Abstract
The early life environment has long-term implications for the risk of developing cardiovascular (CV) disease in adulthood. Fetal responses to changes in maternal nutrition may be of immediate benefit to the fetus, but the long-term effects of these adaptations may prove detrimental if nutrition in postnatal life does not match that predicted by the fetus on the basis of its prenatal environment. We tested this predictive adaptive response hypothesis with respect to CV function in sheep. We observed that a mismatch between pre- and postnatal nutrient environments induced an altered CV function in adult male sheep that was not seen when environments were similar. Sheep that received postnatal undernutrition alone had altered growth, CV function, and basal hypothalamo–pituitary–adrenal axis activity in adulthood. Prenatal undernutrition induced greater weight gain by weaning compared with the prenatal control diet, which may provide a reserve in the face of a predicted poor diet in later life. In an adequate postnatal nutrient environment (i.e., relatively mismatched), these offspring exhibited cardiac hypertrophy and altered CV function in adulthood. These data support the concept that adult CV function can be determined by developmental responses to intrauterine nutrition made in expectation of the postnatal nutritional environment, and that if these predictions are not met, the adult may be maladapted and at greater risk of CV disease. Our findings have substantial implications for devising strategies to reduce the impact of a mismatch in nutrition levels in humans undergoing rapid socio-economic transitions in both developing and developed societies.
Keywords: fetal development, postnatal development, predictive adaptive response
Epidemiological studies have shown that the environment in early life may have long-term effects on the risk of adult-onset diseases such as hypertension and coronary heart disease (reviewed in ref. 1). Studies of adults who were in utero at the time of the Dutch Famine (November 1944 to May 1945) provide direct evidence that maternal undernutrition during early gestation, when the nutrient demands of the conceptus are minimal, leads to increased incidence of coronary heart disease in adult offspring (2). Animal studies support this observation; for example, in sheep, maternal undernutrition in the period after conception influences cardiovascular (CV) function in late gestation in the absence of any changes in birth weight (3, 4). An association between perturbations of the peri-implantation environment and altered postnatal CV function has been confirmed in both rats (5) and sheep (6). The pattern of postnatal growth may also influence later health, as studies in humans have shown that reduced size at birth and accelerated childhood growth confer an increased risk of CV disease in adulthood (7, 8).
Recently, it has been suggested that although responding to changes in maternal nutrition may be of immediate benefit to the fetus, the long-term effects of these adaptations may prove detrimental if nutrition in postnatal life does not match that predicted by the fetus on the basis of its prenatal environment (1). Thus, the biological response to aspects of the prenatal environment conveys fitness in a similar postnatal environment, and such prenatal responses may have arisen by selection for optimal postnatal fitness (9). Such responses are not only manifest in terms of prenatal survival but also reproductive success. Such “predictive adaptive responses” (PARs) are similar to the environmentally induced phenotypes in invertebrates such as the gregarious vs. solitary forms in locusts (10), queens vs. workers in honey bees (11), and seasonal polyphenisms in moths (12). Mammalian examples include seasonal changes in coat thickness in meadow voles (13). If the developmental prediction is incorrect, e.g., a mismatch between pre- and postnatal environment occurs, the phenotype induced by PARs may be poorly adapted to the postnatal environment and result in decreased fitness. Thus in humans, PARs made in response to a suboptimal intrauterine nutrient environment may be inappropriate if a substantial increase in postnatal nutrition arises after economic development or migration. PARs may therefore play a role in the rising incidence of CV and metabolic disease in developing countries. Indeed, Indian children who were small at birth but heavy at 8 years of age, indicating changing nutritional status, have increased risk factors for CV disease, including insulin resistance and increased plasma LDL cholesterol (14). The PARs concept may also apply to placental insufficiency, with or without fetal growth restriction, as opposed to maternal dietary deficiency, when the fetus experiences nutrient deprivation and prepares mistakenly for a life of dietary deprivation. Such mismatches between the pre- and postnatal nutrient environment might, by virtue of inappropriate PARs, therefore underlie the increasing prevalence of CV dysfunction in adulthood in both developed and developing countries.
To investigate the PARs hypothesis in a species with a developmental trajectory comparable to humans, we manipulated separately the pre- and postnatal nutrient environment of sheep during periods equivalent to those identified as critical in determining human CV health. Hypertension associated with low birth weight in rats is predominantly in male offspring (15), and in both rats and humans it is well established that hypertension with renal failure is more likely to occur in males (16), thus we only used male offspring in this study.
Results and Discussion
Ewe and Preweaning Lamb Weight in Response to Early Gestation Undernutrition.
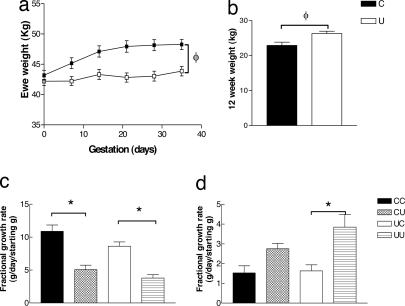
In studies of the famine of the Dutch Hunger Winter, early gestation undernutrition is associated with increased prevalence of CV disease (2); therefore, in this study, ewes received either 100% (C) or 50% (U) of total nutrient intake over the first 31 of 147 days of gestation. During the first 31 days of gestation, U ewes gained less weight than C ewes (Fig. 1a). This reduction in weight gain is comparable to that in normal human pregnancies in which there is a mild reduction in calorific intake, rather than that of severe starvation as in the Dutch Hunger Winter (17). Such a reduction in weight gain during pregnancy is also seen in adolescent pregnancies (18) and when high physical activity is carried out while pregnant (19). The nutritional challenge had no effect on their offspring in terms of birth weight (3.89 ± 0.17 vs. 3.62 ± 0.18 kg) or biometry (data not shown), but U lambs showed greater preweaning growth than C lambs and were heavier at 12 weeks (Fig. 1b). After birth, the composition and quantity of the milk consumed by the lamb will determine early postnatal growth rate (20, 21), although this was not examined in this study. The lambs could also have altered metabolism or appetite, as studies in rat have shown that undernutrition during fetal life leads to a preference for high-fat foods in the offspring (22) and changes in the hypothalamic regulation of food intake (23).
Fig. 1.
Size and growth of ewes and male offspring. (a) Weight between 0 and 35 days of gestation in C (n = 21) and U (n = 26) ewes. (b) Weight at 12 weeks of age in C (n = 24) and U (n = 28) lambs. Fractional growth rate at 12–25 weeks (c) and 25–35 weeks (d) in CC (n = 14), CU (n = 10), UC (n = 14), and UU (n = 14) lambs. φ, P < 0.05, early gestation nutrient-restricted group significantly different from early gestation control group; ∗, P < 0.05, postnatal nutrient-restricted group significantly different from postnatal control group (by two-way ANOVA). Values are mean ± SEM.
Postweaning Lamb Growth After Pre- and Postnatal Undernutrition.
Epidemiological data indicate that early postnatal growth is associated with altered adult CV function (24). We therefore subdivided both C and U groups to receive postnatal nutrient restriction (a level that reduced body weight to 85% of individual target weight predicted from the 0–12 week growth trajectory) (CU and UU) or adequate nutrition (CC and UC) between 12 and 25 weeks of age (immediately postweaning). The nutrient restriction produced a clear reduction in fractional growth rate during this period (Fig. 1c). After the postnatal nutrient challenge (25–35 weeks), UU had a greater fractional growth rate than UC, but CU did not have a significantly greater fractional growth rate than CC (Fig. 1d). Thus, early gestation undernutrition enhanced both early postnatal growth rate (i.e., 0–12 weeks) and recovery from a period of postnatal undernutrition. These findings indicate that these animals develop strategies aimed at protection of body weight from an anticipated period of undernutrition.
CV Dysfunction in Adult Sheep at 1.5 Years of Age After Mismatch of Pre- and Postnatal Nutrition.
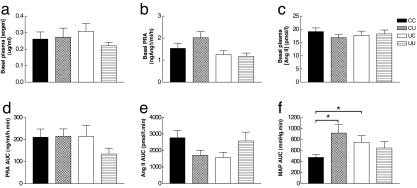
We then assessed CV function in the 1.5-year-old lambs, focusing on the responsiveness of the renin–angiotensin system (RAS) because previous studies have implicated the RAS as a candidate mechanism linking reduced intrauterine nutrition to altered CV function in adulthood (25). The loop diuretic frusemide was used to stimulate the RAS via reduced distal tubular sodium and volume, leading to increased renin and angiotensin II (Ang II) release (26). An initial increase in blood pressure was observed followed by a gradual decrease in blood pressure due to volume depletion. Both nutritionally mismatched groups (UC and CU), but not the matched group (UU), had an increased blood pressure response to frusemide compared with CC (Fig. 2f). The increased blood pressure response to frusemide was not associated with altered basal plasma angiotensinogen (Fig. 2a), plasma renin activity (Fig. 2b), and Ang II (Fig. 2c), nor with an altered plasma renin activity (Fig. 2d) and plasma Ang II (Fig. 2e) response to frusemide. Also, the baroreflex was unaltered by either of the nutritional challenges (unpublished observations). The results therefore suggest either a greater vascular responsiveness to Ang II or an altered blood pressure decrease in response to volume depletion.
Fig. 2.
RAS function in 1.5-year-old male sheep. Basal plasma angiotensinogen (aogen; a), plasma renin activity (PRA; b), and Ang II (c) levels are shown. PRA (d), Ang II (e), and MAP area under the curve (AUC; f) in response to frusemide in CC (n = 14), CU (n = 10), UC (n = 14), and UU (n = 14) lambs are shown. ∗, P < 0.05, significantly different from CC (by three-way ANOVA). Values are mean ± SEM.
CV/Renal Dysfunction in Adult Sheep at 2.5 Years of Age After Postnatal Undernutrition.
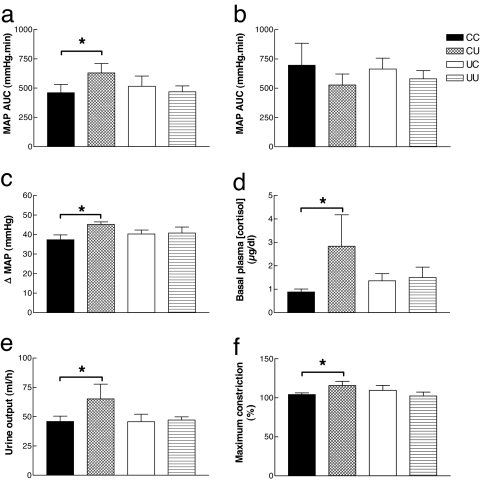
In the same animals at 2.5 years old, we found a similar increased blood pressure response to frusemide in CU (Fig. 3a), but the effect had disappeared in UC. CU also had an increased overnight urine output (Fig. 3e), with no change in sodium concentration (data not shown) after the challenge, suggesting a prolonged response to frusemide. Vascular reactivity was investigated ex vivo in the renal artery by using myography (27). We found that postnatal undernutrition increased the contractile response to phenylephrine in sheep that received prenatal control nutrition (CU vs. CC) but not in those that received prenatal undernutrition (UU vs. UC, Fig. 3f). Also, an increased blood pressure response to a bolus of Ang II was observed in postnatally undernourished sheep with a control prenatal diet (CU vs. CC) but not those with a restricted prenatal diet (UU vs. UC, Fig. 3c). The enhancement of the blood pressure response to frusemide and the exaggerated diuresis was blocked by prior administration of the angiotensin-converting enzyme inhibitor captopril (Fig. 3b). Because captopril blocks Ang II production, this finding suggests that the postnatal nutrient restriction affects the Ang II component of the frusemide response rather than the diuresis component. Cortisol is known to up-regulate ovine arterial blood pressure responses to Ang II (28), and we found that CU vs. CC, but not UU vs. UC, had increased basal plasma cortisol (Fig. 3d). Thus, altered hypothalamo–pituitary–adrenal axis activity may underlie the observed phenotypic changes (29). For all parameters there was no statistical difference between groups CC and UU.
Fig. 3.
CV/renal dysfunction in 2.5-year-old male sheep are absent when the mismatch of pre- and postnatal nutrition is minimized. MAP area under the curve (AUC) in response to frusemide after saline (a) or captopril (b) infusion. (c) MAP response to Ang II. Also shown are basal plasma cortisol (d) and overnight urine output (e) after frusemide administration in CC (n = 14), CU (n = 10), UC (n = 14), and UU (n = 14) lambs. (f) Maximum constriction to phenylephrine in the renal artery; CC (n = 8), CU (n = 7), UC (n = 4), UU (n = 7). ∗, P < 0.05, significantly different from CC (by three-way ANOVA). Values are mean ± SEM.
CV Dysfunction in Adult Sheep at 2.5 Years of Age After Prenatal Undernutrition.
Early gestation nutrient restriction thus appears to confer advantageous phenotypic changes if the animal is faced with a poor postnatal environment. However, if the postnatal environment is mismatched, a disadvantageous phenotype may be observed. Consistent with this observation are findings that in 2.5-year-old sheep, prenatal undernutrition with a control postnatal diet resulted in increased interventricular septal wall thickness (Fig. 4b) and increased mean left ventricular wall thickness (UC, 10.4 ± 0.5 mm vs. CC, 8.5 ± 0.4 mm). However, these effects were not observed after prenatal undernutrition combined with postnatal undernutrition (UU vs. CU). Although we observed no difference between groups in basal blood pressure at 2.5 years of age (CC, 90.4 ± 1.5 mmHg; CU, 87.5 ± 3.5 mmHg; UC, 94.4 ± 1.8 mmHg; UU, 90.8 ± 2.0 mmHg), the altered cardiac wall thickness could be a precursor of longer-term CV dysfunction, because in both sheep (30) and humans (31), left ventricular hypertrophy is associated with hypertension. For all of the above parameters, there was no statistical difference between groups CC and UU. Compared with control lambs (CC), prenatal undernutrition alone (UC), but not when combined with postnatal undernutrition (UU), resulted in increased basal tone and sensitivity to phenylephrine in the left internal thoracic artery (32) and increased constriction (pEC50) to acetylcholine in isolated coronary arteries at 2.5 years of age (Fig. 4d). These observations may indicate longer-term CV dysfunction such as hypertension in UC sheep. Indeed, in the male offspring of protein-restricted pregnant rats (33, 34), altered vascular reactivity is associated with hypertension. A number of potential cellular signaling changes could be hypothesized to underlie the altered vascular reactivity. To investigate this possibility, we focused on myosin light chain kinase (MLCK), a key component of smooth muscle signaling pathways, and acetylcholine receptors (M3) on smooth muscle cells (35). We found that MLCK mRNA levels in the coronary artery were increased in UC, but not UU, compared with CC (Fig. 4c), whereas M3 mRNA levels were unchanged (data not shown). Insufficient PCR and myography data were obtained from CU animals because of difficulty in the dissection of such small vessels. Increased MLCK activity is associated with proliferation and migration of smooth muscle cells (36), and in the coronary artery the resultant wall thickening is associated with increased arterial stiffness (37), which therefore affects vascular function. Increased MLCK is associated with increased fibrogenesis in the smooth muscle (38) and in the present study is consistent with the increased growth rate in response to the early gestation undernutrition. Postnatal undernutrition, which stalls the accelerated postnatal growth induced by early gestation undernutrition (UU), appears to prevent these outcomes, indicating that matching of pre- and postnatal nutrition is beneficial whereas a mismatch is detrimental to long-term CV function.
Fig. 4.
Altered cardiac morphology and coronary function in male adult sheep are absent when the mismatch of pre- and postnatal nutrition is minimized. (a) An echocardiograph showing the right ventricle (RV), interventricular septum (IVS), left ventricle (LV), and left ventricular wall (LVW) of the ovine heart. (b) Thickness of the intraventricular septum; CC (n = 14), CU (n = 10), UC (n = 14), and UU (n = 14). Also shown are MLCK relative mRNA expression in the coronary artery of male sheep as measured by real-time PCR [CC (n = 7), UC (n = 7), and UU (n = 4)] (c) and vascular response to acetylcholine in the coronary artery [CC (n = 10), UC (n = 9), and UU (n = 7)] (d). ∗, P < 0.05, significantly different from CC (by one-way ANOVA). Values are mean ± SEM. Insufficient PCR and myography data were obtained from CU animals.
Conclusion
We have shown that modest nutrient restriction in early gestation produces phenotypic changes in the offspring of a species comparable to humans in terms of maturity at birth. These effects occur without changes in birth weight. They could constitute a postnatal survival strategy by enhancing postnatal growth and also a predictive response to promote growth recovery after an anticipated postnatal nutritional challenge. If the prenatal prediction is not reflected in the postnatal environment, left ventricular hypertrophy, increased coronary artery vascular reactivity, and MLCK mRNA expression are induced in adult life. Conversely, the effects of postnatal undernutrition (increased vasoconstrictor responsiveness and urine output in response to frusemide and elevated basal plasma cortisol) are prevented by prior early gestation undernutrition.
Our data are consistent with the PARs concept (9). However, the PAR induced may not be complete because the responses of the two matched groups (CC and UU) are not always identical. Although we cannot exclude the possibility that the responses can be explained simply as an inappropriate developmental outcome of an appropriate response to an adverse insult in early pregnancy, there were no obvious signs of disruption of development. Our concept of nutritionally induced PARs is supported by other animal studies in which the coronary atherosclerotic (39) or endothelial function (40) effects of a high-fat diet were prevented by prior feeding of a similar diet to the pregnant mother and those in which reduced longevity after a postnatal cafeteria diet was prevented if growth was restricted by nutritional restriction at suckling (41).
The biological response to poor prenatal nutrition induces a phenotype best suited to a similar poor postnatal nutrition. Poor prenatal nutrition followed by adequate postnatal nutrition or adequate prenatal nutrition followed by poor postnatal nutrition (mismatch) leads to adult phenotypes similar to those in human CV and metabolic disease, such as endothelial dysfunction and cardiac hypertrophy, and altered vascular tone, blood pressure control and renal function, and weight gain. This concept may be particularly important in populations in which the mismatch of pre- and postnatal nutritional environments is exaggerated from generation to generation by a rapid socio-economic transition. A nutritional mismatch may also occur in Western society when maternal dietary intake during pregnancy does not meet the energy demands of the conceptus because of high physical activity or dieting prior or during the early stages of pregnancy, or in adolescent pregnancies. Alternatively, the fetal prediction of postnatal environment may be inappropriate because of maternal or placental disease or the greater maternal constraint associated with small stature or primiparous pregnancy. Lastly, neonatal conditions such as feeding high-fat and -calorie infant formula or weaning onto inappropriate foods can exacerbate the mismatch between developmental prediction and later nutrition.
Although the role of environmental mismatch in producing pathophysiology is supported by experimental data, it is now important to elucidate the mechanisms underlying such long-term physiological changes. Evidence is emerging of a role for epigenetic changes to DNA, affecting the expression of both imprinted and nonimprinted genes and induced by nutritional or hormonal factors (42, 43). Indeed, feeding a reduced-protein diet to pregnant rats induces elevated blood pressure and endothelial dysfunction in the offspring and is associated with permanently increased expression in the liver and heart of genes such as the glucocorticoid receptor (GR) and PPARα due to hypomethylation of their respective promoters and associated changes in histone acetylation and methylation (44). The identification of markers for such phenotypic changes in early life will be important for interventions aimed at reducing the substantial global burden of CV morbidity and metabolic disease.
Methods
Experimental Model.
Welsh Mountain ewes (U.K. Animals Scientific Procedures Act 1986) in their second or third parity received 100% (C, n = 21) or 50% (U, n = 26) of total nutrient requirements between days 1 and 31 of gestation and 100% thereafter. The diet consisted of barley, wheat, micronized full-fat soya, grass meal, molasses, chopped straw, calcium carbonate, dicalcium phosphate salt, and sheep vitamin/mineral supplement. As fed, the diet provided 9.6 MJ/kg (metabolizable energy) and 14.75 g of crude protein. Ewes delivered and suckled their lambs naturally until weaning at 12 weeks of age (C, n = 24 [9 single (s), 15 twin (t)] and U, n = 28 [14 s, 14 t] lambs). All lambs were weighed at birth and at 12 weeks of age. Offspring were fed ad libitum [CC, n = 14 (6 s, 8 t) and UC, n = 14 (7 s, 7 t)] or at a level that reduced body weight to 85% of individual target weight (predicted from the 0–12 week growth trajectory) from 12 to 25 weeks postnatal age and ad libitum thereafter [CU, n = 10 (3 s, 7 t) and UU, n = 14 (7 s, 7 t)]. All lambs received 100% of nutritional requirements from 25 weeks of age onwards. The diet consisted of free access to water and hay, and creep pellets (Prestige Lamb Pellets + Decox; BOCM Pauls Ltd., Loughborough, U.K.) were provided each morning and afternoon. As fed, creep pellets provided 10.51 MJ/kg (metabolizable energy) and 18% crude protein. At ≈32 weeks of age, lambs were transferred onto a standard ration of an adult complete pelleted diet (Ewbol 18; BOCM Pauls Ltd.) plus ad libitum hay.
Surgical Procedures.
At 9.9 ± 0.1 months of age, all lambs were vasectomized, and carotid artery loops (externalization of artery within a flap of skin) were created under general anesthesia (3% halothane/O2) to allow temporary catheters to be implanted easily at a later date. At 1.5 (16.5 ± 0.1 months) and 2.5 (29.6 ± 0.2 months) years of age, catheters were inserted into the carotid artery and jugular vein under general anesthesia (3% halothane/O2) using sterile techniques.
Experimental Procedures.
RAS function was assessed at 1.5 and 2.5 years of age using frusemide (5 mg/kg i.v. bolus) and at 2.5 years using captopril (500 μg/kg per h i.v. infusion), angiotensin I (0.05 μg/kg i.v. bolus), and Ang II (0.05 μg/kg i.v. bolus). Mean arterial blood pressure (MAP) was monitored via the carotid artery catheter by using a physiological pressure transducer. At 2.5 years, cardiac morphology and left ventricular function was determined by transthoracic echocardiography under general anesthesia (3% halothane/O2).
Hormone Analysis.
Basal plasma renin and angiotensinogen were measured in duplicate using RIA as described (45). Plasma Ang II was measured in duplicate using a sensitive and specific competitive protein-binding RIA (46). Cortisol was measured (single measurement) in EDTA plasma by using an Immulite analyzer (DPC, Llanberis, U.K.).
Isolated Vascular Assessment.
Vessels were dissected clean of connective tissue, and 2-mm segments were mounted on the Mulvany–Halpern wire myograph (27). Concentration–response curves to acetylcholine (1 nM to 1 mM) in the left anterior interventricular artery and phenylephrine (10 nM to 100 μM) in the right renal artery were carried out as described (47).
Molecular Biology.
Total RNA was extracted from the ovine left anterior interventricular coronary artery by using TRIzol (Sigma, Poole, U.K.) and was reverse transcribed into cDNA. Muscarinic M3 receptor and MLCK mRNA levels were analyzed relative to 18S ribosomal RNA (Applied Biosystems, Warrington, U.K.) by using real-time PCR (48) (Taqman; Applied Biosystems ABL Prism 7700 Sequence Detection System). Coefficient of variation was <15%. Using a geNorm normalizing kit (Primer Design, Southampton, U.K.), we established that 18S is one of the most stable genes with a normalization factor of 0.82 (this must be <1.5) (Hollis, Anthony, M.A.H, and L.R.G, unpublished observations).
Data Analysis.
Data are expressed as mean ± SEM, and a significant difference was accepted at P < 0.05. Vascular contraction in response to phenylephrine or acetylcholine was expressed as percentage of the maximum contraction in response to physiological saline solution with equimolar substitution of K+ for Na+ (125 mM). The pEC50 was calculated by using Prism (Graphpad Software Inc., San Diego, CA) and compared by one-way analysis of variance. For each frusemide experiment, the area under the MAP response curve was calculated (−30 to 120 min). For each Ang II experiment, the maximum MAP response was calculated. Growth and blood pressure data were analyzed by using multifactorial analyses of variance (three-way ANOVA), which tested the effects of prenatal diet, postnatal diet, and number of offspring per pregnancy. Where significant effects of any factor or a significant interaction were found, further analyses were performed having split the data by that factor/s. Statistical analyses were performed by using SPSS version 8 (SPSS, Chicago, IL). Unpaired Student's t test was used to identify differences between two factors. Statistical tests were subject to Bonferroni multiple-comparison correction where appropriate.
Acknowledgments
We thank F. Broughton Pipkin, H. McGarrigle, and R. Henke for biochemical analysis. We are grateful to F. Anthony and P. Boels for advice on the molecular and myography experiments. We thank S. Ohri and J. M. Morgan for supporting the research fellows in this project. This work was supported by The British Heart Foundation.
Abbreviations
- CV
cardiovascular
- PAR
predictive adaptive response
- C
control
- U
undernutrition
- RAS
renin–angiotensin system
- Ang II
angiotensin II
- MLCK
myosin light chain kinase
- MAP
mean arterial blood pressure.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Gluckman PD, Hanson MA. Science. 2004;305:1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- 2.Roseboom TJ, van der Meulen JH, Osmond C, Barker DJ, Ravelli AC, Schroeder-Tanka JM, van Montfrans GA, Michels RP, Bleker OP. Heart. 2000;84:595–598. doi: 10.1136/heart.84.6.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hawkins P, Steyn C, Ozaki T, Saito T, Noakes DE, Hanson MA. Am J Physiol Regul Integr Comp Physiol. 2000;279:R340–R348. doi: 10.1152/ajpregu.2000.279.1.R340. [DOI] [PubMed] [Google Scholar]
- 4.Ozaki T, Hawkins P, Nishina H, Steyn C, Poston L, Hanson MA. Am J Obstet Gynecol. 2000;183:1301–1307. doi: 10.1067/mob.2000.107463. [DOI] [PubMed] [Google Scholar]
- 5.Kwong WY, Wild AE, Roberts P, Willis AC, Fleming TP. Development (Cambridge, UK) 2000;127:4195–4202. doi: 10.1242/dev.127.19.4195. [DOI] [PubMed] [Google Scholar]
- 6.Gardner DS, Pearce S, Dandrea J, Walker R, Ramsay MM, Stephenson T, Symonds ME. Hypertension. 2004;43:1290–1296. doi: 10.1161/01.HYP.0000126991.67203.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eriksson JG, Forsen T, Tuomilehto J, Osmond C, Barker DJ. BMJ. 2001;322:949–953. doi: 10.1136/bmj.322.7292.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singhal A, Cole TJ, Fewtrell M, Deanfield J, Lucas A. Circulation. 2004;109:1108–1113. doi: 10.1161/01.CIR.0000118500.23649.DF. [DOI] [PubMed] [Google Scholar]
- 9.Gluckman PD, Hanson MA. Pediatr Res. 2004;56:311–317. doi: 10.1203/01.PDR.0000135998.08025.FB. [DOI] [PubMed] [Google Scholar]
- 10.Applebaum SW, Heifetz Y. Annu Rev Entomol. 1999;44:317–341. doi: 10.1146/annurev.ento.44.1.317. [DOI] [PubMed] [Google Scholar]
- 11.Elekonich MM, Jez K, Ross AJ, Robinson GE. J Insect Physiol. 2003;49:359–366. doi: 10.1016/s0022-1910(03)00020-9. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki Y, Nijhout HF. Science. 2006;311:650–652. doi: 10.1126/science.1118888. [DOI] [PubMed] [Google Scholar]
- 13.Lee TM, Zucker I. Am J Physiol. 1988;255:R831–R838. doi: 10.1152/ajpregu.1988.255.5.R831. [DOI] [PubMed] [Google Scholar]
- 14.Bavdekar A, Yajnik CS, Fall CH, Bapat S, Pandit AN, Deshpande V, Bhave S, Kellingray SD, Joglekar C. Diabetes. 1999;48:2422–2429. doi: 10.2337/diabetes.48.12.2422. [DOI] [PubMed] [Google Scholar]
- 15.Manning J, Vehaskari VM. Pediatr Nephrol. 2001;16:417–422. doi: 10.1007/s004670000560. [DOI] [PubMed] [Google Scholar]
- 16.Baylis C. J Clin Invest. 1994;94:1823–1829. doi: 10.1172/JCI117531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roseboom TJ, van der Meulen JH, van Montfrans GA, Ravelli AC, Osmond C, Barker DJ, Bleker OP. J Hypertens. 2001;19:29–34. doi: 10.1097/00004872-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Nielsen JN, Gittelsohn J, Anliker J, O'Brien K. J Am Diet Assoc. 2006;106:1825–1840. doi: 10.1016/j.jada.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Giroux I, Inglis SD, Lander S, Gerrie S, Mottola MF. Appl Physiol Nutr Metab. 2006;31:483–489. doi: 10.1139/h06-024. [DOI] [PubMed] [Google Scholar]
- 20.Lawlor MJ, Hopkins SP. Br J Nutr. 1981;45:579–586. doi: 10.1079/bjn19810136. [DOI] [PubMed] [Google Scholar]
- 21.McKusick BC, Thomas DL, Berger YM. J Dairy Sci. 2001;84:1660–1668. doi: 10.3168/jds.S0022-0302(01)74601-2. [DOI] [PubMed] [Google Scholar]
- 22.Bellinger L, Lilley C, Langley-Evans SC. Br J Nutr. 2004;92:513–520. doi: 10.1079/bjn20041224. [DOI] [PubMed] [Google Scholar]
- 23.Zippel U, Plagemann A, Davidowa H. Behav Brain Res. 2003;147:89–94. doi: 10.1016/s0166-4328(03)00140-2. [DOI] [PubMed] [Google Scholar]
- 24.Barker DJ, Osmond C, Forsen TJ, Kajantie E, Eriksson JG. N Engl J Med. 2005;353:1802–1809. doi: 10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- 25.Langley-Evans SC, Sherman RC, Welham SJ, Nwagwu MO, Gardner DS, Jackson AA. Biochem Soc Trans. 1999;27:88–93. doi: 10.1042/bst0270088. [DOI] [PubMed] [Google Scholar]
- 26.Patel A, Smith FG. Can J Physiol Pharmacol. 1997;75:1101–1107. [PubMed] [Google Scholar]
- 27.Mulvany MJ, Halpern W. Circ Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- 28.Tangalakis K, Lumbers ER, Moritz KM, Towstoless MK, Wintour EM. Exp Physiol. 1992;77:709–717. doi: 10.1113/expphysiol.1992.sp003637. [DOI] [PubMed] [Google Scholar]
- 29.Phillips DI. Clin Exp Pharmacol Physiol. 2001;28:967–970. doi: 10.1046/j.1440-1681.2001.03558.x. [DOI] [PubMed] [Google Scholar]
- 30.Dodic M, Samuel C, Moritz K, Wintour EM, Morgan J, Grigg L, Wong J. Circ Res. 2001;89:623–629. doi: 10.1161/hh1901.097086. [DOI] [PubMed] [Google Scholar]
- 31.Berenji K, Drazner MH, Rothermel BA, Hill JA. Am J Physiol Heart Circ Physiol. 2005;289:H8–H16. doi: 10.1152/ajpheart.01303.2004. [DOI] [PubMed] [Google Scholar]
- 32.Khan OA, Torrens C, Noakes DE, Poston L, Hanson MA, Green LR, Ohri SK. Eur J Cardiothorac Surg. 2005;28:811–815. doi: 10.1016/j.ejcts.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 33.Brawley L, Itoh S, Torrens C, Barker A, Bertram C, Poston L, Hanson M. Pediatr Res. 2003;54:83–90. doi: 10.1203/01.PDR.0000065731.00639.02. [DOI] [PubMed] [Google Scholar]
- 34.Torrens C, Brawley L, Anthony FW, Dance CS, Dunn R, Jackson AA, Poston L, Hanson MA. Hypertension. 2006;47:982–987. doi: 10.1161/01.HYP.0000215580.43711.d1. [DOI] [PubMed] [Google Scholar]
- 35.Simonsen U, Prieto D, Rivera L, Hernandez M, Mulvany MJ, Garcia-Sacristan A. Br J Pharmacol. 1993;109:998–1007. doi: 10.1111/j.1476-5381.1993.tb13720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishiguchi F, Fukui R, Hoshiga M, Negoro N, Ii M, Nakakohji T, Kohbayashi E, Ishihara T, Hanafusa T. Atherosclerosis. 2003;171:39–47. doi: 10.1016/j.atherosclerosis.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 37.Vaitkevicius PV, Fleg JL, Engel JH, O'Connor FC, Wright JG, Lakatta LE, Yin FC, Lakatta EG. Circulation. 1993;88:1456–1462. doi: 10.1161/01.cir.88.4.1456. [DOI] [PubMed] [Google Scholar]
- 38.Borisov AB, Raeker MO, Kontrogianni-Konstantopoulos A, Yang K, Kurnit DM, Bloch RJ, Russell MW. Biochem Biophys Res Commun. 2003;310:910–918. doi: 10.1016/j.bbrc.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 39.Norman JF, LeVeen RF. Atherosclerosis. 2001;157:41–47. doi: 10.1016/s0021-9150(00)00668-7. [DOI] [PubMed] [Google Scholar]
- 40.Khan I, Dekou V, Hanson M, Poston L, Taylor P. Circulation. 2004;110:1097–1102. doi: 10.1161/01.CIR.0000139843.05436.A0. [DOI] [PubMed] [Google Scholar]
- 41.Ozanne SE, Hales CN. Nature. 2004;427:411–412. doi: 10.1038/427411b. [DOI] [PubMed] [Google Scholar]
- 42.Waterland RA, Jirtle RL. Nutrition. 2004;20:63–68. doi: 10.1016/j.nut.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 43.Drake AJ, Walker BR. J Endocrinol. 2004;180:1–16. doi: 10.1677/joe.0.1800001. [DOI] [PubMed] [Google Scholar]
- 44.Lillycrop KA, Phillips ES, Jackson AA, Hanson MA, Burdge GC. J Nutr. 2005;135:1382–1386. doi: 10.1093/jn/135.6.1382. [DOI] [PubMed] [Google Scholar]
- 45.Tetlow HJ, Broughton PF. Br J Obstet Gynaecol. 1983;90:220–226. doi: 10.1111/j.1471-0528.1983.tb08612.x. [DOI] [PubMed] [Google Scholar]
- 46.Green LR, McGarrigle HH, Bennet L, Hanson MA. J Physiol. 1998;507(Pt 3):857–867. doi: 10.1111/j.1469-7793.1998.857bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Torrens C, Brawley L, Barker AC, Itoh S, Poston L, Hanson MA. J Physiol. 2003;547:77–84. doi: 10.1113/jphysiol.2002.026120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heid CA, Stevens J, Livak KJ, Williams PM. Genome Res. 1996;6:986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]