Abstract
Parasites often play an important role in modifying the physiology and behavior of their hosts and may, consequently, mediate the influence hosts have on other components of an ecological community. Along the northern Atlantic coast of North America, the dominant herbivorous snail Littorina littorea structures rocky intertidal communities through strong grazing pressure and is frequently parasitized by the digenean trematode Cryptocotyle lingua. We hypothesized that the effects of parasitism on host physiology would induce behavioral changes in L. littorea, which in turn would modulate L. littorea's influence on intertidal community composition. Specifically, we hypothesized that C. lingua infection would alter the grazing rate of L. littorea and, consequently, macroalgal communities would develop differently in the presence of infected versus uninfected snails. Our results show that uninfected snails consumed 40% more ephemeral macroalgal biomass than infected snails in the laboratory, probably because the digestive system of infected snails is compromised by C. lingua infection. In the field, this weaker grazing by infected snails resulted in significantly greater expansion of ephemeral macroalgal cover relative to grazing by uninfected snails. By decreasing the per-capita grazing rate of the dominant herbivore, C. lingua indirectly affects the composition of the macroalgal community and may in turn affect other species that depend on macroalgae for resources or habitat structure. In light of the abundance of parasites across systems, we suggest that, through trait-mediated indirect effects, parasites may be a common determinant of structure in ecological communities.
Keywords: behavior modification, ecosystem functioning, herbivory, intertidal zone, trait-mediated indirect interactions
Parasites can substantially affect host populations by influencing host mortality, fecundity, growth, nutritional status, energetic requirements, and behavior (1–6). Such host–parasite interactions may shape components of an ecological community other than the host population, particularly if the host is abundant or ecologically influential (7–13). For example, parasites may weaken competitively dominant hosts, altering the outcome of competition between the host and its competitors (9, 11, 14–16). Parasites are also known to alter rates of predation, and hence, the feeding ecology of predators and population dynamics of prey (9, 17). However, few studies have documented effects of parasites on the grazing pressure exerted by influential herbivores (18). By indirectly altering the abundance of plant matter, parasites of herbivores could affect the basal food resource and physical structure of a community.
The marine gastropod Littorina littorea is an important grazer in rocky intertidal communities along the east and west coasts of the North Atlantic and exerts strong top-down control on ephemeral macroalgal species in rocky intertidal communities where it is found (19–21). Since its invasion of the New England rocky intertidal zone in the mid-19th century and subsequent spread to its current southern limit of Cape May, NJ (A.M.H.B. and J.E.B., unpublished work), L. littorea has become the most abundant gastropod along the northwestern Atlantic coast (20). Because it strongly prefers ephemeral macroalgae like Ulva lactuca, Porphyra sp., and Neosiphonia harveyi to mechanically and chemically defended taxa like Ascophyllum sp. and Chondrus crispus (20), this herbivorous snail substantially affects the relative abundance of ephemeral versus perennial species, with concomitant changes in the abundance of other intertidal taxa (19–21).
The vast majority of gastropod parasites are digenean trematodes (22), and the most common species infecting L. littorea, in both the northeastern and northwestern Atlantic (23, 24), is Cryptocotyle lingua. Like most digeneans, C. lingua has a complex life cycle with an obligate dependence on three hosts (24); for C. lingua, L. littorea serves as the first intermediate host, in which asexual reproduction takes place (24). The distribution of this parasite among snail host populations is spatially heterogeneous and depends on the distribution of the definitive host (i.e., seabirds). Although snail populations usually have low infection prevalences of C. lingua, prevalences can sometimes reach 50% (A.M.H.B. and J.E.B., unpublished work; J.E.B., A.M.H.B., E. Linder, A. Cooper, and T. McGuire, unpublished work) and, occasionally, as high as 90% at rocky intertidal sites in New England (25, 26). Developing trematode rediae obtain nutrition by consuming the host's visceral hump, which contains the gonad, digestive gland, and some connective tissue (24, 27). Extensive damage is induced in L. littorea's digestive gland during the course of trematode infection (22) as a result of direct consumption by parasite larvae (24), mechanical pressure (22), flooding with parasite wastes (22), loss of glycogen (28) and glucose (29), and autophagic and autolytic activity (22). Additionally, parasitism causes reduced fecundity and, often, a complete cessation of gamete production in the host (30, 31). Infections are sometimes lost, but, in the majority of cases, persist for an entire lifetime (32, 33) of 4–10 years in the field (34).
In light of L. littorea's dominant role in the rocky intertidal, we hypothesized that any effects of the abundant digenean trematode parasite, C. lingua, on the grazing pressure exerted by populations of L. littorea could have important consequences for community composition. Given the apparent severity of the physiological effects of parasitism on the digestive system of the snail, consumption rates of snails seem likely to be altered in response to trematode parasitism (27), although the direction of change is difficult to predict a priori. Snails may respond to infection by increasing the rate of consumption to compensate for a diminished digestive efficiency or for the additional energetic burden of supporting developing parasites (27, 35). Increases in consumption rate could also result from C. lingua's manipulation of its host; manipulation by parasites of host behavioral and physiological processes for the purpose of increasing parasite fitness is well documented in certain host–parasite pairs (2, 36). Alternatively, snails may decrease their rates of consumption in response to parasitism. For example, a compromised digestive system may have a lower maximum efficiency or capacity, or, because parasitized snails are often castrated (24, 31), the decreased need to allocate resources to reproduction (37) may reduce energy demands, permitting a lower consumption rate. Finally, infected snails may be less capable foragers. Field observations suggest that infected L. littorea migrate more slowly and for shorter distances than uninfected snails (38, 39), potentially limiting rates of consumption by reducing the rate of encounter with food items. Regardless of the direction of effect, we hypothesized that parasite-induced changes in grazing rate would affect the community composition of intertidal macroalgae, an important food and habitat resource for many other intertidal organisms (19, 21, 40, 41).
We designed experiments to separately address two research questions: (i) Is consumption rate of L. littorea influenced by infection with C. lingua, and (ii) do differences in consumption rate between infected and uninfected snails influence the composition of the intertidal macroalgal community? A laboratory experiment tested for differences in the consumption rates of infected and uninfected snails that were provided with unlimited, high-quality macroalgal food. In the field, enclosure pens stocked at ambient densities with predominantly infected or predominantly uninfected groups of snails were monitored for changes in community composition of the underlying macroalgal bed.
Results and Discussion
Does Infection Status Influence Consumption Rate?
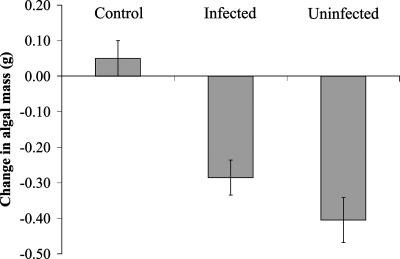
In the laboratory, uninfected snails provided with unlimited, high-quality macroalgal food consumed more macroalgal biomass (mean ± SE = 0.40 ± 0.06 g) in 13 days than infected snails (0.29 ± 0.05 g; Fig. 1), supporting the hypothesis that grazing rates differ between infected and uninfected L. littorea. There was a weak positive relationship between consumption rate and shell length [slope ± SE = 0.03 ± 0.02; F1,50 = 4.07; P = 0.049; supporting information (SI) Table 2] and infected snails (mean shell length ± SE = 24.02 ± 0.44 mm) were significantly larger than uninfected snails (22.30 ± 0.56 mm; t53 = −2.42; P = 0.019). However, trematode infection status of snails remained a significant predictor of the amount of macroalgae consumed over the 13-day trial after we statistically controlled for the effect of shell length (F1,50 = 4.24; P = 0.045; SI Table 2). Because the range of shell lengths for infected (19.13–28.93 mm) and uninfected snails (19.50–28.30 mm) overlapped substantially, statistical inferences are likely to be sound. Furthermore, the potential bias was conservative, because if differences in shell length were responsible for producing the differences in consumption rate between infected and uninfected snails, we would have observed high consumption rates among infected snails, which tend to be larger than uninfected snails. Because the opposite pattern was found, we can be confident that infection status drove the differences in consumption rate between the two groups. In the control replicates that we maintained free of snails, algal biomass increased slightly over the course of the experiment (mean ± SE = 0.05 ± 0.05 g; Fig. 1), suggesting slight growth of macroalgae in the absence of grazing.
Fig. 1.
Change in the mass of macroalgae over the course of a 13-day laboratory experiment in compartments with no snails (i.e., control, n = 4), compartments with a single infected snail (n = 34), and compartments with a single uninfected snail (n = 23). Control compartments were not included in the statistical analysis and are presented here for reference. Columns are means ± 1 SE.
Five mechanisms could have generated the observed depression in consumption rate among infected snails relative to uninfected snails. First, snails with lower consumption rates may be more susceptible to acquiring trematode infection. This explanation, however, is unlikely, because L. littorea becomes infected by incidentally ingesting deposited trematode eggs while grazing in the intertidal zone, and snails with high consumption rates would therefore have the most contact with trematode eggs. Second, reduction in foraging may be the result of host behavior modification by the parasite that reduces movement of host snails; however, this also seems improbable as, in this system, such behavior is unlikely to enhance transmission of released trematode cercariae to their second intermediate hosts (i.e., near-shore fish). Third, parasitic infection may have increased the efficiency of the digestive system, perhaps by increasing the secretion of digestive enzymes (22), thereby reducing the amount of food necessary to meet energetic requirements. Fourth, damage to the digestive gland during the course of parasitic infection may have limited the efficiency or capacity of the snail's digestive system, reducing the rate at which ingested material could be processed and hence, the rate of consumption (35). Rees (35) and James (42) have demonstrated that trematode infection causes substantial damage to digestive tissues in L. littorea, suggesting that a compromised digestive system may explain the diminished consumption rates of infected snails relative to uninfected snails. Finally, various effects of parasitism [e.g., elimination of gamete production (24, 31), retardation of growth (31), and/or breakdown of snail tissues (22)] may have reduced the energetic demands on snail hosts, reducing in turn the amount of food consumed to meet this demand [if these savings are not outweighed by energetic costs associated with trematode cercarial production (27, 35)]. We surmise that damage to the digestive system and/or reduction of energetic demands cause L. littorea with trematode infections to graze macroalgae at lower rates than uninfected snails.
Do Differences in Consumption Rate Between Infected and Uninfected Snails Influence the Composition of the Intertidal Macroalgal Community?
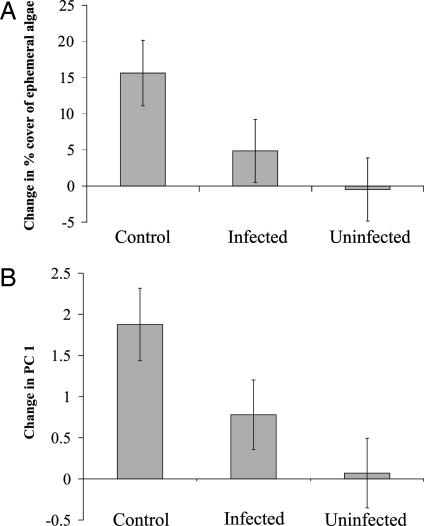
In agreement with previous findings, grazing by L. littorea, regardless of infection status, strongly influenced the abundance of ephemeral algae present in experimental cages installed in the intertidal zone. The percent cover of ephemeral algae increased significantly more in cages without snail grazers than in those with snails (F1,17 = 5.72; P = 0.029; Fig. 2A). Moreover, macroalgal community composition underwent more dramatic shifts toward ephemeral species in cages without snails than in cages with snails, as summarized by the first principal component (F1,17 = 10.95; P = 0.004; Fig. 2B and Table 1). The difference in community composition was primarily caused by increased abundance of the ephemeral alga N. harveyi in treatments without snails (Table 1). N. harveyi grows almost exclusively as an epiphyte on C. crispus. Thus, because we quantified only the top layer of algae, the decrease in C. crispus evident in Table 1 reflects increased colonization by the epiphytic N. harveyi, not a true decline in abundance of C. crispus. These data reaffirm that L. littorea is capable of structuring the intertidal macroalgal community through regulation of the abundance of ephemeral macroalgae.
Fig. 2.
Change in percent cover of ephemeral macroalgae out of total macroalgal abundance (A) and community composition as summarized by the first principal component (B) over the 23–24 days of the field experiment. Columns are means ± 1 SE.
Table 1.
Eigenvectors for a principal components analysis of the percent cover of seven algal species in experimental cages of all three treatments in the field
| Variable | Eigenvector 1 (27.5%) | Eigenvector 2 (18.8%) | Eigenvector 3 (17.0%) |
|---|---|---|---|
| Porphyra sp. | −0.070 | −0.535 | 0.414 |
| N. harveyi | 0.652 | −0.056 | −0.380 |
| Spermothamnion sp. | 0.101 | 0.419 | 0.313 |
| U. lactuca | 0.289 | 0.339 | 0.355 |
| C. crispus | −0.679 | 0.115 | −0.125 |
| M. stellatus | −0.034 | 0.521 | 0.338 |
| C. officinalis | 0.120 | −0.370 | 0.575 |
The percent variance explained by each principal component is indicated.
With confirmation that grazing by L. littorea was a structuring force in this particular system, we then compared the effects of grazing by infected and uninfected snails on macroalgal abundance and community composition. At the end of the experiment, the number of live snails remaining was greater in the uninfected treatment (12.38 ± 0.47) than in the infected treatment (10.77 ± 0.47; t24 = −2.42; P = 0.023). Furthermore, the average snail size was smaller in the uninfected treatment (22.36 ± 0.17 mm) than in the infected treatment (24.02 ± 0.17 mm; t361 = 6.97; P < 0.0001). When we statistically controlled for both of these potential biases, we found that the percent cover of ephemeral algae increased significantly more in the infected treatment than in the uninfected treatment (F1,11 = 9.06; P = 0.012; Fig. 2A; SI Table 3), although this difference was less dramatic than that between treatments with and without snails. This pattern seems to be driven primarily by N. harveyi, as its abundance increased more in the infected treatment than in the uninfected treatment (first principal component, F1,18 = 5.30, P = 0.034; Fig. 2B; SI Table 4).
Our experimental treatments bracket the full range of infection prevalence observed in L. littorea populations in the field. Approximately 10% of snails in the uninfected treatment and 91% of snails in the infected treatment were infected with trematode larvae (as determined by dissection at the end of the experiment; see Materials and Methods). As discussed above, trematode prevalence in L. littorea can become quite high (50–90%), particularly where birds congregate to feed or brood (J.E.B., A.M.H.B., E. Linder, A. Cooper, and T. McGuire, unpublished work; ref. 25). Because our treatments encompass this range of natural variability in infection prevalence, the difference between treatments that we report is an estimate of the maximum possible impact of trematodes on macroalgal communities that we could expect to observe under natural conditions. However, because we were unable to establish a treatment with 0% infection among stocked snails (because of the limitations of the nondestructive technique used to detect infection; see Materials and Methods), this estimate may be somewhat conservative.
The substantial impact of grazing by L. littorea on the structure and function of intertidal communities is well established (19, 21), and any parasite-induced changes in this grazing may therefore strongly affect the larger community. Our data demonstrate that trematode parasites help to structure rocky intertidal macroalgal communities through their influence on L. littorea. As predicted by our laboratory results, percent cover of L. littorea's preferred macroalgal food, ephemeral macroalgae, increased more in the presence of infected snails than in the presence of uninfected snails. Edible algae account for only a very small proportion of the total biomass of macroalgae on rocky shorelines (≈7% of total initial macroalgal cover in our experiment; refs. 43 and 44), but constitute an important food and habitat resource for a variety of intertidal organisms (21, 45, 46). Although the increase in edible algae found in this experiment was modest in terms of total algal abundance, it represents a substantial change in available edible algae. Calculating for just the ephemeral algae, the percent cover of ephemeral algae increased 186% in the no-snails control treatment and 59% in the infected treatment, whereas it decreased by 6% in the uninfected treatment. Infection status of grazing snails may therefore greatly influence the amount of edible algae available to invertebrate grazers in the intertidal zone, underscoring the potential for broader community effects (47).
Importantly, the trematode controlled not just the rate at which a primary community food source was consumed but also the type of food resources and physical structure remaining for utilization by other organisms. Changes in the abundance of ephemeral species can strongly influence the abundance and composition of invertebrate fauna inhabiting a given patch of macroalgae (21, 45, 46). For example, when L. littorea are excluded from exposed intertidal rock habitats, increases in the percent cover of ephemeral macroalgae reduce the availability of substrate suitable for barnacle recruitment, and the resulting absence of barnacle tests reduces blue mussel recruitment (45). Because high parasite prevalence in snails has a similar effect on grazing in a given area as reduction in snail density, high infection levels, like reduced snail density, may exert far-reaching effects in the intertidal community. Moreover, considering that infection prevalence of adult snails can vary from 0% to 90% throughout the northwestern Atlantic, heterogeneity in algal community composition may be influenced by heterogeneity in infection prevalence at various spatial and temporal scales. It is important to bear in mind, however, that in addition to parasite prevalence, other variables (e.g., wave exposure, nutrient availability, recruitment) simultaneously exert strong influence on macroalgal community composition.
What makes our study particularly noteworthy is that we have isolated the indirect, trait-mediated effects of parasites. Obviously, direct mortality effects of C. lingua that depress host populations have repercussions for the remaining community (e.g., refs. 48 and 49). Specifically, C. lingua both castrates L. littorea and increases its mortality rate (present study and ref. 31). Although the rate of loss of infected snails in our field experiment was small and controlled for in analyses, over the long term, the slightly elevated risk of mortality among infected snails would serve to bolster the trematode's positive indirect effect on algae. That is, in addition to altering consumption rates of infected individuals, trematodes, by increasing snail mortality, further mitigate the population-level grazing on algae. Castration would seemingly also mitigate the host's impact on algae by limiting population growth of the snail. However, L. littorea is a broadcast-spawning species and its recruitment (and thus population growth) at a site is largely decoupled from processes like parasitism affecting resident adults.
The literature only sparsely documents community-wide influences of parasites that operate across trophic levels, although many of the well studied host–parasite systems have the potential to strongly influence their respective communities (e.g., refs. 43, 50, and 51). We suggest that biologically important, parasite-induced changes in the grazing patterns of influential herbivores may be pervasive and should be investigated in other systems. The indirect community effects stemming from such nonlethal impacts of parasites on their hosts may make parasites a broadly influential determinant of community composition.
Materials and Methods
Does Infection Status Influence Consumption Rate?
We assessed the effect of snail infection status (i.e., infected or uninfected) on the mass of macroalgae consumed by L. littorea maintained in the laboratory from July 9 through July 22, 2005. Three plastic tackle box containers were modified to house snails individually. Plastic dividers were inserted to create compartments of equal size (5.08 cm × 5.72 cm × 4.45 cm) within each container. Dividers had holes that allowed water to flow through the container when it was partially submerged in a flowing sea water table. We added to each compartment a known amount of U. lactuca (0.8–1.2 g) collected from the intertidal zone on Appledore Island, Maine. Ulva was prepared by placing a small amount (0.5–2.0 g) in a salad spinner and spinning for 30 rotations at three rotations per second. We then measured algal mass to the nearest 0.01 g.
We haphazardly collected 150 adult (>8 mm) L. littorea from the mid-intertidal zone on Appledore Island, searching for snails on rocks, in tide pools, and under Ascophyllum fronds. To achieve roughly equal sample sizes of infected and uninfected snails, we predicted snail infection status by using a method developed by Willey and Gross (52), who found that infected L. littorea usually exhibit darkened (orange) foot coloration and uninfected snails usually possess normal white or light coloration. Although dissection is currently the only definitive method for determining infection status, assessment of foot coloration does not require killing snails and can be fairly accurate (≈90% accuracy). From the initial 150 snails, we chose snails with either very dark or very light foot coloration, and discarded snails with ambiguous coloration, to obtain roughly equal numbers of infected and uninfected snails (i.e., 34 orange-footed snails and 32 white-footed snails). A single snail was added haphazardly to each tackle box compartment. Four compartments were left free of snails to serve as controls for algal growth and degradation in the absence of grazing.
The mass of algae remaining in each compartment was measured, and the infection status and sex of each experimental snail were assessed after 13 days. Snails that died during the experiment (seven orange-footed and two white-footed) were excluded from the final analysis. Using digital calipers, we measured the shell length of the surviving snails by finding the distance from the tip of the spire to the shell aperture. We then dissected each snail, sexed it, and examined the visceral hump (i.e., digestive gland and gonad) in sea water by dissecting it with forceps and searching this tissue thoroughly for trematode rediae and cercariae. If we found rediae or cercariae, we recorded the species of infecting trematode and the relative intensity of the infection based on the abundance of rediae in the visceral hump. In this experiment, C. lingua was the only trematode species found to infect any of the snails.
Of the 57 surviving snails in this experiment, 2/27 snails with orange feet were later found to lack trematode infections and 9/30 snails with white feet were later found to have trematode infections. Although rare in our sample, orange-footed, uninfected snails are likely to be physiologically compromised by some presently unknown, organ-disrupting agent (53). Thus, we excluded these two snails from the analysis.
To test for an effect of infection status on macroalgal consumption, we used a general linear model with the main effect infection status and the covariates snail sex and shell length [included because infected snails tend to be larger than uninfected snails (23, 30, 54)], blocked by container. Sex was later excluded from the original model by backwards elimination because it was not a significant predictor of the response (at α = 0.10). We confirmed that infected snails were larger than uninfected snails by using a two-sample t test.
Do Differences in Consumption Rate Between Infected and Uninfected Snails Influence the Composition of the Intertidal Macroalgal Community?
We assessed the effect of three grazing treatments (i.e., no snails, grazing by uninfected snails at ambient density, and grazing by infected snails at ambient density) on macroalgal species composition in the field. Experimental cages were installed on seven flat ledges (blocks) ranging in tidal height from 0.15 to 0.46 m immediately north of Larus Ledge, a semiexposed site on Appledore Island, Maine; two replicates of each treatment were installed in each block for a total of 42 cages. Each cage (0.30 m × 0.30 m × 0.15 m) and lid (0.43 m × 0.43 m) was constructed of half-inch mesh galvanized hardware cloth and cable ties. Cages had 9- to 15-cm flanges at each side, which were bolted into the substrate and then pounded to conform to the underlying rock. Spaces remaining between the bottom of the cage and rock were plugged with Kop-Coat underwater epoxy (A-788 Splash Zone Compound; Kop-Coat, Pittsburgh, PA). All naturally occurring L. littorea were cleared from the cages after installation was complete.
Immediately before applying treatments, macroalgal species composition was assessed by using a point-contact method. We placed a 3.81-cm × 3.81-cm mesh above the cage and identified the macroalgal species directly beneath each of 49 points in the grid. Only the identity of the uppermost attached alga was recorded. Species identified included ephemeral N. harveyi, U. lactuca, Porphyra sp., and Spermothamnion sp., which are preferred by L. littorea, and perennial C. crispus, Codium fragile subsp. tomentosoides, Mastocarpus stellatus, and Corallina officinalis (20).
We used the nondestructive foot coloration method (52) to create treatments dominated by snails of the appropriate infection status, choosing 630 snails from among a group of 800 adults (>8 mm) collected from the low intertidal zone. Fifteen snails were added to each cage, reflecting the ambient density of 161 snails × m−2 (M.J.D., personal observation). Dissections performed at the end of the experiment confirmed that snails in each treatment were predominantly of the appropriate infection status [χ2(1) = 205.09, P < 0.001]; 131/149 (91.0%) of snails in the infected treatment (i.e., chosen for their orange feet) were infected, and 148/164 (90.2%) of snails in the uninfected treatment (i.e., chosen for their white feet) were uninfected.
The shell length of each snail was measured and shells were marked with numbered bee tags (Queen Marking Kits, The Bee Works, Orillia, Canada). Snails were added to the cages on July 27 and 28, 2005 and cage tops were attached to prevent their escape. Cages were left undisturbed until August 19 and 20, 2005 (23–24 days after the start of the experiment), when we measured final macroalgal species composition in each cage and collected all remaining snails and marked snail shells. This time frame was chosen because it encompassed a portion of the season when ephemeral algal species and L. littorea are both known to be present in the intertidal zone (55).
Four experimental units were not included in the final analysis. Because of time and tide constraints, we were unable to sample two cages at the end of the experiment; in addition, two cages were mislabeled during data collection. After these exclusions, the control treatment had 12 cages, and the uninfected and infected treatments each had 13 cages.
On average, we recovered 88.6% of the snails originally released into each cage. Snails recovered alive were measured and dissected as described above to confirm infection status. Only one snail (0.7%) of the 149 infected individuals deployed into our experimental cages and recovered for dissection was found to be infected with a trematode species other than C. lingua. This snail was classified as infected in our analyses. We compared the number of live and dead snails and the average shell length between the infected and uninfected treatments by using two sample t tests.
To characterize changes in macroalgal community composition, we performed principal components analysis on all before and after percent cover data for seven macroalgal species: the ephemeral taxa N. harveyi, U. lactuca, Porphyra sp., and Spermothamnion sp., and perennial C. crispus, M. stellatus, and C. officinalis. Point-contact categories that occurred extremely infrequently (<2% cover in <3 cages), including C. fragile subsp. tomentosoides, one unidentified green alga, and bare rock or mussel bed, were excluded from this analysis. After ordination, scores corresponding to measurements taken before the experiment were subtracted from scores corresponding to measurements taken after the experiment to find the change in each principal component for each experimental unit.
To confirm that grazing by L. littorea, irrespective of infection status, was an important determinant of macroalgal community composition in this system [as has been shown in other studies (20, 21, 55, 56)], we used a fixed-effects ANOVA with backwards elimination, incorporating experimental units from all three treatments into the data set and starting from a full ANOVA model that included the experimental treatments and potentially influential covariates. The full model included terms for (i) block, (ii) treatment (i.e., infected, uninfected, no-snail control), (iii) the block by treatment interaction, (iv) number of live snails remaining at the end of the experiment (a covariate that may affect grazing potential), and (v) mean shell length of all snails recovered at the end of the experiment (a covariate that may affect individual grazing rates). We analyzed percent cover of ephemeral algae and the first three principal component axes separately. Factors were excluded if they were not significant predictors of the response (at α = 0.10). All effects were considered fixed. We performed planned linear contrasts within the fixed-effects ANOVA to compare the (i) percent cover of ephemeral macroalgae and (ii) the first principal component between cages without snails (i.e., no-snail control treatment) and cages with snails (i.e., infected and uninfected treatments).
To test whether macroalgal abundance and community composition differed between infected and uninfected treatments, we used backwards elimination starting from a full ANOVA model, incorporating only experimental units from the infected and uninfected treatments (i.e., excluding the no-snail control treatment). Apart from this difference in the treatments compared, the ANOVA was set up and performed as in the previous analysis above.
Supplementary Material
Acknowledgments
We thank Art Mathieson for expertise on local algal species, Shoals Marine Laboratory for providing research facilities, and four anonymous referees for valuable comments on this manuscript. C.L.W. was supported by the National Science Foundation and New York and New Hampshire SeaGrants through a Research Experiences for Undergraduates Fellowship and the Dartmouth College Department of Biological Sciences. Support was also provided by National Science Foundation Grant OCE-0503932 and U.S. Department of Agriculture Hatch (to J.E.B.). A.M.H.B. was supported by the Sloan Foundation History of Marine Animal Populations. This paper is scientific contribution 2327 from the New Hampshire Agriculture Experiment Station and contribution 140 from the Shoals Marine Laboratory.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0700062104/DC1.
References
- 1.Price PW. Evolutionary Biology of Parasites. Princeton: Princeton Univ Press; 1980. [Google Scholar]
- 2.Poulin R. Anim Behav. 1994;48:137–146. [Google Scholar]
- 3.Poulin R, Thomas F. Parasitol Today. 1999;15:28–32. doi: 10.1016/s0169-4758(98)01357-x. [DOI] [PubMed] [Google Scholar]
- 4.Sorensen RE, Minchella DJ. Parasitology. 2001;123:S3–S18. doi: 10.1017/s0031182001007843. [DOI] [PubMed] [Google Scholar]
- 5.Lafferty KD, Dobson AP, Kuris AM. Proc Natl Acad Sci USA. 2006;103:11211–11216. doi: 10.1073/pnas.0604755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miura O, Kuris AM, Torchin ME, Hechinger RF, Chiba S. Proc R Soc London Ser B. 2006;273:1323–1328. doi: 10.1098/rspb.2005.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dobson AP, Hudson PJ. Trends Ecol Evol. 1986;1:11–15. doi: 10.1016/0169-5347(86)90060-1. [DOI] [PubMed] [Google Scholar]
- 8.Price PW, Westoby M, Rice B, Atsatt PR, Fritz RS, Thompson JN, Mobley K. Annu Rev Ecol Syst. 1986;17:487–505. [Google Scholar]
- 9.Minchella DJ, Scott ME. Trends Ecol Evol. 1991;6:250–254. doi: 10.1016/0169-5347(91)90071-5. [DOI] [PubMed] [Google Scholar]
- 10.Combes C. Biodiversity Conserv. 1996;5:953–962. [Google Scholar]
- 11.Hudson P, Greenman J. Trends Ecol Evol. 1998;13:387–390. doi: 10.1016/s0169-5347(98)01475-x. [DOI] [PubMed] [Google Scholar]
- 12.Poulin R. Int J Parasitol. 1999;29:903–914. doi: 10.1016/s0020-7519(99)00045-4. [DOI] [PubMed] [Google Scholar]
- 13.Mouritsen KN, Poulin R. Parasitology. 2002;124:S101–S117. doi: 10.1017/s0031182002001476. [DOI] [PubMed] [Google Scholar]
- 14.Park T. Ecol Monogr. 1948;18:265–307. [Google Scholar]
- 15.Pennings SC, Callaway RM. Ecology. 1996;77:1410–1419. [Google Scholar]
- 16.Hatcher MJ, Dick JTA, Dunn AM. Ecol Lett. 2006;9:1253–1271. doi: 10.1111/j.1461-0248.2006.00964.x. [DOI] [PubMed] [Google Scholar]
- 17.Lafferty KD, Morris AK. Ecology. 1996;77:1390–1397. [Google Scholar]
- 18.Arneberg P, Folstad I, Karter AJ. Parasitology. 1996;112:213–219. doi: 10.1017/s003118200008478x. [DOI] [PubMed] [Google Scholar]
- 19.McQuaid CD. Oceanogr Mar Biol Annu Rev. 1996;34:263–302. [Google Scholar]
- 20.Lubchenco J. Am Nat. 1978;112:23–39. [Google Scholar]
- 21.Bertness MD. Ecology. 1984;65:370–381. [Google Scholar]
- 22.Lauckner G. In: Diseases of Marine Animals: General Aspects, Protozoa to Gastropoda. Kinne O, editor. Vol 1. West Sussex, UK: Wiley; 1980. pp. 311–400. [Google Scholar]
- 23.Curtis LA. Parasitology. 2002;124:S43–S56. doi: 10.1017/s0031182002001452. [DOI] [PubMed] [Google Scholar]
- 24.Smyth JD, Halton DW. The Physiology of Trematodes. Cambridge, UK: Cambridge Univ Press; 1983. [Google Scholar]
- 25.Sinderman CJ, Farrin AE. Ecology. 1962;43:69–75. [Google Scholar]
- 26.Lauckner G. In: Diseases of Marine Animals: Introduction—Reptilia, Aves, Mammalia. Kinne O, editor. Vol 4. Hamburg, Germany: Biologische Anstalt Helgoland; 1985. pp. 627–637. [Google Scholar]
- 27.Fretter V, Graham A. British Prosobranch Molluscs: Their Functional Anatomy and Ecology. London: Ray Society; 1994. [Google Scholar]
- 28.Robson EM, Williams IC. J Helminthol. 1971;45:381–401. [Google Scholar]
- 29.McDaniel JS, Dixon KE. Biol Bull. 1967;133:591. doi: 10.2307/1539920. [DOI] [PubMed] [Google Scholar]
- 30.Hughes RN, Answer P. J Mol Stud. 1982;48:321–330. [Google Scholar]
- 31.Huxham M, Raffaelli D, Pike A. J Exp Mar Biol Ecol. 1993;168:223–238. [Google Scholar]
- 32.Rothschild M. J Parasitol. 1942;28:350. [Google Scholar]
- 33.Robson E, Williams IC. J Helminthol. 1970;44:153–168. [Google Scholar]
- 34.Hyman LH. The Invertebrates. Vol VI. New York: McGraw–Hill; 1967. [Google Scholar]
- 35.Rees WJ. Proc Zool Soc London. 1936;2:357–368. [Google Scholar]
- 36.Poulin R. Parasitology. 1994;109:S109–S118. doi: 10.1017/s0031182000085127. [DOI] [PubMed] [Google Scholar]
- 37.Davies MS, Hawkins SJ. Adv Mar Biol. 1998;34:1–100. [Google Scholar]
- 38.Lambert TC, Farley J. Can J Zool. 1968;46:1139–1147. [Google Scholar]
- 39.Williams IC, Ellis C. J Exp Mar Biol Ecol. 1975;17:47–58. [Google Scholar]
- 40.Van Alstyne KL, Ehlig JM, Whitman SL. Mar Ecol Prog Ser. 1999;180:179–185. [Google Scholar]
- 41.Jenkins SR, Coleman RA, Della Santina P, Hawkins SJ, Burrows MT, Hartnoll RG. Mar Ecol Prog Ser. 2005;287:77–86. [Google Scholar]
- 42.James HA. Proceedings of the Third International Congress of Parasitology; Munich: World Federation of Parasitologists; 1974. p. 341. [Google Scholar]
- 43.Thomas F, Renaud F, de Meeus T, Poulin R. Proc R Soc London. 1998;265:1091–1096. [Google Scholar]
- 44.Little C, Kitching JA. The Biology of Rocky Shores. Oxford: Oxford Univ Press; 1996. [Google Scholar]
- 45.Petraitis PS. Ecology. 1983;64:522–533. [Google Scholar]
- 46.Buschbaum C. Hydrobiologia. 2000;440:119–128. [Google Scholar]
- 47.Hudson PJ, Dobson AP, Lafferty KD. Trends Ecol Evol. 2006;21:381–385. doi: 10.1016/j.tree.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 48.Poulin R, Mouritsen KN. J Helminthol. 2006;80:183–191. doi: 10.1079/joh2006341. [DOI] [PubMed] [Google Scholar]
- 49.Lafferty KD. Mar Ecol Prog Ser. 1993;96:229–237. [Google Scholar]
- 50.Mouritsen KN, Poulin R. Mar Biol. 2005;148:1–11. [Google Scholar]
- 51.Chock JS, Mathieson AC. Botanica Marina. 1983;26:87–97. [Google Scholar]
- 52.Willey CH, Gross PR. J Parasitol. 1957;43:324–327. [PubMed] [Google Scholar]
- 53.Zavras ET, James HA. J Invertebr Pathol. 1979;34:276–284. [Google Scholar]
- 54.Curtis LA, Hubbard KM. J Exp Mar Biol Ecol. 1990;143:131–137. [Google Scholar]
- 55.Menge JL. PhD dissertation. Cambridge, MA: Harvard University; 1975. [Google Scholar]
- 56.Lubchenco J. Ecology. 1983;64:1116–1123. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.