Abstract
Recent studies have demonstrated that the LIM homeodomain transcription factor Islet1 (Isl1) marks pluripotent cardiovascular progenitor cells and is required for proliferation, survival, and migration of recently defined second heart field progenitors. Factors that are upstream of Isl1 in cardiovascular progenitors have not yet been defined. Here we demonstrate that β-catenin is required for Isl1 expression in cardiac progenitors, directly regulating the Isl1 promoter. Ablation of β-catenin in Isl1-expressing progenitors disrupts multiple aspects of cardiogenesis, resulting in embryonic lethality at E13. β-Catenin is also required upstream of a number of genes required for pharyngeal arch, outflow tract, and/or atrial septal morphogenesis, including Tbx2, Tbx3, Wnt11, Shh, and Pitx2. Our findings demonstrate that β-catenin signaling regulates proliferation and survival of cardiac progenitors.
Keywords: cardiac morphogenesis, cardiac progenitors, direct target, Isl1
Previous studies have demonstrated a key role for the LIM homeodomain transcription factor Islet1 (Isl1) in cardiac development and as a marker for pluripotent cardiovascular progenitors, which give rise to cardiomyocyte, endothelial, and smooth muscle lineages in vitro (1–5). Isl1 marks proliferating, undifferentiated progenitors of the second heart field, which are located dorsal/medially to the heart (4, 5). Isl1 is required for proliferation, survival, and migration of these progenitors into the forming heart (4). The second heart field migrates in and differentiates later than progenitors of the first heart field. Isl1 expression is down-regulated in second heart lineages as differentiation occurs. The second heart lineage includes cells of the secondary or anterior heart field, which gives rise to the outflow tract or outflow tract and right ventricle, respectively (6–8). Isl1-null mice die embryonically at embryonic day 10 (E10) with hearts missing segments derived from the second heart field, including the outflow tract and right ventricle, and exhibiting severely reduced atrial tissue.
Isl1 also marks cardiac progenitors found within postnatal hearts of rodents and humans (9). These progenitors can be isolated, propagated, and readily differentiated into functional cardiomyocytes when cocultured with neonatal cardiac myocytes. More recent studies have demonstrated that Isl1 marks a pluripotential cardiovascular cell that coexpresses flk1 and Nkx2.5, which can be cloned and amplified from either embryonic stem cells or embryos (1, 2). Amplified clones can give rise to endothelial, smooth muscle, and cardiomyocyte cell types. The pivotal role for Isl1 within cardiovascular progenitors makes it critical to understanding factors that regulate Isl1 expression in this context.
A number of studies have demonstrated a key role for canonical Wnt signaling through β-catenin in stem cells from a variety of cell types (10). The role of β-catenin signaling in cardiovascular stem or progenitor cells, however, has been seemingly contradictory.
Several studies have demonstrated the induction of cardiogenic mesoderm in response to the inhibition of Wnt signaling in chick, Xenopus, and mouse embryos. Wnt antagonists Dickkopf1 and Crescent produced by anterior endoderm in chick embryos stimulate differentiation of a cardiogenic mesoderm (11). In frog embryos, Dickkopf1 and Crescent secreted by Spemann's organizer are also initiators of cardiac differentiation, acting indirectly on anterior mesendoderm to provoke secretion of an as-yet-unidentified cardiogenic induction factor (12, 13). In mouse embryos, ablation of β-catenin using a Cytokeratin19 promoter-driven Cre (K19-Cre) recombinase resulted in ectopic heart formation, which was attributed to ablation of β-catenin in endodermal tissues (14).
In contrast to the foregoing, activation of Wnt signaling is required for cardiogenesis in Drosophila (15) and cell culture systems, including embryonic stem cells and embryonal carcinoma P19 cells (16–18). In these cell culture systems, however, the spatial requirement for Wnt signaling has not been addressed.
To address the temporal and spatial requirements for canonical Wnt signaling through β-catenin in Isl1-expressing cardiac progenitors, we have used a Cre recombinase expressed under the control of the endogenous Isl1 locus. The early expression of Isl1-Cre in cardiogenic progenitors, and in early pharyngeal endoderm (19, 20), a cardiac-inducing tissue, provided us with an opportunity to examine the requirement for β-catenin in these tissues during early cardiogenesis.
Results
Activation of β-Catenin During Early Cardiogenesis.
To investigate where and when β-catenin signaling was occurring in the early embryo, we used a T cell factor (TCF)/Lef-lacZ reporter line (21) (Fig. 1). Results of this analysis demonstrated β-catenin activation in early cardiogenic mesoderm and adjacent endoderm at E7.5 (Fig. 1 A and B). At E8.5 (Fig. 1 C–E), expression of the reporter was observed in pharyngeal mesoderm, outflow tract myocardium, and at low levels in pharyngeal endoderm. At E9.5 (Fig. 1 F–H), expression in pharyngeal mesoderm and outflow tract myocardium was striking, with expression also observed in lateral and ventral pharyngeal endoderm and in proepicardium. At E12.5 (Fig. 1 I–L), TCF/Lef-lacZ reporter expression was observed in epicardium as well as subsets of cells within the outflow tract and ventricles. Expression was also observed in venous valves within the right atrium and in the region of the sinoatrial node.
Fig. 1.
Expression of a TCF/Lef-LacZ transgene in mouse embryos. (A and B) Expression of TCF/Lef-LacZ was detected in cardiogenic mesoderm (arrow) and adjacent endoderm (arrowhead) at E7.5. (C–E) At E8.5, TCF/Lef-LacZ was expressed in pharyngeal mesoderm (D, arrowhead), outflow tract (D, arrows), and myocardium (E, arrow). (F–H) At E9.5, TCF/Lef-LacZ was expressed in foregut endoderm (G and H, arrowheads), outflow tract (G, bigger arrows), pharyngeal mesoderm (G, smaller arrow), and proepicardium (H, arrow). (I–L) At E12.5, TCF/Lef-LacZ was strongly expressed in cells surrounding the outflow tract region, right atria, and sinoatrial node region. (K and L) Section analyses demonstrated that TCF/Lef-LacZ was expressed in the right ventricle, epicardium, pulmonary artery, aorta, and SA node region. END, endoderm; CM, cardiac mesoderm; PM, pharyngeal mesoderm; FE, foregut endoderm; OFT, outflow tract; RA, right atrium; LA, left atrium; RV, right ventricle; LV, left ventricle; PE, proepicardium; EP, epicardium; PA, pulmonary artery; SAN, SA node; LSVC, left superior vena cava.
Ablation of β-Catenin with Isl1-Cre Results in Early Embryonic Lethality and Cardiac and Pharyngeal Arch Artery Defects.
With the exception of the epicardium, regions where β-catenin signaling was active during cardiogenesis were overlapping with Isl1 expression or Isl1-derived lineages (4). We therefore ablated β-catenin by using an Isl1-Cre generated in our laboratory (19, 20). Isl1-Cre;β-catenin mutants were embryonic-lethal. Examination of 12 litters with 89 embryos demonstrated that 100% of Isl1-Cre;β-catenin mutants died at ≈E13.0. In contrast, control littermates of all other genotypes were completely normal and survived postnatally with no apparent phenotypic abnormalities.
Morphological analysis of mutants and control littermates demonstrated abnormal cardiac morphogenesis of mutants at E10.5, with mutant hearts exhibiting dilated outflow tracts, smaller right ventricles, and thin-walled myocardium (Fig. 2 A–F). Measurements performed by using ImageJ software on sections from three mutants and three control littermates at E10.5 demonstrated that outflow tracts in mutants were dilated compared with control littermates. Isl1-Cre;β-catenin mutants also had smaller right ventricles. Hypomorphic mandibles, derived from the first pharyngeal arch where Isl1 is expressed, were also observed in mutants (Fig. 2 A–F). At E12.5, persistent truncus arteriosus, a single undivided outflow tract, was observed in mutants. Formation of atrial septal structures was aberrant, with thickened septum secundum and septum primum primordia, and no apparent septum primum (Fig. 2 G–N). In mutants, the superior atrioventricular cushion was missing, and the inferior atrioventricular cushion was smaller, with ≈30% fewer mesenchymal cells relative to wild-type littermates (Fig. 2 I, J, M, and N). Affected structures arise at least in part from Isl1-expressing progenitors, as indicated by E10.5 cardiac sections from Isl1-Cre;R26R-LacZ lineage-traced animals, demonstrating that >50% of mesenchymal cells within the atrioventricular cushions derive from Isl1-expressing cells (Fig. 2O). The latter observation was confirmed by immunostaining for β-galactosidase protein (data not shown).
Fig. 2.
Ablation of β-catenin with Isl1-Cre results in embryonic lethality, abnormal cardiac morphology, and pharyngeal arch defects. (A–F) Isl1-Cre;β-catenin mutants exhibited a 12.4% increase in diameter of the outflow tract, and right ventricles in mutants were, on average, 9.5% smaller compared to control littermates. Size differences between control and mutant outflow tract and right ventricle were found to be significant with P values <0.05. Hypomorphic mandibles were also observed in mutants (E). (G–N) Whole-mount and section analyses show that, at E12.5, Isl1-Cre;β-catenin mutants exhibited persistent truncus arteriosus. Section analysis demonstrated that Isl1-Cre;β-catenin mutants exhibited thinner ventricular walls, atrial septal defects, a hypomorphic right ventricle, and smaller inferior atrioventricular cushions, and were missing superior atrioventricular cushions. J and N show atrioventricular cushions highlighted in I and J. (O) Lineages studies for the Isl1-expressing progenitors by X-Gal staining counterstained with Eosin on E10.5 cardiac sections. Isl1 cells were observed within endocardium and contributed extensively to atrioventricular cushion mesenchyme at E10.5. (P and Q) Ink injection shows left-sided PAA defects in Isl1-Cre;β-catenin mutants, with smaller third and sixth PAAs and no apparent fourth PAA (arrowhead) at E10.5 embryos. Ao, aorta; PA, pulmonary artery; AS, atria septum; ASD, atrial septal defect; VV, venous valve; AVC, atrioventricular cushion; SAVC, superior atrioventricular cushion; IAVC, inferior atrioventricular cushion; PTA, persistent truncus arteriosus; SP, septum primum; SS, septum secundum; PAA, pharyngeal arch artery.
Ink injections were performed to examine pharyngeal arch artery (PAA) structure and demonstrated left-sided PAA defects in Isl1-Cre;β-catenin mutants, with smaller third and sixth PAAs and no apparent fourth PAA (Fig. 2 P and Q).
Potential Downstream Effector Targets of β-Catenin During Cardiogenesis and Branchial Arch Formation.
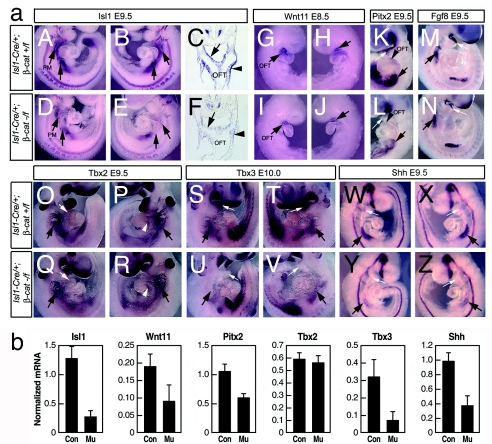
To identify potential downstream effector targets of β-catenin, we examined a number of genes known to be required for branchial arch, outflow tract, and atrial septal morphogenesis by whole-mount RNA in situ hybridization analysis and by real-time quantitative PCR analysis of RNA extracted from cardiogenic regions at comparable stages to those examined by in situ. Genes examined included Isl1, Wnt11, Tbx2, Tbx3, Shh, Pitx2, and Fgf8 (refs. 19, 22–25, 27, and 28; L.L. and S.M.E., unpublished data). Results demonstrated that expression of Isl1, Wnt11, Pitx2, and Tbx3 was selectively down-regulated in Isl1-Cre;β-catenin mutants relative to expression observed in littermate controls (Fig. 3). Tbx2 expression in outflow tract, but not in the region of the aortic arches, was decreased in mutants, whereas Fgf8 was still expressed (Fig. 3 M, O–R).
Fig. 3.
Analysis of potential downstream effector targets in Isl1-Cre;β-catenin mutants and control littermates. (a) Results from whole-mount RNA in situ hybridization assays. Expression of Isl1 (A–F), Wnt11 (G–J), Pitx2 (K and L), Fgf8 (M and N), Tbx2 (O–R), Tbx3 (S–V), and Shh (W-Z) was examined in Isl1-Cre;β-catenin mutants and littermate (A–F) controls. Isl1 was down-regulated in the pharyngeal region posterior to the heart (arrows). (C and F) Section analysis demonstrated decreased Isl1 expression in the foregut endoderm (arrows) and in splanchnic mesoderm (arrow heads) in Isl1-Cre;β-catenin mutants relative to control littlemates. (G–J) In Isl1-Cre;β-catenin mutants, Wnt11 was down-regulated in outflow tract (arrows). (K and L) Pitx2 was down-regulated in outflow tract (arrows) and pharyngeal arches (smaller arrows). (M and N) Expression of Fgf8 was unaffected. (O–R) Expression of Tbx2 was reduced in the outflow tract (smaller arrows) of Isl1-Cre;β-catenin mutants, but was not down-regulated in AV canal (arrowheads) or pharyngeal arches (bigger arrows). (S–V) Tbx3 was down-regulated in pharyngeal arches (smaller arrows) and foregut endoderm (arrows). (W–Z) Shh was down-regulated in anterior foregut endoderm (arrows) and posterior foregut endoderm (smaller arrows). (b) Results from real-time quantitative PCR analyses. Total RNA was prepared from hearts and pharyngeal arches of E9.5 embryos of either control or Isl1-Cre;β-catenin mutants and analyzed by real-time RT-PCR. The mRNA level of each gene was normalized against the mRNA level of hypoxanthine phosphoribosyltransferase. Data obtained from three independent experiments are shown as means ± SD. Con, Isl1-Cre/+;β-catenin+/f; Mu, Isl1-Cre/+;β-catenin−/f.
Isl1 Is a Direct Downstream Target of β-Catenin.
In Isl1-Cre;β-catenin mutants, expression of Isl1 was strongly down-regulated. Ablation of Isl1 in germ-line knockouts results in embryonic lethality at E10 and severely abnormal heart formation, with mutant hearts missing the outflow tract and right ventricle and having severely reduced atrial tissue (4, 29). We have found that a hypomorphic mutant of Isl1 exhibits embryonic lethality at E12.5, with outflow tract defects comparable to those observed with Isl1-Cre;β-catenin mutants (Y. Sun and S.M.E., unpublished data). Because these observations suggested that Isl1 is a key effector target of β-catenin for cardiac morphogenesis, we further investigated whether Isl1 was a direct downstream target of β-catenin.
The observed decreased expression of Isl1 might be reflective of a requirement for β-catenin for Isl1 expression or reflect a selective loss of cells expressing Isl1 in which β-catenin has been deleted. To investigate this issue, we performed coimmunostaining for Isl1 and β-catenin in Isl1-Cre;β-catenin mutants and control littermates [supporting information (SI) Fig. 8]. Results of this analysis demonstrated that β-catenin was efficiently ablated in regions overlapping with Isl1 expression and in descendents of Isl1-expressing cells, the latter including a majority of cells within the outflow tract and right ventricle (4). In cells lacking β-catenin protein, Isl1 expression was still evident, demonstrating their survival, but levels of Isl1 protein were reduced within each cell. No decreases in Isl1 protein levels were observed in littermate controls of all other genotypes, including embryos that were heterozygous-null for Isl1. These data suggested that decreased Isl1 expression in Isl1-Cre;β-catenin mutants is consequent to regulation of Isl1 expression by β-catenin in a direct or an indirect manner.
Bioinformatics analysis using standard parameters in rVISTA to examine potential Isl1 promoter regions revealed two evolutionarily conserved LEF1 sites 5′ of the ATG start site or within intron 1 of Isl1 genomic sequences. ChIP analysis performed by using extracts from E9.5 heart and antibodies to β-catenin demonstrated specific binding to the 5′-conserved LEF1 response element (Fig. 4A).
Fig. 4.
β-catenin acts at early stages of cardiogenesis to directly regulate Isl1 expression. (A) ChIP assay on extracts from E8.5–E9.0 hearts showing in vivo recruitment of β-catenin to the Isl1 5′ promoter with conserved LEF-1-binding sites (lane 1, primers P-2940 and P-2630). ChIP analysis with control primers against distinct promoter regions revealed no recruitment of β-catenin (αβ-Cat, lanes 2, 3, and 4) (see Material and Methods for primer sequences). No recruitment was observed with IgG control. (B) Results of luciferase reporter assays. HEK293 cells were transiently transfected with Isl1 promoter-luciferase reporter (Isl1-Luc) constructs in combination with either control expression vector, LEF-1, or constitutively active β-catenin (aβ-Cat) expression constructs. Isl1p-Luc, wild-type isl1 promoter-luciferase construct; Isl1p (Mu)-Luc, Isl1 promoter-luciferase construct with mutated LEF-1 consensus sites. Data obtained from three independent experiments, each performed in triplicate. Data are presented as fold activity over basal promoter activity (relative activity) and are expressed as mean ± SD of triplicates from a representative experiment. (C) Immunohistochemical analysis with anti-Isl1 antibody on sections from E8.0 embryos demonstrated reduced Isl1 protein levels within Isl1+ cardiovascular progenitors (arrows) and endoderm (arrowheads) in Isl1-Cre;β-catenin mutants when compared with control littermates. Immunostaining analysis was performed in parallel on multiple experimental and control samples, and exposure times were the same for all samples.
To investigate the functional significance of binding, cotransfection assays were performed with a 5-kb Isl1-promoter-luciferase reporter and expression vectors for LEF-1 and activated β-catenin. Results of these assays demonstrated activation of the Isl1-promoter by recruitment of LEF-1 and activated β-catenin (Fig. 4B). Activation was disrupted by mutation of the two conserved LEF-1-binding sites (Fig. 4B), demonstrating that activation by LEF/β-catenin was dependent on the LEF-1 consensus site and that the LEF/β-catenin pathway directly regulates the Isl1 promoter.
Isl1 Protein Is Decreased by E8.0 in Isl1-Cre;β-Catenin Mutants.
Ablation of β-catenin by Isl1-Cre dictates that β-catenin ablation occurs after the expression of Isl1. To investigate whether Isl1 expression was affected at early stages in Isl1-Cre;β-catenin mutants, we performed immunostaining with Isl1 antibody on sections from E8.0 embryos (Fig. 4C). Results of this analysis demonstrated significantly less Isl1 signal both in pharyngeal mesoderm and pharyngeal endoderm in Isl1-Cre;β-catenin mutants relative to control littermates, demonstrating an early requirement for β-catenin. Isl1/Nkx2.5/flk1 multipotent progenitors reside in the pharyngeal mesoderm domain at this stage (1), and Isl1 is reduced in this domain in Isl1-Cre;β-catenin mutants.
Proliferation and Apoptosis in Isl1-Cre;β-Catenin Mutants.
Smaller branchial arch derivatives, outflow tract, and right ventricle in Isl1-Cre;β-catenin mutants suggested that proliferation and/or apoptosis were altered in mutants. To investigate this issue, we performed immunostaining for phosphorylated histone H3 and cleaved activated caspase-3 to investigate proliferation and apoptosis, respectively.
Results of this analysis demonstrated both a reduction in proliferation rate and an increase in apoptosis (Fig. 5). At E9.5 (Fig. 5 A and B), the proliferation rate in outflow tract myocardium was reduced from 4.6% in wild type to 2.1% in mutants and in foregut endoderm from 3.9% in wild type to 1.6% in mutants. At E10.5 (Fig. 5 C and D), the proliferation rate in outflow tract myocardium was reduced from 3.8% in wild type to 1.0% in mutants and in foregut from 3.1% in wild type to 1.5% in mutants. At E10.5 (Fig. 5 E–H), significant increases in apoptotic cells were observed in pharyngeal mesoderm, foregut endoderm, and outflow tract, regions consistent with those similarly affected in Isl1 or hedgehog signaling mutants (4, 30–32).
Fig. 5.
β-catenin is required for proliferation and survival of cardiac progenitors. (A–D) Proliferation was assessed by antibody staining for phosphorylated histone H3 (PHH3). Proliferation rate was significantly decreased in the foregut endoderm and myocardium of outflow tract in Isl1-Cre;β-catenin mutants. (E–H) Cleaved caspase-3 antibody staining showed increased apoptosis in the pharyngeal mesoderm, foregut endoderm, and outflow tract at E10.5 in Isl1-Cre;β-catenin mutants relative to control littermates. Arrows in A–D indicate proliferating cells. Arrows in E–H indicate apoptotic cells.
Analysis of Cardiac Neural Crest Cells in Isl1-Cre;β-Catenin Mutants.
Because cardiac neural crest cells are required for outflow tract septation, we examined Isl1-Cre;β-catenin mutants and littermate controls for expression of the cardiac neural crest marker, PlexinA2 (33). Results demonstrated that PlexinA2-expressing cells were observed in the outflow tract of Isl1-Cre;β-catenin mutants, but were less abundant relative to littermate controls (Fig. 6 A and B).
Fig. 6.
β-catenin is not required for the migration of cardiac neural crest cells. (A and B) Whole-mount RNA in situ analysis was performed for E10.5 Isl1-Cre;β-catenin mutants and littermate controls using a marker specific for cardiac neural crest cells, PlexinA2. Results demonstrated that PlexinA2-expressing cells were observed in the outflow tract of Isl1-Cre;β-catenin mutants, but were less abundant relative to littermate controls (arrows).
Discussion
Isl1 plays a pivotal role in the development of cardiac progenitors of the second heart field, marks postnatal progenitors in postnatal heart, and marks cardiovascular progenitor cells in the early embryo, which are pluripotent in vitro (4, 9). Therefore, understanding factors that regulate Isl1 expression is critical to understanding factors that drive cardiac progenitor proliferation, survival, and migration, both in the context of normal development and for potential application to cell therapies using cardiac progenitors.
Our results have demonstrated that β-catenin directly targets and activates Isl1 expression, and we have observed a striking correspondence between active β-catenin signaling and regions of Isl1 expression; in progenitors of the second heart field, including regions harboring Isl1/flk1/Nkx2.5 progenitors (1); in pharyngeal endoderm; and in regions within the heart where persistent Isl1 expression is observed (3, 9). Isl1-expressing cells isolated from postnatal hearts are capable of expansion in culture and can differentiate to functional cardiomyocytes. Isl1/flk1/Nkx2.5-expressing cells clonally isolated from embryonic stem cells or embryos can be amplified and give rise to endothelial, smooth muscle, or cardiac lineages. Factors that are required upstream of Isl1 for proliferation, however, have not been defined. Our results demonstrate a critical role for β-catenin in proliferation of Isl1-expressing progenitors.
Our results demonstrate a key role for β-catenin in outflow tract morphogenesis, in formation of the atrial septum, and in atrioventricular cushion formation. With regard to the latter, our findings are consistent with the expression of Isl1 in endocardial cells that contribute to cushion formation and previous data which demonstrated that ablation of β-catenin in endothelial cells disrupts epithelial-mesenchymal transformation and cushion formation (34–36). Requirements for β-catenin in cushion formation and septation have also been demonstrated in chick and zebrafish (37). The observed requirement for β-catenin in atrial septation is consistent with down-regulation of Isl1, Shh, and Pitx2 because mutants affecting expression of these genes also have defects in atrial septation (30, 31, 38). The atrial septal defects in the Isl1-Cre;β-catenin mutant, however, appear to be quite distinctive, with two truncated septal primordia observed in the dorsal aspect of the atrial wall.
Decreased proliferation within the secondary/anterior heart field has been demonstrated to result in outflow tract defects (39, 40). Because Isl1 expression is down-regulated in β-catenin mutants and because Isl1 is required for proliferation within the secondary/anterior heart field (4), it is likely that decreased Isl1 expression in the secondary/anterior heart field and its derivatives, as observed here, are contributing to observed outflow tract defects. Indeed, we have observed similar cardiac phenotypes to those of the Isl1-Cre;β-catenin mutants in hypomorphic mutants of Isl1 (Y. Sun and S.M.E., unpublished data).
Cardiac neural crest cells are also required for outflow tract morphogenesis and can affect proliferation of the secondary heart field (41, 42). We have not observed expression of Isl1 in Wnt1-Cre;R26R-lacZ lineage-traced cardiac neural crest cells within the outflow tract, although Isl1 is expressed within intrinsic cardiac ganglia, which derive from the cardiac neural crest (3, 39–41, 43). From this observation, and a comparison of results with Wnt1-Cre and Isl1-Cre (Y. Sun and S.M.E., unpublished data), it is unlikely that Isl1 lineages contribute to the cardiac neural crest population that will contribute smooth muscle cells to the outflow tract, aorta, or pulmonary artery.
From the foregoing, we consider that the outflow tract phenotype of Isl1-Cre;β-catenin mutants is unlikely to be owing to ablation of β-catenin within cardiac neural crest that will directly contribute cells to the aorta and pulmonary artery. However, it is possible that ablation of β-catenin within Isl1-expressing domains secondarily affects this population of cardiac neural crest cells and in this manner contributes to observed outflow tract phenotypes. Consistent with the latter possibility, we observed decreased levels of PlexinA2-expressing cells (33) within the outflow tract of Isl1-Cre;β-catenin mutants relative to littermate controls.
In addition to decreased expression of Isl1 in Isl1-Cre;β-catenin mutants, we also observed down-regulation of a number of other genes required for outflow tract morphogenesis, including Shh, Wnt11, Pitx2, Tbx2, and Tbx3 (refs. 19, 22–25, 27, and 28; L.L. and S.M.E., unpublished data). Selective decreases were observed in Tbx3 expression within the mandibular arch and aortic arch regions, and Tbx2 expression in the outflow tract of Isl1-Cre;β-catenin mutants. Both Tbx2 and Tbx3 are required for outflow tract formation (23) (V. Papaioannou and R. Kelly, personal communication).
Our data do not address whether β-catenin is required within the pharyngeal endoderm, cardiac mesoderm, or both, for cardiogenesis. We observe active β-catenin signaling in both compartments, suggesting a potential role in both. Previous ablation of β-catenin with a Cytokeratin-19 promoter-driven Cre resulted in ectopic heart formation, which was attributed to ablation of β-catenin in the pharyngeal endoderm (14). Despite ablation of β-catenin signaling in early ventral pharyngeal endoderm by Isl1-Cre, we did not observe any ectopic heart formation. The Cytokeratin-19 promoter may be expressed earlier than Isl1 in ventral endoderm or an expression domain of the Cytokeratin-19 promoter, which is distinct from that of Isl1 and is responsible for the observed phenotype.
We found that β-catenin was required within the Isl1 domain for branchial and aortic arch artery formation. In Isl1-Cre;β-catenin mutants, the mandibular arch was severely hypomorphic, as were left aortic arch arteries, and the fourth PAA was absent. A similar phenotype is observed in Isl1 hypomorphs and mutants of the Shh pathway (30, 31). We have previously demonstrated that expression of Shh in pharyngeal endoderm is downstream of Isl1. Similar apoptotic profiles are observed in Shh and Isl1-Cre;smoothened mutants (30, 31), and they are described here in Isl1-Cre;β-catenin mutants. Together these data suggest a genetic cascade with β-catenin upstream of Isl1 and Isl1 upstream of Shh for pharyngeal arch development. β-catenin may also regulate Shh independently of Isl1. Whether these regulatory interactions are direct or indirect remains to be examined.
We have previously shown that Shh is downstream of Isl1 in a signaling pathway required for outflow tract morphogenesis (30). Results of this study demonstrate that β-catenin is a key upstream factor that drives expression of Isl1 in cardiac progenitors and their proliferation and survival, and it is also upstream in genetic cascades that regulate diverse aspects of pharyngeal arch and cardiac morphogenesis (Fig. 7). Wnt ligands, which regulate this pathway, are currently unknown. Wnt2 is expressed in the cardiogenic crescent (44) and may act redundantly with other Wnts upstream of β-catenin in this context.
Fig. 7.
β-catenin is required for Isl1 expression in cardiovascular progenitors. β-catenin directly regulates Isl1, which is required for survival and proliferation of Isl1+ cardiovascular progenitors. β-catenin is upstream of multiple genes, which are required for pharyngeal arch and cardiac morphogenesis. Isl1 is upstream of Shh, but β-catenin may also regulate Shh independently of its regulation of Isl1. Direct regulation is indicated by solid arrow, and direct or indirect regulation is indicated by dotted arrows.
Materials and Methods
Mice.
Floxed β-catenin mice were obtained from the laboratory of Rolf Kemler (45). Isl1-Cre mice were created in our laboratory by a Cre knockin into the endogenous Isl1 locus, replacing the endogenous Isl1 ATG (19, 20). Homozygous floxed β-catenin mice were crossed with Protamine-Cre mice (46) to generate β-catenin +/− mice, which were then crossed with Isl1-Cre mice to produce doubly heterozygous Isl1-Cre;β-catenin +/− mice. These mice were then crossed to β-catenin floxed/floxed homozygous mice to obtain Isl1-Cre;β-catenin−/f mutants for analysis.
Whole-Mount RNA in Situ Hybridization and Histological Analyses.
Whole-mount RNA in situ hybridization was carried out as previously described (26). References for specific RNA in situ probes are as follow: Isl1 (EST; GenBank accession no. AA198791), Tbx2, Tbx3, and Pitx2 were from Marina Campione; Wnt11 was from Andy McMahon; PlexinA2 was from Jon Epstein; Fgf8 was from Gail Martin; and Shh was from Deepak Srivastava. For histological analyses, embryos were fixed in 4% paraformaldehyde, dehydrated in ethanol, embedded in paraffin, 8-μm sections prepared on a microtome, and stained with H&E according to standard protocols. For ink injection, embryos were collected and injected intracardially with India ink. All experiments were repeated a minimum of three times to ensure statistically relevant findings.
RNA Isolation and Real-Time Quantitative PCR Analyses.
Total RNA was isolated from hearts and pharyngeal arches of E9.5 embryos by an RNeasy kit (QIAGEN, Valencia, CA). Semiquantitative PCR was carried out according to the Mx3000P Real-Time PCR Systems manual and the Brilliant qPCR reagent (Stratagene, La Jolla, CA). The mRNA levels of Pitx2, Wnt11, Shh, Isl1, Tbx3, and Tbx2 were normalized to the mRNA levels of hypoxanthine phosphoribosyltransferase to allow comparisons among different experimental groups. Primer sequences are available on request.
X-Galactosidase Staining of Mouse Embryos.
Mouse embryos were fixed in 4% paraformaldehyde for 30 min on ice, permeabilized in PBS containing 0.02% Nadeoxycholate and 0.01% Nonidet P-40 for 4 h at room temperature, and then subjected to X-gal staining for ≈1–2 h.
ChIP Assays.
For in vivo ChIP experiments, extracts were prepared from 20 E9.0–E9.5 wild-type mouse embryo hearts. Embryos were dissected in ice-cold PBS. After gentle pipetting, tissue was cross-linked with 2% formaldehyde for 2 h at room temperature. Chromatin extraction and immunoprecipitations were performed according to the manufacturer's protocols by using a ChIP assay kit (17–295; Upstate Biotechnology, Lake Placid, NY). Protein-DNA cross-linking was reversed by overnight incubation at 65°C. A PCR purification kit (28106; QIAGEN) was used to recover DNA in 50 μl. The following PCR primers against the 5′ Isl1 promoter region were used: primers P-2940 (5′-GCG CCA GGA ACT GTG CTC CAA-3′) and P-2630 (5′-AGG GGC GAC CTC TTG TGT TCA ATG-3′), primers P-850 (5′-GAA CAG GAG ACC TCA CGG GTC GGG-3′) and P-534 (5′-CTA GCA GCG CGC TAC GCG TTA GGG-3′), primers P-15 (5′-GAA GAG AGG TGC CCC GAG CCG TGC-3′) and P-290 (5′-TTT GGT GGA TCG CCC ATG TCT CCC-3′), and primers P790 (5′-CCC GCG TGC TAT TGA AGA ACG TGC-3′) and P1070 (TTG GGA TGG TAA TTG GAG TGT GCC-3′). β-catenin antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Promoter Cloning and Luciferase Transfection Assays.
A 5.0 genomic DNA fragment upstream of an Isl1 start codon was amplified with a high-fidelity DNA polymerase (71086–3; Novagen, Madison, WI) and was cloned into pGL3-basic vector (E1751; Promega, Madison, WI). Primers were 5′ primer 5′-GATGGTACCCTCAACTAA ATGAGGCTAC-3′ and 3′ primer 5′-ATTGTCGACTTGTAAGAGGGAGTAATGTC-3′. The QuikChange sited-directed mutagenesis kit (200518; Stratagene) was used to make point mutation in the conserved LEF-1-binding site in the Isl1 5′-promoter region according to the manufacturer's protocol. Transfections were carried out in HEK293 cells according to standard techniques by FUGENE6 (Roche, Indianapolis, IN). Cells were lysed 48 h after transfection with luciferase, and β-galactosidase activities were measured on a Luminoskan Ascent luminometer (Thermo Electron, Waltham, MA). For luciferase reporters, CMV-β-galactosidase was used to control for transfection efficiency. Normalized luciferase activities were compared with a pGL3 control to calculate the fold of activation. Data are presented as fold activity over basal promoter activity (relative activity) and are expressed as mean ± SD of triplicates from a representative experiment.
Immunohistochemistry.
Mouse embryos were saturated with 20% sucrose and frozen in OCT, and 8-μm sections were prepared on a cryotome. Sections were fixed in 2% paraformaldehyde for 10 min at room temperature, blocked with 5% serum, and stained with antibodies. Reference for antibodies are rabbit anti-phospho-histone H3 antibody (06–570; Upstate Biotechnology), rabbit anti-cleaved caspase-3 (9661; Cell Signaling Technology, Danvers, MA), rabbit anti-β-catenin (ab6302; Abcam, Cambridge, MA), and mouse anti-Isl1 (39.4D5; Hybridoma Bank, Iowa City, IA). Secondary antibodies were goat anti-rabbit 488, donkey anti-mouse 488, and donkey anti-rabbit 594 (all Alexa Fluor was used at 1:250; Molecular Probes, Eugene, OR). All experiments were repeated a minimum of three times to ensure statistically relevant findings.
Statistical Evaluations.
Student's t test was used for statistical comparisons when appropriate, and differences were considered significant at P < 0.05.
Supplementary Material
Acknowledgments
We thank Jon Epstein (University of Pennsylvania School of Medicine, Philadelphia, PA), Andy McMahon (Harvard University, Cambridge, MA), Deepak Srivastava (University of California, San Francisco, CA), Marina Campione (University of Padua, Padua, Italy), and Gail Martin (University of California, San Francisco, CA) for providing us with cDNAs to make riboprobes; Yunqing Shi (University of California at San Diego) for mouse husbandry; and Janet Hightower for figure preparation. This work was supported by National Institutes of Health Grants R01 HL74066 and R01 HL70867 (to S.M.E.) and American Heart Association postdoctoral Fellowship Grant 0525141Y (to L.L.).
Abbreviations
- En
embryonic day
- PAA
pharyngeal arch artery
- TCF
T cell factor.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0700923104/DC1.
References
- 1.Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, Qyang Y, Bu L, Sasaki M, Martin-Puig S, et al. Cell. 2006;127:1151–1165. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Kattman SJ, Huber TL, Keller GM. Dev Cell. 2006;11:723–732. doi: 10.1016/j.devcel.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Sun Y, Liang X, Najafi N, Cass M, Lin L, Cai CL, Chen J, Evans S. Dev Biol. 2007;304:286–296. doi: 10.1016/j.ydbio.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Dev Cell. 2003;5:877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckingham M, Meilhac S, Zaffran S. Nat Rev Genet. 2005;6:826–835. doi: 10.1038/nrg1710. [DOI] [PubMed] [Google Scholar]
- 6.Kelly RG, Buckingham ME. Trends Genet. 2002;18:210–216. doi: 10.1016/s0168-9525(02)02642-2. [DOI] [PubMed] [Google Scholar]
- 7.Abu-Issa R, Waldo K, Kirby ML. Dev Biol. 2004;272:281–285. doi: 10.1016/j.ydbio.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 8.Eisenberg LM, Markwald RR. Dev Biol. 2004;274:225–232. doi: 10.1016/j.ydbio.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 9.Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, et al. Nature. 2005;433:647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reya T, Clevers H. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 11.Marvin MJ, Di Rocco G, Gardiner A, Bush SM, Lassar AB. Genes Dev. 2001;15:316–327. doi: 10.1101/gad.855501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneider VA, Mercola M. Genes Dev. 2001;15:304–315. doi: 10.1101/gad.855601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foley A, Mercola M. Trends Cardiovasc Med. 2004;14:121–125. doi: 10.1016/j.tcm.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Lickert H, Kutsch S, Kanzler B, Tamai Y, Taketo MM, Kemler R. Dev Cell. 2002;3:171–181. doi: 10.1016/s1534-5807(02)00206-x. [DOI] [PubMed] [Google Scholar]
- 15.Zaffran S, Frasch M. Circ Res. 2002;91:457–469. doi: 10.1161/01.res.0000034152.74523.a8. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura T, Sano M, Songyang Z, Schneider MD. Proc Natl Acad Sci USA. 2003;100:5834–5839. doi: 10.1073/pnas.0935626100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pal R, Khanna A. Stem Cells Dev. 2006;15:29–39. doi: 10.1089/scd.2006.15.29. [DOI] [PubMed] [Google Scholar]
- 18.Pandur P, Lasche M, Eisenberg LM, Kuhl M. Nature. 2002;418:636–641. doi: 10.1038/nature00921. [DOI] [PubMed] [Google Scholar]
- 19.Yang L, Cai CL, Lin L, Qyang Y, Chung C, Monteiro RM, Mummery CL, Fishman GI, Cogen A, Evans S. Development (Cambridge, UK) 2006;133:1575–1585. doi: 10.1242/dev.02322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park EJ, Ogden LA, Talbot A, Evans S, Cai CL, Black BL, Frank DU, Moon AM. Development (Cambridge, UK) 2006;133:2419–2433. doi: 10.1242/dev.02367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohamed OA, Clarke HJ, Dufort D. Dev Dyn. 2004;231:416–424. doi: 10.1002/dvdy.20135. [DOI] [PubMed] [Google Scholar]
- 22.Liu C, Liu W, Palie J, Lu MF, Brown NA, Martin JF. Development (Cambridge, UK) 2002;129:5081–5091. doi: 10.1242/dev.129.21.5081. [DOI] [PubMed] [Google Scholar]
- 23.Harrelson Z, Kelly RG, Goldin SN, Gibson-Brown JJ, Bollag RJ, Silver LM, Papaioannou VE. Development (Cambridge, UK) 2004;131:5041–5052. doi: 10.1242/dev.01378. [DOI] [PubMed] [Google Scholar]
- 24.Frank DU, Fotheringham LK, Brewer JA, Muglia LJ, Tristani-Firouzi M, Capecchi MR, Moon AM. Development (Cambridge, UK) 2002;129:4591–4603. doi: 10.1242/dev.129.19.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abu-Issa R, Smyth G, Smoak I, Yamamura K, Meyers EN. Development (Cambridge, UK) 2002;129:4613–4625. doi: 10.1242/dev.129.19.4613. [DOI] [PubMed] [Google Scholar]
- 26.Wilkinson DG. Whole Mount in Situ Hybridization of Vertebrate Embryos. Oxford: IRL; 1992. pp. 75–83. [Google Scholar]
- 27.Kitamura K, Miura H, Miyagawa-Tomita S, Yanazawa M, Katoh-Fukui Y, Suzuki R, Ohuchi H, Suehiro A, Motegi Y, Nakahara Y, et al. Development (Cambridge, UK) 1999;126:5749–5758. doi: 10.1242/dev.126.24.5749. [DOI] [PubMed] [Google Scholar]
- 28.Liu C, Liu W, Lu MF, Brown NA, Martin JF. Development (Cambridge, UK) 2001;128:2039–2048. doi: 10.1242/dev.128.11.2039. [DOI] [PubMed] [Google Scholar]
- 29.Pfaff SL, Mendelsohn M, Stewart CL, Edlund T, Jessell TM. Cell. 1996;84:309–320. doi: 10.1016/s0092-8674(00)80985-x. [DOI] [PubMed] [Google Scholar]
- 30.Lin L, Bu L, Cai CL, Zhang X, Evans S. Dev Biol. 2006;295:756–763. doi: 10.1016/j.ydbio.2006.03.053. [DOI] [PubMed] [Google Scholar]
- 31.Washington Smoak I, Byrd NA, Abu-Issa R, Goddeeris MM, Anderson R, Morris J, Yamamura K, Klingensmith J, Meyers EN. Dev Biol. 2005;283:357–372. doi: 10.1016/j.ydbio.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 32.Brito JM, Teillet MA, Le Douarin NM. Proc Natl Acad Sci USA. 2006;103:11607–11612. doi: 10.1073/pnas.0604751103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown CB, Feiner L, Lu MM, Li J, Ma X, Webber AL, Jia L, Raper JA, Epstein JA. Development (Cambridge, UK) 2001;128:3071–3080. doi: 10.1242/dev.128.16.3071. [DOI] [PubMed] [Google Scholar]
- 34.Liebner S, Cattelino A, Gallini R, Rudini N, Iurlaro M, Piccolo S, Dejana E. J Cell Biol. 2004;166:359–367. doi: 10.1083/jcb.200403050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Person AD, Garriock RJ, Krieg PA, Runyan RB, Klewer SE. Dev Biol. 2005;278:35–48. doi: 10.1016/j.ydbio.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 36.Hurlstone AF, Haramis AP, Wienholds E, Begthel H, Korving J, Van Eeden F, Cuppen E, Zivkovic D, Plasterk RH, Clevers H. Nature. 2003;425:633–637. doi: 10.1038/nature02028. [DOI] [PubMed] [Google Scholar]
- 37.Brade T, Manner J, Kuhl M. Cardiovasc Res. 2006;72:198–209. doi: 10.1016/j.cardiores.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 38.Dagle JM, Sabel JL, Littig JL, Sutherland LB, Kolker SJ, Weeks DL. Dev Biol. 2003;262:268–281. doi: 10.1016/s0012-1606(03)00389-0. [DOI] [PubMed] [Google Scholar]
- 39.Hutson MR, Zhang P, Stadt HA, Sato AK, Li YX, Burch J, Creazzo TL, Kirby ML. Dev Biol. 2006;295:486–497. doi: 10.1016/j.ydbio.2006.02.052. [DOI] [PubMed] [Google Scholar]
- 40.Ward C, Stadt H, Hutson M, Kirby ML. Dev Biol. 2005;284:72–83. doi: 10.1016/j.ydbio.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 41.Hutson MR, Kirby ML. Birth Defects Res C Embryo Today. 2003;69:2–13. doi: 10.1002/bdrc.10002. [DOI] [PubMed] [Google Scholar]
- 42.Waldo KL, Hutson MR, Stadt HA, Zdanowicz M, Zdanowicz J, Kirby ML. Dev Biol. 2005;281:66–77. doi: 10.1016/j.ydbio.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 43.Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Development (Cambridge, UK) 2000;127:1607–1616. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- 44.Monkley SJ, Delaney SJ, Pennisi DJ, Christiansen JH, Wainwright BJ. Development (Cambridge, UK) 1996;122:3343–3353. doi: 10.1242/dev.122.11.3343. [DOI] [PubMed] [Google Scholar]
- 45.Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Development (Cambridge, UK) 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- 46.O'Gorman S, Dagenais NA, Qian M, Marchuk Y. Proc Natl Acad Sci USA. 1997;94:14602–14607. doi: 10.1073/pnas.94.26.14602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.