Abstract
The continuing study of early angiosperms from the Yixian Formation (≈125 Ma) of northeastern China has yielded a second early angiosperm genus. This report is a detailed account of this early flowering plant and recognizes earlier reports of similar fossils from Russia and China. Entire plants, including roots, stems, and branches terminating in fruits are presented and reconstructed. Evidence for a possible aquatic nature of this plant is presented. The relationship of Hyrcantha (“Sinocarpus”) to the eudicots is discussed. The presence of this second early angiosperm genus, now known as a whole plant, is important in the discussion of its systematics and the ecology of the earliest angiosperms.
Keywords: basal angiosperms, first flower, flower evolution, Lower Cretaceous
The Yixian Formation of northeast China has yielded numerous angiospermous plants, such as Archaefructus (1–3) and Sinocarpus (4, 5), and problematic taxa, such as Polygonites, Typhaera, Lilites, Orchidites (6), Archaeamphora (7), and Potamogeton (?) (8). These discoveries have renewed interest in the search for fossils that provide further data about the origin and evolution of early flowering plants during the Early Cretaceous (9, 10). These data, combined with what is known about Archaefructus, should be useful in hypotheses of angiosperm phylogeny (11–14). A species of Hyrcantha, discovered from the Yixian Formation, provides further information about the nature of early angiosperm inflorescence and infructescence, with additional support for the aquatic nature of these early flowering plants.
Hyrcantha has been known as an early infructescence, found in the mid-Albian sediments of Karatsche-Tau Hill in western Kazakhstan, and initially named Carpolithes karatscheensis Vachrameev (15). Further work on the holotype and topotype of this taxon resulted in the generic name, Hyrcantha, and the combination Hyrcantha karatscheensis (Vachrameev) Krassilov et Vachrameev (16). Recent reinvestigation and detailed character analysis of the type material of H. karatscheensis demonstrates its close similarity to Sinocarpus decussatus Leng et Friis published from the Yixian Formation (4, 5). Thus, a combination is proposed as Hyrcantha decussata comb. nov. for this fossil material, and the characters for this fossil are presented.
Systematics
Hyrcantha.
Krassilov et Vachrameev, 1983.
Type–Species.
H. karatscheensis (Vachrameev) Krassilov et Vachrameev.
Emended Generic Diagnosis.
Plant erect, with one to two main slender stems arising from a short taproot. Stems with alternating secondary branches at the dilated nodes. Nodes enlarged, encircled by thin sheathes (ocrea) and may be associated with or attached to small serrate margined leaves. Infructescence open, paniculate, determinate. Ultimate branches bearing one to four terminal fruits. Gynoecium superior and basally syncarpous to apocarpous with two to four carpels fused or appressed ventrally about half their length. Each carpel containing 10–16 anatropous ovules/seeds borne along an adaxial linear placentae.
H. decussata (Leng et Friis) Dilcher, Sun, Ji et Li comb. nov.
Synonymy.
S. decussatus Leng et Friis, 2003, 2006.
Emended Description.
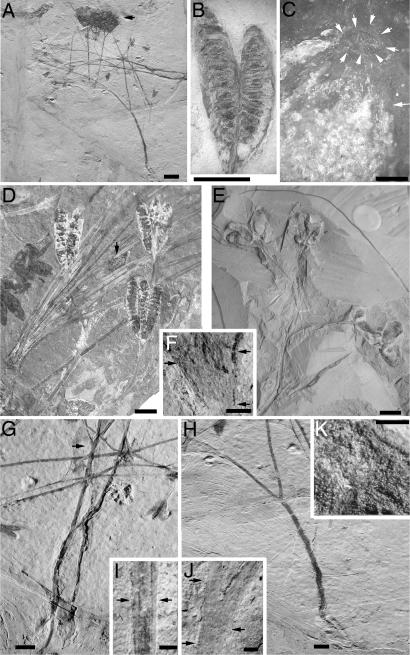
Plant erect, 20–25 cm tall, with predominately alternate branching of 30–45°, rarely ternate branching three to four times (Fig. 1A). Main axis 2.2–2.5 mm wide and lightly striated alternate branches 1–1.2 mm wide. Lower branches with dilated nodes ensheathed by a thin ocrea (Fig. 1J), rarely preserved small leaves may be attached to ocrea at the nodes, and stems characterized by long internodes. Some axes show four to six linear striations in extreme oblique light, and some demonstrate two linear outer zones plus or minus one-quarter of the stem diameter and an apparent central zone plus or minus one-half of the stem diameter (Fig. 1I). Pedicels of the fruits, 1.5–2.7 cm long, ensheathed by an ocrea (Fig. 1F). Gynoecium superior with two to four oval-elongate carpels, in a decussate arrangement, 9–12 mm long by 1.5–3 mm wide, fused or closely pressed ventrally along the lower one-third to one-half of the carpel length (Fig. 1 B and D). The syncarpels terminate the ultimate branches, and the short ocrea covers basal 1.0–1.5 mm of the fruits. Carpels have an enlarged terminal mass that is filled with numerous resinous bodies (Fig. 1K). Two crests extend approximately one-eighth of the carpel length, as well as a well defined adaxial suture extending approximately one-half of the carpel length. Each carpel contains 10–16 ovules/seeds attached along the adaxial linear placentae (Fig. 1B). Ovules/seeds may occur in pairs (5). Ovules/seeds oval to oblong, anatropous, slightly pointed hilar region and rounded to truncate antihilar region (Fig. 1C), 1.2–2.5 mm by 0.6–1 mm in size. Primary root 2.5–4.8 cm long bearing only a few secondary roots (Fig. 1 G and H).
Fig. 1.
Overview of Hyrcantha. (A–D and F–K) H. decussata (A) H. decussata type specimen showing the typical slender branches, branching pattern of pseudodichotomies, and fruits terminal on branches. The dark and densely branched material at the top center of this illustration is sporangial clusters of a fern yet to be described. (B) A pair of fruits terminating a shoot each containing ≈8–12 developing ovules or young seeds attached to the central sides of the fruits. (Specimen NJU-DES02001a.) (C) Micropyle of an ovule or young developing seed as indicated by the arrows. Cells of the outer integument shown as isodiametric cells in the lower half and those of the inner integument (indicated by a single arrow) are small elongate cells. (Specimen NJU-DES02001a.) (D) Seven fruiting terminal axes. Three axes with two fruits (one partially hidden behind a Czekanowskia leafy shoot) two fruit clusters at the extreme left margin, and two axes with three fruits attached. Each fruit contains 8–12 developing ovules or seeds. The terminal pad of tissue can be observed in some of these fruits. (Specimen CB31001.) (E) H. karatscheensis from Karatsche-Tau Hill in western Kazakhstan. The branching axes and terminal fruits are similar to those illustrated in A, B, and D, whereas the fruit shape is different. These fruits are shorter and wider and contain only 7–10 young seeds. (Specimen 3302/43.) (F, I, and J) Specimen NJU-DES02-001b. (F) Base of terminal fruits showing sheath-like covering (left and bottom right arrows mark top and bottom of sheath-like structure) and the zonal section for possible structure connection (upper right arrow). (G) Basal portion of the axes shown in the far right side of A, rotated ≈90°. The axis on the right shows branching of the roots, and the axis on the left shows a single root under the left root of the right axis. A sheath-like covering can be seen enclosing the first branch on the left axis (indicated by arrow). (Specimen JB9901.) (H) An enlargement of the basal portion of the axis shown in the lower half of A. This axis ends in a root that is weakly developed. The root appears to be slightly more carbonized, and one or two short, thin secondary roots may extend a short distance. Cracks across the center and the lower left can be seen. The plant stems are painted in across these repair areas. Only after careful examination could we confirm that the original pieces were cemented back in proper order by the collector/seller. The psuedodichotomous branch may be in fact a lateral branch. (Specimen JB9901.) (I) Portion of a stem showing outer (cortex) and inner (pith) areas of the stem (arrows showing outer portions). (J) Stem node showing sheath-like ocrea (arrows mark top and bottom of sheath-like structure). (K) Resin bodies. (Scale bars: A and H, 2 cm; B and D–E, 5 mm; C, 200 μm; F and J, 1 mm; G, 1 cm; I and K, 0.5 mm.).
Etymology.
Hyrcantha, derived from the ancient name of the Caspian Sea [Hyrcanian (16)], and decussata, from the decussate arrangement of the fruits (4, 5). The specific epithat decussatus of the synonymous genus Sinocarpus is changed to decussata here to comply with rules of nomenclature concerning gender agreement of Latin ending for Hyrcantha.
Localities.
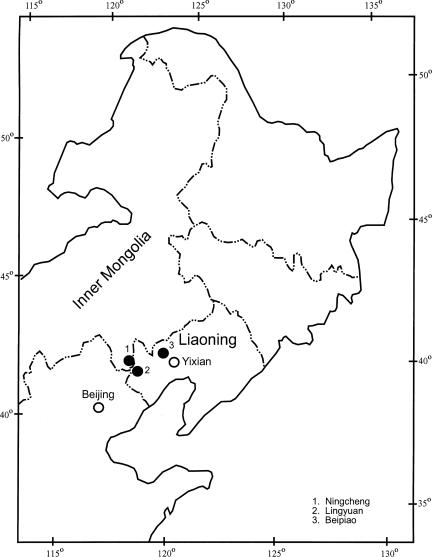
Dawangzhangzi of Lingyuan and Sihetun of Beipiao, western Liaoning; Xidi of Ningcheng, eastern Inner Mongolia; China (Fig. 2).
Fig. 2.
Fossil localities in northeastern China.
Horizon.
Lower part of Yixian Formation; Lower Cretaceous.
Specimens.
JS-003 and NJU-DES02-002 (Xidi of Ningcheng, Inner Mongolia, China); CB31001, 31001A, 31002, 31006H, and NJU-DES02-001 a and b (Dawangzhangzi of Lingyuan, western Liaoning, China); and CB31011 (Sihetun of Beipiao, western Liaoning, China).
Comparison and Comments
The characteristics of H. karatscheensis from western Kazakhstan (ref. 15: pg. 274, pl. 44. figures 1–5; ref. 16: pg. 92, pl. 1, figures 1–5, and pl. 2, figures 1–7) are fundamentally consistent with the species H. decussata, found in northeastern China. These characteristics include the structure of the reproductive organs, including the terminal clustering of multiple carpels, partial basal fusion of individual carpels, and the attachment and orientation of the seeds. The natures of the stem branching and long slender stems also are similar (Fig. 1E). However, the fossil presented in this report is complete; the carpels of H. decussata are almost twice as long, with twice the number of ovules/seeds per carpel; and the ovules/seeds are larger. Krassilov et al. (16) mentioned that the inflorescence is bisexual, and the fruits were thought to contain remains of stamens. However, this finding cannot be confirmed because we saw no evidence of stamens when the type material was examined, although the presence of an ocrea may have been interpreted by Krassilov et al. (16) as stamen attachment scars. In addition, no bracts were observed in the inflorescence as reported by Leng and Friis (4, 5), who also might have confused these with the ocrea. However, there is a zone distal to the ocrea and basal to the carpels that may represent the position where organs such as stamens and bracts may have been attached (Fig. 1F, top right arrow). We could not validate the presence of perianth reported by Leng and Friis (4, 5). Most specimens of H. decussata have carpels that are closely appressed or syncarpous, but a few have a separation that may extend to the base of the carpels, with a 1-mm stalk for each carpel. This observation is in agreement with H. karatscheensis in which the gynoecium was reported as apocarpous (16) with pedicels 1 mm in length (15). The presence of a grooved or striate main axis (16) probably represents the vascular bundles of a herbaceous stem eustele in H. decussata. These vascular bundles are present in the cortex that surrounds a pith or hollow central region of the stem. The centrally darkened midsection seen in Fig. 1I probably represents a pith or infilling of a hollow stem. This midsection is surrounded by lighter-colored striations that are remnants of the concentric vascular strands of the eustele. This finding raises the possibility that the herbaceous stem with a eustele could be the basic ancestral anatomical nature of the angiosperm stem (16). The enlarged terminal area and the crest-like adaxial tissue are similar in both H. karatscheensis (16) (Fig. 1E) and H. decussata (Fig. 1 B and D). Occasionally, we have observed holes in the sides of the fruits and seeds of H. decussata. There may be one or two holes in an individual carpel that correspond to the position of the seeds. A hole in the seed shown in Fig. 1C is only 200 μm in diameter and has some reaction tissue surrounding it. Thus, the hole may have been made by an insect while the plant was living and may represent a hole into which the insect could lay eggs or by which the insect could feed on the rich food source of the young seed, suggesting that this was an aerial part of the plant.
S. decussatus was originally described by Leng and Friis (4) with no mention of or comparisons made with H. karatscheensis (16). Later, they present a brief comparison with H. karatscheensis (5) with no mention made of any observations of the type material. The characteristics presented for S. decussatus by Leng and Friis (4, 5) and this paper are extremely similar to those of the taxon described by Vachrameev (15) and Krassilov et al. (16) except for the phyllotaxy. The decussate nature reported for S. decussatus was not found in the specimens examined in this study, although, in rare cases, ternate branching may be observed, and the carpels appear to be decussate. In most cases, disarticulated branches overlay alternate branching systems, creating an illusion of ternate branching. Thus, we have found that there is great similarity between nearly all of the characteristics of these taxa, which is why S. decussatus should be combined with the genus Hyrcantha
Hyrcantha has several characteristics that suggest it was an aquatic plant with emergent reproductive organs. These characteristics include long stems with elongate internodal stem segments, shallow and weakly developed root system, and the very narrow stems in relation to their length, which would offer little support for the terminal clusters of carpels or fruits in a terrestrial environment. In addition, all of the fruits are borne on short terminal branches that are probably initiated when the growing shoot is exposed above the water surface. Evidence that insects fed on the seeds suggests that these fruits were borne above the surface of the water. The fossils are most often preserved as leafless stems having ocrea at each of the nodes. The ocrea appear to represent the remnants of leaves in shallow underwater stems where the leaves often were lost, leaving little trace of their presence. Finally, specimens of H. decussata are very often closely associated with fossil fish that cooccur with many of the different fossils found in the Yixian Formation.
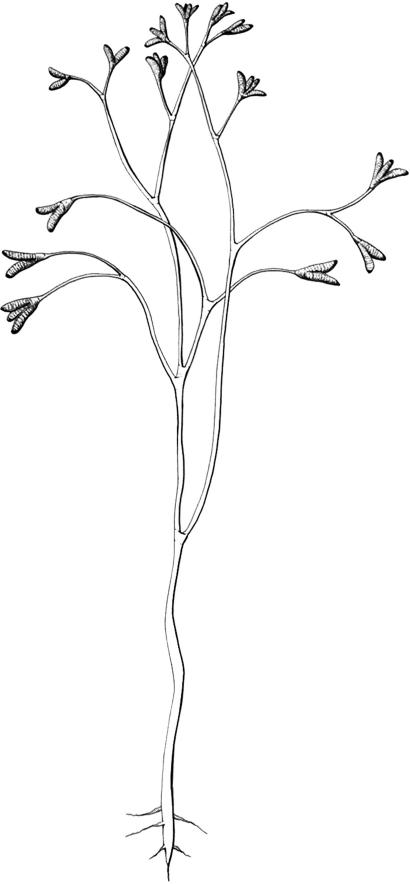
One poorly preserved specimen that appears to have attached leaves was observed. Few details of the leaves are preserved, but they appear to be serrate margined, elliptical, ≈1.2 × 0.6 mm with a midvein and pinnate venation. However, of >20 specimens examined, associated leaves were found only once, so the rare presence of these small leaves attached to fossils of H. decussata indicates their fragile and ephemeral nature. At this point, however, the presence of these leaves is incompletely known and most often missing, so the reconstruction does not reflect this characteristic (Fig. 3). Leng and Friis (5) also report similar associated but unattached leaves for their fossil material.
Fig. 3.
Reconstruction of H. decussata.
Based on comparative considerations of the fruit morphology, Leng and Friis (4, 5) made detailed comparisons of the extant fruits of the Myrothamnaceae, Buxaceae, and Ranunculaceae. They clearly interpret the fossil H. decussata as a basal eudicot based on these comparisons, whereas the eudicots are interpreted as a formal subset of angiosperms established specifically on the presence of tricolpate pollen. There is no pollen associated with Hyrcantha, nor is there any eudicot pollen, although there are three types of basal angiospermous like pollen known from the Yixian Formation (≈125 Ma) (17). This missing character raises a question about the value of trying to match characteristics to extant taxa or families and then using such general similarities to establish the presence of eudicots by 125–122 Ma. The evolution of eudicots is a significant evolutionary node in angiosperm phylogeny. If the eudicots are present by this time, then, according to accepted molecular phylogeny, we can expect that all basal angiosperm taxa should be in place by this time (125–122 Ma). However, in this study, we cannot confirm that Hyrcantha is a eudicot. There are not sufficient numbers of characteristics present that can be included in a combined morphological/molecular analysis (K. C. Nixon, personal communication), as was done with Archaefructus sinensis (3). The lack of characteristics that can be used to discriminate and define the systematic position of Hyrcantha among the angiosperms must be acknowledged here. However, there are a few characteristics that some might use to suggest that Hyrcantha should be placed within the basal eudicots (tricolpates) Ranunculaceae, such as the relationships noted by Krassilov et al. (16) and Leng and Friis (4, 5). The characteristics of H. decussata are not found in any one ranunculoid species but can be found in various taxa over the entire family. Characteristics that are shared include paniculate inflorescence; a unisexual flower; a low number of carpels (1–10) that are free to slightly connate; carpels with very short stalks (18); a stigma with two low crests extending down along the ventral suture; a well defined ventral suture extending down approximately one-half of the carpel length; and a placenta that is linear, lateral, and marginal (19). Associated leaves have been used to further support the basal eudicot ranunculoid nature of H. decussata (5). Other characteristics found in H. decussata, such as the presence of striate and occasionally hollow stems and swollen nodes ensheathed by an ocrea, also are found in the core tricolpate Caryophyllales, specifically the family Polygonaceae (20, 21).
Conclusions
The angiosperm fossil record must become an integral part of the data set used in reconstructing their phylogeny. A second early angiosperm genus is presented here as a whole plant that lived in Asia from ≈125 to 108 Ma. The presence of terminal, many-seeded carpels that have adaxial crests restricted to the distal one half, having anatropous ovules and numerous resin bodies associated with the seeds, are important characteristics of these early angiosperms. The carpels most often are basally fused but may rarely be free on short pedicles. The stems are long and narrow and probably are eusteles typical of herbaceous angiosperms with a pith and cortex. The plants appear to be aquatic, living in shallow water (≈20–40 cm deep), with the terminal fruiting axes exposed above the water.
The systematic affinities are not well placed with any extant angiosperm taxa. H. decussata should be considered an extinct early angiosperm, and it is advisable that it not be used at this time as a reliable node for the origin of the eudicots.
Materials and Methods
The fossil material presented here was collected from the lower part of the Yixian Formation in the Dawangzhangzi, Lingyuan City, and the Sihetun, of Beipiao City, western Liaoning Province, northeastern China, and the Xidi Village of Ningcheng County, eastern Inner Mongolia (Fig. 2). The plant-bearing rocks are mostly light-gray siltstone and dark-gray tuffaceous siltstone, with some light-gray mudstones. The geological age of the fossil-bearing horizons, i.e., the age of the lower part of the Yixian Formation, has previously been considered to range from 122 to 145 Ma, but now a growing consensus accepts an age of ≈125 Ma (2, 22–27).
The holotype specimen, H. karatscheensis (no. 3302/43) and other topotype specimens of this species were examined in detail for comparative work and are housed in the Geological Institute, Russian Academy of Sciences (Moscow, Russia).
The specimens were studied in detail under a STEMI SV8 dissecting microscope (Zeiss, Oberkochen, Germany) and with a Zeiss Axiophot optical binocular microscope with epifluorescence illumination. Specimens CB31001, CB31001A, CB31002-31006, and CB31006H are housed in the Research Center of Paleontology of Jilin University. Specimens JS003, NJU-DES02-001 a and b, and NJU-DES02-002 are housed in the Geological Institute of Chinese Academy of Geosciences.
Acknowledgments
We thank M. A. Akhmeteev (Russian Academy of Sciences) for access to specimens of Hyrcantha; T. A. Lott (University of Florida), W. Crepet (Cornell University, New York, NY), and G. Retallack (University of Oregon, Eugene, OR) for suggestions and reviews; Gerald Gastony (Indiana University, Bloomington, IN) for nomenclatural advise; and Amy Washuta and Lauren Garber for help with reconstruction. This work was supported by Project 111 and National Science Foundation of China Grant 30370096 (to G.S.) and Grant INT0074295 from the National Science Foundation (to D.L.D.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Sun G, Dilcher DL, Zheng SL, Zhou ZK. Science. 1998;282:1692–1695. doi: 10.1126/science.282.5394.1692. [DOI] [PubMed] [Google Scholar]
- 2.Sun G, Zheng SL, Dilcher DL, Wang YD, Mei SW. Early Angiosperms and Their Associated Plants from Western Liaoning, China. Shanghai: Shanghai Sci Technol Educ Press; 2001. pp. 1–227. [Google Scholar]
- 3.Sun G, Ji Q, Dilcher DL, Zheng SL, Nixon KC, Wang XF. Science. 2002;296:899–904. doi: 10.1126/science.1069439. [DOI] [PubMed] [Google Scholar]
- 4.Leng Q, Friis EM. Plant Syst Evol. 2003;241:77–88. [Google Scholar]
- 5.Leng Q, Friis EM. Plant Syst Evol. 2006;262:173–187. [Google Scholar]
- 6.Wu SQ. Paleoworld. 1999;11:7–37. [Google Scholar]
- 7.Li H. Acta Bot Gallica. 2005;152:227–234. [Google Scholar]
- 8.Yabe H, Endo S. Proc Jpn Acad. 1935;11:274–276. [Google Scholar]
- 9.Crepet WL. Science. 1998;282:1653–1654. [Google Scholar]
- 10.Stokstad E. Science. 2002;296:899–904. [Google Scholar]
- 11.Crepet WL, Nixon KC, Gandolfo MA. Am J Bot. 2004;91:1666–1682. doi: 10.3732/ajb.91.10.1666. [DOI] [PubMed] [Google Scholar]
- 12.Soltis PS, Soltis DE. Am J Bot. 2004;91:1614–1626. doi: 10.3732/ajb.91.10.1614. [DOI] [PubMed] [Google Scholar]
- 13.Bremer K. Proc Natl Acad Sci USA. 2000;97:4707–4711. doi: 10.1073/pnas.080421597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bremer K, Friis EM, Bremer B. Syst Biol. 2004;53:496–505. doi: 10.1080/10635150490445913. [DOI] [PubMed] [Google Scholar]
- 15.Vachrameev VA. Reg Stratigr. 1952;1:1–340. (in Russian) [Google Scholar]
- 16.Krassilov VA, Shilin PV, Vachrameev VA. Rev Paleobot Palyn. 1983;40:91–113. [Google Scholar]
- 17.Wang X, Ren D, Wang Y. Act Geol Sin. 2000;74:265–272. [Google Scholar]
- 18.Tamura M. In: The Families and Genera of Vascular Plants. Kubitzki K, Rohwer JG, Bittrich V, editors. Berlin: Springer; 1993. pp. 563–583. [Google Scholar]
- 19.Endress PK, Igersheim A. Bot J Linn Soc. 1999;130:305–393. [Google Scholar]
- 20.Brandbyge J. In: The Families and Genera of Vascular Plants. Kubitzki K, Rohwer JG, Bittrich V, editors. Berlin: Springer; 1993. pp. 531–544. [Google Scholar]
- 21.Haraldson K. Symb Bot (Upsala) 1978;22:1–95. [Google Scholar]
- 22.Swisher CC, Wang YQ, Wang XL, Xu X, Wang Y. Nature. 1999;400:58–61. [Google Scholar]
- 23.Swisher CC, Wang XL, Zhou ZH, Wang YQ, Jin F, Zhang JY, Zhang F, Wang Y. Chin Sci Bull. 2002;47:135–138. [Google Scholar]
- 24.Wang SS, Hu HG, Li PX. Bull Min Petrol Geochem. 2001;20:289–291. [Google Scholar]
- 25.Jin F. Ver PalAsiat. 2001;39:151–156. [Google Scholar]
- 26.Smith PE, Evensen NM, York D, Chang M, Jin F, Li J, Cumbaa S, Russell D. Can J Earth Sci. 1995;32:1426–1431. [Google Scholar]
- 27.Chen PJ, Wang QF, Zhang HC, Gao MZ, Li WB, Wu SQ, Shen YB. Sci China Ser D (Earth Sci) 2005;48:298–312. [Google Scholar]