Abstract
RNA virus replication is an error-prone event caused by the low fidelity of viral RNA-dependent RNA polymerases. Replication fidelity can be decreased further by the use of mutagenic ribonucleoside analogs to a point where viral genetic information can no longer be maintained. For foot-and-mouth disease virus, the antiviral analogs ribavirin and 5-fluorouracil have been shown to be mutagenic, contributing to virus extinction through lethal mutagenesis. Here, we report the x-ray structure of four elongation complexes of foot-and-mouth disease virus polymerase 3D obtained in presence of natural substrates, ATP and UTP, or mutagenic nucleotides, ribavirin triphosphate and 5-fluorouridine triphosphate with different RNAs as template–primer molecules. The ability of these complexes to synthesize RNA in crystals allowed us to capture different successive replication events and to define the critical amino acids involved in (i) the recognition and positioning of the incoming nucleotide or analog; (ii) the positioning of the acceptor base of the template strand; and (iii) the positioning of the 3′-OH group of the primer nucleotide during RNA replication. The structures identify key interactions involved in viral RNA replication and provide insights into the molecular basis of the low fidelity of viral RNA polymerases.
Keywords: 5-fluorouracil, foot-and-mouth disease virus, replication fidelity, ribavirin, RNA elongation
RNA virus populations exist as collections of similar but genetically distinct genomes named quasi-species. Genetic heterogeneity favors adaptation of viruses to environmental changes. The genetic variability of RNA viruses is due, at least in part, to the low fidelity of the RNA-dependent RNA polymerase (RDRP) and the absence of error-repair mechanisms in RNA viruses. One consequence of the low fidelity of RDRPs is the enhanced sensitivity of the viral population to accumulate additional mutations during replication. RNA viruses have evolved to replicate near an error threshold. An increase of genomic mutations beyond this error threshold might force a transition of the virus to error catastrophe, resulting in virus extinction (1–3).
RDRPs use ribonucleotide triphosphates (rNTPs) for synthesis of progeny RNAs. This unique biochemical activity and the high mutation rate of RNA viruses have stimulated the interest in the use of mutagenic rNTP analogs to induce virus entry into error catastrophe. Among them, ribavirin (1-β-d-ribofuranosyl-1,2,3-triazole-3-carboxamide) and 5-fluorouracil have been shown to drive different RNA viruses to extinction through enhanced mutagenesis, including foot-and-mouth disease virus (FMDV) (4–11). Structural studies on replicative complexes of viral RDRPs, template–primers, and rNTP substrates or analogs are needed to understand the molecular basis of the low fidelity of copy of these enzymes and the mutagenic activities displayed by rNTP analogs on viral replication. The analysis may contribute to a rational design of new mutagenic nucleotides with antiviral activity through lethal mutagenesis. We have previously reported the structure and interactions of the FMDV 3D polymerase with a template–primer RNA showing structural evidence of how the physiological substrates bind the large exposed active site of the picornavirus RDRPs (12, 13). In addition, the structure of the complex between the polymerase 3D and its protein–primer VPg revealed the critical interactions involved in the positioning and addition of the first nucleotide (UMP) to the primer molecule, providing insights into the mechanism of initiation of RNA genome replication in picornaviruses (14).
Here, we report the crystallographic analysis of four catalytic complexes of FMDV 3D involving different nucleotides or mutagenic nucleotide analogs: 3D·GCAUGGGCCC·ATP, 3D·GCAUGGGCCC·ATP/UTP, 3D·CGUAGGGCCC·5-fluorouridine triphosphate (FUTP), and 3D·GCAUGGGCCC·ribavirin triphosphate (RTP). The structures determined show high-resolution snapshots of the different events of the RNA elongation process.
Results
Formation of the Elongation Complexes.
Four elongation complexes were obtained, with ATP, ATP/UTP, RTP and FUTP as substrates, by soaking the 3D·template–primer cocrystals (3D·5′-rGCAUGGGCCC-3′ and 5′-rCGUAGGGCCC-3′) in solutions containing the appropriate rNTP substrate or analog in presence of Mn2+. The x-ray structures were determined at 2.7 Å, 3.0 Å, 2.6 Å, and 2.9 Å for the ATP, ATP/UTP, FUTP, and RTP complexes, respectively [supporting information (SI) Table 1]. Analysis of the electron density maps for each of the four different complexes revealed the presence of the template–primer RNA duplex in the front channel of 3D and the 5′ overhang of the template in the template channel (Fig. 1). The dsRNA region appears elongated by 1 bp in the ATP, ATP/UTP, and FUTP complexes. Extra density to accommodate the incoming nucleotide in the polymerase active site was also seen in the ATP/UTP and RTP complexes. The conformations and trajectories of the template–primer RNAs are similar in all complexes and also similar to the previously published structure, obtained in the absence of incoming nucleotides (12). The nucleotide bases at the 5′ end of the template pack against the solvent-exposed hydrophobic residues Phe-162 and Val-181 of the conserved motif F in the fingers subdomain, which form the channel entry (Fig. 2 and SI Fig. 4 to locate the conserved motifs). The walls of this channel are mostly formed by the N-terminal region of the protein and the long loop that connects the helix α4 and the strand β3, where amino acid residues Arg-17, Thr-115, Ala-116, and Arg-128 are hydrogen-bonded to the phosphodiester backbone of the template driving the single stranded RNA toward the active site cavity (Fig. 2). Then, the dsRNA stretches from the active site to the C-terminal end of the protein, exiting through the large central cavity of the polymerase molecule (Fig. 1). The template strand of the duplex product contacts mainly the fingers residues: Asp-109 (loop α4–β3), Arg-193 and His-204 (helix α7), and Gly-216, Cys-217, and Asn-218 (helix α8), whereas the primer strand is adjacent to the thumb domain and forms hydrogen bonds with residues Arg-416 (strand β16) and Glu-422, Lys-423, and Ser-426 (helix α14; Fig. 2).
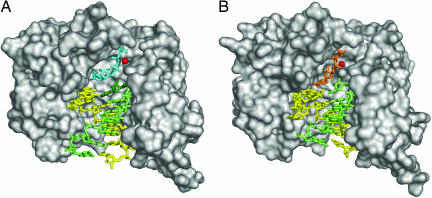
Fig. 1.
Structure of FMDV 3D catalytic complexes. Molecular surface of the polymerase (gray) is shown, with the position of the rNTP substrates and the trajectory of RNA template–primer and duplex product in two different complexes: the 3D·GCAUGGGCCC·ATP/UTP (A) and the 3D·GCAUGGGCCC-RTP (B). The N-terminal residues (residues 34–48) and residues at the top of the NTP tunnel (163–180) of 3D are omitted to show the substrate cavities. RNA molecules are shown in yellow (template strands) and green (primer strands). (A) The UTP substrate is shown in cyan. (B) The position of the antiviral mutagen RTP is shown in orange. Metal ions are shown as red spheres.
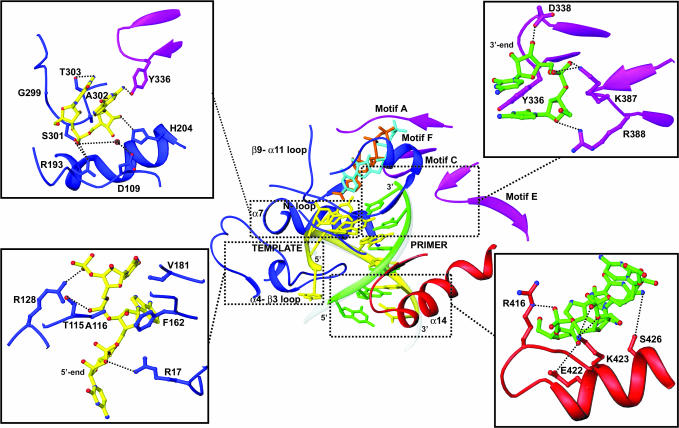
Fig. 2.
Conserved interactions between the FMDV 3D and the different RNA template–primers. The polymerase regions involved in contacts with the RNA molecule are explicitly labeled. The template and primer strands of the RNA molecule are shown in yellow and green, respectively; atoms are displayed in atom-type code, and hydrogen bonds are dashed lines in black. The template strand contacts mainly with residues in the fingers subdomain (blue). The 5′ overhang region of the template binds the template channel, where the different residues of the N-terminal region and the loop α4–β3 of the polymerase drive the ssRNA to the active-site cavity (Lower Left). The template strand of the dsRNA product contacts different residues of helix α7 and the loop β9–α11 in its exit through the central cavity of the enzyme (Upper Left). The primer strand interacts with motifs C and E of the palm subdomain, shown in magenta (Upper Right) and with helix α14 of the thumb, shown in red (Lower Right).
There is no major domain movement in 3D when it binds rNTPs. The structural superimposition of all 476 aa residues in the different elongation complexes onto the 3D·RNA template–primer structure (12) showed root mean square deviation (rmsd) values ranging from 0.42 to 0.52 Å. Noticeable conformational changes include main-chain shifts in loop β9–α11 (rmsd between 1.1 and 1.6 Å) and some side-chain rearrangements in the rNTP-binding region (SI Fig. 5). Subtle conformational changes within the loop β9–α11 allow the accommodation of the incoming substrates and template nucleotides, having different sizes and shapes, to the active site (Fig. 3).
Fig. 3.
Nonconserved interactions established between the FMDV 3D, their template–primer RNAs, and the rNTP substrates in the polymerase active site of the four different complexes 3D·GCAUGGGCCC·ATP (A), 3D·GCAUGGGCCC·ATP/UTP (B), 3D·CGUAGGGCCC·FUTP (C), and 3D·GCAUGGGCCC·RTP (D). The 3D amino acids involved in direct interactions with the template, primer, and incoming nucleotides are shown as sticks in atom-type color code and explicitly labeled. Hydrogen bonds are shown as dashed lines in black. Template and primer RNA strands are shown in yellow and green, respectively. (A–C) Newly incorporated nucleotides are shown in light green. (A and C) Pyrophosphate product (PPi) is also shown in light green. (B) UTP substrate is shown in cyan. (D) Antiviral mutagen RTP is shown in orange. Metal ions are shown as red spheres.
Comparison of the four structures in the active site revealed common contacts mediated by residues of motifs C and E of the palm domain that stabilize the position of the 3′ end of the primer strand in an optimal orientation for RNA elongation (Fig. 2). The comparison also revealed distinct patterns of interactions involving residues of motifs A and B of the palm and motif F in fingers that help the positioning of the acceptor base of the template strand and participate in the recognition and binding of the incoming rNTP (Fig. 3). All of these interactions in the polymerase active site are described in detail below for each of the four complexes.
The 3D·RNA·ATP Complex.
FMDV 3D is active in crystals and catalyzes phosphodiester bond formation. The incubation of cocrystals of 3D·RNA (5′-GCAUGGGCCC-3′) template–primer with ATP in the presence of Mn2+ resulted in the hydrolysis of the triphosphate molecule, incorporation of AMP into the primer molecule, and translocation of the dsRNA product. Interestingly, the pyrophosphate product was also seen in the structure occupying a position in the rNTP entry tunnel and interacting with residues Arg-168 and Ala-243 of motifs F and A of the polymerase, respectively (Fig. 3A). No ions were observed in the active site of this complex.
The newly incorporated adenine (A21) established a Watson and Crick pair with the U4 base of the template strand and provided the new 3′ end of the primer terminus. Its position in the active site of the enzyme was further stabilized by polar bonds mediated by the conserved amino acids Tyr-336, Asp-338, and Lys-387 of motifs C and E, respectively (Fig. 2). Similar interactions were also observed in the active site of the complex 3D·RNA determined in the absence of incoming rNTPs (12). However, the structure of the 3D·RNA·ATP complex also shows significant differences compared with the structure determined in the absence of rNTPs: New polar contacts were formed between the side chain of residue Ser-304 (helix α11) and the base A21 and between the 2′-OH group of A21, and the main-chain oxygen atom of Tyr-336. In addition, the aromatic ring of Tyr-336 makes Van der Waals contacts with the adenine base (Fig. 2). The position of the acceptor base of the template strand (U4) is stabilized by polar and hydrophobic interactions involving residues Thr-115 and Arg-128 (loop α4–β3) and Gly-299, Cys-300, Ser-301, Ala-302, and Thr-303 (loop β9–α11) that contact the phosphate and sugar backbone of U4 (Fig. 3A).
The 3D·RNA·ATP/UTP Complex.
In 3D·RNA·ATP/UTP crystals, the ATP was incorporated into the primer molecule of dsRNA product (Fig. 1A), as in the previous 3D·RNA·ATP complex. However, no clear density to accommodate the pyrophosphate by-product was seen in this second structure. The interactions established between 3D and the new adenine incorporated (A21) are the same that are observed in the 3D·RNA·ATP complex, except for the presence of a new interaction between the side chain of Lys-164 and the adenine base, and the absence of contact with Ser-304 (Fig. 3B). The second nucleotide added (UTP) was also present in the structure; however, this incoming nucleotide is not yet positioned in the active site to form a Watson and Crick pair with the corresponding template acceptor base (Figs. 1A and 3B). The sugar moiety is not located in the correct position; despite its hydroxyl group being hydrogen-bonded to the side chain of Asp-245, the position of this side chain still occludes the ribose-binding pocket. The phosphate groups are located far from the catalytic aspartic acid residues (Fig. 3B). They interact with the conserved basic amino acids Arg-168, Lys-172, and Arg-179 of motif F and also with a metal ion (Fig. 3B). Nevertheless, in this complex, the metal ion that coordinates the triphosphate moiety is located at ≈5 Å of the catalytic Asp-338. The position of the new acceptor nucleotide of the template strand (A3) is stabilized by interactions involving the polymerase residues Thr-115 and Ala-116 (loop α4–β3), Lys-164 and Val-181 (motif F), and Ser-298 and Gly-299 (loop β9–α11). The conformation of the last loop has been changed slightly to accommodate the new acceptor base A3 in the active site. Lys-164 establishes additional polar contacts with the A3 and UTP bases (Fig. 3B).
The 3D·RNA·FUTP Complex.
The formation of a new base pair and translocation of the RNA product were also observed in the 3D·RNA (5′-CGUAGGGCCC-3′) crystals when they were incubated with the mutagenic nucleotide analog FUTP (Fig. 3C). 5-Fluorouridine monophosphate (FUMP) was incorporated into the nascent RNA and occupies the 3′ end of the primer terminus in the active site. The 5-fluorouracil base established a Watson and Crick pair with the acceptor base A4 of the template strand and formed an additional hydrogen bond with the side chain of residue Ser-304 of the polymerase. As in the structure of the 3D·RNA·ATP and 3D·RNA·ATP/UTP complexes, the position of the new primer nucleotide (FUMP) is further stabilized by the polar and hydrophobic interactions mediated by the conserved 3D residues Tyr-336, Asp-338, and Lys-387 (Fig. 2). The pyrophosphate by-product was located at the same position as in the 3D·RNA·ATP structure, in direct contact with residues Ala-243 and Arg-168 of motifs A and F of the polymerase, respectively (Fig. 3C). The amino acid residues Thr-115 and Arg-128 of loop α4–β3 and those of the loop β9–α11 (from Gly-299 to Thr-303) contribute to stabilizing the position of the template nucleotide A4 (Fig. 3C).
The 3D·RNA·RTP Complex.
The crystal structure of the 3D·RNA·RTP complex revealed that the mutagenic nucleotide analog RTP was not incorporated to the nascent RNA (Fig. 1B). However, RTP was positioned at the active site, adjacent to the 3′ terminus of the primer, and base-paired to the acceptor base U4 of the template strand. The position of the RTP base is further stabilized by additional hydrogen bonds with the main-chain atoms of residues Ser-298 and Gly-299, within the loop β9–α11 of 3D (Fig. 3D). The phosphate groups of RTP interact with the side chain of Arg-179 of motif F and the main-chain nitrogen atoms of Ala-243 and Phe-244 of motif A of the polymerase and with one metal ion located at the active site. This ion coordinates the triphosphate moiety of the incoming RTP and also binds the carboxyl group of Asp-338 (Fig. 3D). The sugar moiety of RTP is located at the ribose-binding pocket where the 2′-OH group forms a double hydrogen bond with the specific residues Asn-307 and Asp-245 of motifs B and A, respectively. In addition, the nitrogen atom of the main chain of Asp-245 is hydrogen-bonded to the 3′-OH of RTP (Fig. 3D). The position of the template nucleotide U4 is stabilized by hydrophobic and polar interactions established between the uridine base and sugar and the amino acids Thr-115 and Arg-128 (loop α4–β3), Val-181 and Val-183 (motif F), and Ser-298 and Gly-299 (loop β9–α11) of the polymerase (Fig. 3D). The position of the primer nucleotide C20 is stabilized by the polar and hydrophobic interactions mediated by the conserved 3D residues Tyr-336, Asp-338, and Lys-387 following the same pattern of interactions of the previously described complexes (Fig. 2).
Discussion
Nucleotide Incorporation and RNA Chain Elongation.
In the absence of any proofreading repair activity, the fidelity of copying by viral RDRPs is determined by the template–primer–rNTP–protein interactions in the polymerase active site. The ability of FMDV 3D·RNA template–primer cocrystals to incorporate nucleotides allowed the determination of different structures of the product complexes representing different replication events: (i) The 3D·RNA·RTP complex shows how the antiviral mutagen occupies the polymerase active site in a correct position for the phosphoryl transfer reaction (Figs. 1B and 3D); (ii) the 3D·RNA·ATP complex demonstrates the formation of a new base pair and translocation of the RNA product (Fig. 3A); (iii) the 3D·RNA·ATP/UTP complex illustrates the translocation of the RNA product and the positioning of the new incoming substrate UTP close to the active site (Figs. 1A and 3B); and finally (iv) we captured the 3D·RNA·FUTP complex where the mutagenic nucleotide analog FUTP was also incorporated to the RNA product (Fig. 3C).
The structural comparison of the four complexes revealed the 3D amino acids involved in the correct positioning of the template and primer nucleotides and those responsible of the recognition and positioning of the incoming nucleotide for catalysis (Figs. 2 and 3 and SI Fig. 6). In all complexes, the acceptor base of the template strand is located adjacent to the nucleotide-binding site completely accessible to the incoming nucleotide (Fig. 1). The rest of the ssRNA template (5′ end) turns away from the helical axis of the RNA duplex in the complexes ATP/UTP and RTP (Fig. 1) and is mostly disordered in the ATP and FUTP complexes.
In the active site, the 3′-OH of the primer strand forms a hydrogen bond with the catalytic Asp-338 of motif C in all complexes analyzed here (Fig. 2) as well as in the previously determined 3D·RNA complex (12). In addition, only one metal ion is observed in the catalytic site of the structures 3D·RNA·RTP and 3D·RNA·ATP/UTP. In the 3D·RNA·RTP structure, Asp-338 is bound to this metal ion, which also coordinates the triphosphate moiety of the incoming NTP. The other metal ion that should be presumably located close to the 3′-OH of the primer is absent in our structures. According to the universal mechanism of phosphoryl transfer (15), the formation of the phosphodiester bond is catalyzed by a two-metal mechanism, and two metal sites were observed in the closed conformation of A-type DNA polymerases, in HIV-1 RT and in bacteriophage φ6 and reovirus λ3 RDRPs (16–19). The structures reported here suggest that this second ion has a labile coordination sphere and that the catalytically competent form of the active site of FMDV 3D, which should be finally assembled with the addition of the second catalytic metal, would be stabilized only after the correct rNTP binding at the nucleotide-binding site. As a consequence of this labile metal coordination sphere, the catalytic Asp-338 performs a dual role: in the absence of metal, it forms a hydrogen bond with the 3′-OH that would be subsequently replaced by interaction with the newly introduced cation. Similar observations were made on the terminal transferase human pol β (20), on the Bacillus fragment DNA polymerase (16) and on the HIV-1 RT (21).
Besides contacts involving the catalytic residue Asp-338, the structures determined in this work also reveal three additional conserved interactions that would help the positioning of the primer nucleotide: Tyr-336 (motif C) making both polar and hydrophobic interactions with the primer nucleotide; and Lys-387 and Arg-388 (motif E) which are hydrogen-bonded to the sugar-phosphate backbone of this primer (Fig. 2); the three residues are strictly conserved in picornaviral polymerases. The critical role of Asp-338, Lys-387, and Arg-388 in uridylytation of the VPg primer protein has recently been demonstrated (14). Furthermore, the substitution of either Asp-338 or Lys-387/Arg-388 by Ala in 3D led to noninfectious FMDVs (C.E., A.A., and E.D., unpublished results).
Comparison of the complexes 3D·RNA·ATP/UTP and 3D·RNA·RTP, where the incoming nucleotide is located close to or at the polymerase active site, illustrate the key role of Asp-245 (motif A) and Asn-307 (motif B) in nucleotide recognition and correct positioning of the sugar in the ribose-binding pocket (Fig. 3). Studies on the role of the equivalent 3D positions in poliovirus in the kinetics of incorporation of correct and incorrect nucleotides (22, 23) led Cameron et al. (24) to suggest a two-step model for nucleotide binding. In the first step, the nucleotide was bound in a ground-state configuration in which the ribose moiety could not bind in a productive orientation because the interaction between Asp of motif A, and Asn of motif B occluded the ribose-binding pocket. In the second step, a conformational change occurred that oriented the triphosphate moiety, permitting the phosphoryl transfer reaction. The authors hypothesized that this conformational change step, which required reorientation of Asp (motif A) and Asn (motif B) preceding phosphoryl transfer was a key fidelity checkpoint for the poliovirus RDRP (24). The reoriented residues would stabilize the position of the ribose by direct interactions formed with the 3′- and 2′-OH groups of the incoming nucleotide. The structures determined in this work confirm and extend the model proposed by Cameron and et al., suggesting additional residues (in particular, from Ser-298 to Ser-304) that may play a key role in the control of nucleotide incorporation and chain elongation.
The position occupied by the UTP substrate in the 3D·RNA·ATP/UTP complex illustrates the ground step of nucleotide binding in which the position of Asp-245, hydrogen-bonded to Asn-307, partially blocked the binding site of the ribose. The triphosphate moiety of UTP is located far from the catalytic residues interacting with some of the conserved basic residues of motif F at the nucleotide entry tunnel (Figs. 1A and 3B).
In contrast, in the RTP structure, Asp-245 has changed its rotamer conformation, allowing the positioning of the RTP sugar in the ribose-binding pocket (Fig. 1B). Here, the 2′-OH group of RTP forms a double hydrogen bond with the side chains of residues Asn-307 and Asp-245, whereas the 3′-OH group is hydrogen-bonded to the nitrogen atom of the main chain of Asp-245. In this structure, the phosphate groups of RTP form an extensive network of interactions involving the nitrogen atoms of the main chain of residues Ala-243 and Phe-244 (motif A), the side chain of Arg-179 (motif F), and one metal ion (Fig. 3D). The conformation of the triphosphate moiety is further stabilized by an intramolecular hydrogen bond between the oxygen of the β-phosphate and the 3′-OH group of the nucleotide substrate. The extensive network of hydrogen bonds around the triphosphate moiety was predicted previously by kinetic analysis employing the poliovirus polymerase (23). The correct orientation of the phosphate groups of the nucleotide substrate is critical for nucleotide incorporation not only in the RDRPs but also in other polymerases (16, 25, 26). Stabilization of the triphosphate conformation requires in all cases the conserved structural motif A.
The structures determined in this work have also revealed the key role of additional, previously unidentified amino acids, in nucleotide binding and RNA elongation. In particular, the amino acid residues within the loop β9–α11 (from Ser-298 to Ser-304) establish extensive interactions with the template and incoming nucleotides (Fig. 3). The critical role of this loop in FMDV replication has been further demonstrated by site-directed mutagenesis. Substitutions S298A and T303A resulted in noninfectious transcripts or in the recovery of revertant viruses (C.E., A.A., and E.D., unpublished results).
Incorporation of Mutagenic Nucleotide Analogs by FMDV 3D: Implications for Replication Fidelity.
The 3D·RNA·RTP structure shows the ribavirin pseudobase acting as an adenylate analog paired with the acceptor base U4. Its position is further stabilized by the interactions between the 2′- and 3′-OH groups of the RTP sugar and residues Asp-245 and Asn-307 of motifs A and B of the polymerase and by additional hydrogen bonds involving the conserved residues Ser-298 and Gly-299 (β9–α11 loop) and the RTP base (Fig. 3D). The latter residues are also involved in the positioning of the acceptor base of the template in the four structures determined (Fig. 3). Interestingly, loop β9–α11 contains the site of a mutation (M296I) found in ribavirin-resistant FMDV (27). The structural comparisons of the four FMDV 3D elongation complexes determined in this work as well as the comparisons with the FMDV polymerase structures determined previously (12) revealed that loop β9–α11 is flexible, and its flexibility seems to be required to adapt its conformation and interactions to the size and shape of the different template bases and incoming nucleotides during the RNA elongation process (Fig. 3 and SI Fig. 5). The side chain of Met-296 is not pointing to the RNA-binding cavity, it forms part of a highly hydrophobic cavity also contributed by residues Phe-244, Phe-257, Leu-278, Pro-297, Ile-306, and Leu-310. It is possible that the more rigid Ile side chain in position 296 and its interactions with the hydrophobic cavity could affect the conformation and flexibility of loop β9–α11, altering the interactions either with the incoming rNTP or with the template acceptor base. In different DNA polymerases and HIV RT, substitutions located both, near to, and remote from the active site can have significant effects on fidelity, and such effects appear to be the result of static and/or dynamic changes in the shape and the flexibility of the active site (28–31).
The structural results presented here encourage site-directed mutagenesis of selected residues involved in nucleotide recognition and in positioning of primer and template nucleotides to define further the molecular basis of the error-prone replication in RNA viruses and its relevance to viral pathogenesis.
Methods
Crystallization and Soaking Experiments.
FMDV polymerase (3D) was obtained and purified as described in ref. 12 and stored in a buffer containing 40 mM Tris·HCl, pH 7.5/0.5 M NaCl/0.8 mM DTT/0.8 mM EDTA/8% glycerol at a concentration of 4.6 mg/ml. Two different polymerase·RNA complexes were obtained by using the oligonucleotides r5′-GCAUGGGCCC-3′ and r5′-CGUAGGGCCC-3′ (NWG–Biotech, High Point, NC). Both RNA molecules were able to form 6-bp duplexes by self-complementation, flanked by two 5′ overhangs of four nucleotides each. The oligonucleotides were annealed following the procedure described in ref. 32. Then the 3D polymerase was added slowly in an equimolar proportion in presence of 2 mM MgCl2. The 3D·GCAUGGGCCC complex was crystallized in two different morphologies by using the hanging-drop vapor-diffusion method from crystallization solutions containing PEG 4000 and magnesium acetate as precipitants and buffered at pH 6.0 with sodium cacodylate. Crystals of the complex 3D·CGUAGGGCCC were obtained from a solution containing 33% PEG 4000, 0.2 M ammoniun acetate, 0.1 M sodium citrate (pH 5.6), and 4% γ-butyrolactone. To obtain the ternary complexes containing the antiviral nucleotide analogs RTP and FUTP, the 3D·GCAUGGGCCC and 3D·CGUAGGGCCC crystals were soaked for 6 h in a harvesting solution containing the crystallization buffer, 2 mM MnCl2, and 2 mM RTP or FUTP. The 3D·GCAUGGGCCC/ATP elongation complex was obtained by incubating 3D·RNA complexes for 6 h with 2 mM ATP in the presence of 2 mM MnCl2 in the crystallization buffer. The 3D·GCAUGGGCCC·ATP/UTP elongation complex was obtained as the complex of 3D·RNA·ATP, and then 2 mM UTP was added in the cryobuffer solution immediately before flash freezing in liquid nitrogen.
Data Collection, Structure Determination, and Refinement.
The four data sets were collected at 100 K by using synchrotron radiation at the ESRF. All x-ray data were processed and reduced by using DENZO/SCALEPACK package (SI Table 1) (33). The initial maps for the 3D·GCAUGGGCCC·ATP/UTP and 3D·GCAUGGGCCC·RTP complexes were obtained after a rigid body fitting of the coordinates of 3D protein (PDB ID code 1WNE) (12) to the new unit cells (SI Table 1) by using the program CNS (34). In the two structures, the 2|Fo| − |Fc| and |Fo| − |Fc| difference maps clearly showed the presence of extra density corresponding to RNA template–primer molecules, NTP substrates, and ions. Several cycles of automatic refinement, performed with the program CNS, were alternated with manual model rebuilding by using the program TURBO (35).
Crystals of 3D·GCAUGGGCCC·ATP and 3D·CGUAGGG-CCC·FUTP complexes were not isomorphous to the crystals described above and contained two polymerase complexes in the asymmetric unit (SI Table 1). The structures were solved by molecular replacement with AMoRe (36) by using the isolated 3D protein as the starting model (12). The resulting difference maps also showed the presence of extra density that would correspond to the substrates occupying the central cavity of the polymerase. The refinement of the models was performed with the program REFMAC5 (37), applying noncrystallographic symmetry restraints to the two protein molecules in the asymmetric unit. Automatic refinement was alternated with manual model rebuilding by using the program O (38). The statistics of the refinement for the four complexes are summarized in SI Table 1.
Supplementary Material
Acknowledgments
Work in Barcelona was supported by Grant BFU2005-02376/BMC from the Ministerio de Educación y Ciencia (M.E.C.) (to N.V.) and work in Madrid by Grant BFU2005-00863/BMC from the M.E.C. (to E.D.) and by the Fundación R. Areces. Work in Barcelona and Madrid was further supported by Proyecto Intramural de Frontera (CSIC) (to A.A. and C.F.-O.) and an I3P contract from CSIC (to A.A.). X-ray data were collected at the EMBL protein crystallography beam lines ID14.2 and ID14.4 at European Synchrotron Radiation Facility (ESRF) (Grenoble) within a block allocation group (BAG Barcelona). Financial support was provided by the ESRF.
Abbreviations
- FMDV
foot-and-mouth disease virus
- RDRP
RNA-dependent RNA polymerase
- rNTP
ribonucleotide triphosphate
- FUMP
5-fluorouridine monphosphate
- FUTP
5-fluorouridine triphosphate
- RTP
ribavirin triphosphate.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 2E9R, 2E9T, 2E9Z, and 2ECO).
This article contains supporting information online at www.pnas.org/cgi/content/full/0700518104/DC1.
References
- 1.Eigen M. Proc Natl Acad Sci USA. 2002;99:13374–13376. doi: 10.1073/pnas.212514799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson JP, Daifuku R, Loeb LA. Annu Rev Microbiol. 2004;58:183–205. doi: 10.1146/annurev.micro.58.030603.123649. [DOI] [PubMed] [Google Scholar]
- 3.Domingo E, editor. Virus Res. 2005;107:115–228. doi: 10.1016/j.virusres.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Airaksinen A, Pariente N, Menendez-Arias L, Domingo E. Virology. 2003;311:339–349. doi: 10.1016/s0042-6822(03)00144-2. [DOI] [PubMed] [Google Scholar]
- 5.Pariente N, Sierra S, Lowenstein PR, Domingo E. J Virol. 2001;75:9723–9730. doi: 10.1128/JVI.75.20.9723-9730.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pariente N, Airaksinen A, Domingo E. J Virol. 2003;77:7131–7138. doi: 10.1128/JVI.77.12.7131-7138.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sierra S, Davila M, Lowenstein PR, Domingo E. J Virol. 2000;74:8316–8323. doi: 10.1128/jvi.74.18.8316-8323.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crotty S, Maag D, Arnold JJ, Zhong W, Lau JY, Hong Z, Andino R, Cameron CE. Nat Med. 2000;6:1375–1379. doi: 10.1038/82191. [DOI] [PubMed] [Google Scholar]
- 9.Graci JD, Cameron CE. Rev Med Virol. 2006;16:37–48. doi: 10.1002/rmv.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parker WB. Virus Res. 2005;107:165–171. doi: 10.1016/j.virusres.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Perelson AS, Ribeiro RM. J Hepatol. 2005;43:553–555. doi: 10.1016/j.jhep.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Ferrer-Orta C, Arias A, Perez-Luque R, Escarmis C, Domingo E, Verdaguer N. J Biol Chem. 2004;279:47212–47221. doi: 10.1074/jbc.M405465200. [DOI] [PubMed] [Google Scholar]
- 13.Ferrer-Orta C, Arias A, Escarmis C, Verdaguer N. Curr Opin Struct Biol. 2006;16:27–34. doi: 10.1016/j.sbi.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Ferrer-Orta C, Arias A, Agudo R, Perez-Luque R, Escarmis C, Domingo E, Verdaguer N. EMBO J. 2006;25:880–888. doi: 10.1038/sj.emboj.7600971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brautigam CA, Steitz TA. J Mol Biol. 1998;277:363–377. doi: 10.1006/jmbi.1997.1586. [DOI] [PubMed] [Google Scholar]
- 16.Johnson SJ, Taylor JS, Beese LS. Proc Natl Acad Sci USA. 2003;100:3895–3900. doi: 10.1073/pnas.0630532100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang H, Chopra R, Verdine GL, Harrison SC. Science. 1998;282:1669–1675. doi: 10.1126/science.282.5394.1669. [DOI] [PubMed] [Google Scholar]
- 18.Butcher SJ, Grimes JM, Makeyev EV, Bamford D, Stuart DI. Nature. 2001;410:235–240. doi: 10.1038/35065653. [DOI] [PubMed] [Google Scholar]
- 19.Tao Y, Farsetta DL, Nibert ML, Harrison SC. Cell. 2002;111:733–745. doi: 10.1016/s0092-8674(02)01110-8. [DOI] [PubMed] [Google Scholar]
- 20.Vande Berg BJ, Beard WA, Wilson SH. J Biol Chem. 2001;276:3408–3416. doi: 10.1074/jbc.M002884200. [DOI] [PubMed] [Google Scholar]
- 21.Ding J, Das K, Hsiou Y, Sarafianos SG, Clark AD, Jr, Jacobo-Molina A, Tantillo C, Hughes SH, Arnold E. J Mol Biol. 1998;284:1095–1111. doi: 10.1006/jmbi.1998.2208. [DOI] [PubMed] [Google Scholar]
- 22.Gohara DW, Crotty S, Arnold JJ, Yoder JD, Andino R, Cameron CE. J Biol Chem. 2000;275:25523–25532. doi: 10.1074/jbc.M002671200. [DOI] [PubMed] [Google Scholar]
- 23.Gohara DW, Arnold JJ, Cameron CE. Biochemistry. 2004;43:5149–5158. doi: 10.1021/bi035429s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnold JJ, Vignuzzi M, Stone JK, Andino R, Cameron CE. J Biol Chem. 2005;280:25706–25716. doi: 10.1074/jbc.M503444200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doublie S, Tabor S, Long AM, Richardson CC, Ellenberger T. Nature. 1998;391:251–258. doi: 10.1038/34593. [DOI] [PubMed] [Google Scholar]
- 26.Yin YW, Steitz TA. Science. 2002;298:1387–1395. doi: 10.1126/science.1077464. [DOI] [PubMed] [Google Scholar]
- 27.Sierra M, Airaksinen A, Gonzalez-Lopez C, Agudo R, Arias A, Domingo E. J Virol. 2007;81:2012–2024. doi: 10.1128/JVI.01606-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris D, Kaushik N, Pandey PK, Yadav PN, Pandey VN. J Biol Chem. 1998;273:33624–33634. doi: 10.1074/jbc.273.50.33624. [DOI] [PubMed] [Google Scholar]
- 29.Osheroff WP, Beard WA, Wilson SH, Kunkel TA. J Biol Chem. 1999;274:20749–20752. doi: 10.1074/jbc.274.30.20749. [DOI] [PubMed] [Google Scholar]
- 30.Kim B, Ayran JC, Sagar SG, Adman ET, Fuller SM, Tran NH, Horrigan J. J Biol Chem. 1999;274:27666–27673. doi: 10.1074/jbc.274.39.27666. [DOI] [PubMed] [Google Scholar]
- 31.Kim TW, Brieba LG, Ellenberger T, Kool ET. J Biol Chem. 2006;281:2289–2295. doi: 10.1074/jbc.M510744200. [DOI] [PubMed] [Google Scholar]
- 32.Arnold JJ, Cameron CE. J Biol Chem. 2000;275:5329–5336. doi: 10.1074/jbc.275.8.5329. [DOI] [PubMed] [Google Scholar]
- 33.Otwinowski Z, Minor W. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 34.Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS. Acta Crystallogr D. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 35.Roussel A, Cambillau C. Turbo-Frodo, Silicons Graphics Geometry Partners Directory. Mountain View, CA: Silicons Graphics; 1989. pp. 77–79. [Google Scholar]
- 36.Navaza J. Acta Crystallogr A. 1994;50:150–162. [Google Scholar]
- 37.Murshudov GV, Vagin AA, Dodson EJ. Acta Crystallogr D. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 38.Jones TA, Zou JY, Cowan SW, Kjeldgaard M. Acta Crystallogr A. 1991;47:110. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.