Abstract
Neurodegeneration, a result of multiple dysregulatory events, is a lengthy multistep process manifested by accrual of mutant variants and abnormal expression, posttranslational modification, and processing of certain proteins. Accumulation of these dysregulated processes requires a mechanism that maintains their functional stability and allows the evolution of the neurodegenerative phenotype. In malignant cells, the capacity to buffer transformation has been attributed to heat-shock protein 90 (Hsp90). Although normal proteins seem to require limited assistance from the chaperone, their aberrant counterparts seem to be highly dependent on Hsp90. Whereas enhanced Hsp90 affinity for mutated or functionally deregulated client proteins has been observed for several oncoproteins, it is unknown whether Hsp90 plays a similar role for neuronal proteins and thus maintains and facilitates the transformed phenotype in neurodegenerative diseases. Tauopathies are neurodegenerative diseases characterized by aberrant phosphorylation and/or expression of Tau protein, leading to a time-dependent accumulation of Tau aggregates and subsequent neuronal death. Here, we show that the stability of p35, a neuronal protein that activates cyclin-dependent protein kinase 5 through complex formation leading to aberrant Tau phosphorylation, and that of mutant but not WT Tau protein is maintained in tauopathies by Hsp90. Inhibition of Hsp90 in cellular and mouse models of tauopathies leads to a reduction of the pathogenic activity of these proteins and results in elimination of aggregated Tau. The results identify important roles played by Hsp90 in maintaining and facilitating the degenerative phenotype in these diseases and provide a common principle governing cancer and neurodegenerative diseases.
Keywords: cyclin-dependent protein kinase 5, neurodegeneration, Tau
In cancer cells, heat-shock protein 90 (Hsp90) interacts in a transformation-specific manner with kinases, hormone receptors, and transcription factors that are directly involved in driving multistep malignancy and also with mutated oncogenic proteins required for the transformed phenotype (1–3). Association of Hsp90 with client proteins is regulated both by the activity of its N-terminal ATPase domain, which binds and hydrolyses ATP to mediate a series of association–dissociation cycles between Hsp90 and client substrates, and by cochaperones, which modulate the formation of chaperone/client-specific complexes (4). Association of Hsp90 with these aberrant oncoproteins maintains their ability to function in the deregulated state and appears to be essential for their transforming activity. To evaluate whether Hsp90 plays a buffering role in neurodegenerative diseases similar to that in cancer, we studied the particular case of tauopathy. In this disease, transformation is characterized by abnormalities in the Tau protein, leading to an accumulation of hyperphosphorylated and aggregated Tau (5–7). It has been suggested that Alzheimer disease (AD) and frontotemporal dementia are linked in a genetic spectrum of presenile degenerative brain disorders in which Tau is an important player (8). In AD, Tau hyperphosphorylation is suggested to be a pathogenic process caused by aberrant activation of several kinases, in particular cyclin-dependent protein kinase 5 (cdk5) and glycogen synthase kinase-3β, leading to phosphorylation of Tau on pathogenic sites. Hyperphosphorylated Tau in AD is believed to misfold, undergo net dissociation from microtubules, and form toxic Tau aggregates (9, 10). In a cluster of tauopathies termed “frontotemporal dementia and parkinsonism linked to chromosome 17,” transformation is caused by several mutations in human Tau isoforms on chromosome 17, that result in and are characterized by the accumulation of aggregated Tau similar to that in AD (10, 11). More than 20 distinct pathogenic mutations have been identified, with P301L as the most common mutation in tauopathies. It is currently accepted that these two mechanisms, abnormal phosphorylation of Tau by kinases and mutations in Tau, both causing a failure of the cell to remove aggregated Tau, are key pathogenic factors in these diseases and are implicated as an integral part of disease onset and progression. Of particular importance are cdk5 and mutant Tau (mTau), as demonstrated by the fact that either modulation of cdk5 complex activity or mutant TauP301L expression leads to alleviation of the transformed phenotype. In concordance with these observations, cdk5 inhibitors were found to reduce Tau phosphorylation and apoptosis in neurons (9, 12, 13). A role of the cdk5/p35 complex in the genetic etiology of early onset AD was recently suggested (14). Inhibition of cdk5 in an animal model of Niemann-Pick type C disease significantly attenuated the hyperphosphorylation of neurofilament Tau and ameliorated motor defects in these mice (15). Similarly, the expression of TauP301L was sufficient to produce neuronal loss and generalized forebrain atrophy. Suppression of 90% of mutant TauP301L expression led to remarkable functional improvement and inhibition of neuronal cell death in TauP301L transgenic mice (16). These results support the idea that both cdk5/p35 and TauP301L are involved in tauopathy initiation and progression and portray the kinase complex and the mutant Tau protein as plausible candidates that require Hsp90 assistance for aberrant function and stability. A model of possible roles of Hsp90 in supporting the accumulation of toxic aggregates in neurodegenerative diseases is shown in supporting information (SI) Fig. 6.
Results and Discussions
Inhibition of Hsp90 Reduces Cellular Levels of Both p35 and Mutant Tau.
To examine the roles played by Hsp90 in tauopathy, we made use of both PU24FCl, a synthetic Hsp90 inhibitor developed by our laboratory (17, 18), and 17-(allyllamino)-17-demethoxygeldanamycin (17AAG), a natural product derivative (17), and investigated their effects on both cdk5/p35 and TauP301L. These agents bind selectively to the regulatory pocket of Hsp90, inhibiting chaperone function and altering the association of client proteins with the chaperone complex (17, 18). As a consequence, these proteins do not achieve their mature functional conformation and are degraded by the proteasome (19). Embryonic primary rat cortical neurons and COS-7 cells transfected with cDNAs corresponding to either p35 alone (COS-7/p35) or both p35 and Tau (COS-7/p35/Tau) are relevant experimental systems to study aberrant neuronal kinase activity because phosphorylation of Tau at putative cdk5 sites is both enhanced in these cells and in embryonic and juvenile brains (20, 21) and is similar to that in AD-afflicted brains (21). COS-7 cells transfected with cDNAs corresponding to either human WT Tau (COS-7/Tau) or Tau harboring the P301L mutation characteristic of frontotemporal dementia and parkinsonism linked to chromosome 17 (COS-7/TauP301L) are cellular models that may be used to differentiate the effect of Hsp90 inhibition on a mutant protein compared with its normal counterpart.
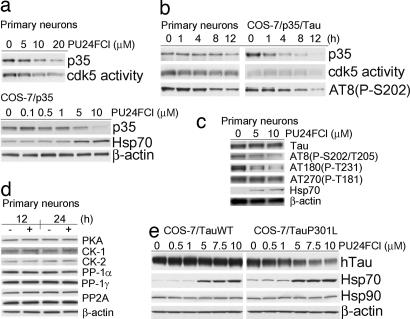
Phosphorylation of Tau by cdk5 is initiated through activation by complex formation with one of the neuron-specific proteins p35 or p39 (22, 23). However, only suppression of p35 by antisense oligonucleotide treatment but not of the highly related isoform p39 selectively reduces cdk5 activity (24). In addition, levels of p35 but not of cdk5 protein are rate-limiting for cdk5 activity (25). In concordance, we assessed the influence of Hsp90 inhibition on p35 cellular expression. A dose- and time-dependent degradation of p35 by PU24FCl was detected in primary neurons by immunoblot (Fig. 1a Upper and b Left) and by immunofluorescence (SI Fig. 7) techniques, as well as in COS-7/p35 (Fig. 1a Lower) and COS-7/p35/Tau cells (Fig. 1b Right and SI Fig. 8). Effects were seen at ≈1–5 μM PU24FCl and were maximal at 10 μM Hsp90 inhibitor, in agreement with the affinity of this compound for Hsp90 (18) (SI Fig. 9a). Exogenously introduced p35 was more sensitive to Hsp90 inhibition than the endogenous protein (Fig. 1 and SI Fig. 8), suggesting that by analogy to Hsp90 oncoproteins, buffering and stabilization of aberrant proteins in tauopathy may be accomplished by co-opting Hsp90. Reduction of p35 levels by Hsp90 inhibition affected the activity of the cdk5/p35 complex, as measured by using a substrate of cdk5, the histone-H1 (Fig. 1 a and b), and lessened Tau phosphorylation at putative cdk5 shown to be phosphorylated in AD brains (26–28) without affecting normal Tau protein expression (Fig. 1 b and c). mTau however, was sensitive to concentrations of PU24FCl that did not interfere with WT Tau expression (Fig. 1e and SI Fig. 8a). The higher sensitivity to Hsp90 inhibition of mTau compared with WT Tau is in agreement with the observed lability of the mutant oncoprotein clients of Hsp90 (1–3). Analogous effects on p35 and mTau were observed with 17AAG (SI Fig. 8b). The effect of PU24FCl on neuronal proteins was well-defined and selective, as the expression of several kinases and phosphatases that regulate normal Tau activity was not affected by the Hsp90 inhibitor (Fig. 1d) (29, 30). Induction of Hsp70 by Hsp90 inhibitors is documented in several neurodegenerative disease models (31–33). Expression of Hsp70 is indirectly regulated by Hsp90 (34). Accordingly, treatment of either primary neurons (Fig. 1c and SI Fig. 7) or transfected COS-7 cells (Fig. 1 a and e and SI Figs. 7 and 8) with PU24FCl led to a dose-dependent increase in Hsp70. Induction of Hsp70 occurred at doses of PU24FCl that also modulated both p35 and mTau, suggesting that degradation of aberrant proteins and induction of a heat-shock response are both direct consequences of Hsp90 inhibition by PU24FCl.
Fig. 1.
Inhibition of Hsp90 specifically decreases both p35 and mTau expression and reduces cdk5 activity in a time- and dose-dependent manner. (a) Western blot analysis of primary embryonic cortical neurons (Upper) and COS-7/p35 cells (Lower) treated with the indicated doses of PU24FCl for 24 h. (b) Western blot analysis of primary embryonic cortical neurons (Left) and COS-7/p35/Tau (Right) treated with PU24FCl (10 μM) for the indicated times. (c and d) Western blot analysis of primary embryonic cortical neurons treated with the indicated doses of PU24FCl for 24 h (c) or with PU24FCl (10 μM) (+) and vehicle (−) for the indicated times (d). (e) Western blot analysis of COS-7/Tau (Left) or COS-7/TauP301L (Right) cells treated with the indicated doses of PU24FCl for 24 h. β-Actin and Hsp90 were used as a protein quantification control. Each experiment was conducted in triplicate, and gels presented here are representative runs. DMSO was used as a vehicle.
Degradation of p35 and mTau by Hsp90 Inhibition Is Mediated by the Proteasome.
Proteins regulated by Hsp90 appear to be degraded via the proteasome pathway upon chaperone inhibition (19). Concordantly, only a proteasome inhibitor (MG132) was able to prevent the degradation of p35 and mTau over a 24-h treatment with the Hsp90 inhibitor (SI Fig. 10). These observations are in agreement with observed effects on Hsp90 oncoprotein clients, which suggest that these proteins are protected through Hsp90 chaperone complex formation from ubiquitination and proteasomal degradation (1–3).
Hsp90 Regulates the Stability of p35 and mTau.
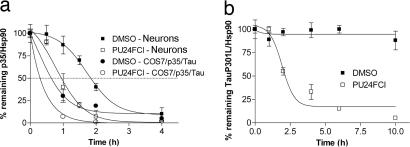
To examine whether Hsp90 plays a direct role in maintaining the stability of these proteins, we tested whether inhibition of Hsp90 function by PU24FCl affected their half-life (Fig. 2 and SI Fig. 11). Primary neuronal cultures were treated with inhibitor or vehicle in the presence of cycloheximide (SI Fig. 11). Quantification of protein levels demonstrated that the half-life of endogenous p35 was ≈120 min in the presence of vehicle and decreased to ≈60 min when PU24FCl was added to the system (Fig. 2a). The exogenous p35 was more labile and had a significantly shorter half-life than the endogenous protein (t1/2 = 60 min in the presence of vehicle and <30 min in the presence of PU24FCl) for both COS-7/p35/Tau cells (Fig. 2a and SI Fig. 11) and primary neurons (not shown). Similar results were observed for mTau: whereas 50% of the protein was degraded at 2–4 h in the presence of the Hsp90 inhibitor, the half-life of mTau in vehicle treated cells exceeded 10 h (Fig. 2b and SI Fig. 11). The inhibitor had no effect on the level of WT Tau (SI Fig. 11). Moreover, mTau and p35 were degraded upon PU24FCl treatment even when induction of Hsp70 was blocked by cycloheximide (SI Fig. 11). These findings strongly position Hsp90 as a direct and important regulator of both p35 and mutant Tau stability.
Fig. 2.
Hsp90 regulates the stability of p35 and mTau. (a) The half-life of p35 was analyzed in primary embryonic cortical neurons or COS-7/p35/Tau cells treated with cycloheximide (100 μg/ml) in the presence of vehicle or PU24FCl (10 μM) for the indicated times. (b) The half-life of mutant Tau was analyzed in COS-7/TauP301L cells treated with 100 μg/ml cycloheximide in the presence of vehicle or 10 μM PU24FCl for the indicated times. Levels of p35 and TauP301L in the presence and absence of the Hsp90 inhibitor were normalized to Hsp90 expression and results plotted against inhibitor treatment time. DMSO was used as a vehicle.
Hsp90 Forms a Complex with and Regulates p35 and mTau.
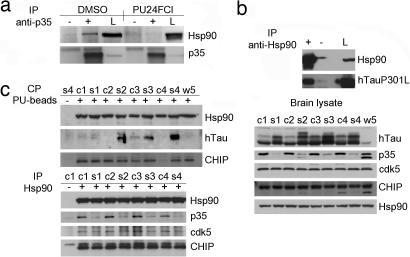
To examine whether Hsp90 regulates the stability of these proteins through protein complex formation, we made use of several chemical and immunological tools that selectively bind either Hsp90 or its putative client proteins. Association of Hsp90 with p35 (Fig. 3a) as well as with mTau, was observed (Fig. 3b). No significant association was observed when cells were immunopurified with a control IgG. Cdc37, a cochaperone of Hsp90 found associated with several chaperone-kinase assemblies (35), was absent in the p35-immunopurified complexes (not shown), in concordance with previous observations of Lamphere et al. (36). Pretreatment of cells with PU24FCl altered the interaction of Hsp90 with p35 (Fig. 3a).
Fig. 3.
mTau and p35 exist in a complex with Hsp90. (a) Immunoprecipitation (IP) of p35-containing protein complexes from COS-7 cells transfected with cDNAs corresponding to myc-His-tagged p35 with a control IgG (−) or a specific anti-p35 antibody (+) in the presence of vehicle or PU24FCl (10 μM). Lysate (L) was added as a control. (b) Immunoprecipitation of Hsp90-containing protein complexes from COS-7 cells transfected with cDNAs corresponding to TauP301L with a control IgG (−) or a specific anti-Hsp90 antibody (+). Lysate was added as a control. (c) Hsp90-containing protein complexes isolated through chemical precipitation (CP) with a solid support immobilized-PU (+) or control beads (−) (Upper Left) and through immunoprecipitation with a specific anti-Hsp90 antibody (+) or a control IgG (−) (Lower Left) from JNPL3 mouse brains. c1–c4 and s1–s4, cortical and subcortical brain homogenates, respectively, extracted from four 10-month-old female mice. w5 is whole-brain homogenate from a nontransgenic control mouse. (Right) Western blot analysis of brain lysate protein content. Hsp90 was used as protein quantification control. DMSO was used as a vehicle. CHIP, C terminus of heat-shock cognate 70-interacting protein.
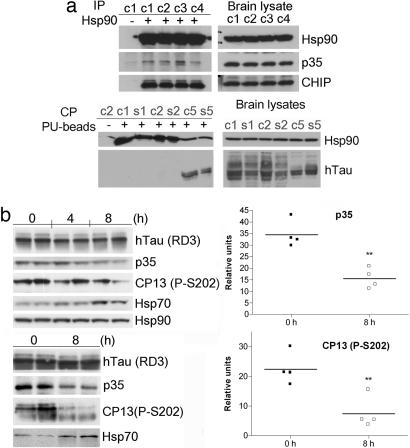
The cellular models presented above demonstrate that an interaction between Hsp90 and aberrant neuronal proteins is possible at a molecular level. However, exogenous introduction of proteins by transfection, may destabilize the cell's protein content and impose a regulation of the alien protein's stability by Hsp90. Therefore, to evaluate the interaction of Hsp90 with TauP301L and p35 in an endogenous environment, we made use of brain homogenates obtained from animal models of tauopathy. The JNPL3 line of mice expressing mutant (P301L) human Tau (hTau) protein (37) exhibit an age, gender and gene dose-dependent increase in Tau phosphorylation and insoluble Tau deposits (37, 38). To isolate proteins associated with Hsp90 in these brains, we made use of brain homogenates obtained from female JNPL3 mice (n = 4) 10 months of age and used either a biotinylated PU derivative immobilized on streptavidin beads or a specific anti-Hsp90 antibody. Hsp90 isolated by PU beads bound mTau specifically (Fig. 3c Upper Left; see also Fig. 5a Lower). The presence of the C terminus of heat-shock cognate 70-interacting protein, an ubiquitin E3 ligase found to collaborate with molecular chaperones in facilitating protein folding, was also identified in the Hsp90 complex, in agreement with findings of Sahara et al. (39). An Hsp90 antibody specifically identified the chaperone in complex with p35 and its kinase partner cdk5 (Fig. 3c Lower). Collectively, these data position Hsp90 as a regulator of p35 and mTau stability through direct protein complex formation.
Fig. 5.
Hsp90 regulates p35 but not WT Tau in vivo in a WT Tau environment. (a) (Upper Left) Immunoprecipitation of Hsp90-containing protein complexes from hTau mouse brains with a specific anti-Hsp90 antibody (+) or a control IgG (−). (Lower Left) Immunoprecipitation of Hsp90-containing protein complexes isolated through chemical precipitation with a solid support immobilized-PU (+) or control beads (−) from hTau (c1, s1, c2, and s2) and JNPL3 (c5 and s5) mouse brains. c1–c5 and s1–s5, cortical and subcortical brain homogenates, respectively, extracted from 10-month-old female mice. (Right) Western blot analyses of brain lysate protein content. (b) (Left) Western blot analysis of soluble (S1) fractions extracted from the cortical brain region of 4-month-old (Upper) and 8- to 10-month-old (Lower) hTau female mice treated for the indicated times with 75 mg/kg PU-DZ8. Representative data are displayed. Hsp90 was used as protein quantification control. (Right) Relative p35 protein expression and Tau phosphorylation at S202 (CP13) in the untreated versus the 8-h-treated mouse group is presented. Specific human Tau isoforms in hTau mice were assessed by immunoblotting with an antibody specific for three-repeat domain Tau (RD3).
In Vivo Degradation of Aberrant Neuronal Hsp90 Clients Leads to a Reduction in Aggregated and Hyperphosphorylated Tau.
To investigate whether release of mTau and p35 from Hsp90 regulation restores normal neuronal activity and results in elimination of toxic Tau aggregates, we made use of the JNPL3 mouse model of tauopathy. Brain tissues of JNPL3 mice contain Tau proteins with different solubilities, and these can be separated into buffer-extractable (S1), high salt-extractable (S2), and sarkosyl-insoluble (P3) fractions. The S1 fractions contain a 50- to 60-kDa hTau protein, whereas sarkosyl-insoluble Tau proteins of 64 kDa and higher molecular masses are detected in the subcortical brain regions of JNPL3 mice as early as 3 months in hemizygous females. These contain insoluble toxic Tau phosphorylated at multiple sites such as T181, S202/T205, T212, and T231 (37, 38).
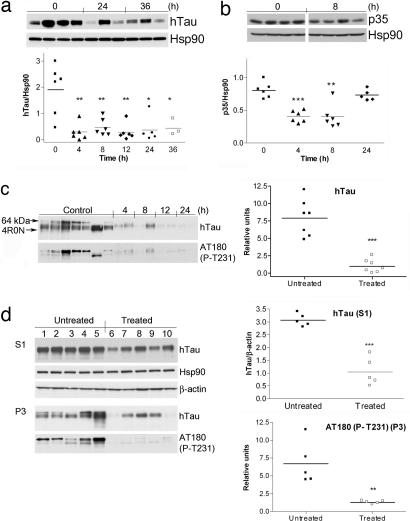
To investigate whether the human TauP301L present in the JNPL3 line of mice is a sensitive target for Hsp90 inhibition, animals were treated with the brain barrier-permeable Hsp90 inhibitor PU-DZ8 (40). This agent is a higher potency water-soluble derivative of PU24FCl (SI Fig. 9a) (EC50JNPL3 brain Hsp90 = 70 nM). One dose of PU-DZ8 (75 mg/kg) was administered i.p. to female mice of 2.5–4 months in age (n = 32), and animals were killed in the interval of 0–36 h (Fig. 4a). Both aggregate-free Tau (S1) and insoluble Tau (P3) fractions were prepared from the subcortical and cortical regions of these mice. Mirroring cell-based data (Fig. 1e), at 4 h postadministration, the Hsp90 inhibitor induced a significant decrease in the soluble precursor pool of mTau as present in the subcortical brain regions (P = 0.0031 at 4 h), with these effects maintained up to 36 h (P = 0.0066 at 8 h, 0.0030 at 12 h, 0.0111 at 24 h, and 0.042 at 36 h) (Fig. 4a). Analogous durable effects on protein levels were previously recorded for tumor Hsp90 targets (17, 40), further positioning mTau as a protein highly dependent on Hsp90 for proper folding and functioning. PU-DZ8 levels in the brain reached 0.35 μg/g (≈700 nM) at 4 h, and the pharmacologically relevant dose was retained for at least 12 h postadministration (0.2 μg/g, ≈390 nM) (SI Fig. 9b). Modulation of p35 levels by the Hsp90 inhibitor was also noted: a significantly relevant reduction of 40–50% in subcortical brain region p35 expression was observed at 4 and 8 h postadministration (P < 0.0001 at 4 h and 0.0012 at 8 h), and the protein recovered to normal levels by 24–36 h (Fig. 4b). No significant changes in cdk5 expression were detected (not shown), indicating that management of cdk5 by Hsp90 in the brain may be limited to regulating the activity of the p35/cdk5 complex. The expressions of Akt and Raf-1, nodal proteins in cell survival and growth pathways, respectively, tightly regulated by Hsp90 in malignant cells (1–3), were not altered by PU-DZ8 (not shown).
Fig. 4.
In vivo degradation of aberrant neuronal Hsp90 clients leads to a reduction in aggregated and hyperphosphorylated Tau. Western blot analysis of brain soluble fractions (S1) extracted from JNPL3 female mice treated for the indicated times with 75 mg/kg PU-DZ8. (a) Subcortical brain region of 2.5- to 4-month-old mice is presented. Human Tau levels were normalized to those of Hsp90. (b) Subcortical brain region of 2.5- to 4-month-old mice is presented. P35 levels were normalized to those of Hsp90. Representative data are displayed. (c) (Left) Western blot analysis of the insoluble Tau (P3) fractions extracted from the subcortical brain region of 6-month-old mice treated with 75 mg/kg PU-DZ8 for the indicated times. The location of 64-kDa Tau (arrowhead) and recombinant Tau containing four-repeat Tau isoforms without the N-terminal inserts (4R0N) is indicated. (Right) Relative hTau protein expression and Tau phosphorylation at T231 in the untreated versus the treated mouse group is presented. (d) Western blot analysis of soluble (S1) and insoluble (P3) subcortical brain fractions extracted from JNPL3 female mice treated for 30 days with Hsp90 inhibitors. β-Actin and Hsp90 were used as protein quantification control.
We next examined in a 4- to 6-month-old mouse group (n = 15) whether the stability of mTau as present in Tau aggregates (P3 fraction) was additionally regulated by Hsp90. As demonstrated in Fig. 4c, a significant reduction of insoluble (P < 0.0001) and hyperphosphorylated (P = 0.001) Tau was observed in treated mice (n = 8), compared with those mice receiving no Hsp90 inhibitor (n = 7). To investigate whether modulation of mTau could be sustained over a longer Hsp90 inhibitor treatment period without being toxic to mice, JNPL3 mice were subjected for 30 days to these agents. Female JNPL3 mice 6.5 months of age (n = 10) were administered i.p. vehicle (n = 5) or one of the Hsp90 inhibitors, PU24FCl (200 mg/kg) or PU-DZ8 (75 mg/kg) (n = 5), on a daily, five times per week schedule, and animals were killed at 8 h after the last administered dose of inhibitor. No toxicity was observed, as evidenced by lack of significant change in animal weight, fur appearance, appetite, and posture. Furthermore, no visible internal organ damage was detected upon gross inspection at death. A significant reduction in Tau expression and phosphorylation in both the precursor protein pool (S1 fraction) (hTau, P < 0.0001) and the toxic aggregate (P3 fraction) (phosphorylated Tau at T231, P = 0.0034) (Fig. 4d), as well as p35 reduction in the S1 fraction (SI Fig. 12), was observed in mice treated with the Hsp90 inhibitor. Collectively, the rapid kinetics of Tau degradation in both the soluble pool and the aggregated form by the Hsp90 inhibitors suggests that Hsp90 regulates the toxic Tau aggregate and facilitates its formation and accumulation. These data also suggest that an Hsp90 inhibitor may both prevent the formation of toxic aggregates and solubilize the already aggregated Tau.
To investigate the in vivo effect of Hsp90 inhibition on p35 in a WT Tau environment, we made use of hTau mice (41). hTau mice develop Tau pathology with a distribution that is comparable to that occurring in the early stages of AD. These mice express six isoforms of nonmutant hTau but develop AD-like Tau pathology. Heat-stable fractions (S1) prepared from cortical homogenates of these mice indicate an age-related accumulation of Tau phosphorylated at putative cdk5 sites. PU beads failed to isolate Hsp90 in complex with WT Tau (Fig. 5a Lower), suggesting that in analogy to Hsp90 client oncoproteins, the chaperone forms tight complexes with aberrant neuronal proteins but not their normal counterparts. The existence of an Hsp90/p35 complex was detected in these mice (Fig. 5a Upper). Therefore, we examined whether inhibition of Hsp90 in these brains may lead to a reduction in p35 expression and a consequent alleviation of Tau phosphorylation. hTau female mice (n = 10) 4 and 8–10 months of age were administered either vehicle or one dose of PU-DZ8 (75 mg/kg) i.p., and animals were killed at 4 or 8 h postadministration (Fig. 5b). By analogy to experiments on primary neuronal cultures and WT Tau transfected cells (Fig. 1), the Hsp90 inhibitor had no effect on soluble WT Tau expression. However, both a significant time-dependent reduction in p35 levels (P = 0.0019) and alleviation of Tau phosphorylation on Ser-202, as detected by antibody CP13 (P = 0.0078), were evident at 8 h postadministration of the Hsp90 inhibitor (Fig. 5b). The monoclonal antibody CP13 is commonly used to detect Tau pathology in both early and more advanced stages of Tau aggregate accumulation (41). Collectively, these data position p35/cdk5 as a kinase complex prone to aberrantly phosphorylate WT and mutant Tau and suggest Hsp90 as a regulator of its activity in both Tau environments. They also confirm cellular data suggesting mutant but not WT Tau as being tightly regulated by Hsp90.
In conclusion, our findings suggest that a neuron undergoing a degenerative process co-opts Hsp90, in a fashion similar to an epithelial cell undergoing malignant transformation to maintain the functional stability of proteins of aberrant capacity, whether mutated or overactivated, providing a common principle governing the two diseases. Whereas in cancer, the buffering effect of Hsp90 permits the existence of a malignant phenotype, in tauopathies it allows and sustains the accumulation of toxic Tau aggregates. Consequently, it is tempting to speculate that by analogy to cancer, Hsp90 inhibition by small molecules may lead to correction of aberrant signaling and elimination of defective proteins and also to solubilization of toxic aggregates, suggesting Hsp90 inhibition as a therapeutic approach for increasing the survival of affected neurons. Taken together with previous findings by Dou et al. (33) that Hsp90 inhibition induces Hsp70, a chaperone able to partition Tau into a productive folding pathway, our data portray Hsp90 inhibition as a double therapeutic modality in tauopathies in particular or neurodegenerative diseases in general.
Materials and Methods
Reagents.
The Hsp90 inhibitors PU24FCl and PU-DZ8 were prepared as described previously (18, 40). 17AAG was a gift of Neal Rosen (Memorial Sloan–Kettering Cancer Center). cDNA constructs expressing human WT Tau (T40) and mTau (T40 P301L) were a gift from Virginia Lee (University of Pennsylvania, Philadelphia, PA), and the pcDNA3.1-myc-His-p35 construct was a gift from L. H. Tsai (Harvard Medical School, Boston, MA).
Antibodies.
The following antibodies were used for immunoblotting at appropriate concentrations as recommended by the manufacturer: anti-p35 and anti-cdk5 rabbit polyclonal antibodies (C-19 and C-8; Santa Cruz Biotechnology, Santa Cruz, CA), anti-human Tau mouse monoclonal antibody HT-7 (MN1000; Pierce Biotechnology, Rockford, IL), anti-RD3 (Upstate USA, Charlottesville, VA), anti-PKA (Cell Signaling Technology, Beverly, MA), anti-PP2A-A (SC-6112; Santa Cruz Biotechnology), CK-1 (2655; Cell Signaling Technology), CK-2 (2656; Cell Signaling Technology), anti-Hsp90 (SC-7947; Santa Cruz Biotechnology), anti-Hsp70 mouse monoclonal antibody (SPA-810; Stressgen Bioreagents, Victoria, BC, Canada), anti-C terminus of heat-shock cognate 70-interacting protein rabbit polyclonal antibody (PC711; Calbiochem, Darmstadt, Germany), and anti-β-actin mouse monoclonal antibody (A1978; Sigma, St. Louis, MO). Antibodies against PP-1α and PP-1γ were generated as reported previously (42). Antibodies recognizing phosphor-specific epitopes on Tau, AT8 for S202/T205, AT180 for T231, and AT270 for T181, were purchased from Pierce Biotechnology. CP13 was a gift from Peter Davies (Albert Einstein College of Medicine, New York, NY).
Primary Neuronal and COS-7 Cell Cultures and Incubation with Hsp90 Inhibitor.
Primary neuronal cultures were derived from the cerebral cortices of embryonic day 17 rat embryos and maintained as described previously (43). To determine the effects of PU24FCl on protein steady-states and on Tau phosphorylation, PU24FCl was added at day 6 of culture, and cells were incubated at 37°C as indicated. COS-7 cells grown in DMEM with 10% FBS and penicillin/streptomycin (50 units and 50 μg/ml, respectively) were transiently transfected by using FuGENE 6 reagent (Roche Molecular Biochemicals, Indianapolis, IN) to overexpress p35 and either WT Tau or Tau harboring a P301L mutation. At 12 h after transfection, cells were incubated for 24 h with the indicated concentration of PU24FCl. After incubation, cells were harvested and lysed in 2% SDS, and the resulting samples were analyzed by Western blotting.
Cdk5 Activity Assay.
Cells were lysed by using a Dounce homogenizer in 1 ml of lysis buffer (150 mM NaCl/20 mM Tris·HCl, pH 7.4, 1 mM EDTA, 0.5% Nonidet P-40, 5 mM NaF, 5 mM Na3VO4, and protease inhibitors). Lysates were centrifuged at 10,000 × g for 10 min at 4°C. The p35/cdk5 complex was immunoprecipitated from the supernatant by using a specific cdk5 antibody. Cdk5 activity assays were performed as described previously (44). Proteins were separated by SDS/PAGE, and data were quantified by using a PhosphorImager (Molecular Dynamics, Sunnyvale, CA).
Cycloheximide Treatments.
Primary cortical cultures or COS-7 transfected cells were treated with cycloheximide (at a final concentration of 100 μg/ml) for the indicated times. Cells were lysed in 2% SDS with an added protease inhibitor mixture, and resulting samples were analyzed by Western blotting. Blots were digitized by UN-SCAN-IT v5.1 software (Silk Scientific, Orem, UT).
Immunoprecipitation.
For isolation of the p35/Hsp90 complexes from cultured cells, COS-7 cells transfected with p35 or empty vector were lysed in TMNSV buffer (50 mM Tris·HCl, pH 7.0, 20 mM Na2MoO4, 0.09% Nonidet P-40, 150 mM NaCl, and 1 mM sodium othovanadate with added protease inhibitor mixture). Samples were immunoprecipitated with anti-p35 antiserum and analyzed by Western blotting. In some experiments, cell cultures were pretreated with DMSO or 10 μM PU24FCl for 6 h. For isolation of Hsp90 complexes from mouse brain tissue, brains were homogenized in a lysis buffer containing 20 mM Tris·HCl (pH 7.4), 25 mM NaCl, 2 mM DTT, 20 mM Na2MoO4, 0.1% Nonidet P-40, 10 μg/ml aprotinin, and 10 μg/ml leupeptin, sonicated and further incubated for 2 h at 4°C. For immunoprecipitation, 500 μg of supernatant protein from each group was incubated overnight with an anti-Hsp90 mouse monoclonal Ab (hHsp90 H9010; a generous gift from David O. Toft and Sara Felts, Mayo Clinic College of Medicine, Rochester, MI) or a control normal mouse IgG (Santa Cruz Biotechnology). Protein G-Sepharose isolated immune complexes were washed three times with assay buffer, boiled in SDS/PAGE sample buffer for 5 min, and further analyzed by Western blot. For chemical precipitation, a similar protocol was conducted with beads containing a streptavidin-immobilized PU-biotin construct (120 μl of 50% bead suspension) or control streptavidin-immobilized biotin, and the protein complexes were processed as described above.
In Vivo Studies.
Transgenic mice, JNPL3 line (37), overexpressing mutant hTau (P301L, 4R0N) and hTau mice (41) as described previously were used in this study. JNPL3 mice were heterozygous and on a mixed hybrid genetic background composed of C57BL/DBA2/SW, as published previously (37). Transgenic and nontransgenic littermates were bred by mating hemizygous JNPL3 mice with Swiss–Webster mice (Taconic, Germantown, NY). Mice were genotyped for the Tau transgene by PCR between exons 9 and 13, using primers directed toward the human Tau 4R0N isoform. hTau mice were purchased from The Jackson Laboratory (Bar Harbor, ME) [strain name: B6.Cg-Mapttm1(EGFP)Klt Tg(MAPT)8cPdav/J]. For experiments designed to test the kinetics of mTau and p35 modulation by Hsp90 inhibitors, animals were administered 75 mg/kg i.p. PU-DZ8 in PBS (6% DMSO). For experiments designed to test the duration and safety of Hsp90 inhibition, mice were injected with either 75 mg/kg PU-DZ8 in PBS (6% DMSO) or 200 mg/kg PU24FCl in PBS/DMSO/EtOH (1:1:1 ratio) i.p. daily for 30 days with weekends off. Mice were killed by CO2 euthanasia 8 h after the last treatment. Hemibrains were separated into corticolimbic and subcortical regions, quickly frozen on dry ice and stored at −80°C, and processed as reported previously (38, 45). Proteins were analyzed by Western blot. Equal protein amounts were used for each S1 fraction, whereas for P3 fractions, appropriate amounts of lysate volume were taken according to individual brain wet weight (38).
Statistical Analysis.
Data were analyzed by unpaired two-tailed t tests as implemented in GraphPad Prism (version 4; GraphPad Software, San Diego, CA).
Supplementary Material
Acknowledgments
We thank Dr. L. H. Tsai for the p35 construct, Dr. V. Lee for the Tau constructs, Dr. P. Davies for the anti-Tau antibodies, Drs. Toft and Felts for the anti-Hsp90 antibody, Drs. J. Lewis and M. Hutton (Mayo Clinic College of Medicine, Jacksonville, FL) for the JNPL3 mice, Ms. Danuta Zatorska for the preparation of PU24FCl and PU-DZ8, and Drs. William Netzer and Gen He for helpful discussions. This work was supported by National Institutes of Health Grant AG09464 (to P.G.), the Experimental Therapeutics Center of Memorial Sloan–Kettering Cancer Center (G.C.), U.S. Department of Health and Human Services Administration on Aging/New York City Department for the Aging Grant 90AZ2791-02 (to P.G.), the F. M. Kirby Foundation (P.G.), and the Fisher Center for Alzheimer's Research Foundation (P.G.).
Abbreviations
- Hsp90
heat-shock protein 90
- AD
Alzheimer disease
- cdk5
cyclin-dependent protein kinase 5
- mTau
mutant Tau
- 17AAG
17-(allyllamino)-17-demethoxygeldanamycin
- hTau
human Tau.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0701055104/DC1.
References
- 1.Neckers L. Handb Exp Pharmacol. 2006;172:259–277. doi: 10.1007/3-540-29717-0_11. [DOI] [PubMed] [Google Scholar]
- 2.McDonald E, Workman P, Jones K. Curr Top Med Chem. 2006;6:1091–1107. doi: 10.2174/156802606777812004. [DOI] [PubMed] [Google Scholar]
- 3.Chiosis G. Expert Opin Ther Targets. 2006;10:37–50. doi: 10.1517/14728222.10.1.37. [DOI] [PubMed] [Google Scholar]
- 4.Young JC, Hartl FU. EMBO J. 2000;19:5930–5940. doi: 10.1093/emboj/19.21.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray B, Lynch T, Farrell M. Biochem Soc Trans. 2005;33:595–599. doi: 10.1042/BST0330595. [DOI] [PubMed] [Google Scholar]
- 6.Kosik KS, Shimura H. Biochim Biophys Acta. 2005;1739:298–310. doi: 10.1016/j.bbadis.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 7.Brandt R, Leschik J. Curr Alzheimer Res. 2004;1:255–269. doi: 10.2174/1567205043332054. [DOI] [PubMed] [Google Scholar]
- 8.Dermaut B, Kumar-Singh S, Rademakers R, Theuns J, Cruts M, Van Broeckhoven C. Trends Genet. 2005;21:664–672. doi: 10.1016/j.tig.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Lau LF, Schachter JB, Seymour PA, Sanner MA. Curr Top Med Chem. 2002;4:395–415. doi: 10.2174/1568026024607526. [DOI] [PubMed] [Google Scholar]
- 10.Goedert M, Jakes R. Biochim Biophys Acta. 2005;1739:240–250. doi: 10.1016/j.bbadis.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Lee VM, Goedert M, Trojanowski JQ. Annu Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 12.Zheng YL, Kesavapany S, Gravell M, Hamilton RS, Schubert M, Amin N, Albers W, Grant P, Pant HC. EMBO J. 2005;24:209–220. doi: 10.1038/sj.emboj.7600441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng YL, Li BS, Amin ND, Albers W, Pant HC. Eur J Biochem. 2002;269:4427–4434. doi: 10.1046/j.1432-1033.2002.03133.x. [DOI] [PubMed] [Google Scholar]
- 14.Rademakers R, Sleegers K, Theuns J, Van den Broeck M, Bel Kacem S, Nilsson LG, Adolfsson R, van Duijn CM, Van Broeckhoven C, Cruts M. Neurobiol Aging. 2005;26:1145–1151. doi: 10.1016/j.neurobiolaging.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Zhang M, Li J, Chakrabarty P, Bu B, Vincent I. Am J Pathol. 2004;165:843–853. doi: 10.1016/S0002-9440(10)63347-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A., DeTure M, Ramsden M, McGowan E, et al. Science. 2005;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiosis G, Caldas Lopes E, Solit D. Curr Opin Invest Drugs. 2006;6:534–541. [PubMed] [Google Scholar]
- 18.Vilenchik M, Solit D, Basso A, Huezo H, Lucas B, He H, Rosen N, Spampinato C, Modrich P, Chiosis G. Chem Biol. 2004;11:787–797. doi: 10.1016/j.chembiol.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Mimnaugh E, Chavany C, Neckers LM. J Biol Chem. 1996;271:22796–22801. doi: 10.1074/jbc.271.37.22796. [DOI] [PubMed] [Google Scholar]
- 20.Kanemaru K, Takio K, Miura R, Titani K, Ihara Y. J Neurochem. 1992;58:1667–1675. doi: 10.1111/j.1471-4159.1992.tb10039.x. [DOI] [PubMed] [Google Scholar]
- 21.Michel G, Mercken M, Murayama M, Noguchi K, Ishiguro K, Imahori K, Takashima A. Biochim Biophys Acta. 1998;1380:177–182. doi: 10.1016/s0304-4165(97)00139-6. [DOI] [PubMed] [Google Scholar]
- 22.Tsai LH, Delalle I, Caviness VS, Jr, Chae T, Harlow E. Nature. 1994;371:419–423. doi: 10.1038/371419a0. [DOI] [PubMed] [Google Scholar]
- 23.Tang D, Yeung J, Lee KY, Matsushita M, Matsui H, Tomizawa K, Hatase O, Wang JH. J Biol Chem. 1995;270:26897–26903. doi: 10.1074/jbc.270.45.26897. [DOI] [PubMed] [Google Scholar]
- 24.Paglini G, Pigino G, Kunda P, Morfini G, Maccioni R, Quiroga S, Ferreira A, Caceres A. J Neurosci. 1998;18:9858–9869. doi: 10.1523/JNEUROSCI.18-23-09858.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi S, Ohshima T, Cho A, Sreenath T, Iadarola MJ, Pant HC, Kim Y, Nairn AC, Brady RO, Greengard P, et al. Proc Natl Acad Sci USA. 2005;102:1737–1742. doi: 10.1073/pnas.0409456102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishiguro K, Omori A, Sato K, Tomizawa K, Imahori K, Uchida T. Neurosci Lett. 1991;128:195–198. doi: 10.1016/0304-3940(91)90259-v. [DOI] [PubMed] [Google Scholar]
- 27.Baumann K, Mandelkow EM, Biernat J, Piwnica-Worms H, Mandelkow E. FEBS Lett. 1993;336:417–424. doi: 10.1016/0014-5793(93)80849-p. [DOI] [PubMed] [Google Scholar]
- 28.Tseng HC, Zhou Y, Shen Y, Tsai LH. FEBS Lett. 2002;523:58–62. doi: 10.1016/s0014-5793(02)02934-4. [DOI] [PubMed] [Google Scholar]
- 29.Singh TJ, Zaidi T, Grundke-Iqbal I, Iqbal K. FEBS Lett. 1995;358:4–8. doi: 10.1016/0014-5793(94)01383-c. [DOI] [PubMed] [Google Scholar]
- 30.Tian Q, Wang J. Neurosignals. 2002;11:262–269. doi: 10.1159/000067425. [DOI] [PubMed] [Google Scholar]
- 31.Sittler A, Lurz R, Lueder G, Priller J, Lehrach H, Hayer-Hartl MK, Hartl FU, Wanker EE. Hum Mol Genet. 2001;10:1307–1315. doi: 10.1093/hmg/10.12.1307. [DOI] [PubMed] [Google Scholar]
- 32.Auluck PK, Bonini NM. Nat Med. 2002;11:1185–1186. doi: 10.1038/nm1102-1185. [DOI] [PubMed] [Google Scholar]
- 33.Dou F, Netzer WJ, Tanemura K, Li F, Hartl FU, Takashima A, Gouras GK, Greengard P, Xu H. Proc Natl Acad Sci USA. 2003;100:721–726. doi: 10.1073/pnas.242720499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou J, Guo Y, Guettouche T, Smith DF, Voellmy R. Cell. 1998;94:471–480. doi: 10.1016/s0092-8674(00)81588-3. [DOI] [PubMed] [Google Scholar]
- 35.Boudeau J, Deak M, Lawlor MA, Morrice NA, Alessi DR. Biochem J. 2003;370:849–857. doi: 10.1042/BJ20021813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lamphere L, Fiore F, Xu X, Brizuela L, Keezer S, Sardet C, Draetta GF, Gyuris J. Oncogene. 1997;14:1999–2004. doi: 10.1038/sj.onc.1201036. [DOI] [PubMed] [Google Scholar]
- 37.Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, Van Slegtenhorst M, Gwinn-Hardy K, Paul Murphy M, Baker M, Yu X, et al. Nat Genet. 2000;25:402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- 38.Sahara N, Lewis J, DeTure M, McGowan E, Dickson DW, Hutton M, Yen SH. J Neurochem. 2002;83:1498–1508. doi: 10.1046/j.1471-4159.2002.01241.x. [DOI] [PubMed] [Google Scholar]
- 39.Sahara N, Murayama M, Mizoroki T, Urushitani M, Imai Y, Takahashi R, Murata S, Tanaka K, Takashima A. J Neurochem. 2005;94:1254–1263. doi: 10.1111/j.1471-4159.2005.03272.x. [DOI] [PubMed] [Google Scholar]
- 40.He H, Zatorska D, Kim J, Aguirre J, Llauger L, She Y, Wu N, Immormino RM, Gewirth DT, Chiosis G. J Med Chem. 2006;49:381–390. doi: 10.1021/jm0508078. [DOI] [PubMed] [Google Scholar]
- 41.Andorfer C, Kress Y, Espinoza M, de Silva R, Tucker KL, Barde YA, Duff K, Davies P. J Neurochem. 2003;86:582–590. doi: 10.1046/j.1471-4159.2003.01879.x. [DOI] [PubMed] [Google Scholar]
- 42.da Cruz e Silva EF, Fox CA, Ouimet CC, Gustafson E, Watson SJ, Greengard P. J Neurosci. 1995;15:3375–3389. doi: 10.1523/JNEUROSCI.15-05-03375.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu H, Gouras GK, Greenfield JP, Vincent B, Naslund J, Mazzarelli L, Fried G, Jovanovic JN, Seeger M, Relkin NR, et al. Nat Med. 1998;4:447–451. doi: 10.1038/nm0498-447. [DOI] [PubMed] [Google Scholar]
- 44.Liu F, Ma XH, Ule J, Bibb JA, Nishi A, DeMaggio AJ, Yan Z, Nairn AC, Greengard P. Proc Natl Acad Sci USA. 2001;98:11062–11068. doi: 10.1073/pnas.191353898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greenberg SG, Davies P. Proc Natl Acad Sci USA. 1990;87:5827–5831. doi: 10.1073/pnas.87.15.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.