Abstract
Fluorescence is increasingly used for in vivo imaging and has provided remarkable results. Yet this technique presents several limitations, especially due to tissue autofluorescence under external illumination and weak tissue penetration of low wavelength excitation light. We have developed an alternative optical imaging technique by using persistent luminescent nanoparticles suitable for small animal imaging. These nanoparticles can be excited before injection, and their in vivo distribution can be followed in real-time for more than 1 h without the need for any external illumination source. Chemical modification of the nanoparticles' surface led to lung or liver targeting or to long-lasting blood circulation. Tumor mass could also be identified on a mouse model.
Keywords: biodistribution, in vivo optical imaging, nanoparticles, phosphorescent nanoparticles
Because of the growing demand for imaging tools for biomedical research and medicine, existing imaging systems have been rapidly improved and new techniques have been developed during the past decades. Nowadays, MRI, microcomputed tomography (microCT), ultrasound, positron emission tomography (PET), optical coherence tomography (OCT), and other major imaging systems are available to scientists and clinicians. Each technique has advantages and limitations, thus making them complementary (1). These techniques are increasingly used for biodistribution studies, because they can significantly reduce the number of required animals and increase the experimental data harvested for each animal in longitudinal studies. However, the high cost of several of these techniques and a number of technological barriers impair their widespread use.
Optical imaging, in which photons are the information source, represents a rapidly expanding field, with direct applications in pharmacology, molecular and cellular biology, and diagnostics. With the recent development of more sensitive optical sensors and new, powerful probes such as semiconductor nanocrystals (2–5), fluorescent proteins (6, 7), or near-infrared fluorescent molecules (8–10), optical imaging can now be considered for in vivo studies.
In vivo optical imaging using fluorescent probes is commonly used (11) but still presents numerous disadvantages. The first one is the autofluorescence (12) from tissue organic components due to constant probe illumination during signal acquisition. This autofluorescence often results in poor signal-to-noise ratio. In addition, deep tissue imaging is difficult because of intrinsic tissue signal attenuation. The probe's emission has thus to be tuned in the tissue transparency window (13) (wavelength from 650 nm to the infrared), in which light attenuation is largely due to scattering rather than to absorption.
To overcome these difficulties, we have presently developed inorganic luminescent nanoparticles (NPs), which are suitable for in vivo imaging and can avoid most of inherent problems encountered in classical optical systems. The key element of this technology is based on a new generation of long luminescent nanoparticles emitting in the red to near-infrared range, which can be optically excited before in vivo local or systemic injection. The long-lasting afterglow (also called persistent luminescence) can reach several hours and permits the removal of the background noise originating from in situ excitation. Thus, the significant signal-to-noise ratio improvement allows detection in rather deep organs and real-time biodistribution monitoring of active elements hours after injection.
Results
NP Synthesis.
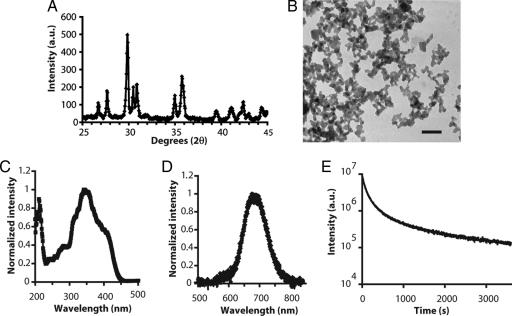
Our starting material composition was the previously described MgSiO3:Eu2+,Dy3+,Mn2+, which was obtained by solid state reaction, and which shows long persistent red luminescence (14). To optimize the emission in the infrared and to prepare NPs, we selected the composition Ca0.2Zn0.9Mg0.9Si2O6, which was doped with the same luminescent ions (Eu2+, Dy3+, Mn2+). The characteristics of this material are summarized in Fig. 1.
Fig. 1.
Physical characteristics of long afterglow nanoparticles. (A) NPs showed a clinoenstatite-like structure. (B) Transmission electronic microscopy images of the synthesized NPs. (Scale bar: 200 nm.) (C) Excitation spectrum. (D) Long afterglow emission spectrum. (E) Time dependence of the luminescence intensity of the NPs. NPs (10 mg) were put in 96-well plates under direct exposure to a 6-W UV lamp for 5 min. The luminous intensity was quantified straightforward by using an intensified charge-coupled device (ICCD) camera (PhotonImager; Biospace). Data analysis was performed by signal integration for 5 s. The luminous decay data were fit by a power law function for time >100 s.
Because persistent luminescent materials are generally synthesized by a solid-state reaction, giving micrometer-sized particles, we developed a Sol-Gel approach synthesis (15) to decrease particles size (see Methods). The resulting crystalline material (Fig. 1A) was ground by using a mortar and a pestle, and the smallest particles were isolated by selective sedimentation. Electronic microscopy analysis showed that the NPs exhibited a quite narrow size distribution, with particles diameter ranging from 50 to 100 nm (Fig. 1B).
In this material, trapped centers are created through the introduction of small amounts of Dy3+, and Mn2+ is the final emitting center receiving energy from electron-hole pair recombination. Rare-earth ions serve as primary acceptors of the energy, which is thermally released to the manganese for several hours. The symmetry and the crystal field strength at the Mn2+ site in the synthesized material are responsible for a Mn2+ emission in the red and the near-infrared part of the spectrum range, corresponding to the transition from the 4T1(4G) excited state to the 6A1(6S) fundamental state. As shown in Fig. 1D, the afterglow emission spectrum was quite large, with maximum intensity ≈690 nm. The broad excitation band in the UV (Fig. 1C) cannot be only assigned to manganese absorption bands, thus demonstrating that the long-lasting afterglow is due to energy transfer within the material.
For signal acquisition, after removal of the excitation source, we used a photon-counting system based on a cooled GaAs intensified charge-coupled device (ICCD) camera (PhotonImager; Biospace, Paris, France) without an external illumination system. The luminous intensity decay was typical of a persistent luminescence material (16) and was detectable for >24 h when kept in the dark. The decay kinetics (Fig. 1E) were found to be close to a power law I ≈ I0 × t−n (n = 0.96, R2 = 0.996) after the first 100 s [supporting information (SI) Fig. 5].
In Vivo NP Local Injection.
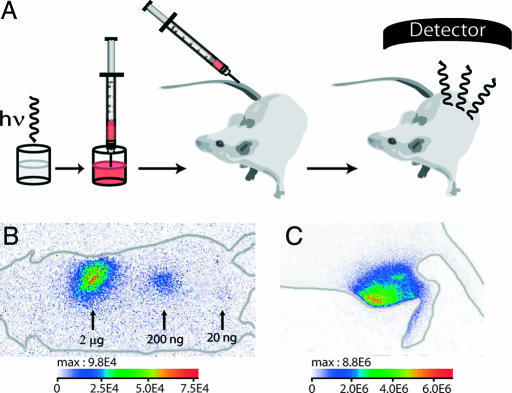
We thus first demonstrated that the light level produced by these NPs was sufficient to yield a localizable signal under a few millimeters of tissues, such as for s.c. or intramuscular injection. A suspension of NPs was injected s.c. to the unshaved back of a Swiss mouse. The excitation of the NP suspension before injection was accomplished by direct exposure to a 6-W UV lamp for 5 min at a distance of 2 cm (Fig. 2A). To test the lowest detectable dose, we injected suspensions (20 μl) at different concentrations (100, 10, and 1 μg/ml) corresponding approximately to 2 × 1010 (2 μg), 2 × 109 (200 ng), and 2 × 108 (20 ng) NPs in three different localizations on the mouse back. The two highest doses (2 μg and 200 ng) were easily monitored by using a 2-min acquisition time. The lowest dose administrated (20 ng) also produced a detectable signal with a satisfactory signal-to-noise ratio superior to 5 (Fig. 2B and SI Fig. 6).
Fig. 2.
Principles of in vivo experiments and first in vivo images. (A) A suspension containing a proper amount of NPs is excited with a 6-W UV lamp and is directly injected to an anesthetized mouse. The signal is then acquired with an intensified charge-coupled device (CCD) camera. (B) Image of three s.c. injections of NPs (2 μg, 200 ng, 20 ng). The different localizations are labeled with arrows, and the corresponding NP amounts are indicated. The acquisition was performed during the 2 min after injection. (C) Image of an intramuscular injection (200 μg) corresponding to a 90-s acquisition. The luminous intensity is expressed in photons per s·cm2·steradians (sr).
To confirm the feasibility of deep-tissue imaging, we then tested intramuscular injections with a higher dose (20 μl at 10 mg/ml). Once again, the signal was clearly detectable in the tibial cranial muscle. As depicted in Fig. 2C, the signal was diffuse throughout the muscle, although the local injection was punctual. Thus, the use of persistent luminescent NPs provides simple observation of light diffusion in tissues by avoiding external illumination. A signal was also noticed originating from the paw of the mouse, where no NPs were injected. This phenomenon was attributed to signal reflection emitted by the NPs in the muscle because of classical light ability to reflect on objects.
Surface Modifications of the Probes.
Modifications on surface of probes have to be possible to ensure and broaden in vivo applications. We used classical coating procedures developed for silicate materials. After thermal treatment, partial erosion of NPs with aqueous base led to surface free hydroxyl groups, which were used for covalent linkage of different functional groups. The free hydroxyl groups gave to NPs a natural, negative, surface electric potential, called Zeta potential, at neutral pH (−34.3 mV). The NPs were then reacted with 3-aminopropyl-triethoxysilane (APTES), which was coupled to the surface. This reaction provided positively charged NPs (referred to as amino-NPs) resulting from the presence of free amino groups at the surface. The success of the grafting procedure was assessed by Zeta potential measurements (+35.8 mV at pH≈7) and a positive test with trinitrobenzene sulfonate (TNBS). The APTES in excess was removed by several sedimentation washing procedures.
The surface charge of the amino-NPs was reversed by reaction with diglycolic anhydride, which reacted with amines to give free carboxyl groups. The Zeta potential of these particles (carboxyl-NPs) at neutral pH was negative (−37.3 mV), as expected. We also conducted peptide coupling of amines with mPEG5,000-COOH [carboxyl-methoxy-polyethyleneglycol formula weight (F.W.):≈5,000 g/mol]. This reaction led to neutral particles (+5.1 mV), partly originating from the charge-shielding effect of PEG. The unreacted reagents were always removed by three or more centrifugation-washing steps.
We have thus obtained three types of particles bearing different surface charges. Although it is well known that surface charges can affect liposomes or nanoparticles biodistribution, real-time biodistribution data are generally not provided. Animals have to be killed at different times to determine biodistribution kinetics. We thus determined whether the persistent luminescent NPs could provide real-time in vivo biodistribution imaging in mice after systemic tail vein injection of 1 mg of NPs (corresponding to 1013 particles).
In Vivo Distribution of Differently Charged Nanoparticles.
For positive amino-NPs, important lung retention was observed (Fig. 3B and SI Movie 1). During the first hour, there was little change in this distribution, except a progressive NP release from lungs to liver and spleen. Two reasons can explain this biodistribution pattern (17). The first one is a nonspecific electrostatic NP interaction with the negative charges displayed by plasmatic membranes of capillary endothelial cells, such as sulfated proteoglycans and glycosylaminoglycans. Indeed, the first highly vascularized organ that is encountered by NPs after an i.v. tail vein injection is the lung, in which blood flow is slower due to the capillary circulation. Thus, the nonspecific interactions become predominant among all forces involved, causing NP to be trapped in the lungs. Another explanation could be provided by NP aggregation with negatively charged blood components, leading to trapping agglomerates of increased size in the narrow lung capillaries.
Fig. 3.
NP surface modification and in vivo biodistristribution. (A) Schematic representation of NP surface modification. (i) Amino-NPs were synthesized by reaction with 3-aminopropyltriethoxysilane. (ii) Carboxyl-NPs resulted from a reaction of amino-NPs with diglycolic anhydride. (iii) PEG-NPs were achieved by a peptidic coupling of amino-NPs with PEG5000COOH (25). (B–F) Optical imaging of mouse with 1-mg tail vein injections of differently charged NPs. (B) Amino-NPs. (C) Carboxyl-NPs. (D) Preinjection of anionic liposomes (6 μmol, 100 μl) 5 min before carboxyl-NP injection. (E) PEG-NPs. (F) Preinjection of anionic liposomes (6 μmol, 100 μl) 5 min before PEG-NP injection. All images correspond to a 2-min signal acquisition performed between the times indicated above each image. The luminous intensity is expressed in photons per s·cm2·steradians (sr). Similar results were obtained in triplicate experiments.
The negatively charged carboxyl-NPs did not present pulmonary sequestration (Fig. 3C and SI Movie 2), probably because negative NPs do not normally interact with negatively polarized capillary endothelial cells and can therefore remain in circulation for a longer time than positive NPs. However, these negative NPs were rapidly cleared from blood flow by liver uptake. This biodistribution pattern presumably resulted from a rapid opsonization and an uptake by endothelial and Kupffer cells of the reticuloendothelial system (RES), as is generally observed for nanoparticles or liposomes (18, 19). Similar images were obtained in rats with the same amount of NPs (data not shown).
We then synthesized PEG-coated-NPs (referred to as PEG-NPs) to avoid rapid clearance from blood and to increase circulation time, because the use of PEG capping has already been shown to increase the circulation time of several colloidal systems (20). Neutral PEG-NPs covered by a PEG shield were able to circulate longer, as assessed by a diffuse signal throughout the mouse body that lasted during all of the acquisition time (Fig. 3E and SI Movie 3). However, at a longer acquisition time (30 min), the distribution clearly showed liver accumulation. These differential distributions are in agreement with published works on other types of NPs (21–23).
Liver and spleen RES uptake is an obvious problem that has to be overcome for efficient targeting to other organs, such as tumors. Several authors have described techniques to avoid or minimize RES uptake (24). We have analyzed the effect of preinjecting polyinosinic acid (data not shown) and anionic liposomes containing equimolar quantity of phosphatidylcholine, cholesterol, and phosphatidylserine. The liposomes were formed by a dry lipid film hydration–extrusion method and had a negative Zeta potential of −31 mV, with a diameter of ≈300 nm, which has been shown to lead to rapid RES removal (25). The i.v. tail vein preinjection of anionic liposomes to mice (6 μmol, 100 μl injected 5 min before NP injection) presaturated RES uptake capacity and greatly improved the circulation time of the negative carboxyl-NPs, even if those NPs still finally localized in the liver and the spleen (Fig. 3D and SI Movie 4).
The RES saturation by anionic liposomes preinjection led to an even more important effect for PEG-NPs in terms of circulation time (Fig. 3F and SI Movie 5). Indeed, the signal was diffuse for a longer period through the whole body, reflecting NP persistence in mouse vasculature. Of note, mouse femoral and carotid arteries could be visualized even if they appeared blurred because of light scattering. After 15 min, the spleen contour was clearly defined, although >30 min were necessary to define liver from the upper body circulation.
Thus, although the preinjection of RES-saturating liposomes does not change NP ultimate distribution, it significantly increased NP circulation time (SI Fig. 7) and thus the NP potential for specific targeting.
Ex vivo optical imaging of organs (liver, spleen, kidneys, and lungs) directly removed from mice after euthanasia corroborate the specific localization of the differently charged NPs (SI Fig. 8). NPs were only detected in blood when PEG-NPs were used.
In Vivo Distribution of PEG-NPs in Tumor-Bearing Mice.
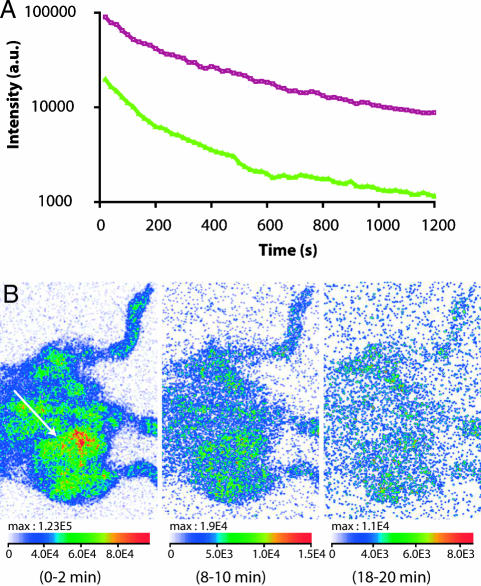
Finally, we used the new luminescent nanoprobes to potentially visualize tumor vascularization. A body-shaved C57BL/6 mouse bearing an s.c. implanted Lewis lung carcinoma (3LL) tumor in the inguinal region was preinjected with liposomes (6 μmol, 100 μl), followed after 5 min by PEG-NPs injection. The presence of melanin in C57BL/6 mice is of particular disadvantage for in vivo optical imaging. Indeed, the attenuation factor of melanin is very high and covers all visible spectrum. Thus, the overall detected intensity was significantly lower (by a 5–7 factor) than for a Swiss mouse (Fig. 4A). However, using our luminescent nanoparticles, the signal was still easily detectable, and biodistribution was optically followed. The tumor region was then clearly revealed by an increase in the local luminous intensity (Fig. 4B), which was attributed to the high vascularization of 3LL tumors. Yet, compared with the experiment with the Swiss mouse in similar RES-saturating conditions, the washout from the tumor and blood clearance of PEG-NPs in C57BL/6 mouse was rapid.
Fig. 4.
Imaging of a tumor-bearing mouse. (A) Overall luminous intensity detected in a Swiss mouse (purple curve) and a body-shaved C57BL/6 mouse bearing a 3LL tumor (green curve). Tail vein i.v preinjection of anionic liposomes (6 μmol, 100 μl injected 5 min before NP injection) was followed by tail vein injection of 1 mg of PEG-NPs. A region of interest covering the whole mouse was manually selected and analyzed by a period of 20 s. (B) Visualization of the hypervascularization of a 3LL tumor (white arrow) with rapid clearance.
Discussion
In the present work, we have synthesized several promising long-lasting luminescent probes. Their persistent luminescent properties not only allow their use for in vivo imaging, but also avoid difficulties in signal analysis linked to external illumination usually necessary for whole-body optical imaging. The advantages provided by this new optical technique are mainly due to the absence of autofluorescence and to the simplicity of the in vivo method of use.
Our approach of using long-lasting luminescent NPs that are excited before in vivo injection was made possible thanks to the use of long-lasting afterglow phosphors that can light up for a long period in the darkness after irradiation with sunlight or UV irradiation. The long-lasting afterglow phenomenon has been known for decades, and materials exhibiting this property are widely used for applications such as emergency guiding signs or luminous paints. However, we have not found any reported use of these types of compounds for in vivo imaging.
Furthermore, the NPs developed in this work, which are particles to have optical properties with nanometric size, are previously unreported, although micrometer-sized particles with red emission have been made available. With a broad afterglow emission spectrum ranging from 600 to 800 nm, with a maximum intensity of ≈690 nm, they are of particular interest, because the emission spectrum falls within the tissue transparency window and can therefore be used for in vivo deep-tissue imaging.
The technique developed here is an alternative approach to the one developed by So et al. (4) on bioluminescent luciferase bound to quantum dots, even if they both address the difficulty of autofluorescence during in vivo optical imaging. The in vivo luminous intensity emitted by our nanoparticles can be roughly compared with those of luciferase-associated quantum dots, although the experiments were performed on different mouse models (Swiss mouse, in our case, versus nude mouse). After intramuscular injection, 10 times more photons per s·cm2·steridian (sr) were detected in the work of So et al. than in our study, but because we injected 10 times fewer particles, luminous intensity per particle was equivalent for both techniques. However, even if the BRET complex is of particular interest for in vivo imaging, real-time biodistribution analysis is easier to handle in our case because there is no need for substrate-protein colocalization.
We have also shown that these long-lasting luminescent NPs are easily amenable to chemical surface modification. This opens a whole area of potential applications for biological applications. The demonstration that differently charged NPs possess markedly altered biodistribution is of particular interest for pharmacological applications. Furthermore, the use of anionic liposomes before NP injection has shown to be a powerful method to improve targeting to specific organs, because it allows NPs to remain longer in blood circulation because of RES saturation. Finally, the demonstration that a mouse tumor can be visualized with the described long-lasting luminescent NPs provides important perspectives for tumor imaging and cancer therapy evaluation. Because the total amount of available light, which is proportional to the amount of injected nanoparticles, could be precisely controlled, persistent luminescent NPs might represent a powerful tool for quantification in optical imaging.
In conclusion, near-infrared, persistent luminescent nanoparticles represent a previously unreported imaging system and in vitro diagnostic tool of broad potential for biologists and physiologist.
Materials and Methods
Animal Ethics.
Experiments were conducted following the NIH recommendations and in agreement with a regional ethic committee for animal experimentation.
NP Synthesis.
All of the reactants are analytical-grade and used without any further purification. The raw materials are magnesium nitrate [Mg(NO3)2,6H2O], zinc chloride (ZnCl2), calcium chloride (CaCl2,2H2O), europium chloride (EuCl3,6H2O), dysprosium nitrate [Dy(NO3)3,5H2O], manganese chloride (MnCl2,4H2O), and tetraethoxysilane (TEOS). All of the salts were dissolved in deionized water acidified at pH 2 by addition of concentrate nitric acid. TEOS was then added rapidly, and the solution was stirred vigorously at room temperature until the solution became limpid (≈1 h). The solution was heated at 70°C until the sol-to-gel transition occured (≈2 h). The wet gel was then dried in an oven at 110°C for 20 h. The resulting opaque gel was directly fired in a zircone crucible in a weak reductive atmosphere [Noxal 4 (Air Liquide, Düsseldorf, Germany): 10% H2, 90% Ar) at 1,050°C for 10 h. The powder was ground with an agate mortar and pestle to obtain the desired nanoparticles.
The powder was dispersed by sonication in 5 mM NaOH solution at a concentration of 10 mg of NP per ml. After neutralization with HCl, the suspension was diluted with distilled water at a concentration of 2.5 mg/ml and gently centrifuged with a SANYO MSE Mistral 1000 (670 × g/15 min) to eliminate the largest particles. Acetone corresponding to 25% of the supernatant volume was added to promote sedimentation of the NPs. The suspension was then centrifuged at 3,400 × g for 30 min. After removal of the supernatant, in which no NPs were detected by using Dynamic Light Scattering, the NPs were dried in a vacuum oven.
The diffractogram was recorded on a Philips (Eindhoven, The Netherlands) RW0830 diffractometer by using CuKα radiation in reflection mode, between 25° and 45° in 2θ (step, 0.05°; accumulation delay, 1 s).
Optical spectra were recorded with a Varian (Palo Alto, CA) Cary-Eclipse Fluorescence spectrophotometer by using the phosphorescence mode with a delay and a gate time of 300 ms (for excitation spectrum: λem, 690 nm; excitation slit; 5 nm; emission slit, 20 nm) (for excitation spectrum: λex, 340 nm; excitation slit, 20 nm; emission slit, 5 nm).
Dynamic light scattering and Zeta potential experiments were performed on a Zetasizer Nano ZS (Malvern Instruments, Southborough, MA) equipped with a 632.8-nm helium neon laser and 5-mW power, with a detection angle at 173° (noninvasive back scattering).
Numbers of NPs per Milligram of Powder.
Because the NPs have a similar RX pattern than clinoenstatite, we used the cell parameter of this compound to approximate particle numbers per milligram (25). A spherical particle of 50 nm diameter has thus ≈7.8 × 104 cells, corresponding to 1.05 × 10−16 g per particle. Thus, 1 mg of NPs corresponds approximatively to 1013 particles.
Surface Coating of NPs.
To obtain amino-NPs, 200 mg of NPs were redispersed in 20 ml of dimethylformamide (DMF), and 100 μl of 3-aminopropyl-triethoxysilane (APTES) was added under constant stirring. The suspension was then stirred overnight at 80°C. A series of centrifugation and redispersion in DMF were performed to wash the excess of APTES.
To obtain the carboxyl-nanoprobes, 52.8 mg of amino-NPs were redispersed in dimethylformamide (DMF) and diglycolic anhydride (12.2 mg, 0.11 mmol) was added. The suspension was stirred overnight at room temperature. Again, the excess of reactant was removed by the washing procedure.
PEG-NPs were synthesized by mixing 53.4 mg of amino-NPs redispersed in 10 ml of dimethylformamide (DMF) with methoxy-PEG5,000-COOH (26) (534 mg, 0.11 mmol), benzotriazol-1-yl-oxy-Tris(dimethylamino)-phosphonium hexafluorophosphate (BOP reagent, 52 mg, 0.12 mmol) and thriethylamine (15 μl). The resulting suspension was stirred overnight at room temperature to provide the desired neutral PEG-NPs. The excess of reactant was removed by the washing procedure.
The different surface modified NPs were dispersed at a concentration of 0.1 mg/ml in 20 mM NaCl to perform Zeta potential measurements.
Synthesis of Anionic Liposomes.
Egg-phosphatidylcholine (11.78 mg, 15.5 μmol), cholesterol (5.99 mg, 15.5 μmol) and lα-phosphatidylserine (12.2 mg, 15.5 μmol) were dissolved in 1 ml of CHCl3. After solvent removal under vacuum, the film obtained was hydrated at 37°C overnight with 1.935 ml of PBS 10 mM. The suspension was extruded through 0.4-μm filters on a miniextruder (Avanti Polar Lipids, Alabaster, AL). Before i.v. injection, the suspension was diluted with 10 mM PBS to obtain liposome suspension at 6 mM lipid concentration.
In Vivo Local and Systemic Injections.
Swiss mice were anesthetized by i.p. injection of a mix of ketamine (85.8 mg/kg) (Centravet, Plancoët, France) and Xylazine (3.1 mg/kg) (Bayer, Leverkusen, Germany) diluted in 150 mM NaCl, and different types of injection were realized. After imaging, mice were euthanized.
Data Analysis of the s.c. Injections.
For the lowest dose, signal intensity was superior to 10 (SI Fig. 6), whereas the background signal had an average intensity of 2.5 and a variance of 1.5. The signal-to-noise ratio was calculated as the ratio of the signal amplitude (≈7.5) by the variance of the noise (1.5) and thus was found to be superior to 5.
Biodistribution in Mice Bearing 3LL s.c. Tumors.
3LL tumors were implanted s.c. in the right flank of 5-week-old female C57BL/6 mice (Janvier, Le Genest St. Isle, France), and 10–14 days later, biodistribution studies were undertaken. After imaging, mice were euthanized.
Supplementary Material
Acknowledgments
We thank G. Chabot and C. Richard for helpfully reviewing this work and N. Mignet for her help with liposome preparation. Q.l.M.d.C was supported by the French Ministry of Defense under a General Delegation for Armaments of France Fellowship.
Abbreviations
- 3LL
Lewis lung carcinoma
- NP
nanoparticle
- PEG
polyethylene glycol.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0702427104/DC1.
References
- 1.Cherry SR. Annu Rev Biomed Eng. 2006;8:35–62. doi: 10.1146/annurev.bioeng.8.061505.095728. [DOI] [PubMed] [Google Scholar]
- 2.Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubertret B, Skourides P, Noriis DJ, Noireaux V, Brivanlou AH, Libchader A. Science. 2002;298:1759–1762. doi: 10.1126/science.1077194. [DOI] [PubMed] [Google Scholar]
- 4.So MK, Xu C, Loening AM, Gambhir SS, Rao J. Nat Biotech. 2006;24:339–343. doi: 10.1038/nbt1188. [DOI] [PubMed] [Google Scholar]
- 5.Ballou B, Lagerholm BC, Ernst LA, Bruchez MP, Waggoner AS. Bioconjugate Chem. 2004;15:79–86. doi: 10.1021/bc034153y. [DOI] [PubMed] [Google Scholar]
- 6.Bhaumik S, Gambhir SS. Proc Natl Acad Sci USA. 2002;99:377–382. doi: 10.1073/pnas.012611099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Contag CH, Bachmann MH. Annu Rev Biomed Eng. 2002;4:235–260. doi: 10.1146/annurev.bioeng.4.111901.093336. [DOI] [PubMed] [Google Scholar]
- 8.Cheng Z, Levi J, Xiong Z, Gheysens O, Keren S, Chen X, Gambhir SS. Bioconjugate Chem. 2006;17:662–669. doi: 10.1021/bc050345c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weissleder R, Tung CH, Mahmood U, Bogdanov A. Nat Biotechnol. 1999;17:375–378. doi: 10.1038/7933. [DOI] [PubMed] [Google Scholar]
- 10.Becker A, Hessenius C, Licha K, Ebert B, Sukowski U, Semmler W, Wiedenmann B, Grötzinger C. Nat Biotechnol. 2001;19:327–331. doi: 10.1038/86707. [DOI] [PubMed] [Google Scholar]
- 11.Ntziachristos V. Annu Rev Biomed Eng. 2006;8:1–33. doi: 10.1146/annurev.bioeng.8.061505.095831. [DOI] [PubMed] [Google Scholar]
- 12.Frangioni JV. Current Opin Chem Bio. 2003;7:626–634. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Cheong WJ, Prahl SA, Welch AJ. IEEE J Quantum Electron. 1990;26:2166–2185. [Google Scholar]
- 14.Wang XJ, Jia D, Yen WM. J Lumin. 2003;103:34–37. [Google Scholar]
- 15.Brinker CJ, Scherer GW. Sol-Gel Science: The Physics and the Chemistry of Sol-Gel Processing. London: Academic; 1990. [Google Scholar]
- 16.Leverenz HW. Science. 1949;109:183–195. doi: 10.1126/science.109.2826.183. [DOI] [PubMed] [Google Scholar]
- 17.Song YK, Liu F, Liu Gene Ther. 1998;5:1531–1537. doi: 10.1038/sj.gt.3300770. [DOI] [PubMed] [Google Scholar]
- 18.Kamps JA, Morselt HWM, Swart PJ, Meijer DKF, Scherphof GL. Proc Natl Acad Sci USA. 1997;94:11681–11685. doi: 10.1073/pnas.94.21.11681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krieger M, Herz J. Annu Rev Biochem. 1994;63:601–637. doi: 10.1146/annurev.bi.63.070194.003125. [DOI] [PubMed] [Google Scholar]
- 20.Moghimi SM, Szebeini J. Prog Lipid Res. 2003;42:463–478. doi: 10.1016/s0163-7827(03)00033-x. [DOI] [PubMed] [Google Scholar]
- 21.Campbell RB, Fukumura D, Brown EB, Mazzola LM, Izumi Y, Jain RK, Torchilin VP, Munn LL. Cancer Res. 2002;62:6831–6836. [PubMed] [Google Scholar]
- 22.Levchenko TS, Rammohan R, Lukyanov AN, Whiteman KR, Torchilin VP. Int J Pharm. 2002;240:95–102. doi: 10.1016/s0378-5173(02)00129-1. [DOI] [PubMed] [Google Scholar]
- 23.Thurston G, McLean JW, Rizen M, Baluk P, Haskell A, Murphy TJ, Hanahan D, McDonald DM. J Clin Invest. 1998;101:1401–1413. doi: 10.1172/JCI965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamps JA, Morselt HWM, Scherphof GL. Biochem Biophy Res Commun. 1999;256:57–62. doi: 10.1006/bbrc.1999.0290. [DOI] [PubMed] [Google Scholar]
- 25.Masson C, Scherman D, Bessodes M. J Polym Sci A. 2001;39:4022–4024. [Google Scholar]
- 26.Morimoto N. Carnegie Inst Washington Ann Rep Dir Geophys Lab. 1959;1958/59:197–198. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.