Abstract
Visual attention provides a means of selecting among the barrage of information reaching the retina and of enhancing the perceptual discriminability of relevant stimuli. Neurophysiological studies in monkeys and functional imaging studies in humans have demonstrated neural correlates of these perceptual improvements in visual cortex during attention. Importantly, voluntary attention improves the discriminability of visual cortical responses to relevant stimuli. Recent work aimed at identifying sources of attentional modulation has implicated the frontal eye field (FEF) in driving spatial attention. Subthreshold microstimulation of the FEF enhances the responses of area V4 neurons to spatially corresponding stimuli. However, it is not known whether these enhancements include improved visual-response discriminability, a hallmark of voluntary attention. We used receiver-operator characteristic analysis to quantify how well V4 responses discriminated visual stimuli and examined how discriminability was affected by FEF microstimulation. Discriminability of responses to stable visual stimuli decayed over time but was transiently restored after microstimulation of the FEF. As observed during voluntary attention, the enhancement resulted only from changes in the magnitude of V4 responses and not in the relationship between response magnitude and variance. Enhanced response discriminability was apparent immediately after microstimulation and was reliable within 40 ms of microstimulation onset, indicating a direct influence of FEF stimulation on visual representations. These results contribute to the mounting evidence that saccade-related signals are a source of spatial attentive selection.
Keywords: cognition, gain control, oculomotor, prefrontal cortex, visual perception
Covert visual attention selectively enhances relevant signals from among the flood of information that enters the eye. Perception of attended stimuli is enhanced in a variety of ways (1–3), but in general discrimination of attended stimuli is improved (4). Neurophysiological studies in monkeys and functional imaging studies in humans have demonstrated neural correlates of these perceptual improvements in visual cortex during attention (5–7). Recent work has implicated saccade-related circuits in driving modulations of visual processing during spatial attention. Specifically, subthreshold microstimulation of the frontal eye field (FEF), an area involved in the control of voluntary saccadic eye movements (8, 9), improves performance on an attention task (10, 11) and transiently enhances visual responses in extrastriate area V4 (12, 13). It is known that voluntary attention improves the discriminability of V4 neuronal responses (14, 15) and that the enhanced discriminability results from selective changes in the magnitude of visual responses but not in their reliability (16). Although the effects of FEF microstimulation on V4 responses mirror those of voluntary attention in some respects (12, 13), how FEF microstimulation affects response discriminability and reliability is not known.
We studied the influence of FEF microstimulation on the ability of V4 neurons to discriminate receptive field (RF) stimuli. We used receiver-operating characteristic (ROC) analysis (17), applied to each neuron's response (18), to quantify how well V4 responses could discriminate two stimuli, and we used this metric to examine how visual-response discriminability in the population of V4 neurons was affected by FEF microstimulation. Several hundred ms after visual stimulus onset, response adaptation had markedly reduced the discriminability of V4 responses to different RF stimuli. However, FEF microstimulation transiently restored response discriminability. Furthermore, the enhancement in discriminability resulted from stimulation-driven changes in response magnitude but not in response reliability. Both effects have been observed in V4 neurons during voluntary attention (16). The enhancement was restricted to responses to RF stimuli appearing at locations that were aligned with the end point of the saccade that could be evoked at the stimulation site. Importantly, enhanced discriminability was apparent immediately after FEF microstimulation, suggesting that the effect of stimulation on visual representations is direct. These results suggest that neural circuits involved in voluntary saccade production also modulate representations in the visual cortex during covert attention.
Results
Visual Response Discriminability.
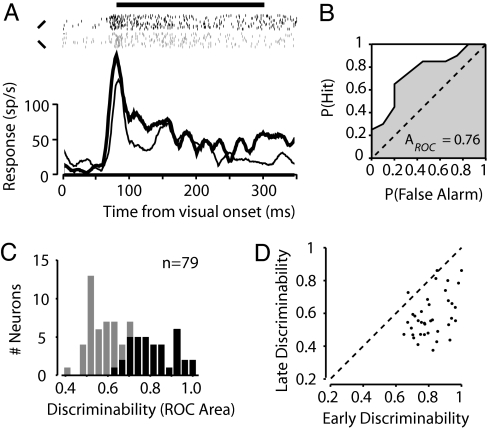
We studied the responses of single V4 neurons to different RF stimuli in monkeys performing a passive fixation task. Fig. 1A shows the response of an example neuron when one of two differently oriented bars was presented to its RF. Shortly after visual stimulus onset (90–290 ms), this neuron responded differently to the two test stimuli (paired t test, P < 0.01). The ROC curve computed from the onset response quantifies the extent to which the response discriminates between the two stimuli tested (Fig. 1B). The area under the ROC curve (AROC), in this case 0.76, is the performance (76%) expected of an ideal observer if she were to make her decision about the RF stimulus orientation based solely on the neuron's response (17), and it is used here as an index of visual-response discriminability (18). For the population of 78 neurons, the mean onset response discriminability varied widely, with a mean AROC of 0.68. We focused our analyses on the subset of neurons that individually showed selective responses to the two RF stimuli (paired t test, P < 0.05; n = 37/78, 47%) (Fig. 1C). For this subset of neurons, response discriminability decayed throughout the trial and had decreased significantly by 500 ms after visual stimulus onset (ΔAROC = −0.23, permutation test, P < 10−12) (Fig. 1D), despite the fact that the RF stimulus remained stable throughout the trial. This degradation in discriminability may reflect the response adaptation often observed in the responses of V4 neurons to stable RF stimuli (19–21).
Fig. 1.
Visual-response discriminability of V4 neurons. (A) Histograms show the average response of an example V4 neuron to the onset of a 45° (thick line) or 135° (thin line) bar presented inside its RF. Rasters (black and gray for 45° and 135° bars, respectively) show action potentials recorded on individual trials. The horizontal line above the rasters indicates the analysis window used to characterize the onset response. (B) ROC curve computed from the onset responses to the two stimuli. The area underneath the curve yields a measurement of how well the V4 neuron's response discriminates between the 45° and 135° bars. This neuron's response reliably discriminated the two stimuli, yielding an ROC area of 0.76. (C) Discriminability values (ROC areas) computed for the population of V4 neurons. Black shading indicates neurons with significant stimulus tuning during the onset analysis window. (D) Comparison of discriminability values computed during early (90 ms after visual onset) versus late (> 500 ms after stimulus onset) analysis windows. The late discriminability values shown here were computed from responses during control trials only.
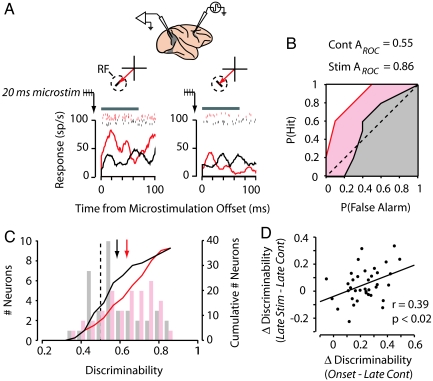
We examined the impact of 20–50 ms of subthreshold FEF stimulation on the visual-response discriminability of simultaneously recorded V4 neurons (Fig. 2). During each experiment, we selected FEF sites with saccade vectors that corresponded spatially with the V4 RF. For the example neuron, microstimulation applied 500 ms after visual onset enhanced responses to the preferred stimulus (paired t test, P < 0.02) but did not reliably affect responses to the nonpreferred stimulus (paired t test, P > 0.5) (Fig. 2A). This stimulus-selective response enhancement produced significant stimulus tuning during microstimulation trials (paired t test, P < 0.02), whereas the responses during control trials could not differentiate the two oriented-bar stimuli (paired t test, P > 0.8). This enhanced discriminability is reflected in the ROC curves generated from stimulation and control trials (AROCstim = 0.86, AROCcont = 0.55, respectively) (Fig. 2B).
Fig. 2.
Subthreshold FEF microstimulation enhances V4 response discriminability. (A) Twenty-millisecond trains of microstimulation were applied to the FEF while visual responses were recorded in an example V4 neuron. FEF and V4 electrodes were positioned so that the saccade that could be evoked at the microstimulation site (red arrow) moved the monkey's gaze to the RF (dotted circle) of the V4 neuron. Histograms and rasters show the response of the V4 neuron to a 45° (Left) and 135° (Right) oriented-bar stimulus for stimulation (red) and control (black) trials (10 repetitions). Histograms and rasters are aligned to the offset of the 20-ms train of subthreshold FEF microstimulation, which was applied 500 ms after visual onset, and to the corresponding period during control trials. The gray bar above the rasters indicates the 70-ms analysis window used to study the effect of microstimulation on neuronal responses. (B) ROC curves computed for the neuron shown in A during the late-analysis window for stimulation (red) and control (black) trials. (C) Discriminability for stimulation (red) and control (black) conditions for the population of stimulus-selective V4 neurons studied. Bar graphs show the distribution of ROC areas (Left ordinate), and colored lines show the corresponding cumulative distribution functions (Right ordinate). Arrows indicate mean ROC area. (D) The effect of subthreshold FEF microstimulation on response discriminability was correlated with the change in discriminability that occurred between the visual-onset and the late-trial analysis periods. Dots show discriminability values for individual neurons, and the line shows the linear best fit.
Microstimulation reliably enhanced response discriminability for the entire population (ΔAROC = 0.05; permutation test, P < 0.003; n = 78) as well as for the neurons that exhibited reliable tuning at visual onset (ΔAROC = 0.05; permutation test, P < 0.02; n = 37) (Fig. 2C). Microstimulation could therefore recover ≈22% of the response discriminability lost during the first half of the trial. For the tuned neurons, the effect of microstimulation on response discriminability was correlated with the drop in discriminability during the trial (Pearson's correlation, r = 0.39, P < 0.02) (Fig. 2D). Thus, FEF microstimulation appeared to compensate for the decay in discriminability observed with stable visual stimuli.
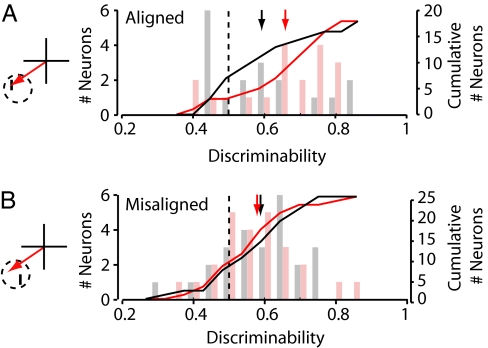
The effect of FEF microstimulation on V4 responses has been shown to depend on the spatial alignment of the visual stimulus and the activated saccade representation within the RF (13). A subset of neurons (n = 49) were tested with RF stimuli that were either spatially aligned or misaligned with the saccade vector that could be evoked at the stimulation site (Fig. 3). As expected, for neurons displaying significant tuning for visual stimuli appearing at the aligned RF position (n = 18), microstimulation enhanced neuronal response discriminability during aligned conditions (AROCcont = 0.59, AROCstim = 0.66, ΔAROC = 0.07; permutation test, P < 0.02) (Fig. 3A). By contrast, during misaligned conditions, microstimulation did not affect the response discriminability of neurons tuned at the misaligned position (AROCcont = 0.59, AROCstim = 0.58, ΔAROC = −0.01; permutation test, P > 0.7; n = 25) (Fig. 3B).
Fig. 3.
Effect of subthreshold FEF microstimulation on response discriminability for aligned and misaligned visual stimulus conditions. (A) (Left) During aligned stimulus conditions, the visual stimulus was positioned within the RF (dotted circle) at the end point of the saccade that could be evoked from the stimulation site (red arrow). (Right) Late-period discriminability is shown for stimulation (red) and control (black) conditions for the population of neurons exhibiting reliable tuning for aligned stimuli. Conventions are as described in Fig. 2C. (B) (Left) During misaligned stimulus conditions, the visual stimulus was positioned within the RF (dotted circle) at a location that was spatially offset from the end point of the saccade that could be evoked from the stimulation site (red arrow). (Right) Late-period discriminability values for stimulation (red) and control (black) conditions for the population of neurons exhibiting reliable tuning for misaligned stimuli. Conventions are as described in Fig. 2C.
Response Reliability.
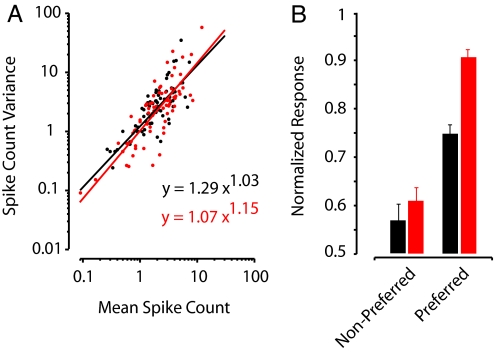
The ability of V4 neurons to discriminate visual stimuli depends on the overall difference in mean responses to different stimuli as well as on response reliability. Stimulation-driven enhancement of discriminability could reflect an increase in the response difference between different stimuli and/or a reduction in variability. To determine whether FEF microstimulation affects response reliability, we examined the relationship between mean response magnitude (spike count) and variance across trials (Fig. 4A). Response magnitude and variance data for the population of tuned neurons (n = 37) was pooled and fit with a power function (16, 22) for stimulation and control conditions. Stimulation and control functions did not differ in their power terms (powerstimulation = 1.15, powercontrol = 1.03; paired t test, P > 0.5) or coefficients (coeffstimulation = 1.07, coeffcontrol = 1.29; paired t test, P > 0.1). However, stimulation did significantly increase the mean response to preferred stimuli (normalized response: stim = 0.91, cont = 0.75; paired t test, P < 0.0005), whereas the mean response to nonpreferred stimuli remained unchanged (stim = 0.61, cont = 0.57; paired t test, P > 0.3) (Fig. 4B). These changes in response magnitude increased the difference between preferred and nonpreferred responses (normalized response difference: stim = 0.29, cont = 0.18; paired t test, P < 0.02). Thus, like voluntary attention, FEF microstimulation improved response discriminability in V4 neurons by selectively enhancing responses to preferred stimuli without altering response reliability.
Fig. 4.
Effect of subthreshold FEF microstimulation on response magnitude and reliability. (A) Relationship between response variance (computed across trials) and average response (spike count) during the late-analysis window for stimulation (red) and control (black) trials. Individual dots show the values for each tuned neuron's response to preferred and nonpreferred stimuli. Power functions were fit to the data for stimulation (red line) and control (black line) conditions. Best-fit equations are shown. (B) Normalized population responses during the late-analysis window for stimulation (red) and control (black) trials. Error bars indicate the SEM.
Timing.
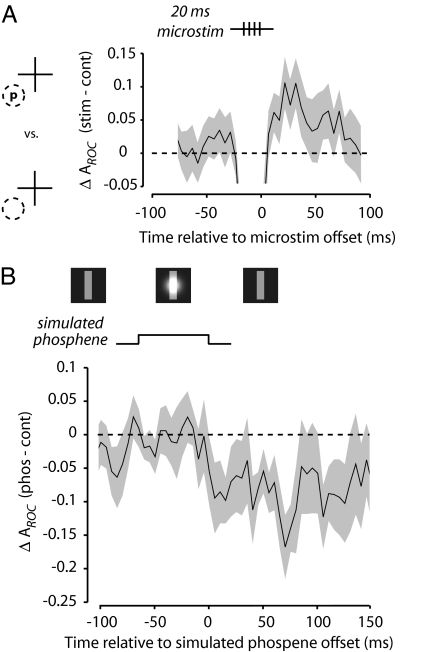
To assess how quickly microstimulation altered V4 response discriminability, we examined the subset of experiments in which very brief trains of microstimulation (20 ms; n = 16) were delivered to the FEF site. For these neurons, we compared the response to an effective visual stimulus with the response to a blank RF to maximize the sensitivity of the ROC analysis. We computed a moving 20-ms window average of ROC areas surrounding the time of FEF microstimulation. Because of the stimulation artifact, response discriminability during microstimulation was transiently disrupted. However, an increase in ROC areas was evident immediately after the offset of the microstimulation train. Already in the first poststimulation time bin, ROC areas were significantly increased above control (ΔAROC = 0.06; permutation test, P < 0.04) (Fig. 5A). Therefore, the discriminability enhancement began within 40 ms of the first FEF microstimulation pulse.
Fig. 5.
Timing of stimulation-driven effects on discriminability and comparison with the effect of a simulated phosphene. (A) A subset of neurons was tested with 20-ms trains of microstimulation (four current pulses at 200 Hz). The average difference in response discriminability (ΔAROC, stimulation minus control) of preferred (p) and blank (background) stimuli in the RF (dotted circle) is shown around the time of FEF microstimulation. Shading indicates SEM. The decrease in discriminability seen during microstimulation is caused by the stimulation artifact. (B) The effect of a simulated phosphene (Gaussian brightness patch superimposed on a RF stimulus; Upper) on response discriminability of preferred and blank stimuli was examined in a population of V4 neurons (n = 11). The difference in ROC area (phosphene minus control) is shown around the time of the simulated phosphene presentation. Shading indicates SEM.
For comparison with the effect of FEF microstimulation, we examined the influence of a simulated phosphene on response discriminability in a small number of V4 neurons (n = 11). The simulated phosphene, depicted in Fig. 5B, consisted of a translucent Gaussian blob (23) transiently superimposed on the stable RF stimulus (or the background) late in the trial. As with the neurons tested with short microstimulation trains, we computed ROC areas from responses to an effective visual stimulus compared with responses to a blank RF. In contrast to the effect of FEF microstimulation, presentation of the simulated phosphene disrupted response discriminability. This disruption began 70 ms after the onset of the simulated phosphene, approximating the visual latency of V4 neurons (24), and persisted for >150 ms. This finding is consistent with the prediction that the simulated phosphene should temporarily mask the stable RF stimulus when presented simultaneously (11, 25) and is consistent with a previous observation that visual cues can interfere with neuronal responses to subsequently presented RF targets (26). By comparison, for the longer (50-ms) FEF stimulation trains, discriminability of an effective stimulus from a blank RF was increased for the duration of the 70-ms analysis window (permutation test, P < 0.03; n = 61).
Discussion
We found that FEF microstimulation increases the ability of V4 neurons to discriminate RF stimuli. V4 response discrimination decays during the presentation of a stable RF stimulus but is transiently restored by FEF microstimulation. The increase in discriminability is restricted to responses to visual stimuli appearing at RF locations aligned with the saccade vector that could be evoked from the FEF site, whereas discriminability of stimuli appearing at misaligned locations remains unchanged. Discriminability enhancement results from changes in response magnitude but not from changes in response reliability. These results, along with previous studies using FEF microstimulation (12, 13), demonstrate that this manipulation modulates visual responses in a manner remarkably similar to the well characterized effects of voluntary attention.
We found that stimulation-driven enhancement of response discriminability is present as soon as it can be measured. By using very brief trains of stimulation pulses, discriminability enhancement is apparent immediately after the offset of FEF microstimulation. Within the relatively small subset of cells tested with 20-ms trains, the enhancement was reliable in the first time bin after microstimulation offset. Thus, the restorative influence of microstimulation on response discriminability takes effect within 40 ms of the first current pulse delivered to the FEF. This finding addresses an important caveat to recent studies reporting attentional benefits from microstimulation of saccade representations during psychophysical tasks. The concern is that the observed effects could be due to an indirect effect of microstimulation (11, 23, 27, 28). Specifically, microstimulation could perhaps induce a spatially localized experience that could subsequently orient attention to the veridical stimulus. Although the possibility of inducing a visual experience, or phosphene, is considered most often (11, 23, 27, 28), microstimulation could conceivably induce a variety of experiences, including highly complex or “psychical” ones (29, 30). However, the rapid enhancement in discriminability implies that rather than producing an experience that subsequently attracts attention, FEF microstimulation activates a neural circuit responsible for modulating visual responses. Moreover, activation of this circuit might underlie the behavioral improvements observed after FEF microstimulation in animals performing an attention task (10, 11). In addition, our failure to produce enhancements of response discriminability with a simulated phosphene is consistent with findings that substitution of microstimulation with simulated phosphenes does not lead to improved attentional performance (23, 28). Therefore, unless our observations were due primarily to antidromic activation of neurons outside the FEF, e.g., in the lateral intraparietal area (LIP), our results imply that FEF neurons, perhaps by way of other structures activated orthodromically, enhance representations in visual cortex during spatial attention. Indeed, the FEF projects to the superior colliculus (SC) and area LIP, two areas known to be involved in both saccade control and visual attention (23, 27, 28, 31, 32). Thus, there are several pathways by which FEF stimulation could act on visual responses.
The conclusion that saccadic representations drive attention is supported by a number of recent studies. One study that recorded neuronal activity in the FEF while monkeys performed covert visual search tasks found that both visual and visuomovement neurons had elevated responses to the search target, a popout stimulus, although no saccades were made to the target (33). By contrast, responses of neurons with purely movement-related activity were suppressed, suggesting that some neurons participate selectively in saccade production and others participate in covert visual selection. Similarly, another study that recorded neuronal activity in the SC while monkeys performed a visual discrimination task found that visuomovement neurons were active during covert shifts of attention (31). Thus, both the FEF and SC contain neurons that signal the locus of attention. Moreover, these results suggest that visuomovement neurons have a dual role in both covert visual selection and saccade production. These findings are complemented by experiments that test whether neuronal signaling in the FEF and SC is necessary for attention by inactivating these areas. Reversible inactivation of FEF neurons not only impairs saccade production (34, 35) but has also been shown to increase reaction time in a covert visual search task that did not require eye movement responses (36). In addition, reversible inactivation of the SC produces deficits in target selection that cannot be attributed to a purely visual or motor impairment (37). Consistent with neurophysiological studies in monkeys, transcranial magnetic stimulation experiments provide causal evidence that the attentional role of the FEF is conserved in humans (38–43). Finally, studies in the barn owl have found that microstimulation of gaze-control circuits homologous with primate FEF produces space-specific modulation of auditory responses (44). Thus, evidence from a diverse set of experimental approaches, species, and modalities, has accrued to indicate that saccade-related mechanisms drive spatial attention in the absence of overt orienting. The improved ability of V4 neurons to discriminate RF stimuli after FEF microstimulation might account for at least some of the perceptual benefits of attention.
Methods
General and Surgical Procedures.
Four male monkeys (two Macaca mulatta and two Macaca fascicularis, 4–10 kg) were used in these experiments. All experimental procedures were in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals, the Society for Neuroscience Guidelines and Policies, and Stanford University Administrative Panel on Laboratory Animal Care. General experimental and surgical procedures have been described previously (45). Each animal was surgically implanted with a head post, a scleral eye coil, and two recording chambers. Surgery was conducted by using aseptic techniques under general anesthesia (isoflurane), and analgesics were provided during postsurgical recovery. Two craniotomies were performed on each animal, allowing access to dorsal V4, on the prelunate gyrus, and FEF, on the anterior bank of the arcuate sulcus.
Visual Stimuli and Behavioral Task.
Monkeys were trained to fixate within a 2–3° diameter error window surrounding a central spot; 250–500 ms after fixation, oriented-bar stimuli (1–4° × 0.25–1.0°) were presented for 1–2 s at locations both inside and outside the RF of the V4 neuron under study. On 50% of experimental trials, subthreshold microstimulation of an FEF site (20–50-ms train) was applied 200–500 ms after visual stimulus presentation. Monkeys were required to maintain fixation throughout the course of visual stimulus presentation, and only correctly completed trials were included in the analyses. Throughout all experiments, eye position was monitored with a scleral search coil and digitized at 200 Hz. All visual stimulus and microstimulation conditions were randomly interleaved and were controlled by the CORTEX system for data acquisition and behavioral control.
Responses to two oriented bar stimuli (0°, 45°, 90°, or 135°) were examined during each experiment, and on each trial visual stimuli were presented to the RF individually. In a subset of experiments, stimuli were presented at one of two positions within the RF: either at the end point of the saccade that could be evoked with suprathreshold FEF stimulation (the aligned position) or at another position (the misaligned position). The misaligned position was chosen to maximize the separation between the aligned and misaligned positions while still evoking a reliable response from the V4 neuron. In all experiments, a second visual stimulus was also presented outside the RF at the mirror-image location in either the ipsilateral or upper hemifield on every trial because the effects of FEF stimulation have been shown to be greatest in the presence of “distracter” stimuli (12). Visual stimuli were most often two grayscale bars of orthogonal orientation (0.5–0.9 Michaelson contrast) but occasionally consisted of two bars of the same orientation but different colors. The experimenter attempted to select two test stimuli that varied in their ability to evoke V4 responses, but stimulus tuning was not characterized before carrying out an experiment. The simulated phosphene stimulus was a two-dimensional Gaussian brightness modulation superimposed on the RF stimulus (either the oriented bar stimulus or the background). Simulated phosphenes were presented for 66 ms at 500 ms after RF stimulus onset, the time when FEF stimulation would have occurred, although no microstimulation was ever applied during the simulated phosphene experiments. Visual stimuli were displayed on LCD (52-cm vertical × 87-cm horizontal, 60 Hz) and CRT (30-cm vertical × 40-cm horizontal, 60 Hz) monitors, positioned 57 cm in front of the monkey, with photopic background illumination.
Single-Neuron Recording in V4.
Single-neuron recordings in awake monkeys were made through surgically implanted cylindrical stainless steel or titanium chambers (20-mm diameter) overlying the prelunate gyrus. Electrodes were lowered into the cortex by using a hydraulic microdrive (Narishige, Tokyo, Japan). Activity was recorded extracellularly with varnish-coated tungsten microelectrodes (FHC, Bowdoinham, ME) of 0.2- to 1.0-MΩ impedance (measured at 1 kHz). Extracellular waveforms were classified as single neurons by using both template-matching and window-discrimination techniques (FHC, Bowdoinham, ME) and Plexon (Dallas, TX). V4 neuron RFs were mapped in a separate behavioral paradigm in which oriented bars were swept across the display while the monkey fixated a central spot. The RFs of V4 neurons studied were in the lower contralateral field with eccentricities between 5° and 16°.
Electrical Microstimulation of the FEF.
Electrical microstimulation consisted of a 20- to 50-ms train of biphasic current pulses (0.2–0.25 ms, 200 Hz) delivered with a Grass stimulator (S88) and two Grass stimulation isolation units (PSIU-6). Current amplitude was measured by the voltage drop across a 1-kΩ resistor in series with the return lead of the current source. All stimulation was delivered by varnish-coated tungsten microelectrodes of 0.2- to 1.0-MΩ impedance (measured at 1 kHz). In each monkey, the FEF was first localized on the basis of its surrounding physiological and anatomical landmarks and the ability to evoke fixed-vector, saccadic eye movements with stimulation at currents of <50 μA (9). During each experimental session, we mapped the saccade vector elicited at the cortical site under study with the use of a separate behavioral paradigm (10). The stimulating electrode was advanced until sites were localized from which saccades could be evoked into the RF of the V4 neuron under study, and the current threshold for evoking saccades was measured for this site. Experimental currents were set at 50% of the FEF site's saccadic threshold (10–13).
Analyses.
Onset responses were characterized by analyzing neural activity during a 90- to 190-ms window, relative to the presentation of visual stimuli. Stimulation and control trials were collapsed in all analyses of visual-onset activity. Analyses of the effects of FEF stimulation on V4 responses were conducted during a 70-ms time window beginning after the offset of microstimulation, adjusted for any stimulation artifact, and during the corresponding period of control trials. This window was chosen to avoid any indirect effects of disturbances in fixation after microstimulation on visual responses (13). For the subset of neurons tested with 20-ms trains of stimulation, a moving 20-ms analysis window was used to examine the effect of stimulation.
Stimulus tuning was determined by performing a paired t test on the responses observed to the two different visual stimuli. ROC areas were used as an index of stimulus tuning and were calculated as in a previous study (18) during both the onset- and late-analysis windows. Specifically, for each trial we computed the average firing rate during the relevant analysis window (early or late) for the two visual stimulus conditions. We then computed the probability that the firing rate in preferred and nonpreferred stimulus conditions exceeded a criterion, P (Hit) and P (False Alarm), respectively. The criterion was incremented from 0 to the maximum firing rate, and the probability of exceeding each criterion was computed. Thus, a single point on the ROC curve is produced for each increment in the criterion, and the entire ROC curve is generated from all of the criteria. The area under the ROC curve is a measure of the separation between the two distributions and provides a measure of how well the neuronal response discriminates the two visual stimuli. Differences in ROC areas, at the population level, were assessed by way of a permutation test on paired samples (46). The permutation test was carried out by randomly assigning a coefficient of +1 or −1 to each difference in ROC area (e.g., stimulation minus control) and computing the mean difference across the population. After generating 1,000 of these random differences, the mean observed difference was compared with the randomly generated distribution to determine whether it fell outside of the 95th percentile.
Population response-variance functions were computed and analyzed as in previous studies (16, 22). Specifically, for all neurons the variance in spike count (across trials) versus the average spike count within the analysis window was plotted, and the data were fit with a power function. Fits were computed for both stimulation and control conditions, and the resulting power and coefficient terms were compared with paired t tests. For the analysis of the effect of microstimulation on response magnitude in the population, each neuron's activity was normalized by its largest average response during all visual stimulus conditions, and a paired t test was performed. All analyses were performed on the combined population of neurons from the four monkeys.
Acknowledgments
We thank D. S. Aldrich for technical assistance and J. K. Fitzgerald for helpful comments on this paper. This work was supported by National Institutes of Health Grant EY14924, the Pew Charitable Trust, the Sloan Foundation (to T.M.), and a Howard Hughes Medical Institute Predoctoral Fellowship (to K.M.A.).
Abbreviations
- AROC
area under the receiver-operating characteristic curve
- FEF
frontal eye field
- RF
receptive field
- ROC
receiver-operating characteristic
- SC
superior colliculus.
Footnotes
The authors declare no conflict of interest.
References
- 1.Posner MI. Q J Exp Psychol. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- 2.Cameron EL, Tai JC, Carrasco M. Vision Res. 2002;42:949–967. doi: 10.1016/s0042-6989(02)00039-1. [DOI] [PubMed] [Google Scholar]
- 3.Carrasco M, Williams PE, Yeshurun Y. J Vis. 2002;2:467–479. doi: 10.1167/2.6.4. [DOI] [PubMed] [Google Scholar]
- 4.Lee DK, Koch C, Braun J. Vision Res. 1997;37:2409–2418. doi: 10.1016/s0042-6989(97)00055-2. [DOI] [PubMed] [Google Scholar]
- 5.Moran J, Desimone R. Science. 1985;229:782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- 6.Kastner S, De Weerd P, Desimone R, Ungerleider LG. Science. 1998;282:108–111. doi: 10.1126/science.282.5386.108. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds JH, Pasternak T, Desimone R. Neuron. 2000;26:703–714. doi: 10.1016/s0896-6273(00)81206-4. [DOI] [PubMed] [Google Scholar]
- 8.Bruce CJ, Goldberg ME. J Neurophysiol. 1985;53:603–635. doi: 10.1152/jn.1985.53.3.603. [DOI] [PubMed] [Google Scholar]
- 9.Bruce CJ, Goldberg ME, Bushnell MC, Stanton GB. J Neurophysiol. 1985;54:714–734. doi: 10.1152/jn.1985.54.3.714. [DOI] [PubMed] [Google Scholar]
- 10.Moore T, Fallah M. Proc Natl Acad Sci USA. 2001;98:1273–1276. doi: 10.1073/pnas.021549498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore T, Fallah M. J Neurophysiol. 2004;91:152–162. doi: 10.1152/jn.00741.2002. [DOI] [PubMed] [Google Scholar]
- 12.Moore T, Armstrong KM. Nature. 2003;421:370–373. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- 13.Armstrong KM, Fitzgerald JK, Moore T. Neuron. 2006;50:791–798. doi: 10.1016/j.neuron.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Spitzer H, Desimone R, Moran J. Science. 1988;240:338–340. doi: 10.1126/science.3353728. [DOI] [PubMed] [Google Scholar]
- 15.McAdams CJ, Maunsell JH. J Neurosci. 1999;19:431–441. doi: 10.1523/JNEUROSCI.19-01-00431.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McAdams CJ, Maunsell JH. Neuron. 1999;23:765–773. doi: 10.1016/s0896-6273(01)80034-9. [DOI] [PubMed] [Google Scholar]
- 17.Green D, Swets DJ. Signal Detection Theory and Psychophysics. New York: Wiley; 1966. [Google Scholar]
- 18.Britten KH, Shadlen MN, Newsome WT, Movshon JA. J Neurosci. 1992;12:4745–4765. doi: 10.1523/JNEUROSCI.12-12-04745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mountcastle VB, Motter BC, Steinmetz MA, Sestokas AK. J Neurosci. 1987;7:2239–2255. doi: 10.1523/JNEUROSCI.07-07-02239.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Motter BC. J Neurophysiol. 1993;70:909–919. doi: 10.1152/jn.1993.70.3.909. [DOI] [PubMed] [Google Scholar]
- 21.Moore T. Science. 1999;285:1914–1917. doi: 10.1126/science.285.5435.1914. [DOI] [PubMed] [Google Scholar]
- 22.Bichot NP, Thompson KG, Chenchal Rao S, Schall JD. J Neurosci. 2001;21:713–725. doi: 10.1523/JNEUROSCI.21-02-00713.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muller JR, Philiastides MG, Newsome WT. Proc Natl Acad Sci USA. 2005;102:524–529. doi: 10.1073/pnas.0408311101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nowak L, Bullier J. The Timing of Information Transfer in the Visual System. New York: Plenum; 1997. pp. 205–241. [Google Scholar]
- 25.Breitmeyer B. Visual Masking: An Integrative Approach. New York: Oxford Univ Press; 1990. [Google Scholar]
- 26.Robinson DL, Kertzman C. J Neurophysiol. 1995;74:713–721. doi: 10.1152/jn.1995.74.2.713. [DOI] [PubMed] [Google Scholar]
- 27.Cavanaugh J, Wurtz RH. J Neurosci. 2004;24:11236–11243. doi: 10.1523/JNEUROSCI.3724-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cavanaugh J, Alvarez BD, Wurtz RH. J Neurosci. 2006;26:11347–11358. doi: 10.1523/JNEUROSCI.2376-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Penfield W, Rasmussen T. The Cerebral Cortex of Man: A Clinical Study of Localization of Function. New York: Macmillan; 1950. [Google Scholar]
- 30.Blanke O, Landis T, Seeck M. Epilepsy Behav. 2000;1:356–361. doi: 10.1006/ebeh.2000.0109. [DOI] [PubMed] [Google Scholar]
- 31.Ignashchenkova A, Dicke PW, Haarmeier T, Thier P. Nat Neurosci. 2004;7:56–64. doi: 10.1038/nn1169. [DOI] [PubMed] [Google Scholar]
- 32.Bisley JW, Goldberg ME. Science. 2003;299:81–86. doi: 10.1126/science.1077395. [DOI] [PubMed] [Google Scholar]
- 33.Thompson KG, Biscoe KL, Sato TR. J Neurosci. 2005;25:9479–9487. doi: 10.1523/JNEUROSCI.0741-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dias EC, Kiesau M, Segraves MA. J Neurophysiol. 1995;74:2744–2748. doi: 10.1152/jn.1995.74.6.2744. [DOI] [PubMed] [Google Scholar]
- 35.Sommer MA, Tehovnik EJ. Exp Brain Res. 1997;116:229–249. doi: 10.1007/pl00005752. [DOI] [PubMed] [Google Scholar]
- 36.Wardak C, Ibos G, Duhamel JR, Olivier E. J Neurosci. 2006;26:4228–4235. doi: 10.1523/JNEUROSCI.3336-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McPeek RM, Keller EL. Nat Neurosci. 2004;7:757–763. doi: 10.1038/nn1269. [DOI] [PubMed] [Google Scholar]
- 38.Grosbras MH, Paus T. J Cogn Neurosci. 2002;14:1109–1120. doi: 10.1162/089892902320474553. [DOI] [PubMed] [Google Scholar]
- 39.Grosbras MH, Paus T. Eur J Neurosci. 2003;18:3121–3126. doi: 10.1111/j.1460-9568.2003.03055.x. [DOI] [PubMed] [Google Scholar]
- 40.Smith DT, Jackson SR, Rorden C. Neuropsychologia. 2005;43:1288–1296. doi: 10.1016/j.neuropsychologia.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 41.Ruff CC, Blankenburg F, Bjoertomt O, Bestmann S, Freeman E, Haynes JD, Rees G, Josephs O, Deichmann R, Driver J. Curr Biol. 2006;16:1479–1488. doi: 10.1016/j.cub.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 42.Silvanto J, Lavie N, Walsh V. J Neurophysiol. 2006;96:941–945. doi: 10.1152/jn.00015.2006. [DOI] [PubMed] [Google Scholar]
- 43.Taylor PC, Nobre AC, Rushworth MF. Cereb Cortex (2007) 2007;17:391–399. doi: 10.1093/cercor/bhj156. [DOI] [PubMed] [Google Scholar]
- 44.Winkowski DE, Knudsen EI. Nature. 2006;439:336–339. doi: 10.1038/nature04411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Graziano MS, Hu XT, Gross CG. J Neurophysiol. 1997;77:2268–2292. doi: 10.1152/jn.1997.77.5.2268. [DOI] [PubMed] [Google Scholar]
- 46.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. Ed 2. Washington, DC: Chapman and Hall/CRC; 1998. pp. 202–219. [Google Scholar]