Abstract
The increasing incidence of serious infections because of Gram-positive pathogens and the rising cost in parenteral administration of antimicrobials has inspired the development of a novel antibiotic. Dalbavancin is the first once a week antibiotic with activity against a broad range of Gram-positive pathogens. A large multicentre, pivotal, Phase III clinical trial, which included 854 patients with complicated skin and skin structure infections, compared 1–2 doses of dalbavancin vs. linezolid. The results demonstrated non-inferiority and a comparable safety profile. With its unique pharmacokinetic profile, ease of use and excellent safety profile, dalbavancin should provide a valuable addition to the armamentarium used to treat infections because of Gram-positive cocci.
Review Criteria
Dalbavancin, with its unique pharmacokinetic profile, ease of use and excellent safety profile should be a valuable addition to the antimicrobial armamentarium used to treat infections because of Gram-positive cocci.
Introduction
The emergence and spread of antimicrobial resistant organisms has made it increasingly difficult to treat serious Gram-positive infections and has dictated the need to develop new antimicrobial agents. Infections because of antimicrobial resistant pathogens have been associated with increased length of stay, healthcare costs, morbidity and mortality (1, 2). Studies have validated the association between increased mortality among critically ill patients and inappropriate antimicrobial selection, with resistance being the primary reason for inappropriate therapy (3, 4). There have been escalating rates of resistance over the last two decades, especially among the Gram-positive pathogens such as Staphylococcus spp., enterococci and streptococci. The efficacy of penicillinase-resistant penicillins, vancomycin and teicoplanin, once the foundation for the treatment of multidrug resistant Gram-positive pathogens, is challenged daily.
The development of glycopeptide-resistant pathogens was initially identified in the late 1980s, when vancomycin-resistant enterococci (VRE) first emerged in hospitals. More recently in 1995, Staphylococcus aureus strains with increased vancomycin minimum inhibitory concentrations (MICs) were reported in the USA (5). Soon after, a heterogeneous vancomycin-intermediate Staphylococcus aureus (VISA) strain was identified in Japan in 1996. In 2002, the first vancomycin-resistant Staphylococcus aureus (VRSA) strain was reported in the USA. To date, there have been six VRSA isolates reported worldwide; all six have been reported in the USA, four of which have been reported in south-east Michigan (6–12). Vancomycin has long been considered the drug of choice for the treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections. Its modest efficacy, coupled with increasing reports of treatment failures as a result of elevated vancomycin MICs seen in a proportionally greater number of isolates, has made it increasingly important to find an alternative agent which is effective in the treatment of resistant Gram-positive infections.
Dalbavancin (formerly BI397) is a novel semisynthetic glycopeptide that was engineered to be an improved alternative to the naturally available glycopeptides, vancomycin and teicoplanin. Preliminary in vitro assays and animal models have demonstrated it to be more active than vancomycin or teicoplanin against Gram-positive bacteria. It is anticipated to be approved by the Food and Drug Administration in the 1st quarter of 2007.
Mechanisms of action and structure
Dalbavancin is characterised as a second-generation bactericidal glycopeptide. Other examples of the glycopeptide class include vancomycin, teicoplanin, oritavancin (formerly LY-333328) and telavancin (formerly TD-6424). Like other glycopeptides, dalbavancin's mechanism of action involves the formation of a complex with the C-terminal d-alanyl-d-alanine of growing peptidoglycan chains, thereby inhibiting bacterial cell wall biosynthesis (13). In addition, dalbavancin appears to have the unique ability to dimerise and anchor its lipophilic side chain in the bacterial membranes (14). This is hypothesised to increase the affinity of dalbavancin for its target and to increase its antimicrobial potency. Consequently, dalbavancin possesses more potent in vitro bactericidal activity than vancomycin or teicoplanin against many resistant Gram-positive organisms such as MRSA (14, 15).
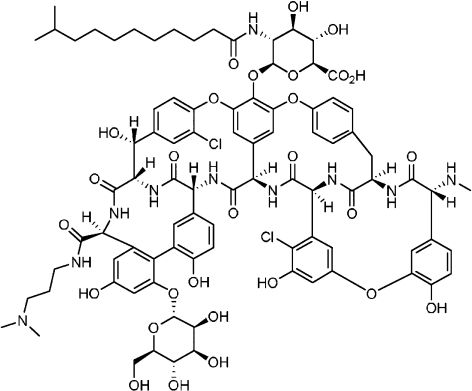
Originally developed by Vicuron Pharmaceuticals Inc., (Fremont, CA, USA) dalbavancin (Figure 1) was chemically derived from parent compound A-40926, a naturally occurring teicoplanin-like glycopeptide produced by the actinomycete Nonomuria spp. Modifications of the parent compound included derivatization of functional groups such as the C-terminus and N-terminus of the peptide, removal of sugars and the addition of acyl moieties (15).
Figure 1.
Chemical structure of dalbavancin
Pharmacokinetics
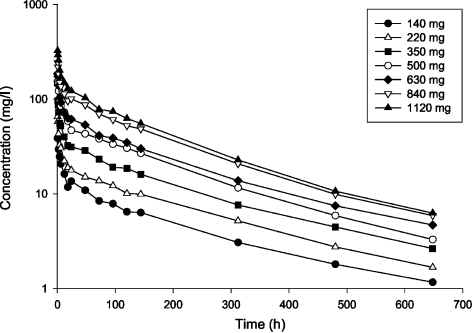
The pharmacokinetics of dalbavancin has been studied in healthy volunteers, and in renally and hepatically impaired subjects. Early Phase I, II and III clinical trials were used to determine pharmacokinetic parameters. Immediately following the end of infusion, maximum concentrations of dalbavancin are achieved. The drug initially distributes into a volume of approximately 8–12 l. Dalbavancin exhibits linear, dose-dependent pharmacokinetics in healthy adults, following the administration of single intravenous doses of dalbavancin 140–1120 mg (Figure 2). The plasma pharmacokinetic profiles are characterised by a rapid decline over 12 h during the distribution phase, followed by a slower terminal elimination phase. It has a half-life of 170–210 h, making once-weekly dosing feasible for dalbavancin (16, 17).
Figure 2.
Mean dalbavancin concentrations in plasma following administration of a single 30-min intravenous infusion (n = 3 per group) (17)
Total protein binding of dalbavancin is concentration independent, reversible and estimated to be 93% (18). Animal studies regarding tissue distribution have demonstrated tissue concentrations reaching maximal levels within 24 h, with the highest concentrations in the liver and kidneys. Two weeks after administration of the drug, more than 1% of the radioactivity was still present in the liver, kidneys, brown fat, skin and skeletal muscle (19).
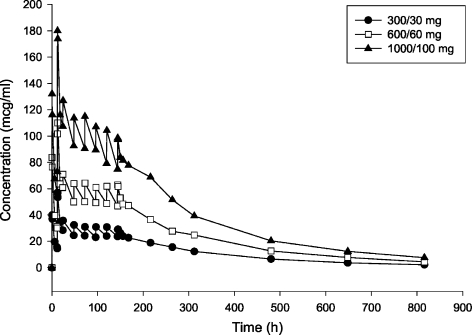
Dalbavancin has been administered and studied in healthy subjects using loading doses of 300–1000 mg given over 30 min (Figure 3), followed by a dose of daily 100 mg/day for 6 days (17). In addition, dalbavancin has also been evaluated using a two-dose regimen in clinical trials (1100 mg as a single intravenous infusion given over 30 min, or a 1000 mg loading dose followed by 500 mg intravenously 1 week later) (20).
Figure 3.
Mean dalbavancin concentrations in plasma following administration of multiple 30-min intravenous infusion doses (n = 3 per group) (17)
Plasma concentrations were determined in a Phase II, randomised, controlled, open-label study of skin and soft-tissue infections (SSTI) caused by Gram-positive pathogens. Subjects that received a single dose of dalbavancin (1100 mg) were able to sustain total plasma concentrations of 30 μg/ml for approximately 1 week. Subjects that received 1000 mg on day 1, followed by 500 mg on day 8 were able to sustain total plasma concentrations of 20 μg/ml for about 20 days. The dalbavancin plasma concentrations were at least equal to the MIC90 values for Gram-positive pathogens 12 days after the second dose, despite the high degree of plasma protein binding. Following a single 1000 mg dose of dalbavancin, penetration into blister fluid was 60%. Blister fluid concentrations of 40 μg/ml are well above the MIC90s of Gram-positive pathogens, and these concentrations are maintained for up to 1 week (21). Although data evaluating dalbavancin's activity against enterococci is scarce, dalbavancin demonstrates excellent in vitro bactericidal activity against staphylococci and streptococci (22–26).
Dalbavancin is not a substrate, inducer or inhibitor of hepatic cytochrome p450 isoenzymes. Forty per cent is eliminated via the renal route. Most of the drug is excreted as intact drug. Concentration was unchanged in patients with mild renal impairment, but further studies are needed to evaluate patients with severe renal impairment. In addition, animal studies have demonstrated that up to 50% of the dalbavancin is excreted into faeces via bile. Total drug clearance, which is influenced by body surface area and the central volume of distribution, is estimated to be approximately 0.04 l/h in healthy adults.
No adjustments are needed in hepatic insufficiency, as concentrations of the drug do not increase with severe hepatic impairment (27, 28). Age, race, gender and serum albumin had no effect on the pharmacokinetics of dalbavancin in clinical trials. At this point, it is still unknown if the drug penetrates the cerebrospinal fluid, or whether the drug is removed during haemodialysis. However, the high protein binding of dalbavancin would suggest both of these scenarios to be unlikely.
In vitro studies
Dalbavancin has a spectrum of activity similar to other glycopeptides, demonstrating bactericidal activity against a variety of Gram-positive pathogens (17, 22, 29–31). Thus far, dalbavancin appears to be more active in vitro than either teicoplanin, vancomycin, linezolid or quinupristin/dalfopristin against all tested Staphylococcus spp. (Tables 1 and 2). In a recent survey of over 1100 MRSA clinical isolates, the MIC50 of dalbavancin was 0.06 μg/ml, compared with 1 μg/ml for vancomycin, and 0.5 μg/ml for teicoplanin. Similar activity was demonstrated against methicillin-resistant coagulase-negative staphylococci (CoNS) with an MIC90 0.06 μg/ml, compared with an MIC90 of 2 μg/ml for vancomycin and 4 μg/ml for teicoplanin. Against isolates with increased MICs to vancomycin and teicoplanin (glycopeptide intermediate Staphylococcus aureus), dalbavancin demonstrates an MIC range of 0.06–1 μg/ml (29). Against linezolid non-susceptible S. aureus, dalbavancin activity is maintained with MICs ranging from 0.03 to 0.06 μg/ml (31). Dalbavancin has also been shown to be active against one of the VRSA strains isolated in the USA (MIC 0.5 μg/ml) (32, 33).
Table 1.
In vitro activity of dalbavancin against Gram-positive and anaerobic organisms
| Organism | Isolates (n) | MIC90 (μg/ml) | MIC range (μg/ml) |
|---|---|---|---|
| Staphylococci | |||
| Quin/dalfo resistant (38) | 8 | NA | 0.03–0.06 |
| Vancomycin intermediate (38) | 10 | 0.06 | 0.06–2 |
| Staphylococcus aureus (25, 39, 40, 42) | 4243 | 0.06 | ≤ 0.008–0.5 |
| Methicillin susceptible (25, 27, 40–44, 47, 48) | 4838 | 0.06–0.5 | ≤ 0.008–0.5 |
| Methicillin resistant (25, 27, 40–44, 47, 48) | 2726 | 0.06–1 | ≤ 0.015–1 |
| Glycopeptide intermediate (25, 41) | 29 | 1–2 | 0.06–16 |
| Linezolid non-susceptible (25) | 5 | NA | 0.03–0.06 |
| Staphylococcus coagulase negative (25, 38, 40, 42) | 1775 | 0.06–0.12 | ≤ 0.008–1 |
| Methicillin susceptible (25, 27, 40–44, 47, 48) | 682 | 0.06–0.5 | ≤ 0.008–0.6 |
| Methicillin resistant (25, 27, 40–44, 47, 48) | 2100 | 0.06–0.5 | ≤ 0.008–1 |
| Vancomycin non-susceptible (25) | 11 | 1 | 0.25–2 |
| Teicoplanin resistant (38) | 15 | 0.25 | 0.03–0.25 |
| Staphylococcus epidermidis | |||
| Methicillin susceptible (27, 41) | 13 | 0.25–0.5 | ≤ 0.03–0.25 |
| Methicillin resistant (27, 41) | 12 | 0.25 | ≤ 0.03–1 |
| Staphylococcus haemolyticus | |||
| Methicillin susceptible (27) | 10 | 0.13 | ≤ 0.03–0.25 |
| Methicillin resistant (27) | 12 | 0.5 | ≤ 0.03–4 |
| Streptococcus pneumoniae (25, 40, 42, 44, 46) | 1422 | ≤ 0.03–0.06 | 0.004–0.125 |
| Penicillin susceptible (25, 27, 40, 42, 48) | 1647 | 0.016–0.06 | 0.004–0.06 |
| Penicillin non-susceptible (25, 27, 38, 40, 42, 48) | 969* | ≤ 0.016–0.03 | ≤ 0.008–0.25 |
| Ceftriaxone resistant (38) | 16 | ≤ 0.016 | ≤ 0.016–0.03 |
| Streptococcus pyogenes (25, 27) | 211 | 0.015 | ≤ 0.002–0.06 |
| Erythromycin susceptible (25) | 161 | 0.015 | ≤ 0.002–0.06 |
| Erythromycin resistant (25) | 45 | 0.015 | ≤ 0.002–0.06 |
| Viridans group streptococci (25, 40, 42, 44) | 313 | 0.016–0.03 | ≤ 0.002–0.06 |
| Penicillin susceptible (25, 48) | 130 | 0.03 | ≤ 0.002–0.06 |
| Penicillin non-susceptible (25, 27, 48) | 6† | 0.03 | ≤ 0.008–0.06 |
| Erythromycin susceptible (24) | 21 | 0.03 | ≤ 0.002–0.03 |
| Erythromycin resistant (25) | 31 | 0.03 | ≤ 0.002–0.06 |
| β-Haemolytic streptococci (25, 40, 42, 44, 48) | 757 | 0.015–0.06 | ≤ 0.002–0.25 |
| Streptococcus agalactiae (25) | 52 | 0.015 | 0.008–0.06 |
| Enterococcus spp. (40, 42) | 2062 | 0.12–16 | ≤ 0.008 to > 16 |
| Vancomycin susceptible (27, 40, 42, 44) | 1606 | 0.06–0.5 | ≤ 0.008–1 |
| Vancomycin resistant (39, 40, 42, 44) | 592 | > 16–32 | ≤ 0.015 to > 32 |
| vanA resistant (27, 38) | 79 | 32 to > 128 | 0.03 to > 128 |
| vanB resistant (27, 38) | 21 | 0.12–1 | 0.02–2 |
| Linezolid resistant (39) | 9 | NA | ≤ 0.015 to > 32 |
| Enterococcus faecalis (48) | |||
| Vancomycin susceptible (48) | 586 | 0.06 | ≤ 0.015–4 |
| Vancomycin resistant (38, 48) | 34 | 32 | ≤ 0.015 to > 32 |
| Enterococcus faecium | |||
| Vancomycin susceptible (48) | 77 | 0.12 | ≤ 0.015–4 |
| Vancomycin resistant (38, 48) | 92 | 32 | 0.03 to > 32 |
| Quin/dalfo resistant (38) | 29 | 0.12‡–8§ | ≤ 0.016 to > 32 |
| Actinomyces spp. (28) | 38 | 0.5 | 0.03–0.5 |
| Bacillus spp. (40, 44) | 25 | 0.12–0.25 | 0.016–2 |
| Clostridium spp. (28) | 16 | 0.5 | ≤ 0.015–1 |
| Clostridium difficile (28) | 26 | 0.25 | 0.125–0.5 |
| Clostridium perfringens (28) | 10 | 0.125 | 0.03–0.125 |
| Corynebacterium spp. (28, 40, 44) | 51 | ≤ 0.03–0.5 | ≤ 0.015–1 |
| Corynebacterium jeikeium (28, 44) | 20 | 0.5 | ≤ 0.03–0.5 |
| Lactobacillus spp. (28) | 23 | > 32 | 0.06 to > 32 |
| Listeria spp. (48) | NA | 0.06 | NA |
| Micrococcus spp. (40) | 13 | 0.03 | ≤ 0.008–0.03 |
| Peptostreptococcus spp. (28) | 30 | 0.25 | ≤ 0.015–0.5 |
| Propionibacterium spp. (28) | 15 | 0.5 | 0.03–0.5 |
Permission for reprint granted by Ann Pharmocother; 2006; 40: 449–60.
Includes penicillin-non-susceptible, penicillin-intermediate and penicillin-resistant isolates.
Includes penicillin-non-susceptible and penicillin-resistant isolates.
vanA negative isolates.
vanA positive isolates. MIC, minimum inhibitory concentration; NA, not available; quin/dalfo, quinupristin/dalfopristin; vanA, vancomycin-resistant enterococci possessing the vanA gene.
Table 2.
Comparative MICs of dalbavancin and other antimicrobials against selected Gram-positive and anaerobic organisms
| MIC90 range (μg/ml)* | ||||||
|---|---|---|---|---|---|---|
| Organism | Dalbavancin | Vancomycin | Linezolid | Teicoplanin | Quin/dalfo | Daptomycin |
| Staphylococcus aureus (25, 40, 42) | 0.06 | 1 | ≤ 2 | 2 | 0.5 | NA |
| Methicillin susceptible (25, 27, 41, 43, 44, 47, 48) | 0.06–0.5 | 1 | 1–4 | 2–4 | 0.25–0.5 | 0.5 |
| Methicillin resistant (25, 27, 41, 43, 44, 47, 48) | 0.06–1 | 1–4 | 0.5–8 | 2–4 | 0.5 | 0.5 |
| Glycopeptide intermediate (25, 39, 41) | 1–2 | 8 | 8–16 | 2 | 1 | NA |
| Staphylococcus coagulase negative (25, 40, 42) | 0.06–0.12 | 2 | 4–8 | 1–2 | 0.5 | NA |
| Methicillin susceptible† (25, 27, 43, 44, 47, 48) | 0.06–0.5 | 2 | 2–8 | 1–2 | 0.25–0.5 | 0.5 |
| Methicillin resistant† (25, 27, 43, 44, 47, 48) | 0.06–0.5 | 2–4 | 2–16 | 1–2 | 0.5–1 | 0.5 |
| Vancomycin non-susceptible (25) | 1 | 8 | > 32 | 2 | 0.5 | NA |
| Teicoplanin resistant (39, 45) | 0.25 | 2 | NA | 1 | 1 | NA |
| Staphylococcus epidermidis | ||||||
| Methicillin susceptible (27, 41) | 0.25–0.5 | 1–2 | 8 | NA | NA | NA |
| Methicillin resistant (27, 41) | 0.25 | 2–4 | 16 | NA | NA | NA |
| Staphylococcus haemolyticus | ||||||
| Methicillin susceptible (27) | 0.13 | 2 | 32 | NA | NA | 2 |
| Methicillin resistant (27) | 0.5 | 4 | 32 | NA | NA | NA |
| Streptococcus pneumoniae (25, 40, 42, 44, 46) | < 0.03–0.06 | 0.5 | 0.125 to ≤ 2 | 1–2 | < 0.5–1 | NA |
| Penicillin susceptible (25, 27, 48) | 0.03–0.06 | 0.5 | 0.06 | 1 | 0.5 | NA |
| Penicillin non-susceptible‡ (25, 27, 38, 48) | ≤ 0.016–0.03 | 0.5 | 0.06 | 1 | 0.5–1 | NA |
| Streptococcus pyogenes (25) | 0.015 | 0.5 | 0.06 | 1 | ≤ 0.12 | NA |
| Erythromycin susceptible (25) | 0.015 | 0.5 | 0.06 | 1 | ≤ 0.12 | NA |
| Erythromycin resistant (25) | 0.015 | 0.5 | 0.06 | 1 | ≤ 0.12 | NA |
| Viridans group streptococci (25, 40, 42, 44) | 0.016–0.03 | 1 | ≤ 2 | 1 | 0.5–1 | NA |
| Penicillin susceptible (25, 48) | 0.03 | 1 | 0.06 | 1 | 1 | NA |
| Penicillin non-susceptible (25, 48) | 0.03 | 0.5–1 | 0.12 | 1 | 1 | NA |
| Erythromycin susceptible (25) | 0.03 | 1 | 0.06 | 1 | 1 | NA |
| Erythromycin resistant (25) | 0.03 | 1 | 0.12 | 1 | 1 | NA |
| β-Haemolytic streptococci (25, 40, 42, 44, 48) | 0.015–0.06 | 0.5 | ≤ 2 | 1 | 0.5 | NA |
| Streptococcus agalactiae (25) | 0.015 | 0.5 | 0.12 | 1 | 0.25 | NA |
| Enterococcus spp. (40, 42) | 0.12–16 | 2 to > 16 | ≤ 2 to > 16 | 2 | > 2 | NA |
| Vancomycin susceptible (44) | 0.5 | 2 | 0.5 | NA | > 8 | NA |
| Vancomycin resistant (44) | 32 | > 16 | > 16 | NA | > 8 | NA |
| vanA resistant (27, 38) | 32 to > 128 | > 128 | > 128 | NA | NA | NA |
| vanB resistant (27) | 0.12–1 | 128 | ≤ 2 | 2 | 8 | NA |
| Enterococcus faecalis | ||||||
| Vancomycin susceptible (48) | 0.06 | NA | 0.5 | 2 | > 8 | NA |
| Vancomycin resistant (38, 48) | 32 | NA | > 16 | 2 | > 8 | NA |
| Enterococcus faecium | ||||||
| Vancomycin susceptible (48) | 0.12 | NA | 0.5 | 2 | 2 | NA |
| Vancomycin resistant (38, 48) | 32 | NA | > 16 | 2 | 1 | NA |
| Quin/dalfo resistant (38) | 0.12§–8¶ | NA | NA | 2 | NA | NA |
| Actinomyces spp. (28) | 0.5 | 1 | NA | 1 | 0.25 | 16 |
| Bacillus spp. (44) | 0.25 | 1 | 2 | NA | 2 | NA |
| Clostridium spp. (28) | 0.5 | 2 | NA | 4 | 0.5 | 8 |
| Clostridium difficile (28) | 0.25 | 2 | NA | 8 | 4 | 2 |
| Clostridium perfringens(28) | 0.125 | 0.5 | NA | 2 | 0.5 | 1 |
| Corynebacterium spp.(28, 44, 48) | ≤ 0.03–0.5 | 0.5–1 | 0.5 | 1 | 0.5–1 | 8 |
| Corynebacterium jeikeium (28) | 0.5 | 0.5 | NA | 0.5 | 0.5 | 0.25 |
| Lactobacillus spp. (48) | > 32 | > 32 | NA | 8 | 2 | > 32 |
| Listeria spp. (40) | 0.06 | NA | NA | NA | NA | NA |
| Peptostreptococcus spp. (28) | 0.25 | 0.5 | NA | 2 | 0.5 | 1 |
| Propionibacterium spp. (28) | 0.5 | 1 | NA | 1 | 0.2 | 16 |
Permission for reprint granted by Ann Pharmocother; 2006; 40: 449–60.
MIC90 range based on MIC90 values reported in different studies that compared dalbavancin with at least one of the comparator agents.
Data from Ref. (27) includes other coagulase-negative staphylococci, but do not include Staphylococcus epidermidis and Staphylococcus haemolyticus.
Includes penicillin-non-susceptible, penicillin-intermediate and penicillin-resistant isolates.
vanA negative isolates.
vanA positive isolates. MICs, minimum inhibitory concentrations; NA, not available; quin/dalfo, quinupristin/dalfopristin; vanA, vancomycin-resistant enterococci possessing the vanA gene.
In pharmacodynamic studies by Lin et al., dalbavancin demonstrated time-kill kinetics against staphylococci that was similar to those of vancomycin and teicoplanin. It exhibited bactericidal activity after 24 h, at four times the MIC (33).
Against Streptococcus spp., dalbavancin is as active as teicoplanin and 4–8 times more active than vancomycin with MIC90 values ranging from 0.03 to 0.06 μg/ml. Dalbavancin has also shown lower MICs for penicillin-resistant and ceftriaxone-resistant isolates of Streptococcus pneumoniae and viridans-group Streptococci, β-haemolytic Streptococci and Streptococcus agalactiae when compared with the activity of either vancomycin, teicoplanin, linezolid or quinupristin/dalfopristin (14, 30).
Dalbavancin inhibits vancomycin-susceptible and resistant enterococcal strains, (MIC range: 0.03–0.12 μg/ml), but has poor activity against vancomycin-resistant (vanA) enterococci (MIC90 value 32 to > 128 μg/ml). This lack of activity against VRE strains that contain the vanA gene differentiates dalbavancin from the other investigational glycopeptides, oritavancin and telavancin. Oritavancin and telavancin have a second mechanism of action, the transglycosylation of the peptidoglycan, which appears to explain their activity against the vanA containing Enterococci (30). It is unknown why dalbavancin shows activity against vanA containing VRSA, but not VRE strains which contain the vanA gene. Possible differences in cell wall between enterococci and staphylococci may need to be explored.
Dalbavancin has variable activity against other pathogens, such as Lactobacillus spp. Its activity against corynebacteria is comparable with the activity of vancomycin. It also has potent activity against some Gram-positive anaerobes and fastidious aerobes including Actinomyces spp., Propionibacterium spp., and Clostriudium spp. excluding Clostridium clostridioforme. Dalbavancin has minimal activity against Gram-negative bacteria, including Gram-negative anaerobic bacilli (34).
Susceptibility breakpoints have not yet been established for dalbavancin. However, the proposed ranges are 0.008–0.03 μg/ml for S. pneumoniae and 0.03–0.12 μg/ml for both Enterococcus faecalis and S. aureus.
In vitro assays have evaluated the potential for spontaneous generation of resistance in vivo. One-step resistance assays in S. aureus have not detected any resistance against dalbavancin. After serial passage, bacterial populations were more homogeneous in their susceptibility to dalbavancin than to vancomycin or teicoplanin. Several investigators have concluded that the selection of dalbavancin resistance might be less likely to develop than resistance in either teicoplanin or vancomycin (35).
In vitro studies
Dalbavancin has been studied extensively in animal models and has successfully demonstrated efficacy in infections caused by MRSA in the rodent pouch model, against penicillin-resistant S. pneumoniae in the lobar pneumonia infections model and against MRSA in the rodent endocarditis model (30, 36, 37).
A single daily dose of dalbavancin was equal to or more active than twice the daily dose of either teicoplanin or vancomycin against staphylococci in experimental endocarditis in rats and in septicaemia models in immunocompetent and neutropenic mice. In addition, dose-dependant killing of MRSA in the rodent endocarditis model was demonstrated with the once-daily dalbavancin (10 mg/kg for 4 days) (30).
Dalbavancin was compared with vancomycin in an attempt to prevent S. aureus colonisation of devices in vivo in a rabbit model. While not statistically significant, there was a trend towards a lower rate of device colonisation with dalbavancin when compared with either vancomycin (p = 0.07) or saline (p = 0.20) (38).
Animal studies using the granuloma pouch model were also important in selecting the once a week dosing in human infections as the most appropriate dosing interval. Dose-dependent reduction of bacterial load and prolonged suppression of regrowth of bacteria were demonstrated. Administration of 2.5, 5 and 10 mg/kg dosages of dalbavancin were administered. No reduction of MRSA bacterial load was observed following the administration of 2.5 mg/kg. A 1 log10 cfu/ml reduction in bacterial load was observed following the administration of 5 mg/kg, and a > 2 log10 cfu/ml reduction was seen following the administration of 10 mg/kg. In addition, bacterial regrowth was inhibited for more than 96 h following treatment with dalbavancin (37).
Clinical efficacy
Two open-label, Phase II clinical trials have been published. Seltzer et al. (20, 39) compared once-weekly dalbavancin vs. standard-of-care antimicrobial therapy for the treatment of SSTI. In this study, patients with a creatinine clearance of <50 ml/min, self-limited infections, compromised vascularity, documented osteomyelitis or glycopeptide hypersensitivity were excluded. Sixty-two adult patients were enrolled and randomly assigned to receive one of three treatment arms: dalbavancin 1100 mg as a single i.v. infusion, dalbavancin 1000 mg i.v., followed by 500 mg i.v. 1 week later, or a defined standard of care antimicrobial (first generation cephalosporin, piperacillin/tazobactam, clindamycin, vancomycin or linezolid, alone or in combination). Patients were allowed to receive additional Gram-negative aerobic or anaerobic coverage if deemed necessary.
The majority of patients had a documented diagnosis of either a deep or complicated infection (> 90%) and most had infections that required surgical drainage (70%). Forty-one (66.1%) patients had one or more pathogens detected at baseline cultures. S. aureus was the most prevalent organism (34/41 pts; 83%), 50% of the S. aureus were MRSA in the dalbavancin group, compared with 20% in the comparator group. Although the numbers are small, analysis of 51 clinically evaluable patients demonstrated clinical success in 16 of 17 (94%) patients treated with two doses of dalbavancin, eight of 13 (62%) treated with one dose of dalbavancin, and 16 of 21 (76%) patients treated with the comparator. The two-dose dalbavancin arm appeared to demonstrate a more favourable response in patients infected with MRSA. Eradication rates of S. aureus among microbiologically evaluable patients were higher in the two-dose dalbavancin group (90%; 9/10 pts.) than in the one-dose dalbavancin group (50%; 5/10 pts.) or in the comparator (60%; 6/10 pts). This suggested that the two-dose regimen of dalbavancin, administered 1 week apart, appears to be more effective than the single-dose dalbavancin or the comparator regimen in the treatment of complicated Gram-positive SSTIs. However, because of the study's small sample size, statistical analysis was not performed (39).
In a second study, Raad et al. (40) conducted a Phase II, open-label, randomised, multicentre clinical trial evaluating dalbavancin vs. vancomycin in adult patients with catheter-related bloodstream infections (CR-BSIs). Dalbavancin was administered as a 1000 mg intravenous loading dose, followed by a 500 mg intravenous dose 1 week later and compared with a 14-day course of intravenous vancomycin at 1000 mg twice daily. Catheter removal was required in all instances of confirmed S. aureus infections. For CoNS, management was at the discretion of the investigator, although catheter removal was recommended. Of the 54 isolates in the 51 patients, the most common pathogens identified in the confirmed intention to treat (microITT) population were CoNS (26 isolates), S. aureus (23 isolates, of which 14 were MRSA) and E. faecalis (five isolates). MRSA was encountered more frequently among isolates in the vancomycin group, nine of 28 (32%), than in the dalbavancin group, five of 26 (19.2%). In the microITT population, overall success rates, defined as the sum of clinical and microbiological success were assessed 18–24 days after the end of therapy. Dalbavancin was superior to vancomycin (p < 0.05) in the microITT population (87% success in the dalbavancin group vs. 50% in the vancomycin group). However, the small number of patients with MRSA infection who received dalbavancin renders it difficult to evaluate the significance of these numbers (40).
Three Phase III clinical trials have been completed evaluating dalbavancin in patients with both uncomplicated or complicated skin and skin structure infections (SSSIs). The New Drug Application (NDA), which was submitted in December, 2004, included results from all three of these clinical trials and included more than 1850 subjects. The results of two of these Phase III trials were recently presented in abstract form (41). In both the phase III clinical trials, each trial met the primary and secondary end-points of non-inferiority when compared with linezolid, cefazolin or vancomycin, three commonly used agents for SSSIs. The most common pathogen isolated in these studies was S. aureus.
In a recently published Phase III trial, Juregui et al. (42) compared once-weekly dalbavancin vs. twice-daily linezolid for the treatment of complicated SSSIs. Eight hundred fifty-four patients were randomised in a double-blind manner (ratio 2 : 1) to receive either dalbavancin (1000 mg administered intravenously on day 1 and 500 mg intravenously on day 8) or linezolid (600 mg administered intravenously or orally every 12 h for 14 days). MRSA was identified in 51% of patients from whom a pathogen was isolated at baseline. Dalbavancin and linezolid demonstrated comparable clinical efficacy in the clinically evaluable population at the test-of-cure visit (88.9% and 91.2% success respectively). The rate of clinical success at the end of therapy was > 90% in both arms. Less than 1.0% of patients in either treatment arm experienced a relapse after the test-of-cure visit. In the microbiologically evaluable patients, microbiological success rates for dalbavancin (89.5%) and linezolid (87.5%) were comparable at the test-of-cure visit. The study met its objective of non-inferiority and demonstrated that two doses of dalbavancin (1000 mg given on day 1 followed by 500 mg given on day 8) were as effective as linezolid given twice daily for 14 days for the treatment of patients with complicated SSSI, including those infected with MRSA (42).
In another Phase III clinical trial, 565 patients were enrolled into the study comparing dalbavancin vs. intravenous cefazolin, followed by oral cephalexin for the treatment of uncomplicated SSSIs. The primary end-point was clinical response at the follow-up visit in the evaluable patient population. Evaluable patients on either dalbavancin or cefazolin demonstrated an 89.1% response vs. an 89.1% response [95% confidence interval (CI); −6.8, 6.8]. In the ITT group, patients on dalbavancin patients showed a 76.0% response rate vs. a 75.8% response rate for those patients receiving cefazolin (95% CI; −7.7, 8.2) (41).
A third Phase III clinical trial was conducted in patients suffering from SSSIs suspected or confirmed to be caused by MRSA. The study was a controlled, open-labelled study and enrolled 156 patients. Patients were randomised to either dalbavancin or vancomycin. Evaluable patients on the dalbavancin arm demonstrated an 89.9% response rate, compared with an 86.7% response rate for vancomycin (95% CI; −13.0, 19.4). In the ITT group, patients that received dalbavancin demonstrated an 86.0% response rate vs. a 65.3% response rate for vancomycin (95% CI; 4.3, 37.0) (41).
Safety and tolerability
Dalbavancin appears to be well tolerated in animal studies, Phase I, II and III clinical trials. At this time, there is no evidence of dose or duration-related toxicities. In randomised, double-blind, placebo-controlled, single- and multiple-dose, dose-escalation studies in healthy adult male and female subjects, dalbavancin was well tolerated without serious adverse events or deaths. In the clinical trials thus far, adverse events have been reported in 67% of subjects and classified as mild in severity. The most commonly reported adverse events included pyrexia (50%), headache (25%) and nausea (6%) (19). In clinical trials thus far, subjects receiving placebo reported similar rates of pyrexia (38%) and headaches (31%). Laboratory findings, physical examinations and electrocardiograms were unchanged from baseline. No auditory or vestibular toxicity was observed in those patients who received dalbavancin dosages as high as 1120 mg or cumulative doses of 1600 mg administered over a 1-week period, respectively (43).
In a separate clinical trial published by Seltzer et al. (39), 62 subjects treated for SSTIs reported drug-related adverse events in 11/20 (55%) patients who received a single dose (1100 mg) of dalbavancin, 10/21 (48%) patients who received two doses (1000 mg on day 1 and 500 mg on day 8) of dalbavancin, and in 12/21 (57%) patients who received a comparator regimen. Laboratory data was unchanged from baseline. In 33 patients with CR-BSIs who received dalbavancin (1000 mg on day 1 and 500 mg on day 8) in the study reported by Raad et al. (40), the most commonly reported, drug-related adverse events were oral candidiasis (12.1%; n = 4), diarrhoea (21.2%; n = 7), constipation (18.2%; n = 6) and pyrexia (18.2%; n = 6). There were no study withdraws or discontinuation of dalbavancin because of any adverse events.
The safety profile reported from the only published Phase III clinical trial also corroborates the relatively good safety profile previously demonstrated by dalbavancin in its other clinical trials (Table 3). Juregui et al. (42) reported the findings of 854 patients that were randomised to receive either dalbavancin or linezolid for the treatment of complicated SSSIs. Overall, the study doses were well tolerated with relatively few side effects. The type and severity of adverse events were comparable between the two groups. Adverse events were more commonly reported in the linezolid group (32.2%) than in the dalbavancin group (25.4%). Gastrointestinal symptoms (e.g. nausea 3.2%, diarrhoea 2.5% and vomiting 1.9%) were the most commonly reported adverse events. There were no cases of red man syndrome reported and few reports of infusion site reactions. Discontinuation rates for each group were similar, 3.9% for dalbavancin and 3.2% for linezolid. Three serious adverse events were reported. One patient in the dalbavancin group developed mild leucopenia which resolved spontaneously. Two patients in the linezolid group experienced a severe adverse event, one patient developed moderate thrombocytopenia, which resolved spontaneously, and one patient developed severe pancytopenia which resolved with treatment.
Table 3.
Adverse events with dalbavacin Phase III clinical trial of 854 patients (42)
| Percentage of patients | ||
|---|---|---|
| Adverse event | Dalbavancin arm (n = 571) | Linezolid arm (n = 283) |
| Any event | 25.4 | 32.2 |
| Nausea | 3.2 | 5.3 |
| Diarrhoea | 2.5 | 5.7 |
| Elevated blood lactate dehydrogenase level | 1.9 | 1.8 |
| Headache | 1.9 | 1.8 |
| Elevated –γ glutamyltransferase level | 1.9 | 1.4 |
| Vomiting | 1.9 | 1.1 |
| Rash | 1.8 | 1.8 |
| Abnormal liver function test results | 1.6 | 1.1 |
| Elevated alanine aminotransferase level | 1.2 | 1.8 |
| Fungal vaginosis | 0.9 | 1.8 |
| Loose stools | 0.4 | 2.1 |
| Thrombocytopenia | 0.2 | 2.5 |
Drug–drug interactions
Dalbavancin does not appear to be metabolised by the cytochrome P450 enzyme system. The administration of cytochrome P450 substrates, inhibitors or inducers do not affect dalbavancin's clearance rates. No drug–drug interactions have been identified. Furthermore, it is unknown whether dalbavancin has any cross-reactivity with glycopeptides as patients with a history of hypersensitivity have been excluded from these clinical trials. Recently, the in vitro drug interaction between dalbavancin in combination with nine different antimicrobial agents (clindamycin, daptomycin, gentamicin, levofloxacin, linezolid, oxacillin, quinupristin/dalfopristin, rifampin and vancomycin) was evaluated for either synergistic or antagonistic interactions. Antagonism was not observed between dalbavancin and any of the nine antimicrobials tested. In addition, there was no evidence of synergy observed between gentamicin and dalbavancin. However, dalbavancin and oxacillin appear to have some degree of synergy or partial synergy against staphylococci, including methicillin-resistant strains, VISA and enterococci. Further testing is needed to determine the clinical significance of these findings (44).
Conclusions
The increase in infections because of the Gram-positive organisms has been described worldwide and across all age groups. Recent outcome studies have demonstrated that a Gram-positive infection may increase the hospital length of stay from 7 to 28 days, thus adding to the rising cost of healthcare. In part, the increasing cost of hospitalisation is frequently for the administration of parenteral antimicrobial agents. Dalbavancin is a novel second generation glycolipopeptide, with excellent activity against a broad spectrum of Gram-positive organisms, including some of the more resistant strains (45–47). Furthermore, dalbavancin's uniqueness is its novel pharmacokinetic profile with a half-life of 170–210 h, which makes the once-weekly dosing optimal. In general, three Phase III studies in subjects with SSTI have been completed. One large, pivotal Phase III study in patients with complicated SSTI demonstrated comparable clinical efficacy vs. linezolid.
Dalbavancin's unique half-life, as well as its excellent activity against Gram-positive organisms, should provide a valuable and economical addition to the current antimicrobial armamentarium used to manage infections because of Gram-positive pathogens. As a new agent dalbavancin should be used judiciously, where a clinical or cost benefit would be anticipated. Possible clinical use of dalbavancin for the treatment of SSSIs and other approved indications could include the following: patients seen in the emergency department that do not require hospital monitoring, completion of inpatient therapy to allow for earlier hospital discharge, patients in whom medical compliance would be an issue, and certain parenteral home-therapy cases. Further studies will need to be performed to determine whether dalbavancin may prove to be a useful alternative to parenteral antimicrobials that are currently used to treat infections that necessitate long courses of therapy such as endocarditis, septic arthritis or osteomyelitis.
References
- 1.Ibrahim EH, et al. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest. 2000;118:146–55. doi: 10.1378/chest.118.1.146. [DOI] [PubMed] [Google Scholar]
- 2.Lodise TP. Clinical and economic impact of methicillin resistance in patients with Staphylococcus aureus bacteremia. Diagn Microbiol Infect Dis. 2005;52:113–22. doi: 10.1016/j.diagmicrobio.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Carmeli Y, et al. Health and economic outcomes of vancomycin-resistant enterococci. Arch Intern Med. 2002;162:2223–8. doi: 10.1001/archinte.162.19.2223. [DOI] [PubMed] [Google Scholar]
- 4.Capitano B, et al. Cost effect of managing methicillin-resistant Staphylococcus aureus in a long-term care facility. J Am Geriatr Soc. 2003;51:10–6. doi: 10.1034/j.1601-5215.2002.51003.x. [DOI] [PubMed] [Google Scholar]
- 5.Sabath L. Cryptic vancomycin-resistant staphylococci (CV-RS) as a cause of treatment failure in Staphylococcus aureus bacteremia. 35th Interscience Conference on Antimicrobial Agents and Chemotherapy; 17–20 September, 1995; San Francisco, CA. (Abstract #LM22) [Google Scholar]
- 6.Hiramatsu K, et al. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet. 1997;350:1670–3. doi: 10.1016/S0140-6736(97)07324-8. [DOI] [PubMed] [Google Scholar]
- 7.Tenover FC, et al. Characterization of staphylococci with reduced susceptibilities to vancomycin and other glycopeptides. J Clin Microbiol. 1998;36:1020–7. doi: 10.1128/jcm.36.4.1020-1027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang S, et al. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N Engl J Med. 2003;348:1342–7. doi: 10.1056/NEJMoa025025. [DOI] [PubMed] [Google Scholar]
- 9.Tiemersma EW, et al. Methicillin-resistant Staphylococcus aureus in Europe, 1999–2002. Emerg Infect Dis. 2004;10:1627–34. doi: 10.3201/eid1009.040069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kacica M. Brief report: vancomycin-resistant Staphylococcus aureus– New York, 2004. MMWR Morb Mortal Wkly Rep. 2004;53:322–3. [PubMed] [Google Scholar]
- 11.Tenover FC, et al. Vancomycin-resistant Staphylococcus aureus isolate from a patient in Pennsylvania. Antimicrob Agents Chemother. 2004;48:275–80. doi: 10.1128/AAC.48.1.275-280.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rudrick JT. [February 2007];Michigan Department of Community Health. http://www.michigan.gov/documents/VRSA_Feb05_HAN_118391_7.pdf.
- 13.Ciabatti R. Semisynthetic glycopeptides: chemistry, structure-activity relationships and prospects. Farmaco. 1997;52:313–21. [PubMed] [Google Scholar]
- 14.Streit JM, et al. Worldwide assessment of dalbavancin activity and spectrum against over 6000 clinical isolates. Diagn Microbiol Infect Dis. 2004;48:137–43. doi: 10.1016/j.diagmicrobio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Malabarba A. Glycopeptide derivatives. Curr Med Chem. 2001;8:1759–73. doi: 10.2174/0929867013371716. [DOI] [PubMed] [Google Scholar]
- 16.Buckwalter M. Population pharmacokinetic analysis of dalbavancin, a novel lipoglycopeptide. J Clin Pharmacol. 2005;45:1279–87. doi: 10.1177/0091270005280378. [DOI] [PubMed] [Google Scholar]
- 17.Leighton A, et al. Tolerability, pharmacokinetics, and serum bactericidal activity of intravenous dalbavancin in healthy volunteers. Antimicrob Agents Chemother. 2004;48:940–5. doi: 10.1128/AAC.48.3.940-945.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dowell JA, et al. The pharmacokinetics and renal excretion of dalbavancin in healthy subjects. 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy; 26–30 September, 2002; San Diego, CA. (Abstract #A-1386) [Google Scholar]
- 19.Cavaleri M, et al. Pharmacokinetics and excretion of dalbavancin in the rat. Antimicrob Chemother. 2005;55(Suppl. 2):ii31–5. doi: 10.1093/jac/dki006. [DOI] [PubMed] [Google Scholar]
- 20.Seltzer E, et al. Once-weekly dalbavancin versus standard-of-care antimicrobial regimens for treatment of skin and soft-tissue infections. Clin Infect Dis. 2003;37:1298–303. doi: 10.1086/379015. [DOI] [PubMed] [Google Scholar]
- 21.Dowell JA, et al. Dalbavancin penetration into skin supports once-weekly dosing. 15th European Congress of Clinical Microbiology and Infectious Diseases; 2005; Copenhagen, Denmark. (Abstract 895) [Google Scholar]
- 22.Andes DR. In vivo pharmacodynamic characterization of dalbavancin (DAL) in the murine thigh infection model. 44th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2004, 30 October–3 November; Washington, DC. (Abstract #A-1872) [Google Scholar]
- 23.Hackbarth CJ, et al. In vitro activity of the glycopeptide BI 397 against Staphylococcus aureus and Staphylococcus epidermidis. 39th Inter-science Conference on Antimicrobial Agents and Chemotherapy; 26–29 September, 1999; San Francisco, CA. (Abstract #1283) [Google Scholar]
- 24.Gales AC. Antimicrobial activity of dalbavancin tested against gram-positive clinical isolates from Latin American medical centres. Clin Microbiol Infect. 2005;11:95–100. doi: 10.1111/j.1469-0691.2004.01051.x. [DOI] [PubMed] [Google Scholar]
- 25.Lin G, et al. Antipneumococcal activity of dalbavancin compared to other agents. Antimicrob Agents Chemother. 2005;49:5182–4. doi: 10.1128/AAC.49.12.5182-5184.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dowell JA, et al. The pharmacokinetics of dalbavancin in subjects with mild, moderate or severe hepatic impairment. 44th Interscience Conference on Antimicrobial Agents and Chemotherapy; 30 October–3 November, 2004; Washington, DC. (Abstract #A-19) [Google Scholar]
- 27.White RJ, et al. Glycopeptide: phase 1 single and multiple-dose placebo controlled intravenous safety, pharmacokinetic, and pharmacodynamic study in healthy subjects. 40th Interscience Conference on Antimicrobial Agents and Chemotherapy; 17–20 September, 2000; Toronto, ON. (Abstract #682) [Google Scholar]
- 28.Streit JM, et al. Worldwide assessment of dalbavancin activity and spectrum against over 6000 clinical isolates. Diagn Microbiol Infect Dis. 2004;48:137–43. doi: 10.1016/j.diagmicrobio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Candiani G, et al. In vitro and in vivo antibacterial activity of BI 397, a new semi-synthetic glycopeptide antibiotic. J Antimicrob Chemother. 1999;44:179–92. doi: 10.1093/jac/44.2.179. [DOI] [PubMed] [Google Scholar]
- 30.Mushtaq S, et al. Activity of dalbavancin against staphylococci and streptococci, assessed by BSAC and NCCLS agar dilution methods. J Antimicrob Chemother. 2004;54:617–20. doi: 10.1093/jac/dkh401. [DOI] [PubMed] [Google Scholar]
- 31.Bozdogan B, et al. Antibacterial susceptibility of a vancomycin-resistant Staphylococcus aureus strain isolated at the Hershey Medical Center. J Antimicrob Chemother. 2003;52:864–8. doi: 10.1093/jac/dkg457. [DOI] [PubMed] [Google Scholar]
- 32.Lin G, et al. Antistaphylococcal activity of dalbavancin, an experimental glycopeptide. Antimicrob Agents Chemother. 2005;49:770–2. doi: 10.1128/AAC.49.2.770-772.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Appelbaum PC, et al. Comparative activities of dalbavancin (DAL) against staphylococci, including a vancomycin-resistant strain of Staphylococcus aureus. 41st Interscience Conference on Antimicrobial Agents and Chemotherapy; 9–12 October, 2003; San Diego, CA. (Abstract #173) [Google Scholar]
- 34.Goldstein EJ, et al. In-vitro activities of dalbavancin and nine comparator agents against anaerobic Gram-positive species and corynebacteria. Antimicrob Agents Chemother. 2003;47:1968–71. doi: 10.1128/AAC.47.6.1968-1971.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopez S, et al. In vitro antistaphylococcal activity of dalbavancin, a novel glycopeptide. J Antimicrob Chemother. 2005;55(Suppl. 2):ii21–4. doi: 10.1093/jac/dki007. [DOI] [PubMed] [Google Scholar]
- 36.Candiani GP, et al. Efficacy of a single dalbavancin (DA) dose compared with multiple linezolid (LN) doses against penicillin-resistant pneumococci (PRSP) in a lobar pneumonia (LP) model in the immunocompetent rat (IR). 41st Interscience Conference on Antimicrobial Agents and Chemotherapy; 16–19 December, 2001; Chicago, IL. (Abstract #B-989) [Google Scholar]
- 37.Jabes D, et al. Efficacy of dalbavancin against methicillin-resistant Staphylococcus aureus in the rat granuloma pouch infection model. Antimicrob Agents Chemother. 2004;48:1118–23. doi: 10.1128/AAC.48.4.1118-1123.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Darouiche RO. Dalbavancin compared with vancomycin for prevention of Staphylococcus aureus colonization of devices in vivo. J Infect. 2005;50:206–9. doi: 10.1016/j.jinf.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 39.Seltzer E, et al. Dalbavancin Skin and Soft-Tissue Infection Study Group. Once-weekly dalbavancin versus standard-of-care antimicrobial regimens for treatment of skin and soft-tissue infections. Clin Infect Dis. 2003;37:1298–303. doi: 10.1086/379015. [DOI] [PubMed] [Google Scholar]
- 40.Raad I, et al. Efficacy and safety of weekly dalbavancin therapy for catheter-related bloodstream infection caused by gram-positive pathogens. Clin Infect Dis. 2005;40:374–80. doi: 10.1086/427283. [DOI] [PubMed] [Google Scholar]
- 41.Goldstein B, et al. Dalbavancin phase III skin and skin structure (SSSI) studies: pathogens and microbiological efficacy. 45th Interscience Conference on Antimicrobial Agents and Chemotherapy; 16–19 December, 2005; Washington, DC. (Abstract #L-1577) [Google Scholar]
- 42.Juregui LE, et al. Randomized, double-blind comparison of a once-weekly dalbavancin versus twice-daily linezolid therapy for the treatment of complicated skin and skin structure infections. Clin Infect Dis. 2005;41:1407–15. doi: 10.1086/497271. [DOI] [PubMed] [Google Scholar]
- 43.Campbell KC, et al. Audiologic monitoring for potential ototoxicity in a phase I clinical trial of a new glycopeptide antibiotic. J Am Acad Audiol. 2003;14:157–68. [PubMed] [Google Scholar]
- 44.Johnson D, et al. In vitro evaluation of dalbavancin in combination with other classes of antimicrobial. 105th General Meeting of the American Society for Microbiology; 20–24 May, 2005; Atlanta, GA. (Abstract #97089) [Google Scholar]
- 45.Streit JM, et al. Dalbavancin activity against selected populations of antimicrobial-resistant Gram-positive pathogens. Diagn Microbiol Infect Dis. 2005;53:307–10. doi: 10.1016/j.diagmicrobio.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Jones RN. Dalbavancin (DAL; formerly BI397) activity against selected populations of antimicrobial-resistant gram-positive pathogens. 41st Annual Meeting of the Infectious Disease Society of America; 9–12 October, 2003; San Diego, CA. (Abstract #172) [Google Scholar]
- 47.Jones RN, et al. Comparative activity of dalbavancin tested against 7,771 isolates from the USA and Europe (2003). 44th Interscience Conference on Antimicrobial Agents and Chemotherapy; 30 October–3 November, 2004; Washington, DC. (Abstract #E-2009) [Google Scholar]
- 48.Beauregard DA, et al. Dimerization and membrane anchors in extracellular targeting of vancomycin group antibiotics. Antimicrob Agents Chemother. 1995;39:781–5. doi: 10.1128/AAC.39.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]