Abstract
Increased levels of Bcr-Abl expression in chronic myelogenous leukemia (CML) cells are associated with disease progression and imatinib (IM) resistance. However, it is not clear if these associations are a direct result of elevated Bcr-Abl expression. We used a human transduction model of CML to directly investigate the role of varying Bcr-Abl expression levels in determining the phenotype and IM sensitivity of hematopoietic cells. CD34+ cells were transduced with vectors coexpressing Bcr-Abl and GFP, and cells expressing low and high levels of GFP and Bcr-Abl (BAlo and BAhi) were selected. BAhi cells demonstrated enhanced activation of downstream proliferative and antiapoptotic signaling and enhanced proliferation and survival compared to BAlo cells. Freshly isolated BAhi CD34+ cells and cell lines demonstrated increased IM-mediated growth inhibition likely reflecting Bcr-Abl dependence for growth and survival. CD34+ cells expressing BCR/ABL kinase-mutant genes demonstrated resistance to IM-mediated inhibition of proliferation and viability, which was not enhanced by increased expression of BCR/ABL kinase-mutant genes. We conclude that Bcr-Abl overexpression results in increased proliferation and antiapoptotic signaling in CD34+ cells, but may not play a direct role in IM resistance in progenitor cells expressing either wild-type or mutant BCR/ABL genes.
Introduction
Chronic myeloid leukemia (CML) is a hematopoietic stem cell disorder characterized by a balanced translocation between chromosomes 9 and 22, also known as the Philadelphia chromosome.1,2 The resulting BCR/ABL fusion oncogene encodes a cytoplasmic protein tyrosine kinase with elevated and dysregulated enzymatic activity.3 The BCR/ABL gene plays a critical role in the pathogenesis of CML.3,4 The clinical course of CML typically progresses over time from an early chronic phase (CP) through an accelerated phase (AP) and terminal blast crisis phase (BC). Disease progression is associated with increased levels of Bcr-Abl expression and acquisition of additional genetic and epigenetic abnormalities, which lead to altered hematopoietic cell growth and differentiation
Imatinib mesylate (IM), a small molecule inhibitor of the c-ABL, Bcr-Abl, c-Kit, and PDGFR kinases, inhibits the growth of Bcr-Abl–expressing cells.5 IM has proven highly effective in treatment of CML. Patients in CP are most likely to benefit from IM treatment.6,7 While responses in CP are usually durable, remissions observed in BC patients are typically transient with relapse occurring despite continued drug treatment.8 Relapse also occurs, though less frequently, in patients in CP and AP. Several groups have investigated mechanisms of resistance to IM in CML in IM-resistant cell line models9–14 and in primary patient samples.15–22 Point mutations in the ABL kinase domain resulting in reduced drug binding is a major mechanism of acquired resistance to IM in CML.23 Other mechanisms implicated as a cause of IM resistance include amplification of the BCR/ABL gene22 and/or overexpression of Bcr-Abl transcripts,20 and activation of non–Bcr-Abl–dependent transformation mechanisms.24–27
It is not clear whether the association of Bcr-Abl overexpression with disease progression and IM resistance directly results from increased expression of the Bcr-Abl protein or reflects coincident occurrence of additional abnormalities contributing to transformation and drug resistance in CML cells, such as kinase domain mutations or activation of non–Bcr-Abl kinase–dependent genetic or epigenetic mechanisms of transformation. In studies analyzing individual subclones of GF-dependent cell lines expressing varying levels of Bcr-Abl, it was observed that the fully transformed phenotype of GF-independent proliferation and survival required high levels of Bcr-Abl expression.9,14 It was felt that increasing levels of Bcr-Abl expression in primary cells could be responsible for the different phenotypic features seen in CP and AP CML. Barnes et al14 showed that cell lines expressing high amounts of Bcr-Abl demonstrated reduced sensitivity to IM and took less time to generate IM-resistant subclones compared to cells with low Bcr-Abl expression levels, suggesting that high Bcr-Abl levels may contribute to rapid development of resistance. However his approach does not allow distinction between direct effects resulting from differences in Bcr-Abl expression levels versus other genetic and epigenetic abnormalities acquired during subcloning. Indeed cells with increased Bcr-Abl expression may be more prone to developing such abnormalities.28–30 Significantly, several of the resistant cell lines had detectable Bcr-Abl kinase mutations. In addition since cell lines may not adequately model human disease, the role of Bcr-Abl expression levels in primary cells remains unclear. Therefore additional studies to elucidate the dose-effect relationships of Bcr-Abl proteins with human hematopoietic cell transformation and drug resistance are required.
In the present study, we directly investigated the role of increased levels of Bcr-Abl expression in cellular transformation and drug sensitivity of primary human progenitor cells, by ectopically expressing the BCR/ABL and GFP genes in normal CD34+ cells and selecting cells for lower and higher levels of Bcr-Abl expression based on GFP expression levels. We also studied the effect of varying levels of gene expression effect on IM resistance of CD34+ cells expressing the M351T and E255K kinase domain mutants, which are known to be associated with moderate and high levels of resistance to kinase inhibition by IM. Our results indicate an important effect of BCR/ABL gene expression levels on the transformed phenotype of Bcr-Abl–expressing CD34+ cells but do not support a direct role for high levels of wild-type and mutant BCR/ABL gene expression in IM resistance.
Patients, materials, and methods
Subjects
Cord blood samples and bone marrow samples from CML patients were collected after obtaining informed consent in accordance with the Declaration of Helsinki using guidelines approved by the institutional review board of the City of Hope National Medical Center and in accordance with the Declaration of Helsinki. The CML samples included 4 from patients with CP and 3 each from patients with AP and BC disease.
Selection of CD34+ progenitors
Bone marrow mononuclear cells (BMMNCs) were isolated by Ficoll-Hypaque (specific gravity 1.077; Sigma Aldrich, St Louis, MO) density gradient centrifugation. CD34+ cells were selected from BMMNCs using immunomagnetic column separation (Miltenyi Biotech, Auburn, CA).
Vectors and virus production
The MIG R1 and MIG 210 retroviral vectors were kind gifts from Dr Warren Pear (University of Pennsylvania, Philadelphia, PA) and have been described previously.31 Bcr-Abl kinase domain mutants were generated by introducing point mutations in p210 Bcr-Abl by site-directed mutagenesis, substituting glutamic acid at position 255 with lysine (E255K) and methionine at position 351 with threonine (M351T). Mutant Bcr-Abl constructs were inserted into MIG R1 vectors to generate MIG E255K and MIG M351T vectors. Replication-incompetent retroviruses were obtained by transient transfection of 293 cells with MIG R1, MIG 210, MIG E255K, or MIG M351T retroviral plasmids and the pCL-ampho plasmid followed by harvesting of supernants and titration of infectious virus as previously described.31
Retroviral transduction
Cord blood CD34+ cells were cultured in fibronectin CH-296 (Retronectin; Pan Vera, Madison, WI)–coated plates in serum-free medium (SFM; Stem Cell Technologies, Vancouver, BC) containing growth factors (GFs) (interleukin-3 [IL-3, 25 ng/mL]; interleukin-6 [IL-6, 10 ng/mL]; Flt-3 ligand [100 ng/mL]; stem cell factor [SCF, 50 ng/mL], and thrombopoietin [100 ng/mL]) at 37°C in 5% CO2. After 48 hours, cells were resuspended in virus supernants (multiplicity of infection [MOI]= 10), with the same GFs. This infection procedure was repeated after 24 hours. After additional culture for 48 hours, CD34+ cells were labeled with anti–CD34-APC antibodies (Becton Dickinson, San Jose, CA) and CD34+GFP+ cells selected using flow cytometry sorting (Dako-Cytomation, Fort Collins, CO). TF1 cells were transduced by culture with virus supernants at MOI of 10 for 2 consecutive days. Cells were maintained in RPMI 1640 medium containing 10% FBS and GM-CSF (2 ng/mL). For cells transduced with MIG 210 vectors, 2 separate populations were selected based on low levels of GFP expression (BAlo, fluorescence intensity between 101 to 102) or high levels of GFP expression (BAhi, fluorescence intensity greater then 102), as shown in Figures 1A and 5A. A similar strategy was used for cells transduced with MIG M351T and MIG E255K vectors. A single population of GFP+ cells was sorted from cells transduced with the control virus (MIG R1).
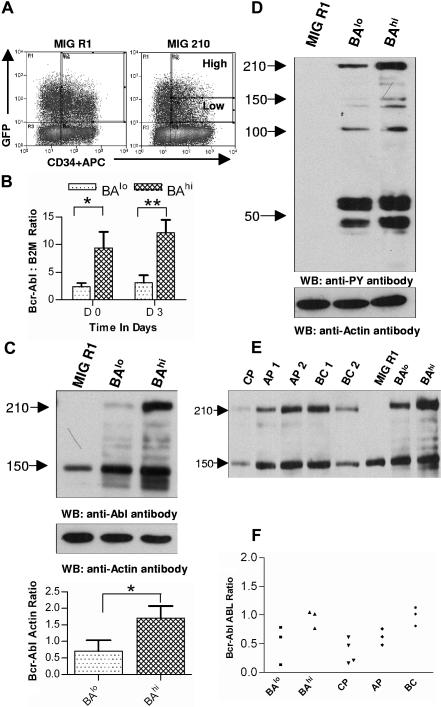
Figure 1.
Selection of CD34+ cells expressing high and low levels of Bcr-Abl. (A) Human CD34+ cells were transduced with MIG R1 and MIG 210 retrovirus as described in “Patients, materials, and methods.” Transduced CD34+ cells were selected by flow cytometry sorting. CD34+GFP-low (BAlo) and CD34+GFP-high (BAhi) populations were selected from MIG 210–transduced cells and a single CD34+GFP+ population was selected from MIG R1–transduced cells as shown. (B) Bcr-Abl mRNA expression in transduced cells was assessed using quantitative RT-PCR and expressed as the ratio of Bcr-Abl/β2M as described in “Patients, materials, and methods.” The results shown are for freshly sorted cells (day 0) and for cells cultured for 72 hours (on day 3). Significance levels for differences between BAlo and BAhi cells were *P < .02 and **P < .036. (C) Bcr-Abl protein expression in transduced cells was assessed by Western blotting. Cells were cultured for 48 hours after sorting and protein extracts prepared as described in “Patients, materials, and methods.” Western blotting was performed using anti-Abl antibodies. Blots were reprobed with an antiactin antibody to confirm equal sample loading. Significance levels between BAlo and BAhi cells is *P < .026. (D) Protein tyrosine phosphorylation in transduced cells was assessed by Western blotting using an antiphosphotyrosine (anti-PY) antibody. (E) Protein extracts were obtained from transduced CD34+ and CD34+ cells isolated from samples obtained from CML patients, and Western blotting was performed with anti-ABL and antiactin antibodies. Results from a representative experiment are shown. (F) Bcr-Abl/ABL ratios obtained by densitometric analysis of BAlo and BAhi cells (n= 3) and primary CML CD34+ cells (n= 10) are shown.
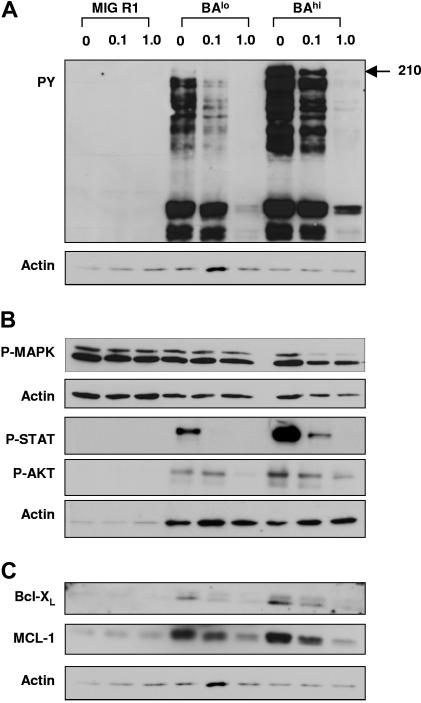
Figure 5.
Effect of IM treatment on Bcr-Abl kinase activity and downstream signaling pathways in CD34+ cells expressing low and high levels of Bcr-Abl. CD34+GFP+ cells were cultured in SFM with low GF with 0, 0.1, and 1.0 μM IM for 16 hours, following which protein extracts were prepared and Western blotting was performed. In each case, blots were reprobed with antiactin antibodies to determine sample loading. Representative blots are shown. Cumulative results of densitometry from multiple experiments are shown in Tables 1–2. (A) Western blotting with antiphosphotyrosine antibodies. (B) Western blotting with anti–P-MAPK, anti–P-AKT, and anti–P-STAT5 antibodies. (C) Western blotting with anti–Bcl-XS/L and anti–Mcl-1 antibodies.
Real-time quantitative RT-PCR (Q-PCR)
Quantitative PCR (Q-PCR) analysis for detection of p210 Bcr-Abl transcripts was performed as previously described using a real-time TaqMan assay and the ABI Prism 7700 sequence detector (Applied Biosystems, Foster City, CA).32 For each sample β2-microglobulin (β2M) levels were also measured as internal controls. Samples were run in duplicate, the amount of Bcr-Abl and β2M was calculated based on the standard curves, and results were expressed as a Bcr-Abl/β2M ratio. The assay was capable of linear detection of Bcr-Abl mRNA across 5 logs of input RNA.
Western blotting
Primary CML CD34+ cells, CD34+GFP+, and TF1+GFP+ cells were cultured with or without IM as indicated, and lysed in buffer containing 50 mM Tris (pH 7.4), 150 mM NaCl, 1 mM EDTA, 0.5% NP40, and 0.5% sodium deoxycholate, supplemented with protease and phosphatase inhibitors. Proteins were resolved on 4% to 20% or 7.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred to nitrocellulose membranes. Membranes were blocked with 10% nonfat dry milk in PBS with 0.1% Tween and labeled with appropriate dilutions of primary antibody anti-Abl: (Abl-3; CalBiochem, OP 20); antiactin (AC-15, A 5441; Sigma-Aldrich); antiphosphotyrosine (4G10; a kind gift from Dr Druker, Oregon Health Sciences University, Portland, OR); antiphosphorylated p42/44 MAPK (sc-7383), anti-p42/44 MAPK (sc-94), and anti-Stat5 (sc-835) (Santa Cruz Biotechnology, Santa Cruz, CA); antiphosphorylated Stat5 (pY694, 611964; BD Biosciences); antiphosphorylated Akt (Ser473, 9271) and anti-Akt (9272) (Cell Signaling Technology); Bcl-XS/L (S-18, sc-634) and Mcl-1 (S-19, sc-819) (Santa Cruz Biotechnology); followed by mouse or rabbit horseradish peroxidase–conjugated secondary antibody (1:6000; Jackson ImmunoResearch Laboratories, Bar Harbor, ME). Proteins bands were visualized using the Superfemto kit (Pierce Biotechnology, Rockford, IL). Relative quantitation of protein levels was performed using densitometric analysis.
Progenitor culture
CD34+GFP+ cells were incubated in SFM supplemented with GF at low concentrations similar to that found in stroma-conditioned medium (granulocyte-macrophage colony-stimulation factor [GM-CSF], 200 pg/mL; granulocyte colony-stimulating factor [G-CSF], 1 ng/mL; SCF, 200 pg/mL; leukemia inhibitory factor [LIF], 50 pg/mL; macrophage inflammatory protein α [MIP-1α], 200 pg/mL; and IL-6, 1 ng/mL)33–35 at 37°C in a humidified atmosphere with 5% CO2. The number of viable cells generated after culture was enumerated. CD34+GFP+ cells cultured for 7 day in SFM with low GF were analyzed for expression of myeloid and erythroid differentiation antigens. Cells were labeled with antibodies to CD33, CD11b, glycophorin A (Gly A), and CD36 and analyzed by flow cytometry (FACScalibur; Becton Dickinson).
Assessment of IM sensitivity
CD34+GFP+ cells were incubated in SFM supplemented with GF at low concentrations for 24 hours followed by exposure to IM (0 to 1.0 μM) in similar conditions for 72 hours. Cells were assayed for expansion in numbers, apoptosis, and proliferation.
MTS assay
CD34+GFP+ cells were cultured in 96-well plates (10 × 103 cells/well for MIG R1 and MIG 210; 5 × 103 cells/well for other cells) under conditions described in “Assessment of IM sensitivity.” TF-1+GFP+ cells were cultured in 96-well plates (10 × 103 cells/well for MIG R1 and MIG 210) with or without GM-CSF. Subsequently, viable cells were quantified using an MTS assay kit (Promega, Madison, WI) in accordance with the manufacturer's instructions. Triplicate determinations were made for each experimental point.
SNARF-1 proliferation assay
CD34+GFP+ cells were labeled with 20 μM succinimidyl ester of SNARF-1 carboxylic acid (Molecular Probes, Eugene, OR) at 37°C in the dark for 30 minutes, followed by washing. Cells were resuspended in SFM supplemented with low concentrations of GF, incubated at 37°C overnight to allow excess unbound dye to diffuse out, and cultured for an additional 72 hours with or without IM, and cell division was analyzed by flow cytometry. Each cell division results in progressive reduction in SNARF-1 intensity. The percentage of cells in each generation of cells was determined using ModFit software (Verity, Topsham, ME), with the position of the parent generation set on the basis of the fluorescence profile of an aliquot of cells treated with colcemid (0.1 μg/mL) immediately after sorting, and a proliferation index (PI) was generated as previously described.36
Assessment of apoptosis
CD34+GFP+ cells were cultured with or without serum and GF withdrawal as well as with or without IM treatment and assayed for apoptosis as described previously.36 Briefly cells were labeled with annexin V–Cy-5 and ViaProbe 7-amino-actinomycin D (7-AAD) (BD PharMingen, San Diego, CA), and analyzed by flow cytometry. Total apoptotic cells were defined as sum of annexin V–Cy-5+7AAD− and annexin V–Cy5+7-AAD+ cells.
Statistics
Results of data obtained from multiple experiments were reported as the mean ± 1 SEM. Significance levels were determined by Student paired t test analysis or where indicated by 1-way or 2-way ANOVA.
Results
Selection of human CD34+ cells with high or low levels of Bcr-Abl expression
Infectious virus particles (MIG R1, MIG 210, MIG M351T, MIG E255K) generated by transient transfection of 293 cells were used to transduce human CD34+ cells. Transduced CD34+GFP+ cells were selected by flow cytometry sorting. Since the MIG 210 vector coexpresses the GFP and BCR/ABL gene, we reasoned that expression levels of Bcr-Abl would correspond to levels of expression of the GFP gene. Two populations of MIG 210 (wild type or mutants)–transduced CD34+GFP+ cells were selected based on low or high levels of GFP expression (BAlo and BAhi) as shown in Figure 1A. For cells transduced with the control MIG R1 vector expressing GFP alone, a single population of CD34+GFP+ cells was selected.
We assessed whether Bcr-Abl expression levels corresponded to GFP gene expression levels in selected CD34+GFP+ cells. Increased expression of Bcr-Abl mRNA was seen in BAhi compared to BAlo cells (4.69 ± 1.09-fold increase in Bcr-Abl/β2M levels in BAhi compared to BAlo cells immediately after sorting [day 0, n= 5], and 5.53 ± 1.90-fold increase in cells cultured for 72 hours after sorting [day 3, n= 3]) (Figure 1B). Western blot analysis confirmed increased Bcr-Abl protein expression in BAhi compared to the BAlo cells (the Bcr-Abl to actin ratio was 4.01 ± 2.07-fold higher in BAhi compared to BAlo cells [n= 3]) (Figure 1C). BAhi cells demonstrated increased tyrosine kinase activity and altered patterns of tyrosine phosphorylation compared to BAlo cells as assessed by Western blot analysis using antiphosphotyrosine antibodies. Both BAlo and BAhi cells exhibited increased phosphotyrosine levels compared to control cells expressing GFP alone (Figure 1D). These results confirm our ability to select cells with different levels of Bcr-Abl expression and allow us to investigate the role of Bcr-Abl expression level in cell transformation and sensitivity to IM.
We evaluated whether Bcr-Abl expression levels in transduced CD34+ cells are comparable to Bcr-Abl expression in CD34+ cells obtained from CML patients. As previously reported, Bcr-Abl protein expression tended to increase with disease progression (the average Bcr-Abl to ABL ratio in was CP 0.36 ± 0.11 [n= 4]; in AP, 0.62 ± 0.081 [n= 3]; and in BC, 0.98 ± 0.094 [n= 3]) (Figure 1E). The range of Bcr-Abl expression in BAlo cells (0.51 ± 0.19, n= 3) was consistent with that in CML CP CD34+ cells, and the range in BAhi (0.95 ± 0.089, n= 3) cells was consistent with that in CML BC patients (Figure 1F).
Effects of higher Bcr-Abl expression levels on CD34+ cell differentiation, proliferation, and apoptosis
Mechanisms proposed to explain myeloid expansion in CML include increased proliferation rate, unregulated proliferation, and reduction in apoptosis.37,38 To determine whether Bcr-Abl expression levels affected CD34+ cell proliferation, we evaluated the expansion of transduced CD34+ cells following culture in media containing low GF for 7 days. As shown in Figure 2A, there was increased expansion in numbers of both BAlo and BAhi cells compared to MIG R1–transduced cells (control cells). However, expansion of BAhi cells was significantly greater than that of BAlo cells (expansion of control cells was 2.1 ± 0.26; BAlo, 20.93 ± 6.27; and BAhi, 115.0 ± 41.55; n= 6).
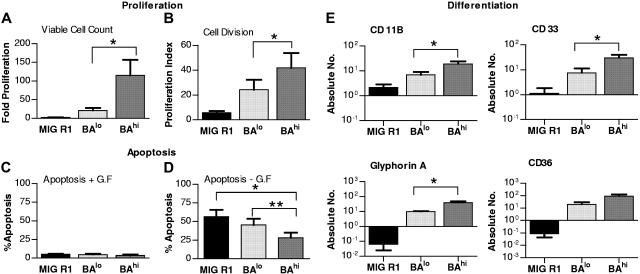
Figure 2.
Effect of high and low Bcr-Abl expression levels on CD34+ cell proliferation, apoptosis, and differentiation. (A) The number of cells generated after culture of control, BAhi, and BAlo CD34+ cells for 7 days in SFM containing low growth factor was determined. The data shown represent the mean ± SEM fold-cell expansion for 6 individual experiments. Significance values for differences between BAhi and BAlo cells is *P < .05. (B) Transduced CD34+ cells (n= 4) were labeled with the fluorescent dye SNARF-1 and cultured for 72 hours followed by assessment of SNARF-1 fluorescence by flow cytometry. Each cell division results in a diminution in SNARF fluorescence. (A) Proliferation index (PI) was calculated from the flow cytometry data using ModFit software. Significance value for differences between BAhi and BAlo cells is *P < .05. (C) Transduced CD34+ cells (n= 5) were cultured in media with or without serum and GF for 48 hours. Cells were labeled with annexin V–Cy-5 and 7-AAD and apoptosis was assessed by flow cytometry. The figure shows total apoptotic cells following culture in serum and GF-containing medium. (D) Total apoptotic cells following culture without serum and GF. Significance values for differences are *P < .003, MIG R1 versus BAhi; **P < .006, BAlo versus BAhi. (E) Transduced CD34+ cells (n= 4) cultured for one week in SFM with low GF and analyzed by flow cytometry for erythroid and myeloid differentiation. The figure shows absolute number (log10 scale) of cells expressing the different phenotypic marker generated per 25 000 input cells. Significance value for differences between BAhi and BAlo cells is *P < .05, for CD11b, CD33, and glycophorin A.
Increased cell expansion may reflect increased cell proliferation or reduced apoptosis. We examined the effects of increased Bcr-Abl expression levels on cell proliferation by labeling transduced cells with the fluorescent-tracking dye SNARF-1, culturing for 72 hours in GF-containing media, and analyzing for cell division by flow cytometry. A marked increase in the proliferation index (PI) was seen in BAlo and BAhi cells compared to controls. As was seen with cell expansion, BAhi cells demonstrated increased proliferation compared to BAlo cells (P.I for MIG R1 transduced controls was 5.25 ± 1.02; BAlo, 24.27 ± 8.1; and BAhi, 41.83 ± 11.94) (Figure 2B).
To determine if Bcr-Abl levels influenced antiapoptotic signaling in CD34+ cells, we examined cell viability following 48-hour deprivation of serum and GF. There were no significant differences in apoptosis between control, BAlo, and BAhi CD34+ cells cultured in serum and GF-containing culture conditions (Figure 2C). On the other hand, BAhi CD34+ cells demonstrated significantly reduced apoptosis following serum and GF deprivation compared to BAlo or control GFP-expressing CD34+ cells (n= 5) (Figure 2D). These results indicate that high levels of Bcr-Abl expression protect cells from apoptosis following GF withdrawal, but that CD34+ cells expressing lower levels of Bcr-Abl, such as CP CML cells, are not protected from apoptosis following GF withdrawal.
Immunophenotypic analyses indicated that Bcr-Abl–expressing cells generated higher number of both myeloid (CD11b and CD33) and erythroid (glycophorin A and CD36) cells compared to control CD34+ cells (Figure 2E). Erythroid progenitors were especially increased compared to control cells, which remained predominantly myeloid (glycophorin A+ erythroid cells: MIG R1, 1.5% ± 0.3%; BAlo, 51.6% ± 6.8%; and BAhi, 65.7% ± 11.2%; P < .001 for MIG R1 compared to BAlo and BAhi cells; CD11b+ myeloid cells: MIG R1, 45.3% ± 8.9%; BAlo, 13.8% ± 3.1%; and BAhi, 7.6% ± 1.3%, P < .001 for MIG compared to BAlo and BAhi cells, significance determined by one-way ANOVA, n= 5). BAhi progenitors generated higher numbers of both myeloid and erythroid cells compared to BAlo cells (P < .05) (Figure 2E). Therefore, Bcr-Abl expression was associated with enhanced erythroid differentiation, which was accentuated by enhanced gene expression levels.
Higher Bcr-Abl expression levels in CD34+ cells are associated with increased STAT5 activity and antiapoptotic signaling
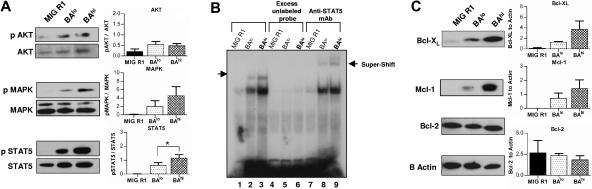
We evaluated the effect of increased levels of BCR/ABL gene expression in CD34+ cells on the activity of candidate downstream signaling mechanisms (Figure 3). BAhi cells demonstrated increased STAT5 phosphorylation (Figure 3A) and increased STAT5 promoter-binding activity (Figure 3B) compared to BAlo cells. A trend toward increased MAPK phosphorylation was seen in BAhi cells. AKT phosphorylation was increased in Bcr-Abl–expressing cells but not consistently increased in BAhi compared to BAlo cells. BAhi cells demonstrated increased expression of the antiapoptotic proteins Bcl-XL and Mcl-1 compared to BAlo cells (Figure 3C). In contrast Bcl-2 levels were similar in control and Bcr-Abl–expressing cells. These results indicate that higher Bcr-Abl expression in CD34+ cells is associated with enhanced activation of downstream proliferative and antiapoptotic signaling mechanisms.
Figure 3.
Downstream molecular mechanisms of CD34+ cells expressing high and low levels of Bcr-Abl. CD34+GFP+ cells were grown in SFM containing low GF for 7 days followed by preparation of total cell lysates for Western blotting and nuclear extracts for EMSA assays. (A) Results of Western blotting for total and phosphorylated AKT (n= 4), MAPK (n= 4), and STAT5 (n= 5) in control and Bcr-Abl–expressing cells. Representative blots are shown on the left, and cumulative results from densitometric analysis of multiple experiments are shown in the graphs on the right. Significance level comparing BAlo and BAhi cells is *P < .014. (B) We further analyzed the promoter-binding activity of STAT5 using electromobility shift assay (EMSA) (lanes 1-3). Specificity of binding is shown by blockage by addition of excess unlabeled probe to the binding reaction (lanes 4-6). The presence of STAT5 protein within the complex is shown by supershift following addition of anti-STAT5 antibodies to the binding reaction (lanes 7-9). (C) Western blot analysis of expression of antiapoptotic proteins Bcl-XS/L, Mcl-1, and Bcl-2. Representative blots are shown on the left and cumulative results from densitometric analysis of multiple experiments are shown in the graphs on the right.
CD34+ cells expressing higher levels of Bcr-Abl demonstrate increased sensitivity to IM
We next investigated the effect of Bcr-Abl expression levels on sensitivity of CD34+ cells to inhibition by IM. CD34+GFP+ cells were cultured in low GF conditions in the presence or absence of IM (0.05 to 1 μM) for 72 hours, followed by quantitation of viable cells using an MTS assay. As shown in Figure 4A, both BAlo and BAhi cells demonstrated enhanced sensitivity to IM compared to control cells. However, at all IM concentrations, BAhi cells were more sensitive to IM than BAlo cells. The IC50 for IM for BAhi cells was 0.025 μm, while the IC50 for BAlo cells was almost 10-fold higher at 0.2 μM.
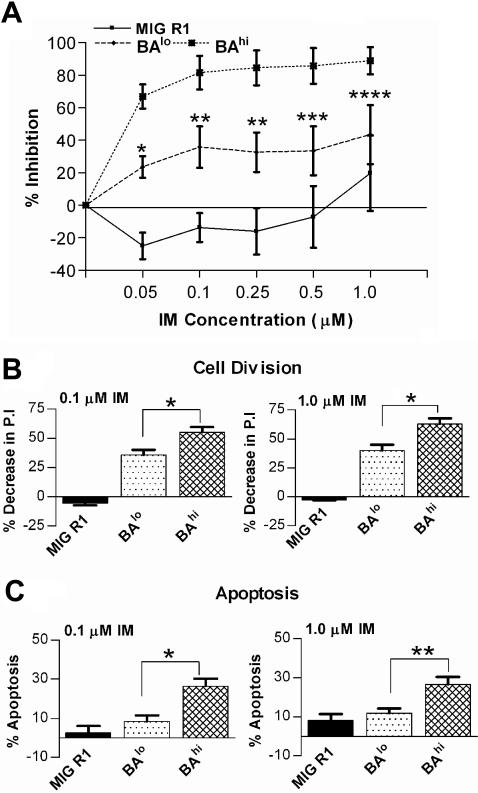
Figure 4.
Effect of IM on growth of CD34+ cells expressing high and low levels of Bcr-Abl. (A) The number of viable cells present after culture of CD34+GFP+ cells for 72 hours with or without IM (0.05-1.0 μM) was determined using an MTS assay. The results shown were obtained from 3 independent experiments in which each experimental point was the mean of triplicate determinations. Significance levels are *P < .001; **P < .005; ***P < .05; and ****P < .016, BAlo versus BAhi. (B) Cell division of CD34+GFP+ cells after culture for 72 hours with or without IM (0.1 and 1.0 μM) was measured using a SNARF-1 labeling assay as described in “Patients, materials, and methods.” A PI was determined using ModFit software. Significance level for BAlo versus BAhi in presence of 0.1 μM and 1.0 μM IM is *P < .001. (C) Apoptosis of CD34+GFP+ cells after culture for 72 hours with or without IM (0.1 and 1.0 μM) was measured using annexin V–Cy5 and 7-AAD labeling and was analyzed by flow cytometry. The data shown represent the mean ± SEM values of results for 5 experiments. Significance levels for BAlo versus BAhi in presence of 0.1 μM and 1.0 μM IM are *P < .002 and **P < .009, respectively.
The effect of IM on cell division was assessed by labeling cells with SNARF-1 and culture with IM (0-1.0 μM) under low GF conditions for 72 hours followed by analysis for cell division by flow cytometry. BAhi cells exposed to 0.1 μM IM had significantly greater reduction of cell division (55.11 ± 4.31) compared with the BAlo cells (35.48 ± 4.42, n= 3) (Figure 4B). Further reduction in cell proliferation was seen on exposure to 1.0 μM IM, with the effect of IM on BAhi cells remaining greater than that on BAlo cells. Cells were also analyzed for induction of apoptosis following culture with IM for 72 hours. Exposure to IM (0.1 μM) resulted in significantly increased apoptosis in BAhi cells compared to BAlo cells or control cells (26.4% ± 3.93%, 8.39% ± 2.98%, and 2.54% ± 3.45% increase in apoptosis, respectively, n= 5) (Figure 4C). These results indicate that inhibition of Bcr-Abl–induced proliferation is an important mechanism for IM-induced inhibition of both BAlo and BAhi cell growth. IM also induces significant apoptosis in BAhi but not BAlo cells, indicating increased dependence on Bcr-Abl kinase activity for survival in CD34+ cells expressing higher levels of Bcr-Abl. Increased sensitivity of BAhi cells compared to BAlo cells to IM is related to both increased inhibition of proliferation as well as increased apoptosis.
Western blotting for tyrosine phosphorylated proteins including Bcr-Abl indicated that IM resulted in similar inhibition of Bcr-Abl kinase activity in BAhi and BAlo cells (Figure 5; Tables 1–2). BAhi and BAlo cells demonstrated reduction in AKT, STAT5, and MAPK phosphorylation on IM treatment, with significantly enhanced inhibition of AKT phosphorylation in BAhi cells compared to BAlo cells. IM treatment also resulted in reduced expression of the antiapoptotic proteins Mcl-1 in BAhi and BAlo cells, with a trend toward enhanced inhibition in BAhi cells. We also observed a trend toward reduced Bcl-XL after IM treatment. These results indicate that IM treatment effectively inhibits Bcr-Abl kinase activity in both BAlo and BAhi CD34+ cells and results in significant inhibition of downstream proliferative and antiapoptotic pathways.
Table 1.
Effect of IM treatment on Bcr-Abl kinase activity and downstream signaling pathways in CD34+ cells expressing low and high levels of Bcr-Abl: relative activity
| IM, μM | MIG R1 |
BAlo |
BAhi |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.00 | 0.10 | 1.00 | 0.00 | 0.10 | 1.00 | 0.00 | 0.10 | 1.00 | |
| P-Bcr-Abl, n = 4 | NE | NE | NE | 1.78 ± 1.06 | 0.46 ± 0.2 | 0.09 ± 0.08 | 5.67 ± 3.46 | 2.06 ± 1.04 | −0.39 ± 0.59 |
| P-Akt, n = 3* | 0.24 ± 0.16 | 0.21 ± 0.07 | 0.31 ± 0.13 | 0.43 ± 0.18 | 0.32 ± 0.09 | 0.32 ± 0.22 | 0.94 ± 0.44 | 0.47 ± 0.23 | 0.15 ± 0.06 |
| P-MAPK, n = 3† | 0.26 ± 0.11 | 0.18 ± 0.03 | 0.27 ± 0.07 | 1.14 ± 0.35 | 0.20 ± 0.09 | 0.27 ± 0.10 | 1.75 ± 0.49 | 0.50 ± 0.16 | 0.20 ± 0.05 |
| P-STAT, n = 4† | 0.28 ± 0.04 | 0.37 ± 0.11 | 0.39 ± 0.12 | 1.66 ± 0.28 | 0.60 ± 0.32 | 0.42 ± 0.22 | 3.01 ± 1.62 | 0.80 ± 0.33 | 0.10 ± 0.03 |
| Bcl-XL, n = 3 | 1.48 ± 0.41 | 1.57 ± 0.51 | 1.52 ± 0.28 | 1.93 ± 0.16 | 1.36 ± 0.54 | 1.81 ± 0.75 | 5.81 ± 2.56 | 3.63 ± 1.46 | 2.49 ± 0.96 |
| Mcl-1, n = 2 | 0.5 ± 0.09 | 0.5 ± 0.03 | 0.71 ± 0.08 | 3.13 ± 0.18 | 1.37 ± 0.67 | 1.56 ± 0.34 | 7.33 ± 0.89 | 2.35 ± 0.29 | 0.91 ± 0.41 |
Western blotting was performed as described in “Patients, materials, and methods” and as shown in Figure 5. Results were normalized to levels of actin on the same blots. Differences related to Bcr-Abl expression levels and IM exposure were analyzed using 2-way ANOVA; values are mean ± SEM.
BAlo indicates Bcr/ABL low; BAhi, BCR/ABL high; IM, imatinib mesylate; and NE, not evaluable.
P < .05 for IM-treated versus untreated cells.
P= .051 for BAlo versus BAhi cells.
Table 2.
Effect of IM treatment on Bcr-Abl kinase activity and downstream signaling pathways in CD34+ cells expressing low and high levels of Bcr-Abl: percentage inhibition with IM treatment
| IM, μM | MIG R1 |
BAlo |
BAhi |
|||
|---|---|---|---|---|---|---|
| 0.10 | 1.00 | 0.10 | 1.00 | 0.10 | 1.00 | |
| P-Bcr-Abl, n = 3* | NE | NE | 68.7 ± 7.6 | 91.8 ± 6.8 | 55.2 ± 9.3 | 97.5 ± 6.1 |
| P-Akt, n = 3†‡ | −45.5 ± 41.7 | −23.12 ± 32.1 | 12.5 ± 16 | 29.1 ± 28.9 | 44.5 ± 9.0 | 80.2 ± 4.7 |
| P-MAPK, n = 3* | 8.5 ± 28.3 | −9.34 ± 4.7 | 69.6 ± 21.4 | 58 ± 28.5 | 66.2 ± 5.5 | 85.6 ± 2.8 |
| P-STAT5, n = 4* | −28 ± 36 | −32 ± 39 | 66.6 ± 13.1 | 73.5 ± 11.5 | 67.2 ± 5.4 | 94.2 ± 2.5 |
| Bcl-XL, n = 3 | −2.03 ± 10.72 | −13.59 ± 23.66 | 23.98 ± 31.93 | 0.51 ± 43.17 | 36.38 ± 8.20 | 53.8 ± 4.6 |
| Mcl-1, n = 2 | −46.41 ± 67.25 | −109.0 ± 103.9 | 61.05 ± 17.89 | 54.97 ± 7.9 | 71.82 ± 3.8 | 89.15 ± 4.77 |
Results are expressed as percentage relative to untreated control cells. Differences related to Bcr-Abl expression levels and IM exposure were analyzed using 2-way ANOVA; values are mean ± SEM.
P < .001[AU21] for IM-treated versus untreated cells.
P < .05 for IM-treated versus untreated cells.
P = .01 for BAlo versus BAhi cells.
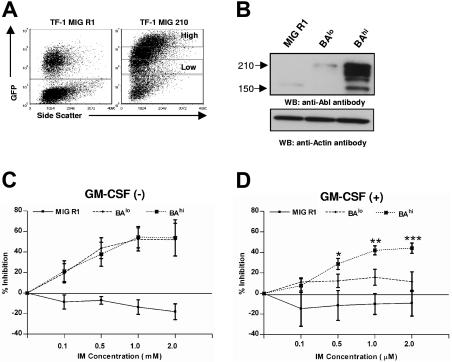
Increased inhibition by IM of CD34+ cells expressing increased levels of Bcr-Abl observed here contrasts with previous studies indicating that hematopoietic cell lines with increased Bcr-Abl expression showed reduced IM sensitivity. However, in these reports, cell lines expressing variable levels of Bcr-Abl were derived by culture and subcloning over long periods of time, which could have been associated with acquisition of additional abnormalities besides altered Bcr-Abl expression levels. We therefore used a similar flow cytometry–based strategy to select TF1 cells expressing low and high levels of GFP and Bcr-Abl, as was used for selection of BAlo and BAhi CD34+ cells (Figure 6A). Western blotting confirmed increased expression of Bcr-Abl in TF-1BAhi compared to TF-1BAlo cells (Figure 6B). TF-1 cells expressing low and high Bcr-Abl levels demonstrated similar sensitivity to IM in the absence of GM-CSF (Figure 6C). However TF-1BAhi cells demonstrated increased sensitivity to IM compared to TF-1BAlo cells in the presence of GM-CSF (1 ng/mL) (Figure 6D). These observations suggest that the viability of IM-treated cells expressing low levels of Bcr-Abl can be at least partially maintained by exogenous GF. In contrast, cells expressing high levels of Bcr-Abl are more dependent on Bcr-Abl kinase activity for survival and cannot be rescued by exogenous GF following Bcr-Abl kinase inhibition.
Figure 6.
Effect of IM on growth of TF-1 BCR/ABL cell line expressing high and low levels of Bcr-Abl. (A) TF-1 hematopoietic cells were transduced with MIG R1 and MIG 210 retrovirus as described in “Patients, materials, and methods.” GFP-low (TF-1-BAlo) and GFP-high (TF-1-BAhi) populations were selected from MIG 210–transduced cells by flow cytometry sorting as shown. A single GFP+ population was selected from MIG R1–transduced cells. Cells were maintained with 2 ng/mL GM-CSF. (B) Bcr-Abl protein expression in transduced cells was assessed by Western blotting using anti-Abl antibodies. Blots were reprobed with an antiactin antibody to check sample loading. A representative blot is shown. The ratio of intensity of Bcr-Abl to actin for TF-1 BAlo and TF-1 BAhi cells from multiple experiments was 0.44 ± 0.20 and 0.93 ± 0.13, respectively (n= 4, P < .012). (C-D) TF1-1/GFP+ cells were plated at density of 10 000 cells/well in absence of GM-CSF or in presence of 1 ng/mL GM-CSF in the presence of graded concentrations of IM as shown and viable cells present after 72 hours culture assessed in a MTS assay. The results shown represent 3 independent experiments in which each experimental point was the mean of triplicate determinations. Significance levels comparing BAlo versus BAhi cells are *P= .078; **P= .025; and ***P= .056.
Effect of level of gene expression levels on IM sensitivity of CD34+ cells expressing Bcr-Abl kinase domain mutants, M351T and E255K
Mutations in the Bcr-Abl kinase domain are a major cause of resistance to kinase inhibition by IM. It has been suggested that enhanced Bcr-Abl expression may be required to develop a fully IM-resistant phenotype in cells expressing Bcr-Abl mutants with intermediate IM resistance.14 To study interactions between kinase mutation and gene expression levels in determining IM sensitivity, we established a novel model for IM-resistant CML by expressing kinase-mutated BCR/ABL genes in CD34+ cells. We investigated whether the expression level of 2 Bcr-Abl kinase domain mutants, M351T and E255K, associated with moderate and high degree of resistance to IM, respectively, in CD34+ cells affected response to IM. CD34+ cells transduced with the MIG M351T and MIG E255K vectors were sorted into GFP-high and GFP-low populations as previously described for MIG 210–transduced cells (M351Thi and M351lo, E255Khi and E255Klo). We confirmed increased levels of BCR/ABL gene expression in M351Thi and E255Khi cells compared to M351lo and E255Klo cells by Western blotting (data not shown). The M351T mutant when expressed in cell lines is associated with moderate resistance to IM (IC50 of 4.38 μM compared to 0.6 μM for wild-type Bcr-Abl).17 Consistent with this, M351Tlo cells demonstrated moderate levels of resistance to IM compared to cells expressing wild-type Bcr-Abl (Figure 7A). M351Thi cells were significantly more sensitive to higher concentrations of IM (0.5 and 1.0 μM) compared to M351Tlo cells. On the other hand, CD34+ cells expressing high and low levels of the E255K mutant, associated with high degree of IM resistance (IC50 for E255K > 10 μM17), demonstrated a higher degree of resistance to inhibition by IM as expected. E255Khi and E255Klo CD34+ cells demonstrated a similar response of IM treatment (Figure 7A).
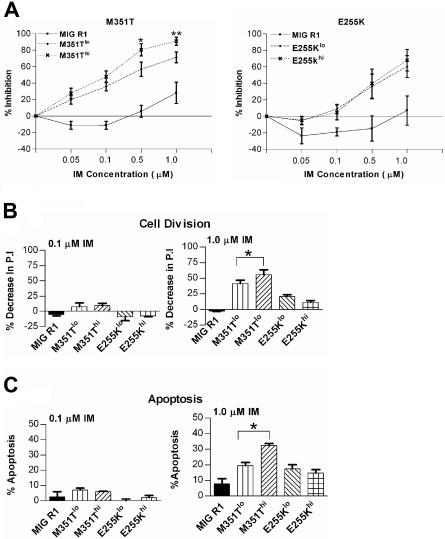
Figure 7.
Effects of IM treatment on growth of CD34+ cells expressing kinase-domain mutant BCR/ABL genes. CD34+ cells were transduced with retroviral vectors expressing the M351T and E255K BCR/ABL kinase-domain mutant genes and CD34+GFPlo (M351T low and E255K low) and CD34+GFPhi (M351T high and E255K high) cells were selected as shown for MIG 210–expressing cells in Figure 1A. (A) The number of viable cells present after culture of M351T- and E255K-expressing cells for 72 hours with or without IM (0.05-1.0 μM) was determined using an MTS assay. Significance levels are for M351Tlo versus M351Thi were *P < .010 and **P < .101; n= 3. (B) Cell division of M351T- and E255K-expressing cells (n= 4) after culture for 72 hours with or without IM (0.1 and 1.0 μM) was measured using a SNARF-1 labeling assay. Significance levels are shown for 1.0 μM IM: *P < .018, comparing M351Tlo and M351Thi. (C) Apoptosis of M351T- and E255K-expressing cells (n= 4) after culture for 72 hours with or without IM (0.1 and 1.0 μM) was measured by annexin V–Cy5 and 7-AAD labeling and flow cytometry. Significance levels are shown for 1.0 μM IM: *P < .009, comparing M351Tlo and M351Thi.
The reduction in proliferation of CD34+ cells expressing M351T and E255K mutants on exposure to 0.1 μM IM as measured by SNARF-1 labeling was much less than that observed for cells expressing wild-type Bcr-Abl. Exposure to higher IM concentrations (1.0 μM) resulted in greater inhibition of proliferation of M351Thi cells (55.86 ± 8.08, n= 4) compared to M351Tlo cells (41.61 ± 5.23, n= 4) (Figure 7B). Exposure to higher IM concentrations (1.0 μM) did not result in significant differences in inhibition of proliferation in cells expressing low and high levels of the E255K mutant (Figure 7B).
Induction of apoptosis observed following exposure to 0.1 μM IM (Figure 7C) was markedly reduced in CD34+ cells expressing both low and high levels of the M351T and E255K mutants compared to wild-type Bcr-Abl–expressing cells. M351Thi cells exposed to 1.0 μM IM (Figure 7C) demonstrated higher rate of apoptosis compared to the M351Tlo cells (32.44% ± 1.31% versus 19.48% ± 2.06% apoptosis, respectively, n= 4), E255Klo cells and E255Khi cells had similar rates of apoptosis (17.58% ± 2.57% and 14.71% ± 2.3% apoptosis, respectively, n= 5) following exposure to 1.0 μM IM.
These results indicate that expression of kinase domain mutant BCR/ABL genes in CD34+ cells was associated with resistance to inhibition by IM, but that IM resistance was not increased as a result of increased gene expression. Increased expression of the M351T mutant, causing intermediate levels of IM resistance, resulted in increased sensitivity to IM-mediated growth inhibition, which was related to both reduced proliferation and increased apoptosis. These results are similar to those obtained with cells expressing wild-type Bcr-Abl. For cells expressing the E255K mutant associated with high levels of resistance to IM, increasing gene expression levels did not change the IM response.
Discussion
Increased Bcr-Abl expression levels in CML cells have been associated with disease progression and IM resistance. In the present study, we used an experimental approach to directly investigate the relationship between BCR/ABL gene expression levels and the transformed phenotype and sensitivity to tyrosine kinase inhibition of primary human progenitor cells. We used a human model of CML hematopoiesis based on ectopic expression of the BCR/ABL gene in human CD34+ cells to select cells for low versus high expression of Bcr-Abl using GFP as a marker of transgene expression levels. We show that increasing levels of Bcr-Abl expression were associated with enhanced proliferation and resistance to apoptosis of CD34+ cells, but with enhanced rather than reduced IM sensitivity. These results have several important implications for our understanding of mechanisms of hematopoietic cell transformation and response to IM in CML.
Our results indicate that Bcr-Abl–induced perturbations in CD34+ cell proliferation and apoptosis are modulated by the levels of BCR/ABL gene expression. Increased Bcr-Abl expression levels result in significantly higher rates of CD34+ cell proliferation and enhanced myeloid and erythroid cell expansion. Protection from apoptosis following GF withdrawal was seen only with higher Bcr-Abl expression levels. Consistent with this, increased Bcr-Abl expression was associated with enhanced expression of antiapoptotic proteins Bcl-XL and Mcl-1, possibly related to increased activation of STAT5 and MAPK in these cells.39,40 These results suggest that CD34+ cells expressing lower levels of Bcr-Abl demonstrate growth properties similar to those observed with primary progenitors from CP CML patients, with enhanced proliferation in response to GF stimulation but without protection from apoptosis on GF withdrawal, whereas the effects of increased Bcr-Abl expression are similar to changes seen with disease transformation from CP to BC. Indeed Bcr-Abl protein levels in CD34+ cells from CP CML patients were comparable to those in BAlo cells; from BC CML patients, comparable to BAhi CD34+ cells; and from AP CML patients, intermediate between BAlo and BAhi cells. Other studies also suggest that disease progression in CML is associated with increased cellular expression of Bcr-Abl mRNA41–43 and protein.44 Taken together, our results support the concept that increased Bcr-Abl expression levels may play a role in progenitor growth alterations associated with disease progression in CML and indicate possible molecular mechanisms underlying this effect.
Another important observation made in this study was that higher levels of Bcr-Abl expression in human hematopoietic cells were associated with increased sensitivity to inhibition by IM rather than with IM resistance. Increased IM sensitivity was related to both increased inhibition of proliferation as well as significant induction of apoptosis in BAhi CD34+ cells, consistent with increased Bcr-Abl–driven proliferation and survival signaling. IM treatment effectively inhibited Bcr-Abl kinase activity in both BAlo and BAhi CD34+ cells and inhibited downstream signaling through AKT, STAT5, and MAPK. In fact, increased inhibition of AKT activity was observed in cells expressing high levels of Bcr-Abl. In addition, Mcl-1 expression is also inhibited, which is possibly related to inhibition of these mechanisms.39,40 TF-1 hematopoietic cells with low Bcr-Abl expression could be partially protected from IM-mediated growth inhibition by GM-CSF, whereas cells expressing high levels of Bcr-Abl were oncogene dependent and could not be rescued by GM-CSF following kinase inhibition. These observations suggest that increased Bcr-Abl expression may lead to increased dependence on Bcr-Abl–dependent signals and thereby increased sensitivity to Bcr-Abl kinase inhibition. Potential mechanism of oncogene dependence include inability of transformed cells to cope with loss of oncogene-conferred survival and proliferation signals or with slower attenuation of intrinsic tumor suppression or negative regulatory pathways activated in response to the oncogene following inhibition.45 Our results are in contrast to other reports that cell lines expressing high levels of Bcr-Abl expression have reduced sensitivity to IM compared to lines with lower Bcr-Abl expression.9–14 Association of IM resistance with higher Bcr-Abl levels in previous studies may reflect acquisition of other abnormalities contributing to drug resistance during the derivation, subcloning, and passage of these lines. Some differences between effects of Bcr-Abl overexpression in CD34+ cells and cell lines may also represent intrinsic differences in Bcr-Abl responses in different cell types, and emphasizes the importance of using the relevant oncogene expression levels and cellular context when studying Bcr-Abl transformation mechanisms and response to therapeutic interventions.
BCR/ABL gene amplification and/or increased Bcr-Abl transcript levels are believed to contribute to drug resistance in a subset of IM-resistant patients. However, our results indicate that increased Bcr-Abl levels may not directly result in IM resistance, in these patients. However, we cannot exclude the possibility that even higher levels of Bcr-Abl expression than were achieved in this study could induce IM resistance in primary hematopoietic cells. Although high Bcr-Abl expression levels may not directly cause drug resistance, persistent elevation of Bcr-Abl kinase activity may indirectly increase the risk of acquiring mutations causing IM resistance by causing an increase in genomic instability.28–30 Besides mutations in the Bcr-Abl kinase domain, genetic alterations leading to Bcr-Abl–independent activation of Src kinase or other growth regulatory pathways could also contribute to IM resistance.24 Increased Bcr-Abl expression levels could also lead to reduced drug sensitivity through mechanisms other than mutation induction. For example, increased Bcr-Abl levels can activate important cellular transformation mechanisms such as MYC through altered mRNA translation of proteins.46 In this context, it is notable that cell lines with increased Bcr-Abl expression levels develop IM resistance more rapidly than cells with low expression.14 Further studies investigating the relationship between elevated Bcr-Abl expression levels and genomic instability in primary progenitor cells are warranted.
Point mutations in the ABL kinase domain may result in varying degrees of reduction in IM binding and kinase inhibition and loss of response to IM in CML patients.23 It has been suggested that enhanced Bcr-Abl expression may be required to develop a fully IM-resistant phenotype in cells expressing Bcr-Abl mutants with intermediate IM resistance.14 To study the interaction of kinase mutation and gene expression levels in determining IM sensitivity in human progenitor cells, we established a novel model for IM-resistant CML based on the ectopic expression of kinase-mutated BCR/ABL genes in CD34+ cells. Expression of the M351T Bcr-Abl mutant, which retains partial IM sensitivity, in CD34+ cells was associated with intermediate level of IM resistance, whereas expression of the highly resistant E255K mutant resulted in a high degree of resistance to IM. These results validate this approach to studying IM-resistant CML. As was observed for cell expressing wild-type BCR/ABL genes, CD34+ cells expressing higher levels of the M351T mutant demonstrated increased sensitivity to IM compared to cells expressing lower levels of the gene. In contrast, cells expressing both low and high levels of the highly resistant E255K mutant had similar degrees of IM resistance. Our results suggest that overexpression of mutant BCR/ABL genes does not enhance IM resistance in primary progenitor cells.
One limitation of IM treatment of CML is that the drug, although highly effective in the inducing remissions, fails to eliminate all leukemia cells. Residual leukemia stem and progenitor cells appear to persist in IM-responsive patients and are a potential source of relapse. Potential mechanisms that may lead to persistence of primitive CML cells in IM-treated patients include the limited induction of apoptosis by IM particularly in nondividing cells,36,47,48 maintenance of viability through microenvironmental signals,49 non–kinase-dependent signaling from Bcr-Abl,24–27 and the presence within a subset of progenitors of Bcr-Abl kinase mutations32 or other molecular abnormalities that can result in resistance to IM. The latter mechanisms are more likely to be present in patients with higher Bcr-Abl expression and more advanced disease. Primitive CD34+CD38− hematopoietic cells express higher levels of Bcr-Abl transcripts and protein than more mature CD34+CD38+ cells.50,51 It remains possible that the effects of Bcr-Abl overexpression may differ in the context of the more primitive CD34+CD38− cells, and the effects of Bcr-Abl expression levels in this specific population need to be evaluated in future studies.
We had previously reported that in our human progenitor model of CML, ectopic Bcr-Abl expression in human CD34+ cells was associated with reduced GF dependence for survival and differed in this respect from primary CML progenitors.31 Our current results indicate that selection of BAlo cells refines and improves the human progenitor model of CML, making it more representative of primary CP CML progenitors. Moreover, the response of BAlo CD34+ cells to IM treatment was similar to that previously observed for primary CML progenitors,36 with dose-dependent growth inhibition primarily related to inhibition of abnormally increased proliferation rather than induction of apoptosis. These observations further suggest the utility of this model for studying mechanisms of Bcr-Abl transformation of human hematopoietic cells, and the response of Bcr-Abl–expressing human progenitors to therapeutic interventions.
In summary, we have used an experimental approach to directly demonstrate that overexpression of Bcr-Abl in CD34+ cells is associated with increased proliferation and increased resistance to apoptosis on growth factor withdrawal, and that increased dependence on Bcr-Abl–driven proliferation and apoptosis in cells expressing high levels of Bcr-Abl is associated with increased susceptibility to withdrawal of the Bcr-Abl–driven proliferative and antiapoptotic stimuli following treatment with Bcr-Abl kinase inhibitors. Our results emphasize the importance of gene expression levels and the cellular context in determining the effects of Bcr-Abl on hematopoietic cell transformation in CML and response to IM. Future studies will explore the relationship of enhanced Bcr-Abl expression and the acquisition of additional abnormalities leading to disease progression and IM resistance in primitive hematopoietic cells.
Acknowledgments
This work was supported by NIH grants R01 HL77847 and R01 CA95684 (R.B.). R.B. is a Scholar in Clinical Research of the Leukemia and Lymphoma Society.
We are grateful to StemCyte for their generous gift of cord blood samples for these studies. We acknowledge the excellent technical support of Lucy Brown and Claudio Spalla and Alex Spalla, Analytical Cytometry Core, who performed all the cell sorting.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC ection 1734.
Authorship
Contribution: H.M. designed and performed research, collected and analyzed data, and wrote the paper; T.M. and S.C. performed research; J.-K.Y. assisted with data interpretation and reviewed the paper; S.J.F. assisted with data interpretation and reviewed the paper; R.B. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ravi Bhatia, Department of Hematopoietic Stem Cell and Leukemia Research Division of Hematology and HCT, City of Hope National Medical Center, Duarte, CA 91010; e-mail: rbhatia@coh.org.
References
- 1.Rowley JD. A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining [letter]. Nature. 1973;243:290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- 2.de Klein A, van Kessel AG, Grosveld G, et al. A cellular oncogene is translocated to the Philadelphia chromosome in chronic myelocytic leukaemia. Nature. 1982;300:765–767. doi: 10.1038/300765a0. [DOI] [PubMed] [Google Scholar]
- 3.Lugo TG, Pendergast AM, Muller AJ, Witte ON. Tyrosine kinase activity and transformation potency of bcr-abl oncogene products. Science. 1990;247:1079–1082. doi: 10.1126/science.2408149. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Ren R. Bcr-Abl efficiently induces a myeloproliferative disease and production of excess interleukin-3 and granulocyte-macrophage colony-stimulating factor in mice: a novel model for chronic myelogenous leukemia. Blood. 1998;92:3829–3840. [PubMed] [Google Scholar]
- 5.Druker BJ, Tamura S, Buchdunger E, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2:561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 6.O'Brien SG, Guilhot F, Larson RA, et al. Imatinib compared to interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 7.Hughes TP, Kaeda J, Branford S, et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med. 2003;349:1423–1432. doi: 10.1056/NEJMoa030513. [DOI] [PubMed] [Google Scholar]
- 8.Druker BJ, Sawyers CL, Kantarjian H, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001;344:1038–1042. doi: 10.1056/NEJM200104053441402. [DOI] [PubMed] [Google Scholar]
- 9.Issaad C, Ahmed M, Novault S, et al. Biological effects induced by variable levels of BCR-ABL protein in the pluripotent hematopoietic cell line UT-7. Leukemia. 2000;14:662–670. doi: 10.1038/sj.leu.2401730. [DOI] [PubMed] [Google Scholar]
- 10.le Coutre P, Tassi E, Varella-Garcia M, et al. Induction of resistance to the Abelson inhibitor STI571 in human leukemic cells through gene amplification. Blood. 2000;95:1758–1766. [PubMed] [Google Scholar]
- 11.Mahon FX, Deininger MW, Schultheis B, et al. Selection and characterization of BCR-ABL positive cell lines with differential sensitivity to the tyrosine kinase inhibitor STI571: diverse mechanisms of resistance. Blood. 2000;96:1070–1079. [PubMed] [Google Scholar]
- 12.Weisberg E, Griffin JD. Mechanisms of resistance imatinib (STI571) in preclinical models and in leukemia patients. Drug Resist Updat. 2001;4:22–28. doi: 10.1054/drup.2001.0180. [DOI] [PubMed] [Google Scholar]
- 13.Keeshan K, Mills KI, Cotter TG, McKenna SL. Elevated Bcr-Abl expression levels are sufficient for a haematopoietic cell line to acquire a drug-resistant phenotype. Leukemia. 2001;15:1823–1833. doi: 10.1038/sj.leu.2402309. [DOI] [PubMed] [Google Scholar]
- 14.Barnes DJ, Palaiologou D, Panousopoulou E, et al. Bcr-Abl expression levels determine the rate of development of resistance to imatinib mesylate in chronic myeloid leukemia. Cancer Res. 2005;65:8912–8919. doi: 10.1158/0008-5472.CAN-05-0076. [DOI] [PubMed] [Google Scholar]
- 15.Branford S, Rudzki Z, Parkinson I, et al. Real-time quantitative PCR analysis can be used as a primary screen to identify patients with CML treated with imatinib who have BCR-ABL kinase domain mutations. Blood. 2004;104:2926–2932. doi: 10.1182/blood-2004-03-1134. [DOI] [PubMed] [Google Scholar]
- 16.von Bubnoff N, Schneller F, Peschel C, Duyster J. BCR-ABL gene mutations in relation to clinical resistance of Philadelphia-chromosome-positive leukaemia to STI571: a prospective study. Lancet. 2002;359:487–491. doi: 10.1016/S0140-6736(02)07679-1. [DOI] [PubMed] [Google Scholar]
- 17.Shah NP, Nicoll JM, Nagar B, et al. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002;2:117–125. doi: 10.1016/s1535-6108(02)00096-x. [DOI] [PubMed] [Google Scholar]
- 18.Roche-Lestienne C, Soenen-Cornu V, Grardel-Duflos N, et al. Several types of mutations of the Abl gene can be found in chronic myeloid leukemia patients resistant to STI571, and they can pre-exist to the onset of treatment. Blood. 2002;100:1014–1018. doi: 10.1182/blood.v100.3.1014. [DOI] [PubMed] [Google Scholar]
- 19.Hofmann WK, Jones LC, Lemp NA, et al. Ph(+) acute lymphoblastic leukemia resistant to the tyrosine kinase inhibitor STI571 has a unique BCR-ABL gene mutation. Blood. 2002;99:1860–1862. doi: 10.1182/blood.v99.5.1860. [DOI] [PubMed] [Google Scholar]
- 20.Hochhaus A, Kreil S, Corbin AS, et al. Molecular and chromosomal mechanisms of resistance to imatinib (STI571) therapy. Leukemia. 2002;16:2190–2196. doi: 10.1038/sj.leu.2402741. [DOI] [PubMed] [Google Scholar]
- 21.Barthe C, Cony-Makhoul P, Melo JV, Mahon JR. Roots of clinical resistance to STI-571 cancer therapy. Science. 2001;293:2163. doi: 10.1126/science.293.5538.2163a. [DOI] [PubMed] [Google Scholar]
- 22.Gorre ME, Sawyers CL. Molecular mechanisms of resistance to STI571 in chronic myeloid leukemia. Curr Opin Hematol. 2002;9:303–307. doi: 10.1097/00062752-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Shah NP, Sawyers CL. Mechanisms of resistance to STI571 in Philadelphia chromosome-associated leukemias. Oncogene. 2003;22:7389–7395. doi: 10.1038/sj.onc.1206942. [DOI] [PubMed] [Google Scholar]
- 24.Donato NJ, Wu JY, Stapley J, et al. BCR-ABL independence and LYN kinase overexpression in chronic myelogenous leukemia cells selected for resistance to STI571. Blood. 2003;101:690–698. doi: 10.1182/blood.V101.2.690. [DOI] [PubMed] [Google Scholar]
- 25.Donato NJ, Wu JY, Stapley J, et al. Imatinib mesylate resistance through BCR-ABL independence in chronic myelogenous leukemia. Cancer Res. 2004;64:672–677. doi: 10.1158/0008-5472.can-03-1484. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto M, Kakihana K, Kurosu T, Murakami N, Miura O. Clonal evolution with inv(11)(p15q22) and NUP98/DDX10 fusion gene in imatinib-resistant chronic myelogenous leukemia. Cancer Genet Cytogenet. 2005;157:104–108. doi: 10.1016/j.cancergencyto.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 27.Villuendas R, Steegmann JL, Pollan M, et al. Identification of genes involved in imatinib resistance in CML: a gene-expression profiling approach. Leukemia. 2006;20:1047–1054. doi: 10.1038/sj.leu.2404197. [DOI] [PubMed] [Google Scholar]
- 28.Canitrot Y, Lautier D, Laurent G, et al. Mutator phenotype of BCR-ABL transfected Ba/F3 cell lines and its association with enhanced expression of DNA polymerase beta. Oncogene. 1999;18:2676–2680. doi: 10.1038/sj.onc.1202619. [DOI] [PubMed] [Google Scholar]
- 29.Salloukh HF, Laneuville P. Increase in mutant frequencies in mice expressing the BCR-ABL activated tyrosine kinase. Leukemia. 2000;14:1401–1404. doi: 10.1038/sj.leu.2401855. [DOI] [PubMed] [Google Scholar]
- 30.Slupianek A, Nowicki MO, Koptyra M, Skorski T. BCR/ABL modifies the kinetics and fidelity of DNA double-strand breaks repair in hematopoietic cells. DNA Repair (Amst) 2006;5:243–250. doi: 10.1016/j.dnarep.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramaraj P, Singh H, Niu N, et al. Effect of mutational inactivation of tyrosine kinase activity on BCR/ABL-induced abnormalities in cell growth and adhesion in human hematopoietic progenitors. Cancer Res. 2004;64:5322–5331. doi: 10.1158/0008-5472.CAN-03-3656. [DOI] [PubMed] [Google Scholar]
- 32.Chu S, Xu H, Shah NP, et al. Detection of BCR-ABL kinase mutations in CD34+ cells from chronic myelogenous leukemia patients in complete cytogenetic remission on imatinib mesylate treatment. Blood. 2005;105:2093–2098. doi: 10.1182/blood-2004-03-1114. [DOI] [PubMed] [Google Scholar]
- 33.Jiang Y, Zhao RC, Verfaillie CM. Abnormal integrin-mediated regulation of chronic myelogenous leukemia CD34+ cell proliferation: BCR/ABL up-regulates the cyclin-dependent kinase inhibitor, p27Kip, which is relocated to the cell cytoplasm and incapable of regulating cdk2 activity. Proc Natl Acad Sci U S A. 2000;97:10538–10543. doi: 10.1073/pnas.190104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gupta P, McCarthy JB, Verfaillie CM. Stromal fibroblast heparan sulfate is required for cytokine-mediated ex vivo maintenance of human long-term culture-initiating cells. Blood. 1996;87:3229–3236. [PubMed] [Google Scholar]
- 35.Bhatia R, McGlave PB, Dewald GW, Blazar BR, Verfaillie CM. Abnormal function of the bone marrow microenvironment in chronic myelogenous leukemia: role of malignant stromal macrophages. Blood. 1995;85:3636–3645. [PubMed] [Google Scholar]
- 36.Holtz MS, Slovak ML, Zhang F, Sawyers CL, Forman SJ, Bhatia R. Imatinib mesylate (STI571) inhibits growth of primitive malignant progenitors in chronic myelogenous leukemia through reversal of abnormally increased proliferation. Blood. 2002;99:3792–3800. doi: 10.1182/blood.v99.10.3792. [DOI] [PubMed] [Google Scholar]
- 37.Clarkson BD, Strife A, Wisniewski D, Lambek C, Carpino N. New understanding of the pathogenesis of CML: a prototype of early neoplasia. Leukemia. 1997;11:1404–1428. doi: 10.1038/sj.leu.2400751. [DOI] [PubMed] [Google Scholar]
- 38.Gordon MY, Dazzi F, Marley SB, et al. Cell biology of CML cells. Leukemia. 1999;13(suppl 1):S65–S71. doi: 10.1038/sj.leu.2401281. [DOI] [PubMed] [Google Scholar]
- 39.Gesbert F, Griffin JD. Bcr/Abl activates transcription of the Bcl-X gene through STAT5. Blood. 2000;96:2269–2276. [PubMed] [Google Scholar]
- 40.Aichberger KJ, Mayerhofer M, Krauth MT, et al. Identification of mcl-1 as a BCR/ABL-dependent target in chronic myeloid leukemia (CML): evidence for cooperative antileukemic effects of imatinib and mcl-1 antisense oligonucleotides. Blood. 2005;105:3303–3311. doi: 10.1182/blood-2004-02-0749. [DOI] [PubMed] [Google Scholar]
- 41.Elmaagacli AH, Beelen DW, Opalka B, Seeber S, Schaefer UW. The amount of BCR-ABL fusion transcripts detected by the real-time quantitative polymerase chain reaction method in patients with Philadelphia chromosome positive chronic myeloid leukemia correlates with the disease stage. Ann Hematol. 2000;79:424–431. doi: 10.1007/s002770000169. [DOI] [PubMed] [Google Scholar]
- 42.Lin F, van Rhee F, Goldman JM, Cross NC. Kinetics of increasing BCR-ABL transcript numbers in chronic myeloid leukemia patients who relapse after bone marrow transplantation. Blood. 1996;87:4473–4478. [PubMed] [Google Scholar]
- 43.Gaiger A, Henn T, Horth E, et al. Increase of bcr-abl chimeric mRNA expression in tumor cells of patients with chronic myeloid leukemia precedes disease progression. Blood. 1995;86:2371–2378. [PubMed] [Google Scholar]
- 44.Guo JQ, Wang JY, Arlinghaus RB. Detection of BCR-ABL proteins in blood cells of benign phase chronic myelogenous leukemia patients. Cancer Res. 1991;51:3048–3051. [PubMed] [Google Scholar]
- 45.Sharma SV, Gajowniczek P, Way IP, et al. A common signaling cascade may underlie “addiction” to the Src, BCR-ABL, and EGF receptor oncogenes. Cancer Cell. 2006;10:425–435. doi: 10.1016/j.ccr.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Notari M, Neviani P, Santhanam R, et al. A MAPK/HNRPK pathway controls BCR/ABL oncogenic potential by regulating MYC mRNA translation. Blood. 2006;107:2507–2516. doi: 10.1182/blood-2005-09-3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holtz MS, Forman SJ, Bhatia R. Nonproliferating CML CD34+ progenitors are resistant to apoptosis induced by a wide range of proapoptotic stimuli. Leukemia. 2005;19:1034–1041. doi: 10.1038/sj.leu.2403724. [DOI] [PubMed] [Google Scholar]
- 48.Jorgensen HG, Allan EK, Jordanides NE, Mountford JC, Holyoake TL. Nilotinib exerts equipotent anti-proliferative effects to imatinib and does not induce apoptosis in CD34+ CML cells. Blood. 2007;109:4016–4019. doi: 10.1182/blood-2006-11-057521. [DOI] [PubMed] [Google Scholar]
- 49.Chu S, Holtz M, Gupta M, Bhatia R. BCR/ABL kinase inhibition by imatinib mesylate enhances MAP kinase activity in chronic myelogenous leukemia CD34+ cells. Blood. 2004;103:3167–3174. doi: 10.1182/blood-2003-04-1271. [DOI] [PubMed] [Google Scholar]
- 50.Jiang X, Z Y, Chan WY, Pang E, Eaves A, Eaves C. Leukemic stem cells of chronic phase CML patients consistently display very high Bcr-Abl transcript levels and reduced responsiveness to imatinib mesylate in addition to generating a rare subset that produce imatinib mesylate-resistant differentiated progeny [abstract]. Blood. 2004;104:204a. [Google Scholar]
- 51.Copland M, Hamilton A, Elrick LJ, et al. Dasatinib (BMS-354825) targets an earlier progenitor population than imatinib in primary CML but does not eliminate the quiescent fraction. Blood. 2006;107:4532–4539. doi: 10.1182/blood-2005-07-2947. [DOI] [PubMed] [Google Scholar]