Abstract
Recent evidence has shown that retinoic acid (RA) signalling is required for early pancreatic development in zebrafish and frog but its role in later development in mammals is less clear cut. In the present study, we determined the effects of RA on the differentiation of the mouse embryonic pancreas. Addition of all-trans retinoic acid (atRA) to embryonic pancreatic cultures induced a number of changes. Branching morphogenesis and exocrine differentiation were suppressed and there was premature formation of endocrine cell clusters (although the total area of β cells was not different in control and atRA-treated buds). We investigated the mechanism of these changes and found that the premature formation of β cells was associated with the early expression of high-level Pdx1 in the endocrine cell clusters. In contrast, the suppressive effect of RA on exocrine differentiation may be due to a combination of two mechanisms (i) up-regulation of the extracellular matrix component laminin and (ii) enhancement of apoptosis. We also demonstrate that addition of fibroblast growth factor (FGF)-10 is able to partially prevent apoptosis and rescue exocrine differentiation and branching morphogenesis in atRA-treated cultures but not in mice lacking the FGF receptor 2-IIIb, suggesting the effects of FGF-10 are mediated through this receptor.
Keywords: retinoic acid, fibroblast growth factor, pancreatic duodenal homeobox-1, pancreas
Introduction
The pancreas contains two distinct populations of cells referred to as endocrine and exocrine cells. The exocrine cells comprise the acinar cells that produce enzymes that aid in the digestion and absorption of food in the gut and the ductal cells that transport the enzymes from the pancreas to the intestine. Endocrine cells are found in the islets of Langerhans which are scattered throughout the exocrine pancreas. Islets contain five endocrine cell types: the insulin-producing β cells, glucagon-producing α cells, somatostatin-producing δ cells, pancreatic polypeptide—producing PP cells and a fifth endocrine cell type, which secretes the hormone ghrelin (Borglio et al., 2003; Murtaugh and Melton, 2003). These hormones function to regulate metabolism.
The three types of pancreatic cells (endocrine, exocrine, and ductal) are derived from dorsal and ventral primordia, which grow together to form the definitive pancreas (Slack, 1995; Kim and Hebrok, 2001). Recombination and lineage labelling studies have shown that both endocrine and exocrine cells arise from the same endodermal rudiment (Percival and Slack, 1999; Gu et al., 2002). Pancreatic morphogenesis requires epithelial–mesenchymal interactions for proper development (Gittes et al., 1996). The candidates for the tropic stimulus from the mesenchyme include follistatin and fibroblast growth factors (FGFs), at least for pancreatic exocrine development (Bhushan et al., 2001; Edlund, 2001).
Retinoids are members of the vitamin A family and are required for vertebrate development (Means and Gudas, 1995). During embryogenesis, retinoids are required for organogenesis of the central nervous system, lung, kidney, intestine, and pancreas (Plateroti et al., 1997; Mendelsohn et al., 1999; Malpel et al., 2000; Maden, 2002; Stafford and Prince, 2002; Chen et al., 2004). There are two isomers of retinoic acid (RA) of particular biological importance, all-trans retinoic acid (atRA) and 9-cis RA. The retinoid-X receptors (RXRs) exclusively bind the 9-cis isomer whereas the retinoic acid receptors (RARs) bind both the 9-cis and atRA isomers (Heyman et al., 1992; Allenby et al., 1993). The distribution of the RAR and RXR receptors has been examined in the developing mouse pancreas. RARα is localized to the pancreatic mesenchyme at E12 whereas RXRα is expressed in the epithelial ducts and acini after E15 (Kadison et al., 2001). Such differences in the expression of RARs suggests that the 9-cis and atRA isomers may exert different effects during development. Conflicting data exist regarding the relative affects of RA on exocrine and endocrine differentiation. This is partly related to the use of different species (e.g., mouse, rat, chick, and Xenopus) as well as differences in model systems (e.g., culture in collagen or Matrigel) and the use of varying concentrations of 9-cis RA or atRA (1 nM–5 μM). For example, addition of atRA was reported to increase the proportion of insulin cells in Xenopus and chick, but not in mouse (Penny and Kramer, 2000; Kadison et al., 2001; Kramer snd Penny, 2003; Chen et al., 2004).
In the present study, we show that addition of atRA to cultures of embryonic pancreas has distinct and separate effects on exocrine and endocrine differentiation: inhibiting branching morphogenesis and exocrine cell differentiation while accelerating endocrine differentiation. The suppressive effects on exocrine differentiation which can be rescued by FGF-10 pretreatment are probably mediated by up-regulation of laminins and inhibition of apoptosis. The effect on endocrine differentiation is probably due to the early appearance of high-level Pdx1 in endocrine cell clusters.
Materials and methods
Culture of embryonic mouse pancreatic buds
Embryonic pancreas were isolated and cultured as described previously (Percival and Slack, 1999; Horb and Slack, 2000; Shen et al., 2000; Shen et al., 2003b). The dorsal buds were isolated from E11.5 mouse embryos. Briefly, coverslips coated with fibronectin (50 μg/ml, Invitrogen) or laminin (50 μg/ml, Invitrogen, Paisley, Scotland, UK) were placed in 35 mm petri plates containing BME medium with Earle's salts, 20% fetal bovine serum and 50 μg/ml gentamycin (Life Technologies, Paisley, Scotland, UK). A stainless-steel ring of 3 mm internal diameter was placed over the fibronectin-coated area, and the pancreatic bud was dropped into the center. To ensure spreading during culture, the buds were turned if necessary with a fine needle so that the cut surface lay face down. Cultures were maintained for up to 7 days at 37°C with 5% CO2, with a change of medium every day. The stainless-steel ring was removed at the second day. Treatment of pancreatic cultures with atRA (100 nM–10 μM, Sigma, Poole, U.K.), activin A (10 μg/ml, R&D, Abingdon, UK), nicotinamide (5 mM, Sigma), FGF-10 (10 μg/ml, R&D), and Caspase inhibitor VI (40 μM, Calbiochem, Nottingham, UK) was performed from the third day. All treatments were applied to at least three to five explants from at least two to three litters of embryos. Typical results are shown in the figures.
Generation of FgfR2-IIIb (−/−) mice
The targeted disruption of FgfR2-IIIb, and the genotyping of offspring has been described previously (Revest et al., 2001). Mutant and wild-type littermates were collected at E11.5, and embryonic pancreas were isolated and cultured as described as above.
Insulin secretion assay
Pancreatic buds were grown in fibronectin-coated 96-well plates for 7 days in medium supplemented with or without 5 days' 1 μM atRA treatment. Each well contained three buds. The medium was removed, and cells were rinsed in 1 ml of Krebs-Ringer buffer (119 mM NaCl, 4.74 mM KCl, 2.54 mM CaCl2, 1.19 mM MgSO4, 1.19 mM KH2PO4, 25 mM NaHCO3, 10 mM HEPES, and 0.1% bovine serum albumin, pH 7.4), and then incubated in 60 μl Krebs-Ringer buffer containing 20 mM glucose for 2 hr. At the end of the incubation period, the buffer was removed, centrifuged briefly, and the supernatant was used for measuring insulin secretion. For the measurement of total protein content, cells were washed twice with PBS, lysed with buffer containing 20 mM HEPES (pH 7.6), 150 mM NaCl, 1 mM EDTA, 2 mM dithiothreitol (DTT), 1% Triton X-100, and 10 μg/ml protease inhibitors (leupeptin, aprotinin, and pepstatin), and then the protein concentration of whole-cell extracts was determined using the Bio-Rad, Hempsted, UK protein assay reagent. Measurement of insulin secretion was carried out with an insulin ELISA kit according to the manufacturer's instructions (Mercodia, Uppsala, Sweden). Statistical analyses were performed on triplicate samples using the Student paired t-test in Excel. Significance was set at p<0.05.
Immunofluorescence analysis
For immunofluorescent staining, the pancreatic buds were fixed in MEMFA (10% formaldehyde, 0.1 M Mops, pH 7.4, EGTA 2 mM, MgSO4 1 mM) for 30–45 min at room temperature or fixed in cold acetone/methanol for 10 min. They were washed three times with PBS and stored in PBS at 4°C for up to a few days. Before immunostaining, pancreatic buds were then permeabilized with 1% (vol/vol) Triton X-100 in PBS for 30 min, and incubated in 2% blocking buffer (Roche, Lewes, Sussex, UK) which contained 0.1% Triton X-100, then incubated sequentially with primary antibodies overnight at 4°C and secondary antibodies for 3 hr at room temperature. After incubation, buds were washed three times with PBS buffer, then mounted in Gelvatol medium (prepared by dissolving 20 g of polyvinyl alcohol, in 80 ml of 10 mM Tris [pH 8.6], and 3 g of n-propyl gallate in 50 ml glycerol followed by mixing and centrifugation at 7,000 × g to remove any undissolved particles). The antibodies were diluted and obtained as follows: rabbit polyclonal anti-amylase (1/300), guinea-pig polyclonal anti-insulin (1/300), mouse monoclonal anti-glucagon (1:100), mouse monoclonal anti-pan cytokeratin (1:300), mouse monoclonal anti-smooth muscle actin (1:200), and rabbit polyclonal anti-guinea pig IgG TRITC conjugate (1/300) all from Sigma Chemical Co. (St. Louis, MO); rat monoclonal anti-E-cadherin (1/200) was purchased from Zymed (San Francisco, CA); rat monoclonal anti-laminin (1/200) was obtained from Biodesign (Abingdon, Oxon, U.K.); rabbit polyclonal anti-RXRα antibody (1/100) from Santa Cruz, Calne, UK; rabbit polyclonal anti-somatostatin (1/200), guinea-pig polyclonal anti-insulin (1/300) and swine polyclonal anti-rabbit IgG TRITC conjugate (1/200) from DAKO (Ely, Cambridge, UK); horse polyclonal anti-mouse IgG FITC conjugate (1/150), horse polyclonal anti-mouse IgG Texas Red conjugate (1/150), horse polyclonal anti-mouse IgG AMCA conjugate (1/150), and goat polyclonal anti-rabbit IgG FITC conjugate (1/150 from Vector Laboratories Inc., Peterborough, UK); rabbit polyclonal anti-mouse IPF/Pdx1 antibody (1/100) was made by JMWS against an 18 amino acid C-terminal peptide conjugated to keyhole limpet haemocyanin. Mouse monoclonal anti-cytokeratin filament seven antibody (undiluted) was a gift from Prof. E. B. Lane (CRC Cell Structure Research Group, Dundee U.K.); the specimens were observed either using a Zeiss confocal microscope (LSM510; Welwyn Garden City, UK) or with a Leica DMRB fluorescent microscope (Milton, Keynes, UK). Image collection from Leica was made with a spot camera (diagnostic instrument) and the images processed with Adobe Photoshop.
For insulin-positive-stained area counting, pancreatic buds were grown for 5 days in culture medium supplemented with or without 1 μM atRA. Counts of total insulin-positive-stained area and total bud area was performed on a Zeiss LSM510 microscope. Determination of the area was carried out using the area tool on the Zeiss LSM510 software. Statistical analyses were performed on triplicate bud cultures using the Student paired t-test in Excel. Significance was set at p<0.01.
RT-PCR
Total RNA was extracted using the TRI reagent (Sigma). Pancreatic buds were cultured on fibronectin-coated coverslips without or with 1 μM atRA for 4 days. Cells were lysed directly on the culture dish by adding 0.05 ml of the TRI reagent. After addition of the reagent, the lysate was passed several times through a pipette to homogenize the cells. The lysate from 10 bud cultures was collected, and then 0.1 ml of chloroform was added to the tube. Covering the sample, it was shaken vigorously for 15 sec and allowed to stand for 15 min at room temperature. It was then centrifuged at 12,000 × g for 15 min at 4°C. The aqueous phase was transferred to a fresh tube; 0.25 ml of isopropanol was added and mixed. The sample was allowed to stand for 5–10 min at room temperature, and then centrifuged at 12,000 × g for 10 min at 4°C. The supernatant was removed and the RNA pellet was washed with 75% ethanol. The RNA pellet was air-dried for 5–10 min, dissolved in water, and then stored in −80°C.
Before carrying out the cDNA synthesis, the RNA samples were digested with RQ-1 DNase (Promega, Southampton, U.K.) to remove any contaminating genomic DNA. First-strand complementary DNA was synthesized using SuperScript II reverse transcriptase (Invitrogen). The reactions were processed in a DNA thermal cycler under the following conditions: denaturation at 94°C for 1 min, annealing at 55°C for 1 min, and extension at 72°C for 1 min. The number of cycles was 31. The primers were for GAPDH (sense primer: 5′-AAGGTCGGTGTGAACGGATT-3′, antisense primer: 5′-TGGTGGTGCAGGATGCATTG-3′) and as described in Shen et al. (2003a).
For real-time PCR, each reaction contained 5 μl of cDNA, 1 × Master Mix solution (Roche) and 0.5 μM PCR primer in a 20 μl volume. The reaction capillaries were processed in a Light Cycler 2.0 System (Roche Diagnostics GmbH, Mannheim, Germany) under the following conditions: preincubation at 95°C for 10 min, denaturation at 95°C for 10 sec, annealing at 58°C for 5 sec, and extension at 72°C for 20 sec. The PCR run was ended with 40°C incubation for 30 sec. The SYBR Green I was monitored at each 72°C incubation. Light Cycler PCR amplification was performed for GAPDH (5′-AAGGTCGGTGTGAACGGATT, 5′-TGGTGGTGCAGGATGCATTG), Amylase (5′-CAGGCAATCCTGCAGGAACAA, 5′-CACTTGCGGATAACTGTGCCA), retinaldehyde dehydrogenase 2 (RALDH2) (5′-ATGCGTCTGAAAGAGG, 5′-TGTCTATGCCCGATGTG), and FGF-10 (5′-TTGGTGTCTTCGTTCCCTGT, 5′-CATTTGCCTGCCATTGTGCT).
Apoptosis assay
Apoptosis was detected in control and treated pancreata using the Vybrant apoptosis assay kit II according to the manufacturer's instructions (Molecular Probes, Paisley, Scotland, UK). In brief, pancreatic buds were cultured in 24-well plates with or without RA and FGF-10 treatment for 3 days. At the end of the treatment period, propidium iodide together with binding buffer was added to each well, and the explants were incubated for 15 min at room temperature. Cells were then washed three times with binding buffer, and staining for dead cells was observed immediately under a Nikon inverted fluorescence microscope (Kingston upon Thames, UK).
Results
Development of pancreatic buds in vitro
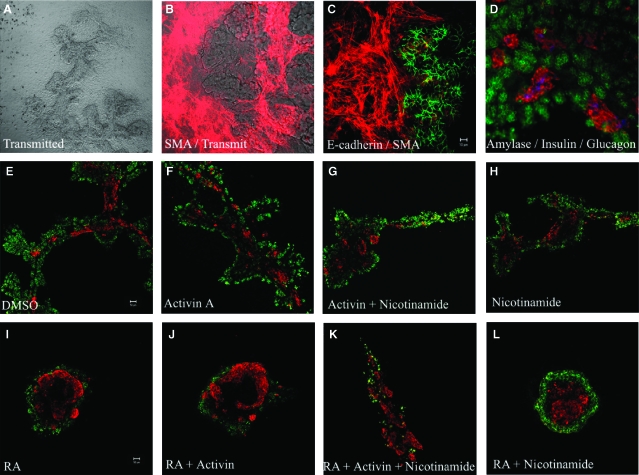
Previously, our lab developed a system for in vitro culture of dorsal pancreatic buds from mouse embryos which enables the organ to grow as a flat branched structure suitable for whole mount immunostaining (Fig. 1A, Percival and Slack, 1999). The dorsal buds were isolated from E11.5 mouse embryos. The buds adhere to the fibronectin substrate within a few hours and gradually flatten out over the culture period. Mesenchymal cells spread rapidly out of the explants to form a monolayer of cells surrounding the epithelial clump in the centre (Fig. 1B, 1C). On the second or third day, branches begin to appear in the epithelium. Over the next 3 days, the epithelium becomes an extended branched structure radiating from the original centre. Exocrine cells can be recognized from day 4, and insulin-producing cells are very few and faintly stained after 1 and 2 days of culture, but become more numerous and strongly stained thereafter. Clumps of endocrine cells resembling nascent islets can be seen scattered throughout the pancreas from around day 7 onwards (Fig. 1D).
Fig. 1.
Differentiation of dorsal pancreatic buds in vitro. (A) Seven-day cultured pancreatic bud, the epithelium forms an extended branched structure. (B, C) The cultured pancreatic buds flatten out onto the substratum and mesenchymal cells spread rapidly out of the explant to form a monolayer of cells which stain for smooth muscle actin (red). The pancreatic epithelium was stained positive for E-cadherin (green). (D) Buds were cultured for 7 days, fixed, and stained for amylase (green), insulin (red), and glucagon (blue). Pancreatic buds were cultured for 2 days and then treated for 5 days with (E) 0.1% DMSO, (F) 10 μg/ml activin A, (G) 10 μg/ml activin A and 5 mM nicotinamide, (H) 5 mM nicotinamide (I) 1 μM all-trans retinoic acid (atRA), (J) 1 μM atRA and 10 μg/ml activin A, (K) 1 μM atRA, 10 μg/ml activin A, and 5 mM nicotinamide (L) 1 μM atRA and 5 mM nicotinamide. Buds were fixed and stained for amylase (green) and insulin (red).
Several factors have been shown to promote β-cell differentiation and insulin production including activin A (a member of the TGF-β superfamily; Penny and Kramer, 2002), nicotinamide (Mngomezulu and Kramer, 2000) and RA (Penny and Kramer, 2000). Initially, we wanted to determine if any of these factors could affect exocrine or endocrine (specifically β cell) differentiation in our culture system. We added 10 μg/ml activin A or 5 mM nicotinamide for 5 days alone and in combination but we did not observe any significant change in the area occupied by insulin-positive cells (Fig. 1E–1H). However, addition of 1 μM atRA to pancreatic cultures caused the cultures to develop with a much higher proportion of insulin positive cells, arranged in large masses (Fig. 1I–1L). Although superficially atRA appeared to increase the proportion of endocrine cells, when the areas of the cell types were measured, the apparent increase in β-cell area was actually due to a suppression of exocrine development. Based on the observations from three sets of area analysis, the total area of insulin positive clusters did not increase significantly on treatment with atRA (Fig. 2A–2H). In contrast, the total area of pancreatic epithelium was reduced by 80% after 5 days of atRA treatment.
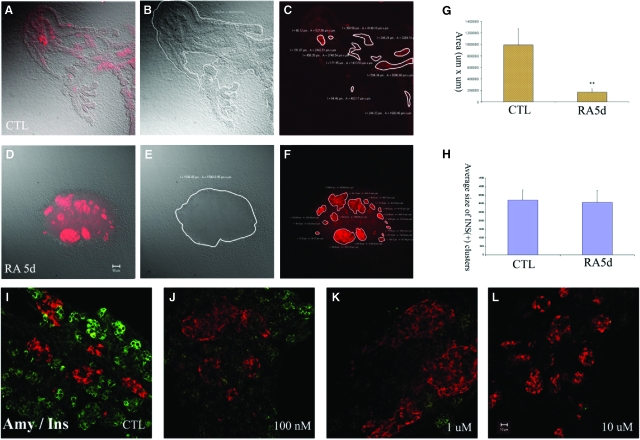
Fig. 2.
Effect of all-trans retinoic acid (atRA) on differentiation and branching morphogenesis of pancreatic buds in culture. Two-day pancreatic buds were cultured for 5 days without (A–C) or with 1 μM retinoic acid (RA) (D–F) and stained for insulin (red). (A) Overlay image of transmitted light image (B) and fluorescence image (C), (D) overlay image of transmitted light image (E) and fluorescence image (F). Area analysis in (A–C) and (D–F) was performed by Zeiss image processing software LSM 5. (G) Area of control and RA-treated pancreas (in μm2). The total areas were calculated from three bud cultures. (H) The area of insulin-expressing clusters was measured by LSM software. Large clusters (>500 μm2) were calculated from three buds and the averaged results are shown as means+SD. (I–L) 2-day pancreatic buds were cultured for 5 days with (I) 0.1% DMSO, (J) 100 nM, (K) 1 μM, (L) 10 μM atRAs and stained for amylase (green) and insulin (red).
Effects of atRA on differentiation of the embryonic pancreas
In order to investigate the dose-dependency of the RA effect on pancreatic differentiation in culture, E11.5d dorsal pancreatic buds were cultured for 2 days and then incubated for 5 days either without or with atRA ranging from 100 nM to 10 μM. Exocrine differentiation, as judged by amylase staining, was found to be suppressed at all concentrations of atRA compared to controls (Fig. 2I–2L). However, the formation of large insulin clusters was only observed at concentrations of 1 μM atRA.
We examined the effect of RA on β-cell differentiation in more detail. Using the 1 μM concentration of atRA, we compared the expression of insulin by RT-PCR and glucose stimulated insulin release in control and atRA-treated dorsal pancreatic buds. Although the insulin-positive clusters sometimes appeared to show more intense staining (Fig. 2D), the expression of insulin did not increase significantly as judged by RT-PCR analysis (Fig. 3I). Glucose-stimulated insulin release was actually reduced by 20% following atRA treatment compared with control cultures (Fig. 3J).
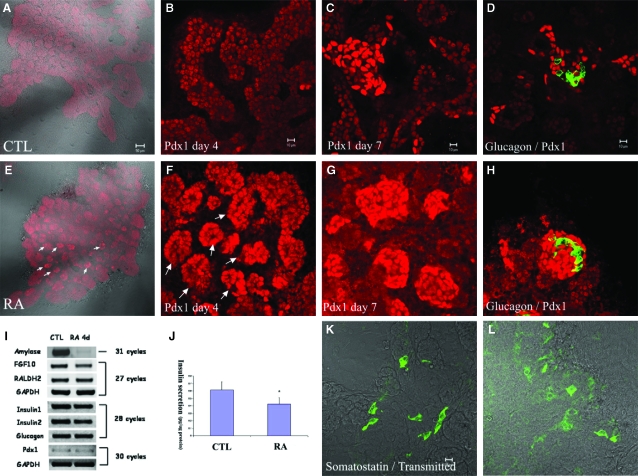
Fig. 3.
Up-regulation of Pdx1 and acceleration of islet formation by retinoic acid. Pancreatic buds were isolated, cultured for 2 days in the absence of any factors and then cultured for a further 2 days (A, B, E, F) or 5 days (C, D, G, H, K, L) without (A–D, K) or with 1 μM retinoic acid (E–H, L) and stained for (A–C, E–G) Pdx1 (red) (D, H) glucagon (green)/Pdx1 (red) (K, L) Somatostatin (green). (I) RT-PCR for amylase, fibroblast growth factor (FGF)-10, retinaldehyde dehydrogenase 2 (RALDH2), insulin 1, insulin 2, glucagon, and pdx1 under control and all-trans retinoic acid-treated conditions. (J) Glucose-stimulated insulin release from buds cells was carried out using an insulin ELISA kit. Results were adjusted for protein concentration of each well, and are shown as means+SD.
RA accelerates the formation of islet-like structures
In order to investigate whether RA affects the differentiation of other endocrine cell types we examined the expression of the endocrine hormones glucagon and somatostatin and the pancreatic transcription factor Pdx1. atRA was added at a concentration of 1 μM to cultures from day 2 until day 7 and the expression of Pdx1 was examined at different time points. Previously, we have shown that Pdx1 is expressed in the whole pancreatic bud at early stages (Shen et al., 2003a), but by 5 days of culture it is mainly expressed in endocrine cell clusters and insulin-producing cells. However, here we found that Pdx1 was up-regulated by atRA at day 4 (Fig. 3A, 3B, 3E, 3F). Pdx1-stained clusters are formed after treatment with atRA for 2 days (arrow in Fig. 3E, 3F). From three sets of experiments, we could find large islet-like clusters intensely stained for Pdx1 in day 7 cultures (Fig. 3C, 3G), but the number of glucagon- or somatostatin-expressing cells was unaffected (Fig. 3D, 3H, 3K, 3L).
In agreement with the immunostaining results, RT-PCR analysis also showed that the expression of glucagon or Pdx1 did not change after 4 days of atRA treatment (Fig. 3I). In contrast, amylase expression was markedly inhibited (Fig. 3I). By real-time quantitative PCR, amylase expression was down-regulated 1,500-fold. To demonstrate that endogenous RA signalling is present in cultured pancreatic buds, we examined the expression of RALDH2, the most effective RA-synthesizing enzyme. We found that RALDH2 was expressed after 6 days of culture in pancreatic explants and the addition of atRA for 4 days of culture did not alter expression of the enzyme further (Fig. 3I).
Pdx1 is a key transcription factor in pancreatic development and is expressed in the whole pancreatic bud at early stages (Edlund, 2002). At 7 days of culture it is mainly expressed in insulin-producing cells and is known to be among the transcription factors regulating insulin expression in mature β cells. Here, we found that treatment of atRA caused an increase in the proportion of cells highly expressing Pdx1 and also accelerates the formation of Pdx1-positive clusters (Fig. 3E–3H).
atRA represses branching morphogenesis and induces formation of cystic ductal structures
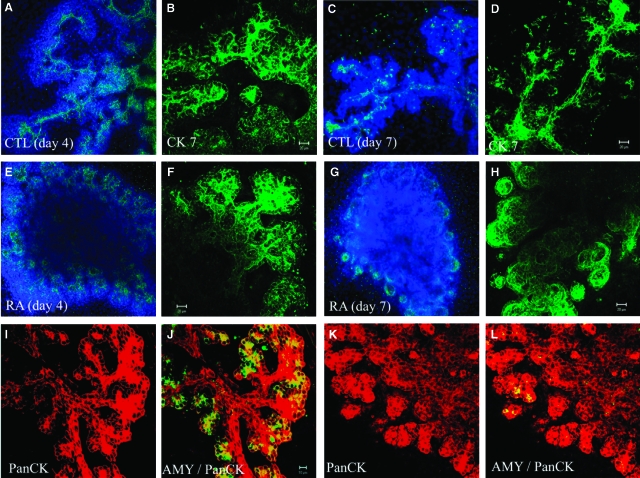
RA has been shown to inhibit the growth of both pancreatic ductal DSL-6A/C1 cells in vitro and azaserine-induced foci in the rat pancreas (Roebuck et al., 1984; Brembeck et al., 1998). Although under the light microscope we found that atRA at concentrations as low as 100 nM can inhibit branching morphogenesis (Figs 1 and 2), we wished to know whether atRA affected ductal cell differentiation. We used CK7 as a marker for the presence of ductal cells. CK7 is an intermediate filament protein found in pancreatic ductal cells (Bouwens, 1998). Under control conditions, CK7 stained ductular structures (Fig. 4A–4D). However, treatment of dorsal buds with atRA for 2 days rapidly suppressed the branching and the elongation of ductular structures based on the loss of cytokeratin staining (Fig. 4E, 4F). After 5 days treatment with atRA, we could find large number of cystic ductal structures in the peripheral region of the epithelium (Fig. 4G, 4H). Acinar-like structures normally form from the termini of the pancreatic ducts in control cultures at day 5 (Fig. 4I, 4J). In contrast, atRA treatment inhibited the formation of the acinar-like structures, and exocrine cells became sparse in cultures (Fig. 4K, 4L).
Fig. 4.
All-trans retinoic acid induces formation of cystic ductal structures. Two-day pancreatic buds were cultured for 2 days (A, B, E, F), 5 days (C, D, G, H), or 3 days (I–L) without (A–D, I, J) or with 1 μM retinoic acid (RA; E–H, K, L), fixed and stained for Cytokeratin 7 (green)/DAPI (blue) (A, C, E, G), Cytokeratin 7 (green) (B, D, E, H), amylase (green)/Pan-cytokeratin (red) (I–L).
Up-regulation of extracellular laminin by RA suppresses exocrine cell differentiation
Recent elegant experiments performed by Gittes and colleagues suggest that 9-cis RAs regulate exocrine lineage selection by changes in expression of mesenchymal laminin-1 (Kobayashi et al., 2002). This observation is not surprising as laminins are up-regulated by retinoid signalling through an RA response element (Vasios et al., 1991). In addition, pancreatic mesenchyme produces laminins and this may play an important role in pancreatic differentiation particularly ductal differentiation (Jiang et al., 1999; Crisera et al., 2000).
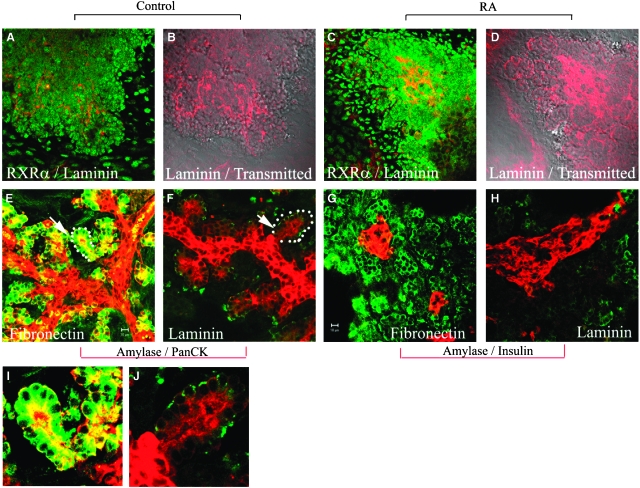
Addition of atRA to the cultures for 3 days resulted in an increase in extracellular laminin staining compared with control cultures (Fig. 5A–5D). Although the nuclear form of RXRα can be seen in both mesenchymal cells and in the peripheral region of the pancreatic epithelium, the expression of RXRα did not increase significantly. In order to determine whether ectopic laminin could alter ductal cell and exocrine cell differentiation, we then cultured E11.5 pancreatic buds on laminin-coated coverslips and compared them to fibronectin-coated coverslips, the ECM component the buds are normally cultured on (Fig. 5F, 5H). The results show that amylase expression was greatly reduced on laminin-coated coverslips compared to fibronectin-coated coverslips (Fig. 5E–5H). However, culture of pancreatic buds on laminin did not suppress the formation of ductular and periductular-like structures (Fig. 5E, 5F, arrows I and J are high-powered images). In agreement with previous work (Jiang et al., 1999), we also found that culture on exogenous laminin was able to induce formation of large β-cell aggregates (Fig. 5G, 5H), similar to culture with atRA. Taken together, the results indicate that both the suppressive effect of RA on exocrine differentiation, and the formation of enlarged islets, but not the inhibition of branching morphogenesis, may result from up-regulation of extracellular laminin.
Fig. 5.
Up-regulation of extracellular laminin by retinoic acid. E11.5d dorsal pancreatic buds were isolated, culture for 2 days and then cultured for 3 days (A–D), or 5 days (E–H) without (A, B, E–H) or with 1 μM retinoic acid (C, D). Buds were then fixed and stained for (A, C) retinoid-X receptorα (RXRα) (green)/laminin (red). (B, D) Overlay picture of laminin staining with transmitted images of (A) and (C). (E, F) Buds were stained for amylase (green)/Pan-cytokeratin (red). (G, H) Buds were stained for amylase (green)/insulin (red). In (F, H) pancreatic buds were cultured on laminin-coated coverslips for 7 days. Laminin mimics the effect of all-trans retinoic acid on exocrine differentiation. I and J are high-powered images from E and F.
The effect of RA on embryonic pancreatic differentiation can be antagonised by FGF-10
FGFs and their receptors (FGFRs1–4) have been identified as mediators of epithelial–mesenchymal interactions in different organs including developing pancreas (Miralles et al., 1999; Hart et al., 2000). FGFR2-IIIb and its ligands FGF-1, FGF-7, and FGF-10 are expressed throughout pancreatic development (Miralles et al., 1999). In addition, FGF-1, FGF-7, and FGF-10 can stimulate growth, morphogenesis, and cytodifferentiation of the exocrine cells of the pancreas in mesenchyme-free pancreatic cultures (Miralles et al., 1999). FGF-10 is a high affinity ligand for the FGFR2-IIIb and has been linked to pancreatic epithelial cell proliferation (Bhushan et al., 2001). In Fgf10−/− embryos, growth, exocrine differentiation and branching morphogenesis of the pancreatic epithelium is arrested. These observations are very similar to the results obtained in the present study on atRA-treated pancreatic bud cultures. Therefore we decided to determine if FGF-10 is able to rescue the suppressive effect of atRA on exocrine differentiation. Addition of FGF-10 in atRA-treated buds culture was able to partially rescue the exocrine differentiation (Fig. 6A–6D) and the formation of ductular structures (Fig. 6E–6H). To demonstrate that endogenous FGF-10 is present in cultured pancreatic buds, we examined the expression by RT-PCR (Fig. 3I). We found that FGF-10 was expressed after 6 days of culture in pancreatic explants and the presence of atRA did not alter expression of FGF-10 significantly.
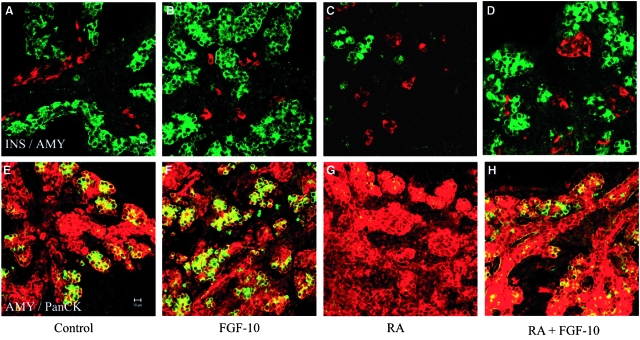
Fig. 6.
Fibroblast growth factor (FGF)-10 antagonizes the effect of retinoic acid (RA) on exocrine differentiation and branching morphogenesis. Two-day pancreatic buds were cultured for 3 days without treatment (A, E), or with 10 μg/ml FGF-10 (B, F), with 1 μM RA (C, G), with 10 μg/ml FGF-10+1 μM RA (D, H). Buds were fixed and stained for (A–D) Insulin (green)/amylase (red), (E–H) Amylase (green)/Pan-cytokeratin (red).
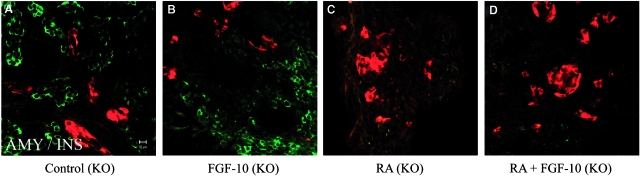
Abrogation of FGFR2-IIIb signalling reduces the size of the pancreas and suppresses development of exocrine cells (Miralles et al., 1999). Recent studies show that knock-out of the IIIb isoform of Fgfr2b resulted in the formation of a smaller pancreas during embryogenesis although both exocrine and endocrine pancreatic differentiation occurred relatively normally (Pulkkinen et al., 2003). In order to determine whether the effect of FGF-10 on exocrine differentiation is directly mediated by the FGFR2-IIIb receptor, we isolated dorsal buds from FGFR2-IIIB−/− embryos. Pancreatic buds from −/− embryos differentiate normally in cultures but are 60% smaller compared with wild-type pancreatic buds (Fig. 7A). Treatment with FGF-10 has no effect on the differentiation of the knockout pancreatic buds (Fig. 7B). The treatment of RA also caused the blockage of exocrine differentiation. As expected, co-treatment with FGF-10 cannot restore the exocrine differentiation in the knockout cultures (Fig. 7C, 7D). These results suggest that FGFR2-IIIb-mediated signalling plays a major role in antagonizing retinoid signalling.
Fig. 7.
Fibroblast growth factor (FGF)-10 antagonizes retinoid signalling E11.5d dorsal pancreatic buds were isolated from FGF receptor 2IIIb isoform homozygote knock-out embryos (A–D), cultured for 2 days and then cultured for a further 3 days without treatment (A), or with: 10 μg/ml FGF-10 (B), 1 μM retinoic acid (RA) (C), or 10 μg/ml FGF-10+1 μM RA (D). Buds were fixed and stained for (A–D) amylase (green)/insulin (red).
RA induces cell death in embryonic pancreas
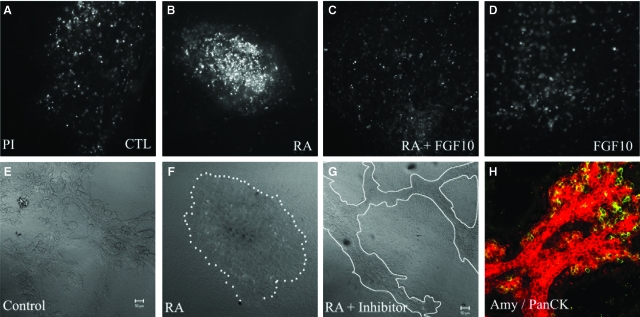
One possible explanation for the inhibition of exocrine cell formation and ductal branching is an increase in apoptosis following atRA treatment. To test this idea we treated pancreata with and without atRA in the absence and presence of FGF-10 and stained for apoptotic cells using propidium iodide (Fig. 8). Addition of atRA increased the number of dead cells as judged by the more intense bright spots (Fig. 8B), and culture with FGF-10 prevented the increase in apoptosis induced by atRA (Fig. 8C). Unfortunately, it was not possible to quantify the amount of apoptosis in the buds for two reasons. First, the cultures are too thick to count the apoptotic cells accurately. Second, when we dissociated the buds into single cell suspensions for FACS analysis, the difference in apoptosis was masked by the cell death induced by the dissociation procedure and the cell debris. Addition of caspase VI inhibitor to atRA cultures partially restored the suppressive effect of RA on branching morphogenesis (Fig. 8E, 8G) and exocrine differentiation (Fig. 8H).
Fig. 8.
Retinoic acid (RA) induces cell death in embryonic pancreas cultures. Dorsal pancreatic buds were cultured initially for 2 days in normal media and then cultured for a further 3 days under control conditions (A), or with the following additions: 1 μM all-trans retinoic acid (atRA) (B), 10 μg/ml fibroblast growth factor (FGF)-10 and 1 μM RA (C), 10 μg/ml FGF-10 (D). Dead cells in pancreatic bud cultures stained positively with propidium iodide (bright spots). The presence of FGF-10 reduces the number of apoptotic cells. (E–G) Transmitted light image of pancreatic bud cultures under control (E), atRA-treated (F) and atRA-treated with 40 μM caspase inhibitor VI (G) conditions. A 5-day bud treated with atRA and caspase inhibitor VI and stained for amylase (green)/Pan-cytokeratin (red) is shown in (H). Addition of caspase inhibitor VI to atRA-treated buds prevented the inhibition of branching and suppression of amylase expression.
Discussion
The mesenchymal factors that control pancreatic differentiation were first described by workers in the 1960s (Golosow and Grobstein, 1962; Wessells and Cohen, 1967). In recent years components of the mesenchymal signal have started to be identified and probably include activins and FGFs (Bhushan et al., 2001; Edlund, 2001). RA is now recognized as a candidate signalling molecule for mesenchymal–epithelial interactions in various organs, and recent studies suggested a specific role in pancreatic specification in zebrafish and frogs (Stafford and Prince, 2002; Chen et al., 2004). The present investigation focused on the effects of atRA on the mouse embryonic pancreas during later development at a stage when the mesenchymal–epithelial interaction is operative. RALDH2 is a key enzyme in the synthesis of RA, and both it and RARs are expressed in the whole pancreatic rudiment after about E12.5 (Tulachan et al., 2003). We observed expression of RALDH2 in cultured pancreatic explants suggesting that endogenous RA signalling is occurring at this stage of pancreatic development (Fig. 3I).
Our results suggest distinct effects of atRA on exocrine and endocrine differentiation. atRA accelerated formation of islet-like structures in vitro probably through up-regulation of the Pdx1 transcription factor. The suppressive effect on exocrine differentiation and formation of branching structures may be due to two mechanisms: (i) increased secretion of the extracellular matrix component laminin and (ii) enhancement of apoptosis.
Laminins are known to be up-regulated by retinoid signalling through the RA response element (Vasios et al., 1991) and a retinoic acid response element (RARE) has been found in the promoter region of the laminin-B1 gene (Ekblom et al., 2003). Laminin expression in the pancreatic mesenchyme may play an important role in regulating differentiation of pancreatic ductal and β cells (Jiang et al., 1999; Crisera et al., 2000). Inhibition of laminin binding to epithelium grown on basement membrane gel inhibits differentiation of ductal cells (Maldonado et al., 2000). The suppression of acinar differentiation by 9-cis RA is mediated by mesenchymal expression of laminin-1 (Kobayashi et al., 2002). Here, we show that treatment of pancreatic buds with atRA up-regulates extracellular laminin and that culture of buds on laminin suppresses exocrine differentiation. Surprisingly, blockage of laminin-1 expression has also been reported to reduce the amount of exocrine differentiation (Kobayashi et al., 2002; Li et al., 2004). The result suggests that there may be a critical level of laminin-1 for acinar differentiation during pancreas development. Either a too-high, or a too-low laminin-1 level prevents acinar differentiation.
One possible mechanism underlying the inhibition of acinar cell differentiation is via apoptosis. Pancreatic cultures treated with atRA showed more apoptotic cells based on propidium iodide staining. Previous observations on pancreatic rudiments treated with atRA showed dysmorphogenesis and overexpression of the proapoptotic protein Bax in the acinar cells (Tulachan et al., 2003). In the present study the demonstration of apoptosis in pancreatic explants could be due to a direct effect of atRA or alternatively it may be mediated through the up-regulation of Pdx1. Persistent up-regulation of Pdx1 has been shown to destroy pancreatic acinar cells by apoptosis (Heller et al., 2001).
The pancreatic progenitor cells present in the early pancreatic anlagen can proliferate and generate the full complement of pancreatic epithelial cell types. FGFR2-IIIb (the high-affinity receptor for FGF-10) has been linked to pancreatic epithelial cell proliferation (Bhushan et al., 2001; Hart et al., 2003). In lung and pancreas, FGF-10 is normally secreted by the mesenchyme, causing outgrowth of those epithelial cells in proximity to the signalling source. Interestingly, the predominant receptor for FGF-10, the III-b-splice form of the FGFR2, is expressed in the pancreas epithelium but not in the adjacent mesenchyme (Miralles et al., 1999; Elghazi et al., 2002). In Fgf10−/− embryos, the growth, exocrine differentiation and branching morphogenesis of the pancreatic epithelium was inhibited. This observation suggests that FGF-10 signalling mediated by the FGFR2-IIIb receptor is required for expansion and differentiation of embryonic pancreas. In the present studies, we show that the pretreatment with FGF-10 was able to partially rescue atRA-mediated suppression of exocrine differentiation as well as branching morphogenesis of normal pancreas. The result also implies that RA is involved in suppression of FGF-10 signalling during normal development of pancreas. However, when we examined the expression of FGF-10 following atRA treatment we could find no significant change in expression (Fig. 3I). This observation indicates FGF-10 is not a direct target of atRA. FGF-10 appears to be an essential factor in branching morphogenesis in several other organs including the prostate gland (Donjacour et al., 2003) and addition of FGF-10 can support branching morphogenesis in prostate organ rudiments (Thomson and Cunha, 1999). Analogous to the present study, FGF-10 is not a direct target of androgens suggesting alternative pathways of regulation exist (reviewed in Thomson, 2001). Therefore, the pancreas may use FGF-10 in a similar way to the prostate.
The development of endocrine- and exocrine-specific cells from a group of equipotent precursor cells is known to be regulated by Notch signalling. Notch signalling has been shown to be involved in regulating differentiation of embryonic pancreas (Apelqvist et al., 1999; Murtaugh et al., 2003). Recent studies provide evidence that the persistent expression of FGF-10 in the developing pancreas is able to maintain Notch activation (Hart et al., 2003; Norgaard et al., 2003). This in turn would inhibit expression of ngn3 within the pancreatic epithelium, and in turn suppresses exocrine differentiation (Hart et al., 2003). Chen et al. (2004) showed that the loss of exocrine cells following application of exogenous RA correlated with an inhibition of Notch signalling activity. The suppressive effect of RA on exocrine cell differentiation (as judged by amylase expression) can be rescued by FGF-10 suggesting it may also involved in activation of Notch signalling.
The data presented here may be of relevance to attempts to produce human β cells for transplantation. Although islet transplantation procedures have recently improved considerably (Ridgway et al., 2003), there are problems of donor supply. One way forward is to augment the supply by producing β cells in vitro, so the generation of pancreatic endocrine tissue for therapeutic transplantation into diabetic patients constitutes an important goal in tissue engineering. However, if the starting material is an embryonic pancreatic rudiment then the problem is to generate endocrine tissue without significant amounts of exocrine tissue. Mouse embryonic pancreas provides a suitable model system for examining the factors that regulate differentiation of the exocrine and endocrine pancreatic components, and our results suggest that RA may be one of the factors that could usefully be used to favor endocrine over exocrine differentiation.
Acknowledgments
This work was funded by the Medical Research Council and the Wellcome Trust (D.T. and J.M.W.S.). The authors wish to thank Antony Iglesias and Gillian Hutchinson (Cancer Research, U.K.) for animal husbandry and Prof. Birgit Lane for supplying the CK7 antibody.
References
- Allenby G. Retinoic acid receptors and retinoid-X receptors—interactions with endogenous retinoic acids. Proc Natl Acad Sci USA. 1993;90:30–34. doi: 10.1073/pnas.90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apelqvist A. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–881. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- Bhushan A. Fgf10 is essential for maintaining the proliferative capacity of epithelial progenitor cells during early pancreatic organogenesis. Development. 2001;128:5109–5117. doi: 10.1242/dev.128.24.5109. [DOI] [PubMed] [Google Scholar]
- Bouwens L. Cytokeratins and cell differentiation in the pancreas. J Pathol. 1998;184:234–239. doi: 10.1002/(SICI)1096-9896(199803)184:3<234::AID-PATH28>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Brembeck FH. Retinoic acid receptor alpha mediates growth inhibition by retinoids in rat pancreatic carcinoma DSL-6A/C1 cells. Br J Cancer. 1998;78:1288–1295. doi: 10.1038/bjc.1998.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broglio F. Ghrelin and the endocrine pancreas. Endocrine. 2003;22:19–24. doi: 10.1385/ENDO:22:1:19. [DOI] [PubMed] [Google Scholar]
- Chen Y. Retinoic acid signaling is essential for pancreas development and promotes endocrine at the expense of exocrine cell differentiation in Xenopus. Dev Biol. 2004;271:144–60. doi: 10.1016/j.ydbio.2004.03.030. [DOI] [PubMed] [Google Scholar]
- Crisera CA. Expression and role of laminin-1 in mouse pancreatic organogenesis. Diabetes. 2000;49:936–44. doi: 10.2337/diabetes.49.6.936. [DOI] [PubMed] [Google Scholar]
- Donjacour AA. FGF-10 plays an essential role in the growth of the fetal prostate. Dev Biol. 2003;261:39–54. doi: 10.1016/s0012-1606(03)00250-1. [DOI] [PubMed] [Google Scholar]
- Edlund H. Factors controlling pancreatic cell differentiation and function. Diabetologia. 2001;44:1071–1079. doi: 10.1007/s001250100623. [DOI] [PubMed] [Google Scholar]
- Edlund H. Pancreatic organogenesis—developmental mechanisms and implications for therapy. Nat Rev Genet. 2002;3:524–532. doi: 10.1038/nrg841. [DOI] [PubMed] [Google Scholar]
- Ekblom P. Expression and biological role of laminin-1. Matrix Biol. 2003;22:35–47. doi: 10.1016/s0945-053x(03)00015-5. [DOI] [PubMed] [Google Scholar]
- Elghazi L. Role for FGFR2IIIb-mediated signals in controlling pancreatic endocrine progenitor cell proliferation. Proc Natl Acad Sci USA. 2002;99:3884–3889. doi: 10.1073/pnas.062321799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittes GK. Lineage-specific morphogenesis in the developing pancreas: role of mesenchymal factors. Development. 1996;122:439–47. doi: 10.1242/dev.122.2.439. [DOI] [PubMed] [Google Scholar]
- Golosow N. Epitheliomesenchymal interaction in pancreatic morphogenesis. Dev Biol. 1962;4:242–255. doi: 10.1016/0012-1606(62)90042-8. [DOI] [PubMed] [Google Scholar]
- Gu G. Direct evidence for the pancreatic lineage: nGN3+ cells are islet progenitors and are distinct from gut progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- Hart A. Fgf10 maintains notch activation, stimulates proliferation, and blocks differentiation of pancreatic epithelial cells. Dev Dyn. 2003;228:185–193. doi: 10.1002/dvdy.10368. [DOI] [PubMed] [Google Scholar]
- Hart AW. Attenuation of FGF signalling in mouse beta-cells leads to diabetes. Nature. 2000;408:864–868. doi: 10.1038/35048589. [DOI] [PubMed] [Google Scholar]
- Heller RS. Improved glucose tolerance and acinar dysmorphogenesis by targeted expression of transcription factor Pdx1 to the exocrine pancreas. Diabetes. 2001;50:1553–1561. doi: 10.2337/diabetes.50.7.1553. [DOI] [PubMed] [Google Scholar]
- Heyman RA. 9-cis retinoic acid is a high affinity ligand for the retinoid-X receptor. Cell. 1992;68:397–406. doi: 10.1016/0092-8674(92)90479-v. [DOI] [PubMed] [Google Scholar]
- Horb LD. Role of cell division in branching morphogenesis and differentiation of the embryonic pancreas. Int J Dev Biol. 2000;44:791–796. [PubMed] [Google Scholar]
- Jiang FX. Laminin-1 promotes differentiation of fetal mouse pancreatic beta-cells. Diabetes. 1999;48:722–730. doi: 10.2337/diabetes.48.4.722. [DOI] [PubMed] [Google Scholar]
- Kadison A. Retinoid signaling directs secondary lineage selection in pancreatic organogenesis. J Paed Surg. 2001;36:1150–1156. doi: 10.1053/jpsu.2001.25734. [DOI] [PubMed] [Google Scholar]
- Kim SK. Intercellular signals regulating pancreas development and function. Genes Dev. 2001;15:11–27. doi: 10.1101/gad.859401. [DOI] [PubMed] [Google Scholar]
- Kobayashi H. Retinoid signaling controls mouse pancreatic exocrine lineage selection through epithelial-mesenchymal interactions. Gastroenterology. 2002;123:1331–1340. doi: 10.1053/gast.2002.35949. [DOI] [PubMed] [Google Scholar]
- Kramer B. Regulation of the proportion of insulin cells in embryonic chick pancreas: effect of a growth factor-reduced extracellular matrix in combination with retinoic acid. In Vitro Cell Dev Biol Anim. 2003;39:196–198. doi: 10.1290/1543-706X(2003)039<0196:ROTPOI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Li Z. Multifaceted pancreatic mesenchymal control of epithelial lineage selection. Dev Biol. 2004;269:252–263. doi: 10.1016/j.ydbio.2004.01.043. [DOI] [PubMed] [Google Scholar]
- Maden M. Retinoid signalling in the development of the central nervous system. Nat Rev Neurosci. 2002;3:843–853. doi: 10.1038/nrn963. [DOI] [PubMed] [Google Scholar]
- Maldonado TS. Basement membrane exposure defines a critical window of competence for pancreatic duct differentiation from undifferentiated pancreatic precursor cells. Pancreas. 2000;2:93–96. doi: 10.1097/00006676-200007000-00057. [DOI] [PubMed] [Google Scholar]
- Malpel S. Regulation of retinoic acid signaling during lung morphogenesis. Development. 2000;127:3057–3067. doi: 10.1242/dev.127.14.3057. [DOI] [PubMed] [Google Scholar]
- Means AL. The role of retinoids in vertebrate development. Ann Rev Biochem. 1995;64:201–233. doi: 10.1146/annurev.bi.64.070195.001221. [DOI] [PubMed] [Google Scholar]
- Mendelsohn C. Stromal cells mediate retinoid-dependent functions essential for renal development. Development. 1999;126:1139–1148. doi: 10.1242/dev.126.6.1139. [DOI] [PubMed] [Google Scholar]
- Miralles F. Signaling through fibroblast growth factor receptor 2b plays a key role in the development of the exocrine pancreas. Proc Natl Acad Sci USA. 1999;96:6267–6272. doi: 10.1073/pnas.96.11.6267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mngomezulu WT. Beneficial effect of nicotinamide on the proportion of insulin cells in developing chick pancreas. Dev Growth Differ. 2000;42:187–193. doi: 10.1046/j.1440-169x.2000.00499.x. [DOI] [PubMed] [Google Scholar]
- Murtaugh LC. Genes, signals, and lineages in pancreas development. Annu Rev Cell Dev Biol. 2003;19:71–89. doi: 10.1146/annurev.cellbio.19.111301.144752. [DOI] [PubMed] [Google Scholar]
- Murtaugh LC. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci USA. 2003;100:14920–14925. doi: 10.1073/pnas.2436557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgaard GA. FGF10 signaling maintains the pancreatic progenitor cell state revealing a novel role of Notch in organ development. Dev Biol. 2003;264:323–338. doi: 10.1016/j.ydbio.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Penny C. The effect of retinoic acid on the proportion of insulin cells in the developing chick pancreas. In Vitro Cell Dev Biol Anim. 2000;36:14–18. doi: 10.1290/1071-2690(2000)036<0014:TEORAO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Penny C. Exogenous activin increases the proportion of insulin cells in the developing chick pancreas in culture. Cell Biol Int. 2002;26:1057–1064. doi: 10.1006/cbir.2002.0965. [DOI] [PubMed] [Google Scholar]
- Percival AC. Analysis of pancreatic development using a cell lineage label. Exp Cell Res. 1999;247:123–132. doi: 10.1006/excr.1998.4322. [DOI] [PubMed] [Google Scholar]
- Plateroti M. Mesenchyme-mediated effects of retinoic acid during rat intestinal development. J Cell Sci. 1997;110:1227–1238. doi: 10.1242/jcs.110.10.1227. [DOI] [PubMed] [Google Scholar]
- Pulkkinen MA. The IIIb isoform of fibroblast growth factor receptor 2 is required for proper growth and branching of pancreatic ductal epithelium but not for differentiation of exocrine or endocrine cells. Mech Dev. 2003;120:167–175. doi: 10.1016/s0925-4773(02)00440-9. [DOI] [PubMed] [Google Scholar]
- Revest JM. Fibroblast growth factor receptor 2-IIIb acts upstream of Shh and Fgf4 and is required for limb bud maintenance but not for the induction of Fgf8, Fgf10, Msx1, or Bmp4. Dev Biol. 2001;231:47–62. doi: 10.1006/dbio.2000.0144. [DOI] [PubMed] [Google Scholar]
- Ridgway DM. Pancreatic islet cell transplantation: progress in the clinical setting. Treat Endocrinol. 2003;2:173–189. doi: 10.2165/00024677-200302030-00004. [DOI] [PubMed] [Google Scholar]
- Roebuck BD. Inhibition by retinoids of the growth of azaserine-induced foci in the rat pancreas. J Natl Cancer Inst. 1984;73:233–236. [PubMed] [Google Scholar]
- Shen CN. Transdifferentiation of pancreas to liver. Mech Dev. 2003a;120:107–116. doi: 10.1016/s0925-4773(02)00337-4. [DOI] [PubMed] [Google Scholar]
- Shen CN. Glucocorticoids suppress beta-cell development and induce hepatic metaplasia in embryonic pancreas. Biochem J. 2003b;375:41–50. doi: 10.1042/bj20030140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen CN. Molecular basis of transdifferentiation of pancreas to liver. Nat Cell Biol. 2000;2:879–887. doi: 10.1038/35046522. [DOI] [PubMed] [Google Scholar]
- Slack JMW. Developmental biology of the pancreas. Development. 1995;121:1569–1580. doi: 10.1242/dev.121.6.1569. [DOI] [PubMed] [Google Scholar]
- Stafford D. Retinoic acid signaling is required for a critical early step in zebrafish pancreatic development. Curr Biol. 2002;12:1215–1220. doi: 10.1016/s0960-9822(02)00929-6. [DOI] [PubMed] [Google Scholar]
- Thomson AA. Role of androgens and fibroblast growth factors in prostatic development. Reproduction. 2001;121:187–195. doi: 10.1530/rep.0.1210187. [DOI] [PubMed] [Google Scholar]
- Thomson AA. Prostatic growth and development are regulated by FGF10. Development. 1999;126:3693–3701. doi: 10.1242/dev.126.16.3693. [DOI] [PubMed] [Google Scholar]
- Tulachan SS. All-trans retinoic acid induces differentiation of ducts and endocrine cells by mesenchymal/epithelial interactions in embryonic pancreas. Diabetes. 2003;52:76–84. doi: 10.2337/diabetes.52.1.76. [DOI] [PubMed] [Google Scholar]
- Vasios G. The late retinoic acid induction of laminin B1 gene transcription involves RAR binding to the responsive element. EMBO J. 1991;10:1149–1158. doi: 10.1002/j.1460-2075.1991.tb08055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessells NK. Early pancreas organogenesis: morphogenesis, tissue interactions and mass effects. Dev Biol. 1967;15:237–270. doi: 10.1016/0012-1606(67)90042-5. [DOI] [PubMed] [Google Scholar]