Abstract
The major physiological role of the vitamin D receptor (VDR) is the maintenance of mineral ion homeostasis. Mutation of the VDR, in humans and mice, results in alopecia. Unlike the effects of the VDR on mineral ion homeostasis, the actions of the VDR that prevent alopecia are ligand-independent. Although absence of the VDR does not prevent the development of a keratinocyte stem cell niche in the bulge region of the hair follicle, it results in an inability of these stem cells to regenerate the lower portion of the hair follicle in vivo and impairs keratinocyte stem cell colony formation in vitro. VDR ablation is associated with a gradual decrease in keratinocyte stem cells, accompanied by an increase in sebaceous activity, a phenotype analogous to that seen with impaired canonical Wnt signaling. Transient gene expression assays demonstrate that the cooperative transcriptional effects of β-catenin and Lef1 are abolished in keratinocytes isolated from VDR-null mice, revealing a role for the unliganded VDR in canonical Wnt signaling. Thus, absence of the VDR impairs canonical Wnt signaling in keratinocytes and leads to the development of alopecia due to a defect in keratinocyte stem cells.
Keywords: alopecia, knockout, Wnt signaling, Lef
Alopecia is a feature of hereditary vitamin D-resistant rickets (1), the molecular basis of which is a mutation of the vitamin D receptor (VDR) (2). Alopecia is also seen in mice with targeted ablation of the VDR (3–6); however, it is not observed in vitamin D-deficient humans or rodents, suggesting that the absence of ligand and the absence of the receptor have different effects on the cells responsible for the cyclic regeneration of the hair follicle (7). Hair follicle development proceeds normally in VDR-null mice (7–9). However, once the morphogenic period ends, during the second week of life, VDR-null mice cannot initiate hair cycles. Thus, when hair is lost, it does not regrow and alopecia ensues.
The hair follicle is an organ composed of epidermal keratinocytes and mesodermal dermal papilla cells (10). VDR expression in the epidermal keratinocyte compartment of the hair follicle, but not the mesodermal dermal papilla, is required to prevent the development of alopecia (7, 11, 12). Primary neonatal keratinocytes from VDR-null mice have no detectable abnormality in proliferation or acquisition of markers of differentiation (8). Furthermore, neonatal keratinocytes lacking the VDR are able to recapitulate hair follicle morphogenesis when implanted into a nude mouse host, along with dermal papilla cells. However, neither the follicles reconstituted using keratinocytes lacking the VDR nor the native follicles of the VDR-null mice are able to undergo postmorphogenic hair cycling (7). Thus, the alopecia observed in VDR-null mice is secondary to an intrinsic keratinocyte defect that is first evident after the period of hair follicle morphogenesis is complete in the second week of postnatal life. Consistent with the observation that alopecia is not observed in states of vitamin D deficiency, keratinocyte-specific expression of a mutant VDR, incapable of ligand binding or ligand-dependent transactivation, maintains postnatal hair cycling in the VDR-null mice (13).
Hair follicle morphogenesis and the regulation of postnatal hair cycling are dependent on reciprocal interactions between the keratinocyte and dermal papilla components of the hair follicle. Postnatally, the epidermal component of the hair follicle, below the sebaceous gland and the bulge, undergoes cycles of growth, apoptosis, and quiescence (14). Keratinocyte stem cells in the bulge are thought to respond to signals from the mesodermal dermal papilla, resulting in the initiation of a new hair cycle (15). In addition to regenerating the lower portion of the hair follicle, these bulge stem cells also play a role in epidermal regeneration and are capable of differentiating into sebocytes that make up the sebaceous glands (16–18).
One of the most extensively studied pathways in hair follicle biology is the Wnt signaling pathway (19). Keratinocyte-specific deletion of β-catenin results in impaired follicle development and lack of hair regrowth after hair follicle morphogenesis (20), whereas overexpression of a constitutively active β-catenin results in de novo hair follicle morphogenesis (21, 22). This same canonical Wnt signaling pathway has been shown to modulate nuclear receptor-dependent gene expression (23–26). Like mutations of the VDR, mutations of the nuclear corepressor Hairless (27) result in alopecia in humans (28) and mice (29). Although the mechanism by which Hairless mutations result in alopecia is still under active investigation, it has been demonstrated that, in addition to interacting with the VDR (30) and suppressing VDR-mediated transactivation in keratinocytes (31), Hairless promotes canonical Wnt signaling (32).
Because the VDR is critical for the maintenance of cutaneous homeostasis and because the effects of the unliganded VDR on canonical Wnt signaling have not been extensively examined, studies were undertaken to address the hypothesis that the ligand-independent actions of the VDR intersect with this key signaling pathway to regulate postmorphogenic hair cycles.
Results
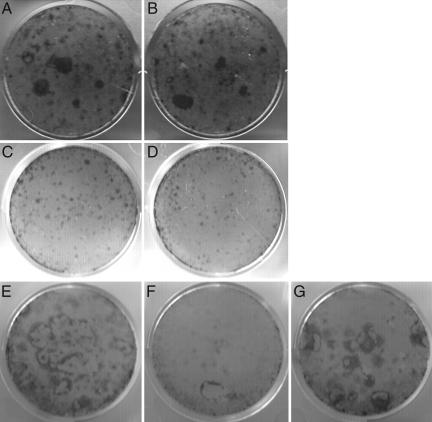
Mutations of the VDR result in alopecia in both humans and mice. In addition to a defect in postmorphogenic hair cycling, the VDR-null mice develop lipid-laden dermal cysts and an increase in oil-red-O-stained sebaceous glands with age (Fig. 1B). Neither of these cutaneous abnormalities is seen in the VDR-null mice with keratinocyte-specific expression of a VDR transgene (Fig. 1C), demonstrating that this phenotype is a direct consequence of impaired VDR action in the keratinocyte. Because keratinocyte stem cells (KSCs) in the bulge of the hair follicle are capable of differentiating into sebocytes, epidermal keratinocytes, and follicle keratinocytes (16–18), and because transcriptional profiling of the bulge stem cells demonstrates enhanced expression of the VDR (16, 18), investigations were undertaken to determine whether the cutaneous consequences of VDR ablation were due to a defect in keratinocyte stem cell self-renewal and/or lineage specification. When plated at low density onto irradiated feeder layers and cultured in media with numerous mineral and vitamin supplements for 2 weeks, ≈5% of epidermal keratinocytes form small colonies (>0.5 mm in diameter). However, most of these colonies are abortive, and by 4 weeks in culture large colonies (>5 mm in diameter) resulting from the high proliferative potential of bulge KSCs are formed by 0.1–0.5% of the cells plated (33, 34). To determine whether the VDR-null mice are born with fewer KSCs, colony formation assays were performed by using neonatal keratinocytes isolated from the VDR-null mice. Neither the number nor the size of the colonies formed by the neonatal VDR-null keratinocytes differed from those observed in cultures from their wild-type littermates after 4 weeks in culture, suggesting that the VDR-null mice are born with a normal number of KSCs (Fig. 2B). When analogous studies were performed in 1-month-old mice, at which point the number of small abortive colonies observed after 2 weeks in culture was not affected by VDR status (Fig. 2 C and D), there was a dramatic reduction in the number of large colonies 4 weeks after plating in cultures of keratinocytes isolated from the VDR-null mice (Fig. 2F; 0.03 ± 0.01% of cells plated), compared with those isolated from their wild-type littermates (Fig. 2E; 0.24 ± 0.04% of cells plated). These data suggest that the VDR is required for the self-renewal or normal lineage specification of bulge KSCs. To demonstrate that the marked reduction in large KSC colony formation by the VDR-null keratinocytes was directly attributable to the lack of a functional VDR, cfu assays were performed on keratinocytes isolated from 4-week-old VDR-null littermates with keratinocyte-specific expression of a VDR transgene. Expression of the VDR transgene corrected the impaired colony formation seen in the transgene-negative VDR-null mice (Fig. 2G). Thus, colony formation by KSCs isolated from neonatal VDR-null mice is indistinguishable from that of their wild-type littermates; however, by 4 weeks of age, in the absence of the VDR, markedly impaired KSC colony formation is observed.
Fig. 1.
VDR-null mice develop lipid-laden dermal cysts and an increase in sebaceous activity. Sections of skin from 8-month-old wild-type (A), VDR-null (B), and keratinocyte-specific VDR transgene-positive VDR-null (C) mice were stained for lipid with oil-red-O and counterstained with hematoxylin. Arrows point to the sebaceous glands. Lipid-laden dermal cysts are indicated by arrowheads in B. Data are representative of those obtained with at least three different mice of each genotype.
Fig. 2.
Evaluation of KSC cfus in VDR-null mice. Keratinocytes isolated from VDR-null mice (B, D, and F), wild-type mice (A, C, and E), and a VDR-null mouse expressing a keratinocyte-specific VDR transgene (G) were plated onto irradiated 3T3 cells and cultured for 2 (C and D) or 4 (A, B, and E–G) weeks. Cells were isolated from 3-day-old (A and B) and 4-week-old (C–G) mice. Staining was performed with rhodanile blue. Data are representative of those obtained with at least three different mice of each age and genotype.
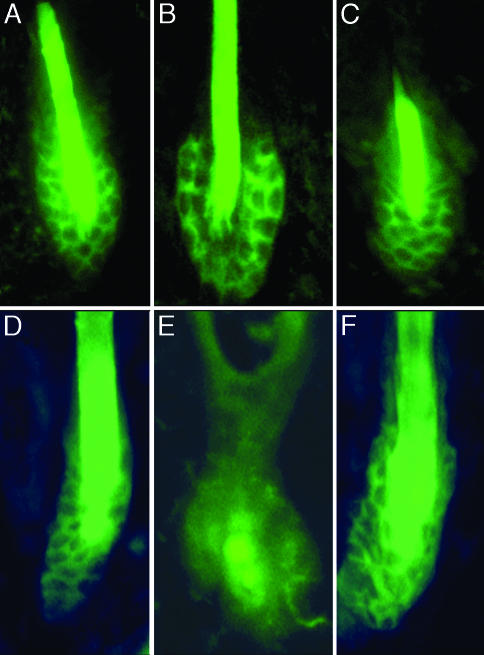
Like other stem cells, the KSCs reside in a special niche known as the bulge region of the hair follicle. This bulge is not present during hair follicle morphogenesis, but is observed at the time of initiation of the first postnatal hair cycle at ≈3 weeks of age (35). Analogous to other stem cells, including those of the hematopoeitic, myogenic, and endothelial lineages, murine bulge stem cells express the cell surface marker CD34 (36). Therefore immunohistochemistry was performed to address whether absence of the VDR interfered with the formation of this stem cell niche in the hair follicle. These studies demonstrate that neither the histological appearance nor the CD34 immunoreactivity of the budge is altered in 4-week-old VDR-null mice (Fig. 3B), compared with that of their wild-type littermates (Fig. 3A) or the VDR-null mice expressing a keratinocyte-specific VDR transgene (Fig. 3C). However, by 9 months of age, while CD34 immunoreactivity was preserved in the bulge region of the hair follicles of the wild-type mice (Fig. 3D), it was not present in VDR-null mice (Fig. 3E). Correlating with the preservation of postmorphogenic hair cycles, the knockout mice expressing the keratinocyte-specific VDR transgene retained CD34 immunoreactivity in the follicle bulge (Fig. 3F).
Fig. 3.
Absence of the VDR does not prevent formation of the hair follicle stem cell niche. Immunohistochemistry with an anti-CD34 antibody was performed to evaluate formation of the KSC niche in the bulge region of the hair follicle. Sections were obtained from wild-type mice (A and D), VDR-null mice (B and E), and VDR-null mice expressing a keratinocyte-specific VDR transgene (C and F) at 4 weeks (A–C) and 9 months (D–F) of age. (D–F) Images are overexposed to permit evaluation of the section from the 9-month-old VDR-null mouse, which fails to reveal membrane-associated fluorescence. Data are representative of those obtained with at least three different mice of each age and genotype.
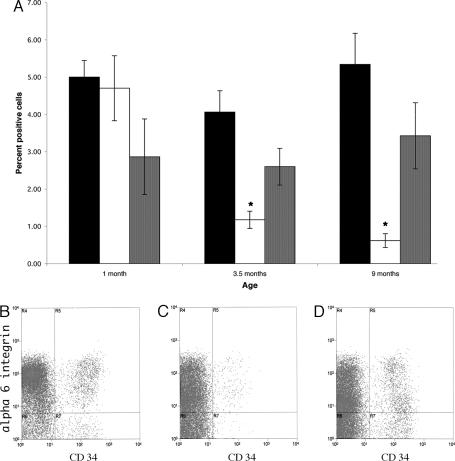
Because these bulge cells make up a small percentage of the keratinocyte population in the skin of mice and are characterized by the expression of both CD34 and α-6 integrin, cell sorting was performed to address whether the marked impairment of colony formation in the keratinocytes of the 28-day-old VDR-null mice was due to a decrease in the number of KSCs residing in the bulge or a functional abnormality of these cells. Consistent with the normal CD34 immunoreactivity (Fig. 3) of the bulge area in the VDR-null mice at 1 month of age, the number of doubly labeled cells detected by FACS analysis at this age was not significantly altered (Fig. 4A). These data strongly suggest that the KSCs in the VDR-null mice have an altered lineage progression or an altered ability to proliferate (or self-renew or survive) because they are unable to give rise to large stem cell colonies in vitro when placed in culture and are unable to generate functional hair follicles in vivo at a point in time when their numbers are apparently unaffected. It is notable that, with aging, there is a progressive decline in the number of doubly labeled cells in the VDR-null mice due to a marked decrease in CD34-positive cells (Fig. 4 A and C), confirming the lack of CD34 immunoreactivity seen at 9 months of age in the skin of the VDR-null mice (Fig. 3E). These data suggest that KSC self-renewal is impaired by the lack of a functional VDR. To determine whether the lack of VDR expression specifically in the keratinocyte component of the hair follicle is responsible for this reduction in CD34/α-6 integrin-positive cells with age, the KSC number was evaluated in VDR-null mice expressing the K-14 VDR transgene. As indicated in Fig. 4 A and D, the number of doubly labeled KSCs in VDR-null mice expressing the K-14-VDR transgene is not significantly different from that of their wild-type littermates at 1, 3.5, or 9 months of age.
Fig. 4.
Quantitation of CD34/α-6 integrin-positive KSCs. (A) Keratinocytes were isolated from wild-type (black bar) and VDR knockout littermates lacking (white bar) and expressing (gray bar) a keratinocyte-specific VDR transgene at 1, 3.5, and 9 months of age. Cells were subjected to FACS analysis to determine the number of doubly labeled cells. Numbers represent the mean ± SEM of cells isolated from at least three different mice of each genotype. ∗, P < 0.05. A representative cell-sorting profile from 9-month-old wild-type (B), VDR-null (C), and keratinocyte-specific VDR transgene-positive, VDR-null (D) mice is presented.
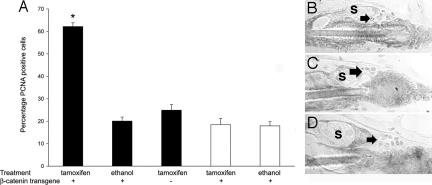
These studies demonstrate that the absence of the VDR leads to a functional abnormality in KSCs, evidenced by impaired colony formation in vitro and an inability to regenerate the lower portion of the hair follicle in vivo by 1 month of age, a time when the number of CD34/α-6 integrin-positive stem cells is normal. However, as the mice age, the KSC number decreases, and the mice develop lipid-laden dermal cysts as well as an increase in sebaceous glands, a phenotype reminiscent of that observed with impaired Wnt signaling. The canonical Wnt signaling pathway has been shown to play a critical role in the lineage determination of KSCs. Attenuation of Wnt signaling and overexpression of a dominant-negative Lef1 impair follicle morphogenesis and result in enhanced sebocyte differentiation (37, 38). In contrast, high levels of Wnt signaling due to constitutive overexpression of β-catenin promote hair follicle formation (20–22). Similarly, induction of a constitutively active β-catenin transgene induces anagen in postmorphogenic hair follicles. To evaluate whether induction of β-catenin could bypass the abnormality in the VDR-null keratinocytes, mice expressing a keratinocyte-specific, tamoxifen-inducible, constitutively active β-catenin transgene (39) were mated into the VDR-null background. VDR-null and control mice carrying this transgene were treated with tamoxifen, to induce transgene expression, at 14 days of age. Studies were specifically performed at this age because it is before the onset of the first postmorphogenic anagen and before the observed decrease in CD34/α-6 integrin-positive KSCs in the VDR-null mice. Induction of the transgene with 4-hydroxytamoxifen failed to induced a proliferative response characteristic of early anagen in the hair follicle keratinocytes of the VDR-null mice carrying the transgene and in control littermates lacking the transgene. A marked increase in proliferating hair follicle keratinocytes, below the bulge region, was observed in the β-catenin transgene-positive control mice in response to tamoxifen treatment (Fig. 5). Treatment with vehicle (ethanol) failed to induce a proliferative response. Analogous studies performed at 14 and 18 days demonstrated that induction of the transgene did not lead to hair regrowth in either shaved or waxed VDR-null mice. These data demonstrate that activation of β-catenin cannot bypass the hair cycling defect in the VDR-null mice at this age, suggesting that nuclear β-catenin is appropriately present in the bulge KSCs in the absence of induction of this transgene. Therefore, immunohistochemistry was performed to evaluate the cellular distribution of β-catenin in the VDR-null mice, their wild-type littermates, and their VDR-null littermates carrying the keratinocyte-specific VDR transgene (all lacking the tamoxifen-inducible transgene). As shown in Fig. 5 B–D, the absence of a functional VDR did not prevent nuclear localization of β-catenin in the bulge KSCs in mice of either genotype, further supporting the data that activation/nuclear localization of β-catenin cannot bypass the defect observed in the VDR-null mice.
Fig. 5.
Activation of β-catenin fails to induce anagen in VDR-null mice. (A) VDR-null mice (open bars) carrying a tamoxifen-inducible β-catenin transgene were treated with 4-hydroxytamoxifen at 14 days of age, as were their transgene-positive and -negative control littermates (filled bars). Skin was harvested 6 days later, and the proliferative response that characterizes anagen induction was quantitated by evaluating the percentage of PCNA-positive follicle keratinocytes in the region below the bulge. Data represent the mean ± SEM of four follicles from each of three mice per genotype and treatment condition. ∗, P < 0.05. Nuclear β-catenin immunoreactivity is present in the bulge cells (arrows) of 18-day-old wild-type (B), VDR-null (C), and VDR transgene-positive, VDR-null (D) mice in the absence of exogenous stimuli. S, sebaceous gland.
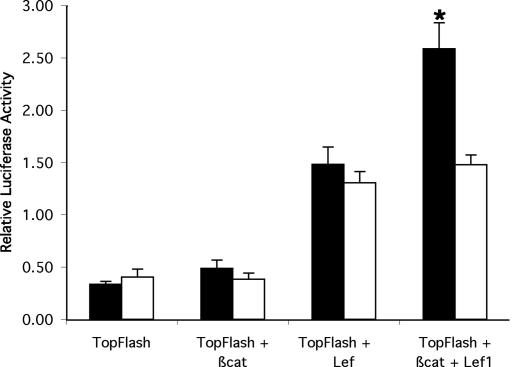
Based on these studies, which suggest that the VDR acts at the level of, or downstream to, transactivation by β-catenin, investigations were undertaken to determine whether absence of the VDR impaired activation of a classical Wnt reporter gene in keratinocytes. Because no culture model of KSCs is available, studies were performed in cultures of primary keratinocytes isolated from VDR-null mice and their wild-type littermates. Transient gene expression assays were performed by using six copies of an optimal TCF/Lef response element upstream of a luciferase reporter gene (TOP-flash). Basal expression of the TOP-flash reporter was unaltered in the VDR-null keratinocytes. However, the absence of the VDR impaired the cooperative interactions of β-catenin and Lef1 on induction of TOP-flash activity (Fig. 6).
Fig. 6.
The VDR is essential for synergistic β-catenin/Lef1 transactivation. Transient gene expression assays were performed in primary neonatal keratinocytes isolated from wild-type (filled bars) mice and their VDR knockout (open bars) littermates. The expression of TOP-flash was corrected for that of cotransfected Renilla luciferase and is expressed as relative luciferase activity. Data are expressed as the mean ± SEM of that obtained with transfections of keratinocytes from three animals of each genotype. ∗, P < 0.05.
Coimmunoprecipitations were, therefore, performed to address whether the VDR could participate in the formation of a complex with β-catenin and/or Lef1 in the absence of ligand. Extracts of COS-7 cells transfected with expression vectors for the VDR, β-catenin, and HA-tagged Lef1 were used for these analyses. Immunoprecipitation of Lef1 resulted in coimmunoprecipitation of both the VDR and β-catenin (Fig. 7), demonstrating that the unliganded VDR forms a complex with these two transcriptional mediators of the canonical Wnt signaling pathway.
Fig. 7.
The unliganded VDR participates in a complex with Lef1 and β-catenin. Proteins present in extracts of COS-7 cells transfected with expression vectors for the VDR, β-catenin, and HA-tagged Lef1 were immunoprecipitated (IP) with α-HA or nonspecific mouse IgG and subjected to Western analyses. Immunoblotting (IB) was performed for β-catenin, Lef1 (Lef1-HA), and the VDR.
Discussion
Several nuclear receptors, including the androgen receptor (AR), retinoic acid, retinoid X, peroxisome proliferator-activated receptor (PPAR), and VDR, have been shown to interact with key effectors of the canonincal Wnt signaling pathway (26). Although these nuclear receptors stratify into different subtypes, they share common domains that are critical for their actions. In contrast to the majority of nuclear receptors, the VDR has a minimal N-terminal transactivation domain. The DNA-binding domain, consisting of two zinc fingers, is required for binding to DNA response elements in target genes. Interactions of cognate ligands with the receptor ligand-binding domains results in the recruitment of nuclear receptor comodulators by a C-terminal AF-2 domain.
The interactions of β-catenin and the AR have been extensively studied in prostate cancer cell models. The binding of β-catenin to the AR requires an intact AR ligand-binding domain, as well as the NH2-terminal transactivation domain, and occurs in a ligand-dependent fashion. The transcriptional consequence of AR/β-catenin interactions is an increase in ligand-dependent transacitvation by the AR (40, 41). Tcf4, another effector of the canonical Wnt signaling pathway, also interacts with the AR in a ligand-dependent fashion. However, these interactions lead to the repression of AR transcriptional activity and are independent of β-catenin (42).
The interactions of the Wnt signaling pathway with PPAR-γ, a key transcriptional regulator of adipogenic differentiation, are somewhat more complex. Sustained activation of β-catenin blocks adipogenesis by inhibiting PPAR-γ-induced gene expression. In a reciprocal manner, activation of PPAR-γ by ligand leads to glycogen synthase kinase 3β-induced β-catenin degradation in preadipocytes (43). However, the interactions of β-catenin with PPAR-γ are dependent on the cellular milieu, in that induction of the Wnt signaling pathway in colon cancer cells leads to increased PPAR-γ expression and PPAR-γ-dependent gene expression (44). The interactions of the VDR with the canonical Wnt signaling pathway are also complex and likely to be dependent on cellular milieu.
Investigations directed at clarifying the interactions of the VDR with the canonical Wnt signaling pathway have largely focused on the downstream consequences of 1,25-dihydroxyvitamin D treatment in a number of cell models. These studies demonstrate that 1,25-dihydroxyvitamin D represses β-catenin signaling. This repression of canonical Wnt signaling by the liganded VDR is dependent on its AF-2 domain (24). Although the molecular mechanisms by which the liganded receptor leads to impaired Wnt signaling are still under active investigation, it has been shown that 1,25-dihydroxyvitamin D promotes the translocation of β-catenin from the nucleus to the plasma membrane (23), thereby inhibiting the expression of β-catenin-responsive genes in colon cancer cell lines. Furthermore, ligand-bound VDR has been shown to compete with TCF4 for β-catenin binding, thereby repressing β-catenin/TCF4-mediated gene transcription. These studies uniformly demonstrate that the ligand-bound VDR impairs Wnt signaling. However, the effects of the VDR on canonical Wnt signaling have been shown to be dependent on cellular milieu: Enhanced β-catenin transcriptional activity is seen when the VDR is overexpressed in human keratinocytes and osteoblasts, whereas attenuated β-catenin transcriptional activity is observed when these same studies are performed in colon cancer cells. The physical interaction of the VDR with β-catenin was shown independent of the ligand binding and AF-2 functions of the VDR (45). Our investigations in a primary keratinocyte cell model demonstrate that the unliganded VDR is critical for the cooperative transcriptional interactions of β-catenin and Lef 1. In vivo correlates of this cooperativity of the VDR and β-catenin were obtained in our investigations in mice with a tamoxifen-inducible, constitutively active β-catenin transgene (39), which demonstrates that overexpression of active β-catenin is insufficient to induce anagen in VDR-null mice. Although the time and/or duration of expression of this transgene may not be ideal for rescuing the hair cycle defect in the VDR-null mice, investigations were performed at the onset of the postmorphogenic phase of the hair follicle, the time when the postdevelopmental VDR-null follicles most closely resemble those of wild-type littermates.
The observation that the VDR requires an intact DNA-binding domain to prevent the development of alopecia (3) suggests that the unliganded VDR regulates the expression or activity of factors that modulate canonical Wnt signaling in bulge KSCs. Although the unliganded VDR could potentially have paracrine actions that regulate canonical Wnt signaling in KSCs, transfection assays in cultured keratinocytes suggest that its actions are, at least in part, autocrine. We were unable to detect the VDR in a complex with β-catenin and/or Lef1 on the TOP response element or on a classical VDR on gel shift analyses. These data suggest that an additional factor that is not ubiquitously expressed interacts with β-catenin /Lef1 and the VDR to promote differentiation of the KSCs into hair follicle keratinocytes, rather than sebocytes or epidermal keratinocytes. Restricted temporospatial expression of this presumptive factor would be consistent with the data in TOPgal mice demonstrating that activation of the canonical Wnt signaling pathway in the bulge KSCs is restricted to the period of anagen initiation (46). Thus, we propose that the unliganded VDR modulates the activity of key effectors of the canonical Wnt signaling pathway in hair follicle KSCs to maintain keratinocyte stem cell self-renewal and to promote differentiation of these cells along the hair follicle keratinocyte lineage.
Methods
Animal Maintenance.
All studies were approved by the institutional animal care committee. Mice were exposed to a 12-h light/12-h dark cycle. The K5/S33Y β-catenin-estrogen receptor transgenic mice (39) were obtained from E. Fearon (University of Michigan, Ann Arbor, MI). Treatment with tamoxifen or ethanol was performed as previously described (39).
Histology and Immunohistochemistry.
Skin obtained from the dorsal region was used for all investigations. Oil-red-O (3% in isopropanol) staining was performed at 37°C for 15 min. Anti-CD34 antibodies were obtained from BD BioSciences (San Jose, CA). Proliferating cell nuclear antigen staining was performed by using a Zymed (South San Francisco, CA) kit according to the manufacturer's instructions.
Cell Culture.
Isolation and transfection of primary neonatal keratinocytes were performed with Lipofectamine (Invitrogen, Carlsbad CA) as previously described (13). Luciferase activity was evaluated by using Stop and Glo (Promega, Madison, WI). Firefly luciferase activity was normalized for a cotransfected Renilla luciferase control. Culture conditions for the evaluation of KSC cfus have been previously described (16, 34). Cells were sorted with FITC-labeled anti-CD34 and phycoerythrin-Cy5 conjugated anti-α-6 integrin antibodies from BD Biosciences (36).
Immunoprecipitation Analyses.
COS-7 cells were transfected with expression vectors for the VDR, β-catenin, and HA-tagged Lef1. Cell lysates were immunoprecipitated with a mouse a-HA antibody (BD Biosciences) and subjected to Western analyses. The same antibody was used to detect immunoprecipitated Lef1. A mouse anti-β-catenin antibody (BD Biosciences) and 9A7 (AbCam) were used to detect β-catenin and the VDR, respectively. Secondary goat anti-mouse and goat anti-rat antibodies were obtained from Santa Cruz Technology (Santa Cruz, CA). Detection was performed by using a chemiluminescence kit (Amersham Biosciences, Piscataway, NJ).
Acknowledgments
The authors thank Dr. Eric Fearon for providing the K5/S33Y-β-catenin-ER transgenic mice and Dr. Hans Clevers (Netherlands Institute for Developmental Biology, Utrecht, The Netherlands) for providing the HA-tagged Lef1 expression vector. This work was supported by National Institutes of Health Grant DK 46974 (to M.B.D.).
Abbreviations
- AR
androgren receptor
- KSC
keratinocyte stem cell
- PPAR
peroxisome proliferator-activated receptor
- VDR
vitamin D receptor.
Footnotes
The authors declare no conflict of interest.
References
- 1.Malloy PJ, Pike JW, Feldman D. Endocr Rev. 1999;20:156–188. doi: 10.1210/edrv.20.2.0359. [DOI] [PubMed] [Google Scholar]
- 2.Hughes MR, Malloy PJ, Kieback DG, Kesterson RA, Pike JW, Feldman D, O'Malley BW. Science. 1988;242:1702–1705. doi: 10.1126/science.2849209. [DOI] [PubMed] [Google Scholar]
- 3.Erben RG, Soegiarto DW, Weber K, Zeitz U, Lieberherr M, Gniadecki R, Moller G, Adamski J, Balling R. Mol Endocrinol. 2002;16:1524–1537. doi: 10.1210/mend.16.7.0866. [DOI] [PubMed] [Google Scholar]
- 4.Li YC, Pirro AE, Amling M, Delling G, Baron R, Bronson R, Demay MB. Proc Natl Acad Sci USA. 1997;94:9831–9835. doi: 10.1073/pnas.94.18.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, Kawakami T, Alioka K, Sato H, Uchiyama Y, et al. Nat Genet. 1997;16:391–396. doi: 10.1038/ng0897-391. [DOI] [PubMed] [Google Scholar]
- 6.Van Cromphaut SJ, Dewerchin M, Hoenderop JG, Stockmans I, Van Herck E, Kato S, Bindels RJ, Collen D, Carmeliet P, Bouillon R, Carmeliet G. Proc Natl Acad Sci USA. 2001;98:13324–13329. doi: 10.1073/pnas.231474698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakai Y, Kishimoto J, Demay M. J Clin Invest. 2001;107:961–966. doi: 10.1172/JCI11676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakai Y, Demay MB. Endocrinology. 2000;141:2043–2049. doi: 10.1210/endo.141.6.7515. [DOI] [PubMed] [Google Scholar]
- 9.Xie Z, Komuves L, Yu QC, Elalieh H, Ng DC, Leary C, Chang S, Crumrine D, Yoshizawa T, Kato S, Bikle DD. J Invest Dermatol. 2002;118:11–16. doi: 10.1046/j.1523-1747.2002.01644.x. [DOI] [PubMed] [Google Scholar]
- 10.Paus R, Cotsarelis G. N Engl J Med. 1999;341:491–497. doi: 10.1056/NEJM199908123410706. [DOI] [PubMed] [Google Scholar]
- 11.Chen C, Sakai Y, Demay M. Endocrinol. 2001;142:5386–5389. doi: 10.1210/endo.142.12.8650. [DOI] [PubMed] [Google Scholar]
- 12.Kong J, Li XJ, Gavin D, Jiang Y, Li YC. J Invest Dermatol. 2002;118:631–638. doi: 10.1046/j.1523-1747.2002.01727.x. [DOI] [PubMed] [Google Scholar]
- 13.Skorija K, Cox M, Sisk JM, Dowd DR, MacDonald PN, Thompson CC, Demay MB. Mol Endocrinol. 2005;19:855–862. doi: 10.1210/me.2004-0415. [DOI] [PubMed] [Google Scholar]
- 14.Dlugosz A. J Clin Invest. 1999;104:851–853. doi: 10.1172/JCI8416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lavker RM, Sun TT, Oshima H, Barrandon Y, Akiyama M, Ferraris C, Chevalier G, Favier B, Jahoda CA, Dhouailly D, et al. J Investig Dermatol Symp Proc. 2003;8:28–38. doi: 10.1046/j.1523-1747.2003.12169.x. [DOI] [PubMed] [Google Scholar]
- 16.Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. Nat Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- 17.Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM. Cell. 2000;102:451–461. doi: 10.1016/s0092-8674(00)00050-7. [DOI] [PubMed] [Google Scholar]
- 18.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alonso L, Fuchs E. Genes Dev. 2003;17:1189–1200. doi: 10.1101/gad.1086903. [DOI] [PubMed] [Google Scholar]
- 20.Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- 21.Gat U, DasGupta R, Degenstein L, Fuchs E. Cell. 1998;95:605–614. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- 22.Lo Celso C, Prowse DM, Watt FM. Development (Cambridge, UK) 2004;131:1787–1799. doi: 10.1242/dev.01052. [DOI] [PubMed] [Google Scholar]
- 23.Palmer HG, Gonzalez-Sancho JM, Espada J, Berciano MT, Puig I, Baulida J, Quintanilla M, Cano A, de Herreros AG, Lafarga M, Munoz A. J Cell Biol. 2001;154:369–387. doi: 10.1083/jcb.200102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah S, Hecht A, Pestell R, Byers SW. J Biol Chem. 2003;278:48137–48145. doi: 10.1074/jbc.M307154200. [DOI] [PubMed] [Google Scholar]
- 25.Shah S, Islam MN, Dakshanamurthy S, Rizvi I, Rao M, Herrell R, Zinser G, Valrance M, Aranda A, Moras D, et al. Mol Cell. 2006;21:799–809. doi: 10.1016/j.molcel.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 26.Mulholland DJ, Dedhar S, Coetzee GA, Nelson CC. Endocr Rev. 2005;26:898–915. doi: 10.1210/er.2003-0034. [DOI] [PubMed] [Google Scholar]
- 27.Thompson CC. J Neurosci. 1996;16:7832–7840. doi: 10.1523/JNEUROSCI.16-24-07832.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmad W, Faiyaz ul Haque M, Brancolini V, Tsou HC, ul Haque S, Lam H, Aita VM, Owen J, deBlaquiere M, et al. Science. 1998;279:720–724. doi: 10.1126/science.279.5351.720. [DOI] [PubMed] [Google Scholar]
- 29.Sundberg JP, King LJ. J Invest Dermatol. 1996;106:368–376. doi: 10.1111/1523-1747.ep12343152. [DOI] [PubMed] [Google Scholar]
- 30.Hsieh JC, Sisk JM, Jurutka PW, Haussler CA, Slater SA, Haussler MR, Thompson CC. J Biol Chem. 2003;278:38665–38674. doi: 10.1074/jbc.M304886200. [DOI] [PubMed] [Google Scholar]
- 31.Xie Z, Chang S, Oda Y, Bikle DD. Endocrinology. 2006;147:314–323. doi: 10.1210/en.2005-1111. [DOI] [PubMed] [Google Scholar]
- 32.Beaudoin GM, III, Sisk JM, Coulombe PA, Thompson CC. Proc Natl Acad Sci USA. 2005;102:14653–14658. doi: 10.1073/pnas.0507609102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris RJ, Potten CS. Cell Prolif. 1994;27:279–289. doi: 10.1111/j.1365-2184.1994.tb01425.x. [DOI] [PubMed] [Google Scholar]
- 34.Wu WY, Morris RJ. Methods Mol Biol. 2005;289:79–86. doi: 10.1385/1-59259-830-7:079. [DOI] [PubMed] [Google Scholar]
- 35.Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 36.Trempus CS, Morris RJ, Bortner CD, Cotsarelis G, Faircloth RS, Reece JM, Tennant RW. J Invest Dermatol. 2003;120:501–511. doi: 10.1046/j.1523-1747.2003.12088.x. [DOI] [PubMed] [Google Scholar]
- 37.Merrill BJ, Gat U, DasGupta R, Fuchs E. Genes Dev. 2001;15:1688–1705. doi: 10.1101/gad.891401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niemann C, Unden AB, Lyle S, Zouboulis Ch C, Toftgard R, Watt FM. Proc Natl Acad Sci USA. 2003;100(Suppl 1):11873–11880. doi: 10.1073/pnas.1834202100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Mater D, Kolligs FT, Dlugosz AA, Fearon ER. Genes Dev. 2003;17:1219–1224. doi: 10.1101/gad.1076103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song LN, Herrell R, Byers S, Shah S, Wilson EM, Gelmann EP. Mol Cell Biol. 2003;23:1674–1687. doi: 10.1128/MCB.23.5.1674-1687.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang F, Li X, Sharma M, Sasaki CY, Longo DL, Lim B, Sun Z. J Biol Chem. 2002;277:11336–11344. doi: 10.1074/jbc.M111962200. [DOI] [PubMed] [Google Scholar]
- 42.Amir AL, Barua M, McKnight NC, Cheng S, Yuan X, Balk SP. J Biol Chem. 2003;278:30828–30834. doi: 10.1074/jbc.M301208200. [DOI] [PubMed] [Google Scholar]
- 43.Liu J, Farmer SR. J Biol Chem. 2004;279:45020–45027. doi: 10.1074/jbc.M407050200. [DOI] [PubMed] [Google Scholar]
- 44.Jansson EA, Are A, Greicius G, Kuo IC, Kelly D, Arulampalam V, Pettersson S. Proc Natl Acad Sci USA. 2005;102:1460–1465. doi: 10.1073/pnas.0405928102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jurutka PW, Hall N, Whitfield GK, Gurevich M, Barthel TK, Hsieh JC, Haussler CA, Haussler MR. J Bone Miner Res. 2004;19(S1):S543. [Google Scholar]
- 46.DasGupta R, Fuchs E. Development (Cambridge, UK) 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]