role of increased cholinergic activity in mediating the generation of hippocampal theta rhythm during exposure to spatial novelty (Vertes, 2005), we found that introduction of stressed animals to the NC significantly augmented theta frequency band and almost completely reversed the stress-induced suppression of basal electrocorticogram activity, which was abolished by atropine treatment before the onset of exploration (Yang Huang & Hsu, unpublished observations). In contrast to the finding of Straube et al. 2003) that novelty exploration-induced strengthening of dentate gyrus LTP is related to increased noradrenergic activity, agents that block noradrenergic receptors had no effect on the novelty exploration-induced reversal of stress effects. The reason for this discrepancy is not clear. This may be attributable partly to the use of a different novel environment stimulus, resulting in activation of different types of neuronal circuits or transmission systems. Another important difference is that Strabue et al. (2003) studied the novelty exploration-induced LTP reinforcement in the dentate gyrus of unstressed rats, whereas we studied the effect of novelty exploration in the CA1 region of stressed rats. Thus, it is possible that the mode of action of novelty exploration on the hippocampal function may vary dynamically according to the recent psychological state of animals and/or hippocampal areas. In fact, at the synaptic level, it is well established that the direction and magnitude of a synaptic change evoked by a given stimulus depend on the recent history of synaptic and cellular activity (Abraham & Bear, 1996).
The present study provides further evidence that the stress-induced sustained activation of ERK1/2 signalling appears to result from an upregulated phosphorylation status of STEP in neurons. Protein tyrosine phosphatases of STEP family have been found to lose their ability to dephosphorylate ERK1/2 when they are phosphorylated by PKA on the regulatory serine residue within their ERK1/2 binding domain (Pulido et al. 1998), and it is conceivable that phosphorylation events regulate the subcellular localization and/or the association with substrates of these phosphatases (Garton & Tonks, 1994). Moreover, we demonstrated that a novelty exploration can effectively increase STEP activity, thereby leading to a complete reversal of stress-induced ERK1/2 hyperphosphorylation. Together, these data suggest that constitutive STEP activity may have a significant role in regulating the duration of ERK1/2 activation and the subsequent downstream signalling cascades in preserving the malleability of synapses which have previously been strengthened or depressed. Consistent with this view, recent work demonstrates that STEP may function as a tonic brake on induction of the hippocampal CA1 LTP (Pelkey et al. 2002).
How might novelty exploration enhance the activation of STEP? Our results clearly demonstrate that NMDA receptor-mediated activation of PP2B during exploration of a novel environment may lie upstream of STEP to regulate its dephosphorylation and activation. This is consistent with previous observations that inhibition of PP2B activity in neuronal cultures abolished the increase in dephosphorylation of STEP induced by glutamate through NMDA receptors (Paul et al. 2003). Together these results suggest a decisive role for PP2B in the physiological regulation of STEP signalling in neurons. Further work will be necessary to elucidate the physical and functional association of PP2B with STEP. Although numerous studies have demonstrated that the levels of phosphorylated ERK1/2 can be influenced by PPs such as PP1 and MAPK phosphatase 3 (MKP3) (Muda et al. 1996; Nika et al. 2004), it seems unlikely that the novelty exploration-induced reversal of ERK1/2 hyperphosphorylation seen in the present study resulted from the activation of these PPs. Indeed, we found that inhibition of PP1/2A activity using okadaic acid did not affect the action of novelty exploration on the stress-induced increase of ERK1/2 phosphorylation, and that the level of MKP3 was not altered during exposure to spatial novelty.
Exploratory behaviour may correspond to a form of learning process (Platel & Porsolt, 1982) or information acquisition (Eichenbaum, 1996). The observation that the reversal of stress effects occurred only during the initial exposure to the NC, whereas re-exposure did not significantly modify stress effects, supports the intriguing possibility that exploratory learning may be associated with the reversal of stress effects. It has been reported that exposure to a novel environment may lead to a widespread and complete depotentiation of the most recently potentiated synapses (Diamond et al. 1994; Xu et al. 1998; Manahan-Vaughan & Braunewell, 1999). This active erasure of synaptic efficacy changes may provide protection from lasting effects of inconsequential inputs and endow the system with enhanced combinatorial plasticity (Dudai, 1996). Although the mechanisms that underlie stress effects on subsequent LTP and LTD are not fully understood, it was hypothesized that stress may proceed by an LTP-like mechanism in the hippocampal network and thereby causes the occlusion of LTP and the facilitation of LTD (Kim & Yoon, 1998; Huang et al. 2005). In support of this view, we have previously found that hippocampal CA1 LTP can usually be elicited by HFS in slices from stressed rats that have previously received depotentiating stimulation (Yang et al. 2004). The present data extend these findings to indicate that novelty exploration may activate an LTD induction mechanism (e.g. PP2B) to reverse previously established synaptic strength and prevent saturation of the information storage capacity of the hippocampal neuronal network.
In contrast to the present experiments, where exposure to a novel environment was found to have no influence on the induction of LTP or LTD in unstressed animals, previous studies report that novelty exploration can facilitate LTP induction in freely moving animals in both CA1 and dentate gyrus areas of hippocampus (Li et al. 2003; Straube et al. 2003; Davis et al. 2004). The reason for this discrepancy is unclear but could be attributable, at least in part, to the different time delay between the start of exploration and the application of the conditioning stimulation. Indeed, the facilitation of LTP was only observed when the novelty exploration started with a time window of about 10 min before the conditioning stimulation (Li et al. 2003).
At the Schaffer collateral–CA1 synapses, the induction of LTP/LTD requires the activation of NMDA receptors (Dudek & Bear, 1992; Bliss & Collingridge, 1993). This leads to an influx of Ca2+ within the dendritic spines, an absolutely necessary trigger for LTP/LTD. We now show, however, that intrahippocampal injection of the NMDA receptor antagonist d-APV blocks the effects of novelty exploration on the stress-induced impairment of LTP and facilitation of LTD but has no effects on the induction of LTP/LTD in unstressed animals. The lack of effects of the in vivod-APV administration on the in vitro LTP/LTD induction could be attributable to the effect of d-APV having worn off by the time the slices were prepared. Two lines of evidence support this conclusion. First, slices obtained from rats injected with d-APV exhibited a normal NMDA receptor-mediated synaptic response (Supplemental Fig. S3A). Second, LTP induction in slices from those rats is NMDA receptor dependent (Supplemental Fig. S3B).
We conclude that the reversal of stress effects on hippocampal CA1 LTP and LTD by novelty exploration is likely to be accounted for by an increase of cholinergic activity to promote NMDA receptor-mediated activation of PP2B, thereby leading to dephosphorylation and activation of STEP. The activated STEP then resets the basal levels of synaptic activity by regulating ERK1/2 activity through direct dephosphorylation of the tyrosine residue in its activation domain in neurons. The results of our experiments here reveal a novel strategy to counter the harmful effect of stress on hippocampal synaptic function. They may have interesting implications for the understanding of the persistent effects of stress on hippocampal function and the way to reverse it independent of drug treatments.
Supplementary Material
Acknowledgments
This work was financially supported by a research grant from the National Health Research Institute (NHRI-EX95-9215NI) of Taipei, Taiwan.
References
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- Acquas E, Wilson C, Fibiger HC. Conditioned and unconditioned stimuli increase frontal cortical and hippocampal acetylcholine release: effects of novelty, habituation, and fear. J Neurosci. 1996;16:3089–3096. doi: 10.1523/JNEUROSCI.16-09-03089.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloisi AM, Casamenti F, Scali C, Pepeu G, Carli G. Effects of novelty, pain and stress on hippocampal extracellular acetylcholine levels in male rats. Brain Res. 1997;748:219–226. doi: 10.1016/s0006-8993(96)01304-2. [DOI] [PubMed] [Google Scholar]
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Blum S, Moore AN, Adams F, Dash PK. A mitogen-activated protein kinase cascade in the CA1/CA2 subfield of the dorsal hippocampus is essential for long-term spatial memory. J Neurosci. 1999;19:3535–3544. doi: 10.1523/JNEUROSCI.19-09-03535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- Davis CD, Jones FL, Derrick BE. Novel environments enhance the induction and maintenance of long-term potentiation in the dentate gyrus. J Neurosci. 2004;24:6497–6506. doi: 10.1523/JNEUROSCI.4970-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond DM, Bennett MC, Stevens KE, Wilson RL, Rose GM. Exposure to a novel environment interferes with the induction of hippocampal primed burst potentiation in the behaving rat. Psychobiology. 1990;18:273–281. [Google Scholar]
- Diamond DM, Fleshner M, Rose GM. Psychological stress repeatedly blocks hippocampal primed burst potentiation in behaving rats. Behav Brain Res. 1994;62:1–9. doi: 10.1016/0166-4328(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Park CR, Woodson J. Stress generates emotional memories and retrograde amnesia by inducing an endogenous form of hippocampal LTP. Hippocampus. 2004;14:281–291. doi: 10.1002/hipo.10186. [DOI] [PubMed] [Google Scholar]
- Dudai Y. Consolidation: fragility on the road to the engram. Neuron. 1996;17:367–370. doi: 10.1016/s0896-6273(00)80168-3. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-d-aspartate receptor blockade. Proc Natl Acad Sci U S A. 1992;89:4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. Is the rodent hippocampus just for ‘place’? Curr Opin Neurobiol. 1996;6:187–195. doi: 10.1016/s0959-4388(96)80072-9. [DOI] [PubMed] [Google Scholar]
- English JD, Sweatt JD. Activation of p42 mitogen-activated protein kinase in hippocampal long term potentiation. J Biol Chem. 1996;271:24329–24332. doi: 10.1074/jbc.271.40.24329. [DOI] [PubMed] [Google Scholar]
- Fibiger HC. The organization and some projections of cholinergic neurons of the mammalian forebrain. Brain Res. 1982;257:327–388. doi: 10.1016/0165-0173(82)90011-x. [DOI] [PubMed] [Google Scholar]
- Garcia R. Stress, hippocampal plasticity, and spatial learning. Synapse. 2001;40:180–183. doi: 10.1002/syn.1040. [DOI] [PubMed] [Google Scholar]
- Garton AJ, Tonks NK. PTP-PEST: a protein tyrosine phosphatase regulated by serine phosphorylation. EMBO J. 1994;13:3763–3771. doi: 10.1002/j.1460-2075.1994.tb06687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemann C, Blank JL. The ups and downs of MEK kinase interactions. Cell Signal. 2001;13:863–875. doi: 10.1016/s0898-6568(01)00220-0. [DOI] [PubMed] [Google Scholar]
- Huang CC, Yang CH, Hsu KS. Do stress and long-term potentiation share the same molecular mechanisms? Mol Neurobiol. 2005;32:223–235. doi: 10.1385/MN:32:3:223. [DOI] [PubMed] [Google Scholar]
- Impey S, Mark M, Villacres EC, Poser S, Chavkin C, Storm DR. Induction of CRE-mediated gene expression by stimuli that generate long-lasting LTP in area CA1 of the hippocampus. Neuron. 1996;16:973–982. doi: 10.1016/s0896-6273(00)80120-8. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Schroder N, Netto CA, Medina JH. Novelty causes time-dependent retrograde amnesia for one-trial avoidance in rats through NMDA receptor- and CaMKII-dependent mechanisms in the hippocampus. Eur J Neurosci. 1999;11:3323–3328. doi: 10.1046/j.1460-9568.1999.00742.x. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Foy MR, Thompson RF. Behavioral stress modifies hippocampal plasticity through N-methyl-d-aspartate receptor activation. Proc Natl Acad Sci U S A. 1996;93:4750–4753. doi: 10.1073/pnas.93.10.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Yoon KS. Stress: metaplastic effects in the hippocampus. Trends Neurosci. 1998;21:505–509. doi: 10.1016/s0166-2236(98)01322-8. [DOI] [PubMed] [Google Scholar]
- Kitchigina V, Vankov A, Harley C, Sara SJ. Novelty-elicited, noradrenaline-dependent enhancement of excitability in the dentate gyrus. Eur J Neurosci. 1997;9:41–47. doi: 10.1111/j.1460-9568.1997.tb01351.x. [DOI] [PubMed] [Google Scholar]
- Klukowski G, Harley CW. Locus coeruleus activation induces perforant path-evoked population spike potentiation in the dentate gyrus of awake rat. Exp Brain Res. 1994;102:165–170. doi: 10.1007/BF00232449. [DOI] [PubMed] [Google Scholar]
- Leung LS. Behavior-dependent evoked potentials in the hippocampal CA1 region of the rat. I. Correlation with behavior and EEG. Brain Res. 1980;198:95–117. doi: 10.1016/0006-8993(80)90347-9. [DOI] [PubMed] [Google Scholar]
- Li S, Cullen WK, Anwyl R, Rowan MJ. Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nat Neurosci. 2003;6:526–531. doi: 10.1038/nn1049. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Braunewell KH. Novelty acquisition is associated with induction of hippocampal long-term depression. Proc Natl Acad Sci U S A. 1999;96:8739–8744. doi: 10.1073/pnas.96.15.8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser E, Mathiesen I, Andersen P. Association between brain temperature and dentate field potentials in exploring and swimming rats. Science. 1993;259:1324–1326. doi: 10.1126/science.8446900. [DOI] [PubMed] [Google Scholar]
- Muda M, Theodosiou A, Rodrigues N, Boschert U, Camps M, Gillieron C, Davies K, Ashworth A, Arkinstall S. The dual specificity phosphatases M3/6 and MKP-3 are highly selective for inactivation of distinct mitogen-activated protein kinases. J Biol Chem. 1996;271:27205–27208. doi: 10.1074/jbc.271.44.27205. [DOI] [PubMed] [Google Scholar]
- Nika K, Hyunh H, Williams S, Paul S, Bottini N, Tasken K, Lombroso PJ, Mustelin T. Haematopoietic protein tyrosine phosphatase (HePTP) phosphorylation by cAMP-dependent protein kinase in T-cells: dynamics and subcellular location. Biochem J. 2004;378:335–342. doi: 10.1042/BJ20031244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Nairn AC, Wang P, Lombroso PJ. NMDA-mediated activation of the tyrosine phosphatase STEP regulates the duration of ERK signaling. Nat Neurosci. 2003;6:34–42. doi: 10.1038/nn989. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- Pelkey KA, Askalan R, Paul S, Kalia LV, Nguyen TH, Pitcher GM, Salter MW, Lombroso PJ. Tyrosine phosphatase STEP is a tonic brake on induction of long-term potentiation. Neuron. 2002;34:127–138. doi: 10.1016/s0896-6273(02)00633-5. [DOI] [PubMed] [Google Scholar]
- Platel A, Porsolt RD. Habituation of exploratory activity in mice: a screening test for memory enhancing drugs. Psychopharmacology. 1982;78:346–352. doi: 10.1007/BF00433739. [DOI] [PubMed] [Google Scholar]
- Pulido R, Zuniga A, Ullrich A. PTP-SL and STEP protein tyrosine phosphatases regulate the activation of the extracellular signal-regulated kinases ERK1 and ERK2 by association through a kinase interaction motif. EMBO J. 1998;17:7337–7350. doi: 10.1093/emboj/17.24.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson MS, Zhang T, Li HL, Mulvaney JM. Activation of the p38 mitogen-activated protein kinase pathway by gonadotropin-releasing hormone. Endocrinology. 1999;140:1310–1318. doi: 10.1210/endo.140.3.6579. [DOI] [PubMed] [Google Scholar]
- Selcher JC, Atkins CM, Trzaskos JM, Paylor R, Sweatt JD. A necessity for MAP kinase activation in mammalian spatial learning. Learn Mem. 1999;6:478–490. doi: 10.1101/lm.6.5.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Dryver E. Effect of stress and long-term potentiation (LTP) on subsequent LTP and the theta burst response in the dentate gyrus. Brain Res. 1994;666:232–238. doi: 10.1016/0006-8993(94)90777-3. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Foy MR, Levine S, Thompson RF. Unpredictable and uncontrollable stress impairs neuronal plasticity in the rat hippocampus. Brain Res Bull. 1989;24:663–667. doi: 10.1016/0361-9230(90)90005-k. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Gallegos RA, Breindl A. Transient and persistent consequences of acute stress on long-term potentiation (LTP), synaptic efficacy, theta rhythms and bursts in area CA1 of the hippocampus. Synapse. 1997;26:209–217. doi: 10.1002/(SICI)1098-2396(199707)26:3<209::AID-SYN2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Thompson RF. Acute stress impairs (or induces) synaptic long-term potentiation but does not affect paired-pulse facilitation in the stratum radiatum of rat hippocampus. Synapse. 1992;11:262–265. doi: 10.1002/syn.890110311. [DOI] [PubMed] [Google Scholar]
- Straube T, Korz V, Balschun D, Frey JU. Requirement of β-adrenergic receptor activation and protein synthesis for LTP-reinforcement by novelty in rat dentate gyrus. J Physiol. 2003;552:953–960. doi: 10.1113/jphysiol.2003.049452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Tiesinga PH, Fellous JM, Jose JV, Sejnowski TJ. Computational model of carbachol-induced delta, theta, and gamma oscillations in the hippocampus. Hippocampus. 2001;11:251–274. doi: 10.1002/hipo.1041. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Hippocampal theta rhythm: a tag for short-term memory. Hippocampus. 2005;15:923–935. doi: 10.1002/hipo.20118. [DOI] [PubMed] [Google Scholar]
- Watabe AM, Zaki PA, O'Dell TJ. Coactivation of β-adrenergic and cholinergic receptors enhances the induction of long-term potentiation and synergistically activates mitogen-activated protein kinase in the hippocampal CA1 region. J Neurosci. 2000;20:5924–5931. doi: 10.1523/JNEUROSCI.20-16-05924.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Anwyl R, Rowan MJ. Behavioural stress facilitates the induction of long-term depression in the hippocampus. Nature. 1997;387:497–500. doi: 10.1038/387497a0. [DOI] [PubMed] [Google Scholar]
- Xu L, Holscher C, Anwyl R, Rowan MJ. Glucocorticoid receptor and protein/RNA synthesis-dependent mechanisms underlie the control of synaptic plasticity by stress. Proc Natl Acad Sci U S A. 1998;95:3204–3208. doi: 10.1073/pnas.95.6.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, McEwen BS, Pavlides C. Site and time dependent effects of acute stress on hippocampal long-term potentiation in freely behaving rats. Exp Brain Res. 2003;152:52–59. doi: 10.1007/s00221-003-1519-0. [DOI] [PubMed] [Google Scholar]
- Yang CH, Huang CC, Hsu KS. Behavioral stress modifies hippocampal synaptic plasticity through corticosterone-induced sustained extracellular signal-regulated kinase/mitogen-activated protein kinase activation. J Neurosci. 2004;24:11029–11034. doi: 10.1523/JNEUROSCI.3968-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CH, Huang CC, Hsu KS. Behavioral stress enhances hippocampal CA1 long-term depression through the blockade of the glutamate uptake. J Neurosci. 2005;25:4288–4293. doi: 10.1523/JNEUROSCI.0406-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.