Abstract
Dietary K intake plays an important role in the regulation of renal K secretion: a high K intake stimulates whereas low K intake suppresses renal K secretion. Our previous studies demonstrated that the Src family protein-tyrosine kinase and mitogen-activated protein kinase (MAPK) are involved in mediating the effect of low K intake on renal K channels and K secretion. However, the molecular mechanism by which low K intake stimulates MAPK is not completely understood. Here we show that inhibitor of growth 4 (ING4), a protein with a highly conserved plant homeodomain finger motif, is involved in mediating the effect of low K intake on MAPK. K restriction stimulates the expression of ING4 in the kidney and superoxide anions, and its related products are involved in mediating the effect of low K intake on ING4 expression. We used HEK293 cells to express ING4 and observed that expression of ING4 increased the phosphorylation of p38 and ERK MAPK, whereas down-regulation of ING4 with small interfering RNA decreased the phosphorylation of p38 and ERK. Immunocytochemistry showed that ING4 was expressed in the renal outer medullary potassium (ROMK)-positive tubules. Moreover, ING4 decreased K currents in Xenopus oocytes injected with ROMK channel cRNA. This inhibitory effect was reversed by blocking p38 and ERK MAPK. These data provide evidence for the role of ING4 in mediating the effect of low K intake on ROMK channel activity by stimulation of p38 and ERK MAPK.
Keywords: ERK, hypokalemia, p38, renal potassium metabolism, superoxide
It is well known that low K intake suppresses renal K secretion by increasing K absorption and inhibiting K secretion (1, 2). The inhibitory effect of low K intake on renal K secretion is partially achieved by removing renal outer medullary potassium (ROMK)-like small conductance K channels from the apical membrane of principal cells in the cortical collecting duct (CCD) through a protein-tyrosine kinase (PTK)-dependent mechanism (3). We have demonstrated previously that low K intake stimulates the production of superoxide anions and its related products, which in turn increase PTK expression and activity (4, 5). Increased PTK activity stimulates tyrosine phosphorylation of ROMK channels and subsequently enhances the internalization of the ROMK channels (6, 7). Furthermore, we have shown that low K intake stimulates ERK and p38 MAPK, which inhibits ROMK channels by a PTK-independent pathway. However, the mechanism by which low K intake stimulates MAPK activity is not completely understood.
Inhibitor of growth 4 (ING4) is a member of the ING family proteins. They have been shown to regulate cell cycle, transcription and oncogenesis (8), and to promote UV-induced apoptosis of skin cells (9). ING4 also plays an important role in inhibiting tumor growth and angiogenesis in brain tumor cells (10). Because K restriction causes renal hypertrophy (11) through stimulation of growth factor levels (12), it is possible that that ING family proteins may also be involved in mediating the effect of low K intake on renal function. The present study tests whether ING4 is a signal molecule mediating the effect of low K intake on K secretion by regulation of ROMK channels.
Results
Low K Intake Stimulates ING4 Expression by a Superoxide-Dependent Mechanism.
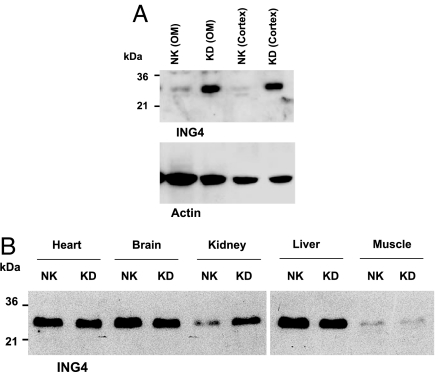
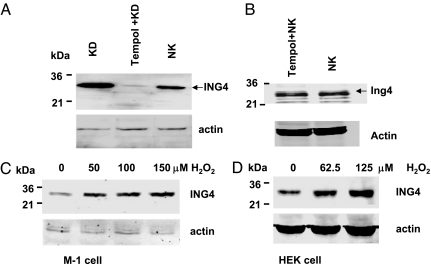
We first examined the effect of K restriction on the renal expression of ING4. Fig. 1 shows a Western blot demonstrating that K restriction (KD) significantly increased the expression of ING4 in both renal cortex and outer medulla by 120 ± 10% (2.2-fold greater than the control value) (P < 0.01, n = 6) compared with that in rats fed with normal K (NK) diet. The effect of K restriction on the renal ING4 expression was specific because it did not affect ING4 expression in heart, brain, liver, and skeletal muscle (Fig. 1B). Next, we explored the role of superoxide anions in mediating the effect of low K intake on the expression of ING4 because K restriction increases the level of superoxide in the renal tubules (4). We examined the effect of K restriction on ING4 expression in rats treated with tempol, an agent that decreases superoxide production in the kidney (4) and other tissues (13). Fig. 2A is a Western blot showing that tempol treatment decreased ING4 expression by 80 ± 10% (P < 0.01, n = 3) compared with that of non-tempol-treated rats fed with KD diet. In contrast, tempol treatment did not significantly affect ING4 expression in the kidney from animals on a normal K diet (Fig. 2B).
Fig. 1.
Western blot showing the effect of K depletion (KD) on the expression of ING4 in renal cortex and outer medulla (OM) (A) and in heart, brain, liver, and skeletal muscle (B). The tissues were collected from rats on a KD diet for 7 days. [NK indicates the tissue from rats on a control K diet (1.1%)]. The kidney sample used for the Western blot shown in B was a mixture of the cortex and outer medulla. The protein sample loaded for each lane was 150 μg.
Fig. 2.
Western blots showing the expression of ING4 in renal cortex and outer medulla (mixture) of rats on a normal K diet (NK, 1.1%), K-deficient diet (KD), or KD + tempol treatment. (A) Western blot demonstrating the effect of tempol on ING4 expression in renal cortex and outer medulla (mixture) from animals on normal K diet (B). The protein sample loaded for each lane was 150 μg. (C and D) Western blots showing the effect of H2O2 on ING4 expression (100 μg of protein per lane) in M-1 cells and in HEK293 cells, respectively. The cells were treated with H2O2 at different concentrations for 60 min. The ING4 expression was detected with rabbit anti-ING4 antibody, and actin served as a load control.
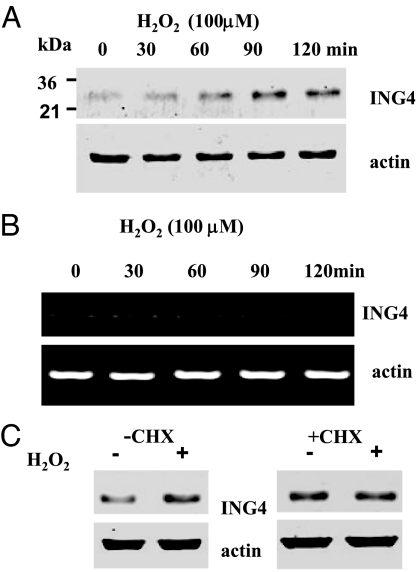
To explore further the role of superoxide anions in mediating the effect of K depletion on the renal expression of ING4, we also used both M-1 cells, a mouse CCD cell line (14, 15), and HEK293 cells to examine the effect of H2O2 on ING4 expression. Fig. 2C is a Western blot showing that application of 50, 100, and 150 μM H2O2 for 60 min increased ING4 expression by 40 ± 7% (1.4-fold greater than the control value), 70 ± 7% (1.7-fold greater than the control) (P < 0.05), and 85 ± 6% (1.85-fold greater than the control) (P < 0.05, n = 6), respectively. The stimulatory effect of H2O2 on ING4 expression was also observed in HEK cells (Fig. 2D), in which application of 62.5 and 125 μM H2O2 increased the ING4 expression by 40 ± 10% (1.4-fold greater than the control) (n = 4) and 90 ± 10% (1.9-fold greater than the control) (P < 0.05, n = 4), respectively. Because the response of HEK cells to H2O2 was similar to that in M-1 cells, we used HEK cells in our further experiments. To determine the time course of the effect of H2O2 on ING4 expression, we incubated HEK cells with 100 μM H2O2 for different periods. Fig. 3A is a representative Western blot demonstrating that incubation of HEK cells with 100 μM H2O2 for 60 min significantly increased the expression of ING4 by 70 ± 20% (1.7-fold greater than the control) (n = 3). The expression of ING4 induced by H2O2 treatment for 90 min and 120 min was 200 ± 20% (3-fold greater than the control) (P < 0.05) and 170 ± 20% (2.7-fold greater than the control) (P < 0.05), respectively.
Fig. 3.
Time course of the effects of 100 μM H2O2 in HEK cells. (A) Effect on ING4 protein expression (100 μg of protein per lane). (B) Effect on mRNA level of ING4. (C) Effect of 100 μM H2O2 (60 min) on ING4 expression (100 μg of protein per lane) in the absence (Left) or in the presence of cyclohexamide (CHX; 50 μg/ml) (Right) in HEK cells. The cells were pretreated for 1 h with CHX before adding H2O2, and CHX was present throughout the treatment (CHX and H2O2-treated group).
H2O2 Stimulates the Protein Translation of ING4.
After demonstrating that increased superoxide anion production was involved in mediating the effect of low K intake on ING4 expression, we investigated whether H2O2-induced increase in ING4 expression was the result of stimulation of ING4 transcription or translation. We used semiquantitative RT-PCR to determine the RNA levels of ING4. Fig. 3B is a representative experiment showing that incubation of HEK cells with 100 μM H2O2 did not alter the mRNA level of ING4. The finding that the superoxide-induced increase in ING4 expression was not the result of stimulation of transcription was also suggested by Northern blot analysis in which the mRNA level of ING4 in the kidney was unchanged in the K-restricted rats (data not shown). Next, we explored whether increases in ING4 expression induced by H2O2 in HEK cells were the result of stimulation of the protein translation. We treated HEK cells with 50 μg/ml cyclohexamide for 60 min before adding 100 μM H2O2 and then examined the effect of H2O2 on ING4 expression. Fig. 3C shows that cyclohexamide treatment abolished the effect of H2O2 on ING4 expression (n = 4) in HEK cells treated with H2O2 for 60 min, which increased ING4 expression in the absence of cyclohexamide. This observation suggests that the effect of H2O2 on ING4 expression is at least partially the result of stimulation of ING4 translation. However, the possibility that H2O2 inhibits the degradation of ING4 cannot be excluded.
ING4 Is Expressed in ROMK-Positive Tubule and Inhibits ROMK.
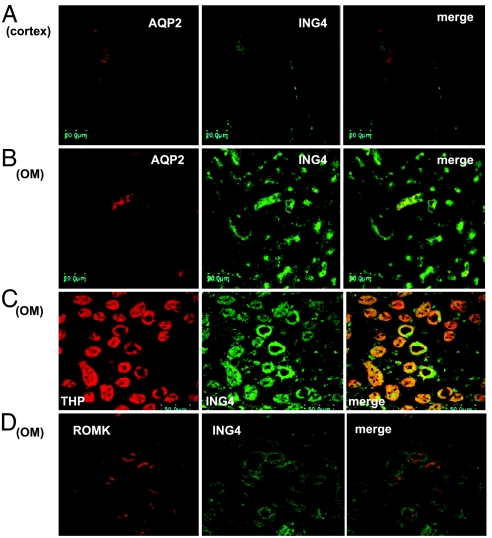
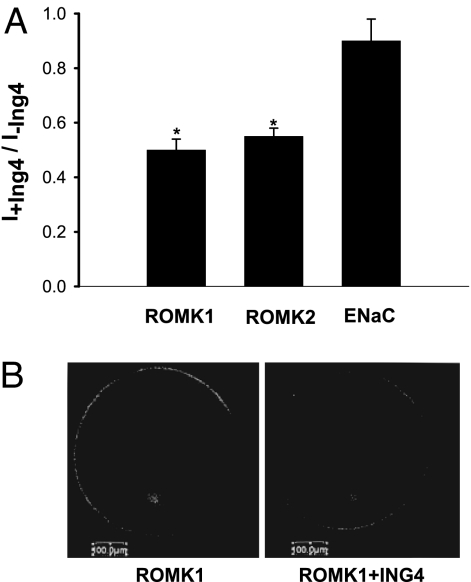
In further studies, we examined the tubule location of ING4 expression in the rat kidney. Fig. 4 shows confocal images demonstrating ING4 immunostaining in renal cortex (A) and outer medulla (B–D). It is apparent that ING4 is expressed in the CCD indicated by positive AQP2 staining (A), outer medullary collecting duct (AQP2-positive) (B), and thick ascending limb (THP-positive) (C). Fig. 4D illustrates ING4 immunostaining in the outer medulla, showing that ING4 is expressed in ROMK-positive tubules. We also used the two-electrode voltage clamp (TEVC) method to examine the effect of ING4 on ROMK channels in oocytes. Results summarized in Fig. 5A demonstrated that expression of ING4 (5 ng per egg) significantly decreased ROMK1 and ROMK2 channel activity by 50 ± 4% (control = 7.8 ± 0.6 μA, ING4 = 3.9 ± 0.3 μA) (P < 0.01, n = 46) and by 46 ± 4% (control = 8.4 ± 0.6 μA, ING4 = 4.5 ± 0.4 μA) (P < 0.01, n = 46), respectively. Furthermore, confocal microscope experiments further showed that coinjection of ING4 decreased the fluorescence intensity of oocytes membrane also by 49 ± 6% (P < 0.05, n = 20) in oocytes injected with GFP-ROMK1 (Fig. 5B). The effect of ING4 on ROMK was specific because ING4 did not significantly affect the endothelial sodium channel (ENaC) activity in oocytes injected with 2 ng of α, 1 ng of β, and 1 ng of γ ENaC subunits.
Fig. 4.
Confocal images show immunostaining of ING4 in renal cortex (A) and outer medulla (OM) (B–D). Double staining (ING4 and AQP2) demonstrates the expression of ING4 in the CCD (A) and outer medullary collecting duct (B). ING4 immunostaining in the THP-positive tubules is shown in C and in ROMK-positive tubules in D.
Fig. 5.
Expression of ING4 inhibits ROMK channels. (A) Effect of ING4 on ROMK1, ROMK2, and ENaC in Xenopus oocytes injected with 5 ng of ING4; 5 ng of ROMK1; 5 ng of ROMK2; or 2 ng of α, 1 ng of β, and 1 ng of γ ENaC. The two-electrode voltage clamp technique was used to measure the current. Data were normalized by calibrating the ration of K or Na currents in oocytes injected with or without ING4. The asterisks indicate that data were significantly different (P < 0.01) from the control value (no ING4). (B) Confocal image showing the effect of ING4 on fluorescence intensity of the cell membrane in oocytes injected with GFP-ROMK1.
ING4 Inhibits ROMK Channels by Activation of MAPK.
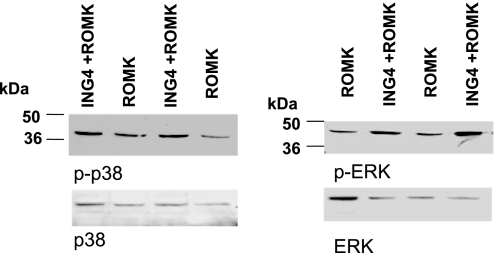
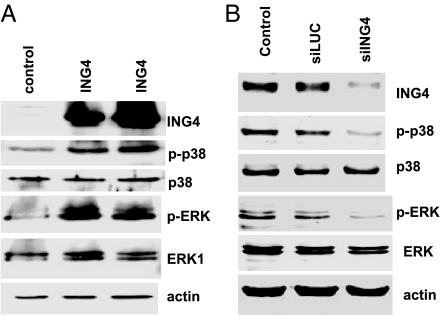
Low K intake has been shown to activate ERK, p38 MAPK, and Src family PTK, which inhibit ROMK channel activity (4, 5). Therefore, we examined whether expression of ING4 stimulates the phosphorylation of p38 and ERK in Xenopus oocytes. Fig. 6 is a typical Western blot showing that expression of ING4 stimulated the phosphorylation of p38 and ERK by 95 ± 10% (1.95-fold greater than the control) (P < 0.01) and 110 ± 10% (2.1-fold greater than the control) (P < 0.05), respectively (n = 4). The finding that ING4 stimulates the phosphorylation of p38 MAPK and ERK is also supported by experiments in which the effect of expression or down-regulation of ING4 on p38 and ERK was investigated. Fig. 7A is a typical Western blot from seven experiments showing that expression of ING4 increased the phosphorylation of ERK and p38 by 98 ± 10% (1.98-fold greater than the control) (P < 0.05) and 96 ± 10% (1.96-fold greater than the control) (P < 0.05), respectively. In contrast, the down-regulation of ING4 expression with siRNA decreased the phosphorylation of ERK by 45 ± 6% (P < 0.05, n = 3) and p38 by 50 ± 6% (P < 0.001, n = 7), respectively (Fig. 7B). The effect of ING4 siRNA on p38 and ERK phosphorylation is specific because siRNA of luciferase had no significant effect on MAPK phosphorylation.
Fig. 6.
Western blots showing that ING4 stimulates the phosphorylation of p38 and ERK in Xenopus oocytes. The protein sample loaded for each lane was 150 μg. The mixture of phosphatase inhibitors was included during the preparation of the tissue samples.
Fig. 7.
Western blotting demonstrates that expression of ING4 (A) stimulates whereas down-regulation of ING4 (B) decreases the phosphorylation of p38 and ERK in HEK cells transfected with pcDNA3-ING4 or siRNA of ING4 and luciferase. The protein sample loaded for each lane was 100 μg. The mixture of phosphatase inhibitors was included in the tissue samples.
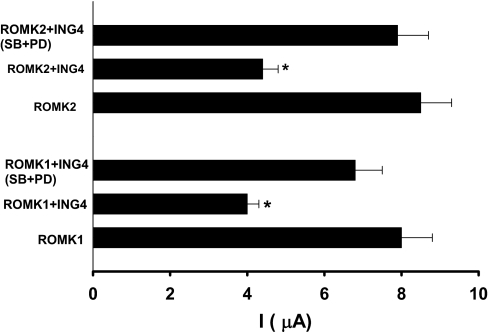
We then used the TEVC method to test whether inhibition of MAPK could reverse the inhibitory effect of ING4 on ROMK channel activity. Oocytes were injected with ROMK1/ROMK2 (5 ng) and ING4 or with ROMK cRNA alone. Twenty-four hours after the injection, oocytes were divided into two groups: a vehicle-treated group and oocytes treated with 50 M PD98059 (ERK inhibitor) + 5 M SB202190 (inhibitor of p38) for 60–120 min. Data summarized in Fig. 8 show that injection of ING4 decreased ROMK1 current from the control value 8 ± 0.7 μA to 4 ± 0.3 μA (n = 15) and ROMK2 current from 8.5 ± 0.8 μA to 4.4 ± 0.4 μA (n = 15). In contrast, inhibition of p38 and ERK increased ROMK1 from 4 ± 0.3 μA to 6.8 ± 0.7 μA (n = 15) and ROMK2 from 4.4 ± 0.4 μA to 7.9 ± 0.8 μA (n = 15), which indicates the role of ERK and p38 in mediating the effect of ING4 on ROMK channels. Without injection of ING4, inhibition of MAPK had no effect on ROMK channel current (n = 10).
Fig. 8.
Inhibition of p38 and ERK abolished the effect of ING4 on ROMK1 and ROMK2 channel activity. The oocytes injected with ING4 (5 ng) and ROMK1/2 (5 ng) were incubated with 50 μM PD098059 (PD) and 5 μM SB202190 (SB) or vehicles (0.4% DMSO) for 60–120 min and the two-electrode voltage clamp method was used to measure K currents. Asterisks indicate that data were significantly different (P < 0.01) from the control value (ROMK alone) or SB + PD-treated groups.
Discussion
ING4 is a member of the ING tumor-suppressor family proteins, which regulate cell cycle (16), transcription, DNA repair, oncogenesis, and apoptosis (8). Although homology among the five members (ING1–5) is low, all ING family proteins have a highly conserved plant homeodomain finger motif that has been demonstrated to modulate ubiquitination (17) and to associate with phosphatidylinositol phosphate (18). ING4 has also been shown to interact with NF-κB and decrease IL-8 production, which is involved in angiogenesis of brain tumors (10). Moreover, it has been reported that ING4 suppresses the activation of hypoxia-induced factors (19). In the present study, we demonstrate a role of ING4 in the regulation of K homeostasis and renal K secretion.
Renal K secretion is regulated by hormones and dietary K intake. It is well established that a high K intake stimulates whereas a low K intake suppresses renal K secretion (1, 20, 21). The effect of high K intake on renal K secretion is mediated by both aldosterone-dependent and aldosterone-independent mechanisms. High K intake raises aldosterone levels, which increases Na absorption and K excretion by augmenting the driving force for K secretion and by stimulating Na+,K+-ATPase-dependent K uptake in the basolateral membrane (20). High K intake also enhances K secretion by an aldosterone-independent mechanism (21, 22). We and others have shown that high K stimulated ROMK channels in the CCD (23, 24) and that the effect of high K intake was not mimicked by infusion of aldosterone and possibly involved in reducing channel retrieval from apical membrane.
Previous studies have demonstrated that low K intake inhibits ROMK channel activity (4, 5) by stimulating internalization of ROMK channels (25, 26). Low K intake increases superoxide anions and its related products, and they are involved in mediating the effect of low K intake on ROMK channels because suppression of superoxide production increased the ROMK channel activity in the CCD from rats fed with KD diet (4). The inhibitory effect of superoxide anions on ROMK channels involves stimulation of the phosphorylation of ERK and p38 MAPK (5) and augmenting the expression of Src family PTK. Increased expression of PTK was shown to enhance the tyrosine phosphorylation and internalization of ROMK. Moreover, we have also shown that p38 and ERK are responsible for mediating the effect of low K intake on ROMK channels by a PTK-independent pathway (5). This view is supported by the observation that inhibition of MAPK increased ROMK channel activity in the CCD treated with PTK inhibitor. It is possible that MAPK-induced inhibition of ROMK channel activity plays an important role in suppressing K secretion in the early stage of K depletion. This notion is supported by two lines of evidence: (i) Increased phosphorylation of ERK and p38 was detected after only 24-h K restriction (5), whereas the expression of Src family PTK had not yet significantly changed until 3 days after K restriction (3); and (ii) patch clamp experiments demonstrated that inhibition of p38 and ERK but not PTK significantly increased ROMK channels in the CCD from rats on KD diet for 24 h (3, 5). Thus, we speculate that MAPK is involved in mediating the early inhibitory effect of low K intake on renal K secretion by a PTK-independent pathway.
Two lines of evidence suggest that ING4 is involved in mediating the effect of low K intake on the MAPK activity: (i) K restriction increased ING4 expression only in the kidney, although it also expressed in the heart, liver, skeletal muscle, and brain; and (ii) phosphorylation of both ERK and p38 MAKP correlates with the expression of ING4. Thus, it is possible that ING4 is involved in mediating the effect of low K intake and superoxide anions on MAPK. However, we cannot exclude the possibility that signaling factors other than ING4 are also involved in mediating the effect of low K intake or superoxide anions on MAPK.
The mechanism by which low K intake stimulates ING4 expression is at least partially mediated by superoxide and its related products because suppression of superoxide levels abolished the effect of low K intake on the ING4 expression. The role of superoxide anions in stimulating ING4 expression in the kidney is also supported by the observation that treatment of HEK cells or M-1 cells with H2O2 increased the expression of ING4. However, the effect of H2O2 on ING4 expression may not be the result of stimulation of protein transcription because H2O2 did not increase mRNA level. The observation that cyclohexamide abolished the effect of H2O2 on ING4 expression suggests that H2O2 stimulates the translation of ING4 protein.
ING family proteins have been demonstrated to regulate gene expression in the nucleus through interaction with chromatin remodeling complexes (8). Also, ING4 regulates NF-κB by binding to its p65 subunit (10) and interacts with p53 (27). However, ING4 is also able to modulate cytoplasmic proteins such as Liprin-α1 and Ras-GAP Src homology 3 domain-binding protein 2 (G3BP2) (28). The mechanism by which ING4 stimulates the phosphorylation of p38 and ERK is not clear. The observation that ING4 stimulates the phosphorylation of MAPK but not the total expression of ERK and p38 indicates that the effect of ING4 on MAPK is the result of posttranslation modification rather than stimulation of the translation of MAPK gene. We speculate that ING4-induced MAPK phosphorylation could be achieved by either stimulation of the upstream protein kinases of MAPK or inhibition of protein phosphatase. In this regard, it has been suggested that association of ING4 with G3BP may modulate Ras signaling, which is involved in the regulation MAPK activity (28). We need further experiments to determine the mechanism by which ING4 regulates MAPK.
In addition to regulation of ROMK channels in the CCD, two lines of evidence suggest that the ING4–MAPK pathway may also be involved in the regulation of K channels in the thick ascending limb (TAL): (i) ING4 is expressed in the TAL; and (ii) expression of ING4 inhibits ROMK2 channels known to be present in the TAL (29). ROMK channels are required for K recycling across the apical membrane in the TAL (30, 31). Thus, ING4-induced inhibition of ROMK2 channels should lead to suppression of K recycling which is essential for the function of Na/K/Cl cotransport. Previous study has shown that low K intake inhibits the activity of the apical K channels in the TAL (32). Therefore, it is possible that ING4 may also regulate Na transport in the TAL by inhibiting apical K channels and K recycling.
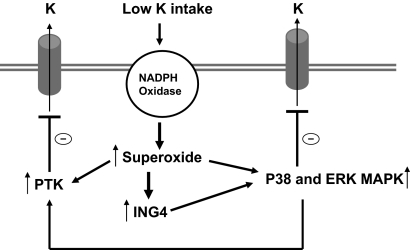
Fig. 9 is a cell model illustrating the possible role of ING4 in mediating the effect of low K intake on ROMK channels. We hypothesize that low K intake increases the production of superoxide anions, possibly through activation of NADPH oxidase, and superoxide and its related product stimulate the expression of ING4. Such increased ING4 expression enhances the phosphorylation of ERK and p38 MAPK, which, in turn, inhibit ROMK channel activity. Activation of MAPK may also be required for stimulation of the expression of nonreceptor type PTK such as c-Src, which enhances tyrosine phosphorylation of ROMK and leads to channel internalization in the collecting duct. We conclude that ING4 is involved in mediating the effect of low K intake on K metabolism and that the effect of ING4 involves regulation of MAPK, which, in turn, inhibits ROMK channels.
Fig. 9.
Cell model illustrating the role of ING4 in mediating the effect of low K intake on ROMK channels.
Materials and Methods
Animals.
Sprague–Dawley rats (6 weeks, either sex) were purchased from Taconic Farms (Germantown, NY). Rats were maintained on either normal K (1.1%) or a K-deficient (KD) diet for 7 days. The tempol-treated rats were also fed with KD diet and had a daily IP injection of tempol (15 mg/kg) for 1 week. Animals (<90 g) were killed by cervical dislocation, and kidneys were removed immediately. The animal use protocol was approved by IACUC of New York Medical College.
Tissue Preparation.
The renal cortex and the outer medulla were separated under a dissecting microscope and suspended in radioimmunoprecipitation assay buffer solution [(1:8 ratio, wt/vol) 1× PBS/1% Nonidet P-40/0.5% sodium deoxycholate/0.1% SDS]. Ten microliters of PMSF (10 mg/ml stock solution in isopropyl alcohol) and 10 μl of a mixture of protease inhibitors (Sigma, St. Louis, MO) were added per ml of buffer at the time of lysis. The samples were homogenized on ice for 15 min with a mortar and pestle. The suspension was incubated at 4°C for 1 h in the presence of 5 μg/ml DNase followed by centrifugation at 500 × g for 10 min, and the resultant supernatant was collected. Protein concentrations were measured in duplicate by using a Dc protein assay kit (Bio-Rad, Hercules, CA).
Preparation of M-1 and HEK293 Cells.
M-1 cells and HEK293 cells were purchased from the American Type Culture Collection (Manassas, VA) and maintained in RPMI medium 1640 supplemented with 10% FBS. Before H2O2 treatment, the cells were cultured in medium containing 1% FBS for 16 h followed by incubation for an additional 30 min in a solution containing 22 mM Hepes (pH 7.4), 124 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1.5 mM CaCl2, 0.16 mM HPO4, 0.4 mM H2PO4, 5 mM NaHCO3, and 5.6 mM glucose. H2O2 (50–200 μM final concentration) was directly added to the cells in Hepes buffer for different time periods. After treatment with H2O2, the cells were washed with ice-cold PBS twice and incubated for 30 min in radioimmunoprecipitation assay lysis buffer. For transfection of HEK293 cells with ING4, the cells at 50–70% confluence (60-mm dishes) were transfected with plasmid DNA containing ING4 by FuGENE HD Transfection Reagent from Roche (Indianapolis, IN) as described by the manufacturer, and the transfected cells were incubated for additional 12 h before harvesting.
Preparation of Xenopus Oocytes.
Xenopus laevis females were obtained from NASCO (Fort Atkinson, WI). The method for obtaining oocytes has been described previously (33). Viable oocytes were selected for injection with different cRNA. The oocytes were incubated at 19°C in a 66% DMEM/F12 medium with freshly added 2.5 mM sodium pyruvate and 50 μg/ml gentamicin. Experiments were performed on days 1–2 after injection with a TEVC.
TEVC Whole-Cell Method
A Warner oocyte clamp OC-725C was used to measure the whole-cell K current. Voltage and current microelectrodes were filled with 1,000 mM KCl and had resistance of <2 MΩ. The current was recorded on a chart recorder (TA240; Gould Electronics, Valley View, OH). To exclude the leaky current, 2 mM Ba2+ was used to determine the Ba2+-sensitive K+ current.
Western Blotting.
Proteins that were homogenized from renal cortex and outer medulla were separated by electrophoresis on 8–10% SDS/polyacrylamide gels and transferred to immunoblot PVDF membrane (Bio-Rad). The membrane was blocked with Odyssey blocking buffer and incubated with the primary antibody at 4°C for 12 h. The membrane was washed four times for 5 min with PBS containing 0.1% Tween 20 and followed by incubation with the secondary antibody for an additional 30 min. The membrane was then washed several times and scanned by Odyssey infrared imaging system (LI-COR, Lincoln, NE) at a 700–800 channel wavelength.
Confocal Microscope.
Surface fluorescence detected by confocal microscopy at the equatorial plane of oocytes expressing GFP-tagged ROMK correlates with channel activity and has been used by us to assess channel expression in the plasma membrane (34). Briefly, GFP fluorescence was excited at 488 nM with an argon laser beam and viewed with an inverted Olympus FV300 confocal system (Middlebush, NJ) equipped with a ×10 lens. We used Scion Image software (Scion Co., Frederick, MD) to determine the fluorescence intensity. All images were acquired and processed with identical parameters.
Immunocytochemistry.
Kidneys were perfused with 50 ml of PBS containing 40 units/ml heparin followed by 200 ml of 4% paraformaldehyde, and they were fixed with 4% paraformaldehyde for 12 h. A Leica 1900 cryostat (Leica, Mussloch, Germany) was used to cut kidney slices which were dried at 42°C for 1 h. After washing with 1× PBS, samples were permeabilized with 0.4% Triton dissolved in 1× PBS buffer containing 1% BSA and 0.1% lysine (pH 7.4) for 15 min. Kidney slices were blocked with 2% goat serum for 1 h at room temperature and then incubated with antibodies to ROMK, Tamm–Horsfall glycoprotein (THP), and ING4 for 12 h at 4°C. Slides were washed with PBS buffer followed by incubation with second antibody for 2 h at room temperature.
RNA and Northern Blotting.
Total RNA was extracted by using the TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Total RNA (10 μg) from rat tissues was separated by electrophoresis in a 1.0% denatured formaldehyde–agarose gel and transferred to Hybond-N+ nylon membrane (Amersham Pharmacia Biotech, Buckinghamshire, U.K.), which was then hybridized with a cDNA fragment of rat ING4 labeled by [α-32P]dCTP by using a random primer DNA-labeling kit (TaKaRa Inc., DaLian, China) and visualized by an SI PhosphorImager (Molecular Dynamics, Sunnyvale, CA). The cDNA fragment was amplified from rat liver RNA with 5′-GTCCTCTTCATTCCCCTTGCTT-3′ and 5′-CGGAGCGCGTACTCGTAATTAC-3′ primers.
RT-PCR.
RT was performed in a 20-μl reaction system with 2 μg of total RNA. Each PCR was generally performed in 25 thermal cycles, and then the PCR products were observed by electrophoresis on 1.5% agarose gel. The human ING4 primers (5′-TGCTTCGAGATGGCTGCG-3′ and 5′-CTATTTCTTCTTCCGTTCTTGGGA-3′) were used for HEK cells.
RNA Interference.
HEK cells were plated into 24-well plates (1 × 105 cells per well) 16 h before the experiment and transfected with 200 nM siRNA duplexes (Shanghai GenePharma Co., Shanghai, China) by using Lipofectamine 2000 reagent (Invitrogen) in serum-free medium. Five hours after transfection, cells were cultured in the culture medium for an additional 72 h. Compositions of the siRNA duplexes of ING4 oligonucleotides were 5′-UGCU C G U GCUCGUUCCAAATT-3′ and 5′-UUUGGAACGAGCACGAGCATT-3′, whereas the sequences of siRNA of luciferase (LUC) were 5′-CUUACGCUGAGUACUUCGATT-3′ and 5′-UCGAAGUACUCAGCGUAAGTT-3′.
Experimental Materials and Statistics.
Antibodies to phospho-p38, p38, phospho-ERK, ERK, AQP2, and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). THP and ROMK antibodies were obtained from ICN Pharmaceutical (Aurora, OH) and Alomone (Jerusalem, Israel). Rabbit polyclonal anti-ING4 antibody described previously (16) was used. Briefly, the antibody was raised against GST-ING4 fusion protein and purified from antiserum with agarose-linked protein A (Amersham). SB202190 and PD98059 were purchased from Sigma. The data are presented as mean ± SEM. We used a paired Student's t test or one-way analysis of variance to determine the statistical significance. If the P value is <0.05, the difference is considered to be significant.
Acknowledgments
This work was supported by Chinese High-Tech Research and Development Program Grants 2006AA02A305 (to X.Z.) and 2006AA02Z193 (to Z.-G.H.), National Natural Science Foundation for Outstanding Youth Grant 30425019 (to Z.-G.H.), and National Institutes of Health Grants DK 54983 (to W.-H.W.) and DK47402 (to W.-H.W.).
Abbreviations
- CCD
cortical collecting duct
- ENaC
endothelial sodium channel
- ING4
inhibitor of growth 4
- KD
potassium-deficient
- PTK
protein-tyrosine kinase
- ROMK
renal outer medullary potassium
- TEVC
two-electrode voltage clamp
- TAL
thick ascending limb
- THP
Tamm–Horsfall glycoprotein.
Footnotes
The authors declare no conflict of interest.
References
- 1.Beck FX, Giebisch G, Thurau K. Kidney Int. 1992;42:272–278. doi: 10.1038/ki.1992.286. [DOI] [PubMed] [Google Scholar]
- 2.Wingo CS. J Clin Invest. 1989;84:361–365. doi: 10.1172/JCI114165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei Y, Bloom P, Lin DH, Gu RM, Wang WH. Am J Physiol. 2001;281:F206–F212. doi: 10.1152/ajprenal.2001.281.2.F206. [DOI] [PubMed] [Google Scholar]
- 4.Babilonia E, Wei Y, Sterling H, Kaminski P, Wolin MS, Wang WH. J Biol Chem. 2005;280:10790–10796. doi: 10.1074/jbc.M414610200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babilonia E, Li D, Wang ZJ, Sun P, Lin DH, Wang WH. J Am Soc Nephrol. 2006;17:2687–2696. doi: 10.1681/ASN.2006050426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin DH, Sterling H, Wang WH. Physiology. 2005;20:140–146. doi: 10.1152/physiol.00044.2004. [DOI] [PubMed] [Google Scholar]
- 7.Lin DH, Sterling H, Lerea KM, Welling P, Jin L, Giebisch G, Wang WH. Am J Physiol. 2002;283:F671–F677. doi: 10.1152/ajprenal.00160.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He GHY, Helbing CC, Wagner MJ, Sensen CW, Riabowol K. Mol Biol Evol. 2005;22:104–116. doi: 10.1093/molbev/msh256. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Li G. J Biol Chem. 2006;281:11887–11893. doi: 10.1074/jbc.M511309200. [DOI] [PubMed] [Google Scholar]
- 10.Garkavtsev I, Kozin SV, Chernova O, Xu L, Winkler F, Brown E, Barnett GH, Jain RK. Nature. 2004;428:328–332. doi: 10.1038/nature02329. [DOI] [PubMed] [Google Scholar]
- 11.Gustafson AB, Shear L, Gabuzda GJ. J Lab Clin Med. 1973;82:287–296. [PubMed] [Google Scholar]
- 12.Rohan RM, Untermann TG, Liu L, Hise MK. Am J Phyiol. 1997;272:F661–F667. doi: 10.1152/ajprenal.1997.272.5.F661. [DOI] [PubMed] [Google Scholar]
- 13.Krishna MC, Grahame DA, Samuni A, Mitchell JB, Russo A. Proc Natl Acad Sci USA. 1992;89:5537–5541. doi: 10.1073/pnas.89.12.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korbmacher C, Segal AS, Fejes-Tóth G, Giebisch G, Boulpaep EL. J Gen Physiol. 1993;102:761–793. doi: 10.1085/jgp.102.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoos BA, Carretero OA, Garvin JL. Am J Physiol. 1992;263:F1–F6. doi: 10.1152/ajprenal.1992.263.1.F1. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Xu LS, Wang ZQ, Wang KS, Li N, Cheng ZH, Huang SZ, Wei DZ, Han ZG. FEBS Lett. 2004;570:7–12. doi: 10.1016/j.febslet.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Coscoy L, Ganem D. Trends Cell Biol. 2003;13:7–12. doi: 10.1016/s0962-8924(02)00005-3. [DOI] [PubMed] [Google Scholar]
- 18.Gozani O, Karuman P, Jones DR. Cell. 2003;114:99–111. doi: 10.1016/s0092-8674(03)00480-x. [DOI] [PubMed] [Google Scholar]
- 19.Ozer A, Wu LC, Bruick RK. Proc Natl Acad Sci USA. 2005;102:7481–7486. doi: 10.1073/pnas.0502716102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giebisch G. Am J Physiol. 1998;274:F817–F833. doi: 10.1152/ajprenal.1998.274.5.F817. [DOI] [PubMed] [Google Scholar]
- 21.Muto S, Sansom S, Giebisch G. J Clin Invest. 1988;81:376–380. doi: 10.1172/JCI113329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muto S, Giebisch G, Sansom S. Am J Physiol. 1987;253:F742–F752. doi: 10.1152/ajprenal.1987.253.4.F742. [DOI] [PubMed] [Google Scholar]
- 23.Wang W. Annu Rev Med. 2003;66:547–569. [Google Scholar]
- 24.Palmer LG, Antonian L, Frindt G. J Gen Physiol. 1994;104:693–710. doi: 10.1085/jgp.104.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chu PY, Quigley R, Babich V, Huang CL. Am J Physiol. 2003;285:F1179–F1187. doi: 10.1152/ajprenal.00150.2003. [DOI] [PubMed] [Google Scholar]
- 26.Lin DH, Sterling H, Yang B, Hebert SC, Giebisch G, Wang WH. Am J Physiol. 2004;286:F881–F892. doi: 10.1152/ajprenal.00301.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X, Wang KS, Wang ZQ, Xu LS, Wang QW, Chen F, Wei DZ, Han ZG. Biochem Biophys Res Commun. 2005;331:1032–1038. doi: 10.1016/j.bbrc.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 28.Unoki M, Shen JC, Zheng ZM, Harris CC. J Biol Chem. 2006;281:34677–34686. doi: 10.1074/jbc.M606296200. [DOI] [PubMed] [Google Scholar]
- 29.Boim MA, Ho K, Shuck ME, Bienkowski MJ, Block JH, Slightom JL, Yang Y, Brenner BM, Hebert SC. Am J Physiol. 1995;268:F1132–F1140. doi: 10.1152/ajprenal.1995.268.6.F1132. [DOI] [PubMed] [Google Scholar]
- 30.Lu M, Wang T, Yan Q, Wang W, Giebisch G, Hebert SC. Am J Physiol. 2004;286:F490–F495. doi: 10.1152/ajprenal.00305.2003. [DOI] [PubMed] [Google Scholar]
- 31.Lu M, Wang T, Yan Q, Yang X, Dong K, Knepper MA, Wang WH, Giebisch G, Shull GE, Hebert SC. J Biol Chem. 2002;277:37881–37887. doi: 10.1074/jbc.M206644200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu RM, Wei Y, Jiang HL, Lin DH, Sterling H, Bloom P, Balazy M, Wang WH. J Gen Physiol. 2002;119:33–44. doi: 10.1085/jgp.119.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Macica CM, Yang YH, Hebert SC, Wang WH. Am J Physiol. 1996;271:F588–F594. doi: 10.1152/ajprenal.1996.271.3.F588. [DOI] [PubMed] [Google Scholar]
- 34.Moral Z, Deng K, Wei Y, Sterling H, Deng H, Ali S, Gu RM, Huang XY, Hebert SC, Giebisch G, et al. J Biol Chem. 2001;276:7156–7163. doi: 10.1074/jbc.M008671200. [DOI] [PMC free article] [PubMed] [Google Scholar]